Submitted:
24 July 2023
Posted:
24 July 2023
You are already at the latest version
Abstract
Keywords:
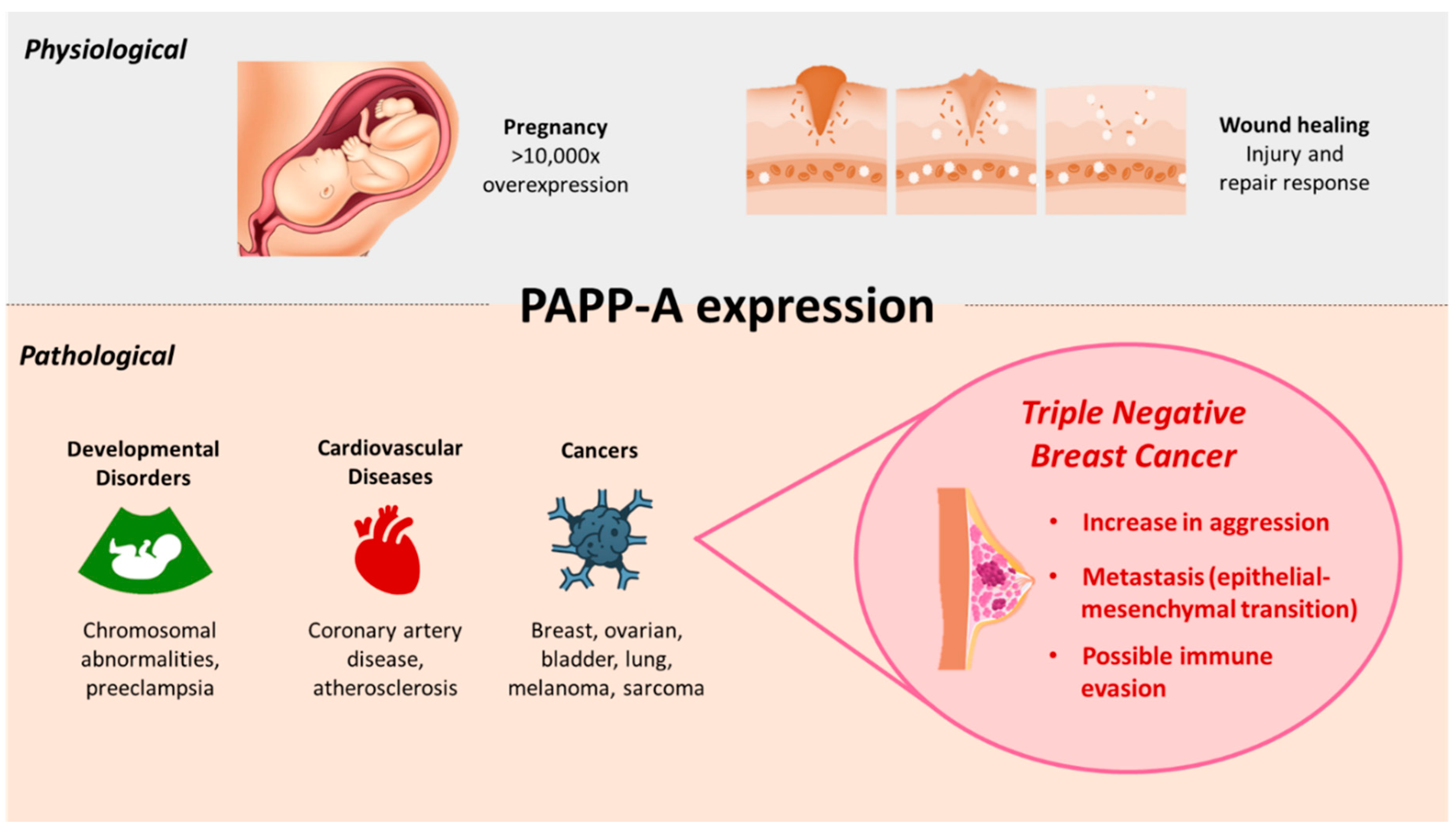
1. Introduction
2. PAPP-A: Structure, Function, and Regulation
2.1. Structure
2.2. Function
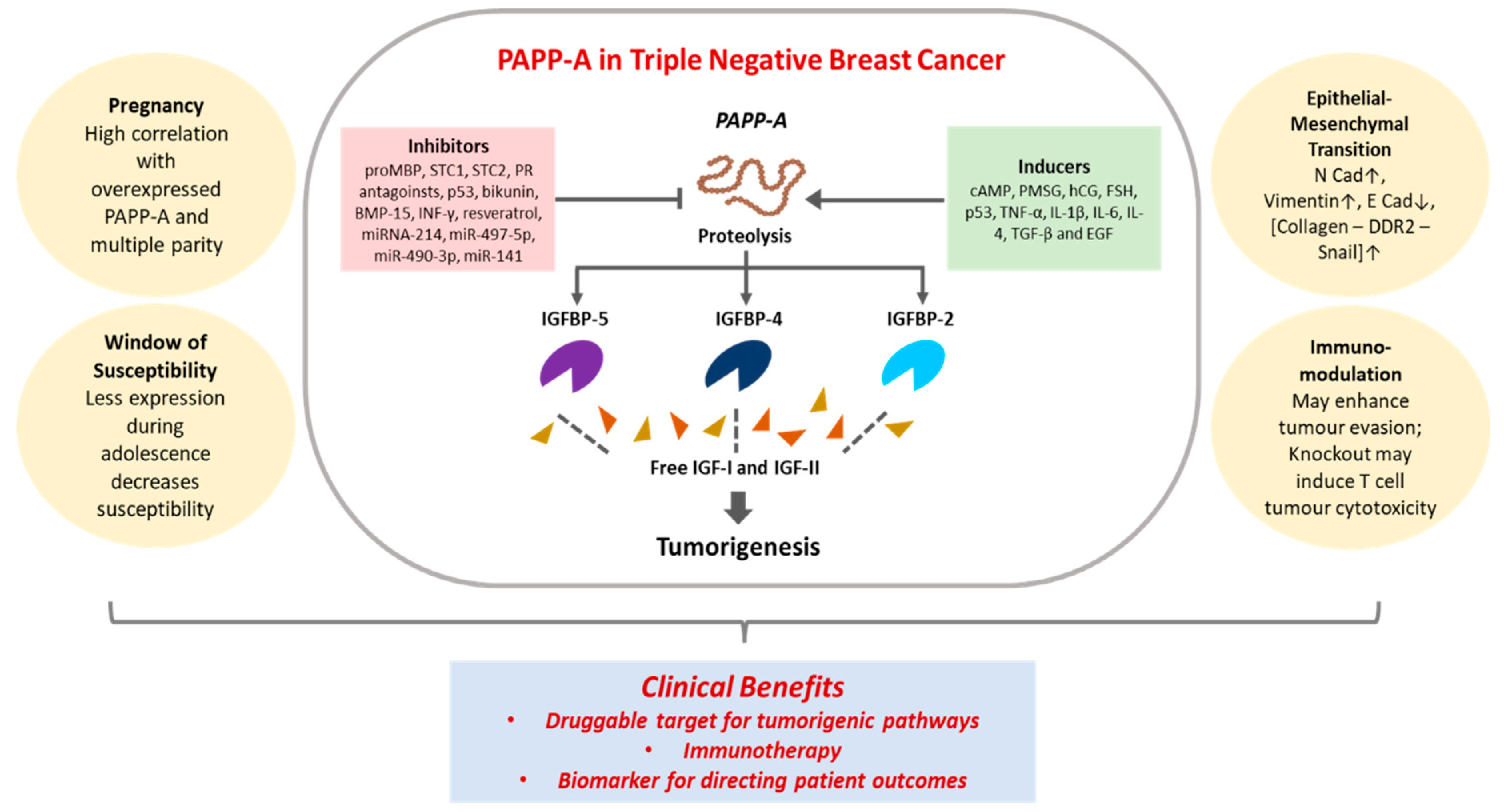
2.3. Regulation
| Regulator | Role | Mode of PAPP-A interaction | Organism | Reference |
|---|---|---|---|---|
| proMBP | Inhibitor | Covalently binds PAPP-A to abrogate its proteolytic activity | Human serum, HEK 293T Cells | [40,64] |
| STC1 | Inhibitor | High affinity binding to PAPP-A rather than covalent binding | HEK 293T Cells | [32] |
| STC2 | Inhibitor | Covalent binding of PAPP-A through Cys-120 residue of STC2 | HEK 293T Cells, transgenic mice, mouse embryonic fibroblasts | [32,33] |
| cAMP | Inducer | cAMP inducible region in 5’ UTR of PAPP-A cDNA | Human placental choriocarcinoma cell line JAR cells | [66] |
| Progesterone antagonist (RU486) | Inhibitor | Inhibition of PAPP-A production rate; PAPP-A production recovered by addition of progesterone | Human trophoblastic and decidual explants, cynomolgus monkey | [67,68] |
| PMSG | Inducer | Transient increase in PAPP-A transcripts | Mouse ovary | [68] |
| hCG | Inducer | Sustained increase in PAPP-A expression after PMSG treatment | Mouse ovary | [68] |
| FSH | Inducer | Increased PAPP-A mRNA expression | Rat granulocytes | [70] |
| BMP-15 | Inhibitor | Reduced PAPP-A expression following FSH stimulation | Rat granulocytes | [70] |
| p53 | Inhibitor or inducer | PAPP-A suppression in TNBC; PAPP-A overexpression in human fibroblasts. | TNBC cell line MDA-MB-157; BJ/ET cell line |
[75,76,77]. |
| Bikunin | Inhibitor | Early suppression of PAPP-A mRNA in response to bikunin treatment | Ovarian cancer cell line HRA | [73] |
| TNF-α, IL-1β, IL-6, IL-4, TGF-β | Inducer | Upregulation of PAPP-A expression | TNF-α and IL-1β: human dermal fibroblasts and human coronary artery endothelial and smooth muscle cells; IL-6: coronary artery smooth muscle cells; TNF-α, IL-1β, IL-4, TGF-β: human osteoblasts |
[72] |
| EGF | Inducer | Upregulation of PAPP-A expression | TNBC cell lines | [25] |
| INF-γ | Inhibitor | Suppression of PAPP-A expression | Human fibroblasts | [74] |
| Resveratrol | Inhibitor | Reduction in cytokine-mediated PAPP-A expression | Coronary artery smooth muscle cells | [74] |
| miRNA-214 | Inhibitor | Targeted suppression of PAPP-A mRNA | NSCLC cell lines U-1810 or H23 | [78] |
| miR-497-5p | Inhibitor | Negative regulator of PAPP-A mRNA | Pregnancy-associated BC tissues and serum, normal breast tissues, BC cell lines MDA-MB-231 and MCF-7 | [34] |
| miR-490-3p | Inhibitor | Targeted suppression of PAPP-A expression | Human coronary artery smooth muscle cells | [79] |
| miR-141 | Inhibitor | Suppression of PAPP-A protein | Vascular smooth muscle cells | [80] |
3. PAPP-A in BC
3.1. PAPP-A in TNBC: Proteolysis of IGFBPs
| IGFBP/IGF | Role | PAPP-A expression | Organism | References |
|---|---|---|---|---|
| IGFBP-4 proteolysis | Leads to increase in IGF-I | Secreted along with IGF-I | Bovine mammary fibroblast cells | [91] |
| PAPP-A resistant IGFBP-4 | Leads to sequestering of IGF-I | Retained tumor suppression; decreased angiogenesis and lung metastasis | 4T1.2, orthotopic model of 4T1.2 BCs | [92,93] |
| IGFBP-4 proteolysis | Due to increased PAPP-A levels from Skp2B overexpression | Putative p53 binding sites in PAPP-A gene | During pregnancy and lactation in the mice mammary glands | [75] |
| IGFBP-4 | Co-expression with PAPP-A | Co-expression with IGFBP-4 | HCC70, MDA-MB-468 and MDA-MB-231 cells | [25] |
| IGF-IR | Co-expression with PAPP-A | Co-expression with IGF-IR | HCC70, MDA-MB-468, HCC1954 and MDA-MB-231 | [25] |
| IGF-IIR | Independent of PAPP-A expression | Expression with and without IGF-IIR | With PAPP-A: MDA-MB-468, HCC1954 and MDA-MB-231 Without PAPP-A: MCF-7, BT474, SKBR3, HCC1569, MDA-MB-453 |
[25] |
| IGFBP-5 proteolysis | Due to increased PAPP-A levels from increased collagen deposition | Higher in parous mice breast than in nulliparous mice | Transgenic mice overexpressing PAPP-A in the mammary gland and MCF-7 BC cells | [96,97] |
3.2. PAPP-A in TNBC: Role in Epithelial-Mesenchymal Transition (EMT)
3.3. PAPP-A in BC: Role in the Window of Susceptibility (WOS)
3.4. PAPP-A in TNBC: Impact of Pregnancy
4. Immunological Relevance of PAPP-A in BC
5. Clinical Relevance of PAPP-A in TNBC
6. Conclusion
7. List of Abbreviations
| 5’ UTR | 5’ untranslated region |
| AFP | Alpha-fetoprotein |
| BC | Breast cancer |
| BME | Bovine mammary epithelial |
| BMF | Bovine mammary fibroblast |
| BMP | Bone morphogenetic protein |
| cAMP | Cyclic adenosine monophosphate |
| CCP | Complement control protein |
| ChIP-seq | Chromatin immunoprecipitation followed by sequencing |
| DDR2 | Discoidin domain receptor 2 |
| DIA | Dimeric inhibin-A |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| EMT | Epithelial-mesenchymal transition |
| ER | Estrogen receptor |
| ERAP1 | Endoplasmic reticulum aminopeptidase 1 |
| FFPE | Formalin-fixed paraffin-embedded |
| FSH | Follicle stimulating hormone |
| FSTL | Follistatin-like |
| hCG | Human chorionic gonadotropin |
| HER2 | Human epidermal receptor 2 |
| IGF | Insulin-like growth factor |
| IGFBP | Insulin-like growth factor dependent insulin-like growth factor-binding proteins |
| IGF-IR | Enhanced type I insulin-like growth factor receptor |
| IGF-IIR | Enhanced type II insulin-like growth factor receptor |
| IHC | Immunohistochemistry |
| IL-1β | Interleukin 1 beta |
| LARP6 | La ribonucleoprotein domain family member 6 |
| LMP | Low molecular mass polypeptides |
| LNR | Linear notch repeat |
| Mcs5c | Mammary carcinoma susceptibility 5c |
| MHC | Major histocompatibility complex |
| miR | microRNA |
| OS | Overall survival |
| PAPP-A | Pregnancy associated plasma protein-A |
| PBMC | Peripheral blood mononuclear cells |
| PMSG | Pregnant mare serum gonadotropin |
| PR | Progesterone receptor |
| proMBP | Proform of the eosinophil major basic protein |
| PSME | Proteasome activator complex subunits |
| qRT-PCR | Real-time quantitative reverse transcription pcr |
| RAS | Rat sarcoma |
| RFS | Recurrence free survival |
| SNP | Single nucleotide polymorphism |
| STC | Stanniocalcins |
| TACS | Tumor-associated collagen signature |
| TCGA | The cancer genome atlas |
| TCR | T cell receptor |
| TGF-β | Transforming growth factor beta |
| TMA | Tissue microarrays |
| TNBC | Triple negative breast cancer |
| TNF-α | Tumor necrosis factor alpha |
| uE3 | Unconjugated estriol |
| WOS | The Window of Susceptibility |
Ethics approval and consent to participate
Consent for publication
Availability of data and materials
Competing Interests
Author Contributions
Funding
Acknowledgements
References
- Lin TM, Galbert SP, Kiefer D, Spellacy WN, Gall S. Characterization of four human pregnancy-associated plasma proteins. Am J Obstet Gynecol. 1974;118(2):223-36. [CrossRef]
- Lawrence, J.B.; Oxvig, C.; Overgaard, M.T.; Sottrup-Jensen, L.; Gleich, G.J.; Hays, L.G.; Yates, J.R., 3rd; Conover, C.A. The insulin-like growth factor (IGF)-dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy-associated plasma protein-A. Proc Natl Acad Sci U S A 1999, 96, 3149–3153. [Google Scholar] [CrossRef]
- Qin, Q.P.; Christiansen, M.; Oxvig, C.; Pettersson, K.; Sottrup-Jensen, L.; Koch, C.; Nørgaard-Pedersen, B. Double-monoclonal immunofluorometric assays for pregnancy-associated plasma protein A/proeosinophil major basic protein (PAPP-A/proMBP) complex in first-trimester maternal serum screening for Down syndrome. Clin Chem 1997, 43, 2323–2332. [Google Scholar] [CrossRef]
- Bonno, M.; Oxvig, C.; Kephart, G.M.; Wagner, J.M.; Kristensen, T.; Sottrup-Jensen, L.; Gleich, G.J. Localization of pregnancy-associated plasma protein-A and colocalization of pregnancy-associated plasma protein-A messenger ribonucleic acid and eosinophil granule major basic protein messenger ribonucleic acid in placenta. Lab Invest 1994, 71, 560–566. [Google Scholar]
- Overgaard, M.T.; Oxvig, C.; Christiansen, M.; Lawrence, J.B.; Conover, C.A.; Gleich, G.J.; Sottrup-Jensen, L.; Haaning, J. Messenger ribonucleic acid levels of pregnancy-associated plasma protein-A and the proform of eosinophil major basic protein: expression in human reproductive and nonreproductive tissues. Biol Reprod 1999, 61, 1083–1089. [Google Scholar] [CrossRef]
- Guo, Y.; Bao, Y.; Guo, D.; Yang, W. Pregnancy-associated plasma protein a in cancer: expression, oncogenic functions and regulation. Am J Cancer Res 2018, 8, 955–963. [Google Scholar]
- Jenkins, E.C.; Brown, S.O.; Germain, D. The Multi-Faced Role of PAPP-A in Post-Partum Breast Cancer: IGF-Signaling is Only the Beginning. J Mammary Gland Biol Neoplasia 2020, 25, 181–189. [Google Scholar] [CrossRef]
- Heitzeneder, S.; Sotillo, E.; Shern, J.F.; Sindiri, S.; Xu, P.; Jones, R.; Pollak, M.; Noer, P.R.; Lorette, J.; Fazli, L.; et al. Pregnancy-Associated Plasma Protein-A (PAPP-A) in Ewing Sarcoma: Role in Tumor Growth and Immune Evasion. J Natl Cancer Inst 2019, 111, 970–982. [Google Scholar] [CrossRef]
- D'Elia, P.; Ionta, V.; Chimenti, I.; Angelini, F.; Miraldi, F.; Pala, A.; Messina, E.; Giacomello, A. Analysis of pregnancy-associated plasma protein A production in human adult cardiac progenitor cells. Biomed Res Int 2013, 2013, 190178. [Google Scholar] [CrossRef]
- Hjortebjerg, R. IGFBP-4 and PAPP-A in normal physiology and disease. Growth Horm IGF Res 2018, 41, 7–22. [Google Scholar] [CrossRef]
- Conover, C.A.; Bale, L.K. Loss of pregnancy-associated plasma protein A extends lifespan in mice. Aging Cell 2007, 6, 727–729. [Google Scholar] [CrossRef]
- Oxvig, C. The role of PAPP-A in the IGF system: location, location, location. J Cell Commun Signal 2015, 9, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Bowers, L.W.; Rossi, E.L.; O'Flanagan, C.H.; deGraffenried, L.A.; Hursting, S.D. The Role of the Insulin/IGF System in Cancer: Lessons Learned from Clinical Trials and the Energy Balance-Cancer Link. Front Endocrinol (Lausanne) 2015, 6, 77. [Google Scholar] [CrossRef]
- Conover, C.A.; Oxvig, C. PAPP-A and cancer. J Mol Endocrinol 2018, 61, T1–t10. [Google Scholar] [CrossRef] [PubMed]
- Kuhajda, F.P.; Eggleston, J.C. Pregnancy-associated plasma protein A. A clinically significant predictor of early recurrence in stage I breast carcinoma is independent of estrogen receptor status. Am J Pathol 1985, 121, 342–348. [Google Scholar] [PubMed]
- Mansfield, A.S.; Visscher, D.W.; Hart, S.N.; Wang, C.; Goetz, M.P.; Oxvig, C.; Conover, C.A. Pregnancy-associated plasma protein-A expression in human breast cancer. Growth Horm IGF Res 2014, 24, 264–267. [Google Scholar] [CrossRef]
- Gadaleta, E.; Thorn, G.J.; Ross-Adams, H.; Jones, L.J.; Chelala, C. Field cancerization in breast cancer. J Pathol 2022, 257, 561–574. [Google Scholar] [CrossRef]
- Azamjah, N.; Soltan-Zadeh, Y.; Zayeri, F. Global Trend of Breast Cancer Mortality Rate: A 25-Year Study. Asian Pac J Cancer Prev 2019, 20, 2015–2020. [Google Scholar] [CrossRef]
- Poddar, A.; Rao, S.R.; Prithviraj, P.; Kannourakis, G.; Jayachandran, A. Crosstalk between Immune Checkpoint Modulators, Metabolic Reprogramming and Cellular Plasticity in Triple-Negative Breast Cancer. Curr Oncol 2022, 29, 6847–6863. [Google Scholar] [CrossRef]
- Emens, L.A.; Cruz, C.; Eder, J.P.; Braiteh, F.; Chung, C.; Tolaney, S.M.; Kuter, I.; Nanda, R.; Cassier, P.A.; Delord, J.P.; et al. Long-term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients With Metastatic Triple-Negative Breast Cancer: A Phase 1 Study. JAMA Oncol 2019, 5, 74–82. [Google Scholar] [CrossRef]
- Ensenyat-Mendez, M.; Llinas-Arias, P.; Orozco, J.I.J.; Iniguez-Munoz, S.; Salomon, M.P.; Sese, B.; DiNome, M.L.; Marzese, D.M. Current Triple-Negative Breast Cancer Subtypes: Dissecting the Most Aggressive Form of Breast Cancer. Front Oncol 2021, 11, 681476. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. Jama 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Almansour, N.M. Triple-Negative Breast Cancer: A Brief Review About Epidemiology, Risk Factors, Signaling Pathways, Treatment and Role of Artificial Intelligence. Front Mol Biosci 2022, 9, 836417. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Zhu, C.; Wang, G.; Gu, J. Treatment for Triple-Negative Breast Cancer: An Umbrella Review of Meta-Analyses. Int J Gen Med 2022, 15, 5901–5914. [Google Scholar] [CrossRef] [PubMed]
- Prithviraj, P.; Anaka, M.; Thompson, E.W.; Sharma, R.; Walkiewicz, M.; Tutuka, C.S.A.; Behren, A.; Kannourakis, G.; Jayachandran, A. Aberrant pregnancy-associated plasma protein-A expression in breast cancers prognosticates clinical outcomes. Sci Rep 2020, 10, 13779. [Google Scholar] [CrossRef]
- Loddo, M.; Andryszkiewicz, J.; Rodriguez-Acebes, S.; Stoeber, K.; Jones, A.; Dafou, D.; Apostolidou, S.; Wollenschlaeger, A.; Widschwendter, M.; Sainsbury, R.; et al. Pregnancy-associated plasma protein A regulates mitosis and is epigenetically silenced in breast cancer. J Pathol 2014, 233, 344–356. [Google Scholar] [CrossRef]
- Park, A.L.; Huang, T.; Meschino, W.S.; Iqbal, J.; Ray, J.G. Prenatal Biochemical Screening and a Woman's Long-Term Risk of Cancer: A Population-Based Cohort Study. JNCI Cancer Spectr 2020, 4, pkz077. [Google Scholar] [CrossRef]
- Laursen, L.S.; Overgaard, M.T.; Weyer, K.; Boldt, H.B.; Ebbesen, P.; Christiansen, M.; Sottrup-Jensen, L.; Giudice, L.C.; Oxvig, C. Cell surface targeting of pregnancy-associated plasma protein A proteolytic activity. Reversible adhesion is mediated by two neighboring short consensus repeats. J Biol Chem 2002, 277, 47225–47234. [Google Scholar] [CrossRef]
- Brahmkhatri, V.P.; Prasanna, C.; Atreya, H.S. Insulin-like growth factor system in cancer: novel targeted therapies. Biomed Res Int 2015, 2015, 538019. [Google Scholar] [CrossRef]
- Kobberø, S.D.; Gajhede, M.; Mirza, O.A.; Kløverpris, S.; Kjær, T.R.; Mikkelsen, J.H.; Boesen, T.; Oxvig, C. Structure of the proteolytic enzyme PAPP-A with the endogenous inhibitor stanniocalcin-2 reveals its inhibitory mechanism. Nat Commun 2022, 13, 6084. [Google Scholar] [CrossRef]
- Glerup, S.; Kløverpris, S.; Laursen, L.S.; Dagnaes-Hansen, F.; Thiel, S.; Conover, C.A.; Oxvig, C. Cell surface detachment of pregnancy-associated plasma protein-A requires the formation of intermolecular proteinase-inhibitor disulfide bonds and glycosaminoglycan covalently bound to the inhibitor. J Biol Chem 2007, 282, 1769–1778. [Google Scholar] [CrossRef]
- Kløverpris, S.; Mikkelsen, J.H.; Pedersen, J.H.; Jepsen, M.R.; Laursen, L.S.; Petersen, S.V.; Oxvig, C. Stanniocalcin-1 Potently Inhibits the Proteolytic Activity of the Metalloproteinase Pregnancy-associated Plasma Protein-A. J Biol Chem 2015, 290, 21915–21924. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, M.R.; Kløverpris, S.; Mikkelsen, J.H.; Pedersen, J.H.; Füchtbauer, E.M.; Laursen, L.S.; Oxvig, C. Stanniocalcin-2 inhibits mammalian growth by proteolytic inhibition of the insulin-like growth factor axis. J Biol Chem 2015, 290, 3430–3439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Li, L.; Nian, Y.; Chen, Y.; Shen, R.; Ma, X. Pregnancy-associated plasma protein-A (PAPPA) promotes breast cancer progression. Bioengineered 2022, 13, 291–307. [Google Scholar] [CrossRef]
- Silahtaroglu, A.N.; Tümer, Z.; Kristensen, T.; Sottrup-Jensen, L.; Tommerup, N. Assignment of the human gene for pregnancy-associated plasma protein A (PAPPA) to 9q33.1 by fluorescence in situ hybridization to mitotic and meiotic chromosomes. Cytogenet Cell Genet 1993, 62, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Sexton, C.; Byun, D.; Strong, D.D.; Baylink, D.J.; Mohan, S. Differential regulation of pregnancy associated plasma protein (PAPP)-A during pregnancy in human and mouse. Growth Horm IGF Res 2002, 12, 359–366. [Google Scholar] [CrossRef]
- Søe, R.; Overgaard, M.T.; Thomsen, A.R.; Laursen, L.S.; Olsen, I.M.; Sottrup-Jensen, L.; Haaning, J.; Giudice, L.C.; Conover, C.A.; Oxvig, C. Expression of recombinant murine pregnancy-associated plasma protein-A (PAPP-A) and a novel variant (PAPP-Ai) with differential proteolytic activity. Eur J Biochem 2002, 269, 2247–2256. [Google Scholar] [CrossRef]
- Bischof, P. Purification and characterization of pregnancy associated plasma protein A (PAPP-A). Arch Gynecol 1979, 227, 315–326. [Google Scholar] [CrossRef]
- Oxvig, C.; Sand, O.; Kristensen, T.; Gleich, G.J.; Sottrup-Jensen, L. Circulating human pregnancy-associated plasma protein-A is disulfide-bridged to the proform of eosinophil major basic protein. J Biol Chem 1993, 268, 12243–12246. [Google Scholar] [CrossRef]
- Overgaard, M.T.; Haaning, J.; Boldt, H.B.; Olsen, I.M.; Laursen, L.S.; Christiansen, M.; Gleich, G.J.; Sottrup-Jensen, L.; Conover, C.A.; Oxvig, C. Expression of recombinant human pregnancy-associated plasma protein-A and identification of the proform of eosinophil major basic protein as its physiological inhibitor. J Biol Chem 2000, 275, 31128–31133. [Google Scholar] [CrossRef]
- Oxvig, C.; Sand, O.; Kristensen, T.; Kristensen, L.; Sottrup-Jensen, L. Isolation and characterization of circulating complex between human pregnancy-associated plasma protein-A and proform of eosinophil major basic protein. Biochim Biophys Acta 1994, 1201, 415–423. [Google Scholar] [CrossRef]
- Weyer, K.; Glerup, S. Placental regulation of peptide hormone and growth factor activity by proMBP. Biol Reprod 2011, 84, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Boldt, H.B.; Glerup, S.; Overgaard, M.T.; Sottrup-Jensen, L.; Oxvig, C. Definition, expression, and characterization of a protein domain in the N-terminus of pregnancy-associated plasma protein-A distantly related to the family of laminin G-like modules. Protein Expr Purif 2006, 48, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Boldt, H.B.; Overgaard, M.T.; Laursen, L.S.; Weyer, K.; Sottrup-Jensen, L.; Oxvig, C. Mutational analysis of the proteolytic domain of pregnancy-associated plasma protein-A (PAPP-A): classification as a metzincin. Biochem J 2001, 358, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Stöcker, W.; Grams, F.; Baumann, U.; Reinemer, P.; Gomis-Rüth, F.X.; McKay, D.B.; Bode, W. The metzincins--topological and sequential relations between the astacins, adamalysins, serralysins, and matrixins (collagenases) define a superfamily of zinc-peptidases. Protein Sci 1995, 4, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Barrios, V.; Chowen, J.A.; Martín-Rivada, Á.; Guerra-Cantera, S.; Pozo, J.; Yakar, S.; Rosenfeld, R.G.; Pérez-Jurado, L.A.; Suárez, J.; Argente, J. Pregnancy-Associated Plasma Protein (PAPP)-A2 in Physiology and Disease. Cells 2021, 10. [Google Scholar] [CrossRef]
- Boldt, H.B.; Kjaer-Sorensen, K.; Overgaard, M.T.; Weyer, K.; Poulsen, C.B.; Sottrup-Jensen, L.; Conover, C.A.; Giudice, L.C.; Oxvig, C. The Lin12-notch repeats of pregnancy-associated plasma protein-A bind calcium and determine its proteolytic specificity. J Biol Chem 2004, 279, 38525–38531. [Google Scholar] [CrossRef]
- Weyer, K.; Overgaard, M.T.; Laursen, L.S.; Nielsen, C.G.; Schmitz, A.; Christiansen, M.; Sottrup-Jensen, L.; Giudice, L.C.; Oxvig, C. Cell surface adhesion of pregnancy-associated plasma protein-A is mediated by four clusters of basic residues located in its third and fourth CCP module. Eur J Biochem 2004, 271, 1525–1535. [Google Scholar] [CrossRef]
- Leguy, M.C.; Brun, S.; Pidoux, G.; Salhi, H.; Choiset, A.; Menet, M.C.; Gil, S.; Tsatsaris, V.; Guibourdenche, J. Pattern of secretion of pregnancy-associated plasma protein-A (PAPP-A) during pregnancies complicated by fetal aneuploidy, in vivo and in vitro. Reprod Biol Endocrinol 2014, 12, 129. [Google Scholar] [CrossRef]
- Bischof, P.; DuBerg, S.; Herrmann, W.; Sizonenko, P.C. Pregnancy-associated plasma protein-A (PAPP-A) and hCG in early pregnancy. Br J Obstet Gynaecol 1981, 88, 973–975. [Google Scholar] [CrossRef]
- Gyrup, C.; Christiansen, M.; Oxvig, C. Quantification of proteolytically active pregnancy-associated plasma protein-A with an assay based on quenched fluorescence. Clin Chem 2007, 53, 947–954. [Google Scholar] [CrossRef]
- Shiefa, S.; Amargandhi, M.; Bhupendra, J.; Moulali, S.; Kristine, T. First Trimester Maternal Serum Screening Using Biochemical Markers PAPP-A and Free β-hCG for Down Syndrome, Patau Syndrome and Edward Syndrome. Indian J Clin Biochem 2013, 28, 3–12. [Google Scholar] [CrossRef]
- Antsaklis, P.; Fasoulakis, Z.; Theodora, M.; Diakosavvas, M.; Kontomanolis, E.N. Association of Low Maternal Pregnancy-associated Plasma Protein A with Adverse Perinatal Outcome. Cureus 2019, 11, e4912. [Google Scholar] [CrossRef] [PubMed]
- Kirkegaard, I.; Uldbjerg, N.; Oxvig, C. Biology of pregnancy-associated plasma protein-A in relation to prenatal diagnostics: an overview. Acta Obstet Gynecol Scand 2010, 89, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Bischof, P.; Mégevand, M. Pregnancy-associated plasma protein-A concentrations in men with testicular and prostatic tumors. Arch Androl 1986, 16, 155–160. [Google Scholar] [CrossRef]
- Boldt, H.B.; Conover, C.A. Pregnancy-associated plasma protein-A (PAPP-A): a local regulator of IGF bioavailability through cleavage of IGFBPs. Growth Horm IGF Res 2007, 17, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Byun, D.; Mohan, S.; Kim, C.; Suh, K.; Yoo, M.; Lee, H.; Baylink, D.J.; Qin, X. Studies on human pregnancy-induced insulin-like growth factor (IGF)-binding protein-4 proteases in serum: determination of IGF-II dependency and localization of cleavage site. J Clin Endocrinol Metab 2000, 85, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Bøtkjær, J.A.; Noer, P.R.; Oxvig, C.; Andersen, C.Y. Author Correction: A common variant of the pregnancy-associated plasma protein-A (PAPPA) gene encodes a protein with reduced proteolytic activity towards IGF-binding proteins. Sci Rep 2019, 9, 17523. [Google Scholar] [CrossRef]
- Laursen, L.S.; Overgaard, M.T.; Søe, R.; Boldt, H.B.; Sottrup-Jensen, L.; Giudice, L.C.; Conover, C.A.; Oxvig, C. Pregnancy-associated plasma protein-A (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: implications for the mechanism of IGFBP-4 proteolysis by PAPP-A. FEBS Lett 2001, 504, 36–40. [Google Scholar] [CrossRef]
- Gaidamauskas, E.; Gyrup, C.; Boldt, H.B.; Schack, V.R.; Overgaard, M.T.; Laursen, L.S.; Oxvig, C. IGF dependent modulation of IGF binding protein (IGFBP) proteolysis by pregnancy-associated plasma protein-A (PAPP-A): multiple PAPP-A-IGFBP interaction sites. Biochim Biophys Acta 2013, 1830, 2701–2709. [Google Scholar] [CrossRef]
- Monget, P.; Mazerbourg, S.; Delpuech, T.; Maurel, M.C.; Manière, S.; Zapf, J.; Lalmanach, G.; Oxvig, C.; Overgaard, M.T. Pregnancy-associated plasma protein-A is involved in insulin-like growth factor binding protein-2 (IGFBP-2) proteolytic degradation in bovine and porcine preovulatory follicles: identification of cleavage site and characterization of IGFBP-2 degradation. Biol Reprod 2003, 68, 77–86. [Google Scholar] [CrossRef]
- Judge, R.A.; Sridar, J.; Tunyasuvunakool, K.; Jain, R.; Wang, J.C.K.; Ouch, C.; Xu, J.; Mafi, A.; Nile, A.H.; Remarcik, C.; et al. Structure of the PAPP-A(BP5) complex reveals mechanism of substrate recognition. Nat Commun 2022, 13, 5500. [Google Scholar] [CrossRef]
- Grimberg, A. Mechanisms by which IGF-I may promote cancer. Cancer Biol Ther 2003, 2, 630–635. [Google Scholar] [CrossRef]
- Overgaard, M.T.; Glerup, S.; Boldt, H.B.; Rodacker, V.; Olsen, I.M.; Christiansen, M.; Sottrup-Jensen, L.; Giudice, L.C.; Oxvig, C. Inhibition of proteolysis by the proform of eosinophil major basic protein (proMBP) requires covalent binding to its target proteinase. FEBS Lett 2004, 560, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Oxvig, C.; Conover, C.A. The Stanniocalcin-PAPP-A-IGFBP-IGF Axis. J Clin Endocrinol Metab 2023, 108, 1624–1633. [Google Scholar] [CrossRef] [PubMed]
- Haaning, J.; Oxvig, C.; Overgaard, M.T.; Ebbesen, P.; Kristensen, T.; Sottrup-Jensen, L. Complete cDNA sequence of the preproform of human pregnancy-associated plasma protein-A. Evidence for expression in the brain and induction by cAMP. Eur J Biochem 1996, 237, 159–163. [Google Scholar] [CrossRef]
- Bischof, P.; Sizonenko, M.T.; Herrmann, W.L. Trophoblastic and decidual response to RU486: effects on human chorionic gonadotrophin, human placental lactogen, prolactin and pregnancy-associated plasma protein-A production in vitro. Hum Reprod 1986, 1, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Sinosich, M.J.; Lee, J.; Wolf, J.P.; Williams, R.F.; Hodgen, G.D. RU 486 induced suppression of placental neutrophil elastase inhibitor levels. Placenta 1989, 10, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Hourvitz, A.; Kuwahara, A.; Hennebold, J.D.; Tavares, A.B.; Negishi, H.; Lee, T.H.; Erickson, G.F.; Adashi, E.Y. The regulated expression of the pregnancy-associated plasma protein-A in the rodent ovary: a proposed role in the development of dominant follicles and of corpora lutea. Endocrinology 2002, 143, 1833–1844. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Sonntag, B.; Hwang, S.S.; Byerly, T.; Hourvitz, A.; Adashi, E.Y.; Shimasaki, S.; Erickson, G.F. Pregnancy-associated plasma protein-a production in rat granulosa cells: stimulation by follicle-stimulating hormone and inhibition by the oocyte-derived bone morphogenetic protein-15. Endocrinology 2004, 145, 3686–3695. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Penny, D.; Monget, P.; Arraztoa, J.A.; Fogelson, L.J.; Bondy, C.A. Insulin-like growth factor binding protein 4 expression parallels luteinizing hormone receptor expression and follicular luteinization in the primate ovary. Biol Reprod 2003, 69, 22–29. [Google Scholar] [CrossRef]
- Conover, C.A. Key questions and answers about pregnancy-associated plasma protein-A. Trends Endocrinol Metab 2012, 23, 242–249. [Google Scholar] [CrossRef]
- Suzuki, M.; Kobayashi, H.; Tanaka, Y.; Hirashima, Y.; Kanayama, N.; Takei, Y.; Saga, Y.; Suzuki, M.; Itoh, H.; Terao, T. Bikunin target genes in ovarian cancer cells identified by microarray analysis. J Biol Chem 2003, 278, 14640–14646. [Google Scholar] [CrossRef]
- Conover, C.A.; Bale, L.K.; Harrington, S.C.; Resch, Z.T.; Overgaard, M.T.; Oxvig, C. Cytokine stimulation of pregnancy-associated plasma protein A expression in human coronary artery smooth muscle cells: inhibition by resveratrol. Am J Physiol Cell Physiol 2006, 290, C183–188. [Google Scholar] [CrossRef] [PubMed]
- Chander, H.; Halpern, M.; Resnick-Silverman, L.; Manfredi, J.J.; Germain, D. Skp2B overexpression alters a prohibitin-p53 axis and the transcription of PAPP-A, the protease of insulin-like growth factor binding protein 4. PLoS One 2011, 6, e22456. [Google Scholar] [CrossRef] [PubMed]
- Melo, C.A.; Drost, J.; Wijchers, P.J.; van de Werken, H.; de Wit, E.; Oude Vrielink, J.A.; Elkon, R.; Melo, S.A.; Léveillé, N.; Kalluri, R.; et al. eRNAs are required for p53-dependent enhancer activity and gene transcription. Mol Cell 2013, 49, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Drost, J.; Mantovani, F.; Tocco, F.; Elkon, R.; Comel, A.; Holstege, H.; Kerkhoven, R.; Jonkers, J.; Voorhoeve, P.M.; Agami, R.; et al. BRD7 is a candidate tumour suppressor gene required for p53 function. Nat Cell Biol 2010, 12, 380–389. [Google Scholar] [CrossRef]
- Salim, H.; Arvanitis, A.; de Petris, L.; Kanter, L.; Hååg, P.; Zovko, A.; Özata, D.M.; Lui, W.O.; Lundholm, L.; Zhivotovsky, B.; et al. miRNA-214 is related to invasiveness of human non-small cell lung cancer and directly regulates alpha protein kinase 2 expression. Genes Chromosomes Cancer 2013, 52, 895–911. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, D.; Cao, L.; Zhang, R.; Zhou, J.; Chen, H.; Li, Y.; Li, M.; Cao, J.; Wang, Z. MiR-490-3p modulates the proliferation of vascular smooth muscle cells induced by ox-LDL through targeting PAPP-A. Cardiovasc Res 2013, 100, 272–279. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, B.; Ming, L.; Qin, H.; Zheng, L.; Yue, Z.; Cheng, Z.; Wang, Y.; Zhang, D.; Liu, C.; et al. MicroRNA-141 inhibits vascular smooth muscle cell proliferation through targeting PAPP-A. Int J Clin Exp Pathol 2015, 8, 14401–14408. [Google Scholar]
- Yu, H.; Rohan, T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst 2000, 92, 1472–1489. [Google Scholar] [CrossRef]
- Neuzillet, Y.; Chapeaublanc, E.; Krucker, C.; De Koning, L.; Lebret, T.; Radvanyi, F.; Bernard-Pierrot, I. IGF1R activation and the in vitro antiproliferative efficacy of IGF1R inhibitor are inversely correlated with IGFBP5 expression in bladder cancer. BMC Cancer 2017, 17, 636. [Google Scholar] [CrossRef] [PubMed]
- Prithviraj, P.; Anaka, M.; McKeown, S.J.; Permezel, M.; Walkiewicz, M.; Cebon, J.; Behren, A.; Jayachandran, A. Pregnancy associated plasma protein-A links pregnancy and melanoma progression by promoting cellular migration and invasion. Oncotarget 2015, 6, 15953–15965. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, N.; Li, Y.; Cheng, H.; Wang, D.; Yang, Q.; Deng, Y.; Yang, Y.; Li, Y.; Ruan, X.; et al. Insulin-like growth factor binding protein 5 (IGFBP5) functions as a tumor suppressor in human melanoma cells. Oncotarget 2015, 6, 20636–20649. [Google Scholar] [CrossRef] [PubMed]
- Kleinberg, D.L. Role of IGF-I in normal mammary development. Breast Cancer Res Treat 1998, 47, 201–208. [Google Scholar] [CrossRef]
- Christopoulos, P.F.; Msaouel, P.; Koutsilieris, M. The role of the insulin-like growth factor-1 system in breast cancer. Mol Cancer 2015, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Akkiprik, M.; Feng, Y.; Wang, H.; Chen, K.; Hu, L.; Sahin, A.; Krishnamurthy, S.; Ozer, A.; Hao, X.; Zhang, W. Multifunctional roles of insulin-like growth factor binding protein 5 in breast cancer. Breast Cancer Res 2008, 10, 212. [Google Scholar] [CrossRef]
- Dittmer, J. Biological effects and regulation of IGFBP5 in breast cancer. Front Endocrinol (Lausanne) 2022, 13, 983793. [Google Scholar] [CrossRef]
- Stacey, S.N.; Manolescu, A.; Sulem, P.; Rafnar, T.; Gudmundsson, J.; Gudjonsson, S.A.; Masson, G.; Jakobsdottir, M.; Thorlacius, S.; Helgason, A.; et al. Common variants on chromosomes 2q35 and 16q12 confer susceptibility to estrogen receptor-positive breast cancer. Nat Genet 2007, 39, 865–869. [Google Scholar] [CrossRef]
- Panagiotou, G.; Papakonstantinou, E.; Vagionas, A.; Polyzos, S.A.; Mantzoros, C.S. Serum Levels of Activins, Follistatins, and Growth Factors in Neoplasms of the Breast: A Case-Control Study. J Clin Endocrinol Metab 2019, 104, 349–358. [Google Scholar] [CrossRef]
- Fleming, J.M.; Leibowitz, B.J.; Kerr, D.E.; Cohick, W.S. IGF-I differentially regulates IGF-binding protein expression in primary mammary fibroblasts and epithelial cells. J Endocrinol 2005, 186, 165–178. [Google Scholar] [CrossRef]
- Ryan, A.J.; Napoletano, S.; Fitzpatrick, P.A.; Currid, C.A.; O'Sullivan, N.C.; Harmey, J.H. Expression of a protease-resistant insulin-like growth factor-binding protein-4 inhibits tumour growth in a murine model of breast cancer. Br J Cancer 2009, 101, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Smith, Y.E.; Toomey, S.; Napoletano, S.; Kirwan, G.; Schadow, C.; Chubb, A.J.; Mikkelsen, J.H.; Oxvig, C.; Harmey, J.H. Recombinant PAPP-A resistant insulin-like growth factor binding protein 4 (dBP4) inhibits angiogenesis and metastasis in a murine model of breast cancer. BMC Cancer 2018, 18, 1016. [Google Scholar] [CrossRef]
- Radke, S.; Pirkmaier, A.; Germain, D. Differential expression of the F-box proteins Skp2 and Skp2B in breast cancer. Oncogene 2005, 24, 3448–3458. [Google Scholar] [CrossRef] [PubMed]
- Espelund, U.; Renehan, A.G.; Cold, S.; Oxvig, C.; Lancashire, L.; Su, Z.; Flyvbjerg, A.; Frystyk, J. Prognostic relevance and performance characteristics of serum IGFBP-2 and PAPP-A in women with breast cancer: a long-term Danish cohort study. Cancer Med 2018, 7, 2391–2404. [Google Scholar] [CrossRef] [PubMed]
- Takabatake, Y.; Oxvig, C.; Nagi, C.; Adelson, K.; Jaffer, S.; Schmidt, H.; Keely, P.J.; Eliceiri, K.W.; Mandeli, J.; Germain, D. Lactation opposes pappalysin-1-driven pregnancy-associated breast cancer. EMBO Mol Med 2016, 8, 388–406. [Google Scholar] [CrossRef]
- Slocum, E.; Craig, A.; Villanueva, A.; Germain, D. Parity predisposes breasts to the oncogenic action of PAPP-A and activation of the collagen receptor DDR2. Breast Cancer Res 2019, 21, 56. [Google Scholar] [CrossRef]
- Jayachandran, A.; Dhungel, B.; Steel, J.C. Epithelial-to-mesenchymal plasticity of cancer stem cells: therapeutic targets in hepatocellular carcinoma. J Hematol Oncol 2016, 9, 74. [Google Scholar] [CrossRef]
- Shrestha, R.; Bridle, K.R.; Crawford, D.H.G.; Jayachandran, A. Immune checkpoint molecules are regulated by transforming growth factor (TGF)-β1-induced epithelial-to-mesenchymal transition in hepatocellular carcinoma. Int J Med Sci 2021, 18, 2466–2479. [Google Scholar] [CrossRef]
- Muqbil, I.; Wu, J.; Aboukameel, A.; Mohammad, R.M.; Azmi, A.S. Snail nuclear transport: the gateways regulating epithelial-to-mesenchymal transition? Semin Cancer Biol 2014, 27, 39–45. [Google Scholar] [CrossRef]
- Valiathan, R.R.; Marco, M.; Leitinger, B.; Kleer, C.G.; Fridman, R. Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metastasis Rev 2012, 31, 295–321. [Google Scholar] [CrossRef]
- Totzkay, D.; Silk, K.J.; Thomas, B.; Walling, B.M.; Smith, S.W. Women's Understanding of Windows of Susceptibility and the Role of the Environment in Breast Cancer Risk. J Cancer Educ 2023, 38, 115–126. [Google Scholar] [CrossRef]
- Veillet, A.L.; Haag, J.D.; Remfert, J.L.; Meilahn, A.L.; Samuelson, D.J.; Gould, M.N. Mcs5c: a mammary carcinoma susceptibility locus located in a gene desert that associates with tenascin C expression. Cancer Prev Res (Phila) 2011, 4, 97–106. [Google Scholar] [CrossRef]
- Samuelson, D.J.; Haag, J.D.; Lan, H.; Monson, D.M.; Shultz, M.A.; Kolman, B.D.; Gould, M.N. Physical evidence of Mcs5, a QTL controlling mammary carcinoma susceptibility, in congenic rats. Carcinogenesis 2003, 24, 1455–1460. [Google Scholar] [CrossRef]
- Henning, A.N.; Haag, J.D.; Smits, B.M.; Gould, M.N. The Non-coding Mammary Carcinoma Susceptibility Locus, Mcs5c, Regulates Pappa Expression via Age-Specific Chromatin Folding and Allele-Dependent DNA Methylation. PLoS Genet 2016, 12, e1006261. [Google Scholar] [CrossRef]
- Lambe, M.; Hsieh, C.; Trichopoulos, D.; Ekbom, A.; Pavia, M.; Adami, H.O. Transient increase in the risk of breast cancer after giving birth. N Engl J Med 1994, 331, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Asztalos, S.; Pham, T.N.; Gann, P.H.; Hayes, M.K.; Deaton, R.; Wiley, E.L.; Emmadi, R.; Kajdacsy-Balla, A.; Banerji, N.; McDonald, W.; et al. High incidence of triple negative breast cancers following pregnancy and an associated gene expression signature. Springerplus 2015, 4, 710. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, R.; Chlebowski, R.T.; Thune, I.; Furberg, A.S.; Hankins, G.D.; Malone, F.D.; D'Alton, M.E. Birth weight, breast cancer and the potential mediating hormonal environment. PLoS One 2012, 7, e40199. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.R.; Tarullo, S.E.; Crump, L.S.; Lyons, T.R. Studies of postpartum mammary gland involution reveal novel pro-metastatic mechanisms. J Cancer Metastasis Treat 2019, 5. [Google Scholar] [CrossRef]
- Allan, G.J.; Beattie, J.; Flint, D.J. The role of IGFBP-5 in mammary gland development and involution. Domest Anim Endocrinol 2004, 27, 257–266. [Google Scholar] [CrossRef]
- Watson, C.J. Involution: apoptosis and tissue remodelling that convert the mammary gland from milk factory to a quiescent organ. Breast Cancer Res 2006, 8, 203. [Google Scholar] [CrossRef]
- Jena, M.K.; Jaswal, S.; Kumar, S.; Mohanty, A.K. Molecular mechanism of mammary gland involution: An update. Dev Biol 2019, 445, 145–155. [Google Scholar] [CrossRef]
- Chen, B.K.; Leiferman, K.M.; Pittelkow, M.R.; Overgaard, M.T.; Oxvig, C.; Conover, C.A. Localization and regulation of pregnancy-associated plasma protein a expression in healing human skin. J Clin Endocrinol Metab 2003, 88, 4465–4471. [Google Scholar] [CrossRef]
- Lyons, T.R.; O'Brien, J.; Borges, V.F.; Conklin, M.W.; Keely, P.J.; Eliceiri, K.W.; Marusyk, A.; Tan, A.C.; Schedin, P. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med 2011, 17, 1109–1115. [Google Scholar] [CrossRef]
- Conklin, M.W.; Eickhoff, J.C.; Riching, K.M.; Pehlke, C.A.; Eliceiri, K.W.; Provenzano, P.P.; Friedl, A.; Keely, P.J. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol 2011, 178, 1221–1232. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Knittel, J.G.; Yan, L.; Rueden, C.T.; White, J.G.; Keely, P.J. Collagen density promotes mammary tumor initiation and progression. BMC Med 2008, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.G.; Jaffee, E.; Pardoll, D.M. Mechanisms of immune evasion by tumors. Adv Immunol 2006, 90, 51–81. [Google Scholar] [CrossRef] [PubMed]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol 2015, 35 Suppl, S185–S198. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, L.; Wan, C.; Sun, Y.; Van der Jeught, K.; Zhou, Z.; Dong, T.; So, K.M.; Yu, T.; Li, Y.; et al. MAL2 drives immune evasion in breast cancer by suppressing tumor antigen presentation. J Clin Invest 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Dersh, D.; Yewdell, J.W. Immune MAL2-practice: breast cancer immunoevasion via MHC class I degradation. J Clin Invest 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Cho, S.W. The Evasion Mechanisms of Cancer Immunity and Drug Intervention in the Tumor Microenvironment. Front Pharmacol 2022, 13, 868695. [Google Scholar] [CrossRef]
- Southall, P.J.; Boxer, G.M.; Bagshawe, K.D.; Hole, N.; Bromley, M.; Stern, P.L. Immunohistological distribution of 5T4 antigen in normal and malignant tissues. Br J Cancer 1990, 61, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Jungbluth, A.A.; Silva, W.A., Jr.; Iversen, K.; Frosina, D.; Zaidi, B.; Coplan, K.; Eastlake-Wade, S.K.; Castelli, S.B.; Spagnoli, G.C.; Old, L.J.; et al. Expression of cancer-testis (CT) antigens in placenta. Cancer Immun 2007, 7, 15. [Google Scholar] [PubMed]
- Silva, W.A., Jr.; Gnjatic, S.; Ritter, E.; Chua, R.; Cohen, T.; Hsu, M.; Jungbluth, A.A.; Altorki, N.K.; Chen, Y.T.; Old, L.J.; et al. PLAC1, a trophoblast-specific cell surface protein, is expressed in a range of human tumors and elicits spontaneous antibody responses. Cancer Immun 2007, 7, 18. [Google Scholar]
- Salanti, A.; Clausen, T.M.; Agerbaek, M.O.; Al Nakouzi, N.; Dahlback, M.; Oo, H.Z.; Lee, S.; Gustavsson, T.; Rich, J.R.; Hedberg, B.J.; et al. Targeting Human Cancer by a Glycosaminoglycan Binding Malaria Protein. Cancer Cell 2015, 28, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, A.; Thiede, M.; Grunewald, T.G.; Alba Rubio, R.; Richter, G.H.; Kirchner, T.; Busch, D.H.; Burdach, S.; Thiel, U. Pappalysin-1 T cell receptor transgenic allo-restricted T cells kill Ewing sarcoma in vitro and in vivo. Oncoimmunology 2017, 6, e1273301. [Google Scholar] [CrossRef]
- Nakasato, M.; Kohsaka, H.; Mizutani, T.; Watanabe, G.; Taya, K.; Nagaoka, K. Pregnancy-associated plasma protein (PAPP)-A expressed in the mammary gland controls epithelial cell proliferation and differentiation. Endocrine 2013, 43, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Prithviraj, P.; Shrestha, R.; Sharma, R.; Anaka, M.; Bridle, K.R.; Kannourakis, G.; Crawford, D.H.G.; Jayachandran, A. Prognostic Role of Immune Checkpoint Regulators in Cholangiocarcinoma: A Pilot Study. J Clin Med 2021, 10. [Google Scholar] [CrossRef]
- Shrestha, R.; Prithviraj, P.; Anaka, M.; Bridle, K.R.; Crawford, D.H.G.; Dhungel, B.; Steel, J.C.; Jayachandran, A. Monitoring Immune Checkpoint Regulators as Predictive Biomarkers in Hepatocellular Carcinoma. Front Oncol 2018, 8, 269. [Google Scholar] [CrossRef]
- Sallusto, F.; Lenig, D.; Forster, R.; Lipp, M.; Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999, 401, 708–712. [Google Scholar] [CrossRef]
- Wieczorek, M.; Abualrous, E.T.; Sticht, J.; Alvaro-Benito, M.; Stolzenberg, S.; Noe, F.; Freund, C. Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Front Immunol 2017, 8, 292. [Google Scholar] [CrossRef]
- Leone, P.; Shin, E.C.; Perosa, F.; Vacca, A.; Dammacco, F.; Racanelli, V. MHC class I antigen processing and presenting machinery: organization, function, and defects in tumor cells. J Natl Cancer Inst 2013, 105, 1172–1187. [Google Scholar] [CrossRef]
- Chattopadhyay, M.; Jenkins, E.C.; Lechuga-Vieco, A.V.; Nie, K.; Fiel, M.I.; Rialdi, A.; Guccione, E.; Enriquez, J.A.; Sia, D.; Lujambio, A.; et al. The portrait of liver cancer is shaped by mitochondrial genetics. Cell Rep 2022, 38, 110254. [Google Scholar] [CrossRef]
- Lodhia, K.A.; Tienchaiananda, P.; Haluska, P. Understanding the Key to Targeting the IGF Axis in Cancer: A Biomarker Assessment. Front Oncol 2015, 5, 142. [Google Scholar] [CrossRef]
- Pohlman, A.W.; Moudgalya, H.; Jordano, L.; Lobato, G.C.; Gerard, D.; Liptay, M.J.; Seder, C.W.; Borgia, J.A. The role of IGF-pathway biomarkers in determining risks, screening, and prognosis in lung cancer. Oncotarget 2022, 13, 393–407. [Google Scholar] [CrossRef]
- Zhou, Q.; Mao, Y.Q.; Jiang, W.D.; Chen, Y.R.; Huang, R.Y.; Zhou, X.B.; Wang, Y.F.; Shi, Z.; Wang, Z.S.; Huang, R.P. Development of IGF signaling antibody arrays for the identification of hepatocellular carcinoma biomarkers. PLoS One 2012, 7, e46851. [Google Scholar] [CrossRef]
- Douglas, J.B.; Silverman, D.T.; Pollak, M.N.; Tao, Y.; Soliman, A.S.; Stolzenberg-Solomon, R.Z. Serum IGF-I, IGF-II, IGFBP-3, and IGF-I/IGFBP-3 molar ratio and risk of pancreatic cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev 2010, 19, 2298–2306. [Google Scholar] [CrossRef]
- Kuhajda, F.P.; Abeloff, M.D.; Eggleston, J.C. Pregnancy-associated plasma protein A: a clinically significant predictor of early recurrence in stage II breast carcinoma. Hum Pathol 1985, 16, 228–235. [Google Scholar] [CrossRef]
- Kuhajda, F.P.; Eggleston, J.C. Pregnancy-associated plasma protein A and extensive necrosis. Clinically significant predictors of early recurrence in stage I estrogen receptor-negative breast carcinoma. Lab Invest 1985, 53, 101–107. [Google Scholar]
- Ekyalongo, R.C.; Yee, D. Revisiting the IGF-1R as a breast cancer target. npj Precision Oncology 2017, 1, 14. [Google Scholar] [CrossRef]
- Hamilton, N.; Austin, D.; Márquez-Garbán, D.; Sanchez, R.; Chau, B.; Foos, K.; Wu, Y.; Vadgama, J.; Pietras, R. Receptors for Insulin-Like Growth Factor-2 and Androgens as Therapeutic Targets in Triple-Negative Breast Cancer. Int J Mol Sci 2017, 18. [Google Scholar] [CrossRef]
| IGFBP | IGF requirement | Role of PAPP-A | Kinetic efficiency | Reference |
|---|---|---|---|---|
| IGFBP-4 | IGF-II (more efficient) or IGF-I | Cleavage at Met-135 and Lys-136 | High | [57] |
| IGFBP-4 | Not required | *Same site | Very low | [57] |
| IGFBP-5 | Not required; inhibited by IGF presence | Cleavage at Ser-143 and Lys-144 | High | [59,60] |
| IGFBP-2 | IGF dependent | Cleavage at Gln-165 and Met-166 | Less than IGFBP-4 | [61] |
| Clinical indicator | Sample Size | Analysis method | References |
|---|---|---|---|
| Independent predictor of early recurrence | Stage II BC: 30 cases (treated with low or standard chemotherapy) | Immunostaining and clinicopathology | [138] |
| Independent predictor of early recurrence | Stage I ER negative: 40 cases | Immunostaining and clinicopathology | [139] |
| Independent predictor of early recurrence; independent of ER status | Stage I ER positive: 30 cases | Immunostaining and clinicopathology | [15] |
| Worse prognosis seen in elevated PAPP-A; Independently prognostic for RFS and OS in the long-term | Early BC (with and without treatment): 301 cases, Non-cancer: 531 cases |
Serum assays on patient samples | [95] |
| Predictor of malignancy presence; positive association with serum activin A, serum activin B, total IGFBP-4, and correlation with total IGF-I; negative association with total cholesterol and triglycerides | Benign tumors: 100 cases, Malignant BC (treatment naïve and chemotherapy): 145 cases Non-cancer: 100 cases |
Serum assays on patient samples | [90] |
| High expression correlated with lymph node metastasis and high-grade tumor; worse prognosis, disease recurrence and poor OS in high-grade BC | BC: 45 cases (with 80% TNBC) | IHC on TMA | [25] |
| PAPP-A/SNAI1/COL1A1 expression panel: High score correlates with distant metastases | Primary BC: dataset of 327 cases | Gene set analysis | [97] |
| Silencing links with distant metastases | Invasive BC: 173 cases Normal breast: 30 cases |
DNA methylation analysis on FFPE | [26] |
| Low serum level in first trimester: greater long-term BC risk | 677,247 pregnancies | Biochemical screening | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




