Submitted:
22 July 2023
Posted:
24 July 2023
You are already at the latest version
Abstract
Keywords:
Introduction
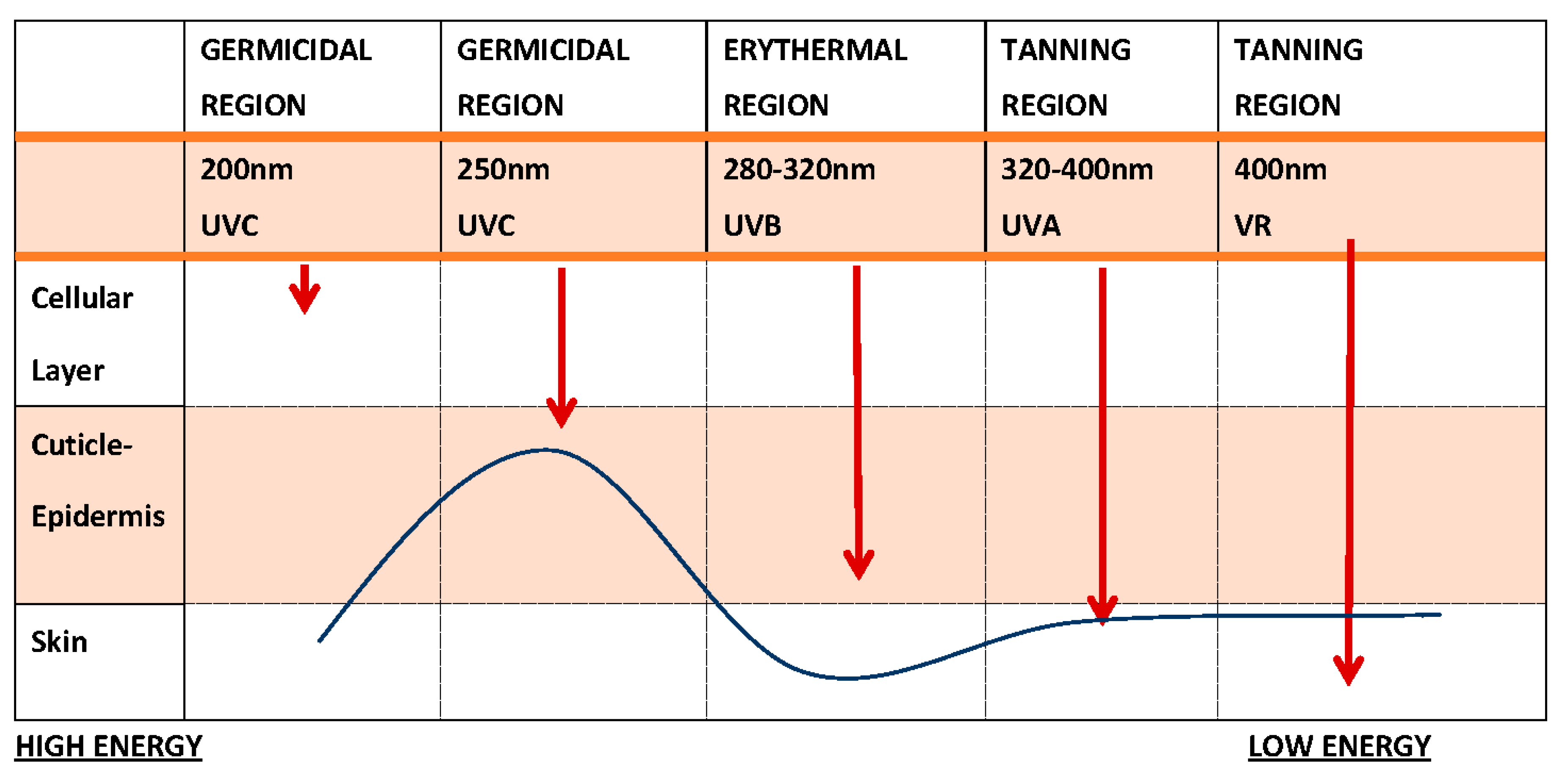
1. UV Radiation Types and Consequences of Exposure
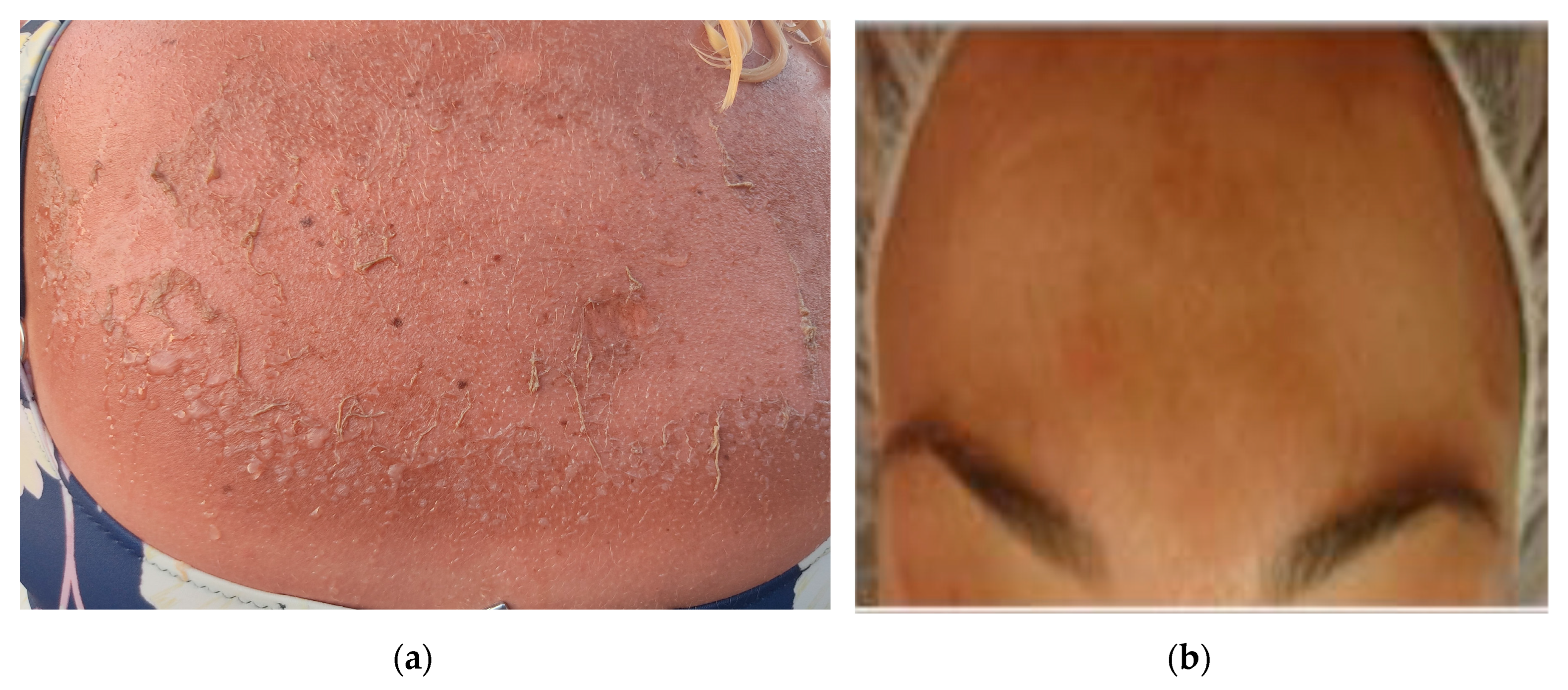
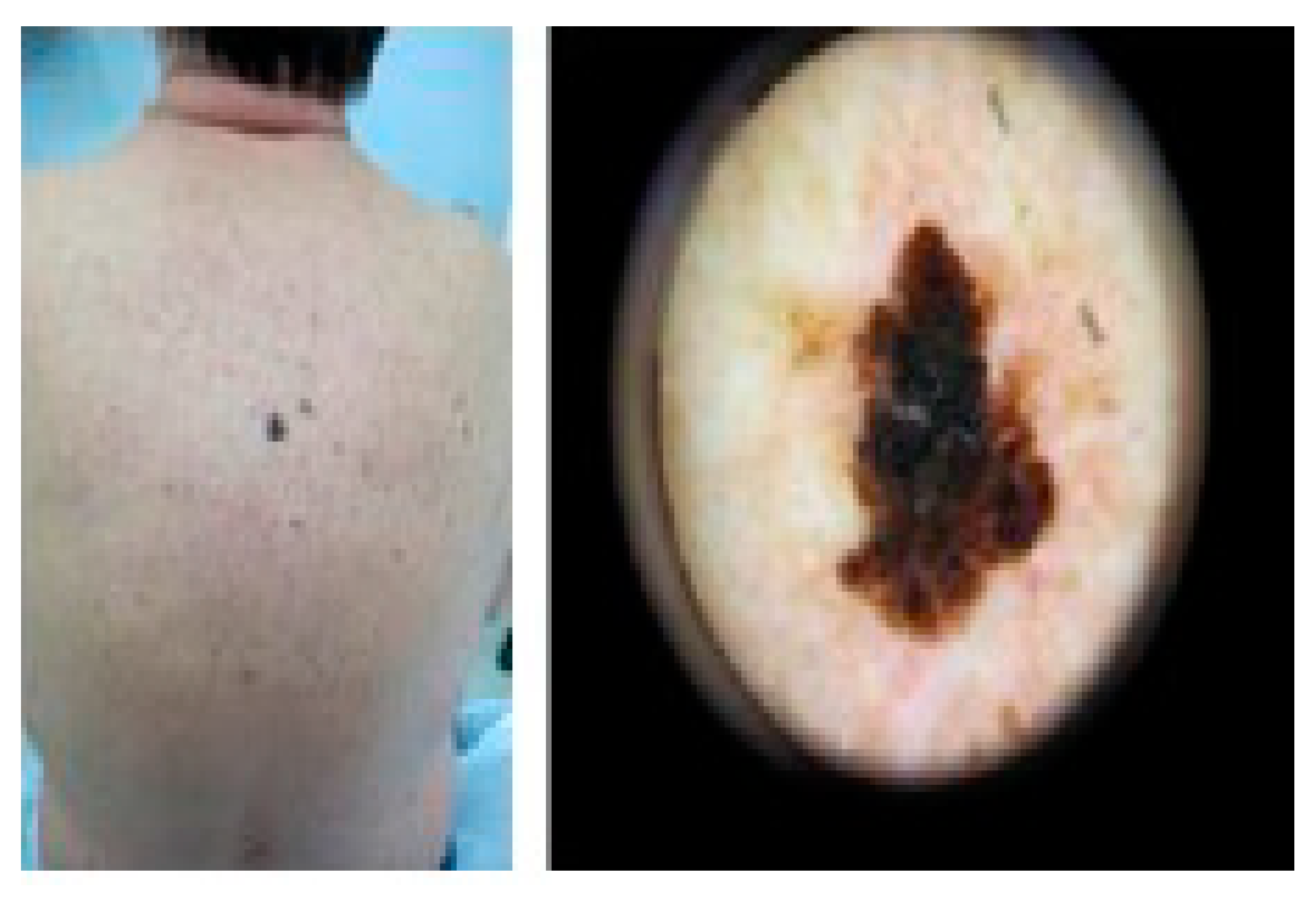
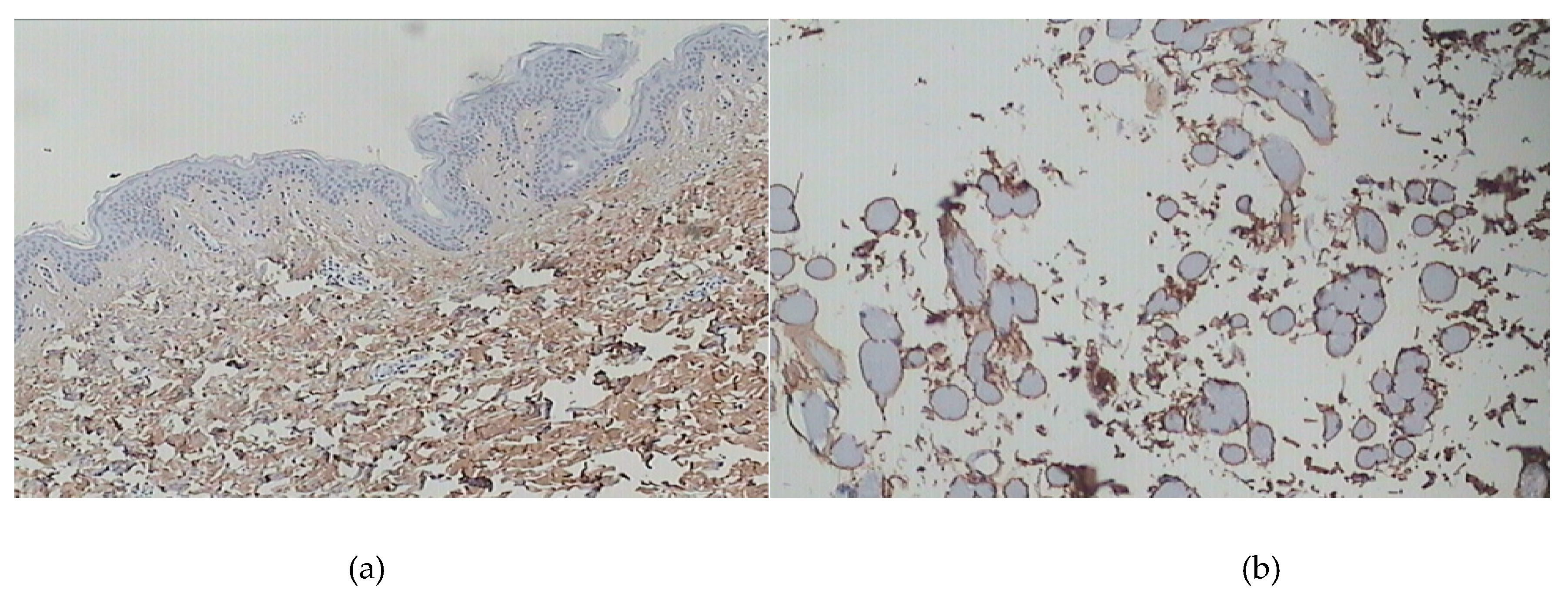
2. Skin Disorders
3. Dietary Carotenoids
4. Carotenoids in the Skin
5. Factors Acting Skin Carotenoid Levels
6. Mechanisms of Skin Protection by Carotenoids
7. Inhibition of Lipid Peroxidation
8. Visible Light-Absorbing Coloured Carotenoids - Photoprotection
9. Carotenoids in Dermoaesthetics
Discussion
Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lai-Cheong, J.E.; McGrath, J.A. Structure and function of skin, hair and nails. Medicine (Baltimore) 2017, 45, 347–351. [Google Scholar] [CrossRef]
- Dąbrowska, A.K.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.D.; Rossi, R.M. The relationship between skin function, barrier properties, and body-dependent factors. Ski. Res. Technol. 2018, 24, 165–174. [Google Scholar] [CrossRef]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Nutraceuticals for skin care: A comprehensive review of human clinical studies. Nutrients 2018, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Geng, R.; Kang, S.-G.; Huang, K.; Tong, T. Boosting the Photoaged Skin: The Potential Role of Dietary Components. Nutrients 2021, 13, 1691. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J. An overview of carotenoids, apocarotenoids and vitamin A in agro-food, nutrition, health and disease. Mol. Nutr. Food Res. 2019, 63, 1801045. [Google Scholar] [CrossRef]
- Igor, V.E.; Werner, G. Optical detection methods for carotenoids in human skin. Arch. Biochem. Biophys. 2015, 572, 101–111. [Google Scholar] [CrossRef]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.-F. Microalgal Carotenoids: A Review of Production, Current Markets, Regulations, and Future Direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef]
- Biskanaki, F. Relationship between Cosmetic and Aesthetic Non-Invasive Machines with Health and the Environment. Proceedings of the 10th Panhellenic Conference Life Sciences in the 21st Century, pp. 65, Panhellenic Association of Bioscientists, 2016. Athens.
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Nutraceuticals for skin care: A comprehensive review of human clinical studies. Nutrients 2018, 10, 403. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D and the skin: Physiology and pathophysiology. Rev. Endocr. Metab. Disord. 2012, 13, 3–19. [Google Scholar] [CrossRef]
- Rittie, L.; Fisher, G.J. Natural and sun-induced aging of human skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef]
- Solano, F. Photoprotection and Skin Pigmentation: Melanin-Related Molecules and Some Other New Agents Obtained from Natural Sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Park, I.; Chang, S.E.; Lim, W.; Kim, M.S.; Park, G.H.; Chae, J.B.; Huh, C.H.; Na, J.I. Augmented Intelligence Dermatology: Deep Neural Networks Empower Medical Professionals in Diagnosing Skin Cancer and Predicting Treatment Options for 134 Skin Disorders. J. Investig. Dermatol. 2020, 140, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Parisi, R.; Symmons, D.P.M.; Griffiths, C.E.M.; Ashcroft, D.M.; Identification and Management of Psoriasis and Associated Comorbidity (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J. Investig. Dermatol. 2020, 133, 377–385. [Google Scholar] [CrossRef]
- Rencz, F.; Gulácsi, L.; Péntek, M.; Baji, P.; Brodszky, V.; Gyula, R.; ... Szegedi, A. Work productivity loss and cost-effectiveness of etanercept in moderate to severe psoriasis in Hungary. Acta Dermato-Venereologica 2018, 98, 560–566.
- Sohn, A.; Qureshi, A.A. The economic burden of skin disease in the United States.
- de Gruijl, F. R.; Rebel, H. G. Photocarcinogenesis: mechanisms, models, and human relevance. Photochemical & Photobiological Sciences 2021, 20, 39–59. [Google Scholar]
- Wang, S. Q.; Dusza, S. W.; Scope, A.; Braun, R. P.; Marghoob, A. A. The biology of sunburn. Journal of the American Academy of Dermatology 2018, 84, 928–946. [Google Scholar]
- Balwani, M.; Desnick, R. J.; Anderson, K. E.; Bloomer, J.; Elias, S.; Gochuico, B. R.; Bissell, D. M. Disease burden and patient-reported outcomes in patients with Erythropoietic Protoporphyria (EPP). Orphanet Journal of Rare Diseases 2020, 15, 1–13. [Google Scholar]
- Wahlin, S.; Asplund, J.; Von Euler, M.; Sandberg, S.; Harper, P.; Andersson, C. Clinical characterization of erythropoietic protoporphyria in Sweden: A population-based study. Journal of the American Academy of Dermatology 2020, 82, 1197–1204. [Google Scholar]
- Warren, M. J.; Elder, G. H. Porphyria and its impact on the skin. Experimental Dermatology 2020, 29(7), 644–653. [Google Scholar]
- Lenglet, H.; Sommer, J. R.; Urech, A.; Gouya, L.; Marzin, K.; Minder, E. I.; Frank, J. Skin manifestations and associated morbidity in inherited porphyrias: experience from the Swiss Porphyria Centre. Journal of the European Academy of Dermatology and Venereology 2020, 34(2), 416–425. [Google Scholar]
- Lim, H. W.; Collins, S. A.; Resnik, S. Photosensitivity reactions and disorders. In Fitzpatrick's Dermatology, 9th Edition 2020; pp. 349-357.
- Young, A. R. Acute and chronic effects of ultraviolet radiation: molecular mechanisms and cellular responses. Physiological Reviews 2018, 98(2), 767–815. [Google Scholar]
- Zhang, L.; Lu, S.; Wei, C.; Quan, T. UVB-induced skin pigmentation: an update. International Journal of Molecular Sciences 2020, 21(14), 5116. [Google Scholar]
- Fisher, D. E.; James, W. D. The effects of ultraviolet radiation on the skin. In Fitzpatrick's Dermatology, 9th Edition 2020; pp. 346-348.
- Heckman, C. J.; Darlow, S.; Cohen-Filipic, J.; Kloss, J. D.; Manne, S. L.; Munshi, T.; Perlis, C. S. Psychosocial Correlates of Sunburn among Young Adult Women. International Journal of Environmental Research and Public Health 2012, 9, 2241–2251 . [Google Scholar] [CrossRef]
- Shipman, G. W.; et al. A protective Langerhans cell–keratinocyte axis that is dysfunctional in photosensitivity. Science Translational Medicine 2018, 10, eaap9527. [Google Scholar] [CrossRef]
- Hassan, S.; Purdie, K. J.; Wang, J.; Harwood, C. A.; Proby, C. M.; Pourreyron, C.; Mladkova, N.; Nagano, A.; Dhayade, S.; Athineos, D.; et al. A Unique Panel of Patient-Derived Cutaneous Squamous Cell Carcinoma Cell Lines Provides a Preclinical Pathway for Therapeutic Testing. International Journal of Molecular Sciences 2019, 20, 3428 . [Google Scholar] [CrossRef]
- Hatakeyama, M.; Fukunaga, A.; Washio, K.; Taguchi, K.; Oda, Y.; Ogura, K.; Nishigori, C. Anti-Inflammatory Role of Langerhans Cells and Apoptotic Keratinocytes in Ultraviolet-B–Induced Cutaneous Inflammation. Journal of Immunology 2017, 199(8), 2937–2947. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Nishioka, K. Action spectrum and minimal erythema dose (MED) for ultraviolet B radiation-induced erythema in human skin: a review. Journal of Dermatological Science 2021, 102(2), 77–81. [Google Scholar]
- Lehmann, M.; Pfahlberg, A. B.; Sandmann, H.; Uter, W.; Gefeller, O. Public Health Messages Associated with Low UV Index Values Need Reconsideration. International Journal of Environmental Research and Public Health 2019, 16, 2067 . [Google Scholar] [CrossRef]
- Vierkötter, A.; Krutmann, J. Environmental influences on skin aging and ethnic-specific manifestations. In Textbook of Aging Skin 2012; pp. 99-112. Springer, Cham.
- Bernard, J. J.; Gallo, R. L.; Krutmann, J. Photoimmunology: how ultraviolet radiation affects the immune system. Nature Reviews Immunology 2019, 19, 688–701 . [Google Scholar] [CrossRef]
- Grandi, C.; D’Ovidio, M. C. Balance between Health Risks and Benefits for Outdoor Workers Exposed to Solar Radiation: An Overview on the Role of Near Infrared Radiation Alone and in Combination with Other Solar Spectral Bands. International Journal of Environmental Research and Public Health 2020, 17, 1357 . [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R. M.; Janjetovic, Z.; Kim, T.-K.; Böhm, M.; Steinbrink, K.; Reiter, R. J.; Kleszczyński, K.; Slominski, A. T. Protective Role of Melatonin and Its Metabolites in Skin Aging. International Journal of Molecular Sciences 2022, 23, 1238 . [Google Scholar] [CrossRef]
- Tewari, A.; Sarkany, R. Ultraviolet radiation and cutaneous infection. British Journal of Dermatology 2018, 178, 449–460. [Google Scholar]
- Tran, A. Q.; Hoeffler, J. P.; Chen, J. G. Artificial light at night and cancer: Global study investigates connection. Environmental Health Perspectives 2018, 126(4), 047002. [Google Scholar]
- Biskanaki, F.; Rallis, E.; Skouras, G.; Stofas, A.; Thymara, E.; Kavantzas, N.; Lazaris, A. C.; Kefala, V. Impact of Solar Ultraviolet Radiation in the Expression of Type I Collagen in the Dermis. Cosmetics 2021, 8(2), 46. [Google Scholar] [CrossRef]
- Dizdaroglu, M. Oxidatively induced DNA damage: Mechanisms, repair and disease. Cancer Letters 2012, 327, 26–47. [Google Scholar] [CrossRef] [PubMed]
- Dong, K. K.; Damaghi, N. Topical cosmeceuticals for photoaging and photodamaged skin. Dermatologic Clinics 2020, 38(2), 167–179. [Google Scholar]
- Lan, C.-C. E.; Hung, Y.-T.; Fang, A.-H.; Ching-Shuang, W. Effects of irradiance on UVA-induced skin aging. Journal of Dermatological Science 2019, 94(1), 220–228. [Google Scholar] [CrossRef]
- Biskanaki, F.; Kefala, V.; Lazaris, A. C.; Rallis, E. Aging and the Impact of Solar Ultraviolet Radiation on the Expression of Type I and Type VI Collagen. Cosmetics 2023, 10(2), 48. [Google Scholar] [CrossRef]
- Zorina, A.; Zorin, V.; Kudlay, D.; Kopnin, P. Molecular Mechanisms of Changes in Homeostasis of the Dermal Extracellular Matrix: Both Involutional and Mediated by Ultraviolet Radiation. Int. J. Mol. Sci. 2022, 23, 6655 . [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.; Kim, M. Structural and Functional Changes and Possible Molecular Mechanisms in Aged Skin. Int. J. Mol. Sci. 2021, 22, 12489 . [Google Scholar] [CrossRef]
- Biskanaki, F.; Kefala, V.; Lazaris, A.C.; Rallis, E. Aging and the Impact of Solar Ultraviolet Radiation on the Expression of Type I and Type VI Collagen. Cosmetics 2023, 10(2), 48. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Photoprotection by dietary carotenoids: Concept, mechanisms, evidence and future development. Mol. Nutr. Food Res. 2012, 56, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Anunciato, T.P.; da Rocha Filho, P.A. Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceuticals. J. Cosmet. Dermatol. 2012, 11, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Stinco, C.M.; Mapelli-Brahm, P. Skin Carotenoids in Public Health and Nutri-cosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene. Nutrients 2019, 11(5), 1093. [Google Scholar] [CrossRef]
- Quideau, St.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. GDCh 2011, 50(3), 586–621. [Google Scholar] [CrossRef]
- Manfred Eggersdorfer, Adrian Wyss. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [CrossRef]
- Dias, M.G.; Olmedilla-Alonso, B.; Hornero-Méndez, D.; Mercadante, A.Z.; Osorio, C.; Vargas-Murga, L.; Meléndez-Martínez, A.J. Comprehensive Database of Carotenoid Contents in Ibero-American Foods. A Valuable Tool in the Context of Functional Foods and the Establishment of Recommended Intakes of Bi-oactives. J. Agric. Food Chem. 2018, 66, 5055–5107. [Google Scholar] [CrossRef]
- Singh, J.; Fan, D.; Banskota, A.H.; Stefanova, R.; Khan, W.; Hafting, J.; Craigie, J.; Critchley, A.T.; Prithiviraj, B. Bioactive components of the edible strain of red alga, Chondrus crispus, enhance oxidative stress tolerance in Caenorhabditis elegans. J. Funct. Foods 2013, 5, 1180–1190. [Google Scholar] [CrossRef]
- Darvin, M.E.; Jung, S.; Schanzer, S.; Richter, H.; Kurth, E.; Thiede, G.; Meinke, M.C.; Lademann, J. Influence of the Systemic Application of Blue–Green Spirulina platensis Algae on the Cutaneous Carotenoids and Elastic Fibers in Vivo. Cosmetics 2015, 2(3), 302–312. [Google Scholar] [CrossRef]
- Rasmussen, H.M.; Muzhingi, T.; Eggert, E.M.R.; Johnson, E. Lutein, zeaxanthin, meso-zeaxanthin content in egg yolk and their absence in fish and seafood. J. Food Compos. Anal. 2012, 27, 139–144. [Google Scholar] [CrossRef]
- Álvarez, R.; Meléndez-Martínez, A.J.; Vicario, I.M.; Alcalde, M.J. Carotenoid and Vitamin A Contents in Biological Fluids and Tissues of Animals as an Effect of the Diet: A Review. Food Rev. Int. 2015, 31, 319–340. [Google Scholar] [CrossRef]
- Lehto, S.; Buchweitz, M.; Klimm, A.; Straßburger, R.; Bechtold, C.; Ulberth, F. Comparison of food colour regulations in the EU and the US: A review of current provisions. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2017, 34, 335–355. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Roriz, C.L.; Morales, P.; Barros, L.; Ferreira, I.C.F.R. Food colorants: Challenges, opportunities and current desires of agro-industries to ensure consumer expectations and regulatory practices. Trends Food Sci. Technol. 2016, 52, 1–15. [Google Scholar] [CrossRef]
- Khachik, F. Analysis of carotenoids in nutritional studies. In Carotenoids. Volume 5: Nutrition and Health; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 2009; pp. 7–44. ISBN 978-3-7643-7501-0. [Google Scholar]
- Meléndez-Martínez, A.; Stinco, C.M.; Liu, C.; Wang, X.-D. A simple HPLC method for the comprehensive analysis of cis/trans (Z/E) geometrical isomers of carotenoids for nutritional studies. Food Chem. 2013, 138, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Burrows, T.; Rollo, M.; Williams, R.; Wood, L.; Garg, M.; Jensen, M.; Collins, C. A Systematic Review of Technology-Based Dietary Intake Assessment Validation Studies That Include Carotenoid Biomarkers. Nutrients 2017, 9, 140. [Google Scholar] [CrossRef]
- Pezdirc, K.; Hutchesson, M.J.; Williams, R.L.; Rollo, M.E.; Burrows, T.L.; Wood, L.G.; Oldmeadow, C.; Collins, C.E. Consuming High-Carotenoid Fruit and Vegetables Influences Skin Yellowness and Plasma Carotenoids in Young Women: A Single-Blind Randomized Crossover Trial. J. Acad. Nutr. Diet. 2016, 116, 1257–1265. [Google Scholar] [CrossRef]
- Van Hoang, D.; Pham, N.; Lee, A.; Tran, D.; Binns, C. Dietary Carotenoid Intakes and Prostate Cancer Risk: A Case-Control Study from Vietnam. Nutrients 2018, 10, 70. [Google Scholar] [CrossRef]
- Nishino, H.; Murakoshi, M.; Tokuda, H.; Satomi, Y. Cancer prevention by carotenoids. Arch. Biochem. Biophys. 2009, 483, 165–168. [Google Scholar] [CrossRef]
- Bonet, M.; Canas, J.A.; Ribot, J.; Palou, A. Carotenoids and their conversion products in the control of adipocyte function, adiposity and obesity. Arch. Biochem. Biophys. 2015, 572, 112–125. [Google Scholar] [CrossRef]
- Mares, J. Lutein and Zeaxanthin Isomers in Eye Health and Disease. Annu. Rev. Nutr. 2016, 36, 571–602. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; Bonet, M.L.; Borel, P.; Keijer, J.; Landrier, J.-F.; Milisav, I.; Ribot, J.; Riso, P.; Winklhofer-Roob, B.; Sharoni, Y.; et al. Mechanistic aspects of carotenoid health benefits – where are we now? Nutrition Research Reviews 2021, 34(2), 276. [Google Scholar] [CrossRef] [PubMed]
- Paula Mapelli-Brahm, Marielle Margier, Charles Desmarchelier, Charlotte Halimi, Marion Nowicki, Patrick Borel, Antonio J. Meléndez-Martínez, Emmanuelle Reboul. Comparison of the bioavailability and intestinal absorption sites of phytoene, phytofluene, lycopene and β-carotene. Food Chemistry 2019, 300, 125232. [CrossRef]
- Imran M, Ghorat F, Ul-Haq I, Ur-Rehman H, Aslam F, Heydari M, Shariati MA, Okuskhanova E, Yessimbekov Z, Thiruvengadam M, et al. Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders. Antioxidants 2020, 9(8), 706. [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [PubMed]
- Linnewiel-Hermoni, K.; Khanin, M.; Danilenko, M.; Zango, G.; Amosi, Y.; Levy, J.; Sharoni, Y. The anti-cancer effects of carotenoids and other phytonutrients reside in their combined activity. Arch. Biochem. Biophys. 2015, 572, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Sterry, W.; Lademann, J.; Vergou, T. The Role of Carotenoids in Human Skin. Molecules 2011, 16, 10491–10506. [Google Scholar] [CrossRef]
- Bohn, T.; Desmarchelier, C.; Dragsted, L.O.; Nielsen, C.S.; Stahl, W.; Rühl, R.; Keijer, J.; Borel, P. Host-related factors explaining interindividual variability of carotenoid bioavailability and tissue concentrations in humans. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D and the skin: Physiology and pathophysiology. Rev. Endocr. Metab. Disord. 2012, 13, 3–19. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids in Marine Animals. Mar. Drugs 2011, 9, 278–293. [Google Scholar] [CrossRef]
- Lehto, S.; Buchweitz, M.; Klimm, A.; Straßburger, R.; Bechtold, C.; Ulberth, F. Comparison of food colour regulations in the EU and the US: A review of current provisions. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2017, 34, 335–355. [Google Scholar] [CrossRef] [PubMed]
- Toh, D.W.K.; Loh, W.W.; Sutanto, C.N.; Yao, Y.; Kim, J.E. Skin Carotenoid Status and Plasma Carotenoids: Biomarkers of Dietary Carotenoids, Fruits and Vegetables for Middle-Aged and Older Singaporean Adults. Br. J. Nutr. 2021, 126(9), 1398. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr. Rev. 2014, 72, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Sansone, R.A.; Sansone, L.A. Carrot Man: A Case of Excessive Beta-Carotene Ingestion. Int. J. Eat. Disord. 2012, 45, 816–818. [Google Scholar] [CrossRef]
- Darvin, M.E.; Sterry, W.; Lademann, J.; Vergou, T. The Role of Carotenoids in Human Skin. Molecules 2011, 16, 10491–10506. [Google Scholar] [CrossRef]
- Meinke, M.C.; Lauer, A.; Taskoparan, B.; Gersonde, I.; Lademann, J.; Darvin, M.E. Influence on the carotenoid levels of skin arising from age, gender, body mass index in smoking/non-smoking individuals. Free Radic. Antioxid. 2011, 1, 15–20. [Google Scholar] [CrossRef]
- Thiviya, P.; Gamage, A.; Piumali, D.; Merah, O.; Madhujith, T. Apiaceae as an Important Source of Antioxidants and Their Applications. Cosmetics 2021, 8, 111 . [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef]
- Tanaka, T.; Morishita, Y.; Suzui, M.; Kojima, T.; Okumura, A.; Mori, H. Chemoprevention of mouse urinary bladder carcinogenesis by the naturally occurring carotenoid astaxanthin. Carcinogenesis 2015, 15(1), 15–19. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef]
- Zerres, S.; Stahl, W. Carotenoids in human skin. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865(11), 158588. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Sterry, W.; Lademann, J.; Vergou, T. The Role of Carotenoids in Human Skin. Molecules 2011, 16, 10491–10506. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Nam, G.-W. Sunscreen Boosting Effect by Solid Lipid Nanoparticles-Loaded Fucoxanthin Formulation. Cosmetics 2020, 7(1), 14. [Google Scholar] [CrossRef]
- Terao, J.; Minami, Y.; Bando, N. Singlet molecular oxygen quenching activity of carotenoids: Relevance to protection of the skin from photoaging. J. Clin. Biochem. Nutr. 2011, 48, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.; Freitas, M.; Silva, A.M.S.; Carvalho, F.; Fernandes, E. Antioxidant and pro-oxidant activities of carotenoids and their oxidation products. Food Chem. Toxicol. 2018, 120, 681–699. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.G.; Olmedilla-Alonso, B.; Hornero-Méndez, D.; Mercadante, A.Z.; Osorio, C.; Vargas-Murga, L.; Meléndez-Martínez, A.J. Comprehensive Database of Carotenoid Contents in Ibero-American Foods. A Valuable Tool in the Context of Functional Foods and the Establishment of Recommended Intakes of Bioactives. J. Agric. Food Chem. 2018, 66, 5055–5107. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J. Carotenoides: Estructura, propiedades y funciones. In Carotenoidesenagroalimentación y salud; Meléndez-Martínez, A., Ed.; Editorial Terracota: Ciudad de México, México, 2017. [Google Scholar]
- Caseiro, M.; Ascenso, A.; Costa, A.; Creagh-Flynn, J.; Johnson, M.; Simões, S. Lycopene in human health. LWT 2020, 127, 109323. [Google Scholar] [CrossRef]
- Kurzawa, M.; Wilczyńska, E.; Brudzyńska, P.; Sionkowska, A. Total Phenolic Content, Antioxidant Capacity and UV Radiation Protection Properties of Marigold (Calendula officinalis), Carrot (Daucus carota), Tomato (Solanum lycopersicum), and Hop (Humulus lupulus) Extracts. Cosmetics 2022, 9, 134 . [Google Scholar] [CrossRef]
- Darvin, M.E.; Jung, S.; Schanzer, S.; Richter, H.; Kurth, E.; Thiede, G.; Meinke, M.C.; Lademann, J. Influence of the Systemic Application of Blue–Green Spirulina platensis Algae on the Cutaneous Carotenoids and Elastic Fibers in Vivo. Cosmetics 2015, 2, 302–312 . [Google Scholar] [CrossRef]
- Rebelo, B.A.; Farrona, S.; Ventura, M.R.; Abranches, R. Canthaxanthin, a Red-Hot Carotenoid: Applications, Synthesis, and Biosynthetic Evolution. Plants 2020, 9, 1039. [Google Scholar] [CrossRef]
- Rasmussen, H.M.; Muzhingi, T.; Eggert, E.M.R.; Johnson, E. Lutein, zeaxanthin, meso-zeaxanthin content in egg yolk and their absence in fish and seafood. J. Food Compos. Anal. 2012, 27, 139–144. [Google Scholar] [CrossRef]
- Barreiro, C.; Barredo, J.L. Carotenoids Production: A Healthy and Profitable Industry. In Microbial Carotenoids; Barreiro, C., Barredo, J.L., Eds.; Humana Press: New York, NY, USA, 2018; pp. 7–22. [Google Scholar]
- European Food Safety Authority (EFSA). Scientific Opinion on the re-evaluation of canthaxanthin (E 161 g) as a food additive. EFSA J. 2010, 8, 1852–1893. [CrossRef]
- Damaziak, K.; Marzec, A.; Riedel, J.; Szeliga, J.; Koczywąs, E.; Cisneros, F.; Michalczuk, M.; Łukasiewicz, M.; Gozdowski, D.; Siennicka, A.; et al. Effect of dietary canthaxanthin and iodine on the production performance and egg quality of laying hens. Poult. Sci. 2018, 97, 4008–4019. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




