Submitted:
20 July 2023
Posted:
25 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study database
2.2. Endpoints and study outcomes
2.3. Statin use
2.4. Statistical analysis
3. Results
| aHR | Lower .95 | Upper .95 | p-value | |
|---|---|---|---|---|
| Statin compliance | 0.6561 | 0.4807 | 0.8954 | < 0.001 |
| Statin use | 0.6329 | 0.5260 | 0.7614 | < 0.001 |
| Sex – female | 0.4311 | 0.3332 | 0.5576 | < 0.001 |
| Age at first diagnosis of IPF | 1.0455 | 1.0357 | 1.0554 | < 0.001 |
| BMI | 1.0057 | 0.9762 | 1.0362 | 0.707 |
| Blood pressure | ||||
| Systolic | 0.9995 | 0.9922 | 1.0069 | 0.366 |
| Diastolic | 0.9995 | 0.9880 | 1.0111 | 0.934 |
| Smoking history (ex + current) | 1.5460 | 1.3911 | 1.7183 | < 0.001 |
| Alcohol frequency | 0.9982 | 0.9315 | 1.0696 | 0.959 |
| Exercise frequency | 0.9537 | 0.8886 | 1.0235 | 0.188 |
| Comorbidities | ||||
| Liver diseases | 1.2332 | 0.6567 | 2.3158 | 0.514 |
| Hypertension | 1.0478 | 0.8229 | 1.3342 | 0.705 |
| Stroke | 1.7280 | 0.7670 | 3.8930 | 0.187 |
| Heart diseases | 1.0514 | 0.6454 | 1.7129 | 0.84 |
| Diabetes | 1.0218 | 0.7330 | 1.4246 | 0.899 |
| Cancers | 0.7224 | 0.3960 | 1.3180 | 0.289 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morofuji, Y.; Nakagawa, S.; Ujifuku, K.; Fujimoto, T.; Otsuka, K.; Niwa, M.; Tsutsumi, K. Beyond Lipid-Lowering: Effects of Statins on Cardiovascular and Cerebrovascular Diseases and Cancer. Pharmaceuticals 2022, 15, 151. [Google Scholar] [CrossRef] [PubMed]
- Davignon, J. Beneficial cardiovascular pleiotropic effects of statins. Circulation 2004, 109, Iii39–Iii43. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.K.; Ridker, P.M. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov 2005, 4, 977–987. [Google Scholar] [CrossRef]
- Schaafsma, D.; McNeill, K.D.; Mutawe, M.M.; Ghavami, S.; Unruh, H.; Jacques, E.; Laviolette, M.; Chakir, J.; Halayko, A.J. Simvastatin inhibits TGFβ1-induced fibronectin in human airway fibroblasts. Respir Res 2011, 12, 113. [Google Scholar] [CrossRef]
- Schaafsma, D.; Dueck, G.; Ghavami, S.; Kroeker, A.; Mutawe, M.M.; Hauff, K.; Xu, F.Y.; McNeill, K.D.; Unruh, H.; Hatch, G.M. , et al. The mevalonate cascade as a target to suppress extracellular matrix synthesis by human airway smooth muscle. Am J Respir Cell Mol Biol 2011, 44, 394–403. [Google Scholar] [CrossRef]
- Li, F.; Liu, G.; Roudi, R.; Huang, Q.; Swierzy, M.; Ismail, M.; Zhao, S.; Rueckert, J.C. Do statins improve outcomes for patients with non-small cell lung cancer? A systematic review and meta-analysis protocol. BMJ Open 2018, 8, e022161. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Kostantinou, A.; Kougias, M.; Kazazis, C. Statins and cancer. Anticancer Agents Med Chem 2014, 14, 706–712. [Google Scholar] [CrossRef]
- Mantha, A.J.; Hanson, J.E.; Goss, G.; Lagarde, A.E.; Lorimer, I.A.; Dimitroulakos, J. Targeting the mevalonate pathway inhibits the function of the epidermal growth factor receptor. Clin Cancer Res 2005, 11, 2398–2407. [Google Scholar] [CrossRef]
- Luttman, J.H.; Hoj, J.P.; Lin, K.H.; Lin, J.; Gu, J.J.; Rouse, C.; Nichols, A.G.; MacIver, N.J.; Wood, K.C.; Pendergast, A.M. ABL allosteric inhibitors synergize with statins to enhance apoptosis of metastatic lung cancer cells. Cell Rep 2021, 37, 109880. [Google Scholar] [CrossRef]
- Fernández, L.P.; Merino, M.; Colmenarejo, G.; Moreno-Rubio, J.; Sánchez-Martínez, R.; Quijada-Freire, A.; Gómez de Cedrón, M.; Reglero, G.; Casado, E.; Sereno, M. , et al. Metabolic enzyme ACSL3 is a prognostic biomarker and correlates with anticancer effectiveness of statins in non-small cell lung cancer. Mol Oncol 2020, 14, 3135–3152. [Google Scholar] [CrossRef]
- Young, R.P.; Hopkins, R.J. The Mevalonate Pathway and Innate Immune Hyper-Responsiveness in the Pathogenesis of COPD and Lung Cancer: Potential for Chemoprevention. Curr Mol Pharmacol 2017, 10, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.H.; Kwon, M.; Jung, H.; Ko, E.; Kim, S.A.; Choi, Y.; Song, S.J.; Kim, S.; Lee, Y.; Kim, G.B. , et al. Statin-mediated inhibition of RAS prenylation activates ER stress to enhance the immunogenicity of KRAS mutant cancer. J Immunother Cancer 2021, 9. [Google Scholar]
- Otahal, A.; Aydemir, D.; Tomasich, E.; Minichsdorfer, C. Delineation of cell death mechanisms induced by synergistic effects of statins and erlotinib in non-small cell lung cancer cell (NSCLC) lines. Sci Rep 2020, 10, 959. [Google Scholar] [CrossRef]
- Takada, K.; Shimokawa, M.; Takamori, S.; Shimamatsu, S.; Hirai, F.; Tagawa, T.; Okamoto, T.; Hamatake, M.; Tsuchiya-Kawano, Y.; Otsubo, K. , et al. A propensity score-matched analysis of the impact of statin therapy on the outcomes of patients with non-small-cell lung cancer receiving anti-PD-1 monotherapy: a multicenter retrospective study. BMC Cancer 2022, 22, 503. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Filetti, M.; Taurelli Salimbeni, B.; Piras, M.; Rizzo, F.; Giusti, R.; Marchetti, P. Statins and immunotherapy: Togetherness makes strength The potential effect of statins on immunotherapy for NSCLC. Cancer Rep (Hoboken) 2021, 4, e1368. [Google Scholar] [CrossRef]
- Keit, E.; Coutu, B.; Zhen, W.; Zhang, C.; Lin, C.; Bennion, N.; Ganti, A.K.; Ernani, V.; Baine, M. Systemic inflammation is associated with inferior disease control and survival in stage III non-small cell lung cancer. Ann Transl Med 2021, 9, 227. [Google Scholar] [CrossRef]
- Vedel-Krogh, S.; Nielsen, S.F.; Nordestgaard, B.G. Statin Use Is Associated with Reduced Mortality in Patients with Interstitial Lung Disease. PLoS One 2015, 10, e0140571. [Google Scholar] [CrossRef]
- Kreuter, M.; Bonella, F.; Maher, T.M.; Costabel, U.; Spagnolo, P.; Weycker, D.; Kirchgaessler, K.U.; Kolb, M. Effect of statins on disease-related outcomes in patients with idiopathic pulmonary fibrosis. Thorax 2017, 72, 148–153. [Google Scholar] [CrossRef]
- Alexeeff, S.E.; Litonjua, A.A.; Sparrow, D.; Vokonas, P.S.; Schwartz, J. Statin use reduces decline in lung function: VA Normative Aging Study. Am J Respir Crit Care Med 2007, 176, 742–747. [Google Scholar] [CrossRef]
- Hubbard, R.; Venn, A.; Lewis, S.; Britton, J. Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am J Respir Crit Care Med 2000, 161, 5–8. [Google Scholar] [CrossRef]
- Ozawa, Y.; Suda, T.; Naito, T.; Enomoto, N.; Hashimoto, D.; Fujisawa, T.; Nakamura, Y.; Inui, N.; Nakamura, H.; Chida, K. Cumulative incidence of and predictive factors for lung cancer in IPF. Respirology 2009, 14, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Lee, S.; Song, J.W. Impact of idiopathic pulmonary fibrosis on clinical outcomes of lung cancer patients. Sci Rep 2021, 11, 8312. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Dickinson, P.; Shrimali, R.K.; Salem, A.; Agarwal, S. Is Thoracic Radiotherapy an Absolute Contraindication for Treatment of Lung Cancer Patients With Interstitial Lung Disease? A Systematic Review. Clin Oncol (R Coll Radiol) 2022, 34, e493–e504. [Google Scholar] [CrossRef]
- Tang, C.; Mistry, H.; Bayman, N.; Chan, C.; Cobben, D.; Faivre-Finn, C.; Harris, M.; Kennedy, J.; Pemberton, L.; Price, G. , et al. Outcomes of curative-intent radiotherapy in non-small cell lung cancer (NSCLC) patients with chronic obstructive pulmonary disease (COPD) and interstitial lung disease (ILD). Radiother Oncol 2021, 160, 78–81. [Google Scholar] [CrossRef]
- Sato, T.; Watanabe, A.; Kondo, H.; Kanzaki, M.; Okubo, K.; Yokoi, K.; Matsumoto, K.; Marutsuka, T.; Shinohara, H.; Teramukai, S. , et al. Long-term results and predictors of survival after surgical resection of patients with lung cancer and interstitial lung diseases. J Thorac Cardiovasc Surg 2015, 149, 64–69, 70.e61-62. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Azuma, K.; Sasada, T.; Okamoto, M.; Hattori, S.; Imamura, Y.; Yamada, K.; Tajiri, M.; Yoshida, T.; Zaizen, Y. , et al. Chemotherapy for non-small cell lung cancer complicated by idiopathic interstitial pneumonia. Oncol Lett 2012, 4, 477–482. [Google Scholar] [CrossRef]
- Yoo, T.K.; Park, S.H.; Do Han, K.; Chae, B.J. Cardiovascular events and mortality in a population-based cohort initially diagnosed with ductal carcinoma in situ. BMC Cancer 2021, 21, 735. [Google Scholar] [CrossRef]
- Jang, S.Y.; Cha, Y.; Yoo, J.I.; Yu, Y.T.; Kim, J.T.; Park, C.H.; Choy, W. Effect of Pneumonia on All-cause Mortality after Elderly Hip Fracture: a Korean Nationwide Cohort Study. J Korean Med Sci 2020, 35, e9. [Google Scholar] [CrossRef]
- Kim, H.J.; Hann, H.J.; Hong, S.N.; Kim, K.H.; Ahn, I.M.; Song, J.Y.; Lee, S.H.; Ahn, H.S. Incidence and natural course of inflammatory bowel disease in Korea, 2006-2012: a nationwide population-based study. Inflamm Bowel Dis 2015, 21, 623–630. [Google Scholar] [CrossRef]
- Cardwell, C.R.; Mc Menamin, Ú.; Hughes, C.M.; Murray, L.J. Statin use and survival from lung cancer: a population-based cohort study. Cancer Epidemiol Biomarkers Prev 2015, 24, 833–841. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Zhang, R.; Xia, Y.; Shao, Z.; Mei, Z. Effects of statin exposure and lung cancer survival: A meta-analysis of observational studies. Pharmacol Res 2019, 141, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.A.; Chang, C.C.; Galvin, C.J.; Wang, Y.C.; An, S.Y.; Huang, C.W.; Wang, Y.H.; Hsu, M.H.; Li, Y.J.; Yang, H.C. Statins use and its impact in EGFR-TKIs resistance to prolong the survival of lung cancer patients: A Cancer registry cohort study in Taiwan. Cancer Sci 2020, 111, 2965–2973. [Google Scholar] [CrossRef] [PubMed]
- Fiala, O.; Pesek, M.; Finek, J.; Minarik, M.; Benesova, L.; Bortlicek, Z.; Topolcan, O. Statins augment efficacy of EGFR-TKIs in patients with advanced-stage non-small cell lung cancer harbouring KRAS mutation. Tumour Biol 2015, 36, 5801–5805. [Google Scholar] [CrossRef]
- Omori, M.; Okuma, Y.; Hakozaki, T.; Hosomi, Y. Statins improve survival in patients previously treated with nivolumab for advanced non-small cell lung cancer: An observational study. Mol Clin Oncol 2019, 10, 137–143. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, K.H.; Lee, G.K.; Lee, S.H.; Lim, K.Y.; Joo, J.; Go, Y.J.; Lee, J.S.; Han, J.Y. Randomized Phase II Study of Afatinib Plus Simvastatin Versus Afatinib Alone in Previously Treated Patients with Advanced Nonadenocarcinomatous Non-small Cell Lung Cancer. Cancer Res Treat 2017, 49, 1001–1011. [Google Scholar] [CrossRef]
- Yoo, H.; Jeong, B.H.; Chung, M.J.; Lee, K.S.; Kwon, O.J.; Chung, M.P. Risk factors and clinical characteristics of lung cancer in idiopathic pulmonary fibrosis: a retrospective cohort study. BMC pulmonary medicine 2019, 19, 149. [Google Scholar] [CrossRef] [PubMed]
- Abu Qubo, A.; Numan, J.; Snijder, J.; Padilla, M.; Austin, J.H.M.; Capaccione, K.M.; Pernia, M.; Bustamante, J.; O'Connor, T.; Salvatore, M.M. Idiopathic pulmonary fibrosis and lung cancer: future directions and challenges. Breathe (Sheff) 2022, 18, 220147. [Google Scholar] [CrossRef]
- Farjah, F.; Wood, D.E.; Mulligan, M.S.; Krishnadasan, B.; Heagerty, P.J.; Symons, R.G.; Flum, D.R. Safety and efficacy of video-assisted versus conventional lung resection for lung cancer. J Thorac Cardiovasc Surg 2009, 137, 1415–1421. [Google Scholar] [CrossRef]
- Yano, M.; Sasaki, H.; Moriyama, S.; Hikosaka, Y.; Yokota, K.; Kobayashi, S.; Hara, M.; Fujii, Y. Post-operative acute exacerbation of pulmonary fibrosis in lung cancer patients undergoing lung resection. Interact Cardiovasc Thorac Surg 2012, 14, 146–150. [Google Scholar] [CrossRef]
- Haasbeek, C.J.A.; Palma, D.; Visser, O.; Lagerwaard, F.J.; Slotman, B.; Senan, S. Early-stage lung cancer in elderly patients: a population-based study of changes in treatment patterns and survival in the Netherlands. Ann Oncol 2012, 23, 2743–2747. [Google Scholar] [CrossRef]
- Kim, H.; Pyo, H.; Noh, J.M.; Lee, W.; Park, B.; Park, H.Y.; Yoo, H. Preliminary result of definitive radiotherapy in patients with non-small cell lung cancer who have underlying idiopathic pulmonary fibrosis: comparison between X-ray and proton therapy. Radiat Oncol 2019, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Conte, P.; Ascierto, P.A.; Patelli, G.; Danesi, R.; Vanzulli, A.; Sandomenico, F.; Tarsia, P.; Cattelan, A.; Comes, A.; De Laurentiis, M. , et al. Drug-induced interstitial lung disease during cancer therapies: expert opinion on diagnosis and treatment. ESMO Open 2022, 7, 100404. [Google Scholar] [CrossRef] [PubMed]
- Minegishi, Y.; Gemma, A.; Homma, S.; Kishi, K.; Azuma, A.; Ogura, T.; Hamada, N.; Taniguchi, H.; Hattori, N.; Nishioka, Y. , et al. Acute exacerbation of idiopathic interstitial pneumonias related to chemotherapy for lung cancer: nationwide surveillance in Japan. ERJ Open Res, 2020, 6.
- Miura, Y.; Saito, T.; Tanaka, T.; Takoi, H.; Yatagai, Y.; Inomata, M.; Nei, T.; Saito, Y.; Gemma, A.; Azuma, A. Reduced incidence of lung cancer in patients with idiopathic pulmonary fibrosis treated with pirfenidone. Respir Investig 2018, 56, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, M.; Costabel, U.; Richeldi, L.; Cottin, V.; Wijsenbeek, M.; Bonella, F.; Bendstrup, E.; Maher, T.M.; Wachtlin, D.; Stowasser, S. , et al. Statin Therapy and Outcomes in Trials of Nintedanib in Idiopathic Pulmonary Fibrosis. Respiration 2018, 95, 317–326. [Google Scholar] [CrossRef]
- Yao, H.W.; Mao, L.G.; Zhu, J.P. Protective effects of pravastatin in murine lipopolysaccharide-induced acute lung injury. Clin Exp Pharmacol Physiol 2006, 33, 793–797. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, D.S.; Kim, E.K.; Choe, K.H.; Oh, Y.M.; Shim, T.S.; Kim, S.E.; Lee, Y.S.; Lee, S.D. Simvastatin inhibits cigarette smoking-induced emphysema and pulmonary hypertension in rat lungs. Am J Respir Crit Care Med 2005, 172, 987–993. [Google Scholar] [CrossRef]
- Johnson, B.A.; Iacono, A.T.; Zeevi, A.; McCurry, K.R.; Duncan, S.R. Statin use is associated with improved function and survival of lung allografts. Am J Respir Crit Care Med 2003, 167, 1271–1278. [Google Scholar] [CrossRef]
- Gibbs, J.B.; Oliff, A.; Kohl, N.E. Farnesyltransferase inhibitors: Ras research yields a potential cancer therapeutic. Cell 1994, 77, 175–178. [Google Scholar] [CrossRef]
- Zhang, X.; Teng, Y.; Yang, F.; Wang, M.; Hong, X.; Ye, L.G.; Gao, Y.N.; Chen, G.Y. MCM2 is a therapeutic target of lovastatin in human non-small cell lung carcinomas. Oncol Rep 2015, 33, 2599–2605. [Google Scholar] [CrossRef]
- Gomes, M.; Teixeira, A.L.; Coelho, A.; Araújo, A.; Medeiros, R. The role of inflammation in lung cancer. Adv Exp Med Biol 2014, 816, 1–23. [Google Scholar]
- Wang, J.; Li, C.; Tao, H.; Cheng, Y.; Han, L.; Li, X.; Hu, Y. Statin use and risk of lung cancer: a meta-analysis of observational studies and randomized controlled trials. PLoS One 2013, 8, e77950. [Google Scholar] [CrossRef]
- Heukels, P.; Moor, C.C.; von der Thüsen, J.H.; Wijsenbeek, M.S.; Kool, M. Inflammation and immunity in IPF pathogenesis and treatment. Respir Med 2019, 147, 79–91. [Google Scholar] [CrossRef]
- Eberlein, M.; Heusinger-Ribeiro, J.; Goppelt-Struebe, M. Rho-dependent inhibition of the induction of connective tissue growth factor (CTGF) by HMG CoA reductase inhibitors (statins). Br J Pharmacol 2001, 133, 1172–1180. [Google Scholar] [CrossRef]
- McGillicuddy, F.C.; O'Toole, D.; Hickey, J.A.; Gallagher, W.M.; Dawson, K.A.; Keenan, A.K. TGF-beta1-induced thrombospondin-1 expression through the p38 MAPK pathway is abolished by fluvastatin in human coronary artery smooth muscle cells. Vascul Pharmacol 2006, 44, 469–475. [Google Scholar] [CrossRef]
- Kim, J.W.; Barrett, K.; Loke, Y.; Wilson, A.M. The effect of statin therapy on disease-related outcomes in idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Respir Med Res 2021, 80, 100792. [Google Scholar] [CrossRef]
- Vainshelboim, B.; Myers, J.; Oliveira, J.; Izhakian, S.; Unterman, A.; Kramer, M.R. Physiological Responses and Prognostic Value of Common Exercise Testing Modalities in Idiopathic Pulmonary Fibrosis. J Cardiopulm Rehabil Prev 2019, 39, 193–198. [Google Scholar] [CrossRef]
- Badenes-Bonet, D.; Rodó-Pin, A.; Castillo-Villegas, D.; Vicens-Zygmunt, V.; Bermudo, G.; Hernández-González, F.; Portillo, K.; Martínez-Llorens, J.; Chalela, R.; Caguana, O. , et al. Predictors and changes of physical activity in idiopathic pulmonary fibrosis. BMC pulmonary medicine 2022, 22, 340. [Google Scholar] [CrossRef]
- Dowman, L.; Hill, C.J.; May, A.; Holland, A.E. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst Rev 2021, 2, Cd006322. [Google Scholar] [CrossRef]
- Nolan, C.M.; Polgar, O.; Schofield, S.J.; Patel, S.; Barker, R.E.; Walsh, J.A.; Ingram, K.A.; George, P.M.; Molyneaux, P.L.; Maher, T.M. , et al. Pulmonary Rehabilitation in Idiopathic Pulmonary Fibrosis and COPD: A Propensity-Matched Real-World Study. Chest 2022, 161, 728–737. [Google Scholar] [CrossRef]
- Vainshelboim, B.; Oliveira, J.; Izhakian, S.; Unterman, A.; Kramer, M.R. Lifestyle Behaviors and Clinical Outcomes in Idiopathic Pulmonary Fibrosis. Respiration 2018, 95, 27–34. [Google Scholar] [CrossRef]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.F.; Flaherty, K.R.; Lasky, J.A. , et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011, 183, 788–824. [Google Scholar] [CrossRef]
- Dempsey, T.M.; Sangaralingham, L.R.; Yao, X.; Sanghavi, D.; Shah, N.D.; Limper, A.H. Clinical Effectiveness of Antifibrotic Medications for Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 2019, 200, 168–174. [Google Scholar] [CrossRef]
- Ali, M.O. Pulmonary complications in diabetes mellitus. Mymensingh Med J 2014, 23, 603–605. [Google Scholar]
- Ehrlich, S.F.; Quesenberry, C.P., Jr.; Van Den Eeden, S.K.; Shan, J.; Ferrara, A. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care 2010, 33, 55–60. [Google Scholar] [CrossRef]
- Enomoto, T.; Usuki, J.; Azuma, A.; Nakagawa, T.; Kudoh, S. Diabetes mellitus may increase risk for idiopathic pulmonary fibrosis. Chest 2003, 123, 2007–2011. [Google Scholar] [CrossRef]
- Hyldgaard, C.; Hilberg, O.; Bendstrup, E. How does comorbidity influence survival in idiopathic pulmonary fibrosis? Respir Med 2014, 108, 647–653. [Google Scholar] [CrossRef]
- American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S111–s124. [Google Scholar] [CrossRef]
- Rangarajan, S.; Bone, N.B.; Zmijewska, A.A.; Jiang, S.; Park, D.W.; Bernard, K.; Locy, M.L.; Ravi, S.; Deshane, J.; Mannon, R.B. , et al. Metformin reverses established lung fibrosis in a bleomycin model. Nat Med 2018, 24, 1121–1127. [Google Scholar] [CrossRef]
- Gou, S.; Zhu, T.; Wang, W.; Xiao, M.; Wang, X.C.; Chen, Z.H. Glucagon like peptide-1 attenuates bleomycin-induced pulmonary fibrosis, involving the inactivation of NF-κB in mice. Int Immunopharmacol 2014, 22, 498–504. [Google Scholar] [CrossRef]
| Characteristics | Statin use | p-value | |
|---|---|---|---|
| No (n = 5810) | Yes (n = 3372) | ||
| Age at diagnosis of IPF | 67.2 ± 11.2 | 64.0 ± 10.2 | < 0.001 |
| Sex | < 0.001 | ||
| Male | 3896 (67.1%) | 2002 (59.4%) | |
| Female | 1914 (32.9%) | 1370 (40.6%) | |
| BMI | 23.0 ± 3.2 | 24.0 ± 3.1 | < 0.001 |
| Total cholesterol | 184.2 ± 34.8 | 199.7 ± 42.6 | < 0.0001 |
| Blood pressure | |||
| Systolic | 125.2 ± 17.1 | 126.6 ± 16.8 | < 0.0001 |
| Diastolic | 76.3 ± 10.6 | 77.3 ± 10.8 | < 0.0001 |
| Smoking history | 0.076 | ||
| Never | 3409 (61.1%) | 2016 (62.3%) | |
| Ex-smoker | 1100 (19.7%) | 574 (17.7%) | |
| Current smoker | 1073 (19.2%) | 644 (19.9%) | |
| Smoking amount | < 0.001 | ||
| Less than a half pack | 258 (38.1%) | 95 (25.7%) | |
| Half pack to less than one pack | 312 (46.1%) | 180 (48.8%) | |
| One pack to less than two packs | 97 (14.3%) | 86 (23.3%) | |
| More than two packs | 10 (1.5%) | 8 (2.2%) | |
| Smoking duration | 0.217 | ||
| Less than five years | 43 (4.1%) | 16 (2.9%) | |
| Five to nine years | 41 (3.9%) | 23 (4.2%) | |
| 10 to 19 years | 146 (13.9%) | 66 (12.1%) | |
| 20 to 29 years | 187 (17.8%) | 120 (22.1%) | |
| 30 years or more | 634 (60.3%) | 319 (58.6%) | |
| Drinking frequency | 0.004 | ||
| None | 2363 (70.0%) | 1285 (69.3%) | |
| Less than once per month | 311 (9.2%) | 155 (8.4%) | |
| Once per month | 347 (10.3%) | 214 (11.5%) | |
| Once per week | 147 (4.4%) | 113 (6.1%) | |
| Daily | 210 (6.2%) | 86 (4.6%) | |
| Drinking amount at a time | 0.001 | ||
| One to two drinks | 499 (50.1%) | 241 (43%) | |
| Three to four drinks | 369 (37.0%) | 208 (37.1%) | |
| Five to six drinks | 72 (7.2%) | 71 (12.7%) | |
| Seven to nine drinks | 57 (5.7%) | 41 (7.3%) | |
| Physical activity frequency | < 0.0001 | ||
| None | 2143 (63.9%) | 1060 (57.5%) | |
| One to two days per week | 582 (17.3%) | 391 (21.2%) | |
| Three to four days per week | 274 (8.2%) | 171 (9.3%) | |
| Five to six days per week | 72 (2.1%) | 41 (2.2%) | |
| Seven days per week | 284 (8.5%) | 179 (9.7%) | |
| Physical activity intensity (per week) |
|||
| Vigorous | 0.8 ± 1.7 | 0.9 ± 1.8 | 0.022 |
| Moderate | 1.0 ± 1.9 | 1.2 ± 2.0 | 0.036 |
| Walk | 2.5 ± 2.6 | 2.6 ± 2.6 | 0.156 |
| Comorbidities | |||
| Liver disease | 65 (1.9%) | 45 (2.4%) | 0.258 |
| Hypertension | 508 (14.7%) | 353 (18.7%) | < 0.0001 |
| Cerebrovascular diseases | 67 (1.9%) | 19 (1.0%) | 0.013 |
| Heart disease | 103 (3.0%) | 79 (4.2%) | 0.026 |
| Diabetes | 241 (7.0%) | 158 (8.4%) | 0.073 |
| Other cancers | 58 (1.7%) | 19 (1.0%) | 0.064 |
| Statin non-user (n=5810) | Stain user (n=3372) | p-value | |
|---|---|---|---|
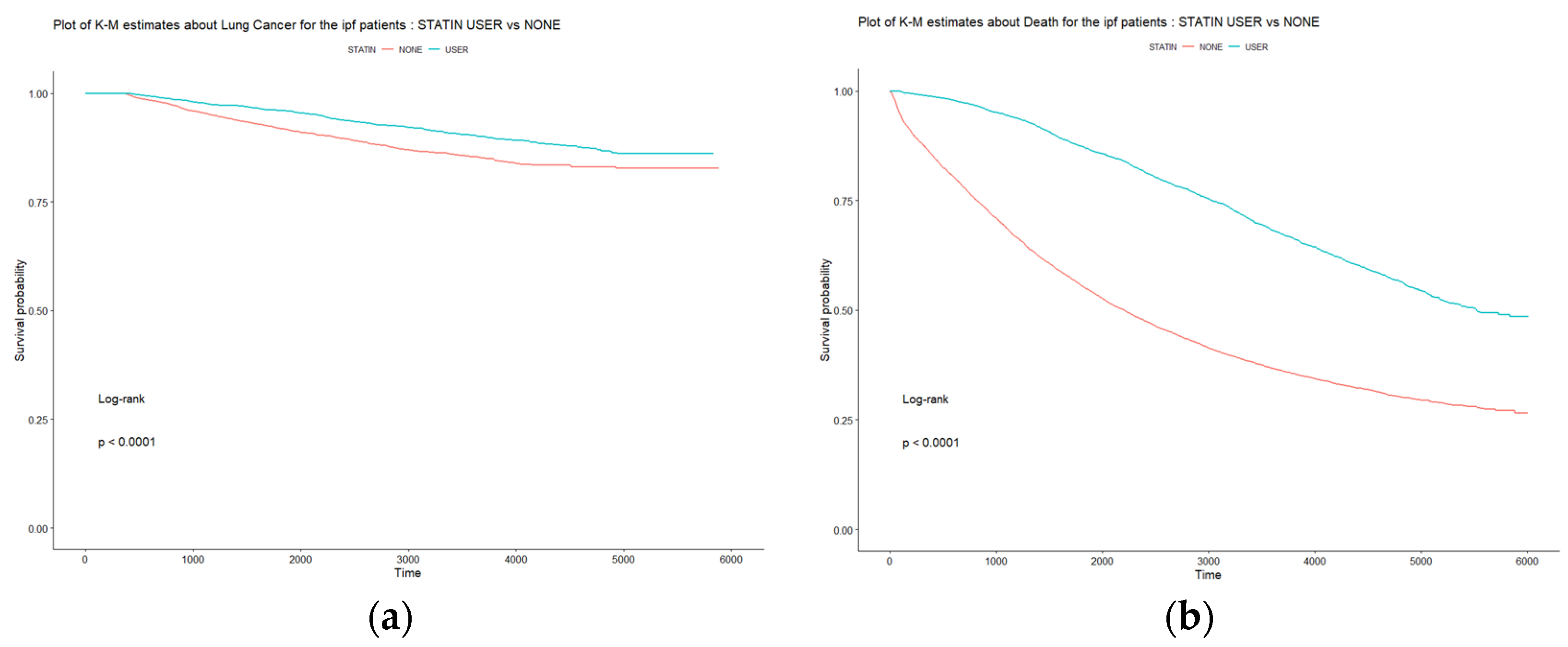
| Lung cancer development in IPF patients | |||
| (n = 850) | 534 (9.2%) | 316 (9.4%) | 0.803 |
| Duration (days) | |||
| Lung cancer development | 2194.6 ± 1601.4 | 3361.0 ± 1331.2 | < 0.0001 |
| IPF diagnosis to death | 2413.9 ±1778.7 | 3741.8 ± 1443.1 | < 0.0001 |
| No. of deaths | 3887 (66.9%) | 1404 (41.6%) | < 0.0001 |
| aHR | Lower .95 | Upper .95 | p-value | |
|---|---|---|---|---|
| Statin use | 0.4254 | 0.3919 | 0.4617 | < 0.001 |
| Sex – female | 0.6668 | 0.6121 | 0.7264 | < 0.001 |
| Ages at first diagnosis of IPF | 1.0714 | 1.0673 | 1.0756 | < 0.001 |
| BMI | 1.0020 | 0.9902 | 1.0139 | 0.7414 |
| Total cholesterol | 1.0005 | 0.9996 | 1.0014 | 0.2637 |
| Blood pressure | ||||
| Systolic | 0.9996 | 0.9968 | 1.0024 | 0.7931 |
| Diastolic | 0.9999 | 0.9954 | 1.0044 | 0.959 |
| Smoking history (ex + current) | 1.0580 | 1.0096 | 1.1087 | 0.0182 |
| Drinking experiences | 0.9884 | 0.9586 | 1.0190 | 0.453 |
| Exercise frequency | 0.9291 | 0.9028 | 0.9563 | < 0.001 |
| Comorbidities | ||||
| Liver diseases | 0.9104 | 0.7143 | 1.1604 | 0.4482 |
| Hypertension | 1.0847 | 0.9868 | 1.1924 | 0.0921 |
| Stroke | 0.9767 | 0.7646 | 1.2477 | 0.8504 |
| Heart diseases | 0.9949 | 0.8323 | 1.1891 | 0.9548 |
| Diabetes | 0.7783 | 0.6885 | 0.8798 | < 0.001 |
| Cancers | 1.0853 | 0.8276 | 1.4233 | 0.5539 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




