1. Introduction
Sweet cherry (
Prunus avium L.) has high nutritional and economic value, and its respiration mode is non-climacteric. It is usually harvested after maturity, and there is no obvious postharvest ripening. The harvest period is concentrated in the high temperature period from May to July. If it is stored at room temperature for 3 to 5 days, it can easily develop withered stems, browning, rotting and flavor deterioration, losing its commercial value [
1].
In recent years, edible coatings have been widely used for postharvest preservation of fruits and vegetables due to their advantages such as ease of use, safety, nontoxicity, biodegradability, good mechanical properties, and barrier properties. Among them, chitosan (CS) has the advantages of low price, safety, non-toxicity, biodegradability, good antibacterial activity, film formation and biocompatibility. CS based edible coating has always been the focus of researchers' attention[
2] and has been used for the preservation of sweet cherries[
3,
4]. However, CS can only be dissolved in acidic solutions with pH below 6.3 and is insoluble in water and general organic solvents, which is not convenient for consumers to clean before consumption. In addition, the bad odor caused by acidic solutions affects the aroma of fruits and vegetables themselves, limiting its use in keeping fruits and vegetables fresh[
5]. Carboxymethyl chitosan (CMCS), an amphoteric derivative of CS, is more water soluble, biocompatible and biodegradable due to its high content of carboxymethyl groups[
6,
7]. The film produced with CMCS has some water and oxygen resistance, which can be used for food packaging films. However, the one-component CMCS film is very fragile and hydrophilic and has poor barrier and mechanical properties, which limits its application in the food industry[
8]. To improve the physical properties of the CMCS film, the CMCS can be mixed with other substances to form membranes. Previous studies have shown that gelatin (GL), as a natural water-soluble protein, can be mixed with CS to improve the properties of edible films[
9,
10]. In addition, the addition of CaCl
2 and/or ascorbic acid (AA) to CS can improve the preservation effect of CS on strawberries[
11], pears[
12], plums[
13] and other fruits and vegetables[
14]. CaCl
2 can also be used as a crosslinking agent to improve the properties of CS edible films[
15,
16]. AA as a safe, inexpensive and efficient antioxidant, CaCl
2 can also effectively control the enzymatic browning of fruits and vegetables[
13,
17]. All these indicate that the addition of CaCl
2 and/or AA to edible films not only improves the mechanical properties of the film, but also improves the preservation effect on fruits and vegetables.
Thus, the main content of this study is the use of CMCS and GL as film-forming substrates, the addition of CaCl2 and AA as crosslinking agents and antioxidants, respectively, the use of the casting method to prepare the film, and the evaluation of the film based on its tensile strength (TS), elongation at break (EAB), water vapor permeability (WVP), and oxygen permeability (OP). The effects of the different components on the mechanical and barrier properties of the membrane were compared using a single factor test. The weights of each performance index were determined by combining principal component analysis and comprehensive membership evaluation method, and the comprehensive membrane performance evaluation formula was determined. The film formula with good mechanical and barrier properties was determined by the optimization test of response surface test. Based on the optimization of film performance, the preservation effect of different CMCS-GL-based edible coatings on sweet cherry was compared.
2. Materials and Methods
2.1. Materials
2.1.1. Plant Materials
The sweet cherry cultivar was red agate harvested from the cherry collection garden of the Fruit Tree Research Institute of Shanxi Agricultural University (Taigu, Shanxi, China; 112 ° 50 'E, 37 ° 34' N). The trees were 17 years old. The fruits that had reached the commercial ripening standard were picked and stored in cold storage (temperature: 0±0.5℃, RH: 85-90%) for pre-chilling. After the core temperature of the fruits decreased to 0±0.5℃, the fruits with the same ripeness, uniform size, no mechanical damage, no pests and diseases were selected and randomly divided into four groups for the experiment.
2.1.2. Chemicals
CMCS (white to pale yellow free-flowing powder, deacetylated≥80%, carboxymethylation≥ 80%), GL (pale yellow solid, ash content < 3%, gel strength ≥ 160 g Bloom), glycerol (purity > 99%), CaCl2 (white powder), and l-ascorbic acid (purity > 99%) were from Macklin Biotechnology Co Ltd (Shanghai, China). All other chemical reagents were of analytical purity.
2.2. Experimental Design
2.2.1. Preparation of CMCS-GL-Based Edible Coating Solutions
CMCS-GL based glare films were prepared according to the method proposed by Martinez Chamacho et al. [
18]with some modifications. Weigh CMCS and GL powders separately and add them to distilled water. Heat them in a constant temperature water bath of 60℃, stir continuously until completely dissolved (~30min), and then cool to 23±1℃ to obtain a final concentration of 2% CMCS and GL solution. Mix CMCS and GL solution in a certain ratio, add Tween-20 as surfactant, AA as antioxidant, and appropriate amounts of glycerol and CaCl
2 powder as plasticizer and crosslinker, respectively. Stir overnight at room temperature, centrifuge at 4000 g for 10 min, and recover the supernatant to remove bubbles and insoluble components. Take 20 ± 0.1 g of the supernatant and spread it evenly in a clean and dry organic glass film former by tape casting method. Dry it by blowing at 60℃, cool it and remove the film. Place it in an incubator with constant temperature and humidity (temperature: 23 ± 1℃, RH: 50%) for at least 48 hours before measuring the various properties of the edible film.
2.2.2. Method for Determination of Mechanical Properties and Barrier Properties of CMCS-GL Based Edible Film
Use a handheld digital micrometer to measure the film thickness (accurate to 0.001 mm). Five different points (one in the center and four at the edge) were taken from each membrane and measured. Results were reported as mean (Mean, M) ± standard deviation (SD). The layer thickness was used to calculate the TS, EAB, WVP, OP of the layer.
- 2.
Determination of TS and EAB
TS and EAB of the film were determined using an electronic universal testing machine (INSTRON-5544, USA). The film was cut into a 70×10 mm rectangle with an initial standard spacing of 50 mm. The film was stretched to break at a rate of 0.1 mm /min at 23±1℃and RH 40-50%. Each sample was measured 3 times and the results were averaged.
The WVP of the film was measured at 23℃and RH 90% using the water vapor permeability tester. Each membrane sample was tested in triplicate using the weight reduction method, and the results were averaged.
The OP of the film was measured by a gas permeability tester (STG -V1, Guangzhou Xitang Electromechanical Technology Co., Ltd, China) at 23℃, the pressure difference between the high- and low-pressure chambers was 50 KPa, and the purity of the oxygen used was 99%. Each membrane was tested in triplicate, and the results were averaged.
- 5.
Determination of comprehensive scores for film mechanical and barrier performance
To obtain a membrane formula with good mechanical and barrier properties, a combination of principal component analysis and a comprehensive membership evaluation method was used in this experiment to determine the comprehensive membrane performance evaluation. Use principal component analysis in SPSS software to determine the weights of each membrane performance index to avoid subjective errors caused by artificial assignment of weights. Use the comprehensive membership evaluation method to comprehensively evaluate the TS, EAB, WVP, and OP of the slide. In practice, the better the mechanical performance indicators of the film - TS and EAB - the larger they are expected to be. TS and EAB are called positive indicators, and their membership degree is calculated according to equation (1). However, the smaller the expected barrier performance - WVP and OP - the better. WVP and OP are called negative indicators, and their degree of belonging is calculated according to equation (2):
Where P is the degree of membership of a particular indicator; Ai is the indicator value; Amin is the minimum value of the same indicator; Amax is the maximum value of the same indicator.
The overall evaluation of the mechanical and barrier properties of the film is calculated using equation (3):
Where S is the overall rating of the edible film performance; P1, P2, P3, and P4 are the film grades of TS, EAB, WVP, and OP, respectively; a, b, c, and d are the weights of TS, EAB, WVP, and OP.
2.2.3. Single Factor Test for Performance Optimization of CMCS-GL-Based Edible Film
According to the steps described in 2.2.1, the edible film was prepared, and the TS, EAB, WVP, and OP of the film were used as evaluation indices to determine the best values of each factor, and the factors that have less influence on the performance of the film were removed. All experiments were repeated 3 times, and the results are expressed as M±SD. The factor levels of each test are shown in
Table 1.
2.2.4. Response Surface Optimization Test of Performance of CMCS-GL-Based Edible Film
Based on the results of the one-factor experiment, a three-factor, three-level response surface experiment was designed using Design Expert software. CMCS: GL (w: w), glycerol addition, and CaCl
2 addition were selected as independent variables, and the comprehensive score of membrane performance Y was used as the response value. The experimental results were fitted into a quadratic regression model, and the optimized membrane formula was finally obtained. The table of response surface analysis factors is shown in
Table 2.
2.2.5. Experimental Design of the Fresh-Keeping Effect of Edible Coating on Sweet Cherry
1. Sweet cherry coating treatment and Grouping
The selected sweet cherries were rinsed in tap water, dried at 23±1℃, and soaked in coating solutions (CMCS-GL, CaCl
2- CMCS-GL, AA-CaCl
2-CMCS-GL) for 2 minutes before being pulled out. The control group was rinsed with tap water only, and the treated sweet cherries were dried at 23±1℃ before being packed in a perforated plastic package (length×width×height: 157×118×72 mm
3, thickness: 0.2 mm) and then refrigerated at 0±0.5℃, RH 85-90% for 30 days. Samples were taken every 6 days to determine the quality indicators (decay rate, weight loss rate, firmness, and skin color) of the different treatment groups. After 30 days of refrigeration, the remaining samples were stored at 23±1℃and RH 40-50% for another 3 days, and then samples were taken to determine the quality indicators. The classification of sweet cherries according to different CMCS-GL -based edible coatings is shown in
Table 3.
2. Determination of fruit quality of sweet cherries during storage
(1) Determination of fruit rot rate: using sensory evaluation method, 3×100 fruits (3 repeated groups) were randomly selected from each group for fruit rot rate evaluation. The number of rotten fruits was recorded after 30 days of cold storage and at the end of storage (33rd day), the rotting rate was calculated as the ratio of the number of rotten fruits to the total number (%).
(2) Determination of weight loss rate: using the weighing method, 3×300g of sweet cherries from each group were randomly selected to determine the fruit weight loss rate. An electronic balance (± 0.01 g) was used to weigh the sweet cherries regularly. The weight loss rate of sweet cherries was expressed as a percentage of the original total weight of the sample.
(3) Measurement of fruit firmness: A texture analyzer (TA-TX Plus; Stable Microsystems, Godalming, UK) equipped with a cylindrical probe (P/36R) was used to measure fruit firmness. The compression speed was 2 mm/s and the compression depth was 5 mm. Ten fruits from each treatment group were tested and the mean value was calculated.
(4) Measurement of skin color characteristics: A colorimeter (model CM -5; Konica-Minolta, Japan) was used to measure the skin color (L*, a*, and b*) of sweet cherries on opposite sides of 30 fruits. The L*, chroma, and hue angle values were used to describe the color characteristics of the skin using the following calculations: chroma = (a*2+ b*2)1/2, hue angle = tan−1(b*/a*).
2.3. Statistical Analysis
Experimental results were expressed as M ± SD, and software such as Design Expert, SPSS, and Origin were used for experimental design and data processing. Significance analysis of the data was performed using the Duncan New Complex Range method with p < 0.05.
3. Results and Discussion
3.1. Univariate Tests Results and Analysis
3.1.1. Effect of CMCS: GL (w:w) on Mechanical and Barrier Properties of Edible Film
Table 4 shows the influence of the different factors on the properties of the edible film.
Table 4 shows that the TS of the pure CMCS film is significantly higher than that of the pure GL film. With decreasing CMCS:GL (w:w), the TS of the film first increases and then decreases, which might be due to the fact that the addition of GL decreases the crystallization ability of CMCS in the film and makes the composite film softer and more elastic. This is consistent with the result that the addition of GL improves the mechanical properties of CS. However, the EAB of pure GL films was significantly higher than that of pure CMCS films. The EAB first increased and then decreased with decreasing CMCS: GL (w:w), which was attributed to the limited binding sites between CMCS and GL molecules. When the ratio of the two molecules is 4:2, they can be completely bonded together, and the bond between the molecules is the tightest, and the EAB value is the largest. The results showed that the mechanical properties of pure CMCS and GL films were deficient to some extent. The composite membrane prepared by mixing CMCS and GL films in a ratio of 4:2 could overcome the deficiencies of both films and obtain a membrane with better mechanical properties.
Compared to CMCS-only films, GL -only films exhibited lower WVP and OP, which was consistent with the results of Pereda et al[
19]. In addition, the WVP and OP values of edible films showed a downward trend with the decrease of CMCS: GL (w:w), which was attributed to GL molecules penetrating into the film cavity, making the film more compact, reducing the transfer rate of H
2O and O
2 in the film, and then reducing the WVP and OP values [
10]. When the CMCS:GL ratio was 4:2 and 3:3, there was no significant difference in the WVP of the films. There was no significant difference between CMCS:GL 3:3, 2:4 and pure GL films. At CMCS:GL ratios of 4:2, 3:3, and 2:4, there was no significant difference in the OP values of the prepared edible films, but they were all lower than those of the pure CMCS films.
In summary, CMCS:GL=4:2 (2:1) was chosen as the central level for the response surface test.
3.1.2. The Effect of Glycerol Addition on the Mechanical and Barrier Properties of Edible Films
From
Table 4, it can be seen that the TS of the film decreases with increasing amount of added glycerol. This is because glycerol is a small molecule that can easily insert into the chains of CMCS and GL molecules, leading to the destruction of the dense structure of the film. This effect weakens the interaction between or within the molecules of CMCS and GL, resulting in a decrease of TS. However, the EAB of the membrane increased with the increase of glycerol supply because glycerol softened the rigid structure of the CMCS-GL film and increased the fluidity of the chain. The structure of the film was loosened to some extent, which improved the flexibility of the membrane and increased the EAB.
The WVP and OP of the film first decreased and then increased with the increase of glycerol addition. At 1% glycerol addition, the WVP and OP reached the minimum value ((1.41±0.03) ×10
-12 g-cm/(cm-s-Pa), (5.18±0.13) ×10
-11 cm
3-cm/(m-s-Pa)). This is due to the formation of a large number of hydrogen bonds in the molecular structure of the film with appropriate glycerol addition, and the bond between CMCS and GL molecules is tighter, which hinders the penetration of H
2O and O
2. However, when the addition of glycerol increases further, the WVP of the film shows an upward trend, which is due to the change in hydrogen bonds between and within the molecules of CMCS and GL caused by excessive glycerol. This leads to increased vacancy in the film structure, loosened film structure, and increased WVP and OP of the film[
20].
In summary, 1% glycerol was selected as the central value for the response surface experiment.
3.1.3. Effect of CaCl2 Addition on the Mechanical and Barrier Properties of Edible Films
Table 4 shows that the TS of the film first increases and then decreases with increasing CaCl
2 addition. When CaCl
2 addition increases to 2%, TS reaches its maximum value (16.22±0.49 MPa). However, when the CaCl
2 addition continues to increase, the TS of the film decreases. This is because CaCl
2 as a crosslinking agent at a suitable concentration can make the connection between CMCS and GL molecules tighter, increase the crosslinking density between the molecular chains, and increase the TS of the film. But if the added amount of CaCl
2 is too high, the film becomes brittle and hard and TS decreases[
21]. The EAB of the film decreases with increasing CaCl
2 addition, which is due to the fact that the ductility of the membrane decreases with increasing CaCl
2 addition, leading to a decrease in membrane flexibility and EAB. At 1-2% addition, the EAB value of the film decreased slightly (from 78.66±1.83% to 71.92±1.74%), but with increasing addition, the EAB value of the film decreased rapidly (from 71.92±1.74% to 46.82±1.86%). In addition, studies have shown that the floating powder phenomenon occurs when the added amount of CaCl
2 is too large, which affects the appearance of the film[
11,
16]. Therefore, the appropriate amount of added CaCl
2 should be selected by combining various factors.
The WVP and OP of the film showed a trend that first decreased and then increased with increasing CaCl2 addition. When the CaCl2 addition reached 2%, the WVP and OP values both reached the minimum value of (1.45 ± 0.07) × 10-12 g-cm/(cm2-s-Pa), (5.20 ± 0.12) × 10-11 cm3-cm/(m2-s-Pa). This is because when an appropriate amount of CaCl2 is added to the film as a crosslinking agent, a dense network structure is formed between CMCS and GL molecules, which reduces the diffusion rate of H2O and O2 and reduces the WVP and OP values of the film. Excess CaCl2 also damages the dense structure of the film, again increasing the WVP and OP of the film. In summary, adding an appropriate amount of CaCl2 as a crosslinking agent can improve the mechanical and barrier properties of the film. However, if too much CaCl2 is added, it will have negative effects on the properties of the film.
Therefore, after extensive consideration, 2% CaCl2 was selected as the central value for the response surface experiment.
3.1.4. Effect of Tween-20 Addition on the Mechanical and Barrier Properties of Edible Films
Table 4 shows that there is no significant difference in the thickness, TS, EAB, WVP, and OP of the edible membrane films with different Tween-20 addition amounts, i.e., the addition amount of Tween-20 does not have a great influence on the mechanical and barrier properties of the film, which are determined by its own properties. As a surfactant, Tween-20 can be added to the edible coating solution to reduce the surface tension of the coating solution, so that the coating solution can be evenly applied to the surface of fruits and vegetables with low surface tension. The results of the contact angle test of different coating solutions on the surface of sweet cherries show that the contact angle of the coating solution on the surface of sweet cherries can be reduced from 86.7° to 63.6° when 0.1% Tween-20 is added to the coating solution, so that the coating solution can spread better on the epidermis of sweet cherries with strong hydrophobicity. In addition, Tween-20 has some inherent odor, so under the premise of reducing the surface tension of the film coating solution, the less Tween-20 is added, the less the effect on the sensory properties of the film. Therefore, in conjunction with the conclusion of this test, the amount of Tween-20 added in this test was set at 0.1%. In addition, the amount of added Tween-20 was no longer considered as a response surface factor for further analysis.
3.1.5. Effect of AA Addition on the Mechanical and Barrier Properties of Edible Films
Table 4 shows that there is no significant difference in the thickness, EAB, WVP, and OP of films prepared with different AA addition amounts. When the additional amount of AA was 4%, the TS of the film was significantly lower than that of the film with the additional amount of AA of 1 and 2%, indicating that the additional amount of AA to 4% would decrease the TS of the film. There was no significant difference in the TS of the films prepared with 0, 1, 2 and 3% AA addition, and there was no significant difference between the films prepared with 0, 3 and 4% AA addition, indicating that the amount of AA addition had little effect on the properties of the edible membrane. This is due to the fact that AA was added to the edible membrane as an antioxidant and did not have much effect on the mechanical and barrier properties of the membrane. Therefore, after reviewing the relevant literature [
22]and combining the results of this one-factor test, the added amount of AA was fixed at 2% and the added amount of AA was no longer used as a factor in the response surface test for further analysis.
In summary, CMCS: GL (w/w), the addition of glycerol and CaCl2 have a great influence on the mechanical properties and barrier properties of the edible membrane. Therefore, these three factors were selected as objects for the analysis of the response surface test.
3.2. Determination of the Comprehensive Scores of the Mechanical and Barrier Properties of the Edible Film
3.2.1. Results of Principal Component Analysis
Using the four membrane performance indicators (TS, EAB, WVP, OP) as objects of analysis, two data sets were randomly selected from three single factor test results (CMCS: GL (w: w), the amount of added glycerol, and the amount of added CaCl
2. SPSS software was used to perform principal component analysis for the six selected data sets (see
Table 5). Due to the different dimensions of the four indicators and the inclusion of two positive indicators (TS, EAB) and two negative indicators (WVP, OP), the four indicators were standardized according to the formulas (1) and (2) before analysis. The standardized data are shown in
Table 5, and the eigenvalues and contribution rates of the relevant components are shown in
Table 6.
Table 6 shows that the eigenvalues (2.619, 1.008) of the first two principal components are greater than 1, the variance contribution of the first principal component (
Z1) is 65.475%, the variance contribution of the second principal component (
Z2) is 25.188%, and the cumulative variance contribution of the two principal components is 90.663%, exceeding 85%. Therefore, the first two principal components can essentially reflect the overall information of the film performance index and replace the original four indicators.
The factor loading matrix of the two principal components is shown in
Table 7. The magnitude of the factor loading may reflect the contribution of each index to the principal components. The magnitude of Z
1 is mainly determined by TS and OP, with OP having the largest loading on Z
1. The magnitude of Z
2 is mainly determined by TS and EAB, with EAB having the largest loading on Z
2.
Note: Only extract the principal component factors whose eigenvalue exceeds 1
According to the factor loading matrix of principal components, the linear relationship between
Z1,
Z2 and the performance indices of CMCS-GL film can be constructed as follows (4) (5):
3.2.2. Determination of Comprehensive Scores of Mechanical and Barrier Properties of Edible Membranes
Using the factor loading matrix of the two principal components (
Table 7) and the eigenvalues of the two principal components (
Table 6), the coefficient
Y was calculated in the linear combination (= the number of loadings of the index/square root of the corresponding eigenvalues of the principal components). Then, the coefficient
H in the comprehensive score model was calculated from the variance contribution fraction of
Y and the principal components = (
Z1 variance contribution fraction ×
Y1+
Z2 variance contribution fraction ×
Y2)/(
Z1 variance contribution fraction +
Z variance contribution fraction)). After normalizing
H for each index, the weight
W for each index is obtained, see
Table 8. The weights of TS, EAB, WVP, and OP were 0.251, 0.068, 0.334, and 0.347, respectively.
According to formula (3), the calculation formula for the overall grade of the film performance is as follows:
3.3. Response Surface Optimization Test Results and Analysis
Based on the results of the one-factor experiment, CMCS: GL (w: w,
X1), glycerol additive (
X2), and CaCl
2 additive (
X3) were selected as independent variables, and the comprehensive values of the mechanical properties and barrier of the film were used as response values (
Y). A three-factorial and three-stage response surface experiment was conducted. The experimental design and results are shown in
Table 9.
Applying Design Expert to perform a multiple regression fit on the experimental data in
Table 10 yielded the following regression model:
Perform an analysis of variance and significance test for the above regression model, and the results are shown in
Table 10. From
p < 0.0001, it can be seen that the regression of this model is highly significant. From the
p-value of the misfit term=0.8240 (> 0.05), it can be seen that the misfit of the model is not significant, which indicates that other factors have less influence on the model. The experimental results are in good agreement with the regression model. From R
2=0.9959,
, and the coefficient of variation CV =3.11%, it can be seen that the predicted value of this experiment has high correlation with the experimental value and the error is small. Therefore, this model can be used to analyze and predict the comprehensive result
Y of edible film performance based on CMCS-GL. According to the F value of each factor (X
2 > X
1 > X
3), the factors affecting the overall evaluation of the mechanical and barrier performance of the membrane are ranked as follows: X
2 (addition of glycerol) > X
1 (addition of CaCl
2) > X
3 (CMCS: GL). From the significance results of each element of the regression model, the primary term X
1 (
p=0.0248 < 0.05) has a significant effect on membrane performance, X
2 (
p=0.0012 < 0.01) has a very significant effect, and X
3 (
p=0.2427 > 0.05) has no significant effect. The interaction between X
1X
2 (
p=0.7652 > 0.05) was not significant, while the interaction between X
1X
3 (
p < 0.0001) and X
2X
3 (
p=0.0002) was significant.
3.4. Response Surface Optimization Test Graph Analysis
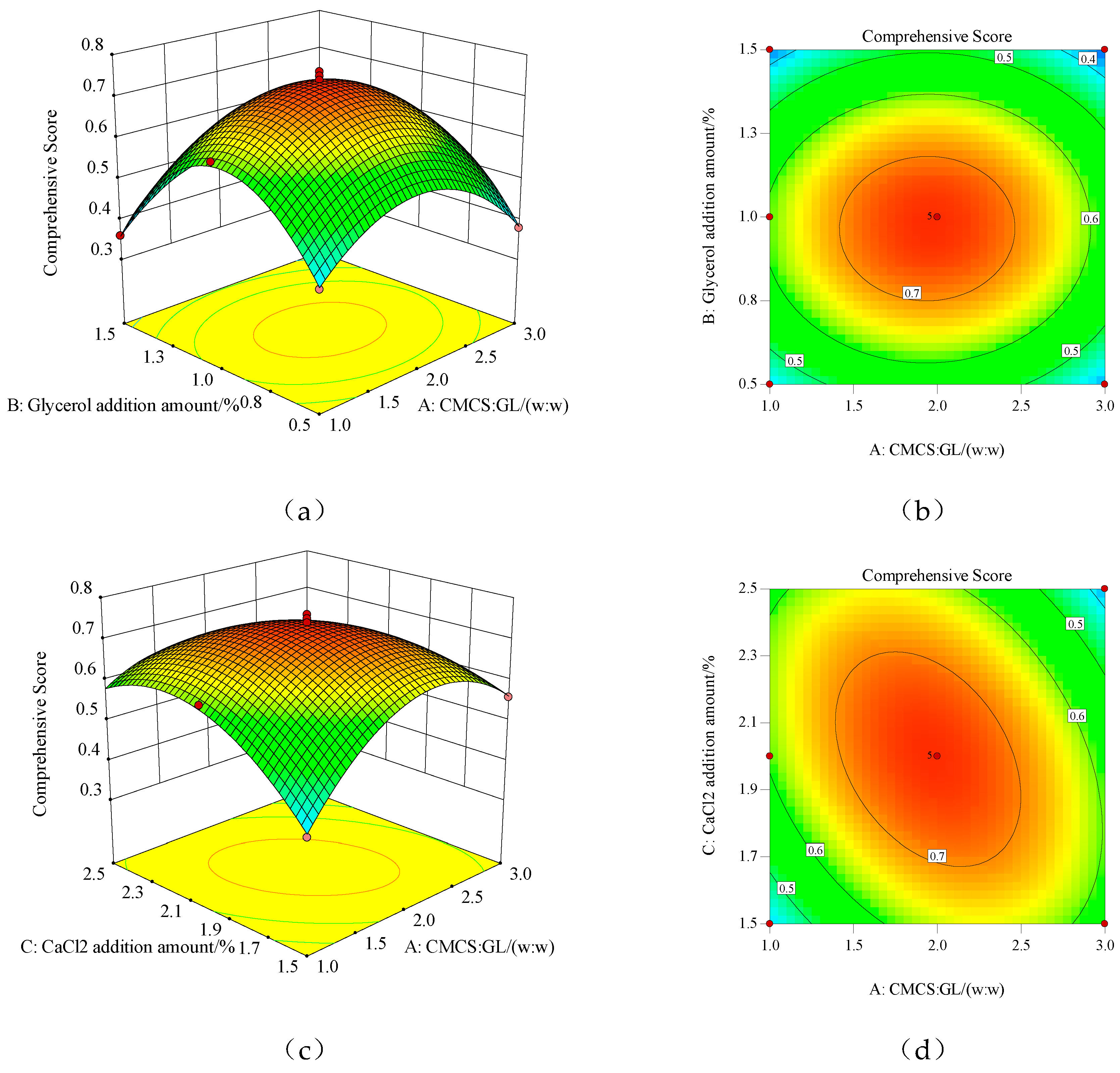
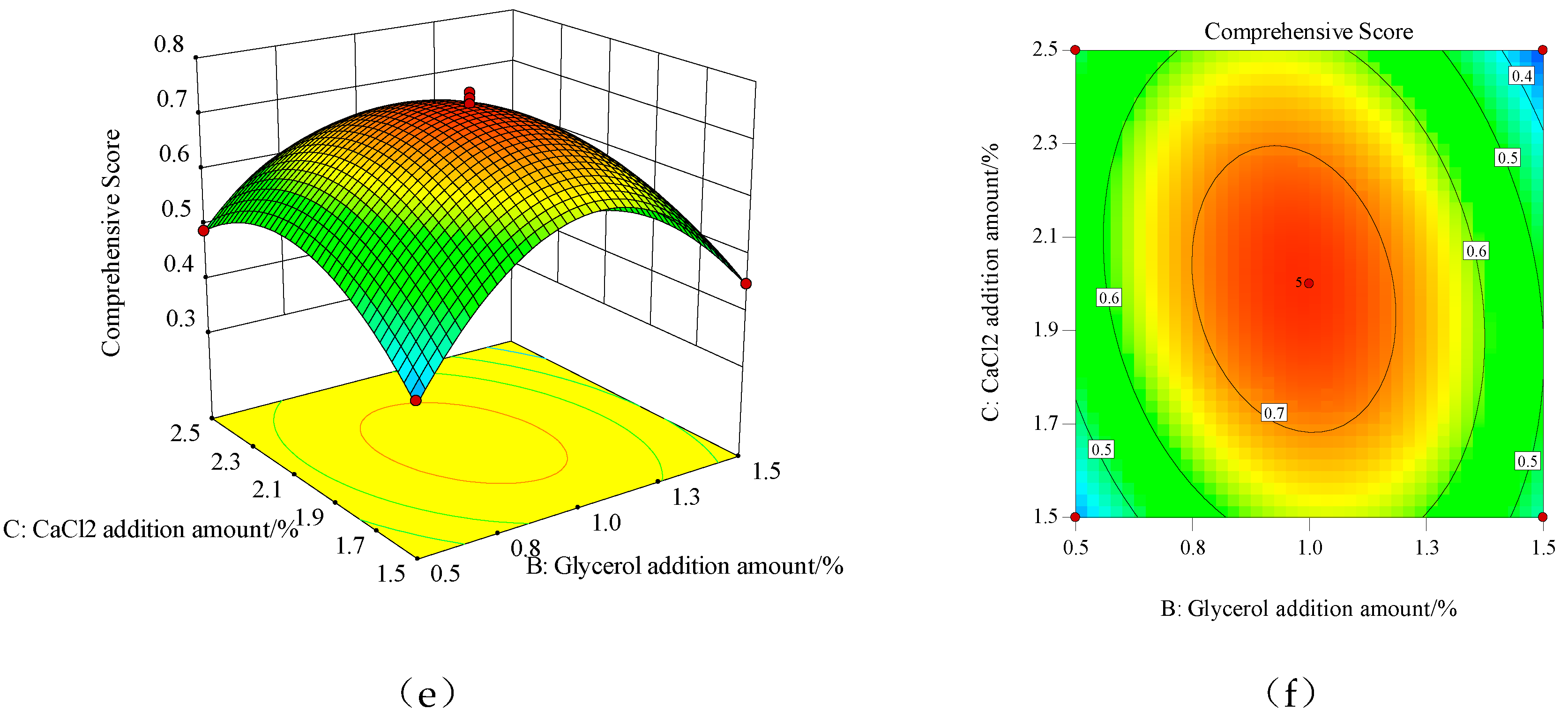
The three-dimensional surface and contour plots of the reaction surface for the interaction of the various factors are shown in
Figure 1. From
Figure 1a,c,e, the interaction of the three experimental factors can be intuitively seen. In the three-dimensional surface plot, the change in color from blue to red indicates a change in response value from small to large. The faster the change, the steeper the slope of the reaction surface and the flatter the reaction surface. This indicates that this factor has a smaller effect on the overall evaluation of the mechanical and barrier performance of the membrane. Conversely, the steeper the reaction surface, indicating that this factor has a greater impact on the overall evaluation of membrane performance. In the contour plots, the center of the smallest circle is the maximum value of the response value, while the circle represents a weak interaction between factors, while the ellipse represents a strong interaction between factors. The contour lines in
Figure 1b are nearly circular, indicating a weak interaction between CMCS: GL and the amount of glycerol addition, while the contour plots in
Figure 1d,e are elliptical, indicating a strong interaction between CMCS: GL and CaCl
2 addition, glycerol addition, and CaCl
2 addition.
3.5. Determination and Verification of the Optimal Formulation of CMCS-GL-Based Edible Film
The regression model was analyzed using response surface analysis. When the mechanical and barrier properties of the edible membrane based on CMCS-GL were better, the formula was CMCS:GL=2:1, the addition of glycerol was 1%, the addition of CaCl2 was 2.002%, and the predictive value of the comprehensive score of membrane performance was 0.741. Under these conditions, the film was prepared and three parallel tests were performed. The TS, EAB, WVP and OP were measured as follows: 16.28 MPa, 71.46%, 1.39×10-12g-cm/(cm2-s-Pa), 5.10×10-11 cm3-cm/(m2-s-Pa). After standardization according to formula (6), the comprehensive evaluation of membrane performance was 0.73, which shows that the verification test results were close to the predicted value of the model. It can be seen that the model can well simulate and predict the comprehensive evaluation of the edible membrane based on CMCS-GL. The formulation of the edible film obtained by response surface optimization has some practical significance when its mechanical and barrier properties are good.
3.6. Fresh-Keeping Effects of Different CMCS-GL-Based Edible Films on Sweet Cherries
3.6.1. Effects of Different CMCS-GL-Based Edible Coatings on Fruit Decay Rate of Sweet Cherry during Storage
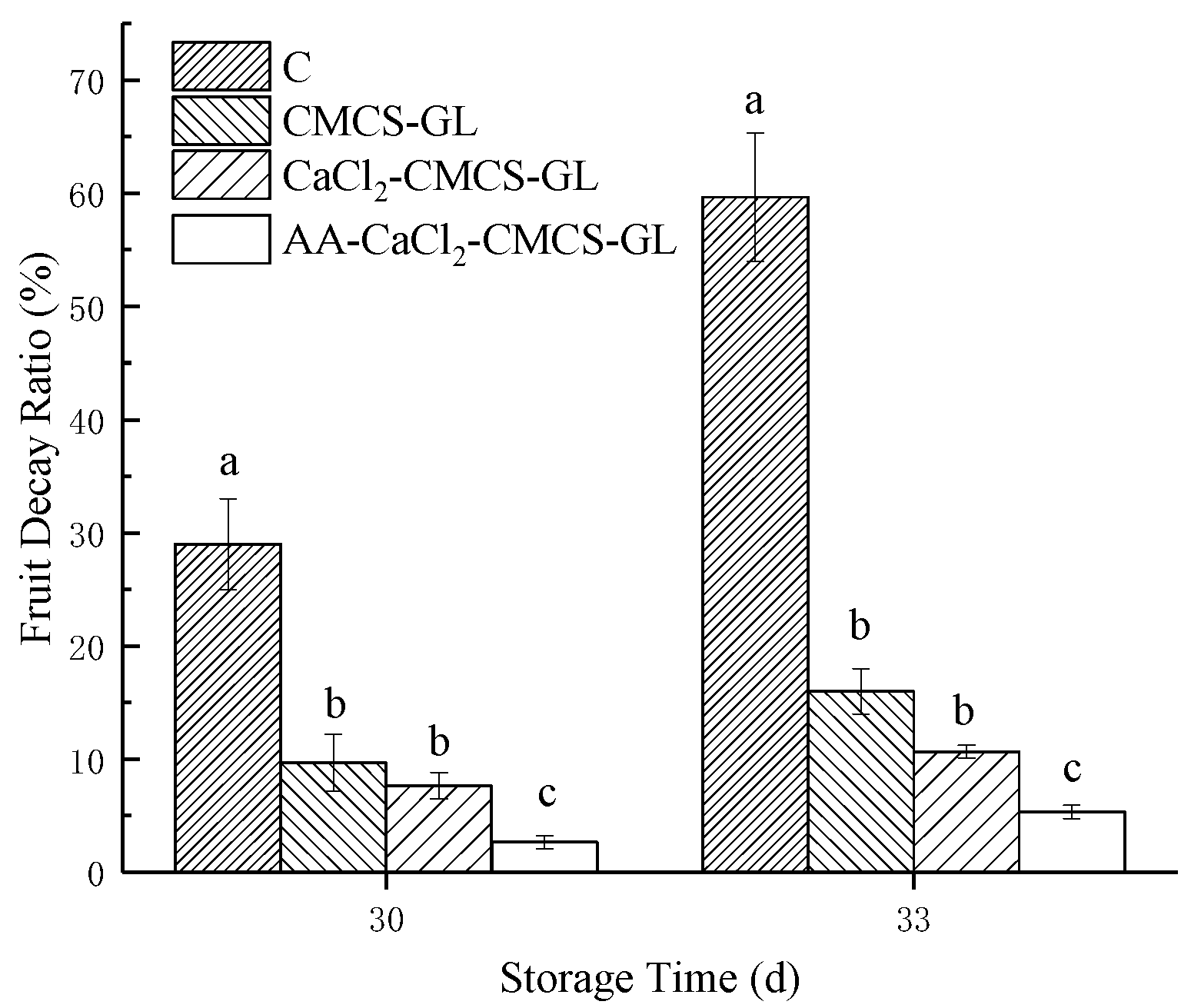
Sweet cherries rot quickly after harvest because they have high respiration intensity and are susceptible to pathogens. As shown in
Figure 2, the rot rate of C (29.0%) was significantly higher than that of each film treatment group after 30 days of storage. The fruit rot rate of AA -CaCl
2- CMCS-GL treatment group was the lowest (2.7%), which was significantly lower than that of CMCS-GL and CaCl
2- CMCS-GL treatment groups. At the end of storage (day 33), C had the highest decay rate (59.7%). The decay rate of AA -CaCl
2- CMCS-GL was still the lowest (5.3%), and there was no significant difference between CMCS-GL and CaCl
2- CMCS-GL, but both were significantly lower than C.
In conclusion, various CMCS-GL based edible coatings were effective in reducing the rotting rate of sweet cherries during storage, and AA -CaCl2- CMCS-GL coating had the best effect.
CMCS-GL and CaCl
2-CMCS-GL could also play a role in reducing the rotting rate of sweet cherries during storage. This could be related to the antibacterial property of CMCS and the addition of Ca
2+ and AA [
23] in the coating solution. The inhibitory effect of Ca
2+ on fruit rot rate is due to the fact that Ca
2+ can make the cell wall less susceptible to the degradation enzymes produced by pathogens, thus improving the stability [
24] of the cells. The significant reduction in fruit rotting rate by AA may be related to its antioxidant activity, which needs further investigation.
3.6.2. Effects of Different CMCS-GL-Based Edible Coatings on the Weight Loss Rate and Hardness of Sweet Cherries during Storage
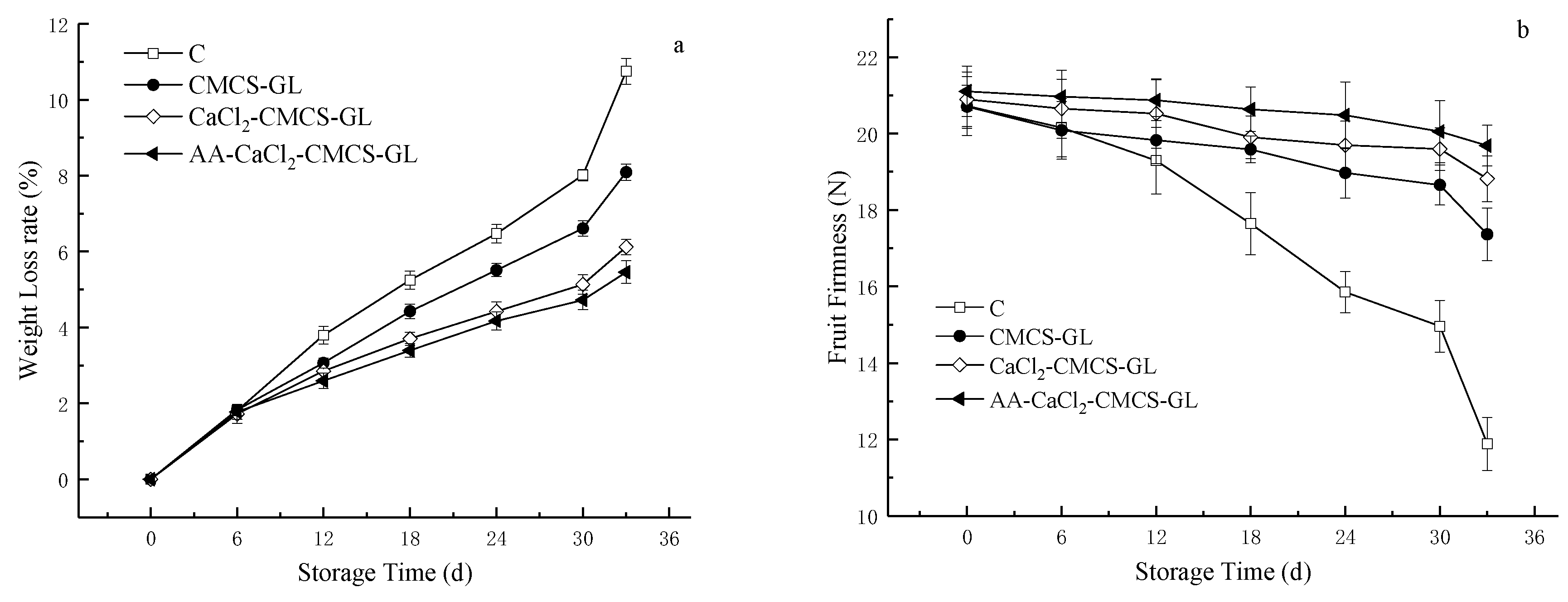
The effects of different edible coatings based on CMCS-GL on the weight loss and decay rate of sweet cherries during storage are shown in
Figure 3. During the storage period, the weight loss rate increased continuously in each treatment group and in the control group. Postharvest weight loss is mainly caused by water loss and nutrient consumption during respiration and transpiration [
25]. Previous studies have shown that CS coating with AA can reduce the weight loss rate of plums and fresh-cut apples during storage [
17,
26]. The results of our experiment are consistent with those of these studies. Compared with C, all three coating treatments inhibited the fruit weight loss rate, and the AA-Cacl
2- CMCS-GL coating group had the lowest weight loss rate. This might be related to the formation of an improved network structure between AA and hydrophilic compounds (such as phenols), which can form a semipermeable film through hydrogen bonding and reduce the permeability of the film to water vapor. In this way, the weight loss of the fruits was effectively controlled.
The change in the degree of hardness of sweet cherries determines their shelf life during storage, while reducing the rate of fruit rot and susceptibility to mechanical damage [
27]. As fruits ripen, cell wall degrading enzymes such as β-galactosidase, polygalactosidase, and pectin methylesterase reduce the intercellular adhesion strength and the mechanical strength of the cell wall, which eventually leads to a decrease in fruit hardness [
22,
28]. As shown in
Figure 3b, the fruit hardness of C decreased rapidly during storage and lost 42.3% of its hardness at the end of storage. The hardness of fruits treated with CMCS-GL, CaCl
2- CMCS-GL and AA -CaCl
2- CMCS-GL also showed a decreasing trend during storage, but all of them were significantly lower than that of C, indicating that the CMCS-GL -based edible coating could help to maintain the hardness of fruits during storage. Compared with other treatments, the hardness of fruits coated with AA -CaCl
2- CMCS-GL was always the highest and only decreased by 6.7% at the end of storage, which effectively slowed down the softening process of fruits. The softening process of fruits treated with CMCS-GL and CaCl
2- CMCS-GL was also retarded. The effect of CaCl
2- CMCS-GL coating was better than that of CaCl
2- CMCS-GL. The different effects of the three treatment groups on the maintenance of fruit firmness could be due to the change in O
2 permeability of the edible membrane caused by the addition of CaCl
2 or/and AA, resulting in a decrease in the corresponding pectinase activity, which needs further investigation.
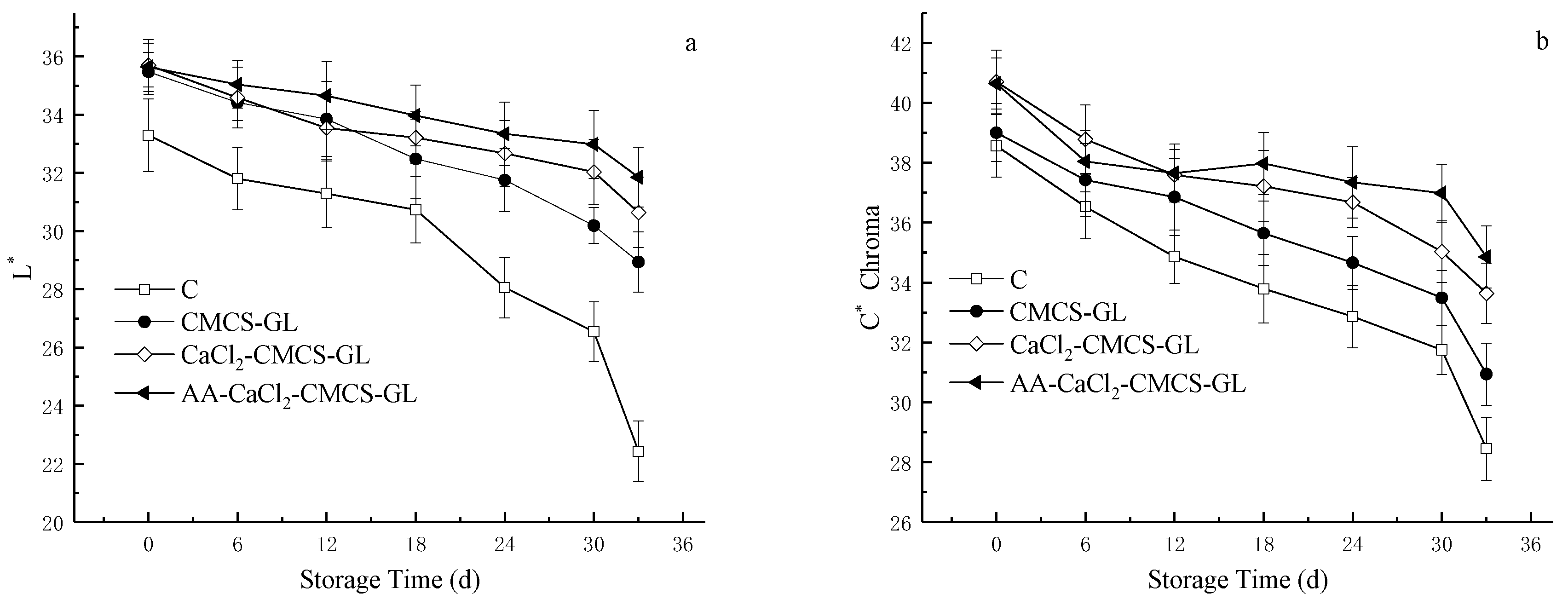
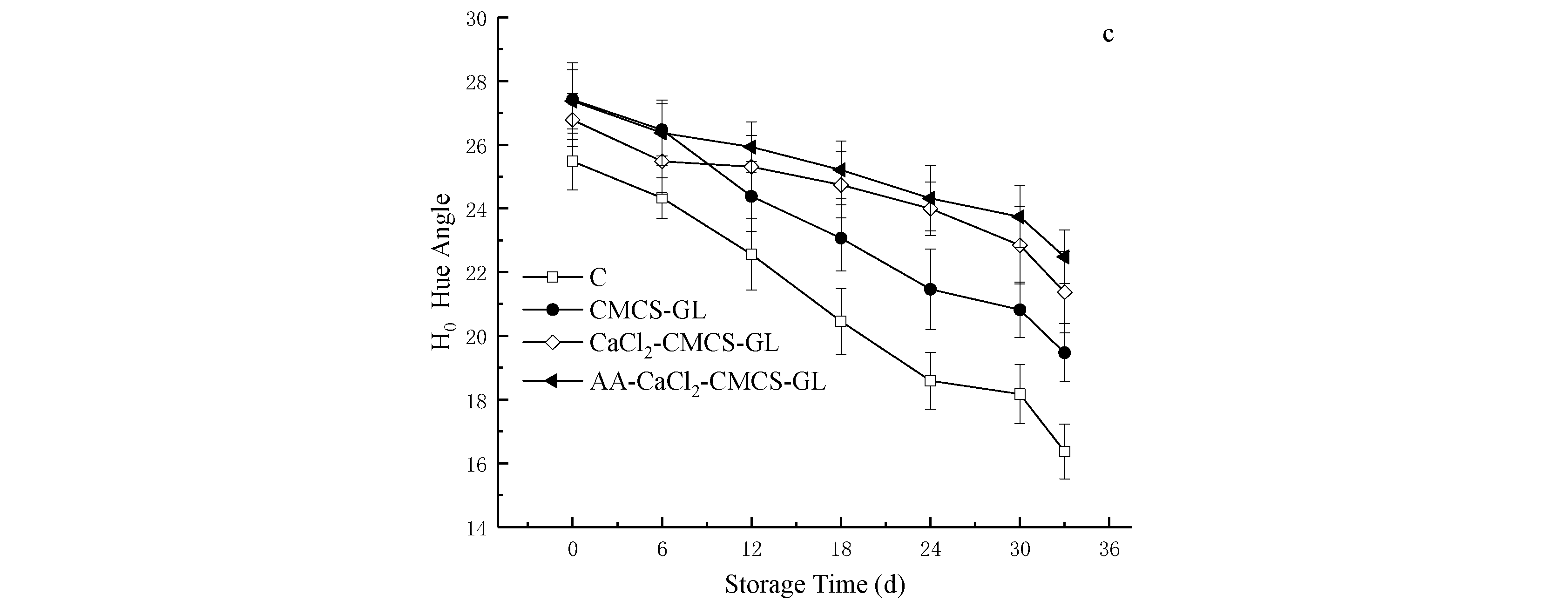
3.6.3. Effects of Different CMCS-GL-Based Edible Coatings on Peel Color of Sweet Cherries during Storage
The skin color of sweet cherries is an important indicator of their quality and maturity, which directly affects consumer acceptance. During the ripening and aging process, the synthesis and degradation of anthocyanins change the concentration and distribution of anthocyanins in the skin, resulting in a change in skin color [
29,
30]. As shown in
Figure 4, the color of pericarp in C and all treatment groups showed a trend of darkening during storage, and the values of L
*, C
* and H
0 all showed a downward trend. The L
*, C
*, and H
0 values of the foil-treated fruit were significantly higher than those of C because the foil decreased the O
2 concentration in the fruit, inhibiting respiration and reducing the activities of two key enzymes for the synthesis of anthocyanins (phenylalanine ammonia lyase and flavone synthase) [
31]. Moreover, the L
*, C
*, and H
0 values of CaCl
2- CMCS-GL and AA -CaCl
2- CMCS-GL were significantly higher than those of CMCS-GL. The L
* values of the AA -CaCl
2- CMCS-GL group remained the highest, and the H
0 and C
* values were the highest from the 12th to the 33rd day of storage, which is consistent with the conclusions of other researchers [
13].
4. Conclusions
Based on the single-factorial test, this study developed a three-factorial test at three levels using the response surface method. Considering the overall evaluation of the mechanical and barrier properties of the edible film as the response value, the order of the factors affecting the overall evaluation of the edible membrane (glycerol addition > CaCl2 addition > CMCS: GL) and the formula with the best performance of the edible membrane were determined as follows: CMCS:GL (w:w) =2:1, and the addition amounts of glycerol, CaCl2, Tween-20 and AA were 1%, 2%, 0.1% and 2%, respectively. Under these conditions, the average values for TS, EAB, WVP, and OP of the edible film were as follows: 16.28 MPa, 71.46% 1.39×10-12g-cm/(cm2-s-Pa), 5.10×10-11cm3-cm/(m2-s-Pa), respectively. The comprehensive score was 0.73, which was close to the predicted value of 0.74. The formula of the edible film based on CMCS-GL, which was obtained by the response surface method, has some practical significance if its mechanical and barrier properties are good. Various edible films based on CMCS-GL (CMCS-GL, CaCl2- CMCS-GL and AA -CaCl2- CMCS-GL) were used for postharvest preservation of sweet cherries. It was found that all three CMCS-GL based edible coatings could effectively reduce the rotting rate and weight loss during storage. Among them, AA -CaCl2- CMCS-GL coating has the best effect. It can be used as a new method for postharvest preservation of sweet cherries. However, the mechanism of preservation is still unclear, and further research is needed.
Author Contributions
Conceptualization, Q.-L. C., Y. W., and J.-L. L.; methodology, Y.-L. Z. and J.-L. L.; software, Y.-L. Z.; validation, Q.-L. C. and Y.-Q. Z.; formal analysis, Y.-L. Z.; resources, Y.-L. Z. and Q.-L. C.; data curation, Y.-L. Z. and Y.-Q. Z.; writing—original draft preparation, Y.-L. Z.; writing—review and editing, Y.-L. Z. and Q.-L. C.; supervision, Q.-L. C., Y. W.; funding acquisition, Y.-L. Z. and Y. W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Shanxi Doctoral Graduates, Postdoctoral Researchers to Work in Jin Award Funding Research Projects, grant number SXBYKY2022028 and Shanxi Agricultural University doctoral research project, grant number 2021BQ89.
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors would like to express their gratitude to Key Technology and Equipment of dry farming agricultural machinery Shanxi Key Laboratory for providing test equipment and site,and EditSprings (
https://www.editsprings.cn ) for the expert linguistic services provided.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Blando, F. and B.D. Oomah, Sweet and sour cherries: Origin, distribution, nutritional composition and health benefits. Trends in Food Science & Technology, 2019. 86: p. 517-529. [CrossRef]
- Mujtaba, M., et al., Current advancements in chitosan-based film production for food technology; A review. International Journal of Biological Macromolecules, 2019. 121: p. 889-904. [CrossRef]
- Zhang, C., H. Gong, and Y. Liu, Effects of postharvest coating using chitosan combined with natamycin on physicochemical and microbial properties of sweet cherry during cold storage. International Journal of Biological Macromolecules, 2022. 214: p. 1-9. [CrossRef]
- Tokatli, K. and A. Demirdoven, Effects of chitosan edible film coatings on the physicochemical and microbiological qualities of sweet cherry (Prunus avium L.). Scientia Horticulturae, 2020. 259: p. 7. [CrossRef]
- Cazón, P., et al., Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocolloids, 2017. 68: p. 136-148. [CrossRef]
- Zhao, J., et al., Carboxymethyl chitosan incorporated with gliadin/phlorotannin nanoparticles enables the formation of new active packaging films. International Journal of Biological Macromolecules, 2022. 203: p. 40-48. [CrossRef]
- Bai, R.Y., et al., Development and characterization of antioxidant active packaging and intelligent Al3+-sensing films based on carboxymethyl chitosan and quercetin. International Journal of Biological Macromolecules, 2019. 126: p. 1074-1084. [CrossRef]
- Dayarian, S., et al., Physico-Mechanical Properties of Films of Chitosan, Carboxymethyl Chitosan, and Their Blends. Journal of Polymers and the Environment, 2014. 22(3): p. 409-416. [CrossRef]
- Fan, S., et al., Incorporation of cinnamon essential oil-loaded Pickering emulsion for improving antimicrobial properties and control release of chitosan/ gelatin films. Food Hydrocolloids, 2023. 138. [CrossRef]
- Yadav, S., et al., Preparation, physicochemical and biological evaluation of quercetin based chitosan-gelatin film for food packaging. Carbohydr Polym, 2020. 227: p. 1-9. [CrossRef]
- Nguyen, V.B., D.H.H. Nguyen, and H.H. Nguyen, Combination effects of calcium chloride and nano-chitosan on the postharvest quality of strawberry (Fragaria x ananassa Duch.). Postharvest Biology and Technology, 2020. 162(1): p. 1-8. [CrossRef]
- Kou, X.H., et al., Effects of Chitosan, Calcium Chloride, and Pullulan Coating Treatments on Antioxidant Activity in Pear cv. "Huang guan" During Storage. Food and Bioprocess Technology, 2014. 7(3): p. 671-681. [CrossRef]
- Liu, K.D., et al., Combined effects of ascorbic acid and chitosan on the quality maintenance and shelf life of plums. Scientia Horticulturae, 2014. 176: p. 45-53. [CrossRef]
- Aleryani-Raqeeb., A., et al., Effects of Calcium Infiltration and Chitosan Coating on Storage Life and Quality Characteristics During Storage of Papaya (Carica papaya L.). International Journal of Agricultural Research, 2008. 3(4): p. 296-306. [CrossRef]
- Tian, Z., et al., Nano Calcium-Deficient Hydroxyapatite/O-carboxymethyl Chitosan-CaCl2 Microspheres Loaded with Rhein for Bone Defect Repair. Journal of Bionic Engineering, 2022. 19(4): p. 1087-1099. [CrossRef]
- Tuan Mohamood, N.F.A.-Z., A.H. Abdul Halim, and N. Zainuddin, Carboxymethyl Cellulose Hydrogel from Biomass Waste of Oil Palm Empty Fruit Bunch Using Calcium Chloride as Crosslinking Agent. Polymers, 2021. 13(23). [CrossRef]
- Ozdemir, K.S. and V. Gokmen, Effect of Chitosan-Ascorbic Acid Coatings on the Refrigerated Storage Stability of Fresh-Cut Apples. Coatings, 2019. 9(8): p. 1-12. [CrossRef]
- Martinez-Camacho, A.P., et al., Chitosan composite films: Thermal, structural, mechanical and antifungal properties. Carbohydrate Polymers, 2010. 82(2): p. 305-315. [CrossRef]
- Pereda, M., et al., Chitosan-gelatin composites and bi-layer films with potential antimicrobial activity. Food Hydrocolloids, 2011. 25(5): p. 1372-1381. [CrossRef]
- Rocha, G.O., et al., Biodegradable Composite Films Based on Cassava Starch and Soy Protein. Polimeros-Ciencia E Tecnologia, 2014. 24(5): p. 587-595. [CrossRef]
- Zugravu, M.V., et al., Physical properties and in vitro evaluation of collagen-chitosan-calcium phosphate microparticle-based scaffolds for bone tissue regeneration. Journal of Biomaterials Applications, 2013. 28(4): p. 566-579. [CrossRef]
- Maringgal, B., et al., Recent advance in edible coating and its effect on fresh/fresh-cut fruits quality. Trends in Food Science & Technology, 2020. 96: p. 253-267. [CrossRef]
- Wang, Y., X.B. Xie, and L.E. Long, The effect of postharvest calcium application in hydro-cooling water on tissue calcium content, biochemical changes, and quality attributes of sweet cherry fruit. Food Chemistry, 2014. 160: p. 22-30. [CrossRef]
- Conway, W.S., et al., Inhibition of Penicillium expansum polygalacturonase activity by increased apple cell wall calcium. Phytopathology, 1988. 78(8): p. 1052-1055. [CrossRef]
- Sogvar, O.B., M. Koushesh Saba, and A. Emamifar, Aloe vera and ascorbic acid coatings maintain postharvest quality and reduce microbial load of strawberry fruit. Postharvest Biology and Technology, 2016. 114: p. 29-35. [CrossRef]
- Chiabrando, V. and G. Giacalone, Effect of alginate edible coating on quality and antioxidant properties in sweet cherry during postharvest storage. Italian Journal of Food Science, 2015. 27(2): p. 173-180. [CrossRef]
- Riva, S.C., U.O. Opara, and O.A. Fawole, Recent developments on postharvest application of edible coatings on stone fruit: A review. Scientia Horticulturae, 2020. 262: p. 1-10. [CrossRef]
- Maftoonazad, N., H.S. Ramaswamy, and M. Marcotte, Shelf-life extension of peaches through sodium alginate and methyl cellulose edible coatings. International Journal of Food ence & Technology, 2008. 43(6): p. 951-957. [CrossRef]
- Goncalves, B., et al., Effect of ripeness and postharvest storage on the evolution of colour and anthocyanins in cherries (Prunus avium L.). Food Chemistry, 2007. 103(3): p. 976-984. [CrossRef]
- Zhao, H., et al., Enhancement of quality and antioxidant metabolism of sweet cherry fruit by near-freezing temperature storage. Postharvest Biology & Technology. 2019. 147: p. 113-122. [CrossRef]
- Kumar, P., et al., Effect of chitosan coating on postharvest life and quality of plum during storage at low temperature. Scientia Horticulturae, 2017. 226: p. 104-109. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).