Submitted:
21 July 2023
Posted:
25 July 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
| Study | Year | Design | Number of patients(PCSK9/ control) | Inhibitor PCSK9 | Control | Underlying disease | Follow-up duration (weeks) |
|---|---|---|---|---|---|---|---|
| IVUS | |||||||
| ODYSSEY-J | 2019 | RCCT | 93/89 | Alirocumab 75/150mg + statin (atorvastatin ≥10mg or rosuvastatin ≥5mg) | Atorvastatin ≥10mg or Rosuvastatin ≥5mg | ACS | 36 |
| GLAGVO | 2016 | RCCT | 484/484 | Evolocumab 420mg + statin | Statin + placebo | chrIHD | 78 |
| PACMAN-AMI | 2022 | RCCT | 148/152 | Alirocumab 150mg + rosuvastatin 20mg | Rosuvastatin 20mg + placebo | MI | 52 |
| OCT | |||||||
| HUYGENS | 2021 | RCCT | 80/81 | Evolocumab 420mg + atorvastatin ≥40mg | Placebo + atorvastatin ≥40mg | MIwST | 52 |
| Yanoet al. | 2019 | 18/40 | Evolocumab 140mg + rosuvastatin 5mg | Rosuvastatin 5mg | ACS | 12 | |
| ALTAIR | 2020 | RCCT | 12/12 | Alirocumab 75mg + rosuvastatin 10mg | Rosuvastatin 10mg | chrIHDor ACS | 36 |
| Gao et al. | 2021 | RCCT | 30/3 | Alirocumab 75mg + atorvastatin 20mg or rosuvastatin 10mg | Atorvastatin 20mg or Rosuvastatin 10mg | IHD orACS | 36 |
| CTCA | |||||||
| ARCHITECT | 2023 | Single group study | 104 | Alirocumab 150mg /2 weeks+ high-intensity statin therapy | - | Familial hypercholesterolemia without clinical manifestations of atherosclerotic CVD | 78 |
Stable and unstable AP
The Role of Imaging Methods in the Diagnostics of Vulnerable APs
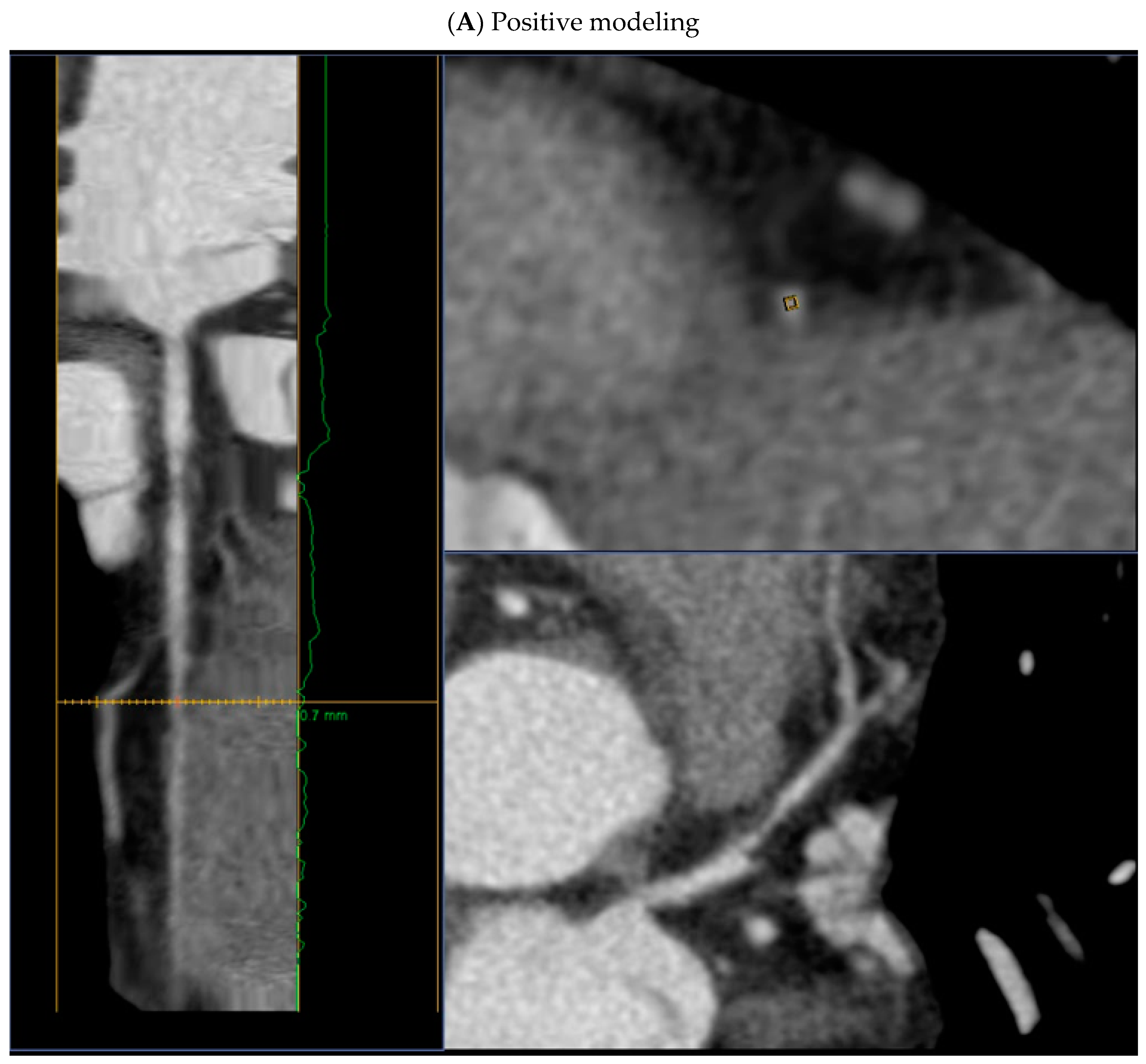
- positive remodeling (foci of vascular dilatation) - increase in the total volume of the AP, leading to a relative expansion of the diameter of the coronary artery. To assess it, a quantitative indicator is used - the remodeling index (RI), which is calculated by the formula RI = D1/D2, where D1 is the diameter of the vessel at the level of the plaque, D2 is the diameter of the intact segment proximal to the plaque. Positive remodeling is considered to be an excess of the vessel diameter at the plaque level by more than 10% compared to the reference segment;
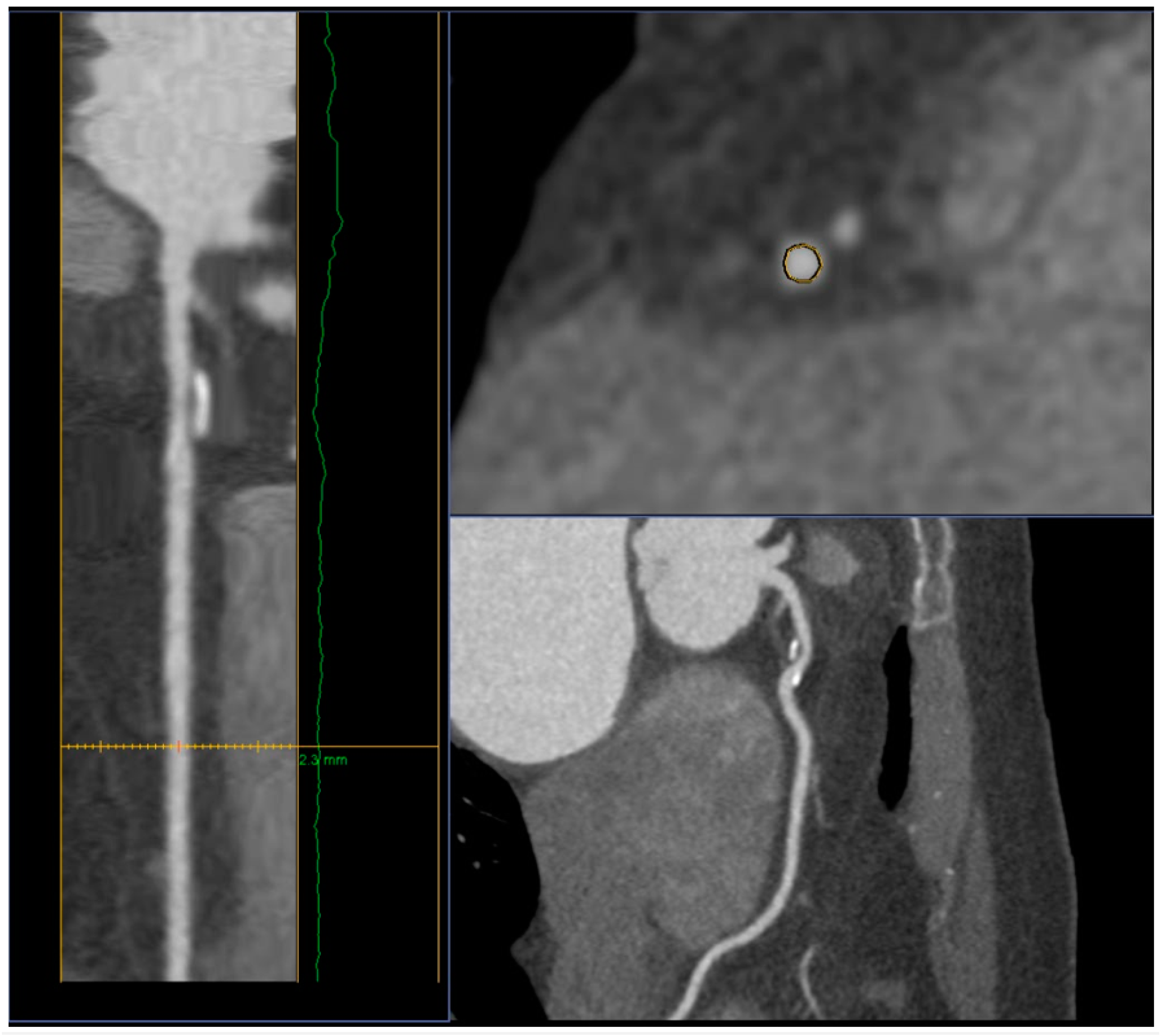
- presence of a low-density area within the plaque (less than 30 HU);
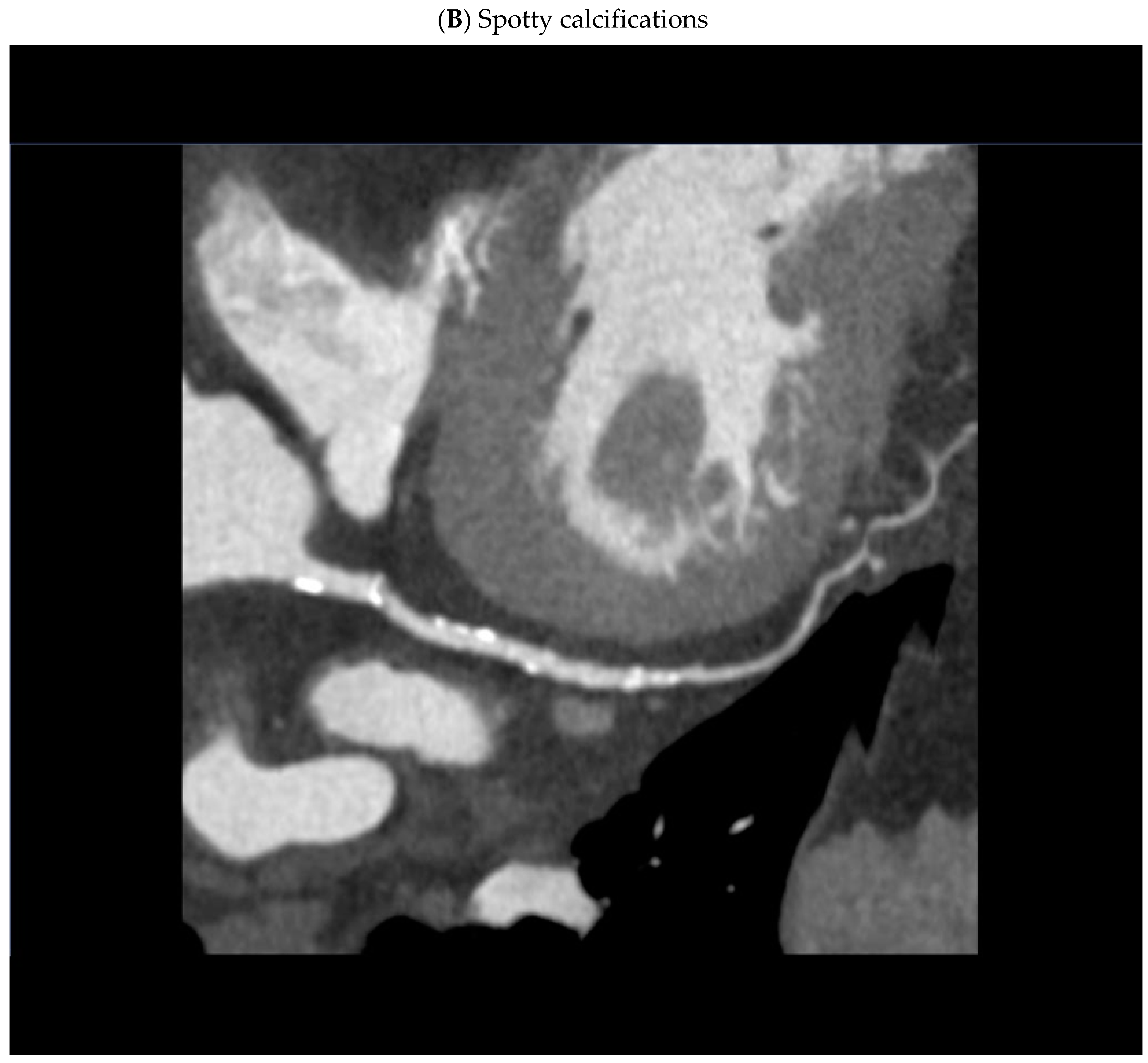
- spotty calcifications within the plaque - uneven inclusions of small calcium deposits less than 3 mm;
- napkin-ring sign - a ring-shaped increase in X-ray density along the periphery of the plaque, not exceeding 130 HU;
- distance from the ostium of the vessel to the stent - the smaller the distance from the ostium to the stent, the higher the risk of developing ACS, (<39 mm for the left coronary artery and <60 mm for the right coronary artery);
- tortuosity - presence of one bend more than 90° or three bends from 45° to 90° using a 3-point angle inside the lesion;
- bifurcation lesion.
The role of imaging methods in evaluating the effectiveness of lipid-lowering therapy
- if there is a significant increase of LDL in patients with a very high risk (above 4.0 mmol/l), it is recommended to consider an initiation of a statin and ezetimibe, preferably in one tablet or capsule.
- if there is a significant increase of LDL in patients with extreme or very high risk (above 5.0 mmol/l), it is recommended to consider an initiation of a statin at the maximum tolerated dose + ezetimibe + PCSK9 inhibitor: Alirocumab, Evolocumab or Inclisiran.
Conclusion
References
- Timmis, A.; Vardas, P.; Townsend, N.; et al. European Society of Cardiology: cardiovascular disease statistics 2021. Eur Heart J. 2022, 43, 716–799. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Yoo, W.; Alesh, I.; et al. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: a Mendelian randomization analysis. J Am CollCardiol 2012, 60, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Domanski, M.J.; Tian, X.; Wu, C.O.; et al. Time course of LDL cholesterol exposure and cardiovascular disease event risk. J Am CollCardiol 2020, 76, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Metelskaya, V.A.; Shalnova, S.A.; Deev, A.D.; et al. Analysis of the prevalence of indicators characterizing the atherogenicity of the spectrum of lipoproteins among residents of the Russian Federation (ESSE-RF study). Preventive Medicine 2016, 19, 15–23. [Google Scholar] [CrossRef]
- Shah, P.K. Mechanisms of plaque vulnerability and rupture. J Am CollCardiol. 2003, 41 (4 Suppl S), 15S–22S. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.M.; Davies, M.J. Vulnerable plaque. Relation of characteristics to degree of stenosis in human coronary arteries. Circulation. 1996, 94, 928–31. [Google Scholar] [CrossRef]
- Ako, J.; Hibi, K.; Tsujita, K.; et al. Effect of alirocumab on coronary atheroma volume in japanese patients with acute coronary syndrome - The ODYSSEY J-IVUS trial. Circ J 2019, 83, 2025–2033. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; PuriR, *!!! REPLACE !!!*; Anderson, T.; et al. Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients: The GLAGOV Randomized Clinical Trial. JAMA. 2016, 316, 2373–2384. [Google Scholar] [CrossRef]
- Räber, L.; Ueki, Y.; Otsuka, T.; et al. Effect of alirocumab added to high-intensity statin therapy on coronary atherosclerosis in patients with acute myocardial infarction: the PACMAN-AMI randomized clinical trial. JAMA 2022, 327, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Wang, Z.J.; Ma, X.T.; et al. Effect of alirocumab on coronary plaque in patients with coronary artery disease assessed by optical coherence tomography. Lipids Health Dis. 2021, 20, 106. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Kataoka, Y.; Nissen, S.E.; et al. Effect of evolocumab on coronary plaque phenotype and burden in statin-treated patients following myocardial infarction. JACC Cardiovasc Imaging 2022, 15, 1308–1321. [Google Scholar] [CrossRef] [PubMed]
- Yano, H.; Horinaka, S.; Ishimitsu, T. Effect of evolocumab therapy on coronary fibrous cap thickness assessed by optical coherence tomography in patients with acute coronary syndrome. J Cardiol 2020, 75, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Sugizaki, Y.; Otake, H.; Kawamori, H.; et al. Adding alirocumab to rosuvastatin helps reduce the vulnerability of thin-cap fibroatheroma: an ALTAIR trial report. JACC Cardiovasc Imaging 2020, 13, 1452–1454. [Google Scholar] [CrossRef] [PubMed]
- Pérez de Isla, L.; Díaz-Díaz, J.L.; Romero, M.J.; et al. Alirocumab and Coronary Atherosclerosis in Asymptomatic Patients with Familial Hypercholesterolemia: The ARCHITECT Study. Circulation. 2023, 147, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, M.C. The vulnerable and unstable atherosclerotic plaque. CardiovascPathol. 2010, 19, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Kolodgie, F.D.; Burke, e.t. al. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Anatomic features in victims of sudden coronary death: coronary artery pathology. Circulation. 1992, 85, 119–124 PMID: 1728500. [Google Scholar] [PubMed]
- Sidharta, S.L.; Baillie, T.J.; Howell, S.; et al. Evaluation of human coronary vasodilator function predicts future coronary atheroma progression. Heart 2018, 104, 1439–1446. [Google Scholar] [CrossRef]
- Malaiapan, Y.; Leung, M.; White, A.J. The role of intravascular ultrasound in percutaneous coronary intervention of complex coronary lesions. Cardiovasc Diagn Ther. 2020, 10, 1371–1388. [Google Scholar] [CrossRef] [PubMed]
- Räber, L.; Mintz, G.S.; Koskinas, K.C.; et al. Intracoronary Imaging Part 1: Guidance Optimization of Coronary Interventions An Expert Consensus Document of the European Association of Percutaneous Cardiovascular Interventions /, L. Räber [et al.]. Eur. Heart J. 2018, 39, 3281–3300. [Google Scholar] [CrossRef]
- Kume, T.; Akasaka, T. ; KawamotoT; et al Assessment of coronary arterial thrombus by optical coherence tomography. Am, J. Cardiol. 2006, 97, 1713–1717. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Imanishi, T.; Takarada, S.; et al. Assessment of culprit lesion morphology in acute mycardial infarction: ability of optical coherence tomography compared with intravascular ultrasound coronary angioscopy. J. Am. Coll. Cardiol. 2007, 50, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; et al. Lessons From Sudden Coronary Death: A Comprehensive Morphological Classification Scheme for Atherosclerotic Lesions. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef]
- Gutiérrez-Chico, J.L.; Alegría-Barrero, E.; Teijeiro-Mestre, R.; et al. Optical Coherence Tomography From Research to Practice Eur Heart, J. Cardiovasc. Imaging. 2012, 13, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Kume, T. ; UemuraS Current Clinical Applications of Coronary Optical Coherence Tomography / T. Kume, S. Uemura. Cardiovasc. Interv. Ther. 2018, 33, 1–10. [Google Scholar] [CrossRef]
- Maehara, A.; Matsumura, M.; Ali, Z.A.; et al. IVUS-Guided Versus OCT-Guided Coronary Stent Implantation: A Critical Appraisal. JACC Cardiovasc Imaging. 2017, 10, 1487–1503. [Google Scholar] [CrossRef] [PubMed]
- Bourantas, C.V.; Jaffer, F.A.; Gijsen, F.J.; et al. Hybrid intravascular imaging: recent advances, technical considerations, and current applications in the study of plaque pathophysiology. Eur. Heart J. 2017, 38, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Cury, R.C.; Abbara, S.; Achenbach, S.; et al. CAD-RADS(TM) Coronary Artery Disease - Reporting and Data System. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr. 2016, 10, 269–81. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Lin, A.; Kuronuma, K.; et al. Association of Plaque Location and Vessel Geometry Determined by Coronary Computed Tomographic Angiography With Future Acute Coronary Syndrome-Causing Culprit Lesions. JAMA Cardiol. 2022, 7, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Leber, A.W.; Becker, A.; Knez, A.; et al. Accuracy of 64-Slice Computed Tomography to Classify and Quantify Plaque Volumes in the Proximal Coronary System. J Am CollCardiol. 2006, 47, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, M.; Tanaka, A.; Kitabata, H.; et al. Comparison of diagnostic accuracy between multidetectorcomputed tomography and virtual histology intravascular ultrasound for detecting optical coherence tomography-derived fibroatheroma. CardiovascIntTher. 2013, 29, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, U.; Moselewski, F.; Nieman, K.; et al. Noninvasive Assessment of Plaque Morphology and Composition in Culprit and Stable Lesions in Acute Coronary Syndrome and Stable Lesions in Stable Angina by Multidetector Computed Tomography. J Am CollCardiol. 2006, 47, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Pundziute, G.; Schuijf, J.D.; Jukema, J.W.; et al. Head-to-Head Comparison of Coronary Plaque Evaluation Between Multislice Computed Tomography and Intravascular Ultrasound Radiofrequency Data Analysis. JACC: Cardiovascular Interventions. 2008, 1, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Rudd, J.H.; Myers, K.S.; Bansilal, S.; et al. Atherosclerosis inflammation imaging with 18F-FDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med. 2008, 49, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; On, Y.K.; Lee, E.J.; et al. Reversal of vascular 18F-FDG uptake with plasma high-density lipoprotein elevation by atherogenic risk reduction. J Nucl Med. 2008, 49, 1277–1282. [Google Scholar] [CrossRef] [PubMed]
- Rogers, I.S.; Nasir, K.; Figueroa, A.L.; et al. Feasibility of FDG imaging of the coronary arteries: comparison between acute coronary syndrome and stable angina. JACC Cardiovasc Imaging. 2010, 3, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Rudd, J.H.; Narula, J.; Strauss, H.W.; et al. Imaging atherosclerotic plaque inflammation by fluorodeoxyglucose with positron emission tomography: ready for prime time? J Am CollCardiol. 2010, 55, 2527–2535. [Google Scholar] [CrossRef] [PubMed]
- Ezhov, M.V.; Kukharchuk, V.V.; Sergienko, I.V.; et al. Disorders of lipid metabolism. Clinical Guidelines 2023. Russian Journal of Cardiology. 2023, 28, 5471. [Google Scholar] [CrossRef]
- Zyryanov, S.K.; Butranova, O.I. New opportunities for lowering lowdensity lipoprotein cholesterol: comparative characteristics of PCSK9-targeted therapy. Russian Journal of Cardiology. 2022, 27, 5271. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





