1. Introduction
Middle Cerebral Arteries are the most accessible cerebral vessel in fetal Doppler examination because it carries 80% of cerebral blood flow. The right and left cerebral arteries are the main branches of the Circulus Willis that originate from the carotid and vertebral arteries.[
1] Fetal cerebral circulation normally has a high impedance; in the event of fetal hypoxemia, impedance decreases, resulting in the central redistribution of blood flow to vital organs which maintains the oxygen delivery to the brain. A decrease in impedance manifests with a decrease in cerebral arterial resistance which is an early marker of autoregulation in hypoxemic States.[
2] This redistribution is known as the brain-sparing effect and plays an important role in the adaptation of the fetus to the lack of oxygen.[
1]
Reverse end-diastolic flow in the MCA is a rare fetal condition and a terminal hemodynamic event.[
3] PREDF of the MCA occurs as a result of increased intracranial or extracranial pressure or as a result of cerebral edema and severe hypoxemia.[
2,
3] Transient MCA-REDF may occur due to excessive external probe pressure, which signifies an artifact without clinical consequence.[
4] We reported a rare case of abnormal colour Doppler sonography findings indicating a persistent REDF MCA in a 33-year-old female at 30 weeks gestation who was referred to our hospital with severe preeclampsia, IUGR, and oligohydramnios.
2. Case Presentation
Patient G2P1A0, age 33 years, was pregnant for seven months and referred to Hasan Sadikin Hospital because of high blood pressure. High blood pressure was detected in the seventh month of pregnancy. The presence of high blood pressure was accompanied by severe headaches, blurred vision, and heartburn. There was no complaint of labour contraction nor a history of profuse discharge from the birth canal. The fetal motion was still reported by the mother. A previous history of high blood pressure was denied; there was neither a history of high blood pressure in the family nor a history of other chronic diseases. The history of contact with COVID-19 patients was denied. The patient had a two-dose vaccination for COVID-19.
During the physical examination, the patient was found to be fully alert, and the blood pressure was 180/110 mmHg, heart rate 87 beats/min, respiration 20 breaths/min, and the temperature was 36.6 0C. Obstetric examination revealed the height of the uterine fundus was 22 cm, the abdominal circumference was 97 cm, the fetal heart rate was 144-148 beats/min, and the longitudinal position of the fetus was 5/5 left back. The urine analysis was performed with proteinuria +2.
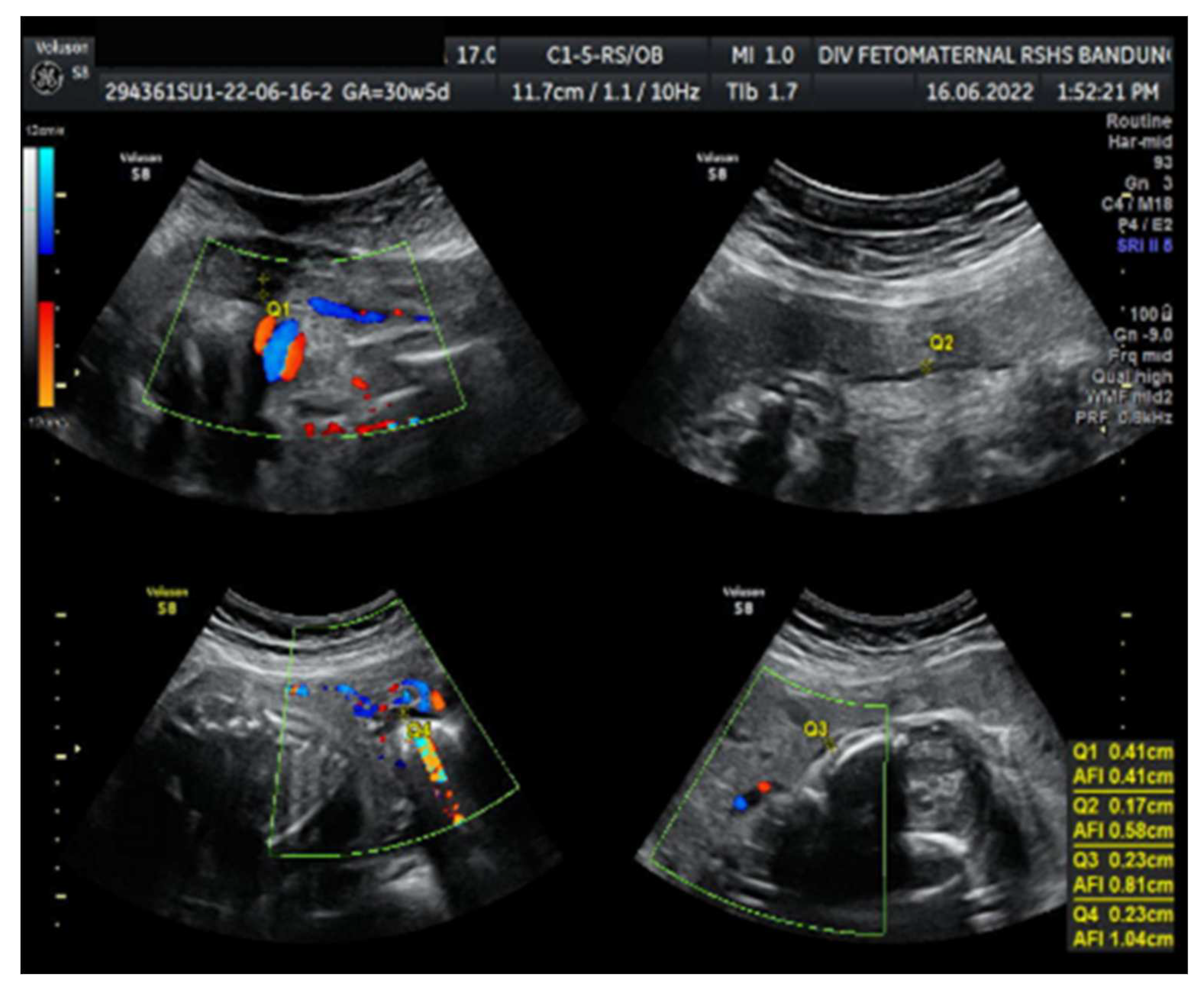
Ultrasound examination showed a fetus with biometry Biparietal Diameter (BPD), Head Circumference (HC), Abdominal Circumference (AC), and Femur Length (FL) corresponding to 26-27 weeks of gestation; the gestational age based on the last menstrual period was 30 weeks. Transcerebellum diameter corresponded to 29-30 weeks of gestation; amniotic fluid volume suggested oligohydramnios with Amniotic Fluid Index (AFI) equal to 1.04 cm, with an estimated fetal weight (EFW) of 956 g. The structure of both kidneys and urinary vesica was within a normal range, and fetal structural abnormalities were difficult to assess. The placenta was located anteriorly.
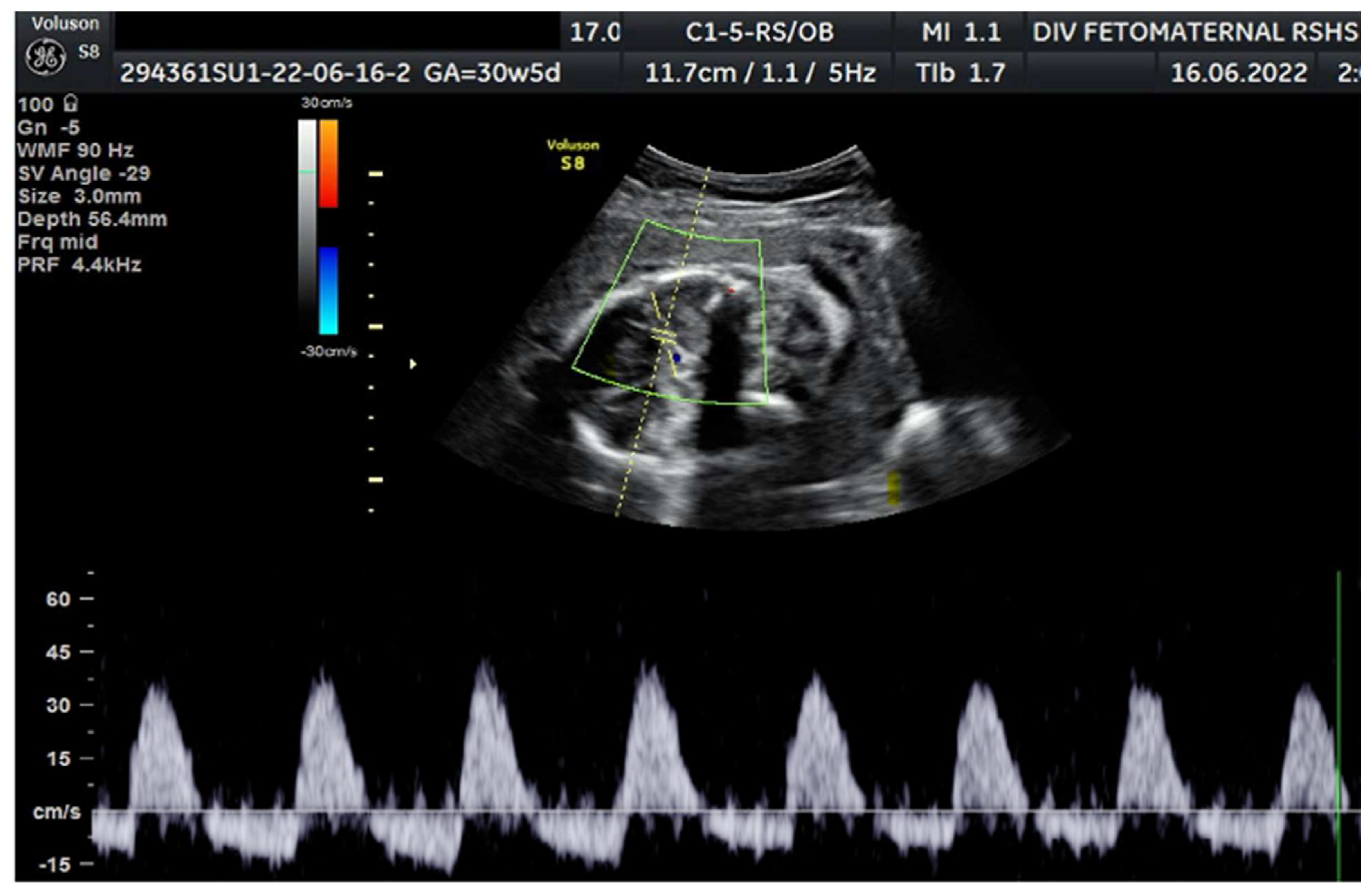
Colour Doppler examination of the MCA revealed a peak systolic velocity of 42.33 cm/s, 1.045 Multiple of Median (the normal range 40.5 cm/s for this gestational age) with persistent reversed end Diastolic Flow (
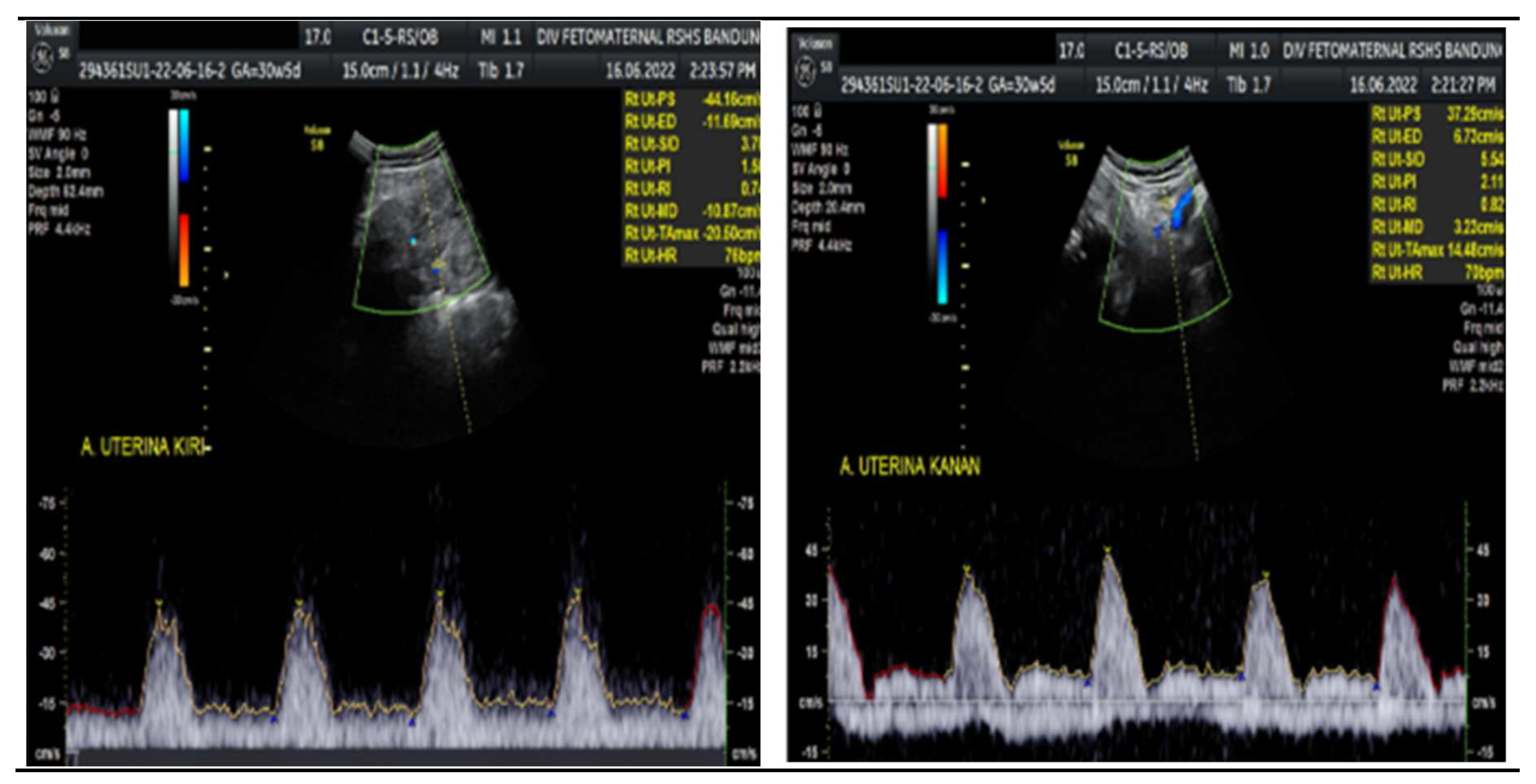
Figure 1). The umbilical artery pulsatility index (PI) was 0.99, and RI was 0.63, with an S/D ratio of 2.71. The right uterine Artery is notching with a PI of 2.11, left Uterine artery is notching with a PI of 1.58. Laboratory results were within a normal range.
A cesarean section was performed due to severe preeclampsia with a low bishop score. Before the procedure, the patient's condition was stabilized with given a loading dose of magnesium sulphate 4 g IV over 15 to 20 minutes followed by a maintenance dose of 1 to 2 g/hour and given antihypertensive drugs to reduce the mean arterial pressure by no more than 25 percent. A male baby was then born with a birth weight of 1100 g, and the baby's length was 37 cm; the baby was in poor condition with APGAR score = 1 in min 1 and min 5. Resuscitation was performed; however, the baby only survived for 10 minutes.
Figure 3.
Amniotic Fluid index ( AFI 1,04 cm ): Oligohydramnios.
Figure 3.
Amniotic Fluid index ( AFI 1,04 cm ): Oligohydramnios.
3. Discussion
Currently, Doppler ultrasound is widely used to evaluate fetal circulation in both normal and pathological pregnancy. Fetal arterial Doppler studies are useful in determining the differential diagnosis of small-for-gestational-age fetuses. Due to impaired placental perfusion, the PI in the umbilical artery is increased and in the fetal MCA, the PI is decreased. Hemodynamic changes occur in fetal arterial vessels during hypoxia and acidemia induced by uteroplacental insufficiency.[
5,
6]
MCA assessment is performed to assess fetal well-being at ultrasound. In the hypoxic fetus, the cerebral vasculature dilates and this causes a reduction in the MCA pulsatility index in a phenomenon known as “brain sparing”. [
7,
8,
9] With disease progression the fetus is no longer able to compensate, and resistance in the MCA increases.[
8,
10] In the preterm (<32 weeks) small for gestational age fetus, MCA Doppler has limited accuracy in predicting acidaemia in the absence of other Doppler abnormalities. In the term small for gestational age fetus with normal umbilical artery Doppler, an abnormal MCA Doppler (PI < 5th centile) has moderate predictive value for acidosis at birth and should be used to time delivery.[
4]
Reverse flow in the middle cerebral artery (MCA) is a rare condition found in fetuses. Some reports suggested severe neonatal morbidity or rapid intrauterine fetal death in association with intrauterine growth restriction or intrauterine pathology.[
2,
3] Small-for-gestational age fetuses may be constitutionally small, with no increased perinatal death or morbidity; or their growth may be restricted due to either low growth potential, the result of genetic disease or environmental damage, or due to reduced placental perfusion and uteroplacental insufficiency. Pre-eclampsia and intrauterine growth restriction are associated with inadequate quality and quantity of the maternal vascular response to placentation. In both conditions, there are characteristics of pathological findings in the placental bed. In pre-eclampsia, there was a necrotizing lesion with foam cells in the wall of the basal and spiral arteries, which was referred to as acute atherosclerosis. In essential hypertension, there were hyperplastic lesions in the basal and spiral arteries.[
5]
The exact pathophysiology of Reverse flow in the MCA is still not fully understood. Failure of cerebral circulation can be caused by extracranial pressure such as external artificial pressure, oligohydramnios, and anhydramnios; or increased intracranial pressure due to hydrocephalus, cerebral edema, or cerebral hemorrhage.[
3,
6,
7] Transient REDF MCA can occur due to an artifact produced by excessive external pressure by the probe, which has no impact or clinical consequences. If such an artifact occurs, the examination should be repeated and confirmed by the second examiner cautiously to minimize the pressure by the transducer. Persistent REDF MCA is diagnosed if the examination was conducted properly with an appropriate technique and following the description of persistent REDF MCA.[
7,
8]
REDF in the MCA is a terminal hemodynamic disorder. Mari and Wassertrum were the first to report the loss of brain sparing before death in fetuses with IUGR conditions, and the observation results were also confirmed by several other examiners. These findings signify increased intracranial pressure due to cerebral edema associated with severe hypoxemia, as indicated by Vyas et al. who demonstrated the correlation of PI in the MCA and blood gas level from fetal blood sampling. In mild-to-moderate hypoxemia, there is a decrease in the PI of MCA with cerebral vasodilation. However, in the prolonged hypoxemia condition, the brain-sparing effect autoregulation mechanism cannot sufficiently compensate due to increased intracranial pressure following cerebral edema. Thus, the PI of MCI decreases in fetuses with highly severe hypoxemia.[
8]
Hypoxemia is compensated by a central redistribution of blood flow, resulting in increased blood flow to the brain, heart, and adrenals; and decreased blood flow to the peripherals.[
1,
5] This mechanism allows preferential delivery of nutrients and oxygen to vital organs. Increased blood flow of MCA in doppler findings is characterized by a decrease in PI with manifestations of cerebral vasodilation as a response to fetal hypoxemia.[
2,
9,
10] Compensation through cerebral vasodilation is limited, and a plateau corresponding to the base of PI level is reached at least 2 weeks before the development of the fetus is compromised.[
5]
In advance cases, this autoregulation process does not adequately perform, and there is an increase in resistance to cerebral blood flow, leading to a poor fetal state. Reverse flow in MCA is a rare case indicating the loss of brain-sparing autoregulation, which is strongly associated with the impending death of the fetus.
There have been several cases of PREDF in MCA (
Table 1). The etiologies include intracranial hemorrhage, severe anemia due to feto-maternal hemorrhage or severe fetal growth restriction, and a rare intrahepatic bile duct malformation. All cases lead to severe outcomes for the fetus or neonate, such as intrauterine death or morbidity. Fetal morbidities include a grade III intraventricular hemorrhage, periventricular leukomalacia, hemorrhagic parietal infarct, and bilateral ischaemic changes in the basal ganglia.
Sevulpeda et al. previously reported PREDF in MCA caused by IUGR with intrauterine fetal death outcomes, meanwhile, Kawakita et al. reported PREDF in MCA but without recorded neonatal follow-up outcomes. In this case, a pregnancy with severe preeclampsia, IUGR, and oligohydramnios has Doppler findings indicating PREDF, resulting in early neonatal death (within 10 minutes). This is in line with previous case reports where PREDF in MCA in fetal with IUGR has very poor outcomes leading to intrauterine/early neonatal death.
The Doppler examination also revealed a notching of the right and left uterine arteries, which is a marker of spiral artery remodelling failure, causing early-onset severe preeclampsia. In this case, inadequate early prevention worsened the patient's condition, leading to severe preeclampsia. The pregnancy did not present with hydrocephalus, cerebral hemorrhage, or mechanical compression of the fetal head.
4. Conclusions
The findings of PREDF, in this case, can be caused by oligohydramnios, IUGR, and severe preeclampsia, which all contributed to severe hypoxemia, cerebral edema, and ultimately increased intracranial pressure. PREDF in MCA in this pregnancy indicated a terminal condition with a poor outcome. Operative delivery was performed in favour of ameliorating the mother's condition presenting with severe preeclampsia and a low bishop score after the stabilization phase. Further research is necessary to outline various causes precipitating PREDF in MCA and its association with fetal/neonatal conditions, so we can formulate the most suitable treatment for this condition.
Author Contributions
writing—original draft preparation, D.S, J.C.M, N.K, F.Z.; writing—review and editing, D.S, J.C.M, N.K, F.Z , M.G.D; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Acknowledgments
We would like to thank for Department of Obstetrics and Gynaecology of Padjadjaran University for supporting this study.
Conflicts of Interest
There were no conflicts of interest in this study
References
- Mlynarczyk M, Romary L, Abuhamad AZ (2017) Role Of Doppler Sonography in Obstetrics. In: Callen's (ed) Ultrasonography in Obstetrics and Gynecology, Elsevier, p 753-59.
- Hirshberg A, Levine L, Schwartz N,Durnwald CP (2013) Reversed End-Diastolic Flow in the Middle Cerebral Artery Preceding Death in a Normally Grown Fetus. Obstet Gynecol 122:507-9. [CrossRef]
- Hjortøa S, Skibsted L, Lykke JA. Reverse flow in the middle cerebral artery in a severely intrauterine growth restricted fetus (2013) Eur J Obstet Gynecol 70:575-80. [CrossRef]
- Brownfoot FC, Cluver CA, Walker SP (2015) Persistent reversed end diastolic flow in the fetal middle cerebral artery: an ominous finding. Ultrasound 23:186-89.
- Nicholaides K, Rizzo G, Hecher K, Ximenes R (2002) Doppler studies in fetal hypoxemic hypoxia. In: Doppler in Obstetrics. The Fetal Medicine Foundation And ISUOG Educational Series 62-8.
- Jakobovits AA (1997) Reverse flow in the fetal middle cerebral artery. Am J Obstet Gynecol 176, 2:497. [CrossRef]
- Respondek M, Kaczmarek P, Borowski D (2000) Reversal of diastolic flow in the middle cerebral artery of the fetus during the second half of pregnancy. Ultrasound Obstet Gynecol 9:324-9. [CrossRef]
- Sepulveda W, Shennan A, Peek MJ (1996) Reverse end-diastolic flow in the middle cerebral artery: An agonal pattern in the human fetus. Am J Obstet Gynecol 74, 5:1645-7. [CrossRef]
- Giancotti A, Spagnuolo A, Pasquali G,et al (2011) Reverse end-diastolic flow in a fetus with a rare liver malformation: a case report. J Med Case Rep 5:37. 2011;5:1-4. [CrossRef]
- Medicine SfM-F. Doppler assessment of the fetus with intrauterine growth restriction (2012) Am J Obstet Gynecol 301-8. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).