1. Introduction
The microbiome is critical to the function of it’s host and changes fall broadly into three categories. The first are negative changes such as those seen in infection, an excellent example of this is
clostridium difficile (C. Diff) infection where the elimination of the normal microbiome allows for colonization of a pathological entity[
1]. The second outcome is that there is no discernible change, this occurs on a regular basis as there are shifts in the microbiome due to changes in diet geographic location and time[
2]. The last type of change is one of improved function of the organism and the majority of evidence for this comes from experimental replacement of microbiome in laboratory animals, however we know that this holds true in humans as well going back to the example of C. Diff infection[1, 3-7]. If a healthy microbiome is transplanted into the patient, then normal gastrointestinal (GI) and organismal health is restored. What we currently do not know is which of these categories changes to the microbiome induced by spaceflight fall into.
Previous reports on the spaceflight microbiome led to the conclusion that spaceflight, as an environment, has an impact on the composition of not only the gastrointestinal microbiome but that of the skin, nasal cavities, etc. in humans[8-11]. Rodents have been shown to be effected in a similar manner (gastrointestinal) to varying degrees across studies and missions[
12]. However, there have been no studies to examine if these changes are pathological in nature or not.
Typically, the microbiome interacts with a host through the metabolome which is the sum of the byproducts produced by all the constituents of the microbiome and has effects on the immediate tissue surrounding them such as the intestinal epithelial or in some cases can have effects on distal tissues such as the lymph nodes, brain, and/or cardiovascular system[5, 13-17]. Certain amino acids such as histidine, are only produced by the bacterial components of the microbiome and serve as precursors to factors made by the host. One such factor is histamine which has been found to be critical for regulating the activity and survival of subsets of gut resident macrophages and T-cells[13, 18-21].
Interestingly, histamine not only regulates immune function in the gut but also changes the function of the lymphatic vasculature in the gut[22, 23]. The lymphatic system serves as transport and spatial organization system for immune cells as well as a transport system for nutrients (lipids), extracellular fluids, signaling molecules (cytokines, chemokines, etc. both free and packaged), antigens, and cellular debris[24-29]. The mesenteric lymph nodes, through which all lymph collected in the gut passes, are the sites for the coordination of the adaptive immune response of the gut[27, 30, 31]. This immune coordination is extremely complex in the context of the gastrointestinal system where the adaptive immune response must tolerate the normal Microbiome constituents well responding to pathogenic bacteria, fungi, and parasites.
Also of interest are short chain fatty acids, these are produced exclusively by the host bacterial constituent of the microbiome. Short chain fatty acids impact immune function, the gut brain axis, and more[4, 32]. Examples of this are butyrate’s (butyric acid) action to reduce inflammation in models of inflammatory bowel disease (IBD) and the tie between isovaleric acid levels and clinical depression[14, 16, 33]. The variety of targets, endogenous production, and the balance of abundance of these factors makes them an interesting target for interventions across multiple issues. Short chain fatty acids are classically described as being absorbed by the portal circulation entering the blood in the colon however the circulation of these factors will eventually lead to the deposition and action in beds of immunological function such as the lymph nodes, Peyer’s patches and even as far as the thymus[34-36].
We hypothesize that changes to the spaceflight GI microbiome leads to changes in physiology and immunology of the GI tract. To assess the impact of the microbiome alone in the absence of other confounding factors of spaceflight (radiation, microgravity, etc.) we have chosen to perform fecal microbiota transfer from spaceflight and ground (habitat control) animals into healthy donor animals.
2. Results
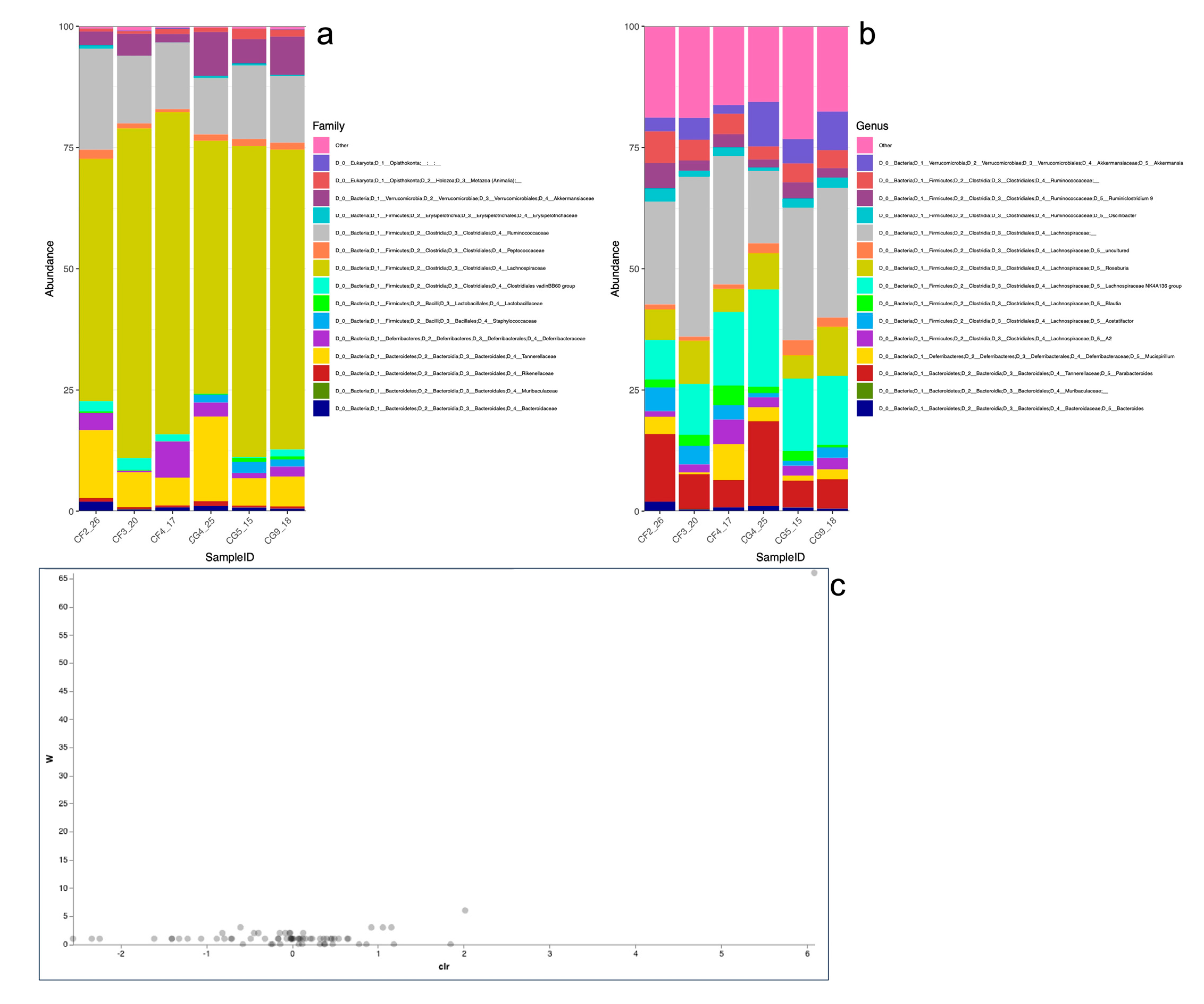
16s sequencing of the cecal contents from RR9 revealed that there were no major detected changes in either family or genus (
Figure 1a,b) and ANCOM analysis revealed that there was only a single point of significant variation. The staphylococcaceae family (genus staphylococcus) was significantly elevated in flight animals over habitat controls (
Figure 1c).
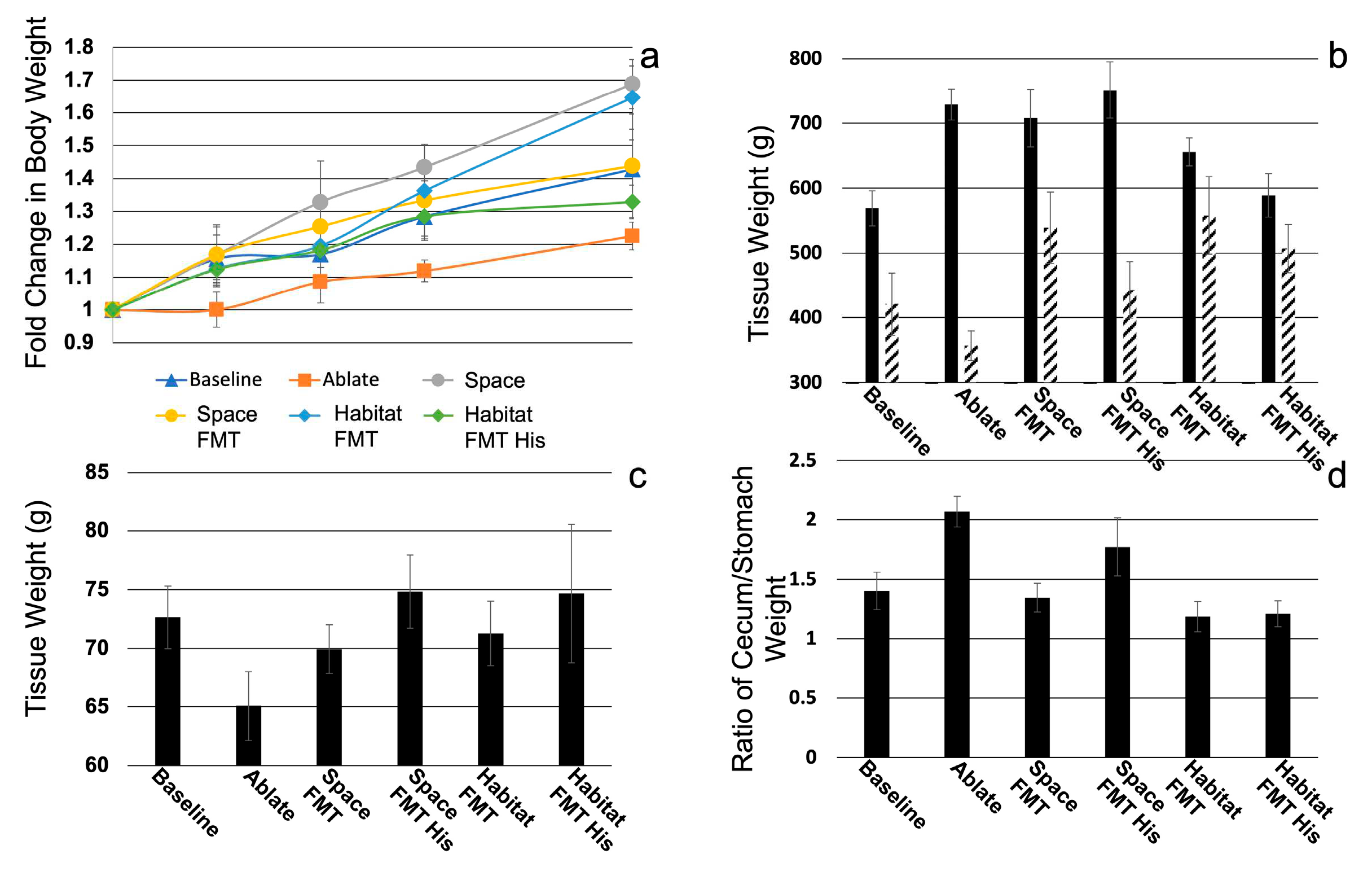
There was no significant difference in body weight between baseline animals in animals that received FMT, the ablation controls however lagged in weight gain compared to baseline animals (
Figure 2a). There was evidence of cecal retention in space FMT and space FMT + histidine animals though not significantly elevated over habitat controls (
Figure 2b solid bars) except in the case of space FMT + histidine cecal/stomach ratio (
Figure 2d). Spleen weights were not significantly different between space and habitat FMT groups but histidine treatment did show trends for elevating spleen weight in both groups compared to non-treated groups (
Figure 2c).
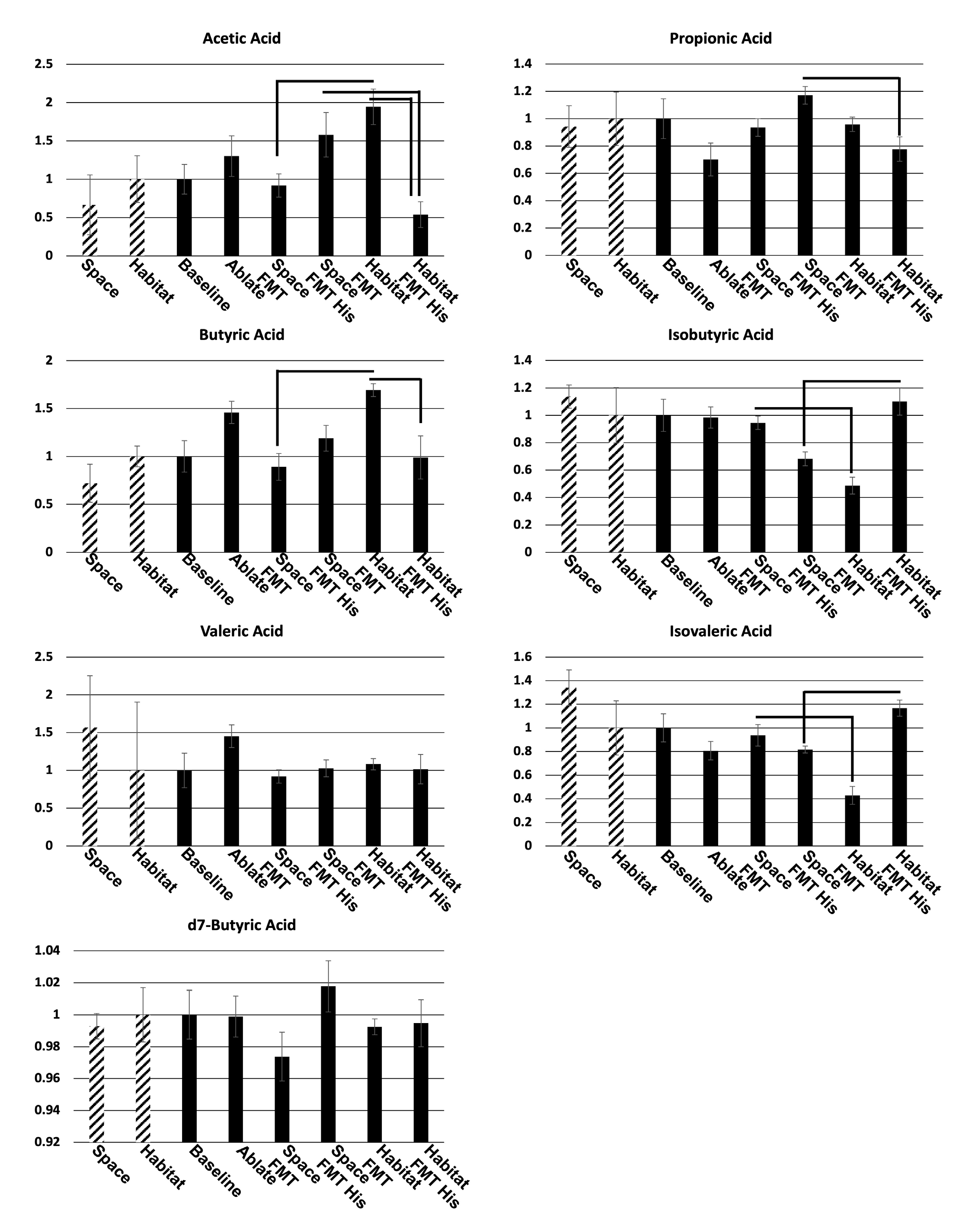
Metabolomic (lipidomic) short chain fatty acid analysis (
Figure 3) revealed that there were several compounds that were significantly different between spaceflight and habitat FMT samples and the spike control d7-butyric acid provided consistent (not significantly different) readings in all samples. There were significant decreases in both butyric and acetic acids in spaceflight FMT compared to habitat FMT while isobutyric and isovaleric acids were both significantly elevated. Almost universally histidine had opposite effects on the production of SCFAs in flight vs. habitat FMT where it trended to elevate butyric and acetic acids in flight FMT and significantly decrease them in habitat FMT, conversely histidine decreases in isobutyric (significantly) and isovaleric acids (trend) in flight FMT and significantly elevated them in habitat FMT animals. Furthermore, histidine significantly reversed trends for SCFA abundance in acetic acid, isobutyric acid, and isovaleric acid. One last note was that in many cases FMT mimicked the pattern of SCFA level changes from the donors (acetic acid, propionic acid, butyric acid, isobutyric acid, and isovaleric acid) but not the magnitude of those differences which trended to be larger in FMT.
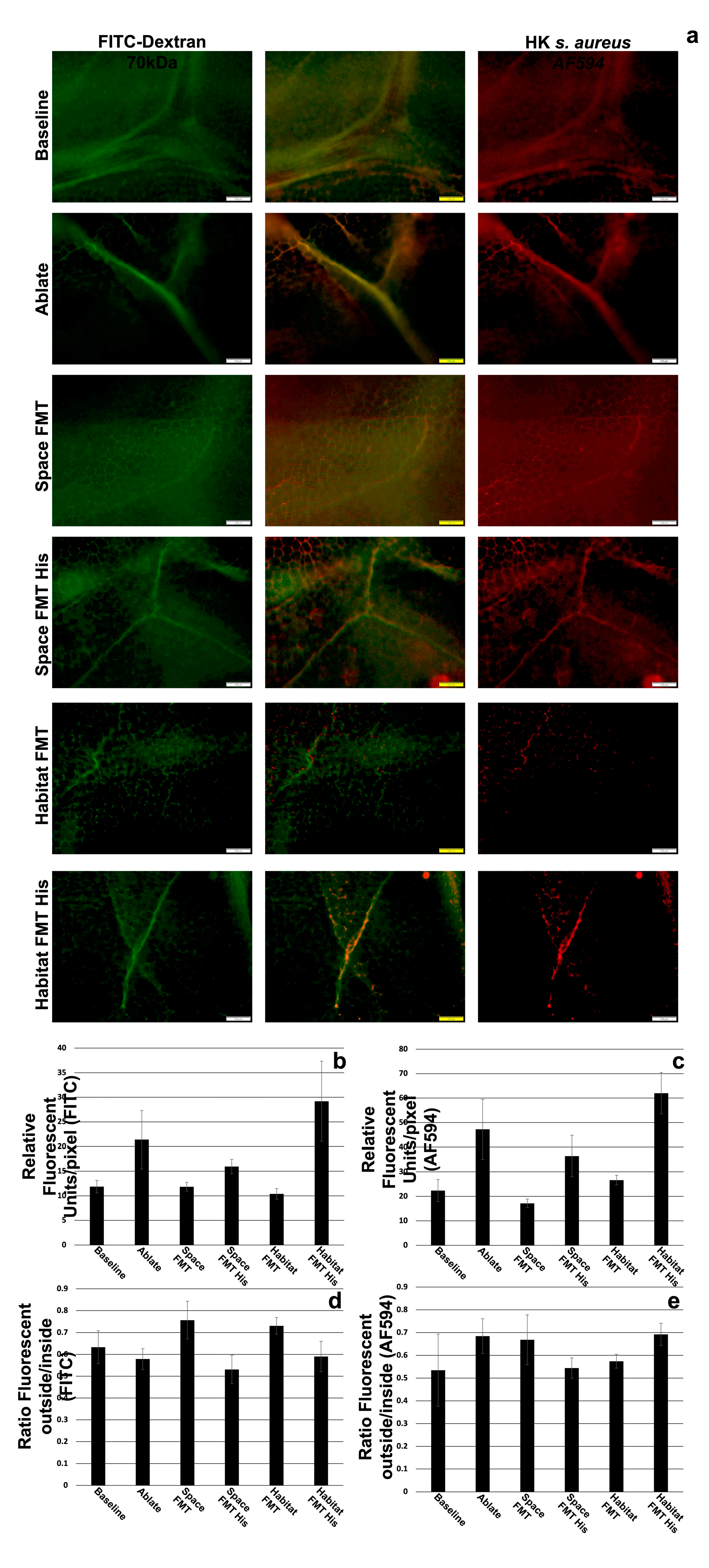
The origin of the microbiome transplant did not appear to have significant impacts on the transport or leakage of small (70kDa dextran) or large (heat killed
s. aureus) between baseline, space FMT, and habitat FMT (
Figure 4b,c). The ablation and non-restoration of the microbiome did significantly increase the transport of both small and large tracers into the lymphatic vessels but did not significantly alter the leakage of those molecules into the surrounding tissue (
Figure 4d,e). The addition of histidine in both spaceflight and habitat FMT significantly elevated the levels of both tracers in the lymphatic vessels (transport) while in the case of the smaller tracer decreasing the leakage into the tissue in spaceflight FMT significantly and trending to do so in the habitat FMT as well while having no effect on the larger tracer.
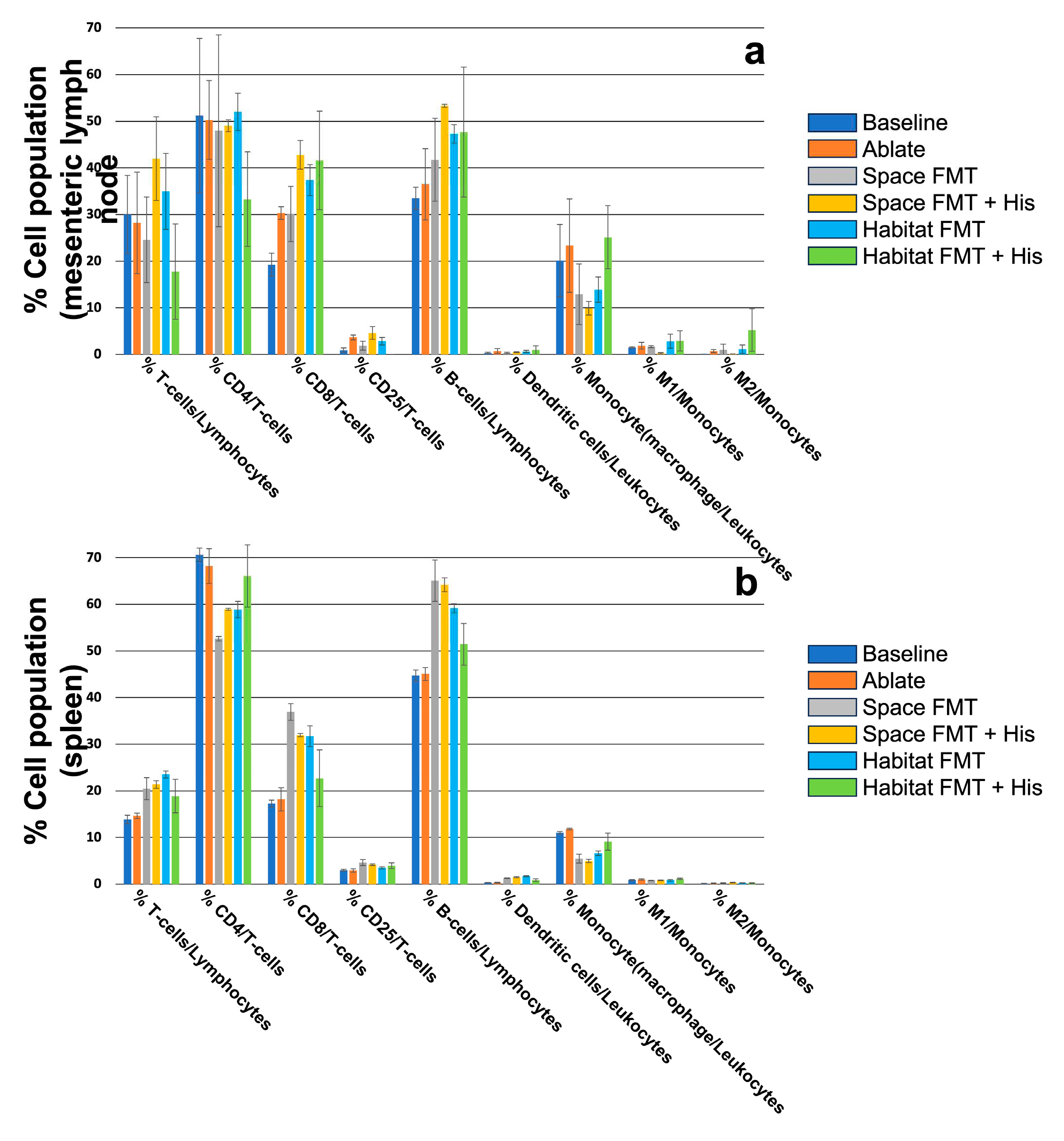
The immune composition of the local (mesenteric lymph nodes,
Figure 5a) and more global (spleen,
Figure 5b) populations showed a mixed response to FMT from either spaceflight or habitat sources. The only significant differences between the groups that could be detected were in the spleen population of CD4
+ T-cells. Likewise, treatment with histidine had little effect on either FMT source again significantly elevating CD4+ T-cells in spaceflight FMT spleens while simultaneously decreasing CD8+ T-cells in the same compartment. The only significant effect in the mesentery node was the reduction of M1 polarized macrophages in spaceflight FMT treated with histidine and there were no significant effects of histidine on habitat FMT in either compartment.
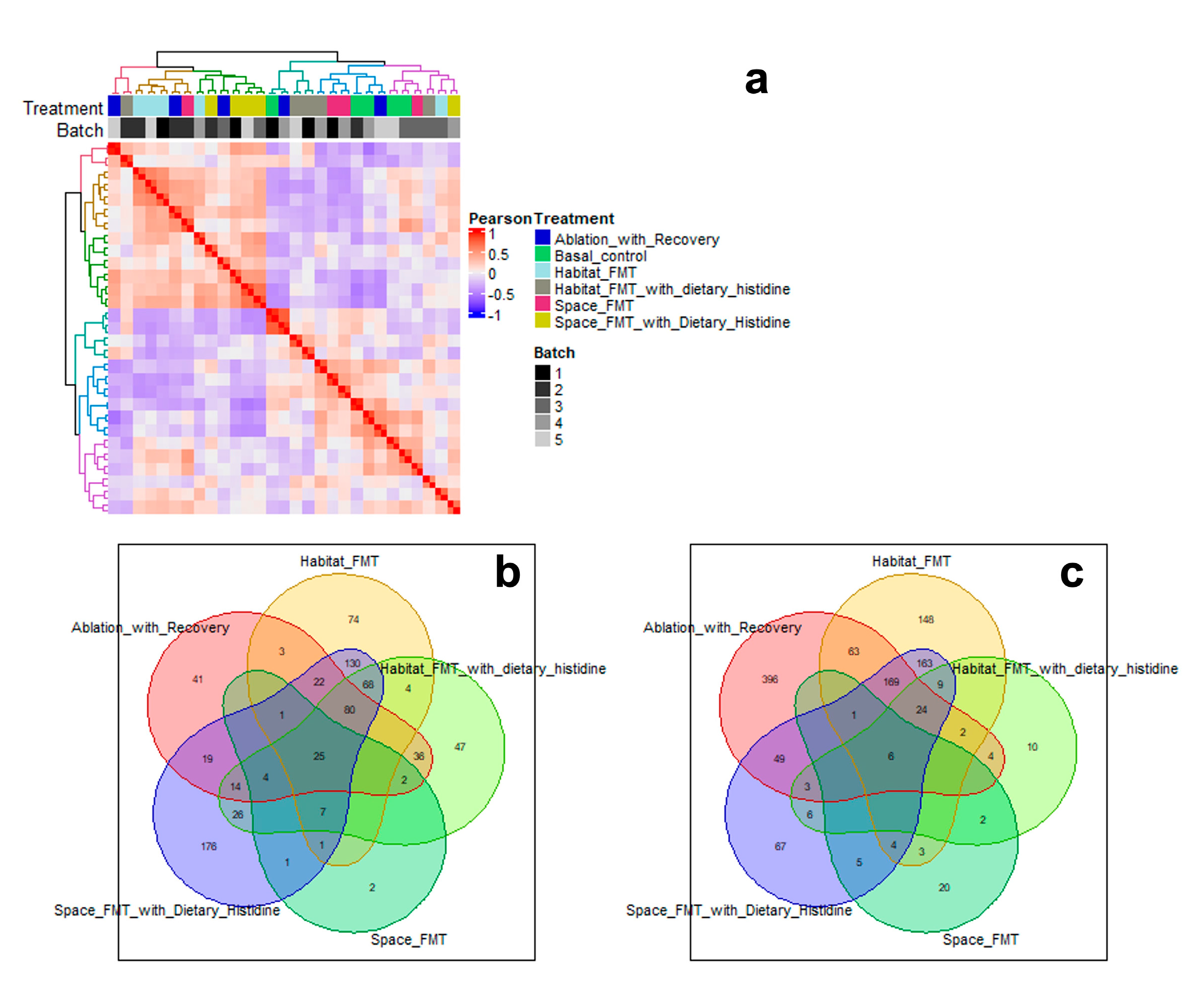
Transcriptomic analysis revealed all treatment groups had numerous genes that significantly diverged from baseline animals (supplementary
Figure 1). Habitat FMT and spaceflight FMT + histidine shared the most up (
Figure 6b) and down (
Figure 6c) regulated genes and clustered closest together in hierarchical analysis (
Figure 6a). There were also 25 genes upregulated and 6 downregulated shared in all treated groups.
3. Discussion
Here we have shown that there are impacts of the spaceflight microbiome in the absence of the environment of spaceflight. Interestingly we found no significant difference in the donor microbiomes with the exception one family of staphylococcaceae, this does not fit with previous studies that have shown numerous times that there are significant changes in the microbiome of flight animals. Despite a lack of compositional changes in the this there were in fact functional differences between spaceflight and habitat microbiomes.
The most obvious change that occurred was the change in the SCFA composition between spaceflight and habitat FMT. While FMT did not precisely match the original space and habitat lipid profiles the were close in direction and magnitude. This suggested the functional aspects of the microbiomes transferred with the FMT procedure. The relative lack of acetic acid in flight FMT matched the direction if not the magnitude of abundance in actual flight samples. It is consistent with findings in stressed and “depressed” rodent models and may be indicative of stress the flight animals undergo and is transferable with the microbiome[
37]. Acetic acid is converted to acetate in tissues and is an important regulator of immune function (an example is the regulation of TNF secretion by monocytes after LPS stimulation) and neurological function (regulating levels of glutamate, glutamine, and GABA) with other effects on general tissue metabolism[38, 39]. Likewise, reduction of butyrate in spaceflight FMT animals recapitulated what is seen in actual flight samples. Butyrate is a major player in the regulation of monocyte derived cell function in the gut wall as well as regulating T-cell homeostatic in the same compartment and reduction of this SCFA would suggest that tissues were primed for an inflammatory state[14, 35]. Histidine treatment elevated levels of both acetic and butyric acid in spaceflight FMT and curiously reduced the same in habitat FMT. Both isovaleric and isobutyric acids were elevated in spaceflight FMT and reduced by histidine treatment. The levels previous 2 SCFAs have a strong positive correlation with stress and depression this suggests again that the space flight microbiome and metabolome is likely experiencing stress that can persist long after the stressor is removed[33, 37]. This may also be a potential target for the treatment of isolation stress given the effect of histidine supplementation in this model. These groups started as compositionally similar, but the function diverged as well as the response to interventions which was curious.
When we examined lymph transport of large and small molecules there was no discernable difference between spaceflight and habitat FMT animals, which in turn were the same as baseline animals. Ablation without immediate restoration (ablation control) showed greater levels of lymph uptake and transport of the tracers which may be due to the relation of the microbiome with intestinal epithelial integrity[17, 40, 41]. Other studies have shown that simply removing the microbiome causes disruption of the junctional components of intestinal epithelial cells and culturing intestinal epithelial cell lines in the presence of a microbiome simulant can increase the expression of junctional components[
42]. In the cases histidine supplementation we also saw increased lymph uptake and transport of the tracers, however, we believe that this is due to another phenomenon unrelated to intestinal epithelial integrity. We know that histidine is converted to histamine which causes relaxation of lymphatic muscles, leading to vessel dilation and reduced resistance to flow. If this is the case then there may be an application for the supplementation of diets with histidine in lymphatic congestive disorders, but in the case of spaceflight there appears to be no specific lymphatic benefit.
It was interesting to note that despite numerous changes in SCFA production there were few significant changes in the immune cell complements of either the mesenteric lymph nodes or spleen between spaceflight and habitat FMT animals. Histidine also appeared to have a muted effect on the immune composition compared to other measures (lymph transport and SCFA levels). There could be a number of reasons for this, the main and most likely explanation is that the animals were not given an “immunological challenge”. Despite FMT being a shift in the host microbiome the source material came from otherwise healthy animals and had no significant population changes that would lead us to suspect either spaceflight or habitat microbiomes were inflammatory or pathological. There was also a significant level of variation, especially in the mesenteric lymph nodes, between animals within groups which may have masked some more subtle changes. There were however defined FMT changes detectable in the spleen populations of T-cells, B-cells, and monocytes with FMT decreasing CD4+ T-cells, increasing CD8+ T-cells and total B-cells, and again decreasing the total population of monocytes. Some of these changes were similar in the mesenteric lymph nodes but were not significant.
Despite there being few significant alterations in the immune cell populations of the lymph nodes there were large shifts in the gene expression of the stromovascular fraction of the nodes. Clustering analysis of gene expression profiles revealed that space FMT with histidine clustered close to habitat FMT, closer to each other than their respective treatment pairs (space FMT and habitat FMT with histidine). This is similar to some of the responses that were seen in SCFA production. We take this to mean that histidine treatment would be an effective way to reverse or block the transcriptomic effects of the spaceflight microbiome. However, in the absence of a challenge and the lack of evidence that the spaceflight microbiome is pathological our conclusions are limited in scope.
Overall, our data shows that the spaceflight microbiome does have an impact on several factors that contribute to GI and whole-body health, but these changes do not appear to be pathological. Some changes, such as SCFA production and differential responses to histidine, raise the questions of how these different microbiomes would effect the host’s response to challenges (stress, infection, radiation, etc.) and the host responses to drugs and supplements. As an example the elevation of isovaleric and isobutyric acids may predispose the host to aberrant stress responses. Additionally, the changes that were noted in the transcriptomic profile of node stromovascular fractions may alter the ability of the host to respond to infection, but until it is tested we cannot be sure. While our study is a first step in the examination of the isolated space microbiome it is limited in scope and there needs to be further studies to determine how it impacts the host in situations other than basal conditions.
4. Materials and Methods
4.1. Collection of Transplant material:
The material for the FMT procedure was collected from mice flown for the rodent research 23 (RR923) mission at Texas A&M which consisted of male C57/Bl6 mice flown for 34 days on the international space station or animals housed in habitat controls (matched cage, food, light dark cycles, and CO2 levels). Whole cecum were isolated from each animal intact to preserve obligate anaerobic populations and snap frozen immediately after harvest. Material from 3 cecum were pooled for each transplant round (corresponding to the appropriate group) and were made into a slurry at 1g/mL in PBS/glycerol (90/10%) while on ice prior to storage at -80C.
4.2. Microbiome Analysis:
Cecal samples from flight and habitat control groups were sent for 16S sequencing at the TAMU Molecular Genomics Core (MGC) and compared via pairwise ANCOM analysis.
4.3. Fecal Microbiome Transplant (FMT):
All animal procedures conformed to federal, state and institutional animal use and care protocols. Animals (male C57Bl6 mice, 20g) that were used for fecal transplant or ablation controls were put on a regiment of 1g/L ampicillin, 0.5g/L vancomycin, 0.5g/L neomycin in their drinking water for 14 days (changed every other day) with daily cage changes (to prevent self-recolonization). At the end of the antibiotic regiment animals used for FMT (spaceflight or habitat controls) were given 2 enemas of the corresponding cecal slurry (10uL/g bodyweight) 7 days apart, simultaneously cage changes were returned to weekly changes to promote coprophagic recolonization. Ablation controls were allowed to recover the native microbiome of the animal facility. One week after the final transplant enema one group each of flight and habitat control transplants were put on L-histidine (1g/L) in the drinking water (changed every day as a intervention test. The weight of the animals was monitored for the duration of the study and organ weights (spleen, stomach and cecum) were taken at harvest.
4.4. Metabolomic (lipidomic analysis):
Short chain fatty acid analysis was performed on unfixed, snap frozen cecal contents. The cecum (n=5) were submitted to the TAMU Integrated Metabolomics analysis core (IMAC). Levels of SCFAs (acetate, propionate, and butyrate) and branched chain fatty acids (BCFAs: isobutyrate, isovalerate, and valerate) in cecal contents were measured using gas chromatography-mass spectrometry (GC-MS) following the Integrated Metabolomics Analysis Core’s standard analysis protocol. The samples were lyophilized overnight, powdered, and weighed. Fifty mg of each sample was diluted in 800μL extraction solution (30 mM hydro-chloric acid) and spiked with 200mM heptadeuterated butyric acid (d7-Butyric acid) internal standard to the final concentration of 0.1mM, to normalize for extraction efficiency. Samples were homogenized using a Precellys homogenizer, then centrifuged for 10min at 15,000× g at 4°C. Supernatants were collected and mixed with equal volume of ethyl acetate, vortexed for 10sec to emulsify, incubated on ice for 5min, then centrifuged for 1min at 15,000× g at 4°C. From each sample, 150μL of supernatant were transferred to sample vials and maintained at room temperature on an autosampler before injection. One μL of extracted sample was injected with a split ratio of 20:1, into a gas chromatography triple quadrupole mass spectrometer (TSQ EVO 8000, Thermo Scientific, Waltham, MA, USA) for chromatographic separation and quantification. The ionization was carried out in the electron impact (EI) mode at 70eV. Separation was achieved using a ZB WAX Plus capillary column (30m × 0.25mm, 0.25μm film thickness, Phenomenex). The MS data and retention times were acquired in full scan mode from mass-to-charge ratios (m/z) 40–500 for the individual target compounds. The target compounds were quantified in the Selected Ion Monitoring (SIM) mode using the following product ions in positive-ion mode (compound: product ions in m/z): acetic acid: 43, 45, 60; propionic acid: 43, 73, 74; isobutyric acid: 41, 43, 73; butyric acid: 42, 60, 73; isovaleric acid: 43, 60, 87; valeric acid: 41, 60, 73; D7-butyric acid: 45, 63, 77. The injector, MS transfer line and ion source were maintained at 230 °C, 240°C and 240°C, respectively. The flow rate of helium carrier gas was kept at 1mL/min. Absolute levels of SCFAs in μM were calculated, and then normalized to mg dried sample weights. The samples were extracted in ethyl acetate, and the standard curve was prepared in ethyl acetate. Sample acquisition and analysis was performed with TraceFinder 3.3 (Thermo Scientific).
4.5. Lymphatic Uptake and Transport:
1cm sections of the terminal ileum containing no apparent luminal contents and bridging a section of mesentery containing 1 neurovascular bundle (NVB) were tied off above and below the NVB (oral and rectal orientation) immediately after euthanasia. The mesentery was cut along the clear windows flanking the NVB and the NVB was cut at the root of the mesenteric lymph node. This tissue section was transferred to warmed (37*C) Kreb’s solution containing HEPES in 15mL centrifuge tubes. A fine gauge (30 gauge) needle was used to introduce 200uL of PBS containing 2% BSA and a combination of 70kDa AF488 dextran and heat killed staphylococcus aureus (AF594) (1mg/mL each). These tissues were allowed to incubate for 30 minutes in warmed Kreb’s solution after which time the mesentery was cut away from the intestinal tissue and spread on 25X60mm coverslips and allowed to air dry for 2 hours in a dark environment prior to imaging. Images were taken on a wide field Olympus microscope no closer than 3mm to the mesentery/gut border and no farther than 6mm from the gut border focused on lymphatic vessels that were apparent above the layer of adipose cells. Fluorescent intensity was measured in FIJI for both tracers within the bounds of the identified lymphatic vessel and in a 30um box immediately on the outside boundary of the vessel. The mean intensity was recorded and the ratio of the mean fluorescent intensity inside and outside the vessel was calculated to estimate lymphatic leakage.
4.6. Flow Cytometry:
Mesenteric lymph nodes and spleens from each group (n=5) were extracted at the termination of the experiment. Mesenteric lymph nodes were pressed through a 70um cell strainer and washed with 1mL of PBS repeatedly, the remaining portion of the node (the stromovascular fraction was) was removed from the strainer and frozen for transcriptomic analysis. Spleens were repeatedly nicked with a fresh sterile scalpel prior to pressing through a 70um cell strainer and washed repeatedly with 1mL of PBS. The cells were spun at 300g for 5 minutes and supernatant was removed, the resulting pellets were resuspended in 1mL RPMI-1640 media. The cell suspensions were mixed 1:1 with RPMI-1640 containing 20% DMSO while gently agitating prior to freezing. The cell samples were transported to UTMB for analysis where they were thawed and stained for flow cytometric analysis (antibodies listed in
Table 1).
4.7. Transcriptomic Analysis:
The tissues (node stromovascular fractions) were transferred to the Texas A&M Experimental Genomics Core where the tissues were processed for RNA extraction and transcriptomic analysis using a NextSeq500 (Illumina). The resulting data was then submitted to the Texas A&M IBT High Throughput Research and Screening Center where the transcriptomic reads were aligned to an appropriate mouse reference genome (C57BL/6) prior to clustering, enrichment, and shared gene expression analysis.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
Dr. Cromer provided overall study design, collated data from other investigators, provided funding for the study and performed lymph transport studies; Dr. Powel performed data analysis on transcriptomic assays in this manuscript; Dr. Endsley performed flow cytometric analysis of spleens and mesenteric lymph nodes.
Funding
This Study was funded by NASA grant 80NSSC19K0438 Use of Dietary Histidine to Counteract Lymphatic and Mucosal Immune Dysfunction Due to Space Dysbiosis.
Institutional Animal Care and Use Committee Approval
All animal studies in this work were approved by the Texas A&M IBT IACUC under Animal Use Protocol 2020-0045.
Data Availability Statement
Transcriptomic data will be available from the NASA Gene lab data repository. All other data will be available from the NASA Life Science Data Archive. All data will be listed under the PI name and grant name.
Acknowledgments
Use of the TAMU Integrated Metabolomics Analysis Core is acknowledged.
Conflicts of Interest
None
References
- Garza-Gonzalez, E., et al., Intestinal Microbiome Changes in Fecal Microbiota Transplant (FMT) vs. FMT Enriched with Lactobacillus in the Treatment of Recurrent Clostridioides difficile Infection. Can J Gastroenterol Hepatol, 2019, 2019, 4549298. [CrossRef]
- Walters, W.A., et al., Epidemiology and associated microbiota changes in deployed military personnel at high risk of traveler’s diarrhea. PLoS One, 2020, 15, e0236703. [CrossRef] [PubMed]
- Dargahi, N., et al., Immunomodulatory effects of probiotics: Can they be used to treat allergies and autoimmune diseases? Maturitas, 2019, 119, 25–38. [CrossRef] [PubMed]
- Liu, Y., J.J. Alookaran, and J.M. Rhoads, Probiotics in Autoimmune and Inflammatory Disorders. Nutrients 2018, 10. [CrossRef]
- Ganesh, B.P., et al., Diacylglycerol kinase synthesized by commensal Lactobacillus reuteri diminishes protein kinase C phosphorylation and histamine-mediated signaling in the mammalian intestinal epithelium. Mucosal Immunol, 2018, 11, 380–393. [CrossRef] [PubMed]
- Bianchi, F., et al., In vitro modulation of human gut microbiota composition and metabolites by Bifidobacterium longum BB-46 and a citric pectin. Food Res Int, 2019, 120, 595–602. [CrossRef]
- Gutin, L., et al., Fecal microbiota transplant for Crohn disease: A study evaluating safety, efficacy, and microbiome profile. United European Gastroenterol J, 2019, 7, 807–814. [CrossRef]
- Tesei, D., et al., Understanding the Complexities and Changes of the Astronaut Microbiome for Successful Long-Duration Space Missions. Life (Basel) 2022, 12. [CrossRef]
- Mikelsaar, M.and R. Mandar, Commentary: Gut Microbiome and Space Travelers’ Health: State of the Art and Possible Pro/Prebiotic Strategies for Long-Term Space Missions. Front Physiol, 2021, 12, 651977. [CrossRef]
- Siddiqui, R., N. Akbar, and N.A. Khan, Gut microbiome and human health under the space environment. J Appl Microbiol, 2021, 130, 14–24. [CrossRef]
- Voorhies, A.A., et al., Study of the impact of long-duration space missions at the International Space Station on the astronaut microbiome. Sci Rep, 2019, 9, 9911. [CrossRef] [PubMed]
- Ritchie, L.E., et al., Space Environmental Factor Impacts upon Murine Colon Microbiota and Mucosal Homeostasis. PLoS One, 2015, 10, e0125792. [CrossRef] [PubMed]
- Thomas, C.M., et al., Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One 2012, 7, e31951. [CrossRef]
- Bach Knudsen, K.E., et al., Impact of Diet-Modulated Butyrate Production on Intestinal Barrier Function and Inflammation. Nutrients 2018, 10. [CrossRef]
- Martin-Gallausiaux, C., et al., Butyrate produced by gut commensal bacteria activates TGF-beta1 expression through the transcription factor SP1 in human intestinal epithelial cells. Sci Rep, 2018, 8, 9742. [CrossRef]
- Martin-Gallausiaux, C., et al., Butyrate Produced by Commensal Bacteria Down-Regulates Indolamine 2,3-Dioxygenase 1 (IDO-1) Expression via a Dual Mechanism in Human Intestinal Epithelial Cells. Front Immunol, 2018, 9, 2838. [CrossRef] [PubMed]
- Grosheva, I., et al., High-Throughput Screen Identifies Host and Microbiota Regulators of Intestinal Barrier Function. Gastroenterology 2020. [CrossRef]
- Gao, C., et al., Histamine H2 Receptor-Mediated Suppression of Intestinal Inflammation by Probiotic Lactobacillus reuteri. MBio, 2015, 6, e01358–15. [CrossRef]
- Case, L.K., et al., Histamine H(1) receptor signaling regulates effector T cell responses and susceptibility to coxsackievirus B3-induced myocarditis. Cell Immunol, 2012, 272, 269–74. [CrossRef]
- del Rio, R., et al., Histamine H4 receptor optimizes T regulatory cell frequency and facilitates anti-inflammatory responses within the central nervous system. J Immunol, 2012, 188, 541–7. [CrossRef]
- Schnedl, W.J. and D. Enko, Considering histamine in functional gastrointestinal disorders. Crit Rev Food Sci Nutr 2020, 1–8.
- Kurtz, K.H., et al., Involvement of H1 and H2 receptors and soluble guanylate cyclase in histamine-induced relaxation of rat mesenteric collecting lymphatics. Microcirculation, 2014, 21, 593–605. [CrossRef] [PubMed]
- Tsoy Nizamutdinova, I., et al., Histamine as an Endothelium-Derived Relaxing Factor in Aged Mesenteric Lymphatic Vessels. Lymphat Res Biol 2017. [CrossRef]
- Santambrogio, L.and H.G. Rammensee, Contribution of the plasma and lymph Degradome and Peptidome to the MHC Ligandome. Immunogenetics, 2019, 71, 203–216. [CrossRef]
- Santambrogio, L., The Lymphatic Fluid. Int Rev Cell Mol Biol, 2018, 337, 111–133.
- Cromer, W.E., et al., Burn Injury-Associated MHCII(+) Immune Cell Accumulation Around Lymphatic Vessels of the Mesentery and Increased Lymphatic Endothelial Permeability Are Blocked by Doxycycline Treatment. Lymphat Res Biol, 2018, 16, 56–64. [CrossRef]
- Clement, C.C., et al., Quantitative Profiling of the Lymph Node Clearance Capacity. Sci Rep, 2018, 8, 11253. [CrossRef]
- Santambrogio, L.and L.J. Stern, Carrying yourself: self antigen composition of the lymphatic fluid. Lymphatic research and biology, 2013, 11, 149–54. [CrossRef]
- Clement, C.C.and L. Santambrogio, The Lymph Self-Antigen Repertoire. Frontiers in immunology, 2013, 4, 424. [CrossRef]
- Kedl, R.M., et al., Migratory dendritic cells acquire and present lymphatic endothelial cell-archived antigens during lymph node contraction. Nat Commun, 2017, 8, 2034. [CrossRef]
- Kedl, R.M.and B.A. Tamburini, Antigen archiving by lymph node stroma: A novel function for the lymphatic endothelium. Eur J Immunol, 2015, 45, 2721–9. [CrossRef]
- Morris, G., et al., The Role of the Microbial Metabolites Including Tryptophan Catabolites and Short Chain Fatty Acids in the Pathophysiology of Immune-Inflammatory and Neuroimmune Disease. Mol Neurobiol, 2017, 54, 4432–4451. [CrossRef]
- Szczesniak, O., et al., Isovaleric acid in stool correlates with human depression. Nutr Neurosci, 2016, 19, 279–83. [CrossRef]
- Hegedus, R., et al., Enhanced cellular uptake and in vitro antitumor activity of short-chain fatty acid acylated daunorubicin-GnRH-III bioconjugates. Eur J Med Chem, 2012, 56, 155–65. [CrossRef]
- Astbury, S.M.and B.M. Corfe, Uptake and metabolism of the short-chain fatty acid butyrate, a critical review of the literature. Curr Drug Metab, 2012, 13, 815–21. [CrossRef]
- Mascolo, N., V.M. Rajendran, and H.J. Binder, Mechanism of short-chain fatty acid uptake by apical membrane vesicles of rat distal colon. Gastroenterology, 1991, 101, 331–8. [CrossRef]
- Wu, M., et al., Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Transl Psychiatry, 2020, 10, 350. [CrossRef]
- Silva, Y.P., A. Bernardi, and R.L. Frozza, The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne), 2020, 11, 25. [CrossRef]
- Yao, Y., et al., The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit Rev Food Sci Nutr, 2022, 62, 1–12. [CrossRef]
- Shin, W., et al., A Robust Longitudinal Co-culture of Obligate Anaerobic Gut Microbiome With Human Intestinal Epithelium in an Anoxic-Oxic Interface-on-a-Chi. Front Bioeng Biotechnol 2019, 7, 13. [CrossRef]
- Guzman, J.R., V.S. Conlin, and C. Jobin, microbiome, and the intestinal epithelium: an essential triumvirate? Biomed Res Int, 2013, 2013, 425146. [CrossRef] [PubMed]
- Preethy, S., et al., Role of Gut Microbiome Homeostasis, Integrity of the Intestinal Epithelial Cells, and the (Endogenous) Butyrate in Enduring a Healthy Long Life. Georgian Med News 2023, 73–78.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).