Submitted:
20 July 2023
Posted:
24 July 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
Statistical Analysis
Results
Entire cohort
NSCLC cohort
Discussion
Conclusion
Author Contributions
Funding
Study approval number
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Drudge-Coates, L.; Rajbabu, K. Diagnosis and management of malignant spinal cord compression: part 2. Int. J. Palliat. Nurs. 2008, 14, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Robson, P. Metastatic spinal cord compression: a rare but important complication of cancer. Clin. Med. (Lond). 2014, 14, 542–545. [Google Scholar] [CrossRef]
- Perrin, R.G.; Laxton, A.W. Metastatic spine disease: epidemiology, pathophysiology, and evaluation of patients. Neurosurg. Clin. N. Am. 2004, 15, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Cooke, D.; Hayward, C.; Kanellos, F.S.; Tsiouris, A.K.; Chatziantoniou, A.A.; Zakynthinakis-Kyriakou, N.; Karathanasi, A. Metastatic Spinal Cord Compression: Unraveling the Diagnostic and Therapeutic Challenges. Anticancer. Res. 2018, 38, 4987–4997. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Hayward, C.; Cooke, D.; Zakynthinakis-Kyriakou, N.; Tsiouris, A.K.; Chatziantoniou, A.A.; Kanellos, F.S.; Karathanasi, A. Spinal Ewing Sarcoma Debuting with Cord Compression: Have We Discovered the Thread of Ariadne? Anticancer. Res. 2018, 38, 5589–5597. [Google Scholar] [CrossRef]
- Lee, K.; Tsou, I.; Wong, S.; Yu, C.; Ming, Z.; Loh, Y.; Shakespeare, T.; Mukherjee, R.; Back, M. Metastatic spinal cord compression as an oncology emergency: getting our act together. Int. J. Qual. Health. Care. 2007, 19, 377–381. [Google Scholar] [CrossRef]
- Rades, D.; Douglas, S.; Veninga, T.; Stalpers, L.J.; Hoskin, P.J.; Bajrovic, A.; Adamietz, I.A.; Basic, H.; Dunst, J.; Schild, S.E. Validation and simplification of a score predicting survival in patients irradiated for metastatic spinal cord compression. Cancer. 2010, 116, 3670–3673. [Google Scholar] [CrossRef]
- Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine. (Phila Pa 1976). 2005, 30, 2186–2191.
- Levack, P.; Graham, J.; Collie, D.; Grant, R.; Kidd, J.; Kunkler, I.; Gibson, A.; Hurman, D.; McMillan, N.; Rampling, R.; et al. Scottish Cord Compression Study Group. Don't wait for a sensory level--listen to the symptoms: a prospective audit of the delays in diagnosis of malignant cord compression. Clin. Oncol. (R Coll Radiol). 2002, 14, 472–480. [Google Scholar] [CrossRef]
- McLinton A, Hutchison C. Malignant spinal cord compression: a retrospective audit of clinical practice at a UK regional cancer centre. Br. J. Cancer. 2006, 94, 486–491. [CrossRef]
- Loughrey GJ, Collins CD, Todd SM, Brown NM, Johnson RJ. Magnetic resonance imaging in the management of suspected spinal canal disease in patients with known malignancy. Clin. Radiol. 2000, 55, 849–855.
- Husband, D.J.; Grant, K.A.; Romaniuk, C.S. MRI in the diagnosis and treatment of suspected malignant spinal cord compression. Br. J. Radiol. 2001, 74, 15–23. [Google Scholar] [CrossRef]
- 2019 surveillance of metastatic spinal cord compression in adults: risk assessment, diagnosis and management (NICE guideline CG75). National. Institute. For. Health. And. Care. Excellence. (NICE). 2019.
- Rades, D.; Heidenreich, F.; Karstens, J.H. Final results of a prospective study of the prognostic value of the time to develop motor deficits before irradiation in metastatic spinal cord compression. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 975–979. [Google Scholar] [CrossRef]
- Quraishi, N.A.; Esler, C. Metastatic spinal cord compression. BMJ. 2011, 342, d2402. [Google Scholar] [CrossRef] [PubMed]
- Nakata, E.; Sugihara, S.; Sugawara, Y.; Nakahara, R.; Furumatsu, T.; Tetsunaga, T.; Kunisada, T.; Nakanishi, K.; Akezaki, Y.; Ozaki, T. Multidisciplinary treatment system for bone metastases for early diagnosis, treatment and prevention of malignant spinal cord compression. Oncol. Lett. 2020, 19, 3137–3144. [Google Scholar] [CrossRef] [PubMed]
- Souchon, R.; Wenz, F.; Sedlmayer, F.; Budach, W.; Dunst, J.; Feyer, P.; Haase, W.; Harms, W.; Sautter-Bihl, M.L.; Sauer, R.; German Society of Radiation Oncology (DEGRO). DEGRO practice guidelines for palliative radiotherapy of metastatic breast cancer: bone metastases and metastatic spinal cord compression (MSCC). Strahlenther. Onkol. 2009, 185, 417–424. [CrossRef]
- Ribas, E.S.; Schiff, D. Spinal Cord Compression. Curr. Treat. Options. Neurol. 2012, 14, 391–401. [Google Scholar] [CrossRef]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Payne, R.; Saris, S.; Kryscio, R.J.; Mohiuddin, M.; Young, B. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005, 366, 643–648. [Google Scholar] [CrossRef]
- Shah, S.; Kutka, M.; Lees, K.; Abson, C.; Hadaki, M.; Cooke, D.; Neill, C.; Sheriff, M.; Karathanasi, A.; Boussios, S. Management of Metastatic Spinal Cord Compression in Secondary Care: A Practice Reflection from Medway Maritime Hospital, Kent, UK. J. Pers. Med. 2021, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Morgen, S.S.; Lund-Andersen, C.; Larsen, C.F.; Engelholm, S.A.; Dahl, B. Prognosis in patients with symptomatic metastatic spinal cord compression: survival in different cancer diagnosis in a cohort of 2321 patients. Spine. (Phila Pa 1976). 2013, 38, 1362–1367. [CrossRef]
- Tang, Y.; Qu, J.; Wu, J.; Li, S.; Zhou, Y.; Xiao, J. Metastatic Spinal Cord Compression from Non-Small-Cell Lung Cancer Treated with Surgery and Adjuvant Therapies: A Retrospective Analysis of Outcomes and Prognostic Factors in 116 Patients. J. Bone. Joint. Surg. Am. 2015, 97, 1418–1425. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, K.; Cao, S.; Hou, S.; Wang, T.; Guo, W.; Wu, Z.; Jia, Q.; Liu, T.; Xiao, J. Surgical Treatment of Spinal Cord Compression Caused by Metastatic Small Cell Lung Cancer: Ten Years of Experience in a Single Center. Cancer. Manag. Res. 2020, 12, 3571–3578. [Google Scholar] [CrossRef]
- da Silva, G.T.; Bergmann, A.; Santos Thuler, L.C. Prognostic factors in patients with metastatic spinal cord compression secondary to lung cancer: a systematic review of the literature. Eur. Spine. J. 2015, 24, 2107–2113. [Google Scholar] [CrossRef]
- Rades, D.; Weber, A.; Karstens, J.H.; Schild, S.E.; Bartscht, T. Prognostic role of the number of involved extraspinal organs in patients with metastatic spinal cord compression. Clin. Neurol. Neurosurg. 2014, 118, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Conway, R.; Graham, J.; Kidd, J.; Levack, P.; Scottish Cord Compression Group. What happens to people after malignant cord compression? Survival, function, quality of life, emotional well-being and place of care 1 month after diagnosis. Clin. Oncol. (R Coll Radiol). 2007, 19, 56–62. [CrossRef]
- Tabouret, E.; Cauvin, C.; Fuentes, S.; Esterni, B.; Adetchessi, T.; Salem, N.; Madroszyk, A.; Gonçalves, A.; Casalonga, F.; Gravis, G. Reassessment of scoring systems and prognostic factors for metastatic spinal cord compression. Spine J. 2015, 15, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Weigel, B.; Maghsudi, M.; Neumann, C.; Kretschmer, R.; Müller, F.J.; Nerlich, M. Surgical management of symptomatic spinal metastases. Postoperative outcome and quality of life. Spine. (Phila Pa 1976). 1999, 24, 2240–2246. [CrossRef]
- Tokuhashi, Y.; Uei, H.; Oshima, M.; Ajiro, Y. Scoring system for prediction of metastatic spine tumor prognosis. World. J. Orthop. 2014, 5, 262–271. [Google Scholar] [CrossRef]
- Leithner, A.; Radl, R.; Gruber, G.; Hochegger, M.; Leithner, K.; Welkerling, H.; Rehak, P.; Windhager, R. Predictive value of seven preoperative prognostic scoring systems for spinal metastases. Eur. Spine. J. 2008, 17, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Lee, C.S.; Chung, S.S. Surgical results of metastatic spinal cord compression (MSCC) from non-small cell lung cancer (NSCLC): analysis of functional outcome, survival time, and complication. Spine. J. 2016, 16, 322–328. [Google Scholar] [CrossRef]
- Rades, D.; Douglas, S.; Veninga, T.; Bajrovic, A.; Stalpers, L.J.; Hoskin, P.J.; Rudat, V.; Schild, S.E. Metastatic spinal cord compression in non-small cell lung cancer patients. Prognostic factors in a series of 356 patients. Strahlenther. Onkol. 2012, 188, 472–476. [Google Scholar] [CrossRef]
- Bakar, D.; Tanenbaum, J.E.; Phan, K.; Alentado, V.J.; Steinmetz, M.P.; Benzel, E.C.; Mroz, T.E. Decompression surgery for spinal metastases: a systematic review. Neurosurg. Focus. 2016, 41, E2. [Google Scholar] [CrossRef]
- Tancioni, F.; Navarria, P.; Pessina, F.; Attuati, L.; Mancosu, P.; Alloisio, M.; Scorsetti, M.; Santoro, A.; Baena, R.R. Assessment of prognostic factors in patients with metastatic epidural spinal cord compression (MESCC) from solid tumor after surgery plus radiotherapy: a single institution experience. Eur. Spine. J. 2012, 21, S146–S148. [Google Scholar] [CrossRef]
- Rades, D.; Lange, M.; Veninga, T.; Stalpers, L.J.; Bajrovic, A.; Adamietz, I.A.; Rudat, V.; Schild, S.E. Final results of a prospective study comparing the local control of short-course and long-course radiotherapy for metastatic spinal cord compression. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 524–530. [Google Scholar] [CrossRef]
- Chaichana, K.L.; Pendleton, C.; Sciubba, D.M.; Wolinsky, J.P.; Gokaslan, Z.L. Outcome following decompressive surgery for different histological types of metastatic tumors causing epidural spinal cord compression. Clinical article. J. Neurosurg. Spine. 2009, 11, 56–63. [Google Scholar] [CrossRef]
- Silva, G.T.; Bergmann, A.; Thuler, L.C. Incidence, associated factors, and survival in metastatic spinal cord compression secondary to lung cancer. Spine. J. 2015, 15, 1263–1269. [Google Scholar] [CrossRef]
- Ogihara, S.; Seichi, A.; Hozumi, T.; Oka, H.; Ieki, R.; Nakamura, K.; Kondoh, T. Prognostic factors for patients with spinal metastases from lung cancer. Spine. (Phila Pa 1976). 2006, 31, 1585–1590. [CrossRef]
- Meyer, H.S.; Wagner, A.; Raufer, A.; Joerger, A.K.; Gempt, J.; Meyer, B. Surgery in Acute Metastatic Spinal Cord Compression: Timing and Functional Outcome. Cancers. (Basel). 2022, 14, 2249. [Google Scholar] [CrossRef]
- Younsi, A.; Riemann, L.; Scherer, M.; Unterberg, A.; Zweckberger, K. Impact of decompressive laminectomy on the functional outcome of patients with metastatic spinal cord compression and neurological impairment. Clin. Exp. Metastasis. 2020, 37, 377–390. [Google Scholar] [CrossRef]
- Helweg-Larsen, S.; Sørensen, P.S.; Kreiner, S. Prognostic factors in metastatic spinal cord compression: a prospective study using multivariate analysis of variables influencing survival and gait function in 153 patients. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Li, J.; Liu, Y.; Jiang, W.; Liu, S.; Zhou, S. Who are the Best Candidates for Decompressive Surgery and Spine Stabilization in Patients With Metastatic Spinal Cord Compression?: A New Scoring System. Spine. (Phila Pa 1976). 2016, 41, 1469–1476. [CrossRef]
- Lei, M.; Liu, Y.; Liu, S.; Wang, L.; Zhou, S.; Zhou, J. Individual strategy for lung cancer patients with metastatic spinal cord compression. Eur. J. Surg. Oncol. 2016, 42, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Liu, Y.; Yan, L.; Tang, C.; Yang, S.; Liu, S. A validated preoperative score predicting survival and functional outcome in lung cancer patients operated with posterior decompression and stabilization for metastatic spinal cord compression. Eur. Spine. J. 2016, 25, 3971–3978. [Google Scholar] [CrossRef]
- Reinmuth, N.; Meister, M.; Muley, T.; Steins, M.; Kreuter, M.; Herth, F.J.; Hoffmann, H.; Dienemann, H.; Thomas, M. Molecular determinants of response to RTK-targeting agents in nonsmall cell lung cancer. Int. J. Cancer. 2006, 119, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Carper, M.B.; Claudio, P.P. Clinical potential of gene mutations in lung cancer. Clin. Transl. Med. 2015, 4, 33. [Google Scholar] [CrossRef]
- Chen, X.; Xu, B.; Li, Q.; Xu, X.; Li, X.; You, X.; Yu, Z. Genetic profile of non-small cell lung cancer (NSCLC): A hospital-based survey in Jinhua. Mol. Genet. Genomic. Med. 2020, 8, e1398. [Google Scholar] [CrossRef]
- Oldenburger, E.; Brown, S.; Willmann, J.; van der Velden, J.M.; Spałek, M.; van der Linden, Y.M.; Kazmierska, J.; Menten, J.; Andratschke, N.; Hoskin, P. ESTRO ACROP guidelines for external beam radiotherapy of patients with complicated bone metastases. Radiother. Oncol. 2022, 173, 240–253. [Google Scholar] [CrossRef]
- Bilsky, M.H.; Laufer, I.; Fourney, D.R.; Groff, M.; Schmidt, M.H.; Varga, P.P.; Vrionis, F.D.; Yamada, Y.; Gerszten, P.C.; Kuklo, T.R. Reliability analysis of the epidural spinal cord compression scale. J. Neurosurg. Spine. 2010, 13, 324–328. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | N |
Median OS (months) |
Crude estimates | Adjusted estimates | ||
| HR (95% CI) | P | HR (95% CI) | P | |||
|
Age ≤55 years > 55 years |
29 45 |
5.6 5.1 |
0.67 (0.39 – 1.16) 1 |
0.153 |
Not included |
|
|
Gender Female Male |
29 45 |
5.8 5.1 |
0.50 (0.28 – 0.90) 1 |
0.021 |
0.97 (0.46 – 2.03) 1 |
0.934 |
|
Smoker Yes No |
54 20 |
5.1 7.0 |
2.83 (1.38 – 5.84) 1 |
0.005 |
1.00 (0.39 – 2.55) 1 |
0.997 |
|
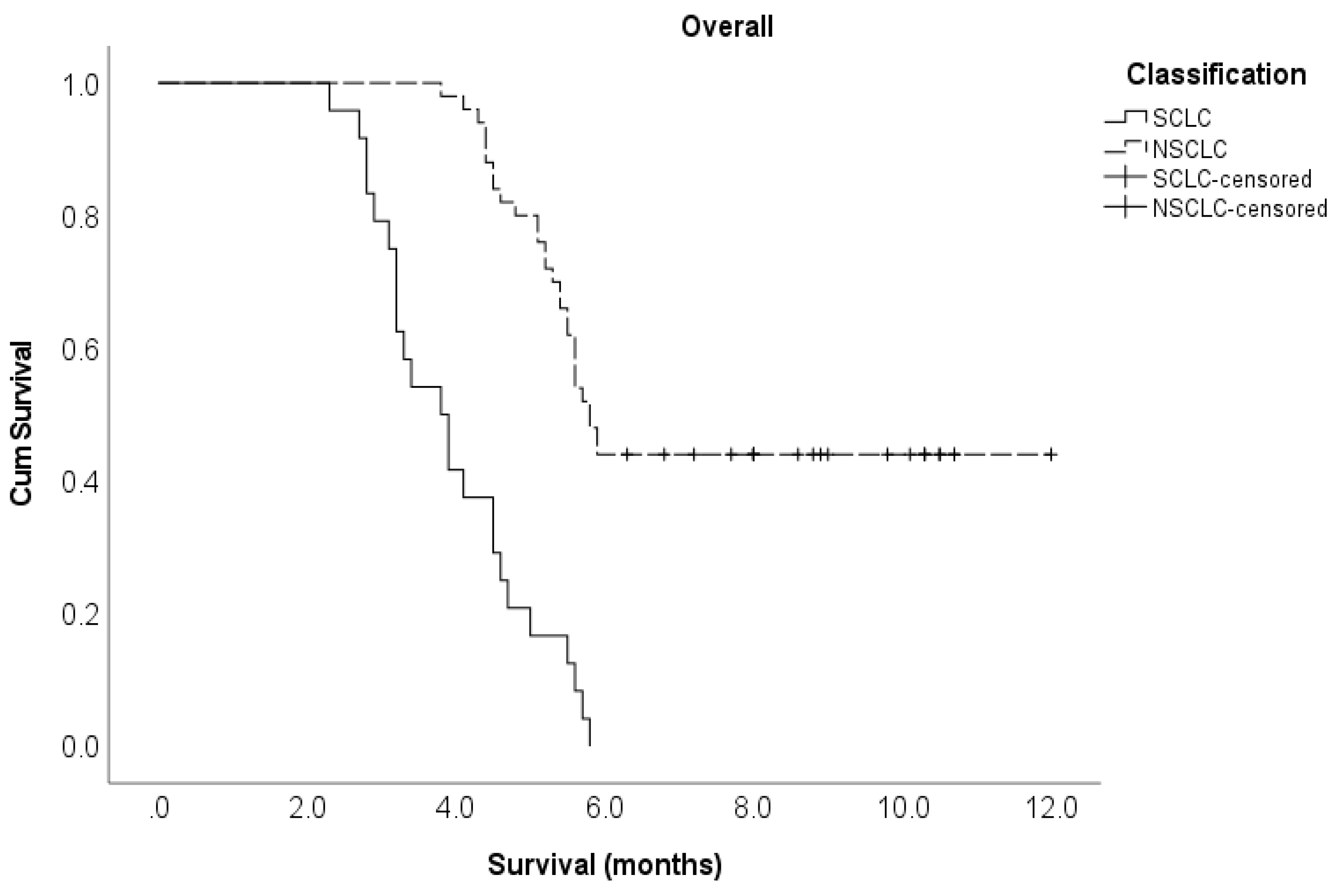
Lung cancer subtype Non-small cell Small cell |
50 24 |
5.8 3.9 |
0.18 (0.10 – 0.32) 1 |
<0.001 |
0.18 (0.08 – 0.39) 1 |
<0.001 |
|
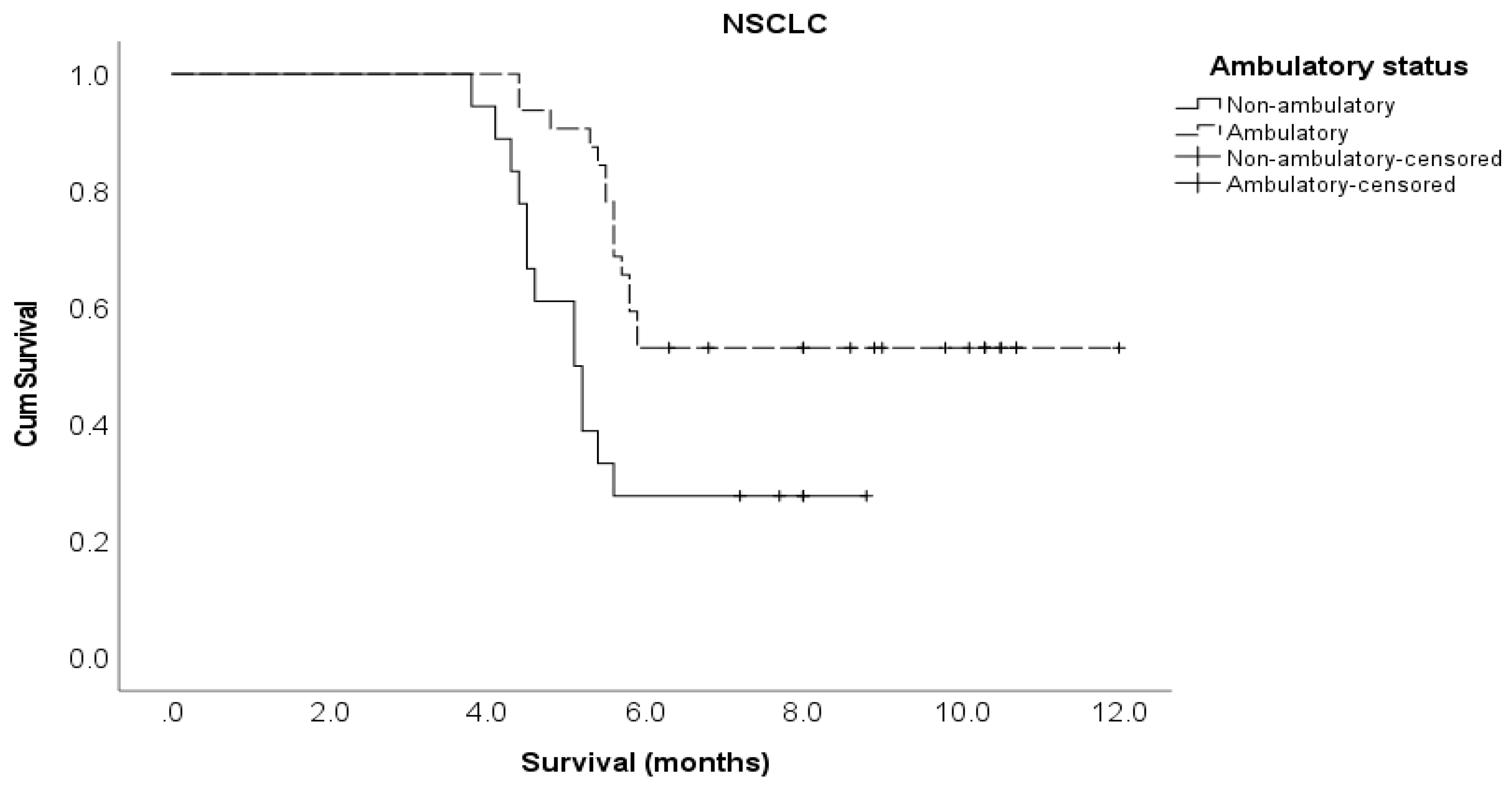
Pre-treatment ambulatory status Ambulatory Not ambulatory |
41 33 |
5.8 4.5 |
0.37 (0.21 – 0.65) 1 |
<0.001 |
0.53 (0.26 – 1.12) 1 |
0.096 |
|
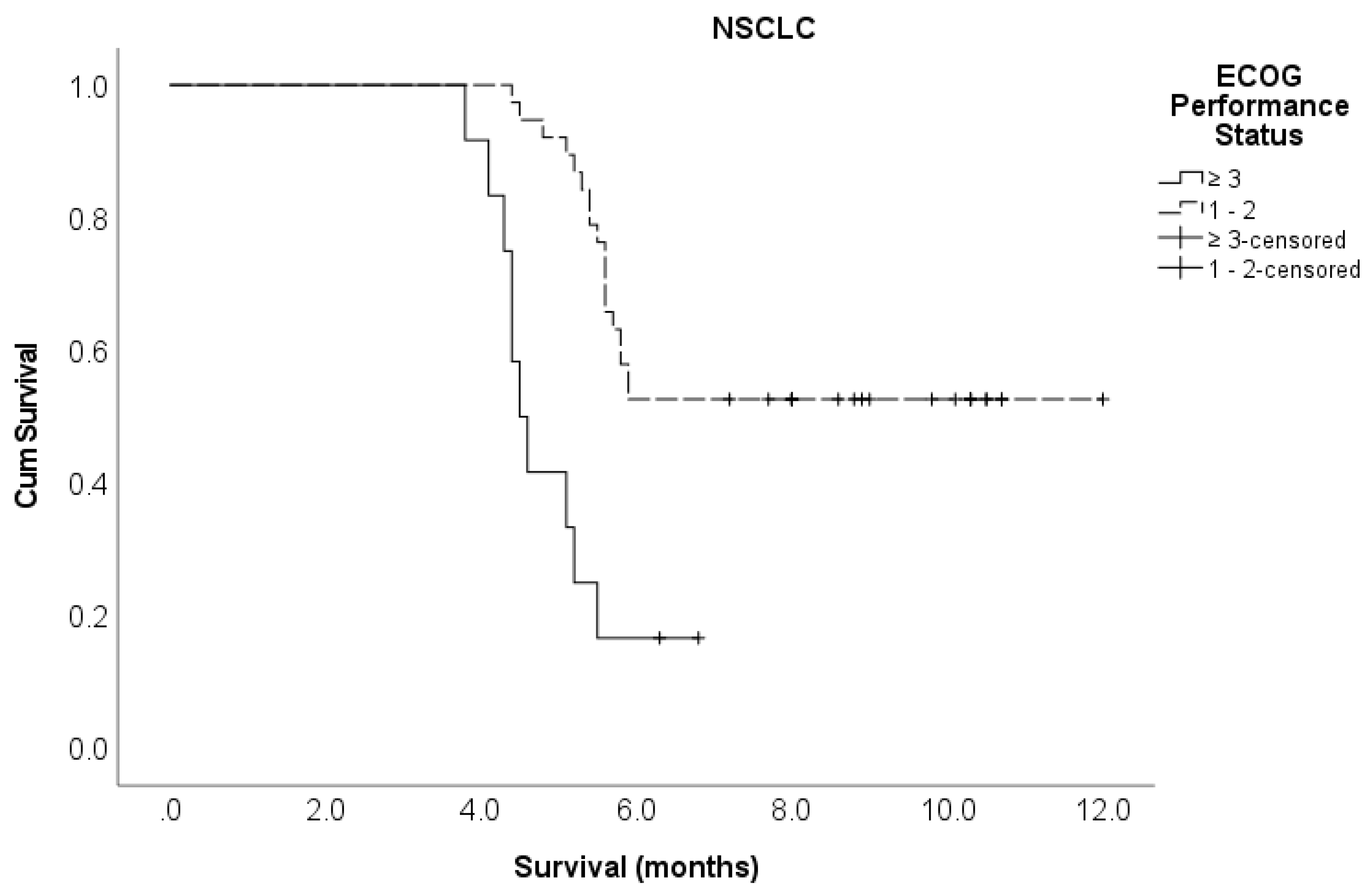
ECOG PS 1–2 ≥ 3 |
50 24 |
5.7 4.2 |
0.27 (0.16 – 0.49) 1 |
<0.001 |
0.78 (0.35 – 1.77) 1 |
0.556 |
|
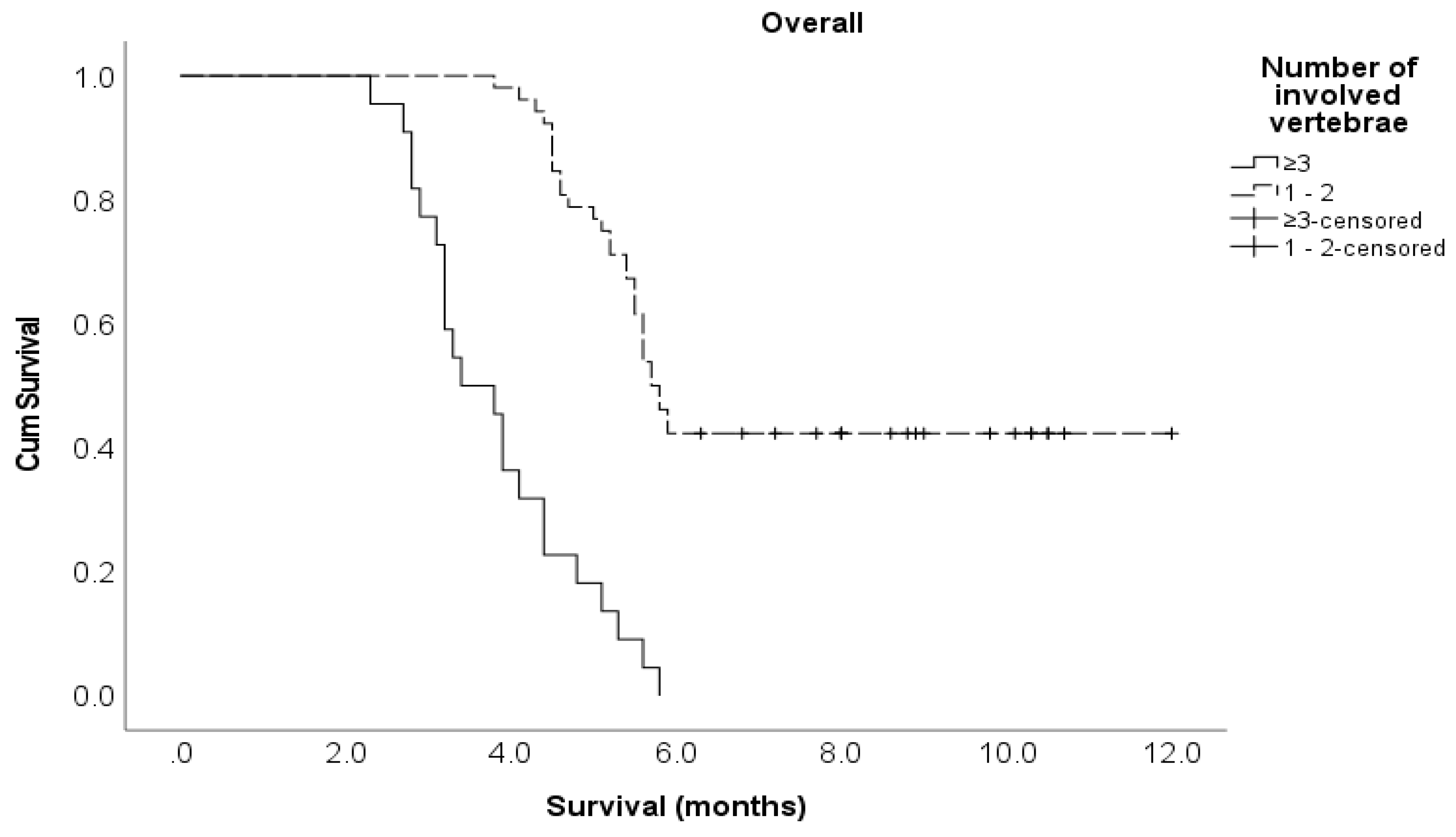
Number of involved vertebrae 1–2 ≥ 3 |
52 22 |
5.8 3.6 |
0.15 (0.09 – 0.28) 1 |
<0.001 |
0.36 (0.15 – 0.87) 1 |
0.024 |
|
Pre-treatment chemotherapy Yes No |
34 40 |
7.0 4.5 |
0.24 (0.13 – 0.45) 1 |
<0.001 |
0.72 (0.33 – 1.58) 1 |
0.411 |
|
Interval from suspicion of MSCC to MRI ≤ 24 hours > 24 hours |
33 41 |
5.9 4.6 |
0.37 (0.20 – 0.66) 1 |
<0.001 |
0.60 (0.29 – 1.24) 1 |
0.165 |
|
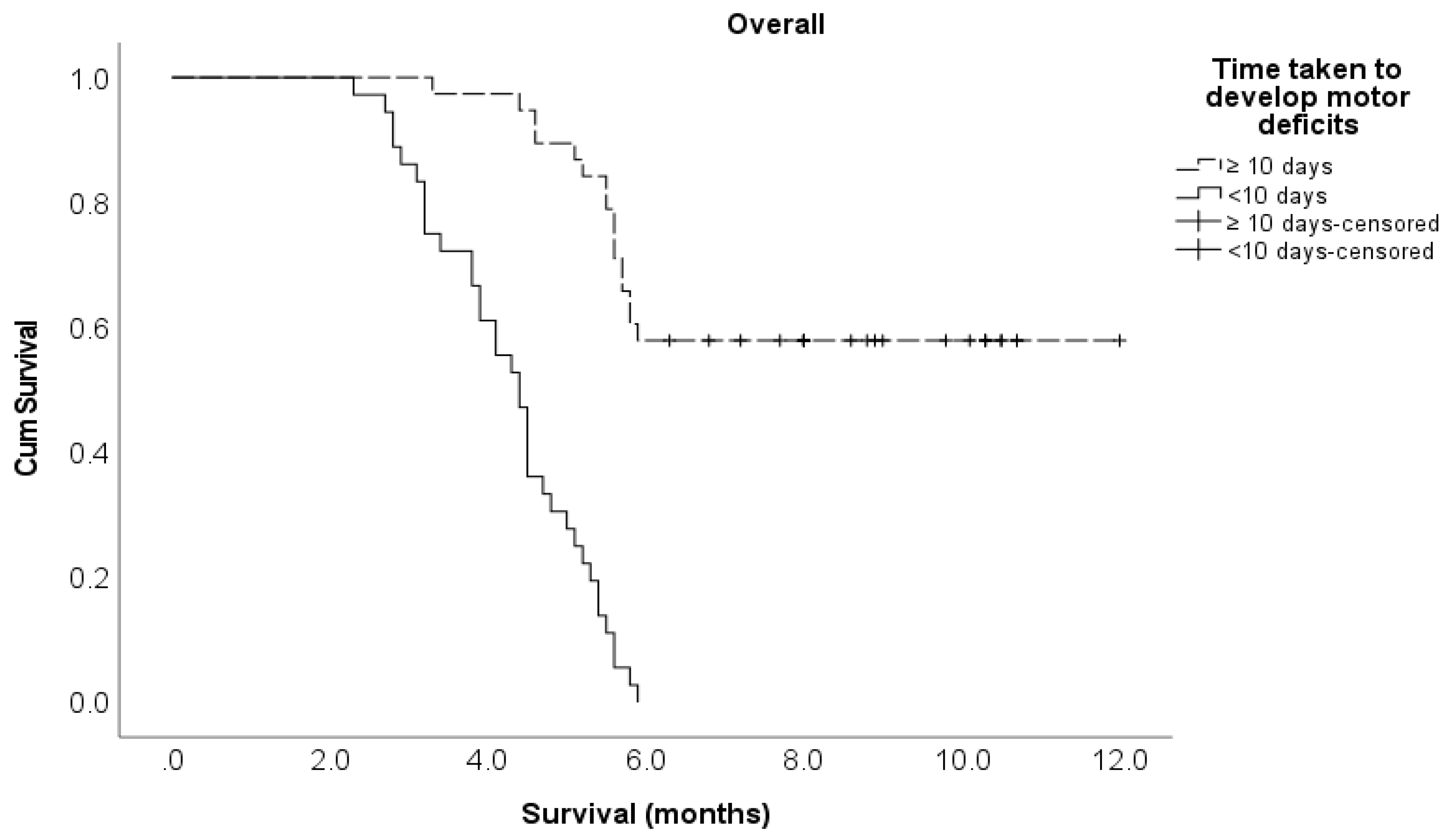
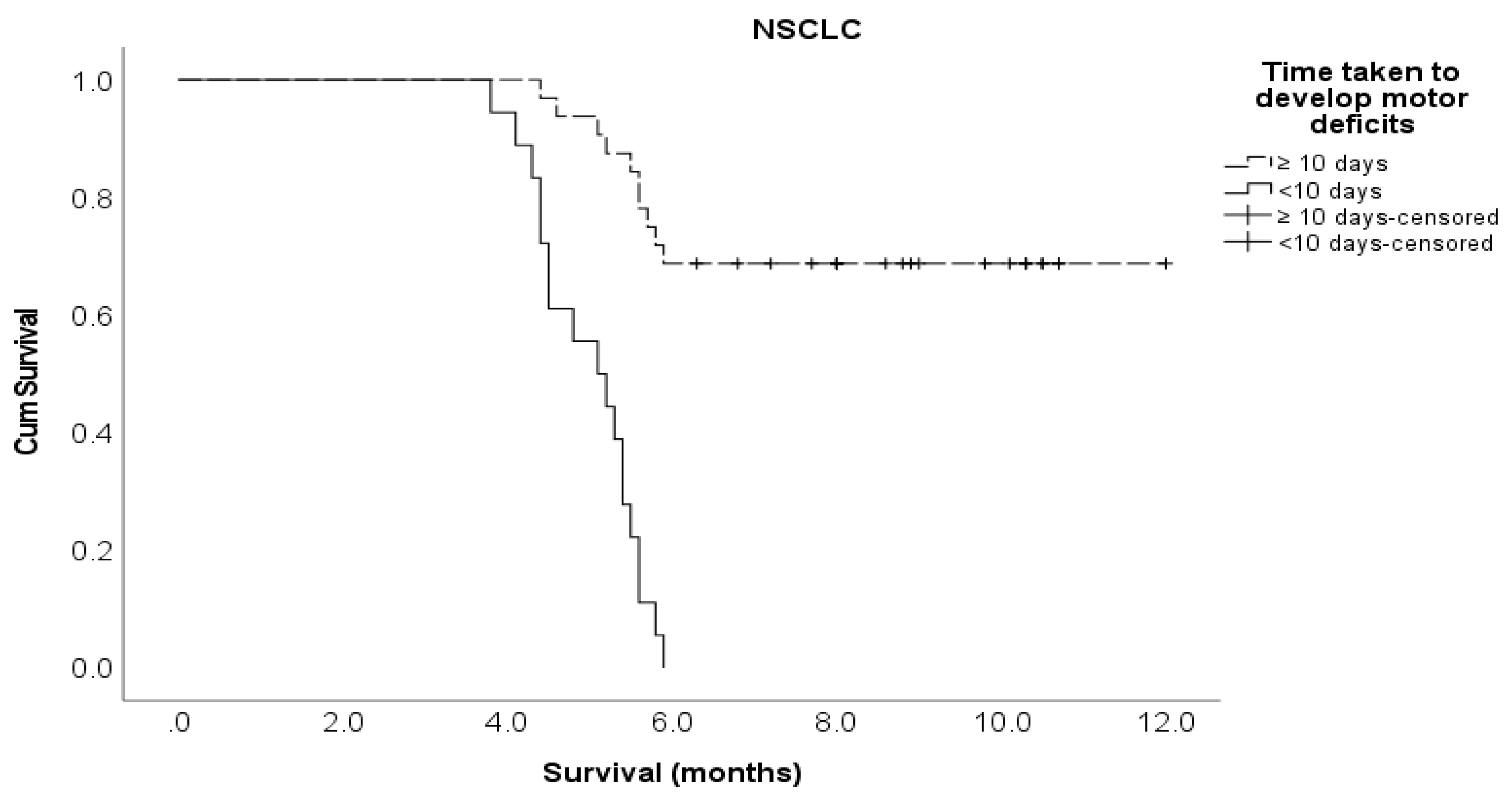
Time developing motor deficits ≤ 10 days > 10 days |
36 38 |
4.4 7.5 |
7.95 (4.20 – 15.1) 1 |
<0.001 |
6.06 (2.59 – 14.2) 1 |
<0.001 |
|
Interval from confirmed MSCC to radiotherapy ≤ 24 hours No radiotherapy > 24 hours |
12 34 28 |
7.4 5.5 4.8 |
0.25 (0.10 – 0.66) 0.56 (0.31 – 0.99) 1 |
0.009 |
1.34 (0.40 – 4.51) 0.74 (0.36 – 1.52) 1 |
0.529 |
|
Interval from confirmed MSCC to surgery ≤ 6 days No surgery > 6 days |
7 59 8 |
8.9 5.1 6.9 |
(0.00 – ) 2.72 (0.98 – 7.57) 1 |
0.159 |
Not included |
|
| Characteristics | N | Median OS (months) | Crude estimates | Adjusted estimates | ||
| HR (95% CI) | P | HR (95% CI) | P | |||
|
Age ≤55 years > 55 years |
32 18 |
5.6 5.1 |
0.76 (0.35 – 1.62) 1 |
0.473 |
Not included |
|
|
Gender Female Male |
22 28 |
5.8 5.1 |
0.48 (0.22 – 1.06) 1 |
0.068 |
Not included |
|
|
Smoker Yes No |
30 20 |
5.1 7.0 |
1.68 (0.76 – 3.73) 1 |
0.199 |
Not included |
|
|
Histology of NSCLC Adenocarcinoma Non-adenocarcinoma |
30 20 |
6.5 5.7 |
0.78 (0.37 – 1.64) 1 |
0.511 |
Not included |
|
|
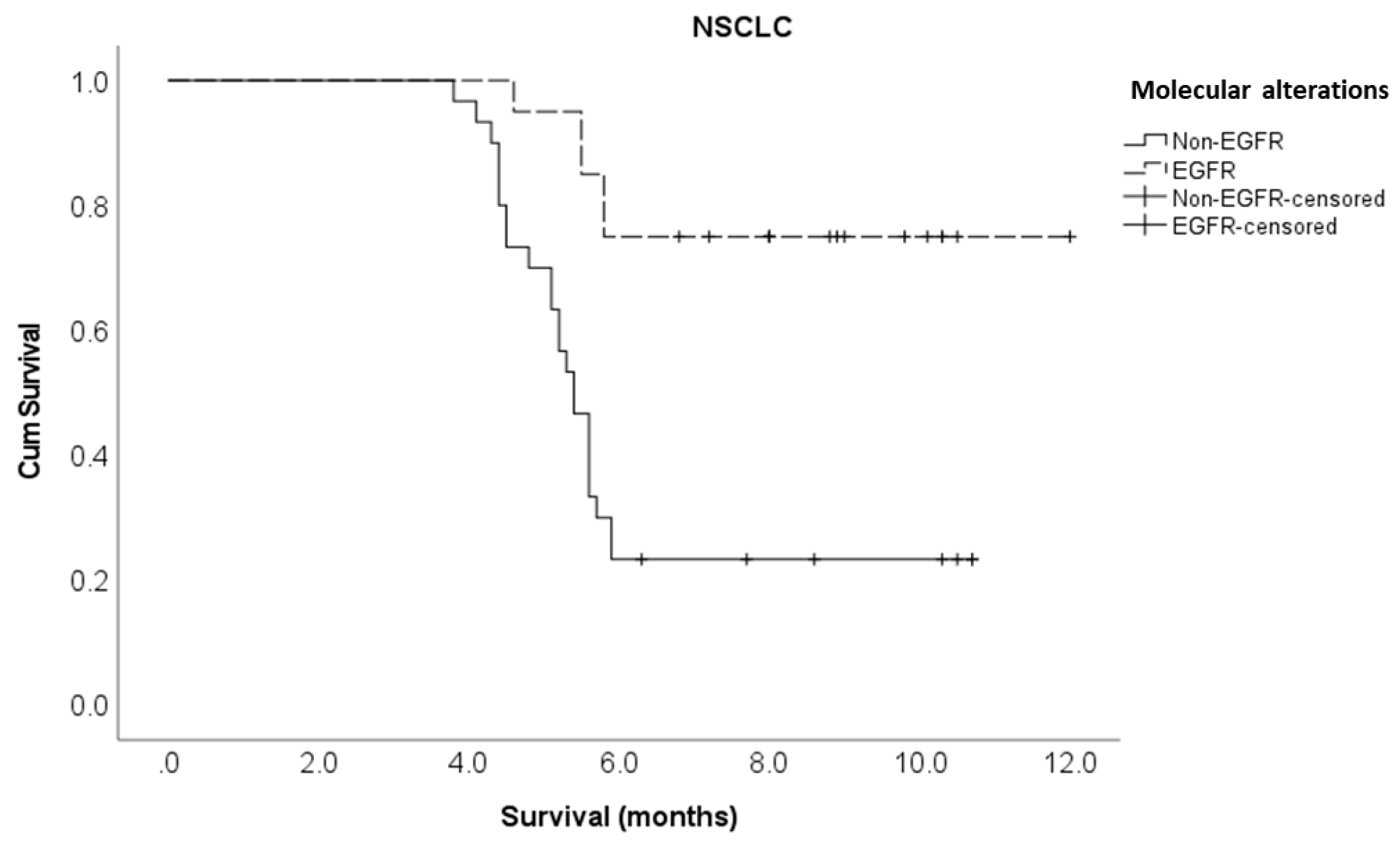
Driver molecular alterations in NSCLC EGFR Non-EGFR |
20 30 |
8.0 3.9 |
0.20 (0.07 – 0.52) 1 |
0.001 |
0.10 (0.01 – 0.70) 1 |
0.021 |
|
Pre-treatment ambulatory status Ambulatory Not ambulatory |
32 18 |
5.8 4.5 |
0.37 (0.18 – 0.79) 1 |
0.010 |
0.36 (0.14 – 0.92) 1 |
0.032 |
|
ECOG PS 1 – 2 ≥ 3 |
38 12 |
5.7 4.2 |
0.23 (0.11 – 0.52) 1 |
<0.001 |
0.29 (0.11 – 0.80) 1 |
0.017 |
|
Number of involved vertebrae 1 – 2 ≥ 3 |
41 9 |
5.8 3.6 |
0.19 (0.08 – 0.42) 1 |
<0.001 |
1.08 (0.32 – 3.68) 1 |
0.900 |
|
Pre-treatment chemotherapy Yes No |
31 19 |
7.0 4.5 |
0.33 (0.16 – 0.70) 1 |
0.004 |
0.49 (0.17 – 1.39) 1 |
0.178 |
|
Interval from suspicion of MSCC to MRI ≤ 24 hours > 24 hours |
29 21 |
5.9 4.6 |
0.52 (0.25 – 1.09) 1 |
0.084 |
Not included |
|
|
Time developing motor deficits ≤ 10 days > 10 days |
18 32 |
4.4 7.5 |
8.31 (3.65 – 18.9) 1 |
<0.001 |
6.17 (2.14 – 17.8) 1 |
<0.001 |
|
Interval from confirmed MSCC to RT ≤ 24 hours No radiotherapy > 24 hours |
12 23 15 |
7.4 5.5 4.8 |
0.33 (0.12 – 0.93) 0.42 (0.18 – 0.95) 1 |
0.043 |
0.99 (0.29 – 3.45) 0.82 (0.30 – 2.19) 1 |
0.902 |
|
Interval from confirmed MSCC to surgery ≤ 6 days No surgery > 6 days |
7 35 8 |
8.9 5.1 6.9 |
0 (0 – ) 1.83 (0.63 – 5.29) 1 |
0.536 |
Not included |
|
|
Targeted therapy for NSCLC Yes No |
22 28 |
8.0 5.4 |
0.27 1 |
0.003 |
2.70 (0.49 – 15.0) 1 |
0.257 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





