1. Introduction
Tropical forests contain diverse ecosystems and provide a home for people, flora and faunal species. Forests provide habitats for 80 per cent of amphibian species, 75 per cent of bird species and 68 per cent of mammal species. About 60 per cent of all vascular plants are found in tropical forests. Additionally, they are essential in regulating the climate through oxygen production and carbon storage, and provide livelihoods for millions of people (Bennet, 2004; Bharucha & Pretty, 2010; Blackman & Veit, 2018). Consequently, understanding the dynamics of tropical forests and their conservation has gained prominence. Forests are increasingly recognized for their role as a nature-based solution to many sustainable development challenges, as manifested in increased political will and global commitments to reduce rates of deforestation and enhance restoration of degraded forest ecosystems (Beatty et al., 2018; Bastin et al., 2019). Most of the terrestrial biodiversity is contained in tropical forests. Consequently, the conservation of global biodiversity is dependent on how we interact with and use the world’s tropical forests.
Deforestation and forest degradation remain the biggest challenges in forest conservation (Zhu et al., 2004; Agrawal et al., 2008). These two processes contribute significantly to the current loss of biodiversity. Over the last 30 years, it is estimated that some 420 million hectares of forest have been lost through conversion to other land uses (Chomba et al., 2014). Agricultural expansion and urbanization are currently the main drivers of deforestation, forest fragmentation and the accompanying loss of forest biodiversity (Chan et al., 2007). Large-scale commercial agriculture and local subsistence agriculture account for most tropical deforestation. The resilience of human food systems and their capacity to adapt to future change depend on the biodiversity that include; dry land shrub and tree species that are key in combating desertification, forest insects, bats and bird species that are useful in crop pollination, trees with extensive root systems which mitigate soil erosion, and mangrove species that provide resilience against flooding and erosion in coastal areas (Ahenkan et al., 2011; Barros et al., 2019). With climate change exacerbating the risks to food systems, the role of forests in capturing and storing carbon and mitigating climate change is of ever-increasing importance for the agricultural sector (CBD 2010b; Ding et al., 2016).
Data and information are key to sustainable management of forests (Wenger, 2013; Dau et al., 2015). Such information is obtained mainly through forest inventories. Forest inventories are crucial in forest management because, they provide the data for planning, monitoring, evaluation, research, growth and yield, biodiversity, and timber sale (Monserud, 2013; Zerihun & Yemir 2013). An inventory of tree species that provides information on diversity and abundance helps in identifying species of special concern that may be heavily impacted by forest deforestation and degradation (Mendoza & Prabhu, 2000).
Tropical rainforest biodiversity is threatened by intense anthropogenic pressure that includes, deforestation, habitat degradation and fragmentation, exploitation, invasive species, pollution, and global climate change (Marengo 2020; Lawrence & Vandecar, 2015). Besides, synergies among these drivers have had a major negative impact on biodiversity (Kissinger et al., 2012). These threats may alter the stand structure of the forest ecosystems (Krupnick, 2013; Fischer et al., 2016). Tropical forest restoration which models the natural regeneration has been adopted as a strategy for restoring degraded forests (Aide, 2000). Regeneration is a central component of forest ecosystem dynamics and the restoration of degraded forest lands (Chadzon & Uriarte, 2016). The patterns of regeneration drive the structure and composition of the forest (Wangda, 2003; Mori and Takeda, 2004). These factors influence the species composition of tropical forests at different spatial scales (Peña-Claros et al., 2012; Danková & Saniga 2013). One outcome is the enhancement of the stability, resilience and diversity of forest ecosystems (Liira et al., 2011). Successful regeneration requires sufficient seedlings survival, which is a function of the prevailing microclimate of the site and the level of anthropogenic activities. The success of the different growth stages as well as the size-class distribution of a tree population is important in the recovery of the forest following disturbances (Chadzon 2003). Seedling densities in the forest understory are dynamic and rates may vary among species and in the gap and shade environments (Bazzaz 1991; Khumbongmayum et al., 2006; Saikia & Khan, 2013; Strassburg 2019). Where regeneration is continuous, the size-class distribution of species cohorts will tend to exhibit a reverse J shape curve (Teketay, 2005). Understanding the knowledge of species composition and stand structure together with the regeneration patterns of the forest ecosystem is important in evaluating the sustainability, species diversity, conservation and management of the forest ecosystems (Kacholi, 2014). There are a number of studies that have determined patterns of tree species composition and diversity in different forest ecosystems globally emphasizing the determination of floristic similarity and diversity gradients (Wittmann et al., 2006; Silva et al., 2011; Gotelli & Chao, 2013).). Similar studies have been reported in the Kakamega tropical rain forest in Kenya (Schleuning 2011; Osewe, 2022) and Mau Forest (Tarus & Nadir, 2020) among others. No study has been done yet on the woody species composition, tree diversity and regeneration status of Londiani Forest. Therefore, this study sought to determine: i) tree abundance, species diversity and distribution in Londiani Forest, ii) species composition and stand structure of Londiani Forest, and iii) the regeneration status of the Londiani Forest.
2. Materials and Methods
Study Area
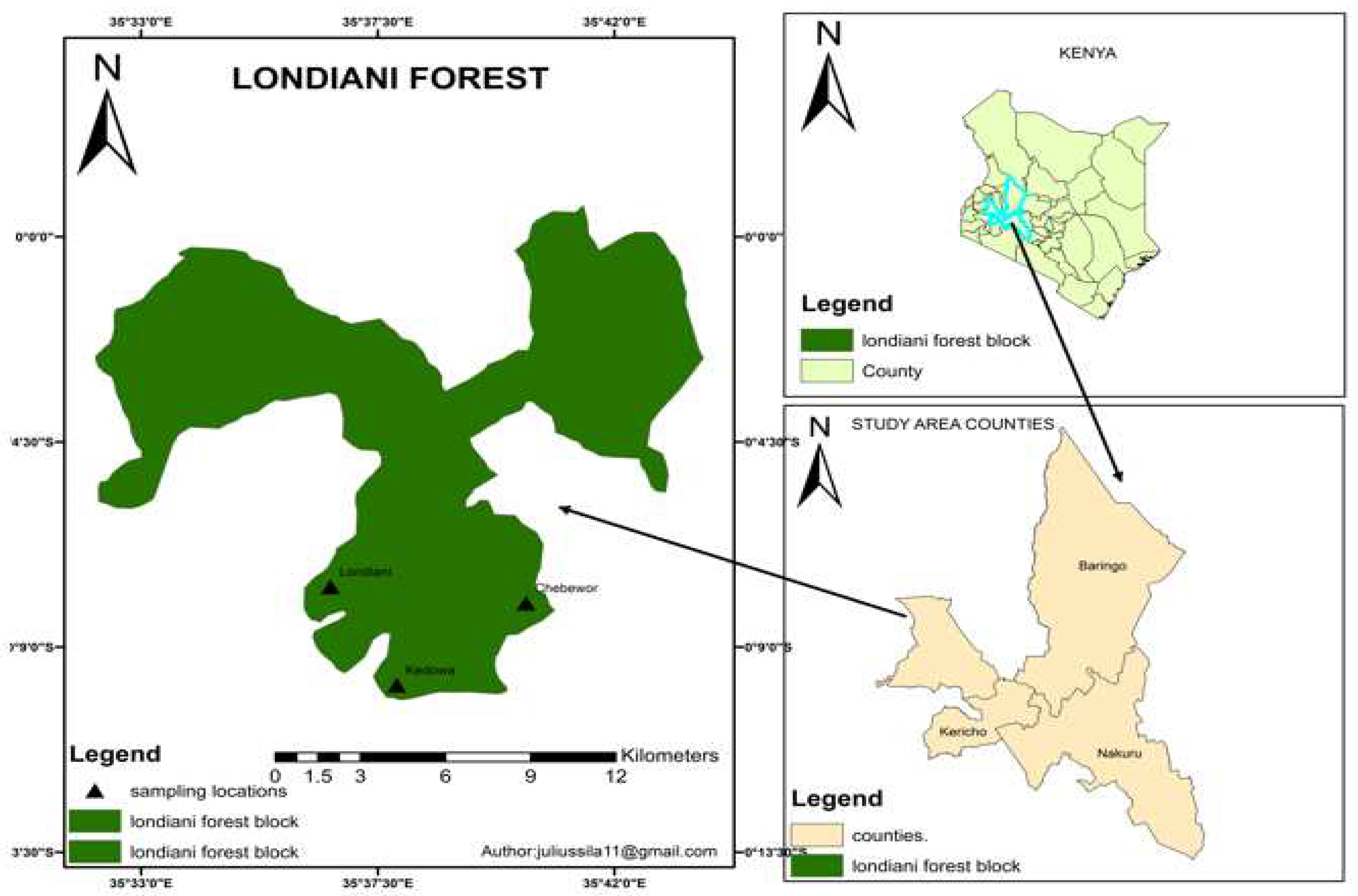
The Londiani Forest, located in Kericho County covers some 18,938 ha (GoK, 2009). It lies to the West of Nakuru town, East of Bomet County and along the Kericho-Nakuru highway. Londiani town is about 260 km from the capital city of Nairobi, with a Latitude of 0.17º South, a Longitude of 35.6º East and an elevation of 2326 m above the sea level (GoK, 2000) (
Figure 1). The forest was gazetted via legal notice No. 44 of 1932 with the objective of conservation. Londiani Forest and its environments receive rainfall that varies between 1500 and 17000 mm per year that is bimodally distributed (GoK, 2019). Long rains fall between mid-March and June while short rains fall between mid-October and December. There is a mean difference in precipitation of 133 mm between the driest and the wettest month (GoK, 2019). The mean maximum temperature is 24°C and the lowest mean minimum temperature is 10°C. The region is a source for several rivers that drain into Lake Victoria (GoK, 2000). The main economic activity is farming, which involves crop production and livestock keeping. All these economic practices have a direct impact on the Londiani Forest (KFS, 2010).
Research Design
A cross-sectional research design was applied in this study. Data was collected in the three forest blocks of the Londiani Forest; Londiani, Kedowa and Chebewor. Sampling areas were established using GPS and coordinates noted down.
Data Collection
In each of the three forest blocks, belt transects 25 m wide and 1 km long were established 100 m from the edge into the forest. At every 200 m along the transect, 25 m × 25 m quadrats were set up. An inventory of all trees was made recording data on diameter at breast height (DBH), tree height, the species name and the number per plot in a data sheet. For tree species that could not be identified in the field, the local name was provided by para-taxonomists who participated in the data collection and the species name was later identified with the help of a taxonomist or a manual of woody tree species of the Londiani Forest (KFS, 2018). The tree species abundance was scaled to a hectare. The diameter at breast height (DBH) was measured at 1.3 m from the ground using a diameter tape. Tree height was measured using a Suunto angular clinometer. A nested quadrat 5 m x 5 m quadrat within the 25 m x 25 m quadrat was used to sample saplings while a 1 m x 1 m quadrat was used for sampling seedlings. All saplings and seedlings within the nested quadrat were identified and matched to the existing tree species. Regeneration status was assessed using the number of seedlings and saplings. Anthropogenic disturbances were assessed in each quadrat by counting tree stumps that were identified to species level and recording evidence of charcoal burning.
Data Analysis and Presentation
Data from the quadrats were entered into Microsoft Excel. The population structure of the tree species was analyzed across fifteen DBH classes with an interval of 10 cm apart and also across eleven Height classes with an interval of 5 m apart. Total stem density, species density, basal area, species basal area, relative density, species diversity, evenness and richness were determined. Relative densities were extrapolated to per hectare.
1. Stem Density (trees/ha) = x 10000 m2/ha
Spp. Stem density (trees/ha) = x 10000 m2/ha
2. Total Basal Area (m2/ha) = ∑ x 10000 m2/ha
Where: Individual tree basal area (m2) = π x (tree radius) 2 x (1m2/10,000cm2)
Spp. Basal Area (m2/ha) = ∑ x (10000 m2/ha).
3. Relative density = No. of trees of a species / Total no. of trees of all species
= ∑
(Plot area (m2) x (10000 m2/ha)
4. Species Diversity
Diversity was calculated using Shannon Diversity index as shown below
H = -Σ [(𝑝𝑖) ×𝑙𝑛 (𝑝𝑖)]
Where Σ: A Greek symbol that means “sum” (ln) is Natural log, and (pi) is the proportion of the entire community made up of species i. ( Roswell et al., 2021)
5. Evenness
Hmax = ln(S) = Maximum diversity possible
E= Evenness = H/Hmax
S = number of species, = species richness
6. Jaccard Similarity Index
The Jaccard similarity index (Hancock, 2004) was determined using the formula shown below
SJ = c / (a + b + c)
Where SJ is the similarity index, c is the number of shared species between the two sites and a and b are the number of species unique to each site (Hancock, 2004).
7. The Importance Value Index (IVI), was used to determine the ecological importance of each tree species.
IVI = RD + RF + RDO
Where,
RD is Relative density,
RF is the relative frequency
RDO is relative dominance where:
RD =
RF =
RDO =
The quantifiable data was tested for normality using Kolmogorov-Smirnov test and analyzed statistically using techniques for descriptive and inferential statistics through the aid of Predictive Analytics Software (PASW) version 25. Analysis of variance (ANOVA) was used to determine differences in the parameters among the three forest blocks.
3. Results
Forest Stand Structure and Composition of Londiani Forest
Woody species richness and (IVI)
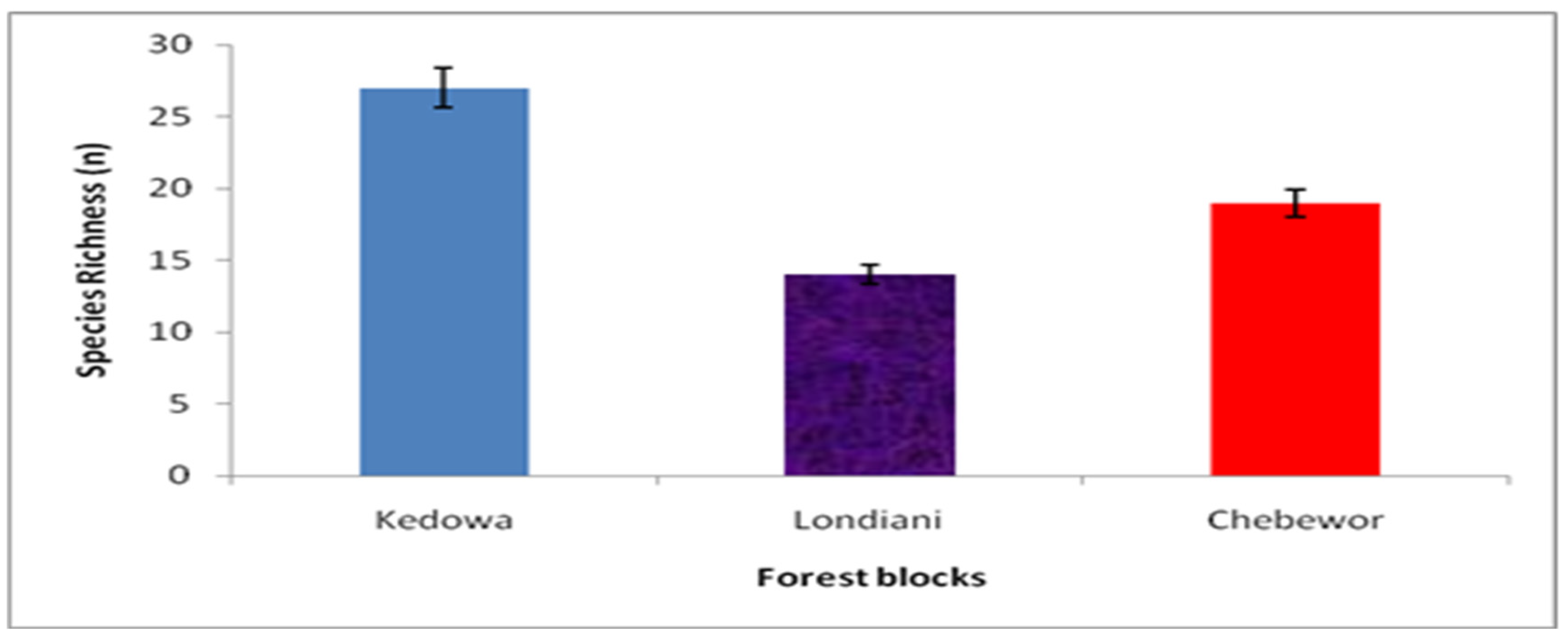
A total of 1308 trees were sampled that represented thirty-four (N=34) woody tree species in 24 families. Trees’ species richness ranged from 14 to 27 (
Figure 2). The Kedowa Forest block had the highest species richness (n= 27, 45%) while the Londiani Forest block had the least (n= 14, 23.3%). There was a statistically significant difference in richness distribution among the three forest blocks
p= 0.034. Indigenous tree species comprised 45.1% (n=591) of all the trees sampled while exotics comprised 54.9% (n=717). Kedowa Forest block accounted for 54.4% (n=235) of the indigenous trees and 45.6% (n=197) of the exotics, Chebewor Forest block accounted for 53.5% (n=231) of indigenous trees and 46.5% (n=201) of the exotics while Londiani Forest block accounted for 27.4% (n=118) of indigenous trees and 72.6% (n=312) of the exotics. The family
Cupressaceae had the highest number of woody plants (32%; n= 421), followed by
Pinaceae (15%; n= 196). The family with the least woody plants were
Proteaceae and
Rhamnaceae each representing 0.2% (n= 2). Analysis of importance value indices of woody species (IVI) for the three forest blocks ranged from 31.48% to 48.25%. Kedowa Forest block had the highest (IVI) of 48.25%, followed by the Londiani Forest block at 42.86% and then the Chebewor Forest block at 31.48%.
Diameter at Breast Height Distribution
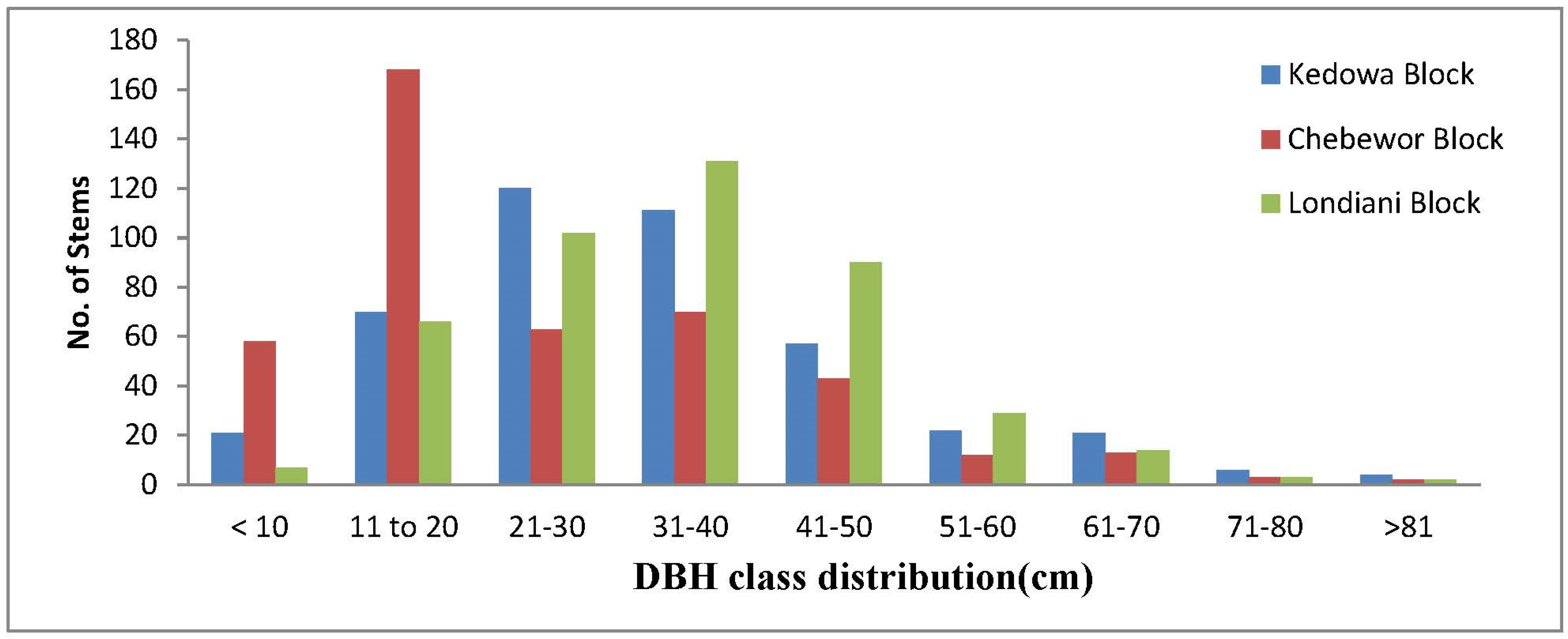
The DBH ranged between 10 cm and 150 cm in Londiani Forest. The mean DBH for the Chebewor Forest block was 24.8 cm, for the Kedowa Forest block 32.4 cm and Londiani Forest block 34.2 cm (
Figure 3).
Kedowa and Londiani Forest blocks had more trees in the DBH range of (21-30cm), (31-40cm) and (41-50cm). Chebewor on the other hand had more trees in (11-20cm) DBH range. In all the forest blocks, there were trees in the DBH <10 cm which were mainly saplings. A total of (n=1,177(89.9%) trees out of (N=1,308) trees recorded from the entire forest recorded a small DBH below 50 cm showing that the forests consist of young still growing trees. Results of a one-way ANOVA found no statistically significant differences in the mean DBH distribution among the three forest blocks (F=0.560; p=0.729).
Height Distribution of Trees
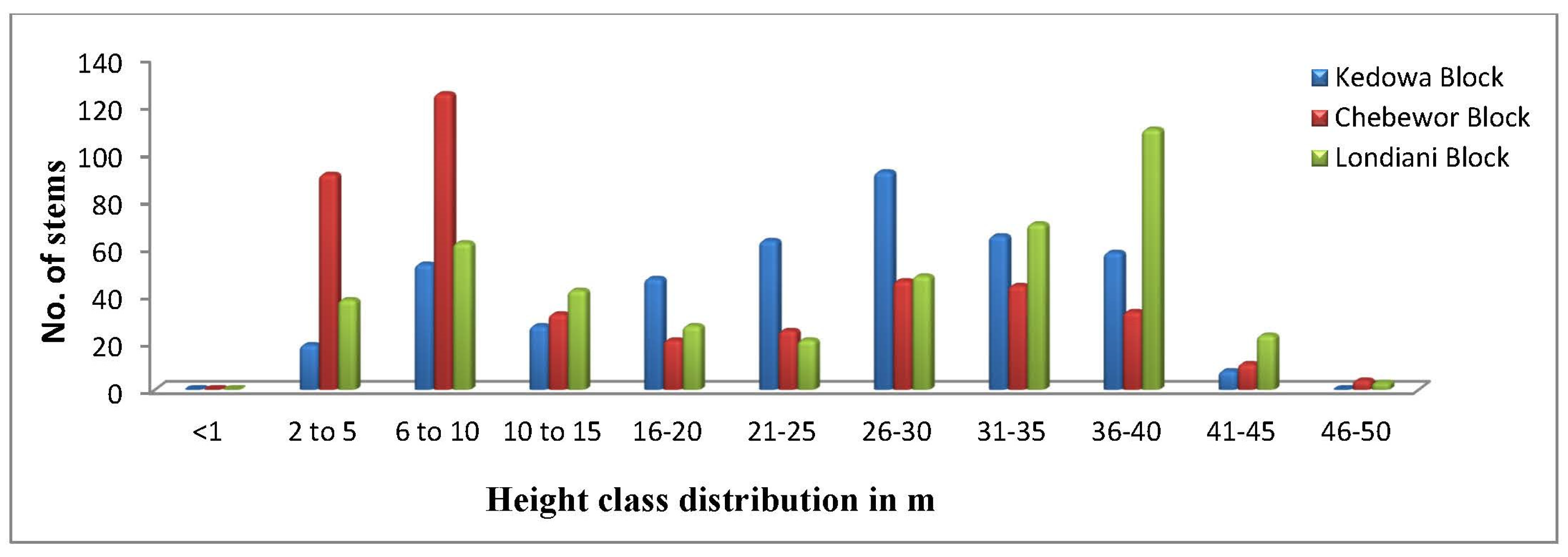
The height of trees in Londiani Forest ranged from 1 m to 50 m. The mean height for the Chebewor Forest block was 16.9 m, the Kedowa Forest block had a mean height of 23.9 m and the Londiani Forest block had a mean height of 25 m (
Figure 4).
The height of trees in the Kedowa Forest block ranged from 11 m to 45 m while the height of trees in Chebewor and Londiani Forest blocks ranged from 11 m to 50 m respectively. One-way ANOVA analysis found no statistically significant difference in height distribution classes between the three forest blocks (F= 0.821, p=.558).
Density and Relative Density
The overall stem density in the Londiani forest varied from 0.33 stems/ha to 45.5 stems ha-1. Cupressus lusitanica had the highest density of 45.5 stems ha-1, followed by Pinus patula with a density of 32.7 stems ha-1 then Eucalyptus globulus and Juniperus procera were third in the row with a density of 24.7 stems ha-1 each Grevillea robusta had the least density of 0.33 stems ha-1. There was a statistically significant difference in stem density between the three forest blocks (F=12.22; p=0.005).
Basal Area, Dominance and Relative Dominance
The total basal area for all species recorded in the entire forest was 122.6419 m2. Cupressus lusitanica had the highest relative dominance of 33.684 m2 and the species with the least relative dominance of 0.038 m2 was Grevillea robusta. There was no statistically significant difference in basal area distribution within the three forest blocks (X2 = 12.000 df = 9 p = .213). Cupressus lusitanica recorded the highest species basal area of 2.295m2 ha-1, followed by Pinus patula with 0.992m2 ha-1, Juniperus procera with 0.985m2 ha-1. The species recording the lowest species basal area was Grevillea robusta with 0.0026 m2 ha-1
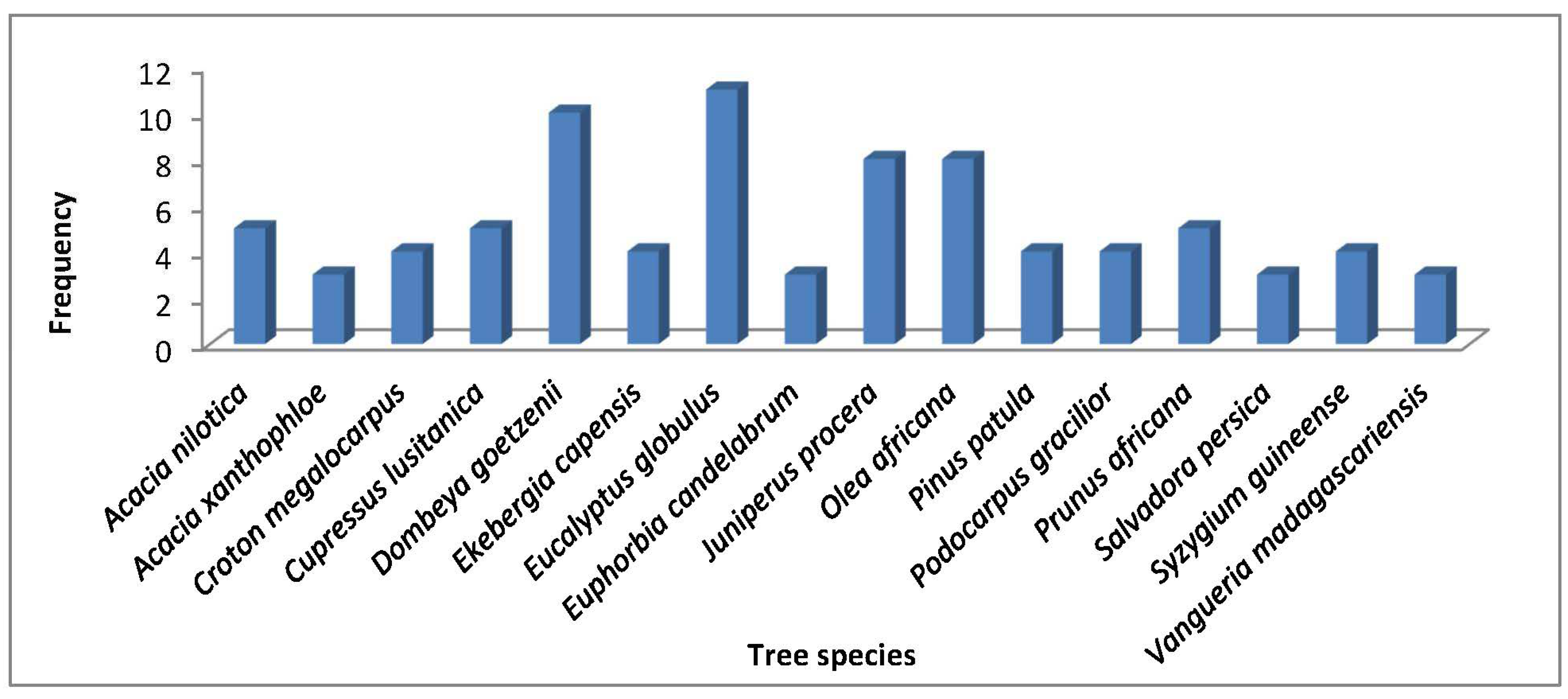
Frequency and Relative frequency
Eucalyptus globulus had the highest frequency of 11 appearances and a relative frequency of 61.1%, followed by
Dombeya goetzenii appearing 10 times with a relative frequency of 55.5%,
Juniperus procera and
Olea africana each had 8 frequency and a relative frequency of 44.4%,
Acacia nilotica,
Cupressus lusitanica and
Prunus africana each had a frequency of 5 with a relative frequency of 27.7% (
Figure 5).
Arundiana alpina, Brassica actinophylla, Croton macrostchyus, Grevillea robusta, Maesopsis eminii, Ocotea usambarensis among others had the least frequencies of 1 and a relative frequency of 5.6%.
Woody Species Diversity and Evenness
The species diversity for the three forest blocks ranged from 0.792 to 0.864. Kedowa block had the highest Diversity H´= 0.864 followed by Chebewor Forest block with H´= 0.855 and finally, Londiani block which had the least diversity of H´= 0.792 (
Table 1). There were significant statistical differences in woody species diversity among the three forest blocks (F=0.32;
p=0.001). Chebewor Forest block had the highest evenness (1±0.32) while Londiani block had the lowest (0.41±0.04).
Similarity between Sites
Table 2 shows the Jaccard similarity indices among the three forest blocks. The similarity index ranged from 0.34 to 0.47. Kedowa and Chebewor forest blocks had a relatively higher similarity index of 0.47 which implied that the two vegetation types shared more woody species than between Londiani and Kedowa. This was followed by a lower similarity index between Londiani and Chebewor forest blocks.
Regeneration Status of Londiani Forest
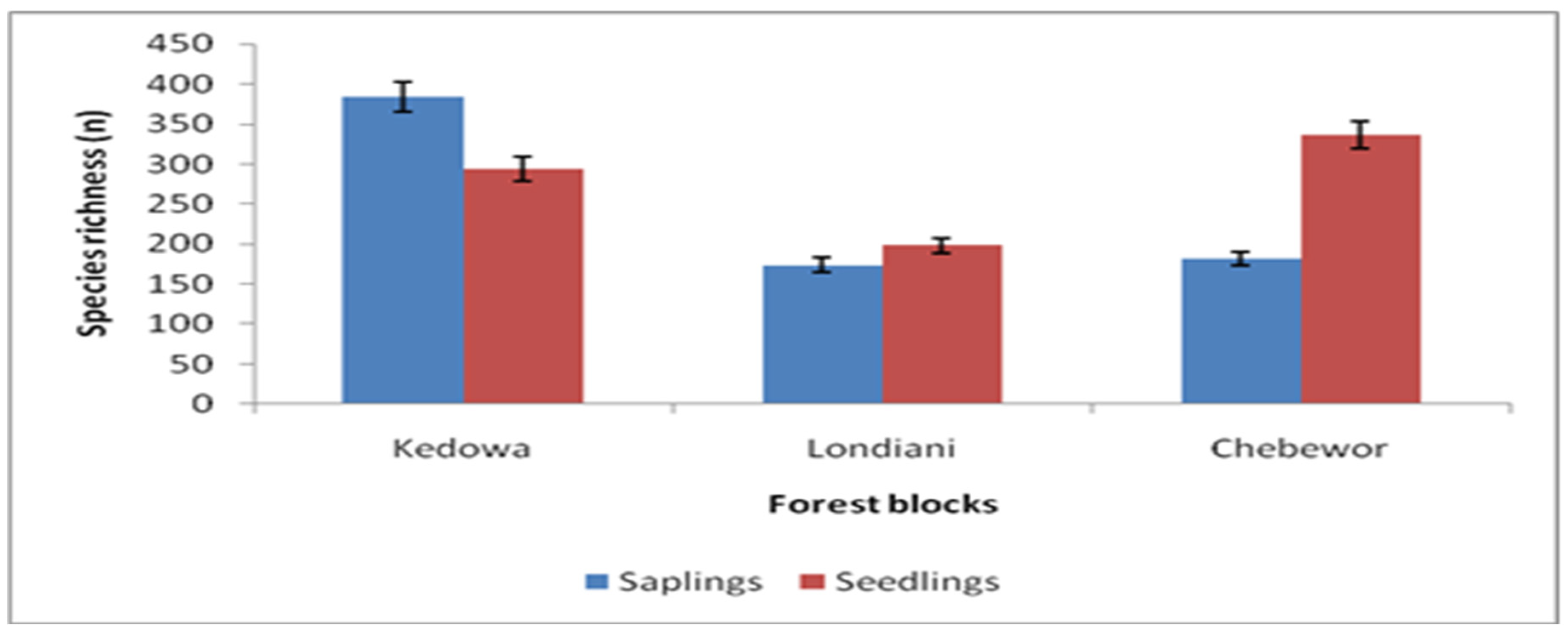
Londiani Forest recorded a total of (N=740) saplings and (N=832) seedlings. Kedowa Forest block recorded saplings abundance of (n=384) and seedlings (n=294), Chebewor had saplings abundance of (n=182) and seedlings (n=337) while Londiani block recorded saplings abundance of (n=174) and seedlings (n=198) (
Figure 6).
In Kedowa Forest block Cupressus lusitanica accounted for most of the saplings (33.4%; n=128) while Vangueria madagascariensis (0.5%; n=2) had the least number of saplings. Likewise, Cupressus lusitanica had the highest number of seedlings (66.7%; n=198), followed by Croton megalocarpus (4.4%; n=13) and, Eucalyptus globulus (4%; n=12). Pinus patula did not record any seedlings since the trees were still young and had not reached maturity stage where they can produce seeds which would later become seedlings.
In Chebewor Forest block, Juniperus procera had the highest numbers of saplings (34.6%; n=63), followed by Eucalyptus globulus with (21.4%; n=39), Acacia mearnsii (16.5%; n=30), Rhus natalensis (5.5%; n=10). Cupressus lusitanica, Anthocleista vogelii, Grevillea robusta, Podocarpus falcatus, Salvadora persica, Tamarindus indica and Vangueria madagascariensis had zero saplings. Juniperus procera had the most seedlings (45.4%; n=153), followed by Eucalyptus globulus at (23.7%; n=80). The rest of the species had no seedlings.
In the Londiani Forest block the saplings ranged from 0-121. Acacia xanthophloe had the most saplings (69.5%; n=121), Juniperus procera (10.3%; n=18), Eucalyptus globulus (1.3%; n=2). The rest of the tree species had no saplings. Acacia xanthophloe had the most seedlings (n=130; 65.7%) Juniperus procera (n=29; 14.7%) seedlings, and Dombeya goetzenii (n=2; 1.01%). The rest of the species had no seedlings.
Abundance, Richness and Diversity of saplings and seedlings in Londiani Forest
Londiani Forest recorded a total of (740) saplings and (832) seedlings (
Table 1). There was no statistically significant difference
p= .082 in abundance distribution of saplings and seedlings
p= .238 within the three forest blocks.
Table 3
4. Discussion
Forest Stand Structure and Species Composition
The results of this study revealed that the three forest blocks of Londiani Forest recorded a total of 34 different species. Other studies done in the same area like by the Kenya Forest Service (KFS) in 2018 (GoK, 2018) reported a total of 27 species while Mutiso et al., (2011) identified 38 plant species. The species composition recorded in Londiani Forest was comparable to those of another dry Afromontane forest of Ethiopia (36 species) (Girma and Mosandl, 2012) and Mopane woodland in northern Botswana (35 species) (Teketay et al., 2018). However, it was lower than tropical forests (55 species) of Kenya (Mutiso et al., 2015). The different species within such ecosystems according to Getaneh et al., (2019), helps to fulfill critical ecological functions which encompass provision of habitats for other plants and wild animals, as well as economic functions. The high number of species recorded in Londiani Forest could be attributed to the past and present disturbances mostly occasioned as a result of anthropogenic activities and well as natural causes. These factors stimulate the establishment of varied species (Kinyanjui, 2013). There were ongoing disturbances observed in the study site which further affects forest diversity. Livestock were grazing freely and unsupervised, charcoal burning was witnessed ongoing in Chebewor and Kedowa blocks at the remaining indigenous portion of the forest. These disturbances have led to decline in climax economic important species. Similarly, Sapkota et al., (2010) noted species diversity reduction in response to disturbances.
The findings show that Kedowa block was rich in tree species compared to the other two blocks same sentiments were shared by KFS, (GoK, 2018). There were many indigenous tree species fully matured and with saplings and seedlings unlike Londiani block which had a small area with indigenous tree species same observations were recorded by Mutiso et al., (2009). Chebewor block had above 50% of indigenous tree species but had faced numerous anthropogenic impacts. Efforts of restoration could be seen where many secondary planted indigenous tree species some matured while other plots were still young trees growing under Plantation Establishment for Livelihood Improvement Scheme (PELIS). The DBH for the forest ranged from 10 cm to 150 cm, an indication that the forest consists of young trees which are still growing. Translating to carbon sequestration potential implied the forest is actively sequestering carbon. The small DBH ranges in the trees sampled in the forest could also be an indication that bigger trees are being harvested for timber and charcoal leaving behind small ones.
Cupressus lusitanica was the most dominant species in Londiani Forest. It is a secondary exotic species planted under Plantation Establishment for Livelihood Improvement Scheme (PELIS) program to replace the indigenous trees after the forest experienced disturbances. Mutiso et al., (2013) recorded Cupressus lusitanica as the most abundant exotic species in the Mau Forest Complex which Londiani Forest is part of. Among the conifers introduced, Cupressus lusitanica and Pinus patula have adapted well to local growing conditions and are now the key species widely planted in commercial plantations (Joram et al., 2023, Kuria et al., 2019, Mbugua, 2005). The two conifer species Cupressus lusitanica and Pinus patula currently make up around 80% of the 186,000 ha state-owned forest plantation estate (Kuria et al., 2019). Additionally, because the species have existed in the Kenyan landscape for a considerable amount of time, their management regimes, silviculture, and other growth features are well recognized (Kuria et al., 2019). This study also agrees with Henry et al., (2011) and Agevi, (2020) who found out high abundance of these species on farms neighboring Kakamega tropical rainforest. The only indigenous conifer species, Juniperus procera, was recorded in Londiani Forest but had a very low abundance. The results of this study are consistent with those of Cheboiwo et al., (2015), who discovered that Juniperus procera is a slow-growing conifer when compared to exotic conifers and that it grows naturally in Western rainforests and high-altitude montane forest types, such as the Kenya Mau Forest Complex. According to Cheboiwo et al., (2015), it achieves a production of 33.73 m3/ha/year for Mean Annual Increment (MAI).
Analysis of importance value indices of woody species (IVI) for the three forest blocks ranged from 31.48% to 48.25% indicating that some of the tree species are commonly found in the forests. Based on Jaccard similarity indices, it is very clear that the three forest blocks have very low similarity index less than 0.5. The important thing worth noting is that in all the three forest blocks there is a certain degree of species sharing especially between Chebewor and Kedowa block. This can be greatly attributed to endemism and neighborhood effects. Neighbor plant interaction is cited by Huang et al., (2003) as a factor in post-disturbance plant forms. Conversely, the small variations in their topography and levels of disturbance might have contributed to the high similarity in Kedowa and Chebewor Forest blocks.
Population Structure and Regeneration Status of Londiani Forest
The population structure and regeneration status varied among the tree species because of poor forest management that has exposed the trees to illegal harvesting and hence the observed cut tree stumps in the three forest blocks. The variations of species richness of adult trees remained insignificant among the three forest blocks, but the abundance and diversity of species of adult tree species was much higher than saplings and seedlings. The overall numbers of seedlings and saplings varied among forests. Similar to the densities of adult tree species, a few species accounted for the number of species of seedlings and saplings. These findings are in agreement with those of Mutiso et al., (2013) and Mutiso et al., (2015) in studies done in Mau complex who asserted that ongoing disturbances on the sites have impacted negatively on the regeneration and recruitment processes. Such regeneration patterns promote mono-dominant forests with low redundancy (Gaaf, 1999). The degrees of harvesting in the forests were the main source of variations in species composition between the adults and saplings and seedlings. Therefore, human interventions are required to reduce the effects of disturbances, maintain the integrity of the forests as well as enhance their composition and structure (Getaneh, et al., 2019).
5. Conclusions
The objective of this study was to determine the Woody Species Composition, Tree diversity and Regeneration Status of Londiani Forest, Kenya. The findings revealed gains and losses in forest cover over the years. Physical observation revealed a low floristic composition in the Londiani Forest at mature, seedling, and sapling stages, indicating poor establishment and recruitment as a result of continuing disturbances, primarily anthropogenic. Anthropogenic factors stemming from population pressure and ineffective implementation of relevant forest laws, policies, and regulations were shown to be the main causes of forest cover reduction.
Funding
There was no funding obtained for this study.
Acknowledgments
I thank Kenya Forest Service Londiani for giving me permit to collect data in the forest. I also appreciate Gilbert Koskei, Irine Cherotich, Erick Bii, Mercy Chelangat, Peacemark Kipkorir and Willy Cheruiyot for their help in data collection.
References
- Agevi, H. Determination of Species Abundance, Diversity and Carbon Stocks in Kakamega Forest Ecosystem. Ph.D. Dissertation, Moi University, Eldoret. 2020. http://ir.mu.ac. 8080. [Google Scholar]
- Aide, T.M. Tropical Forest Restoration. Restor. Ecol. Spec. Issue 2000, 8, 327–424. [Google Scholar] [CrossRef]
- Agrawal, A. , Chhatre, A., Hardin, R. 2008. Changing governance of the world’s forests. Science 2008, 320, 1460–1462. [Google Scholar] [CrossRef] [PubMed]
- Ahenkan, A. & Boon, E. Improving nutrition and health through non-timber forest products in Ghana. Journal of Health, Population and Nutrition 2011, 29, 141–148. [Google Scholar] [PubMed]
- Bazzaz, F. “Regeneration of Tropical Forests: Physiological Responses of Pioneer and Secondary Species.” In Rainforest Regeneration and Management, edited by A. Gomez-pompa, T. Whitmore, and M. Hadley, 1991, 91–118. Paris: Parthenon Publishing, UNES.
- Barros, F.M. , Peres, C.A., Pizo, M.A. & Ribeiro, M.C. Divergent flows of avian-mediated ecosystem services across forest-matrix interfaces in human-modified landscapes. Landscape Ecology 2019, 35, 879. [Google Scholar] [CrossRef]
- Bastin, J.-F. , Finegold, Y., Garcia, C., Mollicone, D., Rezende, M., Routh D., Zohner, C.M. & Crowther, T.W. The global tree restoration potential. Science 2019, 365, 76–79. [Google Scholar]
- Beatty, C.R. , Cox, N.A. & Kuzee, M.E. 2018. Biodiversity guidelines for forest landscape restoration opportunities assessments. 1st edition. Gland, Switzerland, IUCN.
- Bennett, G. 2004. Integrating biodiversity conservation and sustainable use: lessons learned from ecological networks, Gland, Switzerland, IUCN.
- Bharucha, Z. & Pretty, J. The roles and values of wild foods in agricultural systems. Philosophical Transactions of the Royal Society B: Biological Sciences 2010, 365, 2913–2926.
- Blackman, A. & Veit, P. Titled Amazon indigenous communities cut forest carbon emissions. Ecological Economics 2018, 153, 56–67. [Google Scholar]
- CBD. 2010b. Linking Biodiversity Conservation and Poverty Alleviation: A State of Knowledge Review. CBD Technical Series No: 55. Montreal, Canada, Secretariat of the Convention on Biological Diversity.
- 13. CBD. 2012b. Cities and biodiversity outlook. Montreal, Canada, Secretariat of the Convention on Biological Diversity.
- Chan, K.M.A., Pringle, R.M., Ranganathan, J., Boggs, C.L., Chan, Y.L., Ehrlich, P.R., Haff, P.K., Heller, N.E, Al-Khafaji, K. & Macmynowski, D.P. When agendas collide: human welfare and biological conservation. Conservation Biology 2007, 21, 59–68.
- Cheboiwo, K., Joshua, M., Robert, O., Mbinga, J., and Festus, M. Potential Growth, Yields and Socioeconomic benefits of Four Indigenous Species for Restoration in Moist Forest, Mau Kenya. Journal of Environment and Earth Science. 2015, 5, 6.
- Chomba, B.M. , Tembo, O., Mutandi, K., Mtongo, C.S. & Makano, A. 2014. Drivers of deforestation, identification of threatened forests and forest co-benefits other than carbon from REDD+ implementation in Zambia. A consultancy report prepared for the Forestry Department and the Food and Agriculture Organization of the United Nations under the national UN-REDD Programme. Lusaka, Ministry of Lands, Natural Resources and Environmental Protection. [also available at http://landforlions.org/data/documents/drivers-deforestation-Zambia-WEB_final.pdf].
- Chazdon, R.L. & Uriarte, M。 Natural regeneration in the context of large-scale forest and landscape restoration in the tropics. Biotropica 2016, 48, 709–715.
- Chazdon, R.L. Tropical forest recovery: legacies of human impact and natural disturbances, Perspectives in Plant Ecology, Evolution and Systematics 2003, 6, 51-71. ISSN 1433–8319.
- Cheboiwo, K., Joshua, M., Robert, O., Mbinga, J., and Festus, M. Potential Growth, Yields and Socioeconomic benefits of Four Indigenous Species for Restoration in Moist Forest, Mau Kenya. J. Environ. Earth Science. 2015, 5, 6.
- Dau, J.H., Mati A, and Dawaki, S.A. Role of Forest Inventory in Sustainable Forest Management: A Review. International Journal of Forestry and Horticulture 2015, 1, 33–40.
- Ding, H. , Veit, P.G., Blackman, A., Gray, E., Reytar, K., Altamirano, J.C. & Hodgdon, B. Climate benefits, tenure costs: the economic case for securing indigenous land rights in the Amazon; WRI: Washington, DC, USA, 2016. [Google Scholar]
- Erick, O. Osewe, Mihai Daniel Niţă, Ioan Vasile Abrudan. Assessing the Fragmentation, Canopy Loss and Spatial Distribution of Forest Cover in Kakamega National Forest Reserve, Western Kenya. Forests 2022, 13, 2127. [Google Scholar]
- Fischer R, Bohn F, de Paula MD, Dislich, C, Groeneveld, J., Gutiérrez, A.G., Kazmierczak, M., Knapp, N., Lehmann, S., Paulick, S, Pütz, S. Lessons learned from applying a forest gap model to understand the ecosystem and carbon dynamics of complex tropical forests. Ecol Modell 2016, 326, 124–133. [Google Scholar] [CrossRef]
- Gaaf, N., Poels, H., Rompaey, R. Effect of silvicultural treatment on growth and mortality of rainforest in suriname over long periods. Forest Ecology and Management 1999, 124, 123–135. [Google Scholar] [CrossRef]
- Getaneh, G. Getaneh, G., Teshome, S., Tesfaye, B. and Demel, T. Species composition, stand structure, and regeneration status of tree species in dry Afromontane forests of Awi Zone, northwestern Ethiopia. Ecosystem Health and Sustainability 2019, 5, 199–215. [Google Scholar] [CrossRef]
- Girma, A. & Mosandl, Reinhard. Structure and potential regeneration of degraded secondary stands in Munessssa-Shashemene Forest, Ethiopia. Journal of Tropical Forest Science 2012, 24, 46–53.
- Gotelli, N. J. , & Chao, A. Measuring and estimating species richness, species diversity, and biotic similarity from sampling data. 2013.
- Hancock, P. R. Book Reviews. Review of Radical Political Economics 2004, 36, 260–263. [Google Scholar] [CrossRef]
- Kacholi DS Edge-interior disparities in tree species and structural composition of the Kilengwe forest in Morogoro region, Tanzania. ISRN Biodiversity 2014, 2014, 1–8. [CrossRef]
- Khumbongmayum, A. D., M. Khan, and R. Tripathi. “Biodiversity Conservation in Sacred Groves of Manipur, Northeast India: Population Structure and Regeneration Status of Woody Species.”. Human Exploitation and Biodiversity Conservation Springer 2006, 15, 2439–2456. [CrossRef]
- Kissinger, G., M. Herold, V. De Sy. Drivers of Deforestation and Forest Degradation: A Synthesis Report for REDD+ Policymakers. 2012; Lexeme Consulting, Vancouver Canada.
- Krupnick, G.A. “Conservation of tropical plant biodiversity: what have we done, where are we going? Wiley online library,”. Biotropica 2013, 45, 693–708. [CrossRef]
- Kuria, N. C., Balozi, K and Kipkore, W. ‘Growth and Yield Models for Plantation-Grown Cupressus lusitanica for Central Kenya’. African Journal of Education, Science and Technology 2019, 5, 34–58.
- Lawrence, D., and Vandecar, K. Effects of tropical deforestation on climate and agriculture. Nat. Clim. Change 2015, 5, 27–36. [CrossRef]
- Liira J, Sepp T, Kohv K The ecology of tree regeneration in mature and old forests: combined knowledge for sustainable forest management. J For Res 2011, 16, 184–193. [CrossRef]
- Marengo J Drought, Floods, Climate Change, and Forest Loss in the Amazon Region: A Present and Future Danger? Front. Young Minds. 2020, 8, 147. [CrossRef]
- Mendoza, G.A. and R. Prabhu. Qualitative multi-criteria approach to assessing indicators of sustainable forest resource management. For. Ecol. Manag. 2000, 131, 107–126. [Google Scholar] [CrossRef]
- Monserud, R.A. Evaluating Forest Models in a Sustainable Forest Management Context; FBMIS 2003; Volume1; pp: 35-47; ISSN: 1740-5955; http://www.fbmis.info/A/3_1_ MonserudR_1.
- Mori, A., and H. Takeda. “Effects of Undisturbed Canopy Structure on Population Structure and Species Coexistence in an Old-growth Subalpine Forest in Central Japan.”. Forest Ecology and Management 2004, 89–100. [CrossRef]
- Mutiso, F., Hitimana, J., Kiyiapi, J., Sang, F., & Eboh, E. Recovery of Kakamega tropical rainforest from anthropogenic disturbances. Journal of Tropical Forest Science 2013, 25, 566–576, Forest Research Institute Malaysia.
- Mutiso,F.M., Mugo, M.J., Cheboiwo, J., Sang,F. and Tarus, G.K. Floristic Composition, Affinities and Plant Formations in Tropical Forests: A Case Study of Mau Forests in Kenya. International Journal of Agriculture and Forestry 2015, 5, 79–91. [CrossRef]
- Peña-Claros, M.L.; Poorter, A. Alarcón, G. Blate, U. Choque, T. S. Fredericksen, M. J. Justiniano, C. Leaño, J. C. Licona, and W. Pariona. “Soil Effects on Forest Structure and Diversity in a Moist and a Dry Tropical Forest.”. Biotropica 2012, 44, 276–283. [CrossRef]
- Sapkota, I., Tigabu, M., Oden, P. Changes in tree species diversity and dominance across disturbance gradient in Napelese Sal (Shorea robusta Gaertn. f.) forests. Journal of Forestry Research 2010, 21.
- Schleuning, M., Farwig, N., Peters, M. K., Bergsdorf, T., Bleher, B., Brandl, R.,... & Böhning-Gaese, K. (2011). Forest fragmentation and selective logging have inconsistent effects on multiple animal-mediated ecosystem processes in a tropical forest. PloS ONE 2011, 6, e27785.
- Strassburg, B.B. N, H. L. Beyer, R. Crouzeilles, A. Iribarrem, F. Barros, M. F. de Siqueira, A. Sánchez-Tapia, A. Balmford, J. B. B. Sansevero, P. H. S. Brancalion, E. N. Broadbent, R. L. Chazdon, A. O. Filho, T. A. Gardner, A. Gordon, A. Latawiec, R. Loyola, J. P. Metzger, M. Mills, H. P. Possingham, R. R. Rodrigues, C. A. M. Scaramuzza, F. R. Scarano, L. Tambosi, M. Uriarte Strategic approaches to restoring ecosystems can triple conservation gains and halve costs. Nat. Ecol. Evol. 2019, 3, 62–70. [Google Scholar]
- Tarus, G.K. & Nadir, S.W. Effect of Forest Management Types on Soil Carbon Stocks in Montane Forests: A Case Study of Eastern Mau Forest in Kenya. Int. J. For. Res. 10 pages. 2020. [Google Scholar] [CrossRef]
- Teketay, D. “Seed and Regeneration Ecology in Dry Afromontane Forests of Ethiopia: I Seed Production-population Structures.”. Tropical Ecology 2005, 46, 29–44. [Google Scholar]
- Teketay, D., K. Kashe, J. Madome, M. Kabelo, J. Neelo, M. Mmusi, and W. Masamba. “Enhancement of Diversity, Stand Structure and Regeneration of Woody Species through Area Exclosure: The Case of a Mopane Woodland in Northern Botswana.”. Ecological Processes 2018, 7, 5. [CrossRef]
- Wangda, P. Wangda, P. (2003). Forest zonation along the complex altitudinal gradients in a dry valley of Punatsang Chu. M. Sc. Thesis, Tokyo: Graduate School of Frontier Sciences, Laboratory of Biosphere Functions, University of Tokyo, Japan.
- Wenger, K.F. (2013): Forestry Handbook. John Wiley and Sons, Inc., New York. 1,335 pp.
- Wittmann, F., Schöngart, J., Montero, J.C. Motzer, T. Junk, W.J., Pieadade, M.T.F., Queiroz, H.L. and Worbes, M. Tree Species Composition and Diversity Gradients in the White-Water Forests across the Amazon Basin. J. Biogeogr. 2006, 33, 1334–1347. [CrossRef]
- Zhu, H., Xu, Z.F., Wang, H. & Li, B.G. 2004. Tropical rain forest fragmentation and its ecological and species diversity changes in southern Yunnan. Biodiversity and Conservation 2004, 13, 1355–1372.
- Zerihun, A. and Yemir T. (2013): Hawassa University: Wondo Genet Colleg of Forestry and Natural Resources, Training Manual on: Forest Inventory and Management in the Context of SFM & REDD. Pp. 1-67.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).