Submitted:
20 July 2023
Posted:
24 July 2023
You are already at the latest version
Abstract
Keywords:
Introduction
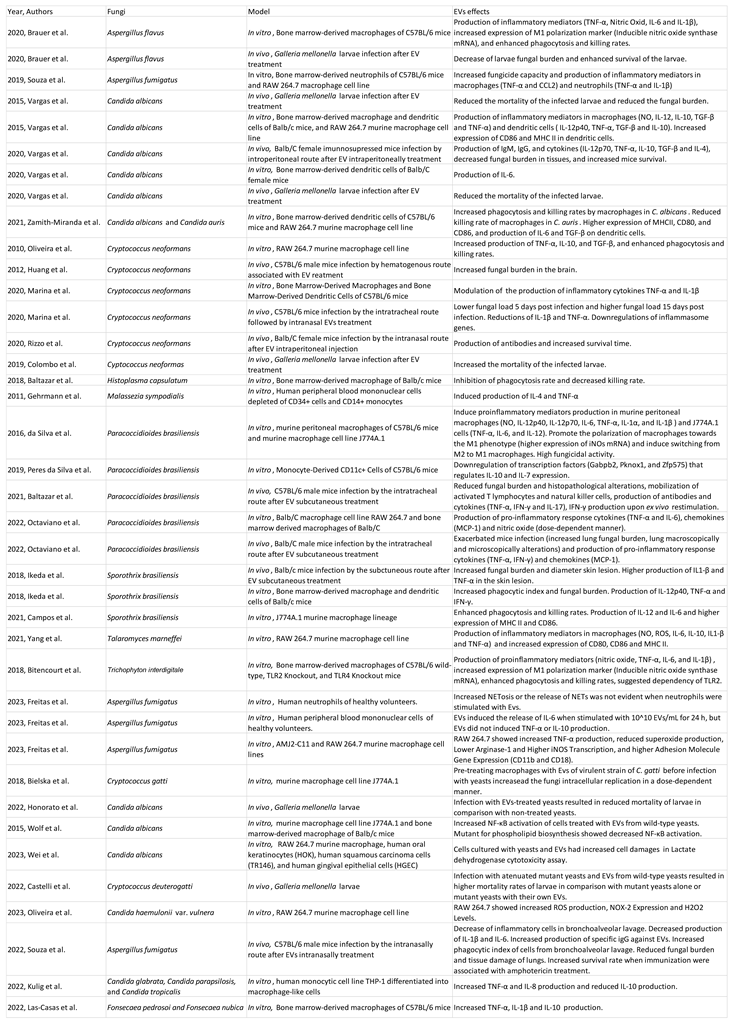
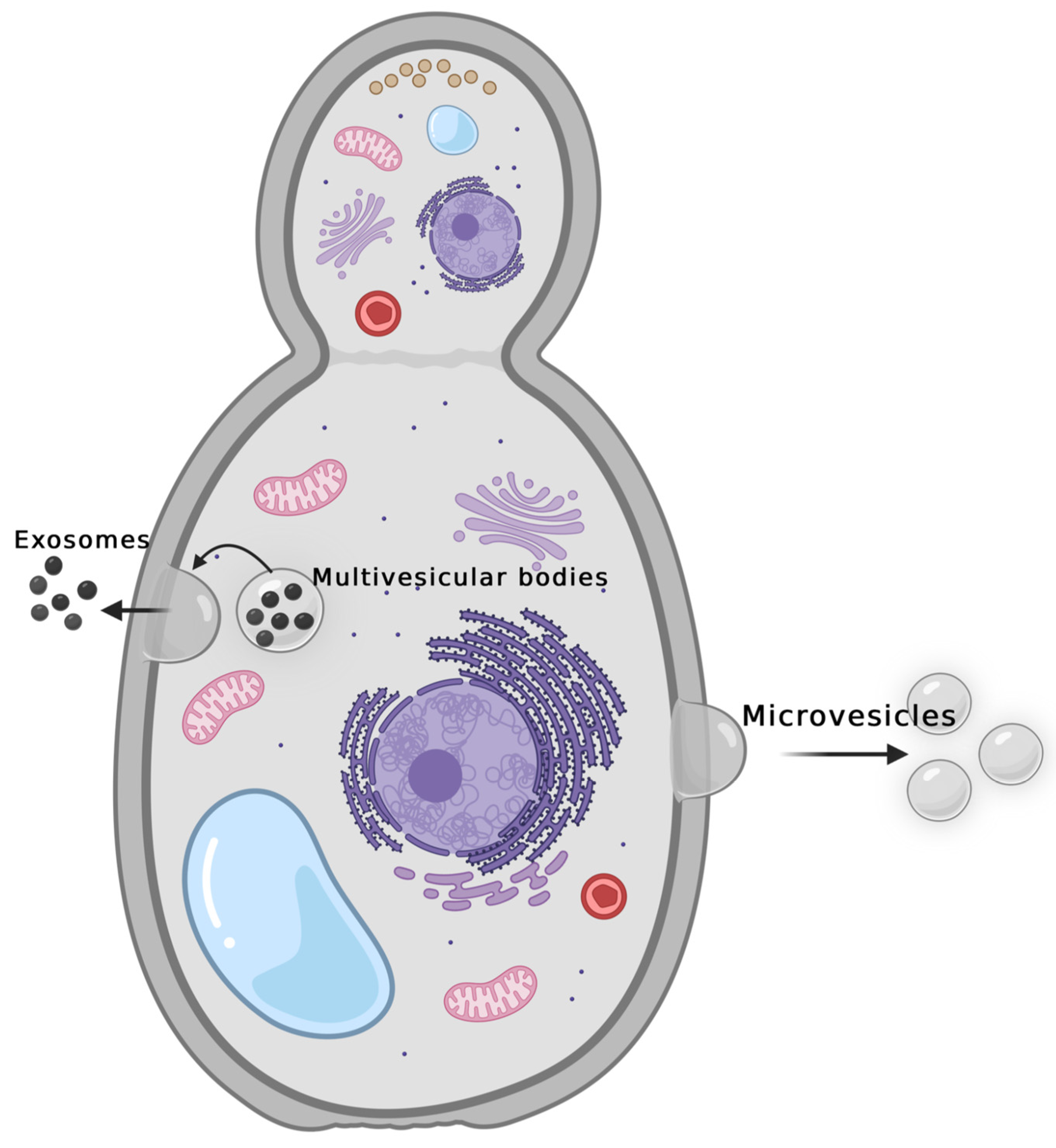
- Fungal Extracellular Vesicles
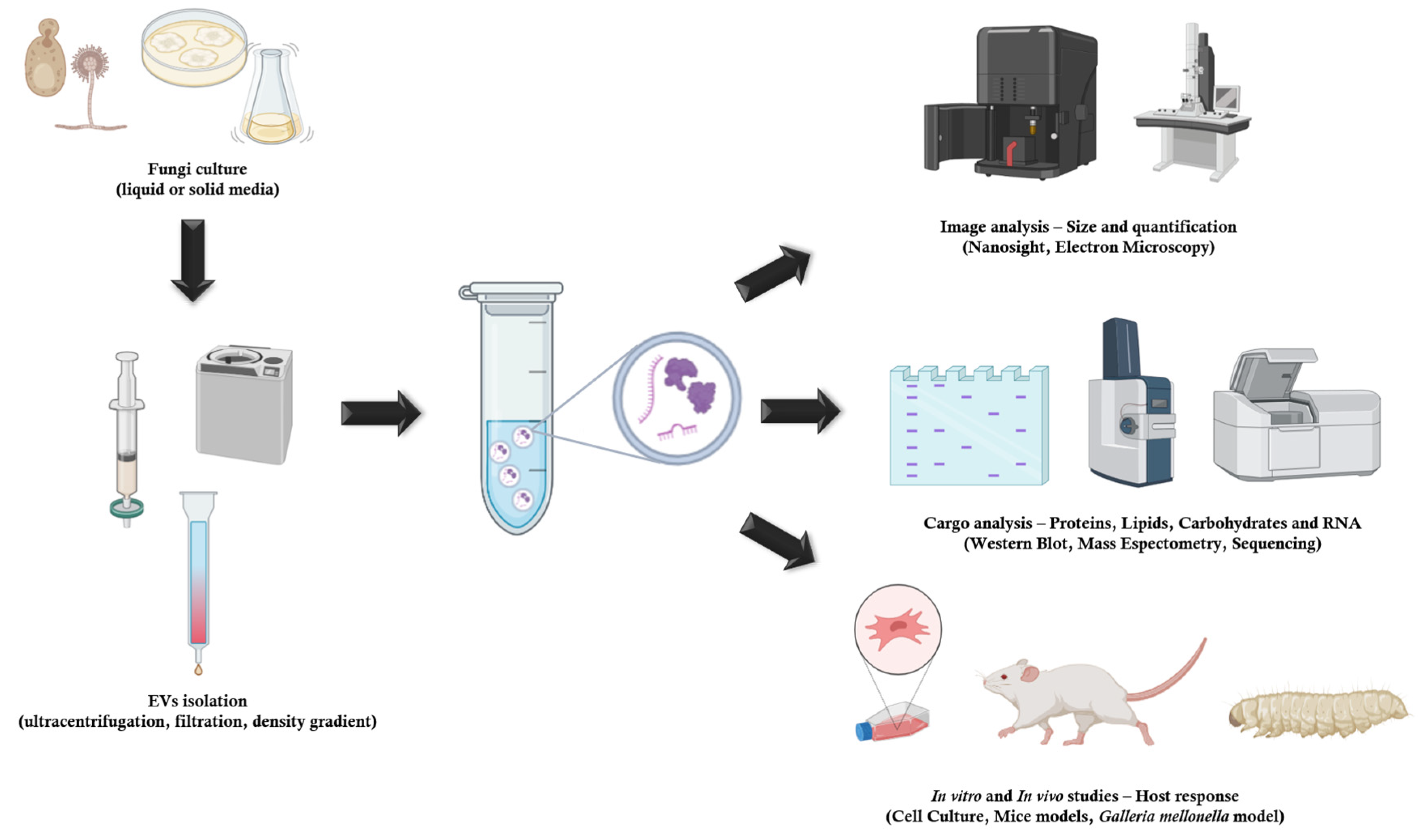
- Fungal EVs Methods of Extraction
- Cargo of Fungal Extracellular vesicles
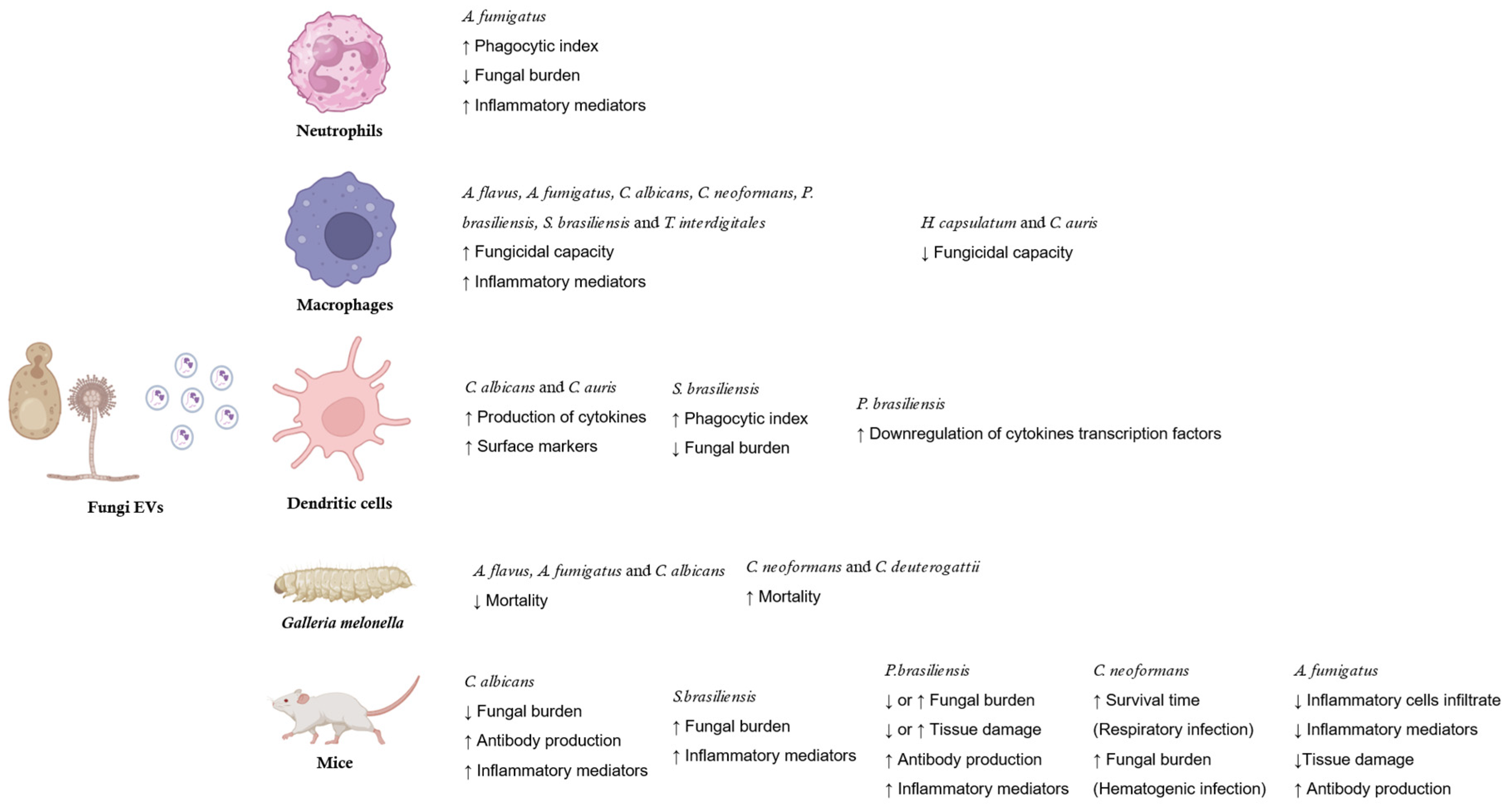
- Fungi EVs and host immune system
 |
References
- Albuquerque, P. C., Nakayasu, E. S., Rodrigues, M. L., Frases, S., Casadevall, A., Zancope-Oliveira, R. M., Almeida, I. C., & Nosanchuk, J. D. (2008). Vesicular transport in Histoplasma capsulatum: an effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cellular microbiology, 10(8), 1695–1710. [CrossRef]
- Abramowicz, A., Widlak, P., & Pietrowska, M. (2016). Proteomic analysis of exosomal cargo: the challenge of high purity vesicle isolation. Molecular BioSystems, 12(5), 1407-1419. [CrossRef]
- Alves, L. R., Peres da Silva, R., Sanchez, D. A., Zamith-Miranda, D., Rodrigues, M. L., Goldenberg, S., Puccia, R., & Nosanchuk, J. D. (2019). Extracellular Vesicle-Mediated RNA Release in Histoplasma capsulatum. mSphere, 4(2), e00176-19. [CrossRef]
- Amatuzzi, R. F., Zamith-Miranda, D., Munhoz da Rocha, I. F., Lucena, A. C. R., de Toledo Martins, S., Streit, R., Staats, C. C., Trentin, G., Almeida, F., Rodrigues, M. L., Nosanchuk, J. D., & Alves, L. R. (2022). Caspofungin Affects Extracellular Vesicle Production and Cargo in Candida auris. Journal of fungi (Basel, Switzerland), 8(10), 990. [CrossRef]
- B R Da Silva, L., P Taborda, C., & D Nosanchuk, J. (2020). Advances in Fungal Peptide Vaccines. Journal of fungi (Basel, Switzerland), 6(3), 119. [CrossRef]
- Baltazar, L. M., Nakayasu, E. S., Sobreira, T. J., Choi, H., Casadevall, A., Nimrichter, L., & Nosanchuk, J. D. (2016). Antibody Binding Alters the Characteristics and Contents of Extracellular Vesicles Released by Histoplasma capsulatum. mSphere, 1(2), e00085-15. [CrossRef]
- Baltazar, L. M., Zamith-Miranda, D., Burnet, M. C., Choi, H., Nimrichter, L., Nakayasu, E. S., & Nosanchuk, J. D. (2018). Concentration-dependent protein loading of extracellular vesicles released by Histoplasma capsulatum after antibody treatment and its modulatory action upon macrophages. Scientific reports, 8(1), 8065. [CrossRef]
- Bielska, E., Sisquella, M. A., Aldeieg, M., Birch, C., O'Donoghue, E. J., & May, R. C. (2018). Pathogen-derived extracellular vesicles mediate virulence in the fatal human pathogen Cryptococcus gattii. Nature communications, 9(1), 1556. [CrossRef]
- Bitencourt, T. A., Rezende, C. P., Quaresemin, N. R., Moreno, P., Hatanaka, O., Rossi, A., Martinez-Rossi, N. M., & Almeida, F. (2018). Extracellular Vesicles From the Dermatophyte Trichophyton interdigitale Modulate Macrophage and Keratinocyte Functions. Frontiers in immunology, 9, 2343. [CrossRef]
- Bitencourt, T. A., Pessoni, A. M., Oliveira, B. T. M., Alves, L. R., & Almeida, F. (2022). The RNA Content of Fungal Extracellular Vesicles: At the "Cutting-Edge" of Pathophysiology Regulation. Cells, 11(14), 2184. [CrossRef]
- Bleackley, M. R., Samuel, M., Garcia-Ceron, D., McKenna, J. A., Lowe, R. G. T., Pathan, M., Zhao, K., Ang, C. S., Mathivanan, S., & Anderson, M. A. (2020). Extracellular Vesicles From the Cotton Pathogen Fusarium oxysporum f. sp. vasinfectum Induce a Phytotoxic Response in Plants. Frontiers in plant science, 10, 1610. [CrossRef]
- Brauer, V. S., Pessoni, A. M., Bitencourt, T. A., de Paula, R. G., de Oliveira Rocha, L., Goldman, G. H., & Almeida, F. (2020). Extracellular Vesicles from Aspergillus flavus Induce M1 Polarization In Vitro. mSphere, 5(3), e00190-20. [CrossRef]
- Brennan, K., Martin, K., FitzGerald, S. P., O'Sullivan, J., Wu, Y., Blanco, A., Richardson, C., & Mc Gee, M. M. (2020). A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Scientific reports, 10(1), 1039. [CrossRef]
- Campos, R., Jannuzzi, G. P., Ikeda, M., de Almeida, S. R., & Ferreira, K. S. (2021). Extracellular Vesicles From Sporothrix brasiliensis Yeast Cells Increases Fungicidal Activity in Macrophages. Mycopathologia, 186(6), 807–818. [CrossRef]
- Castelli, R. F., Pereira, A., Honorato, L., Valdez, A., de Oliveira, H. C., Bazioli, J. M., Garcia, A. W. A., Klimeck, T. D. F., Reis, F. C. G., Staats, C. C., Nimrichter, L., Fill, T. P., & Rodrigues, M. L. (2022). Extracellular Vesicle Formation in Cryptococcus deuterogattii Impacts Fungal Virulence and Requires the NOP16 Gene. Infection and immunity, 90(8), e0023222. [CrossRef]
- Cleare, L. G., Zamith, D., Heyman, H. M., Couvillion, S. P., Nimrichter, L., Rodrigues, M. L., Nakayasu, E. S., & Nosanchuk, J. D. (2020). Media matters! Alterations in the loading and release of Histoplasma capsulatum extracellular vesicles in response to different nutritional milieus. Cellular microbiology, 22(9), e13217. [CrossRef]
- Colombo, A. C., Rella, A., Normile, T., Joffe, L. S., Tavares, P. M., de S Araújo, G. R., Frases, S., Orner, E. P., Farnoud, A. M., Fries, B. C., Sheridan, B., Nimrichter, L., Rodrigues, M. L., & Del Poeta, M. (2019). Cryptococcus neoformans Glucuronoxylomannan and Sterylglucoside Are Required for Host Protection in an Animal Vaccination Model. mBio, 10(2), e02909-18. [CrossRef]
- Costa, J. H., Bazioli, J. M., Barbosa, L. D., Dos Santos Júnior, P. L. T., Reis, F. C. G., Klimeck, T., Crnkovic, C. M., Berlinck, R. G. S., Sussulini, A., Rodrigues, M. L., & Fill, T. P. (2021). Phytotoxic Tryptoquialanines Produced In Vivo by Penicillium digitatum Are Exported in Extracellular Vesicles. mBio, 12(1), e03393-20. [CrossRef]
- Deregibus, M. C., Figliolini, F., D'Antico, S., Manzini, P. M., Pasquino, C., De Lena, M., Tetta, C., Brizzi, M. F., & Camussi, G. (2016). Charge-based precipitation of extracellular vesicles. International journal of molecular medicine, 38(5), 1359–1366. [CrossRef]
- Desai, J. V., & Lionakis, M. S. (2018). The role of neutrophils in host defense against invasive fungal infections. Current clinical microbiology reports, 5(3), 181–189. [CrossRef]
- Freitas, M. S., Bonato, V., Pessoni, A. M., Rodrigues, M. L., Casadevall, A., & Almeida, F. (2019). Fungal Extracellular Vesicles as Potential Targets for Immune Interventions. mSphere, 4(6), e00747-19. [CrossRef]
- Gardiner, C., Di Vizio, D., Sahoo, S., Théry, C., Witwer, K. W., Wauben, M., & Hill, A. F. (2016). Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. Journal of extracellular vesicles, 5, 32945. [CrossRef]
- Gehrmann, U., Qazi, K. R., Johansson, C., Hultenby, K., Karlsson, M., Lundeberg, L., Gabrielsson, S., & Scheynius, A. (2011). Nanovesicles from Malassezia sympodialis and host exosomes induce cytokine responses--novel mechanisms for host-microbe interactions in atopic eczema. PloS one, 6(7), e21480. [CrossRef]
- Gil-Bona, A., Llama-Palacios, A., Parra, C. M., Vivanco, F., Nombela, C., Monteoliva, L., & Gil, C. (2015). Proteomics unravels extracellular vesicles as carriers of classical cytoplasmic proteins in Candida albicans. Journal of proteome research, 14(1), 142–153. [CrossRef]
- Guerreiro, E. M., Vestad, B., Steffensen, L. A., Aass, H. C. D., Saeed, M., Øvstebø, R., Costea, D. E., Galtung, H. K., & Søland, T. M. (2018). Efficient extracellular vesicle isolation by combining cell media modifications, ultrafiltration, and size-exclusion chromatography. PloS one, 13(9), e0204276. [CrossRef]
- Honorato, L., de Araujo, J. F. D., Ellis, C. C., Piffer, A. C., Pereira, Y., Frases, S., de Sousa Araújo, G. R., Pontes, B., Mendes, M. T., Pereira, M. D., Guimarães, A. J., da Silva, N. M., Vargas, G., Joffe, L., Del Poeta, M., Nosanchuk, J. D., Zamith-Miranda, D., Dos Reis, F. C. G., de Oliveira, H. C., Rodrigues, M. L., … Nimrichter, L. (2022). Extracellular Vesicles Regulate Biofilm Formation and Yeast-to-Hypha Differentiation in Candida albicans. mBio, 13(3), e0030122. [CrossRef]
- Huang, S. H., Wu, C. H., Chang, Y. C., Kwon-Chung, K. J., Brown, R. J., & Jong, A. (2012). Cryptococcus neoformans-derived microvesicles enhance the pathogenesis of fungal brain infection. PloS one, 7(11), e48570. [CrossRef]
- Ikeda, M., de Almeida, J., Jannuzzi, G. P., Cronemberger-Andrade, A., Torrecilhas, A., Moretti, N. S., da Cunha, J., de Almeida, S. R., & Ferreira, K. S. (2018). Extracellular Vesicles From Sporothrix brasiliensis Are an Important Virulence Factor That Induce an Increase in Fungal Burden in Experimental Sporotrichosis. Frontiers in microbiology, 9, 2286. [CrossRef]
- Ikeda, M., & Ferreira, K. S. (2021). Extracellular Vesicles from Sporothrix Yeast Cells. Current topics in microbiology and immunology, 432, 35–44. [CrossRef]
- Ingato, D., Lee, J. U., Sim, S. J., & Kwon, Y. J. (2016). Good things come in small packages: Overcoming challenges to harness extracellular vesicles for therapeutic delivery. Journal of controlled release: official journal of the Controlled Release Society, 241, 174–185. [CrossRef]
- Kim, J., Shin, H., Kim, J., Kim, J., & Park, J. (2015). Isolation of High-Purity Extracellular Vesicles by Extracting Proteins Using Aqueous Two-Phase System. PloS one, 10(6), e0129760. [CrossRef]
- Kulig, K., Karnas, E., Woznicka, O., Kuleta, P., Zuba-Surma, E., Pyza, E., Osyczka, A., Kozik, A., Rapala-Kozik, M., & Karkowska-Kuleta, J. (2022). Insight Into the Properties and Immunoregulatory Effect of Extracellular Vesicles Produced by Candida glabrata, Candida parapsilosis, and Candida tropicalis Biofilms. Frontiers in cellular and infection microbiology, 12, 879237. [CrossRef]
- Lamparski, H. G., Metha-Damani, A., Yao, J. Y., Patel, S., Hsu, D. H., Ruegg, C., & Le Pecq, J. B. (2002). Production and characterization of clinical grade exosomes derived from dendritic cells. Journal of immunological methods, 270(2), 211–226. [CrossRef]
- Las-Casas, L. O., Marina, C. L. F., de Castro, R. J. A., Coelho, L. C., Báo, S. N., de Hoog, G. S., Vicente, V. A., Fernandes, L., & Bocca, A. L. (2022). Pathogenicity and Growth Conditions Modulate Fonsecaea Extracellular Vesicles' Ability to Interact With Macrophages. Frontiers in cellular and infection microbiology, 12, 879018. [CrossRef]
- Lavrin, T., Konte, T., Kostanjšek, R., Sitar, S., Sepčič, K., Prpar Mihevc, S., Žagar, E., Župunski, V., Lenassi, M., Rogelj, B., & Gunde Cimerman, N. (2020). The Neurotropic Black Yeast Exophiala dermatitidis Induces Neurocytotoxicity in Neuroblastoma Cells and Progressive Cell Death. Cells, 9(4), 963. [CrossRef]
- Leone, F., Bellani, L., Muccifora, S., Giorgetti, L., Bongioanni, P., Simili, M., Maserti, B., & Del Carratore, R. (2018). Analysis of extracellular vesicles produced in the biofilm by the dimorphic yeast Pichia fermentans. Journal of cellular physiology, 233(4), 2759–2767. [CrossRef]
- Liebana-Jordan, M., Brotons, B., Falcon-Perez, J. M., & Gonzalez, E. (2021). Extracellular Vesicles in the Fungi Kingdom. International journal of molecular sciences, 22(13), 7221. [CrossRef]
- Liu, M., Bruni, G. O., Taylor, C. M., Zhang, Z., & Wang, P. (2018). Comparative genome-wide analysis of extracellular small RNAs from the mucormycosis pathogen Rhizopus delemar. Scientific reports, 8(1), 5243. [CrossRef]
- Mathieu, M., Martin-Jaular, L., Lavieu, G., & Théry, C. (2019). Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nature cell biology, 21(1), 9–17. [CrossRef]
- Merchant, M. L., Powell, D. W., Wilkey, D. W., Cummins, T. D., Deegens, J. K., Rood, I. M., McAfee, K. J., Fleischer, C., Klein, E., & Klein, J. B. (2010). Microfiltration isolation of human urinary exosomes for characterization by MS. Proteomics. Clinical applications, 4(1), 84–96. [CrossRef]
- Munhoz da Rocha, I. F., Martins, S. T., Amatuzzi, R. F., Zamith-Miranda, D., Nosanchuk, J. D., Rodrigues, M. L., & Alves, L. R. (2021). Cellular and Extracellular Vesicle RNA Analysis in the Global Threat Fungus Candida auris. Microbiology spectrum, 9(3), e0153821. [CrossRef]
- Musante, L., Tataruch, D. E., & Holthofer, H. (2014). Use and isolation of urinary exosomes as biomarkers for diabetic nephropathy. Frontiers in endocrinology, 5, 149. [CrossRef]
- Nimrichter, L., de Souza, M. M., Del Poeta, M., Nosanchuk, J. D., Joffe, L., Tavares, P.deM., & Rodrigues, M. L. (2016). Extracellular Vesicle-Associated Transitory Cell Wall Components and Their Impact on the Interaction of Fungi with Host Cells. Frontiers in microbiology, 7, 1034. [CrossRef]
- Octaviano, C. E., Abrantes, N. E., & Puccia, R. (2022). Extracellular Vesicles From Paracoccidioides brasiliensis Can Induce the Expression of Fungal Virulence Traits In Vitro and Enhance Infection in Mice. Frontiers in cellular and infection microbiology, 12, 834653. [CrossRef]
- Oliveira, D. L., Freire-de-Lima, C. G., Nosanchuk, J. D., Casadevall, A., Rodrigues, M. L., & Nimrichter, L. (2010). Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions. Infection and immunity, 78(4), 1601–1609. [CrossRef]
- Oliveira, B. T. M., Dourado, T. M. H., Santos, P. W. S., Bitencourt, T. A., Tirapelli, C. R., Colombo, A. L., & Almeida, F. (2023). Extracellular Vesicles from Candida haemulonii var. vulnera Modulate Macrophage Oxidative Burst. Journal of fungi (Basel, Switzerland), 9(5), 562. [CrossRef]
- de Paula, R. G., Antoniêto, A. C. C., Nogueira, K. M. V., Ribeiro, L. F. C., Rocha, M. C., Malavazi, I., Almeida, F., & Silva, R. N. (2019). Extracellular vesicles carry cellulases in the industrial fungus Trichoderma reesei. Biotechnology for biofuels, 12, 146. [CrossRef]
- Peres da Silva, R., Heiss, C., Black, I., Azadi, P., Gerlach, J. Q., Travassos, L. R., Joshi, L., Kilcoyne, M., & Puccia, R. (2015). Extracellular vesicles from Paracoccidioides pathogenic species transport polysaccharide and expose ligands for DC-SIGN receptors. Scientific reports, 5, 14213. [CrossRef]
- Peres da Silva, R., Puccia, R., Rodrigues, M. L., Oliveira, D. L., Joffe, L. S., César, G. V., Nimrichter, L., Goldenberg, S., & Alves, L. R. (2015). Extracellular vesicle-mediated export of fungal RNA. Scientific reports, 5, 7763. [CrossRef]
- Peres da Silva, R., Longo, L., Cunha, J., Sobreira, T., Rodrigues, M. L., Faoro, H., Goldenberg, S., Alves, L. R., & Puccia, R. (2019). Comparison of the RNA Content of Extracellular Vesicles Derived from Paracoccidioides brasiliensis and Paracoccidioides lutzii. Cells, 8(7), 765. [CrossRef]
- Rayner, S., Bruhn, S., Vallhov, H., Andersson, A., Billmyre, R. B., & Scheynius, A. (2017). Identification of small RNAs in extracellular vesicles from the commensal yeast Malassezia sympodialis. Scientific reports, 7, 39742. [CrossRef]
- Reales-Calderón, J. A., Vaz, C., Monteoliva, L., Molero, G., & Gil, C. (2017). Candida albicans Modifies the Protein Composition and Size Distribution of THP-1 Macrophage-Derived Extracellular Vesicles. Journal of proteome research, 16(1), 87–105. [CrossRef]
- Reis, F. C. G., Borges, B. S., Jozefowicz, L. J., Sena, B. A. G., Garcia, A. W. A., Medeiros, L. C., Martins, S. T., Honorato, L., Schrank, A., Vainstein, M. H., Kmetzsch, L., Nimrichter, L., Alves, L. R., Staats, C. C., & Rodrigues, M. L. (2019). A Novel Protocol for the Isolation of Fungal Extracellular Vesicles Reveals the Participation of a Putative Scramblase in Polysaccharide Export and Capsule Construction in Cryptococcus gattii. mSphere, 4(2), e00080-19. [CrossRef]
- Reis, F. C. G., Gimenez, B., Jozefowicz, L. J., Castelli, R. F., Martins, S. T., Alves, L. R., de Oliveira, H. C., & Rodrigues, M. L. (2021). Analysis of Cryptococcal Extracellular Vesicles: Experimental Approaches for Studying Their Diversity Among Multiple Isolates, Kinetics of Production, Methods of Separation, and Detection in Cultures of Titan Cells. Microbiology spectrum, 9(1), e0012521. [CrossRef]
- Rizzo, J., Chaze, T., Miranda, K., Roberson, R. W., Gorgette, O., Nimrichter, L., Matondo, M., Latgé, J. P., Beauvais, A., & Rodrigues, M. L. (2020). Characterization of Extracellular Vesicles Produced by Aspergillus fumigatus Protoplasts. mSphere, 5(4), e00476-20. [CrossRef]
- Rizzo, J., Wong, S., Gazi, A. D., Moyrand, F., Chaze, T., Commere, P. H., Novault, S., Matondo, M., Péhau-Arnaudet, G., Reis, F., Vos, M., Alves, L. R., May, R. C., Nimrichter, L., Rodrigues, M. L., Aimanianda, V., & Janbon, G. (2021). Cryptococcus extracellular vesicles properties and their use as vaccine platforms. Journal of extracellular vesicles, 10(10), e12129. [CrossRef]
- Rodrigues, M. L., Nakayasu, E. S., Oliveira, D. L., Nimrichter, L., Nosanchuk, J. D., Almeida, I. C., & Casadevall, A. (2008). Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryotic cell, 7(1), 58–67. [CrossRef]
- Rodrigues, M. L., Nakayasu, E. S., Oliveira, D. L., Nimrichter, L., Nosanchuk, J. D., Almeida, I. C., & Casadevall, A. (2008). Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryotic cell, 7(1), 58–67. [CrossRef]
- Rodrigues, M. L., & Nimrichter, L. (2022). From fundamental biology to the search for innovation: The story of fungal extracellular vesicles. European journal of cell biology, 101(2), 151205. [CrossRef]
- Rutter, B. D., Chu, T. T., Dallery, J. F., Zajt, K. K., O'Connell, R. J., & Innes, R. W. (2022). The development of extracellular vesicle markers for the fungal phytopathogen Colletotrichum higginsianum. Journal of extracellular vesicles, 11(5), e12216. [CrossRef]
- Santavanond, J. P., Rutter, S. F., Atkin-Smith, G. K., & Poon, I. K. H. (2021). Apoptotic Bodies: Mechanism of Formation, Isolation and Functional Relevance. Sub-cellular biochemistry, 97, 61–88. [CrossRef]
- Silva, B. M., Prados-Rosales, R., Espadas-Moreno, J., Wolf, J. M., Luque-Garcia, J. L., Gonçalves, T., & Casadevall, A. (2014). Characterization of Alternaria infectoria extracellular vesicles. Medical mycology, 52(2), 202–210. [CrossRef]
- da Silva, T. A., Roque-Barreira, M. C., Casadevall, A., & Almeida, F. (2016). Extracellular vesicles from Paracoccidioides brasiliensis induced M1 polarization in vitro. Scientific reports, 6, 35867. [CrossRef]
- Souza, J., Baltazar, L. M., Carregal, V. M., Gouveia-Eufrasio, L., de Oliveira, A. G., Dias, W. G., Campos Rocha, M., Rocha de Miranda, K., Malavazi, I., Santos, D. A., Frézard, F., de Souza, D., Teixeira, M. M., & Soriani, F. M. (2019). Characterization of Aspergillus fumigatus Extracellular Vesicles and Their Effects on Macrophages and Neutrophils Functions. Frontiers in microbiology, 10, 2008. [CrossRef]
- de Sousa, H. R., de Oliveira, G. P., Jr, Frazão, S. O., Gorgonha, K. C. M., Rosa, C. P., Garcez, E. M., Lucas, J., Jr, Correia, A. F., de Freitas, W. F., Borges, H. M., Brito Alves, L. G., Paes, H. C., Trilles, L., Lazera, M. D. S., Teixeira, M. M., Pinto, V. L., Jr, Felipe, M. S. S., Casadevall, A., Silva-Pereira, I., Albuquerque, P., … Nicola, A. M. (2022). Faster Cryptococcus Melanization Increases Virulence in Experimental and Human Cryptococcosis. Journal of fungi (Basel, Switzerland), 8(4), 393. [CrossRef]
- Souza, J. A. M., Gurgel, I. L. D. S., Malacco, N. L. S. O., Martins, F. R. B., Queiroz-Junior, C. M., Teixeira, M. M., & Soriani, F. M. (2022). Pre-Exposure With Extracellular Vesicles From Aspergillus fumigatus Attenuates Inflammatory Response and Enhances Fungal Clearance in a Murine Model Pulmonary Aspergillosis. Frontiers in cellular and infection microbiology, 12, 898619. [CrossRef]
- Takeo, K., Uesaka, I., Uehira, K., & Nishiura, M. (1973). Fine structure of Cryptococcus neoformans grown in vitro as observed by freeze-etching. Journal of bacteriology, 113(3), 1442–1448. [CrossRef]
- Taylor, D. D., & Shah, S. (2015). Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods (San Diego, Calif.), 87, 3–10. [CrossRef]
- Théry, C., Amigorena, S., Raposo, G., & Clayton, A. (2006). Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Current protocols in cell biology, Chapter 3,. [CrossRef]
- Vallejo, M. C., Matsuo, A. L., Ganiko, L., Medeiros, L. C., Miranda, K., Silva, L. S., Freymüller-Haapalainen, E., Sinigaglia-Coimbra, R., Almeida, I. C., & Puccia, R. (2011). The pathogenic fungus Paracoccidioides brasiliensis exports extracellular vesicles containing highly immunogenic α-Galactosyl epitopes. Eukaryotic cell, 10(3), 343–351. [CrossRef]
- Vallejo, M. C., Nakayasu, E. S., Longo, L. V., Ganiko, L., Lopes, F. G., Matsuo, A. L., Almeida, I. C., & Puccia, R. (2012). Lipidomic analysis of extracellular vesicles from the pathogenic phase of Paracoccidioides brasiliensis. PloS one, 7(6), e39463. [CrossRef]
- Vallejo, M. C., Nakayasu, E. S., Matsuo, A. L., Sobreira, T. J., Longo, L. V., Ganiko, L., Almeida, I. C., & Puccia, R. (2012). Vesicle and vesicle-free extracellular proteome of Paracoccidioides brasiliensis: comparative analysis with other pathogenic fungi. Journal of proteome research, 11(3), 1676–1685. [CrossRef]
- Vargas, G., Rocha, J. D., Oliveira, D. L., Albuquerque, P. C., Frases, S., Santos, S. S., Nosanchuk, J. D., Gomes, A. M., Medeiros, L. C., Miranda, K., Sobreira, T. J., Nakayasu, E. S., Arigi, E. A., Casadevall, A., Guimaraes, A. J., Rodrigues, M. L., Freire-de-Lima, C. G., Almeida, I. C., & Nimrichter, L. (2015). Compositional and immunobiological analyses of extracellular vesicles released by Candida albicans. Cellular microbiology, 17(3), 389–407. [CrossRef]
- Vargas, G., Honorato, L., Guimarães, A. J., Rodrigues, M. L., Reis, F., Vale, A. M., Ray, A., Nosanchuk, J. D., & Nimrichter, L. (2020). Protective effect of fungal extracellular vesicles against murine candidiasis. Cellular microbiology, 22(10), e13238. [CrossRef]
- Wolf P. (1967). The nature and significance of platelet products in human plasma. British journal of haematology, 13(3), 269–288. [CrossRef]
- Wolf, J. M., Espadas-Moreno, J., Luque-Garcia, J. L., & Casadevall, A. (2014). Interaction of Cryptococcus neoformans extracellular vesicles with the cell wall. Eukaryotic cell, 13(12), 1484–1493. [CrossRef]
- Wolf, J. M., Espadas, J., Luque-Garcia, J., Reynolds, T., & Casadevall, A. (2015). Lipid Biosynthetic Genes Affect Candida albicans Extracellular Vesicle Morphology, Cargo, and Immunostimulatory Properties. Eukaryotic cell, 14(8), 745–754. [CrossRef]
- Yáñez-Mó, M., Siljander, P. R., Andreu, Z., Zavec, A. B., Borràs, F. E., Buzas, E. I., Buzas, K., Casal, E., Cappello, F., Carvalho, J., Colás, E., Cordeiro-da Silva, A., Fais, S., Falcon-Perez, J. M., Ghobrial, I. M., Giebel, B., Gimona, M., Graner, M., Gursel, I., Gursel, M., … De Wever, O. (2015). Biological properties of extracellular vesicles and their physiological functions. Journal of extracellular vesicles, 4, 27066. [CrossRef]
- Yang, B., Wang, J., Jiang, H., Lin, H., Ou, Z., Ullah, A., Hua, Y., Chen, J., Lin, X., Hu, X., Zheng, L., & Wang, Q. (2021). Extracellular Vesicles Derived From Talaromyces marneffei Yeasts Mediate Inflammatory Response in Macrophage Cells by Bioactive Protein Components. Frontiers in microbiology, 11, 603183. [CrossRef]
- Zamith-Miranda, D., Nimrichter, L., Rodrigues, M. L., & Nosanchuk, J. D. (2018). Fungal extracellular vesicles: modulating host-pathogen interactions by both the fungus and the host. Microbes and infection, 20(9-10), 501–504. [CrossRef]
- Zamith-Miranda, D., Heyman, H. M., Couvillion, S. P., Cordero, R., Rodrigues, M. L., Nimrichter, L., Casadevall, A., Amatuzzi, R. F., Alves, L. R., Nakayasu, E. S., & Nosanchuk, J. D. (2021). Comparative Molecular and Immunoregulatory Analysis of Extracellular Vesicles from Candida albicans and Candida auris. mSystems, 6(4), e0082221. [CrossRef]
- Zamith-Miranda, D., Peres da Silva, R., Couvillion, S. P., Bredeweg, E. L., Burnet, M. C., Coelho, C., Camacho, E., Nimrichter, L., Puccia, R., Almeida, I. C., Casadevall, A., Rodrigues, M. L., Alves, L. R., Nosanchuk, J. D., & Nakayasu, E. S. (2021). Omics Approaches for Understanding Biogenesis, Composition and Functions of Fungal Extracellular Vesicles. Frontiers in genetics, 12, 648524. [CrossRef]
- Zhang, L., Zhang, K., Li, H., Coelho, C., de Souza Gonçalves, D., Fu, M. S., Li, X., Nakayasu, E. S., Kim, Y. M., Liao, W., Pan, W., & Casadevall, A. (2021). Cryptococcus neoformans-Infected Macrophages Release Pro-inflammatory Extracellular Vesicles: Insight into Their Components by Multi-omics. mBio, 12(2), e00279-21. [CrossRef]
- Zhu, L., Sun, H. T., Wang, S., Huang, S. L., Zheng, Y., Wang, C. Q., Hu, B. Y., Qin, W., Zou, T. T., Fu, Y., Shen, X. T., Zhu, W. W., Geng, Y., Lu, L., Jia, H. L., Qin, L. X., & Dong, Q. Z. (2020). Isolation and characterization of exosomes for cancer research. Journal of hematology & oncology, 13(1), 152. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





