2. Results
2.1. The stomach
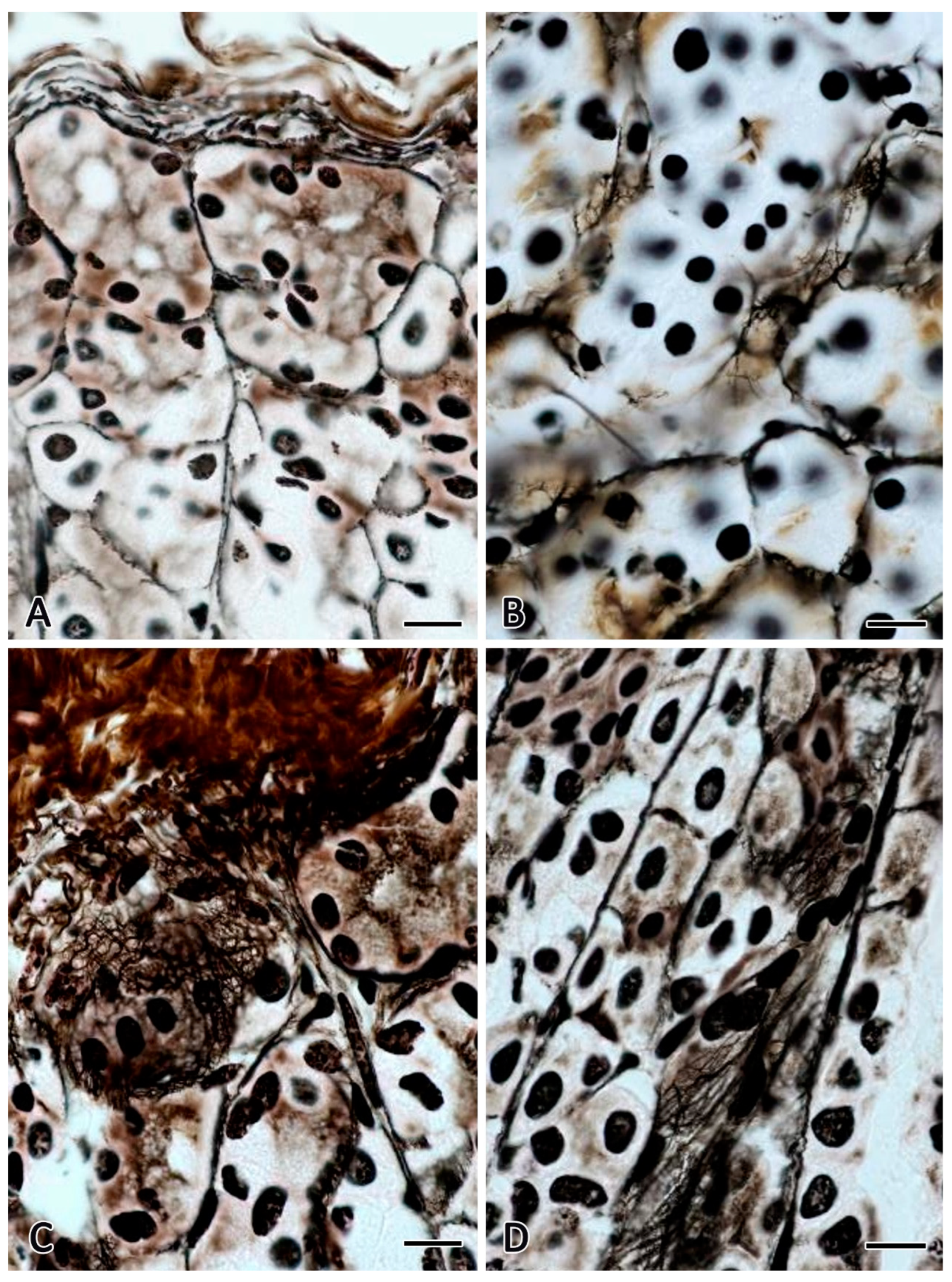
The fibrous component of the connective tissue was adequately detected in all structures of the stomach wall of mice from the vivarium control group (
Figure 1A and
Figure 2A). In the lamina propria of the mucous membrane, mainly reticular fibers located between the fundic glands of the stomach were detected (
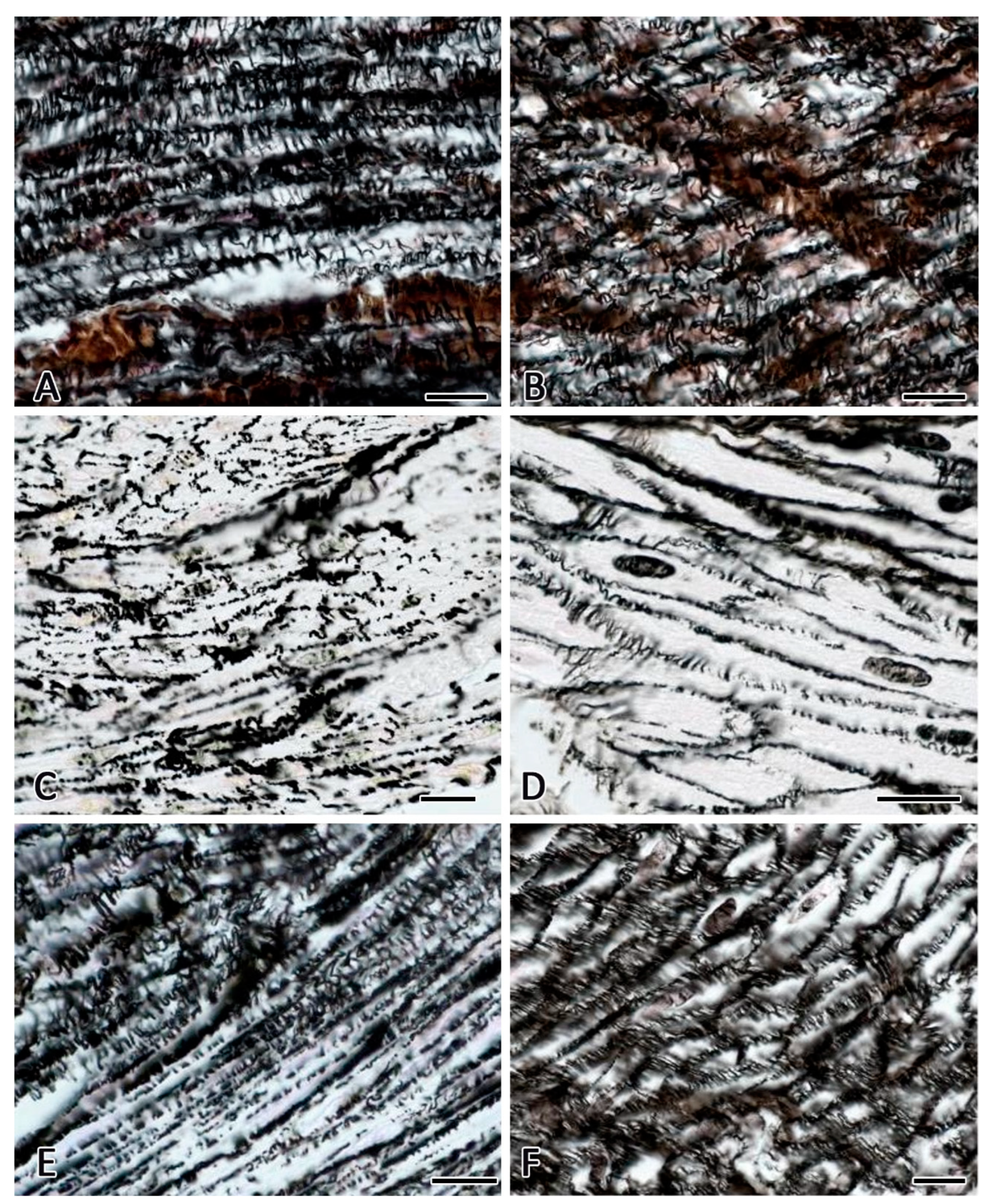
Figure 1A,B). Some of them repeated histotopography of the basement membrane of the proper gland, extending almost the entire thickness of the gastric mucosa. Within the mucosal muscularis lamina, reticular fibers were accompanied by smooth myocytes, forming a network of fine intertwining fibers with mild argyrophilia. In the submucosa, there was a greater number of reticular fibers of various calibers located next to the bundles of fibrous structures, mainly represented by type I collagen. It should be noted that, upon impregnation with silver, these fibers were stained in more brownish-yellow shades compared to almost black impregnated reticular fibers. A high content of reticular fibers was detected in the muscular membrane of the stomach (
Figure 2A). Layers of smooth myocytes were separated by accumulations of loose fibrous connective tissue containing reticular fibers of various thicknesses.
Within the muscle layer, reticular fibers contacting with the myocyte plasmalemma were arranged in a specific sequence and formed a dense network entwisting the contractile items. The reticular fibers in the muscular membrane of the organ was oriented both in the direction of the long axis of smooth myocytes and transverse (
Figure 2A). Sometimes unevenly distributed argyrophilic material in the form of pulverized grains or larger clumps contacted with the myocyte plasmalemma. Collagen fibers, including reticular ones, were constantly detected in the serous membrane.
After the orbital flight, there were found various transformations of collagen fibers related to the parameters of animals from the vivarium control group, their severity depended on the histotopography in the stomach wall. The processes of reticular fiber disorganization combined with changes in their quantitative parameters became evident. The increased representation of connective tissue in the mucosa was due to the formation of microloci with a high content of reticular fibers (
Figure 1C). These areas often extended through the entire thickness of the mucous membrane. However, the main trend was a locally increased number of impregnated fibrous structures within limited areas of the mucosal surface. Histotopographically, this was typical of the lower third of the gastric proper glands or the area of the fundus. The most probable reason for such changes seem to be the specific trophism of the gastric mucosa under space flight conditions, this specificity determines the extent of spread. There were determined microloci of the gastric proper glands with a violated integrity of the argyrophilic fibrous structures composing the basement membrane. As for the tinctorial features of the reticular fibers, there was observed an increased degree of their argyrophilia in the gastric interstitium of C57BL/6N mice, whereas after staining according to van Gieson, an increased intensity of fuchsin staining of the fibers was more often detected, and in some cases there was an increased picrinophilia in sites manifesting morphological signs of homogenization of fibers or their bundles. There was an evidentially decreased representation of reticular fibers in the muscle plate of the gastric mucosa. Similar alterations were detected in the area of the submucosa. The number of large-caliber reticular fibers decreased, granular-like accumulations of impregnated material and intensely stained individual fiber fragments were often observed. The reduction of bundles of collagen fibers was combined with an increased fuchsinophilia and signs of edematous events. There were detected areas of homogenization of collagen fibers, some of them acquired picrinophilia. This fact evidences dystrophic changes leading to partial disorganization of collagen fibers within microloci, or implementation of a directed mechanism of their lysis.
The most noticeable post-flight changes in the fibrous structures of the extracellular matrix were found in the muscular membrane of the stomach. The number of reticular fibers significantly decreased (
Table 1,
Figure 2C,D). These data coincided with a decrease in the expression of type III collagen in the pericellular matrix of smooth muscle cells (
Table 2).
A similar trend in the representation of connective tissue was observed in the study of micropreparations after staining according to Masson-Goldner (
Figure 3).
In the muscular membrane there were sometimes formed rather large loci, in which fibrous connective tissue structures, including argyrophilic, were almost completely absent (
Figure 1C). In endomysium, the mutual arrangement of fibers relative to each other changed. The number of reticular fibers localized transversely to the long axis of smooth myocytes was significantly reduced (
Figure 2C,D). Reduced impregnated fibers were histotopographically characterized by a predominantly parallel orientation related to the long axis of smooth myocytes. Loci of endomysium with accumulation of granular argyrophilic material were often detected. Changes in the affinity for the dye were detected throughout the reticular fibers, this manifested as a pronounced variability from areas with a low degree of staining to the formation of microloci with a high degree of impregnation. In addition, there were identified formations represented by conglomerates of impregnated material without an ordered structure, having an elongated shape and sometimes reaching rather large sizes.
The results obtained in C57BL/6N mice from the 7-day recovery group after the orbital flight demonstrated no return of the connective tissue state in the gastric mucous membrane to the level of the vivarium control group. First, it related to the quantitative parameters of reticular fibers. In the places where cells of the gastric proper glands, characterized by the most pronounced signs of dystrophy under space flight conditions, were localized, an increased content of argyrophilic structures remained (
Figure 1D). An increased number of reticular fibers was also observed in the layers of connective tissue separating the proper glands of the stomach (
Figure 1D). There was an increased argyrophilia in all micropreparations.
Notably, the number of reticular fibers relevantly increased in the muscularis mucosa and, especially, in the muscular membrane (
Table 1,
Figure 2E,F). Increased content of type III collagen in the extracellular matrix around smooth muscle cells (
Table 2). Argyrophilic fibers with a transverse direction towards the long axis of smooth myocytes were restored. To a lesser extent, the content of reticular fibers increased due to fibrous structures localized in the endomysium parallel to the long axis of smooth myocytes, and those located in the perimysium. Concurrently, attention was drawn to the high content of impregnated granular formations in the muscle membrane, which could evidence incomplete processes of the reticular fibers lysis combined with active biogenesis under conditions of adaptation to the usual level of Earth's gravity. In addition, there was a restoration of the content of endomysium argyrophilic structures contacting with the basement membrane of smooth myocytes, which was especially clearly observed in cross sections (
Figure 1E,F). The frequency of detection of gastric smooth muscle loci with pronounced reduction of the network of reticular fibers in the endomysium was significantly reduced compared to that in animals from the space flight group, although such areas were being constantly detected.
In terms of the changes detected in the fibrous extracellular matrix of the stomach in C57BL/6N mice from the space flight group, the study results of animals from the ground-based space-flight-simulated biological control group were crucial. The analysis of the biomaterial performed immediately after a 30-day stay in the BIOS-MLZH flight equipment model and in 7 days after the space flight simulation demonstrated that the main changes in the fibrous component of the connective tissue occurred in the mucous membrane and affected mainly the state of the reticular fibers. However, the trend towards a decreased representation of reticular fibers was not relevant compared to the parameters of animals from the vivarium control group (
Figure 1B). The changes mainly concerned the tinctorial features of the reticular fibers. Dystrophic changes in the integumentary epithelium and the proper glands of the stomach were rare and were observed within microareas. In the muscularis mucosa, the topography of fibrous elements practically did not differ from the patterns observed in mice from the vivarium control group. The study of the fibrous skeleton of the submucosa demonstrated a high content of reticular fibers in close contact with fibrous structures formed by type I collagen. In the muscular coat, there was a tendency to the increased index of the content of reticular fibers compared to the parameters of animals from the control group; however, no statistical significance was obtained. The fibrous structure histotopography did not undergo significant changes (
Figure 2B). The results obtained in animals examined in 7 days after the simulated experiment demonstrated that revealed microscopic pictures did not differ in most signs from the parameters of animals from the vivarium control group.
2.2. The intestine
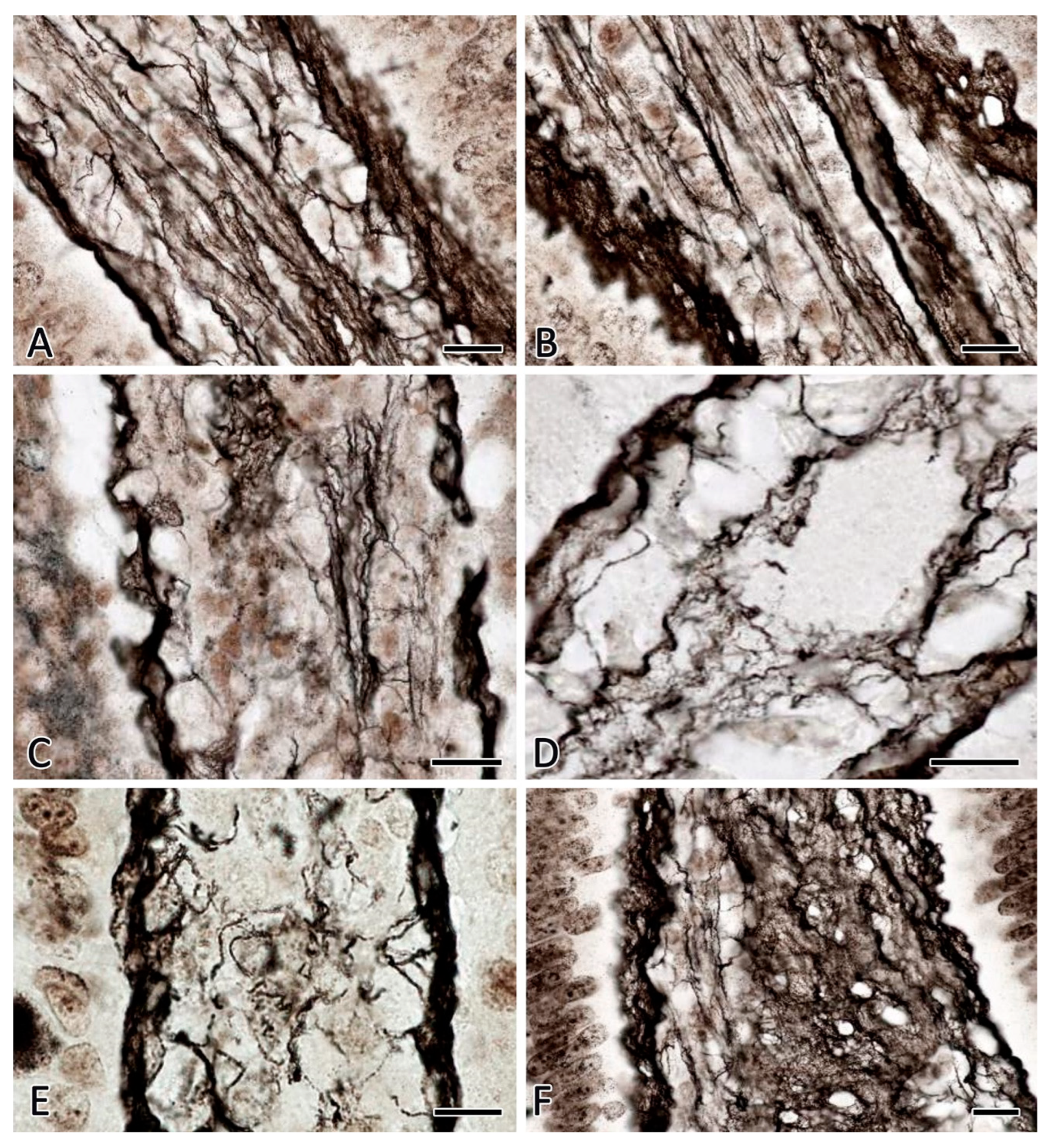
In the wall of the jejunum in animals from the vivarium control group, there were reticular fibers identified in all membranes, while collagen fibers were predominantly located in the submucosa and serous membrane. In the serous membrane, reticular fibers were localised in the subserous layer, had a large caliber and a high level of argyrophilia. In the muscle membrane, impregnated fibrous elements were detected both in the circular layer and in the longitudinal layer (
Figure 4A). Concurrently, reticular fibers created a network oriented mainly along the long axis of smooth myocytes (
Figure 4A). On cross sections, the basement membrane of smooth myocytes contacting with the structures of endomysium was well defined.
Within the submucosa, the largest caliber reticular fibers were detected. As in the stomach, they were located together with bundles of collagen fibers, which were well identified after staining by van Gieson and Masson-Goldner techniques.
In the lamina propria of the mucous membrane, reticular fibers, which formed the skeleton of the villi and were freely located in the intercryptal stroma, were predominantly detected. In the villi, reticular fibers could form a fine-mesh network with varying degrees of expressiveness (
Figure 5А).
After the space flight, quantitative and qualitative changes in the fibrous component of the connective tissue were detected in all membranes of the jejunum compared to the parameters of animals from the vivarium group. In the subserous layer, the reticular fibers acquired a different-sized appearance with a variable level of argyrophilia throughout. In the muscular coat, a complete loss of reticular fibers was detected in some loci due to a decreased argyrophilia (
Figure 4C,D). The caliber of reticular fibers simultaneously decreased along with their number (
Table 1). The content of type III collagen around the smooth muscle membrane myocytes was significantly reduced (
Table 2). However, sometimes there were impregnated fibrous fragments of considerable thickness, supporting signs of a specific disintegration of the stromal component. A large amount of impregnated material in the form of grains, as well as fragments of reticular fibers with high argyrophilia, were detected (
Figure 4C). Histotopographic features of the impregnated fibrous structure localization changed, if compared with the patterns typical for animals from the vivarium control group. In particular, the ordered reticular fiber localization was lost in the muscular coat, the fibers became sparser and changed direction (
Figure 4C,D). Similar patterns were detected in the lamina propria of the mucous membrane of the jejunum. The reduction of reticular fibers was marked in the stroma of the villi (
Figure 5C,D,E).
The intercryptal stroma was also characterized by a decreased representation of argyrophilic fibers, which was combined with their decreased caliber and accumulation of a large number of fragments with a high degree of impregnation. In the submucosa, the content of collagen fibers decreased, they acquired a higher level of fuchsinophilia.
The study of the biomaterial taken from the jejunum of C57BL/6N mice in 7 days after the biological satellite landing demonstrated significant qualitative and quantitative changes in the system of the fibrous extracellular matrix of the connective tissue compared to the parameters of animals from the space flight group. This was foremost due to an increased expressiveness of the reticular skeleton in the lamina propria and the muscle membrane (
Table 1;
Figures 4E,F and 5F). There was formed a network of thickened fibrous structures, its topography took the form typical of the animals from the vivarium control group. Concurrently, there was found a large amount of granular impregnated material with high argyrophilicity, and fragments of reticular fibers, this, obviously, being a reflection of the processes of incomplete readaptation of the jejunal stroma to the standard level of the Earth's gravity. The content of collagen fibers in the submucosa and serous membranes increased.
The study results demonstrated that the fibrous extracellular matrix of the connective tissue was present in all membranes of the large intestine in animals from the vivarium group. Reticular fibers were located mainly in the lamina propria and the muscularis propria.
In the smooth muscles of the colon, a more pronouncedly developed network of reticular fibers was noted compared to the muscular membrane of the jejunum. These fibers had a reasonably large caliber and were characterised by uniform argyrophilia throughout. After a 30-day space flight, argyrophilic fragments, accumulations of impregnated granular material, were found in the lamina propria of the colon mucosa. In general, the content of reticular fibers decreased in all membranes of the organ. In animals from the flight group, in the muscular membrane of the colon there were detected loci without argyrophilic structures, in some cases extending over significant areas of the colon wall. Notably, the detected reticular fibers were characterized by uneven staining throughout, they were thickened or thinned. Concurrently, in 7 days of ground adaptation after space flight, there was observed a pronouncedly increased number of reticular fibers.
Collagen fibers in the colon wall were predominantly distributed in the submucosa, although they were observed in a small amount in the subserous layer of the serosa. Within the submucosa, collagen fibers formed bundles of various sizes. In animals from the group after the space flight, attention was drawn to an increased fuchsinophilia along with a trend towards a decreased content of collagen fibers in the submucosa compared to parameters of animals from the biological control group. There were formed signs of edema unevenly spreading in the area of the submucosa. Concurrently, there were detected areas of homogenization of collagen fiber bundles, their increased fuchsinophilic properties; picrinophilia loci were detected in the submucosa. In seven days after the space flight, the level of fuchsinophilia of collagen fiber bundles decreased, and the tinctorial properties of fibrous structures approached the level typical of animals from the vivarium control group. Notably, signs of edematous events within the submucosa were being detected.
The analysis of the biomaterial taken from the small and large intestines in animals from the biological control groups after flight simulation demonstrated that the main changes in the state of the fibrous connective tissue occurred in the mucous membrane and affected the tinctorial properties of the reticular fibers. In particular, reticular fibers of different sizes which had unequal argyrophilia throughout were found in the intercryptal stroma of the jejunum of mice. In the stroma of villi, there was a tendency to increased representation of the network of reticular fibers along with signs of a decreased argyrophilic properties, although their number did not differ from that detected in animals of the vivarium control group (
Figure 5B). In some animals, reticular fiber fragments were detected with an increased intensity. However, this was not as pronounced as in animals from the spaceflight group. It is noteworthy that the state of the reticular fibers in the muscular membrane did not undergo significant alterations (
Figure 4B). The amount of the fibrous component in the large intestine practically did not change neither after the completed simulated experiment nor in 7 days of stay in conditions of simulated readaptation (
Table 1).
3. Discussion
Before discussing the results obtained, we would like to note that the role of altered gravity in the formation of morphological equivalents of the body's response has been the subject of numerous scientific discussions for a long time [
1,
2,
3,
4,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17]. The data obtained in this study are consistent with the research results studying the digestive organs of Mongolian gerbils after a 12-day space flight on the Foton-M3 spacecraft [
1,
2,
3,
4]. Intraorganic connective tissue can be considered as a gravity-dependent system that largely determines the specifics of the developing morphological and functional changes in other organ components in orbital flight [
1,
2,
3,
4,
13,
18].
As in the study investigating Mongolian gerbils, the data obtained in the experiment on C57BL/6N mice evidence intensive adaptive remodeling of the extracellular matrix of the digestive connective tissue under space flight conditions, which can be mediated by a decreased efficiency of fibrillogenesis and violated processes of the intercellular substance restoration. This assumption is also consistent with the results of an embryological experiment on the ‘Mir’ orbital station involving Japanese quail chicks, which demonstrated a weakly developing connective tissue of the gastrointestinal stroma under conditions of weightlessness, including a looser arrangement of fibers in embryos and chicks [
19].
It can be assumed that the fundamental reasons for alterations in histoarchitectonics and tinctorial properties of the fibrous extracellular matrix of the studied connective tissue are the features of the amorphous component and acid-base balance that are formed during space flight. Notably, an altered biosynthesis of collagen proteins and fibrillogenesis can ultimately affect the properties of individual reticular fibers and the spatial structures arranged by these reticular fibers - loops, networks, etc [
18,
20,
21,
22]. Perhaps this is the reason for the decrease in the content of type III collagen in the extracellular matrix of the muscular membrane of both the stomach and the small. This is of particular importance for the endomysium of the gastric and intestinal muscular membrane. The results obtained demonstrate a high potential of fibrous elements of the connective tissue for readaptation processes in animals after the space flight. However, based on the morphological features, they cannot be considered completed in 7 days of the post-flight period.
Under conditions of weightlessness, the digestive connective tissue undergoes specific structural and functional rearrangements, reflecting both adaptive and alternative gravity-induced processes. Signs of increased interaction of selective dyes with collagen proteins may evidence depolymerization of fibrous structures, which results in the release of a significant number of reactive groups. This allows admitting the presence of either direct effects of cosmic radiation on the crosslinking of amino acids in collagen molecules, or the phenomena of disorganization of fibrous structures resulted from the developing trophic disorders. In addition, it is impossible to exclude the influence of matrix metalloproteinases, which can be actively functioning under conditions of gravitational unloading, including the influence of mast cell proteases [
23,
24,
25]. In particular, the bioeffects of cosmic radiation were demonstrated in an experiment on a cultured human fibroblasts exposed for 14 days aboard the ISS [
26]. It should be taken into account that under the conditions of orbital flight, due to the development of hemodynamic changes, the digestive connective tissue functions in an environment with different properties [
27,
28]. Obviously, under weightlessness, a decreased efficiency of extracellular fiber assembly in the connective tissue is due to a significant restructuring of the microenvironment in the amorphous component of the interstitium: a change in the pH level, the content of hyaluronan, other glycosaminoglycans, proteins, water, etc. Collagen fibrillogenesis in the extracellular matrix is accompanied by the aggregation of molecules into supramolecular structures: protofibrils, microfibrils, fibrils, and fibers [
20]. In the initial stage of the developing interstitium fibrous phase, tropocollagen molecules form pericellular accumulations (mesophases), in these accumulations the distance between the molecules is filled with liquid. This liquid crystal state of the initial stage of fiber formation is called a tactoid [
20]. Tropocollagen molecules occupy an energetically favorable position in relation to each other in tactoids, and the shape of the molecules takes part in the stabilization of this state together with the action of electrostatic forces, the energy of molecular kinetic motion, and the influence of the surrounding liquid phase. To initiate the onset of developing supramolecular aggregates of collagen, it is necessary to bring tropocollagen molecules closer to a certain distance, which requires a decreased volume of the aqueous medium between them. This is achieved either as a result of an increased concentration of tropocolagen molecules by cellular secretion growth, or by increased liberalization into the extracellular space of glycosaminoglycan molecules that can interact with water from tactoids by the osmotic mechanism. Therefore, this initiates the assembly of protofibrils from tropocollagen molecules. Microfibrils, fiber fibrils or collagen bundles are further formed. However, to successfully form a collagen fiber, well-defined conditions are requisites, they depend on water content, tropocollagen concentration, osmotic pressure, temperature, ionic strength, pH, and many other factors. Moreover, even when the necessary requirements are met, the effect of fiber formation will depend on the amount of proteoglycans, complexing ions, ATP, ascorbic acid, enzymes, etc. [
18,
20,
21]. There is no doubt that in weightlessness the processes of fiber formation occur under conditions of a specific tissue microenvironment that are different from those on the ground [
18,
21]. That is why, apparently, in orbital flight, the processes of physiological regeneration of collagen fibers cannot be realised in full, since the consequences of venous stasis in the organs of the digestive system are accompanied by modified parameters of the integrative-buffer metabolic environment.
Moreover, under conditions of weightlessness, apparently, the process of remodeling or adaptation of the stroma of the organ is actively developed. In this case, the processes of disorganization of collagen fibers can be of great significance due to both the formation of specific trophic disorders and removal of the static load that occurs under the conditions of the Earth's gravity. Thus, the processes of accelerated reduction combined with decelerated formation of new fibers lead to a decreased volume of fibrous structures in the interstitium of the digestive system under conditions of weightlessness.
The dynamics of the content of the fibrous extracellular matrix in the wall of the digestive organs during space flight is expressed mainly by reduction processes decreasing the integrative role of the extracellular phase of the gastrointestinal connective tissue. The processes of accelerated lysis of fibrous structures, together with their decelerated formation, result in a decreased volume in the interstitium of the stomach and intestines, the fact potentially considered as one of the manifestations of the connective tissue intercellular matrix remodeling in accordance with the achieved state of the adaptive, or "cosmic", norm [
29]. In the stomach (excepting the lamina propria) and intestines, weightlessness caused a reduction in the fibrous phase of the connective tissue, which was accompanied by decreased morphometric parameters of their structures, including the muscular membrane. A decreased representation of reticular fibers in the wall of the stomach and intestines correlated with the results of morphometric analysis [
1,
2]. This suggests that the indicated morphological changes in the interstitium, primarily associated with the loss of fibrous collagens by the muscular layer, may have an impact on the weakening motor function of the gastrointestinal tract, noted in a number of studies, including those conducted on board orbital stations [
30,
31,
32,
33,
34].
The results obtained related to the state of connective tissue structures under conditions of weightlessness evidence that the cell and the extracellular matrix surrounding it are a single formation with a very high ability to detect the level of gravity in various environmental conditions. This emphasizes the existence of the cell in combination with other structural components of a specific tissue microenvironment as an integral mobile entity with powerful potentials for implementing adaptive responses under the influence of microgravity. The findings on alterations of the collagen fibrous structure and metabolism in the extracellular matrix of the gastric and intestinal connective tissue obtained in mice and Mongolian gerbils as a result of a long flight of animals in the near-Earth orbit support potential similar changes in other organs, including vessels.
A number of experimental studies allow assuming that the state of the intercellular substance of the connective tissue of various gastrointestinal organs after a space flight or under ground-based simulated physiological effects of weightlessness is closely related to the features of mast cell population [
3,
4,
35]. Notably, the active secretion of proteases, tryptase and chymase, whicah are capable of activating matrix metalloproteinases and, as a result, accelerating the degradation of collagen fibers, seems to be especially important [
18,
21,
23,
24,
25,
36]. The ability of mast cells to migrate in all layers of the walls of hollow organs and to actively secrete various factors determines their potential active participation in the gastric and intestinal stroma remodeling during space flight.
Figure 1.
Gastric mucosa of C57BL/6N mice. Fixation: 10% neutral formalin. Technique: The Foot’s silver nitrate impregnation. Groups of vivarium control animals for the 30-day space flight (A), biological control – ground-based experiment simulated 30-day space flight in the flight equipment model “BIOS-MLZH” (B), space flight group of animals that spent 30 days under conditions of weightlessness (C) and readaptation group including animals examined in 7 days after a 30-day space flight (D). Notes: (A) Reticular fibers are localized between the fundic glands of the stomach of the lamina propria. (B) The number of reticular fibers in the stroma does not change compared to the vivarium control group. (C) High content of reticular fibers in the lower sections of the proper glands of the stomach. (D). The stroma of the mucosa retains an increased content of impregnated fibrous structures. Scale bar: 10 μm.
Figure 1.
Gastric mucosa of C57BL/6N mice. Fixation: 10% neutral formalin. Technique: The Foot’s silver nitrate impregnation. Groups of vivarium control animals for the 30-day space flight (A), biological control – ground-based experiment simulated 30-day space flight in the flight equipment model “BIOS-MLZH” (B), space flight group of animals that spent 30 days under conditions of weightlessness (C) and readaptation group including animals examined in 7 days after a 30-day space flight (D). Notes: (A) Reticular fibers are localized between the fundic glands of the stomach of the lamina propria. (B) The number of reticular fibers in the stroma does not change compared to the vivarium control group. (C) High content of reticular fibers in the lower sections of the proper glands of the stomach. (D). The stroma of the mucosa retains an increased content of impregnated fibrous structures. Scale bar: 10 μm.
Figure 2.
Gastric muscular membrane of C57BL/6N mice. Fixation: 10% neutral formalin. Technique: The Foot’s silver nitrate impregnation. Notes: (А,B) - a developed network of reticular fibers in the endomysium of the gastric muscular membrane in animals from the vivarium control for the 30-day space flight group (A) and animals from the group of the ground-based experiment simulated a 30-day space flight in the BIOS-MLZH flight equipment model (B). (C,D) Reduction of reticular fibers in the gastric muscular membrane in mice after space flight. (E,F) An increased representation of impregnated fibrous structures in the endomysium of the gastric muscular membrane in mice in 7 days after the BION-M 1 biosatellite landing. Areas with a reduced content of reticular fibers are preserved (E). Scale bar: 10 μm.
Figure 2.
Gastric muscular membrane of C57BL/6N mice. Fixation: 10% neutral formalin. Technique: The Foot’s silver nitrate impregnation. Notes: (А,B) - a developed network of reticular fibers in the endomysium of the gastric muscular membrane in animals from the vivarium control for the 30-day space flight group (A) and animals from the group of the ground-based experiment simulated a 30-day space flight in the BIOS-MLZH flight equipment model (B). (C,D) Reduction of reticular fibers in the gastric muscular membrane in mice after space flight. (E,F) An increased representation of impregnated fibrous structures in the endomysium of the gastric muscular membrane in mice in 7 days after the BION-M 1 biosatellite landing. Areas with a reduced content of reticular fibers are preserved (E). Scale bar: 10 μm.
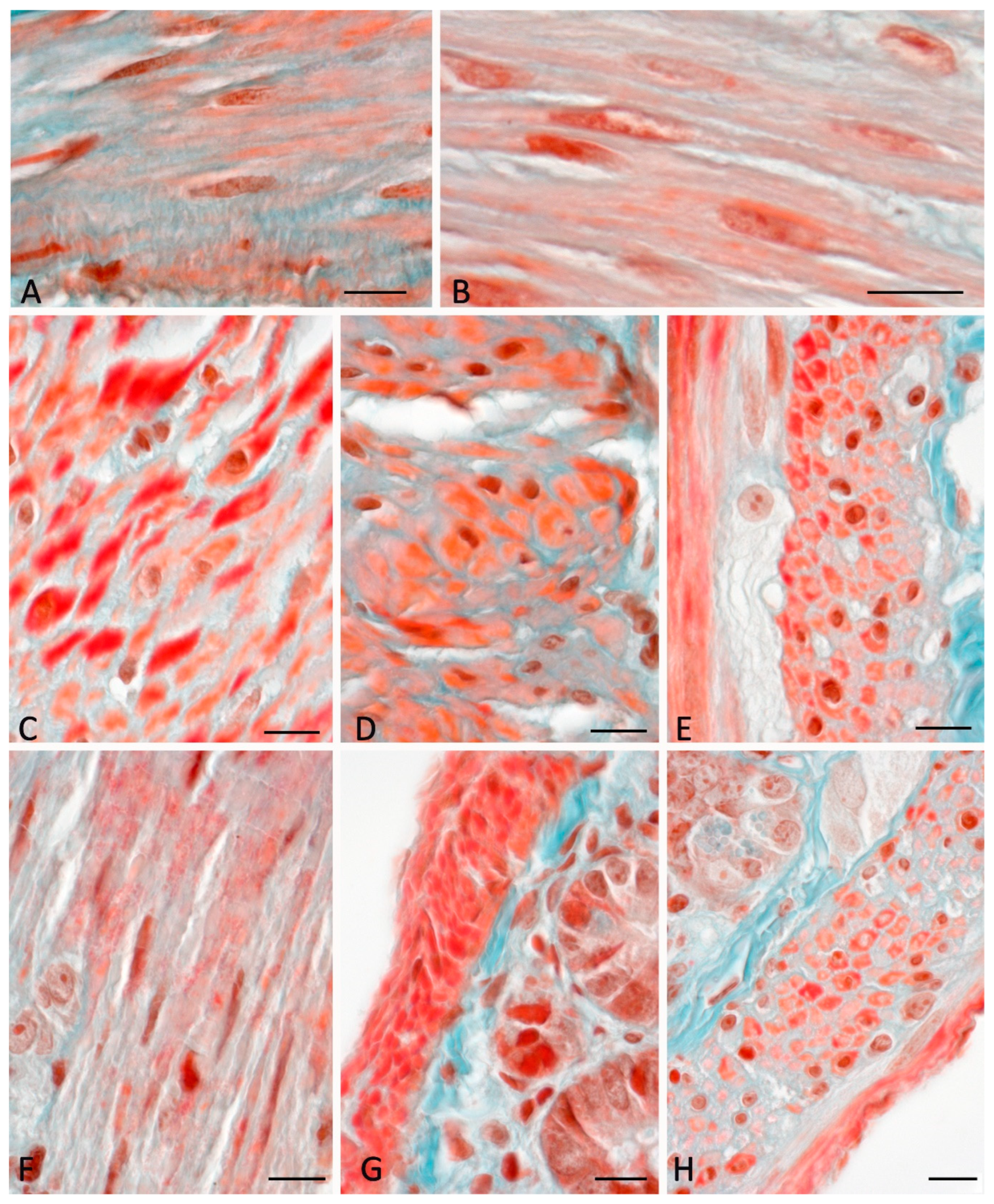
Figure 3.
Muscular layer of the gastrointestinal hollow organs in C57BL/6N mice. Fixation: 10% neutral formalin. Technique: Masson's trichrome stain (Goldner variant). (A-D) stomach, (E-H) jejunum. If compared with animals from the vivarium control group (A,E), there is a decreased content of the connective tissue in the stroma of the muscular layer (B,F,G) after a 30-day space flight. Seven-day post-flight rehabilitation is accompanied by an increased representation of the fibrous component in smooth muscle tissues both in the stomach (C,D) and in the jejunum (H). Scale bar: 10 μm".
Figure 3.
Muscular layer of the gastrointestinal hollow organs in C57BL/6N mice. Fixation: 10% neutral formalin. Technique: Masson's trichrome stain (Goldner variant). (A-D) stomach, (E-H) jejunum. If compared with animals from the vivarium control group (A,E), there is a decreased content of the connective tissue in the stroma of the muscular layer (B,F,G) after a 30-day space flight. Seven-day post-flight rehabilitation is accompanied by an increased representation of the fibrous component in smooth muscle tissues both in the stomach (C,D) and in the jejunum (H). Scale bar: 10 μm".
Figure 4.
Histoarchitectonics of reticular fibers in the muscular layer of the jejunum of C57BL/6N mice. Fixation: 10% neutral formalin. Technique: The Foot’s silver nitrate impregnation. Notes: (A) The group of vivarium control for the 30-day space flight group. Reticular fibers are clearly visible on longitudinal and transverse sections of smooth muscle layers. (B) The group of the ground-based experiment simulated a 30-day space flight in the "BIOS-MLZH" flight equipment model (biological control). The state of histoarchitectonics of reticular fibers does not change compared to animals from the vivarium group. (C, D) The group of 30-day space flight. The reduction of reticular fibers in the interstitium of the muscular layer with signs of fragmentation and formation of granular impregnated material is detected. (E,F) The group of readaptation including animals examined 7 days after the space flight. There is an increased number of reticular fibers in the muscular membrane. Scale bar: 10 μm.
Figure 4.
Histoarchitectonics of reticular fibers in the muscular layer of the jejunum of C57BL/6N mice. Fixation: 10% neutral formalin. Technique: The Foot’s silver nitrate impregnation. Notes: (A) The group of vivarium control for the 30-day space flight group. Reticular fibers are clearly visible on longitudinal and transverse sections of smooth muscle layers. (B) The group of the ground-based experiment simulated a 30-day space flight in the "BIOS-MLZH" flight equipment model (biological control). The state of histoarchitectonics of reticular fibers does not change compared to animals from the vivarium group. (C, D) The group of 30-day space flight. The reduction of reticular fibers in the interstitium of the muscular layer with signs of fragmentation and formation of granular impregnated material is detected. (E,F) The group of readaptation including animals examined 7 days after the space flight. There is an increased number of reticular fibers in the muscular membrane. Scale bar: 10 μm.
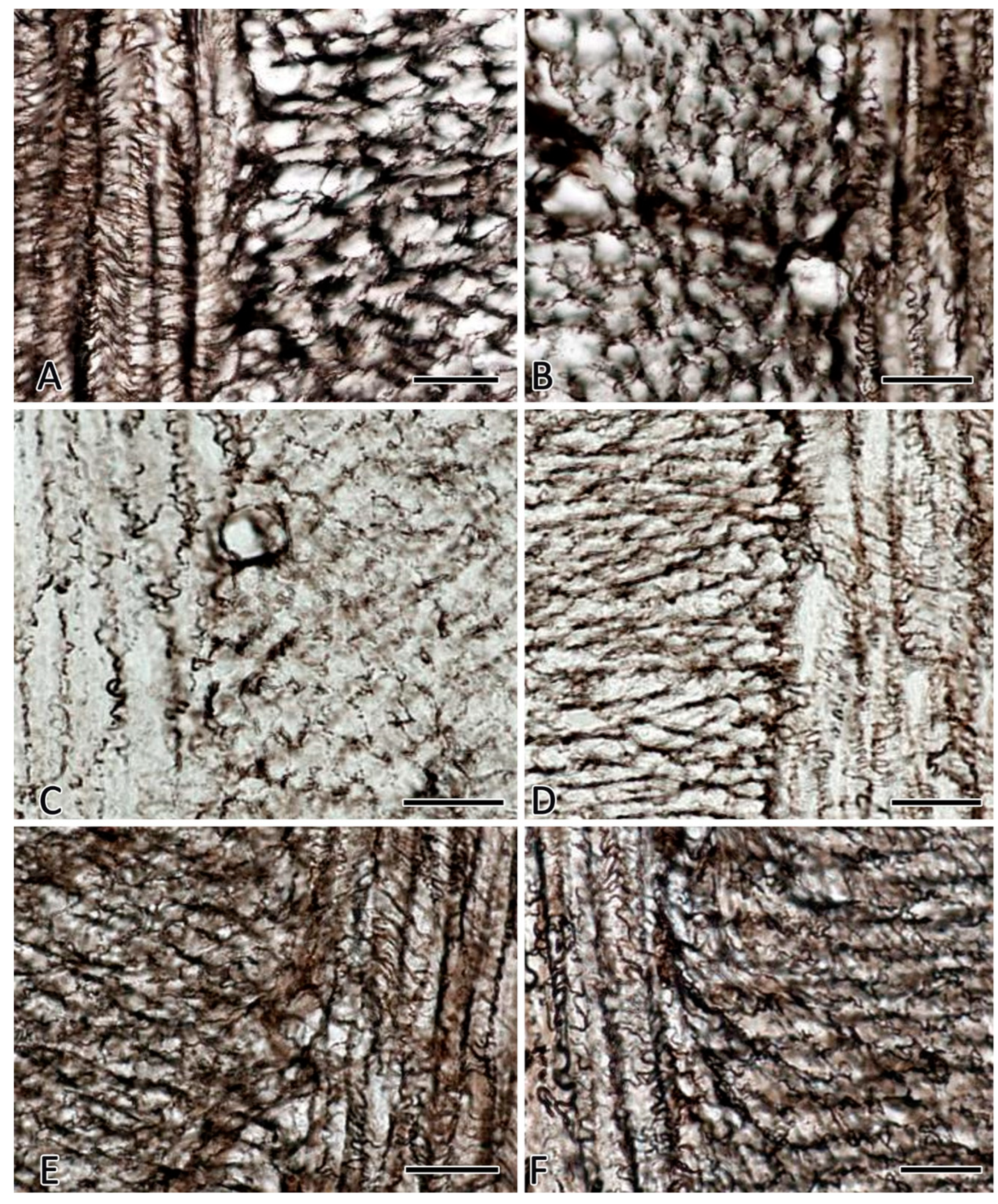
Figure 5.
Histoarchitectonics of reticular fibers in the villi stroma of the jejunum of C57BL/6N mice. Fixation: 10% neutral formalin. Technique: The Foot’s silver nitrate impregnation. (A) The group of vivarium control for animals from the 30-day space flight group. (B) The group of the ground-based experiment simulated a 30-day space flight in the "BIOS-MLZH" flight equipment model (biological control). In the stroma of the villi, a network of reticular fibers is well defined. (C,D,E) The space flight group. The reduction of reticular fibers in the stroma of the villi, the presence of impregnated material in the form of grains and fragments is detected. (F) The readaptation group including animals examined in 7 days after a 30-day space flight. In the stroma of the villi, a high content of different-sized reticular fibers is detected.
Figure 5.
Histoarchitectonics of reticular fibers in the villi stroma of the jejunum of C57BL/6N mice. Fixation: 10% neutral formalin. Technique: The Foot’s silver nitrate impregnation. (A) The group of vivarium control for animals from the 30-day space flight group. (B) The group of the ground-based experiment simulated a 30-day space flight in the "BIOS-MLZH" flight equipment model (biological control). In the stroma of the villi, a network of reticular fibers is well defined. (C,D,E) The space flight group. The reduction of reticular fibers in the stroma of the villi, the presence of impregnated material in the form of grains and fragments is detected. (F) The readaptation group including animals examined in 7 days after a 30-day space flight. In the stroma of the villi, a high content of different-sized reticular fibers is detected.
Table 1.
The index of the reticular fibers content in the muscular membrane of the digestive organs in C57BL/6N mice (relative units).
Table 1.
The index of the reticular fibers content in the muscular membrane of the digestive organs in C57BL/6N mice (relative units).
| Groups of animals |
Stomach |
Jejunum |
Colon |
| Experiment on board the "BION-M" 1 biological satellite |
VC-SF |
0.214±0.019 |
0.156±0.011 |
0.188±0.012 |
| SF |
0.154±0.011 *,** |
0.084±0.002 *,** |
0.112±0.007 *,** |
| VC-RSF |
0.202±0.012 |
0.144±0.014 |
0.173±0.013 |
| RSF |
0.244±0.017*,** |
0.179±0.011* |
0.208±0.018*,** |
| Ground-based experiment simulated conditions on board the "BION-M" 1 biological satellite for animals staying in the flight equipment model "BIOS-MLZH" |
VC-BC |
0.208±0.022 |
0.165±0.018 |
0.178±0.020 |
| BC |
0.214±0.017 |
0.173±0.021 |
0.171±0.024 |
| RBC |
0.197±0.023 |
0.154±0.018 |
0.170±0.014 |
| VC-RBC |
0.212±0.018 |
0.152±0.016 |
0.182±0.022 |
Table 2.
Expression of type III collagen in the muscular membrane of the digestive organs in C57BL/6N mice (relative units).
Table 2.
Expression of type III collagen in the muscular membrane of the digestive organs in C57BL/6N mice (relative units).
| Groups of animals |
Pericellular matrix of smooth myocytes of the stomach |
Pericellular matrix of smooth myocytes of the jejunum |
| Expression Intensity (%) |
Expression Intensity (%) |
| Not identified |
+
(M±m)
|
++
(M±m)
|
+++
(M±m)
|
Not identified |
+
(M±m)
|
++
(M±m)
|
+++
(M±m)
|
| Experiment on board the "BION-M" 1 biological satellite |
VC-SF |
2,2±0,2 |
56,4±3,2 |
34,2±3,3 |
7,2±0,7 |
3,4±0,4 |
51,3±4,2 |
40,1±3,5 |
5,2±0,4 |
| SF |
5,4±0,4*∆
|
66,3±4,7*∆
|
24,9±2,1*∆
|
3,4±0,2*∆
|
7,6±0,4*∆
|
63,3±5,3*∆
|
26,3±2,2*∆
|
2,8±0,3*∆
|
| VC-RSF |
1,7±0,2 |
52,3±4,4 |
37,6±3,1 |
8,4±0,7 |
3,3±0,3 |
54,3±3,7 |
36,2±4,2 |
6,2±0,3 |
| RSF |
2,3±0,3 |
44,1±4,1*∆
|
42,3±3,8*∆
|
11,3±1,0*∆
|
4,7±0,4*∆
|
43,9±3,4*∆
|
45,6±4,3* |
5,8±0,6∆
|
| Ground-based experiment simulated conditions on board the "BION-M" 1 biological satellite for animals staying in the flight equipment model "BIOS-MLZH" |
VC-BC |
1,9±0,2 |
57,4±4,1 |
33,9±2,9 |
6,8±0,4 |
4,1±0,5 |
46,3±4,4 |
43,4±3,8 |
6,2±0,4 |
| BC |
1,8±0,3 |
58,3±4,3 |
32,8±3,3 |
7,1±0,6 |
3,9±0,2 |
44,2±4,4 |
47,6±4,7 |
4,3±0,4 |
| RBC |
2,3±0,3 |
57,4±5,1 |
34,7±2,2 |
5,6±0,5 |
2,8±0,3 |
49,6±5,1 |
40,2±3,5 |
7,4±0,4 |
| VC-RBC |
2,8±0,3 |
60,3±5,4 |
31,4±1,7 |
5,5±0,7 |
3,3±0,4 |
52,6±6,2 |
39,5±3,1 |
4,6±0,2 |
Table 3.
Experimental groups of C57BL/6N mice.
Table 3.
Experimental groups of C57BL/6N mice.
| Experiment |
Groups of animals |
Number of animals |
| Experiment on board the "BION-M" 1 biological satellite |
VC-SF |
8 |
| SF |
5 |
| VC-RSF |
8 |
| RSF |
5 |
| Ground-based experiment simulated conditions on board the "BION-M" 1 biological satellite for animals staying in the flight equipment model "BIOS-MLZH" |
BC |
8 |
| VC-BC |
8 |
| RBC |
8 |
| VC-RBC |
8 |
| Total number of experimental animals |
58 |
Table 4.
Reagents used for histochemical and immunohistochemical staining of mouse gastrointestinal tract.
Table 4.
Reagents used for histochemical and immunohistochemical staining of mouse gastrointestinal tract.
| Dyes |
Catalogue number |
Provider |
Dilution |
Manufacturer |
| Anti-Collagen III Rabbit polyclonal antibodies |
ab7778 |
Abcam |
1:100 |
Abcam |
| Masson’s trichrome stain (Goldner variant) |
1.00485 |
Sigma-Aldrich Co. |
Ready to-use |
Sigma-Aldrich Co., Germany |
| Silver impregnation |
21-026 |
Biovitrum |
Ready to-use |
ErgoProduction LLC, Russia |
Table 5.
Secondary antibodies and other reagents.
Table 5.
Secondary antibodies and other reagents.
| Antibodies and Other Reagents |
Source |
Dilution |
Label |
| AmpliStain™ anti-Mouse 1-Step HRP (#AS-M1-HRP) |
SDT GmbH, Baesweiler, Germany |
ready-to-use |
HRP |
| DAB Peroxidase Substrat Kit (#SK-4100) |
Vector Laboratories, Burlingame, CA, USA |
ready-to-use |
DAB |
| Mayer’s hematoxylin (#MHS128) |
Sigma-Aldrich |
ready-to-use |
w/o |