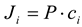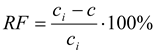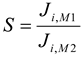1. Introduction
Selective removal of metal ion from aqueous solutions using polymer inclusion membranes (PIMs) can be an effective method of separation. This technique is studied for various application, i.e. hydrometallurgical recovery of metals from electronic waste. We are seeing an ever-increasing consumption of lithium-ion batteries and accumulators that power mobile phones, electronic equipment and electric cars. The utilization of the stream of spent lithium–ion batteries (LIBs) will be a big challenge. The hydrometallurgical processes play an important role in recycling of spent batteries, accumulators and catalysts which are a rich source of strategic metals needed in many industries. One of the main process in this recycling technology is acid leaching of metallic waste in order to transfer metal ions (cations or anions) into the aqueous solutions. Separation of metals is the next important stage in hydrometallurgical process. We know many various methods of separation such as solvent extraction, ion exchange, adsorption, precipitation and membrane processes [
1]. The proposal to use polymer membranes for separation of metal ions is noteworthy due to a number of advantages of this method, such as: a low cost of producing membranes and a simple design of the system consisting of a source phase/a membrane/and a receiving phase. The synthesis of PIM is very easy and requires small amounts of reagents. PIM consist of an ion carrier/extractant immobilized within a polymer matrix (i.e. cellulose triacetate CTA, poly(vinyl chloride) PVC, poly(vinylidene fluoride-
co-hexafluoropropylene) PVDF-HFP) [
2]. The choice of an ion carrier depends on the metal species present in aqueous solutions [
3].
An additional substance applied for PIM synthesis is a plasticizer, which gives the membrane softness and flexibility (i.e. nitrofenyl octyl ether NPOE, nitrofenyl pentyl ether NPPE, etc.) [
3,
4]. Various types of extractants/ion carriers are used for membrane synthesis. It depends on the form of the metal species present in the aqueous solution [
1]. The transport of metal ion is related to the chemical composition of the polymer membrane as well as the composition of the aqueous solutions. Many authors have proposed various mechanisms of the facilitated transport of metal ion, such as (a) carrier-diffusion, (b) fixed site jumping and mobile-site jumping mechanism wherein the extracted species jumps between fixed carrier sites [
2,
5,
6,
7]. Due to the very different proposals, Hu et al. [
4] reported that the mechanism for transport of metal ions through PIMs is still open to be verified, especially for membranes containing new ion carriers such as ionic liquids (ILs) called “green solvents” [
9]. They have many advantages such as non-volatility, high thermal stability, extracting properties [
10]. ILs are used both as the extractants and the ion carriers of metal ion [
9,
10,
11,
12,
13,
14,
15,
16].
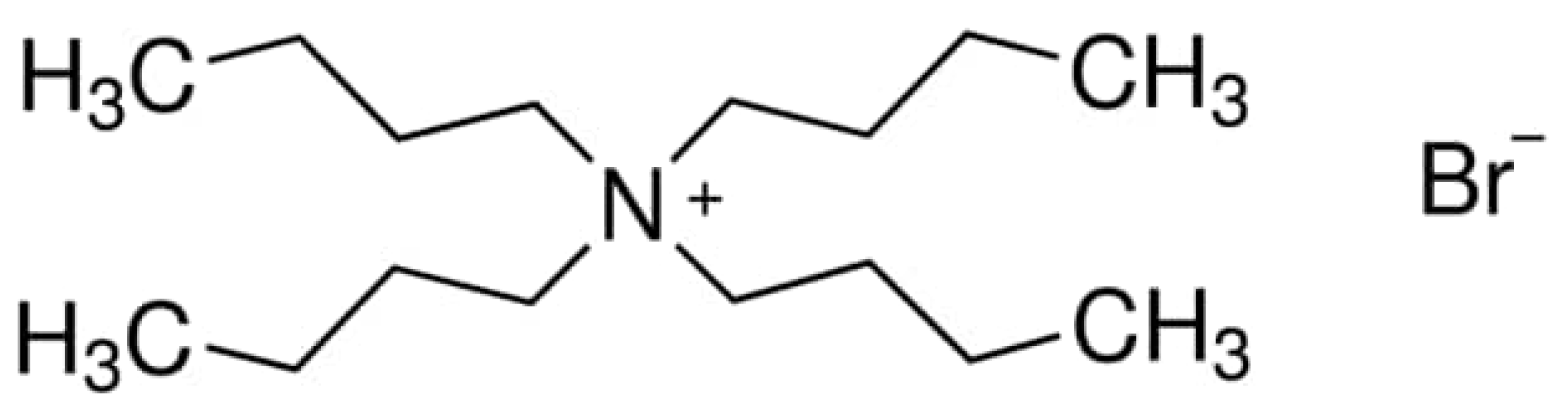
Tetrabutylammonium bromide (TBAB) is a quaternary ammonium salt, which can dissolve in both aqueous as well as in organic solvents [
17]. This reagent is cheap, non-toxic, non-corrosive and environmentally friendly. Its melting points is over 100° C and this compound is classified as an ionic liquid.
Figure 1 shows the structure of TBAB [
18]. As can be seen, this salt contains organic cation and inorganic anion. This compound was used as the extractant of Fe(III) [
19], Cr(VI) [
20,
21] and anionic dyes (e.g. golden yellow), etc. [
21].
Taking into account the extracting properties of TBAB and existing need of selective metal ion separation from various leach liquors (e.g. leach liquor of spent lithium-ion batteries, LIBs), the main aim of this work is to develop an alternative separation technique of Fe(III) from Ni(II), Co(II) and Li(I) from aqueous solutions using PIMs with TBAB as the ion carrier.
2. Materials and Methods
2.1. The inorganic chemicals
i.e. iron(III) chloride (FeCl3), cobalt(II) chloride (CoCl2·6H2O), nickel(II) chloride (NiCl2·6H2O), lithium(I) chloride (LiCl), hydrochloric acid (HCl), sulfuric acid (H2SO4 ) were of analytical grade and were purchased from POCh (Poland).
2.2. The organic chemicals
i.e. tetrabutylammonium bromide (TBAB) (ACROS, Germany, purity≥98.0%), cellulose triacetate (CTA), o-nitrophenyl octyl ether (NPOE), and dichloromethane were of analytical grade (Aldrich) and used without further purification.
2.3. Synthesis of polymer inclusion membranes (PIMs)
PIMs were prepared similar as reported in the earlier papers [
11,
12,
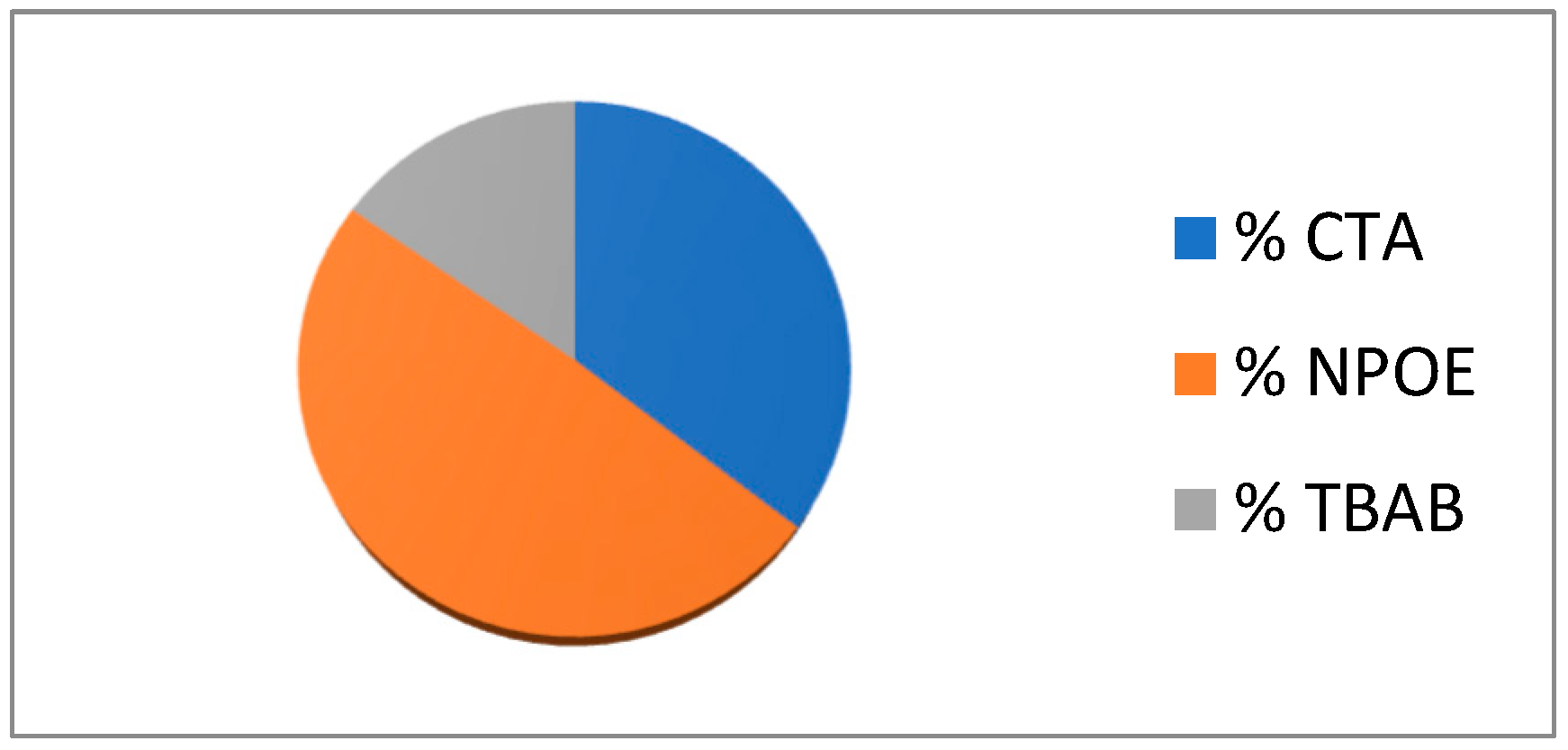
23]. The mixture containing appropriate amounts of CTA, TBAB and NPOE in dichloromethane was prepared. This solution was poured into a Petri dish. The dichloromethane was evaporated and the resulting membrane was separated by immersion in distilled water. The composition of PIM was following: 35.5% w/w CTA, 49.2% w/w NPOE and 15.3 % w/w TBAB.
Figure 2.
Composition of PIM.
Figure 2.
Composition of PIM.
2.4. Transport of metal ions experiments
The transport experiments were conducted similar as reported in the earlier papers [
11,
12,
23]. The membrane module was used for the transport of Fe(III), Ni(II), Co(II) and Li(I) from the source phase across PIM into the receiving phase. The membrane separated the two phases. The both aqueous phases were pumped with a peristaltic pump (PP1B-05A, Zalimp, Poland). The volumes of source and receiving phases were 100 cm
3, respectively. The source phase contained 0.01 M Fe(III), 0.01 M Co(II), 0.01 M Ni(II) and 0.01 M Li(I) in a hydrochloric acid solution. Sulfuric acid was used as the receiving phase. The effective membrane area was 12.56 cm
2. The aqueous phases were stirred by the magnetic stirrers. The concentration of metal ion was monitored by sampling of the source phase at regular intervals. The concentration of metal ions in aqueous phases was analyzed by means of a plasma emission spectrometer MP-AES 4200 (Agilent). The PIM transport experiments were conducted at room temperature (22°C). The kinetics of PIM transport process was described by a first-order reaction according to the following equation [
24]:
were:
c – the metal ion concentration (mol/dm3) in the source phase at some given time,
ci – the initial metal ion concentration in the source phase (mol/dm3),
t – the time (s),
k – the rate constant (s-1).
In order to calculate rate constant (k), a diagram of the dependence of ln(c/c
i) vs. time was prepared for each transport of metal ion [
23]. Permeability coefficient (P) and initial flux (J
i) were calculated according to the equations presented in previous works [
11,
12,
23]. The permeability coefficient (P) can be calculated as follows:
where V – volume of the aqueous source phase,
A – an area of effective membrane.
The initial flux (Ji) was determined as [
11,
12,
23]:
The recovery factor (RF) of metal was calculated as [
11,
12,
23]:
The selectivity coefficient (S) was defined as the ratio of initial fluxes for M
1 and M
2 metal ion according to the equation [
11,
12,
23]:
3. Results
3.1. Effect of hydrochloric acid concentration on the removal of Fe(III) across PIM from aqueous solution
One of many parameters affecting the transport of metal ion across PIM is the concentration of inorganic acid in the source phase containing various metals such as Fe(III), Ni(II), Co(II) and Li(I). In hydrochloric acid solutions metal can exist as cationic or anionic chlorocomplexes in depending on chloride ion concentration. From this reason, the effect of HCl concentration in this phase was studied. This parameter can significantly affect on the efficiency and selectivity transport of metal ion. The concentration of HCl in the source phase was varied between 1.0 mol·dm
-3 to 6 mol ·dm
-3.
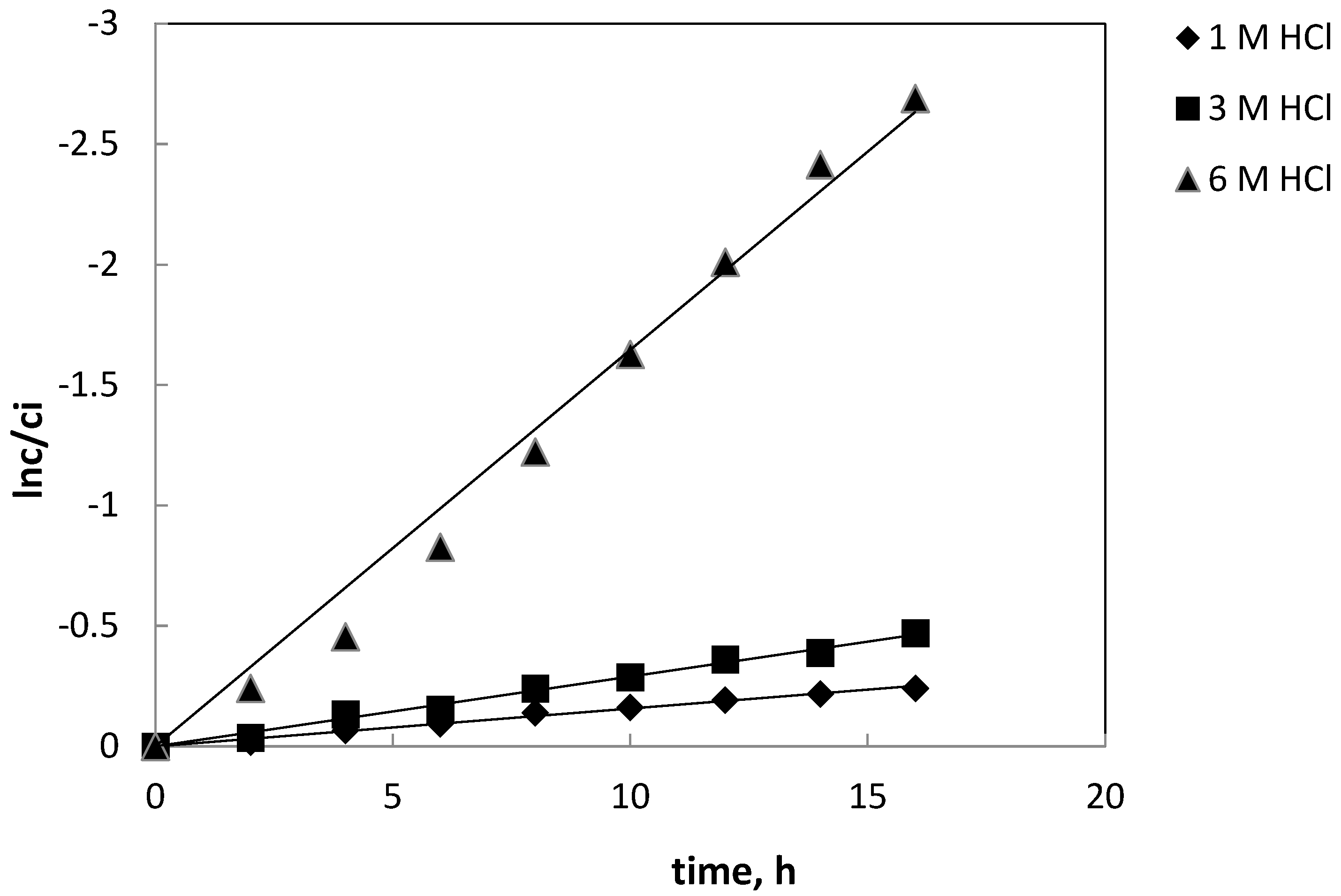
Figure 3 shows the plot of lnc/c
i versus HCl concentration for Fe(III). Other metals (i.e. Ni(II), Co(II), Li(I)) were not transferred into 1 M H
2SO
4 used as the receiving phase.
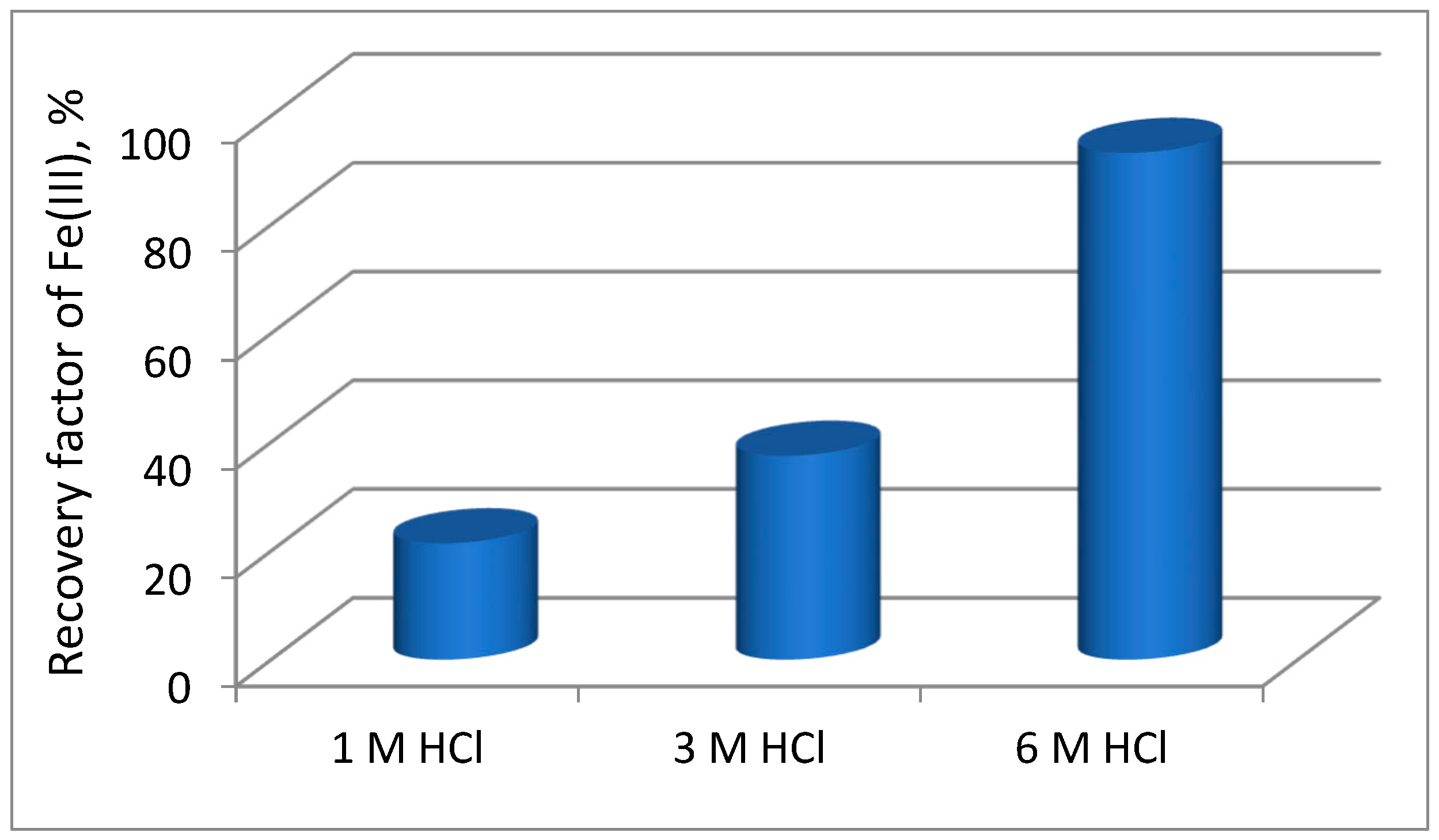
Figure 4 presented the dependence of the of the iron(III) recovery (RF, %) versus time for the transport of metal ion at various hydrochloric acid concentration. Ni(II), Co(II) and Li(I) were not transported into the receiving phase. Thus, it can be concluded that this process is very selective and allows the removal of impurities in the form of iron(III) from aqueous solutions.
Table 1 presents the kinetic parameters of the transport of Fe(III) across PIM in depending on HCl concentration in source phase. The parameter were the following: rate constant (k) and permeability coefficient (P) for different HCl concentration in the receiving phase. Based on the presented data, it can be observed that an increase in acid concentration has a significant effect on the rate constant (k). Its increase was observed from the value of 0.0157 h
-1 at 1 M HCl concentration to the value of 0.1650 h
-1 at 6 M hydrochloric acid.
The initial flux is an important parameter characterizing the transport of metal ions from aqueous solutions using PIM.
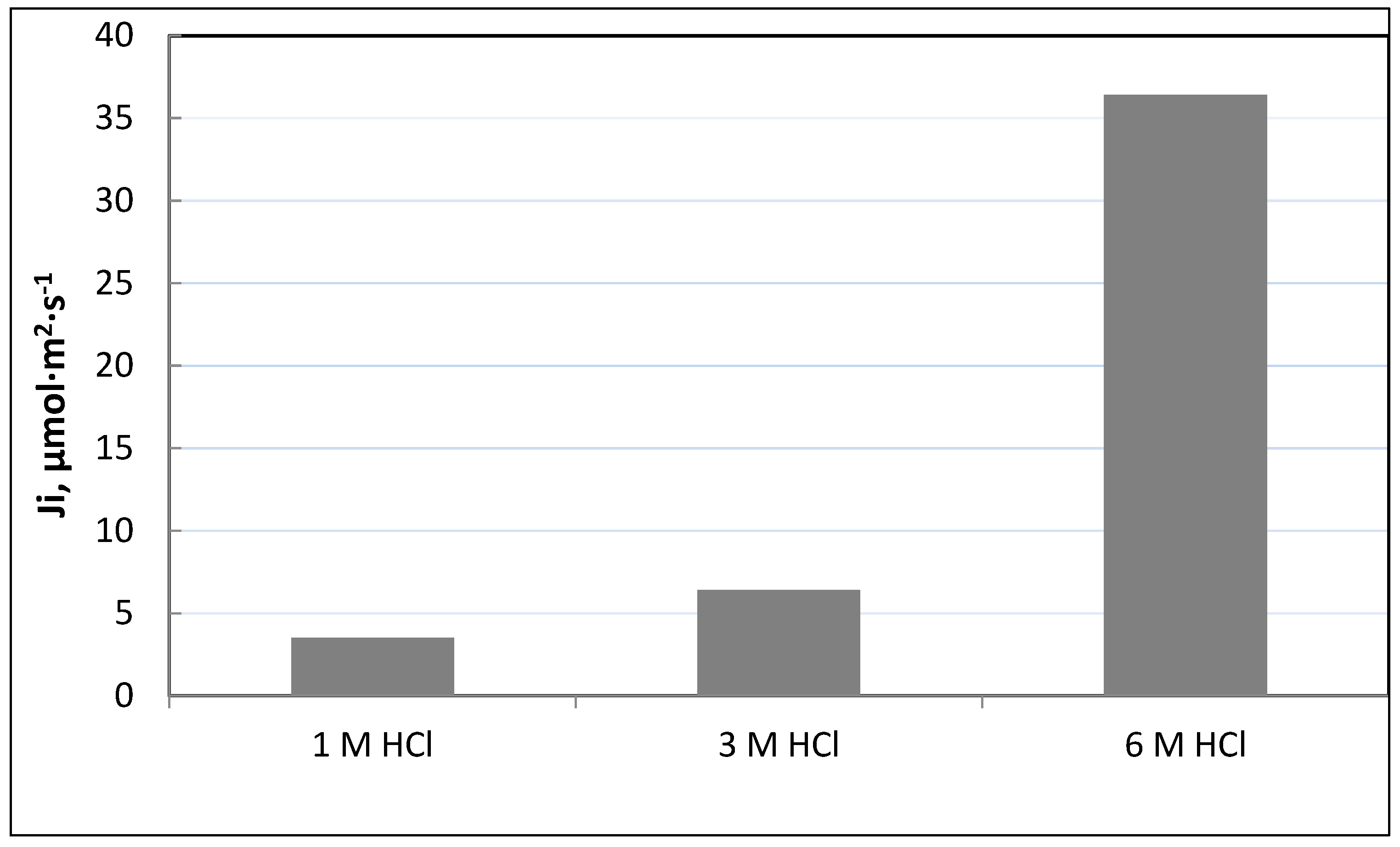
Figure 5 shows the effect of HCl concentration in the source phase on initial flux of Fe(III). As can be seen from this figure, initial flux (J
i) for the transport of Fe(III) across PIM with TBAB increases with an increasing hydrochloric acid concentration up to 6 mol·dm
3. It can be concluded that a high concentration of acid has a positive effect on the transport efficiency of Fe(III) ions and enables their selective removal.
3.2. Influence of the PIM composition on the kinetic parameter of the transport of Fe(III)
In order to ensure good properties of the membrane, it is first necessary to determine its appropriate chemical composition [
25]. This is a key issue. The studied PIM contains CTA as the polymer support, TBAB as the ion carrier and NPOE used as the plasticizer. The content of these substances significantly affects the transport and separation properties of PIMs. The content and type of an ion carrier and plasticizer is especially prominent. A number of studies have confirmed that one of the best plasticizers is NPOE, because polymer membranes containing this plasticizer show much better permeability, are flexible and soft [
26]. There are no literature reports on the effect of TBAB concentration on the transport and separation properties of the membrane. Hence, there is a need to determine the optimal concentration of this carrier in the PIM. Moreover, it is worth noting that there are no literature reports on the application of TBAB as the ion carrier of Fe(III) for production of PIM. We do not have knowledge about the possibility of using this compound for the synthesis of membranes. Hence, there is a need to determine the optimal concentration of this carrier in the PIM.
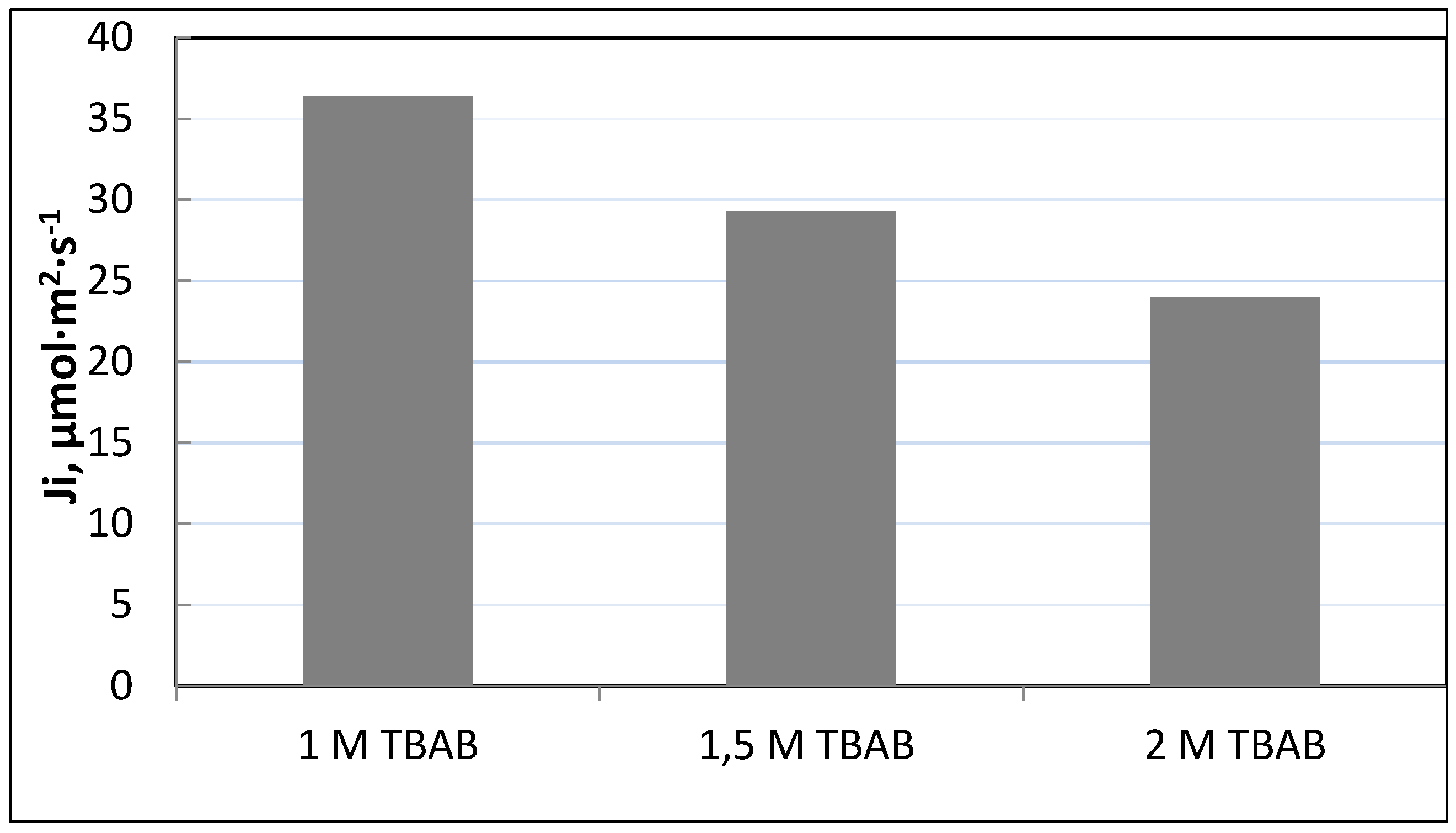
The aim of the next series of studies will be the characteristic of the influence of TBAB concentration on the transport and separation of Fe(III) from 6 M HCl. The concentration of TBAB was varied from 1 to 2 mol·dm
-3 (based on the volume of plasticizer).
Table 2 shows the composition of PIMs. 1 M sulfuric acid was used as the receiving phase. The source phase contained 0.0 1 M Fe(III), 0.0 1 M Ni(II), 0.0 1 M Co(II) and 0.0 1 M Li(I) in 6 M HCl.
The results indicated that only Fe(III) was transported from the source phase, while Co(II), Ni(II) and Li(I) ions remained in the feed solution. As can be observed from
Figure 6, initial flux (J
i) decreases with an increasing TBAB concentration (based on the volume of plasticizer). It can be concluded that too high a concentration of this carrier is unfavorable for the transport of iron(III) ions. The optimal TBAB concentration in PIM was determined as 1.0 mol/dm
3. Based on the obtained results, it was found that the optimal composition of the membrane is as follows: 35.5% w/w CTA, 49.2% w/w NPOE and 15.3 % w/w TBAB.
3.3. Influence of H2SO4 concentration in receiving phase on transport of Fe(III)
Determining the optimal concentration of sulfuric acid in the feeding phase was the next stage of this investigation. H
2SO
4 is often used to the stripping (re-extraction) of various metals from organic phase/membrane phase with good results. Nevertheless, it is important to determine the concentration of this acid that will ensure good efficiency of the selective removal of iron(III) from the membrane phase. It is worth mentioning that the entire membrane process can be divided into several stages [
26]:
(I) Fe(III) reacts with TBAB at the source solution/interface of PIM to form the metal-carrier complexes
(II) The metal-carrier complexes diffuse through membrane towards the receiving phase ("the stripping solution")
(III) At the interface of membrane(PIM)/receiving phase, the complexes metal-carrier are dissociated by hydrogen ions and Fe(III) is released into receiving phase.
It can be summarized that the extraction and re-extraction in the membrane process occur simultaneously.
Therefore, in the next study, the effect of the concentration of H
2SO
4 on the transport of Fe(III), Ni(II), Co(II), Li(I) was studied. The concentration of H
2SO
4 was varied from 0.1 to 2 mol∙dm
-3.
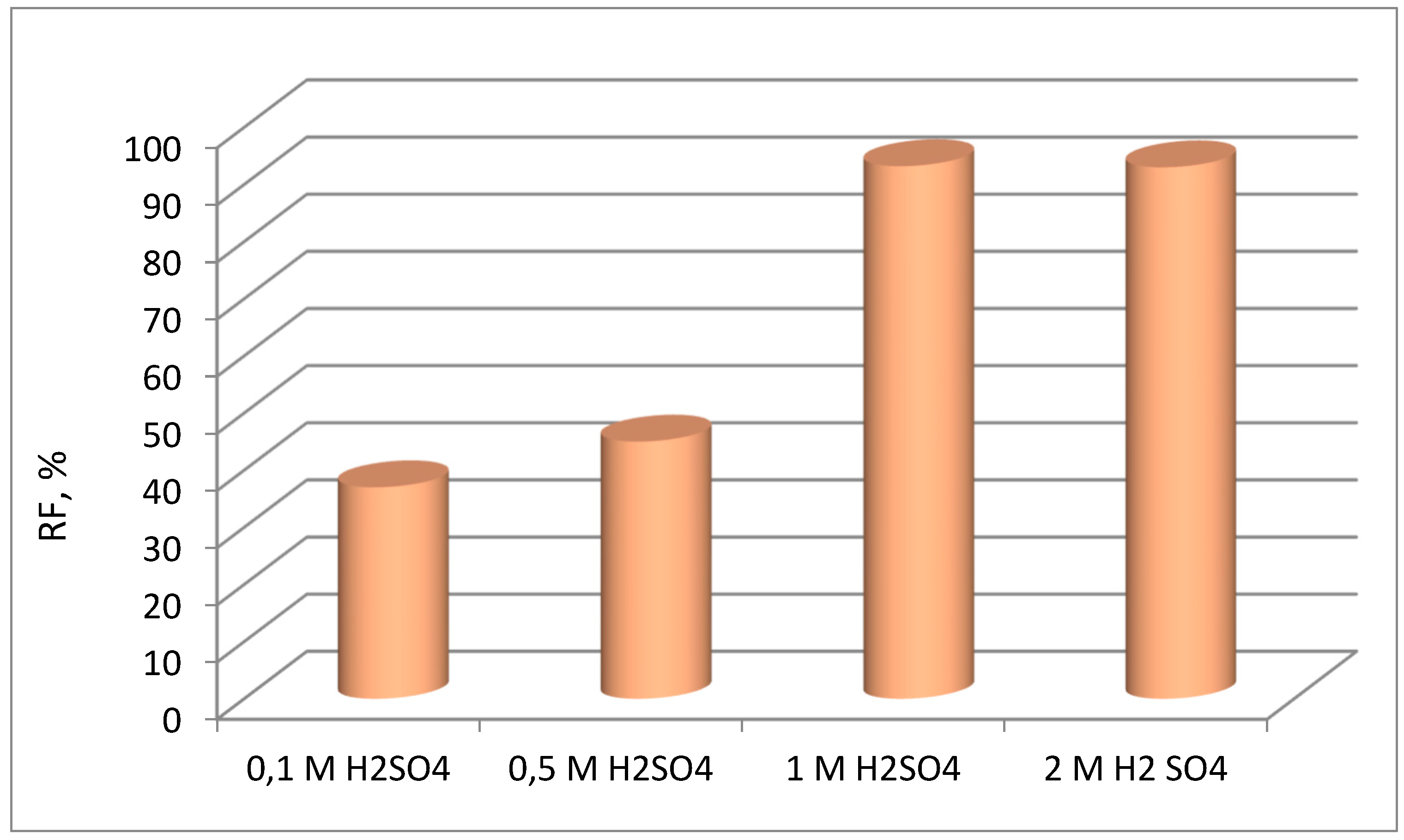
Figure 7 shows the dependence of the recovery factor (RF, %) of Fe(III) at various sulfuric acid concentration. As can be observed from this figure Ni(II), Co(II), Li(I) were not transported into the receiving phase. Whereas, the recovery factor (RF, %) of Fe(III)increases in an increasing acid concentration up to 1 mol∙dm
-3 and the its value this value remained practically unchanged. Thus, based on the obtained results, it can be concluded that 1 M H
2SO
4 is suitable as the receiving phase in the transport of Fe(III) from 6 M HCl.
4. Discussion
The presented research results show that both the composition of the initial feed phase, the receiving phase and the composition of the membrane have a decisive influence on the efficiency of the Fe(III) ion transport process. It is worth pointing out that the appropriate composition of PIM ensures its flexibility and stability. We can conclude that too high concentration of TBAB used as the ion carrier adversely affect the transport of Fe(III) reducing the initial flux of the ion transfer from the source phase into the receiving phase. The conducted studies allows the optimal TBAB concentration in PIM to be determined as 1.0 mol/dm
3. The resulting membrane consisted of 35.5% w/w CTA, 49.2% w/w NPOE and 15.3 % w/w TBAB. We know from review of literature, that ILs may have plasticizing properties. However, as can be seen from the test results, the conveyor used requires the use of an appropriate amount of plasticizer. Too little plasticizer content also had an adverse effect on the kinetic parameters of the process. The previous research showed that at high concentration of the ion carrier and the plasticizer we can observed forming a film on the membrane surface. This phenomenon causes the viscosity to increase which limits the transport of metal ions (e.g. Fe(III)) across PIM. These relationships are confirmed by studies by other authors [
26,
27].
The concentration of HCl hydrochloric acid had a significant influence on the kinetic parameters of iron(III) transport through and the obtained recovery rate (RF, %) of iron(III) from the multicomponent solution. It turns out that only a high concentration of HCl enables effective iron transport. It is worth noting that at high concentrations of chloride ions and HCl acid, iron occurs in the form of anion complexes of the FeCl
4 - type [
29,
30] and this phenomenon probably enables the removal of Fe(III) by PIM with TBAB. from concentrated HCl.This method is also very selective towards Ni(II), Co(II) and Li(I).
On the other hand, the concentration of sulfuric acid used as the receiving phase is also very important parameter influencing the efficiency of iron(III) transport. In a previous study [
11,
12,
23] this acid was used as the efficient stripping phase. Many other authors have also used sulfuric acid as an effective receiving phase [
1,
28]. For example, Baczynska et. al. [
1] and Kogelnig et. al. [
28] confirmed that solutions of H
2SO
4 were very effective as the stripping phases for Zn(II) transport through PIM containing ionic liquids (ILs) as the ion carriers, such as Cyphos IL 101 (trihexyl (tetradecyl)phosphonium chloride) and Cyphos IL 104 (trihexyl(tetradecyl)phosphonium bis(2,4,4-trimethylpentyl)phosphinate).
5. Conclusions
So far, there are no literature reports on the use of TBAB as a carrier of metal ion for obtaining polymer membranes. In this work, TBAB was applied as the ion carrier for the synthesis of PIMs. This membrane was used in order to selective separation of Fe(III) from Co(II), Ni(II) and Li(I) from the aqueous solution containing Co(II), Ni(II) and Li(I) . The composition of this source phase was very similar to leach liquor of the spent LIBs. The results show that CTA as the base polymer provides mechanical strength to the PIM, NPOE improves the flexibility and TBAB provides transport and separation properties of the polymer membrane. The optimal membrane composed of 35.5% w/w CTA, 49.2% w/w NPOE and 15.3 % w/w TBAB. The high concentration of HCl enables effective and selective transport of Fe(III) from the source phase into 1 M H2SO4. This method can be used to remove iron(III) impurities from solutions with Ni(II), Co(II) and Li(I).
Author Contributions
Conceptualization, B.P. ; methodology, B.P; B.P.; validation, B.P; formal analysis, B.P.; investigation, B.P.; resources, B.P.; writing—original draft preparation, B.P.; writing—review and editing, B.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by statutory research: BS/PB-200-301/ZB-202-12/2023 funds from the Faculty of Production Engineering and Materials Technology, Czestochowa University of Technology, Poland.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Baczynska, M.; Slomka, Z.; Rzelewska, M.; Waszak, M.; Nowicki, M.; Regel-Rosocka. Characterization of polymer inclusion membranes (PIM) containing phosphonium ionic liquids as their application for separation of Zn(II) from Fe(III). J. Chem. Techol. Biotechnol. 2018, 93, 1767–1777. [Google Scholar] [CrossRef]
- Nagul, E.A.; Croft, Ch.F.; Cattrall, R.W. Nanostructural characterization of polymer inclusion membranes using X-ray scattering. J. Membr. Sci. 2019, 588, 117208. [Google Scholar] [CrossRef]
- Staszak, K.; Kruszelnicka, I.; Ginter-Kramarczyk, D.; Gora, W.; Baraniak, M.; Lota, G.; Regel-Rosocka, M. Advances in the removal of Cr(III) from spent industrial effluents – a Review. Materials 2023, 16, 378. [Google Scholar] [CrossRef] [PubMed]
- Nagul, E.A.; Croft, Ch.F.; Cattrall, R.W. Nanostructural characterization of polymer inclusion membranes using X-ray scattering. J. Membr. Sci. 2019, 588, 117208. [Google Scholar] [CrossRef]
- Hedwig, S.; Kraus, M.; Amrein, M.; Stiehm, J. , Costable, E., C. Recovery of scandium from acidic waste solutions by means of polymer inclusion membranes. Hydrometallurgy 2022, 213, 105916. [Google Scholar] [CrossRef]
- Hu, F.; Hu, H.; Tang, J.; Qiu, X.; Jin, W.; Hu, J. Plasticization-induced oriented micr-channels within polymer inclusion membranes for facilitating Cu(II) transport. J. Mol. Liq. 2020, 301, 112457. [Google Scholar] [CrossRef]
- Cussler, E.; Aris, R.; Bhown, A. On the limits of facilitated diffusion. J. Membr. Sci. 1989, 43, 149–164. [Google Scholar] [CrossRef]
- Noble, R.D. Analysis of facilitated transport with fixed site carrier membranes. J. Membr. Sci. 1990, 50, 207–214. [Google Scholar] [CrossRef]
- Fontas, C.; Tayeb, R.; Dhahbi, M.; Gaudichet, E.; Thominette, F.; Roy, P.; Steenkeste, K.; Fontaine-Aupart, M.P.; Tingry, S.; Tronel-Pyroz, E. ; Polymer inclusion membranes: the concept of fixed sites membrane revised. J. Membr. Sci. 2007, 290, 62–72. [Google Scholar] [CrossRef]
- Carner, C.A.; Croft, Ch.F.; Kolev, S.D.; Almeida, I.G.S. Green solvents for the fabrication of polymer inclusion membranes (PIMs). Sep. Purif. Technol. 2020, 239, 116486. [Google Scholar] [CrossRef]
- Pospiech, B.; Kujawski, W. Ionic liquids as selective extractants and ion carriers of heavy metal ions from aqueous solutions utilized in extraction and membrane separation. Rev. Chem. Eng. 2015, 31, 179–191. [Google Scholar] [CrossRef]
- Pospiech, B. Studies on extraction and permeation of cadmium(II) using Cyphos IL 104 as selective extractant and ion carrier. Hydrometallurgy 2015, 154, 88–94. [Google Scholar] [CrossRef]
- Makowka, A.; Pospiech, B. Studies on extraction and competitive permeation of cerium(III) and lanthanum(III) using Cyphos IL104 as selective extractant and ion carrier. Sep. Sci. Technol. 2020, 55, 2193–2203. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Nowak, L.; Wisniewski, M. Removal of Zn(II) and iron ions from chloride solutions with phosphonium ionic liquids. Sep. Purif. Technol. 2012, 97, 158–163. [Google Scholar] [CrossRef]
- Guo, L.; Liu, Y.; Zhang, C.; Chen, J. Preparation of PVDF-based polymer inclusion membrane using ionic liquid plasticizer and Cyphos IL 104 carrier for Cr(VI) transport. J. Membr. Sci. 2011, 372, 314–321. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Wisniewski, M. Selective removal of zinc(II) from spent pickling solutions in the presence of iron ions with phosphonium ionic liquid Cyphos IL 101. Hydrometallurgy 2011, 110, 85–90. [Google Scholar] [CrossRef]
- Rybka, P.; Regel-Rosocka, M. Nickel(II) and cobalt(II) extraction from chloride solutions with quaternary phosphonium salts. Sep. Sci. Technol 2012, 47, 1296–1302. [Google Scholar] [CrossRef]
- Banik, B.K.; Banerjee, B.; Kaur, G.; Saroch, S.; Kumar, R. Tetrabutylammonium bromie (TBAB) catalyzed synthesis of bioactive heterocycles. Molecules 2020, 25, 5918. [Google Scholar] [CrossRef]
-
https://www.sigmaaldrich.com/PL/pl/product/sial/426288 (14.07.2023).
- Hariharan, A.V.; Sudhakar, Ch.; Rao, B.V. Solvent extraction of iron(III) with tetrabutylammonium bromide from aqueous acid solutions. Int. J. Anal. Bioanal. Chem. 2013, 3, 78–81. [Google Scholar]
- Venkateswaran, P.; Palanivelu, K. Solvent extraction of hexavalent chromium with tetrabutyl ammonium bromide from aqueous solution. Sep. Purif. Technol. 2004, 40, 279–284. [Google Scholar] [CrossRef]
- Kalidhasan, S.; Rajesh, N. Simple and selective extraction process for chromium (VI) in industrial wastewater. J. Hazard. Mater. 2009, 170, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Muthuraman, G.; Palanivelu, K. Selective extraction and separation of textile anionic dyes from aqueous solution by tetrabutyl ammonium bromide. Dyes Pigm. 2005, 64, 251–257. [Google Scholar] [CrossRef]
- Pospiech, B. Separation of Co from Ni and Li from chloride media using polymer inclusion membrane system with thiosalicylate based ionic liquid. Physicochem. Probl. Miner. Process. 2022, 58, 152997. [Google Scholar] [CrossRef]
- Danesi, P.R. Separation of metal species by supported liquid membranes. Sep. Sci. Technol. 1984, 19, 857–894. [Google Scholar] [CrossRef]
- Garcia-Rodriguez, A.; Fontas, C.; Matamoros, V.; Ines, M.; Almeida, G.S.; Cattral, R.W.; Kolev, S.D. Development of a polymer inclusion membrane-based passive sampler for monitoring of sulfamethoxazole in natural waters. Minimizing the effect of the folw pattern of the aquatic system. Microchem. J. 2016, 124, 175–180. [Google Scholar] [CrossRef]
- Wang, D.; Hu, J.; Li, Y.; Fu, M.; Liu, D.; Chen, Q. Evidence on the 2-nitrophenyl octyl ethr (NPOE) facilitating copper(II) transport through polymer inclusion membranes. J. Membr. Sci. 2016, 501, 228–235. [Google Scholar] [CrossRef]
- Kozlowski, C.A. Facilitated transport of metal ions through composite and polymer inclusion membranes. Desaliantion 2006, 198, 132–140. [Google Scholar] [CrossRef]
- Kogelnig, D.; Regelsberger, A.; Stojanovic, A.; Jirsa, F. , Krachler, R.; Keppler, B.K. A polymer inclusion membrane based on the ionic liquid trihexyl(tetradecyl)phosphonium chloride and PVC for solid–liquid extraction of Zn(II) from hydrochloric acid solution. Monatsh. Chem. 2011, 142, 769–772. [Google Scholar] [CrossRef]
- Biswas, R.K.; Begum, D.A. Solvent extraction of Fe3+ from chloride solution by D2EHPA in kerosene. Hydrometallurgy, 1998, 50, 153–168. [Google Scholar] [CrossRef]
- Staszak, K.; Cierpiszewski, R.; Prochaska, K. Equilibrium and rate of iron(III) extraction from chloride solutions by individual hydrophobic extractants and their mixtures. Pol. J. Chem. Technol. 2011, 13, 1–5. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
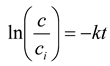
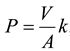 ,
,