1. Introduction
Fractures of the orbit alone and in combination with other facial skeleton fractures are common in cases of midface trauma: the orbital floor is affected by up to 57% of midface fractures [
1].
They can lead to entrapment of extraocular muscles and/or intermuscular septum and loss of orbital volume with different clinical symptoms and/or complications, such as dyplopia, enophthalmos, ocular dystopia, subconjunctival bleeding, ocular contusion, etc., which can lead to functional and morphological deficits, resulting in social and occupational problems.
Complete restoration of orbital contents with anatomically correct reconstruction of orbital walls is necessary to avoid functional deficits and for restoration of anatomical relations and morphology [
2]. The main aims of post-traumatic orbital reconstruction include both release and reduction of herniated or prolapsed soft tissue, accurate and stable reconstruction of the orbital walls, restoration of native orbital volume, avoidance of damage to vital structures such as muscles and nerves [
2].
Orbital dystopia and enophthalmos are the most important sequelae of blow-out orbital floor fractures.
Significant enophthalmos after orbital injury and floor fracture is usually not immediately apparent because of edema and swelling of the surrounding orbital tissues. This swelling and hemorrhage could even cause proptosis on the affected side. A retrospective noncomparative study by Hawes and Dortzbach [
3] reported that CT-scan is useful tool in predicting significant postinjury enophthalmos. The authors recommended surgical repair within 2 weeks if greater than one half of the orbital floor is depressed. They reported less satisfactory enophthalmos repair with later correction because of presumed fat atrophy and scarring of the orbital fat to the maxillary antrum, making late repair unsatisfactory [
4].
However, medical literature highlights that immediate surgical treatment is needed in cases involving extraocular muscle function within a trapdoor fracture, acute enophthalmos, and hypoglobus. Otherwise, observation can be recommended, to allow for resolution of edema before definitive evaluation of the need for treatment. If required, surgery is usually performed within 7-14 days of injury. Otherwise, it is recommended that surgery be delayed to allow tissues to heal before further intervention [
5].
Reconstruction of orbital wall(s) is also required during or after midface oncologic surgery following radical surgery for tumors involving the orbit, and it can be considered uniquely complex. Secondary procedures can be challenging in correcting structural derangements, especially if adjuvant radiation is administered, and so care must be taken to select the optimal reconstructive option at the same operative time of demolition, whenever it is possible.
The main aim of secondary orbital reconstruction is to restore symmetrical globe position to recover function and morphology.
Orbital floor reconstruction can be achieved using a variety of different methods and materials: a review made by Avashia et al. [
6], including 3457 distinct orbital floor reconstructions, showed inconclusive data on any biomaterial/implant absolute best fit for orbital floor reconstruction. As demonstrated, each material has its unique characteristics, and the choice of one over another is often related to the kind of defect, other than surgeon’s preference.
For example, the use of biological materials such as fervens bones, autogenous bones or collagen membranes (Lyoplant®) offers the potential advantages of better biocompatibility, but come at the cost of donor-site morbidity.
Conversely, the use of synthetic implants, such as titanium meshes or high density polyethylene foils or implants, has historically been associated with higher rates of complications.
Non-biologic materials are indeed capable of providing strength, durability, are not prone to resorption, and do not require increased surgery to obtain nor they increase morbidity; however, they are prone to infection as they never mucosalize when they abut sinuses [
7], and can undergo superficilization and/or cutaneous extrusion over time.
Advantages of titanium mesh plates include availability, biocompatibility, ease of intraoperative contouring, and rigid fixation [
8].
Unfortunately, these implants are not easily positioned: placement of the mesh deep within the orbital cone is relatively difficult, especially if scarring has obscured identification of certain stable anatomical landmarks.
Custom-made titanium implants using computer-assisted designs have enabled surgeons to achieve optimal reconstruction in areas of limited visibility and protection of vital structures [
9].
Computer-assisted design and computer-assisted manufacturing today are largely applied in maxillo-facial reconstructive surgery increasing the accuracy and allowing to achieve better clinical outcomes [10-11].
Consequently, from the early 2000’s, there has been a growing use of individual implants in orbital floor reconstruction, clearly demonstrating a to be less time consuming and more accurate if compared to ‘free hand’ modelling of the implant. [
12].
With the increasing interest of Computer-Assisted Surgery (CAS), there has been a large application of patient-specific reconstruction of orbital floor defects and a large number of research papers have demonstrated the advantages existing in application of patient-specific implants (PSI) for orbital reconstructions [
13,
14].
CAS for orbital reconstruction can be achieved through manual preoperative molding of the implant based on the patient’s stereolithographic 3D model (produced on the basis of CT scans). This technology increases the orbital surgeon’s options in managing complex orbital pathology [
15].
Another form of CAS in orbital reconstruction are Computer Aided Design - Computer Assisted Manufacturing (CAD-CAM) procedures, which come in form of preoperative digital design of the implant based on the mirroring of the uninjured 3D reconstruction of the orbit on the contralateral affected side; the digital implant is then manufactured thanks to computer assisted technique, such as direct metal laser sintering or computer numerical control.
The aim of the present prospective clinical study is assess the clinical outcomes after CAD-CAM delayed reconstruction of the floor of the orbit, more specifically addressing restoration of globe position and resolution of enophthalmos.
2. Materials and methods
This study was approved by local ethical board and conducted on 12 patients who underwent orbital floor reconstruction using computer-assisted design-computer-assisted manufacturing (CAD-CAM) method. Only delayed orbital floor reconstruction were enrolled in this study. Immediate orbital floor repair was excluded.
All the patients attended Oral and Maxillofacial Unit at IRCCS – Azienda Ospedaliero-Universitaria di Bologna from December 2009 to March 2023.
The patients were divided into three pathological groups: post-traumatic, post-oncological resection and syndromic.
Patients’ characteristics are detailed in
Table 1.
The reconstructive protocol involved 4 steps: mirroring of the healthy orbit on the affected side, virtual design of a patient-specific non resorbable orbital floor mesh, CAM procedures for producing the customized implant, and surgical insertion of the device.
2.1. Virtual planning and computer-aided design
Planning began by acquiring a high-resolution CT scan of the patient’s craniofacial skeleton. Imaging was performed using a multidetector CT scanner (HiSpeed CT scanning station; General Electric, Milan, Italy). Volumetric, DICOM-format data (0.625-mm slice thickness, 0.312 slice spacing, 08 gantry tilt, 512x512 pixel resolution) were processed using Proplan and Mimics software (Materialise, Lueven, Belgium). After setting a suitable threshold value, this software allows creation of three-dimensional virtual models of the maxillofacial skeleton.
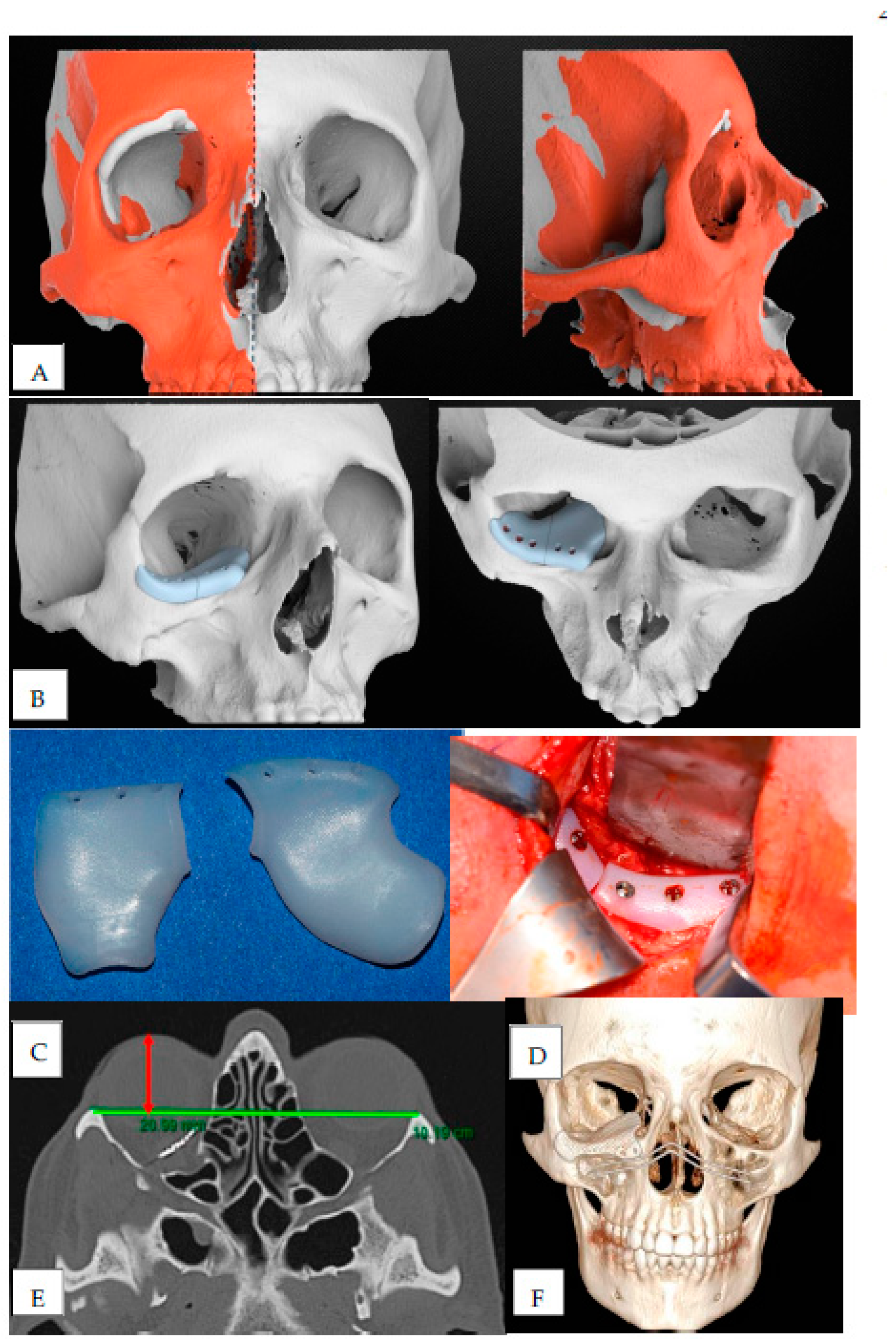
The CT scan was evaluated, and the normal uninjured side of the craniofacial skeleton was reflected onto the contralateral injured side by a mirroring technique using Geomagic Freeform (3D Systems, Rock Hill, South Carolina) (
Figure 1A). A reconstructive orbital floor implant was then designed virtually on the mirrored orbital bone surface (
Figure 1B).
2.2. Manufacturing of customized implants
Customised titanium meshes were surgically implanted in 10 cases.
High-density polyethylene (HDPE) was used in 2 patients (
Figure 1C).
Solid-to-layer files were used to manufacture the titanium mesh by direct metal laser-sintering (Sintac srl, Trento, Italy), which resolves the shaping and bending biases inherent in the indirect method (using an anatomical model) [
16].
Computer Numerical Control (CNC) machining, a manufacturing process in which pre-programmed computer software dictates the movement of factory tools and machinery, was instead used for the manufacturing of the HDPE implants.
2.3. Surgical procedure
Transconjunctival lower-eyelid approach, associated with lateral canthotomy and cantholysis to obtain a wider surgical field, was performed in 9 cases (
Figure 1D); in just 1 case isolated transconjunctival approach alone was executed.
Weber-Ferguson incision was performed in 2 complex post-oncological reconstuctive cases, of which one was associated with Lynch subciliary extension and the other one with Dieffenbach subciliary extension.
After dissection of the inferior orbital rim and opening of the periosteum, the patient-specific implant was fixed onto the intact facial buttress. After exposure of the orbital floor defect, the customized device was placed on the defect and further fixed with 5-mm screws as needed.
2.4. Outcomes evaluation
Pre-operative and post-operative globe protrusions were assessed as described by Ramli et al. [
17]. All patients underwent CT scans of the orbit after surgery. Each patient had their head strapped in the supine position during the scan to ensure reliability of the measurements. Their positions were centered by aligning the midsagittal line perpendicular to the laser line. The coronal line was centered 1 cm above the external auditory meatus.
An interzygomatic line was first drawn on the axial-view image in which the lens was best seen. A perpendicular line was then drawn from the inner corneal surface to the interzygomatic line, bisecting the lens. The length of this perpendicular line was taken as the primary measurement (
Figure 1E). As proposed by Ramli [
17], Hertel exophthalmometer and CT measurements of globe position are similar and strongly correlated.
We have decided to use the millimetric difference (Δ) of globe protrusion between the affected and the healthy side detected in CT scans according to Ramli’s method as a parameter of predictability and reproducibility of the whole CAD-CAM procedure.
Postoperative 3D CT scans were evaluated also to verify the correct positioning of the implant (
Figure 1F).
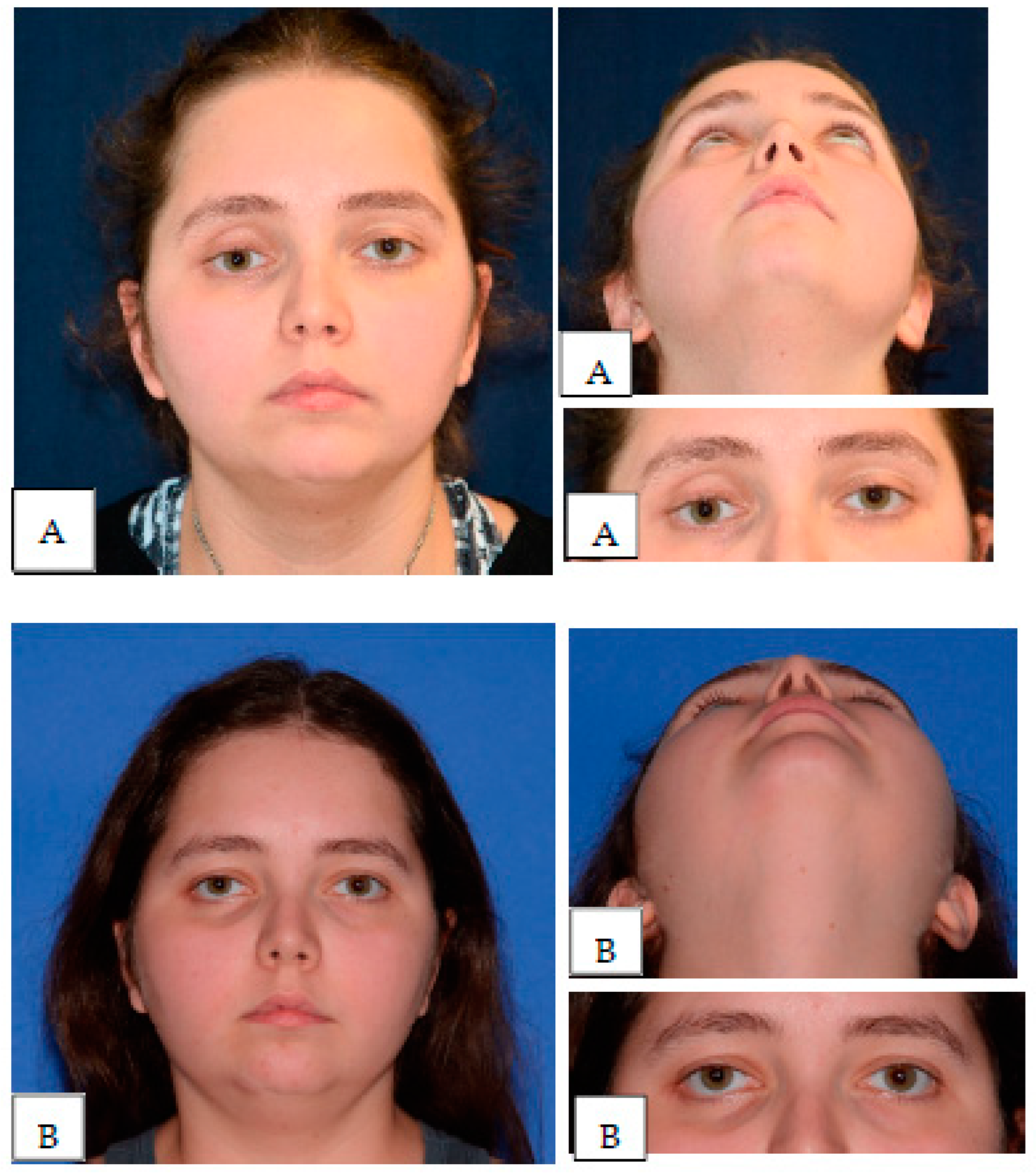
Aesthetical outcomes were assessed using 3dMD photogrammetry (3dMD Inc, Atlanta, GA). Faces were captured using the 3dMD photogrammetric system with patients keeping their eyes open.
The three-dimensional facial surface was calculated using 3dMD patient software (version 3.0). The Virtual Reality Modeling Language file with colored texture was used for the final virtual face display. Measurements of facial symmetry, globe protrusion, and dystopia were clinically analysed.
3. Results
The average age of patients undergoing surgery was 36.5 years old (range 18-75).
Mean follow-up duration was 31.75 months (range 5-120).
No acute or delayed implant-related complications, including wound site infection, dehiscence, hematoma, foreign body reaction, and diplopia, were recorded.
Clinical assessments showed that three-dimensional mirroring technique and PSI were effective in obtaining high precision in orbital floor restoration if compared with pre-operative situation.
Globe protrusion, assessed using Ramli method, appeared to be improved in all cases treated (
Table 1): we have calculated this parameter, obtaining an improvement of
3,04 mm (range 0,3-6 mm).
The Δ of postoperative globe protrusion between uninjured and treated eye was calculated: the mean Δ measured was
1,22 mm (range 0,27-3,69 mm), thus showing a mean difference between healthy and affected size
<2 mm in all cases, which is considered the minimum distance clinically detectable and evident by inspection [
18].
4. Discussion
Materials used for orbital reconstruction can be categorized into resorbable and not resorbable; we only used non-resorbable materials in this study because of the long-term duration and predictability over time that they can give.
Titanium mesh for orbital walls reconstruction is largely used with predictable results over the time [
6].
Also porous polyethylene has been used over the past decades as one of the main materials for craniomaxillofacial surgery procedures, thanks to its high biocompatibility, duration and malleability: technically, it is easy to work and yet strong, so it offers the possibility of obtaining a precise three-dimensional shape. Orbital floor reconstruction remains one of the most common applications of porous polyethylene shaped as thin (1,5 mm) or ultrathin (0,85 mm) sheets.
In this study we propose the use of both titanium implants and high-density polyethylene implants obtained from a computer-assisted manufacturing process.
Despite a short-term follow-up, we have observed that HDPE implants are easy to manufacture, being a medium-cost material when compared to titanium implants.
They are robust and adaptable at the same time, giving the chance to minimally reshape them intraoperatively, if it is necessary.
They appear to be biocompatible: we have not observed any acute or late-onset rejection, infection or exposure of the implants.
The main advantages derived from application of CAD-CAM technologies in orbital reconstruction are the sparing of operative time and the greater accuracy in positioning, thus leading to better functional and morphological outcomes; moreover, CAD-CAM procedures require an accurate preoperative planning, determing a larger amount of time spent by clinicians studying and preparing for the surgery on the single clinical case.
However, after an initial learning curve, the procedural time is significantly reduced. A study made by Zieliński et al. showed shorter operative time in patients in whom individual implants were used and less intraoperative bleeding time in patients in whom individual implants were used compared to patients who were treated using intraoperative bending titanium mesh [
19].
Functional and aesthetic results were significantly satisfactory; all patients had obtained a valid globe repositioning assessed using 3dMD photogrammetry.
Moreover, our results confirms the reliability and efficacy of the titanium implants, which appear to be strong, biocompatible and capable of osseointegration over the years: they have also a high rate of predictability thanks to the absence of resorption and remodeling rate.
This study comes after a preliminary one [
20] realised in our Center that represented a first step in the development of a wider experimental protocol for orbital floor reconstruction using computer-assisted design-computer-assisted manufacturing technology. It showed good results in terms of reliability and accuracy in orbital floor reconstruction using patient-specific implants.
In our center’s experience, over the past few years we have studied and observed that CAD-CAM technologies guarantee a high standard of accuracy and result in a very high degree of reproducibility in various fields of Oral and Maxillo-Facial reconstructive surgery.
They have been used for the reconstruction of complex areas following mandibular resections [
21,
22].
Nowadays, with the spread of digital technologies applied in surgical planning thanks to an early training for Residents as well, it appears increasingly easy to manage and obtain a tailored planning and treatment for the patients, and it should be considered as a valid option whenever a reconstructive procedure is required.
Finally, costs could be considered as a limit of the procedure. However, although the price for PSIs exceed the total cost of freehand reconstructions, when all aspects related to the quality of the results, intraoperative time gain, and the time of recovery for the patient were considered, CAD-CAM procedures appears to economically viable [
23].
5. Conclusion
In conclusion, the CAD-CAM technique described in this study appears to be a viable method to correct complex orbital floor defects needing delayed reconstruction.
We have confirmed the feasibility of a method for the 3D preoperative planning of custom-made implants thanks to the mirroring technique, which is currently the gold standard to obtain a hypothetical model of a patient’s healthy anatomy.
The use of patient-specific orbital implants has demonstrated high level of accuracy and predictability, showing a very low difference between postoperative uninjured vs injured globe protrusion, resulting clinically undetactable for most patients.
The manufacturing of these custom-made reconstructive implants appears to be easily obtained thanks to the active collaboration with Biomedics Engineers and to the improvement of the production times: we can thus obtain precision and efficiency of reconstruction.
We strongly believe that late or secondary corrections of the orbital floor should be performed using CAD-CAM technology. It allows more predictable results thanks to computer-aided virtual planning, construction of a customized implant. Moreover, the virtual planning of the reconstruction plate and its manufacture using direct metal laser-sintering and CNC technique allow the surgeon to obtain better procedural control and to reduce operative time.
References
- Blumer, M.; Rostetter, C.; Johner, J.P.; et al. : Associated ophthalmic injuries in patients with fractures of the midface. Craniomaxillofacial Trauma Reconstr 2020, 13, 168. [Google Scholar] [CrossRef]
- Seen, S.; Young, S.; Lang, S.S.; Lim, T.C.; Amrith, S.; Sundar, G. Orbital Implants in Orbital Fracture Reconstruction: A Ten-Year Series. Craniomaxillofac Trauma Reconstr. 2021, 14, 56–63. [Google Scholar] [CrossRef]
- Hawes, M.J.; Dortzbach, R.K. Surgery on orbital floor fractures. Influence of time of repair and fracture size. Ophthalmology 1983, 90, 1066–70. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.; Desmangles, P.; Rossillion, B. Le traitement pre´coce des impotences musculaires secondaires aux fractures du plancher de l’orbite. J Fr Ophtalmol 1999, 22, 645–650. [Google Scholar] [PubMed]
- Pidro, A.; Jovanovic, N.; Kadribašic, E.; Barucija, N.; Leto, N.; Kahana, A. Delayed Management of an Orbital Floor Blow-out Fracture. Beyoglu Eye J. 2021, 6, 249–253. [Google Scholar] [CrossRef]
- Avashia, Y.J.; Sastry, A.; Fan, K.L.; Mir, H.S.; Thaller, S.R. Materials Used for Reconstruction After Orbital Floor Fracture. Journal of Craniofacial Surgery 2012, 23, S49–S55. [Google Scholar] [CrossRef] [PubMed]
- Manolidis, S.; Weeks, B.H.; Kirby, M.; Scarlett, M.; Hollier, L. Classification and Surgical Management of Orbital Fractures: Experience With 111 Orbital Reconstructions. Journal of Craniofacial Surgery 2002, 13, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Ellis, E., 3rd; Tan, Y.H. Assessment of internal orbital reconstructions for pure blowout fractures: cranial bone grafts versus titanium mesh. J Oral Maxillofac Surg 2003, 61, 442Y453. [Google Scholar] [CrossRef] [PubMed]
- Lieger, O.; Richards, R.; Liu, M.; et al. Computer-assisted design and manufacture of implants in the late reconstruction of extensive orbital fractures. Arch Facial Plast Surg 2010, 12, 186Y191. [Google Scholar] [CrossRef]
- Tarsitano, A.; Battaglia, S.; Ciocca, L.; Scotti, R.; Cipriani, R.; Marchetti, C. Surgical reconstruction of maxillary defects using a computer-assisted design/computer-assisted manufacturing-produced titanium mesh supporting a free flap. J Craniomaxillofac Surg. 2016, 44, 1320–6. [Google Scholar] [CrossRef] [PubMed]
- Tarsitano, A.; Ciocca, L.; Scotti, R.; Marchetti, C. Morphological results of customized microvascular mandibular reconstruction: A comparative study. J Craniomaxillofac Surg. 2016, 44, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Schön, R.; Metzger, M.C.; Zizelmann, C.; Weyer, N.; Schmelzeisen, R. Individually preformed titanium mesh implants for a true-to-original repair of orbital fractures. Int J Oral Maxillofac Surg. 2006, 35, 990–5. [Google Scholar] [CrossRef] [PubMed]
- Chepurnyi, Y.; Chernogorskyi, D.; Kopchak, A.; Petrenko, O. Clinical efficacy of peek patient-specific implants in orbital reconstruction. J Oral Biol Craniofac Res. 2020, 10, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Baumann, A.; Sinko, K.; Dorner, G. Late Reconstruction of the Orbit With Patient-Specific Implants Using Computer-Aided Planning and Navigation. J Oral Maxillofac Surg. 2015, 73 (12 Suppl), S101–6. [Google Scholar] [CrossRef]
- Holck, D.E.; Boyd EM Ng, J., Jr.; Mauffray, R.O. Benefits of stereolithography in orbital reconstruction. Ophthalmology. 1999, 106, 1214–8. [Google Scholar] [CrossRef] [PubMed]
- Leiggener, C.; Messo, E.; Thor, A.; et al. A selective laser sintering guide for transferring a virtual plan to real time surgery in composite mandibular reconstruction with free fibula osseous flaps. Int J Oral Maxillofac Surg 2009, 38, 187–192. [Google Scholar] [CrossRef]
- Ramli, N.; Kala, S.; Samsudin, A.; Rahmat, K.; Abidin, Z.Z. Proptosis--Correlation and Agreement between Hertel Exophthalmometry and Computed Tomography. Orbit. 2015, 34, 257–62. [Google Scholar] [CrossRef] [PubMed]
- Rootman, J. Diseases of the Orbit: A Multidisciplinary Approach. 2nd ed. Philadelphia: Lippincott Williams & Wilkins. 2003. [Google Scholar]
- Zieliński, R.; Malińska, M.; Kozakiewicz, M. Classical versus custom orbital wall reconstruction: Selected factors regarding surgery and hospitalization. J Craniomaxillofac Surg. 2017, 45, 710–715. [Google Scholar] [CrossRef]
- Tarsitano, A.; Badiali, G.; Pizzigallo, A.; Marchetti, C. Orbital Reconstruction: Patient-Specific Orbital Floor Reconstruction Using a Mirroring Technique and a Customized Titanium Mesh. J Craniofac Surg. 2016, 27, 1822–1825. [Google Scholar] [CrossRef]
- Tarsitano, A.; Mazzoni, S.; Cipriani, R.; Scotti, R.; Marchetti, C.; Ciocca, L. The CAD-CAM technique for mandibular reconstruction: an 18 patients oncological case-series. J Craniomaxillofac Surg. 2014, 42, 1460–4. [Google Scholar] [CrossRef]
- Tarsitano, A.; Battaglia, S.; Ricotta, F.; Bortolani, B.; Cercenelli, L.; Marcelli, E.; Cipriani, R.; Marchetti, C. Accuracy of CAD/CAM mandibular reconstruction: A three-dimensional, fully virtual outcome evaluation method. J Craniomaxillofac Surg. 2018, 46, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Tarsitano, A.; Battaglia, S.; Crimi, S.; Ciocca, L.; Scotti, R.; Marchetti, C. Is a computer-assisted design and computer-assisted manufacturing method for mandibular reconstruction economically viable? J Craniomaxillofac Surg. 2016, 44, 795–9. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).