Submitted:
24 July 2023
Posted:
25 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study site and organisms
2.2. Field conditions
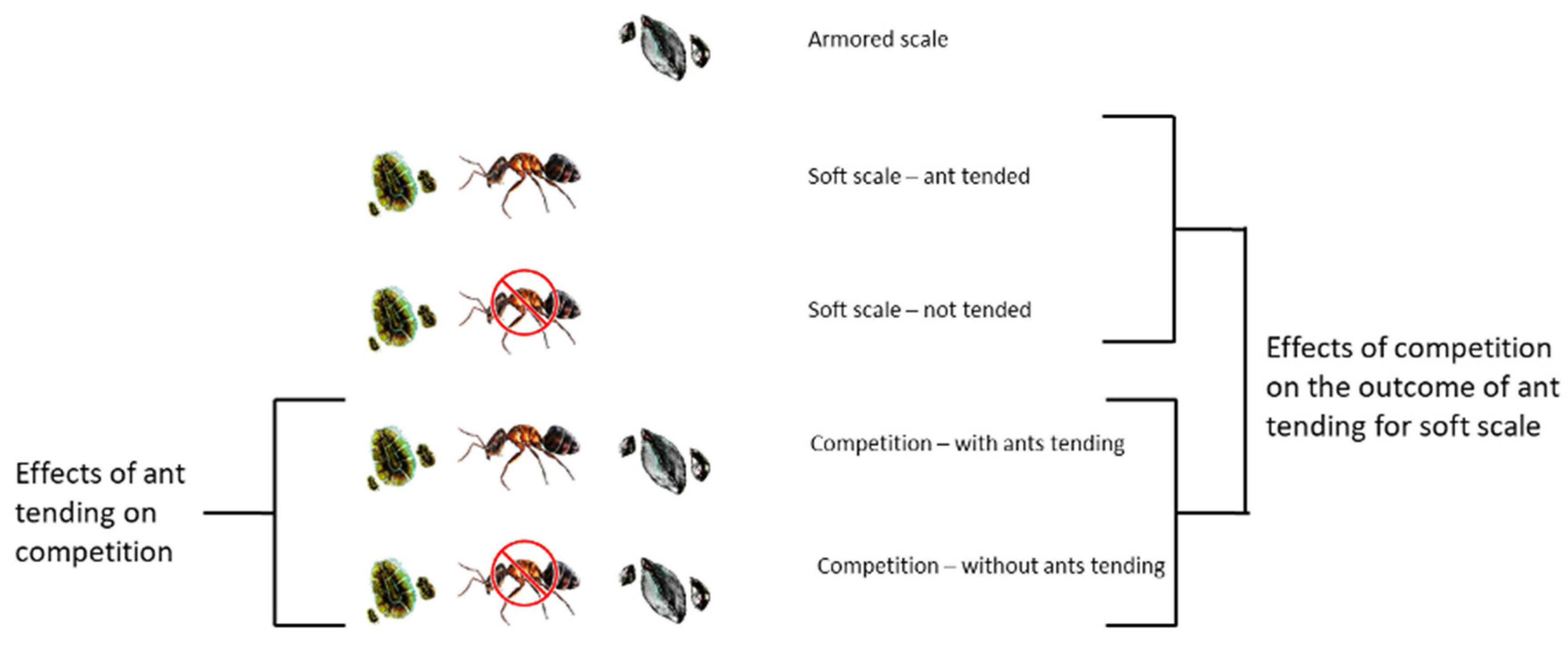
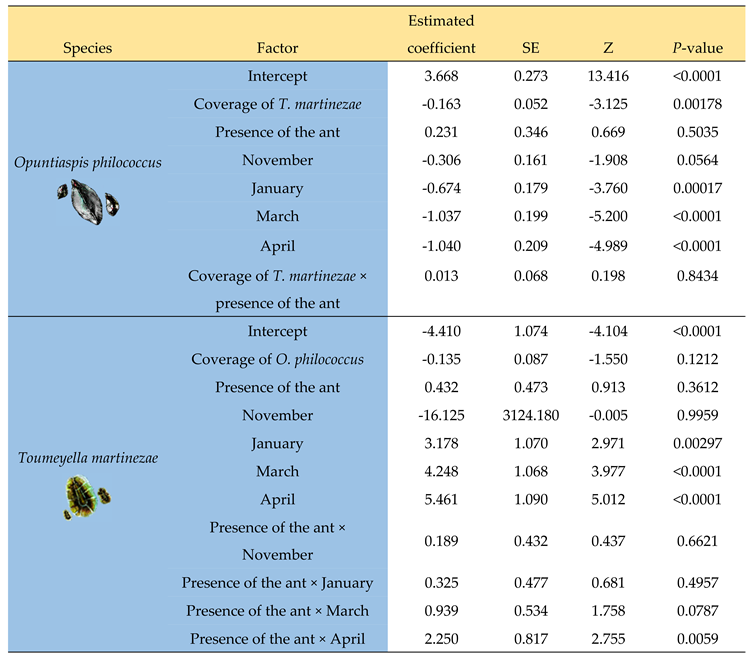
2.3. Measures of competition and statistical analyses
2.4. Population structure
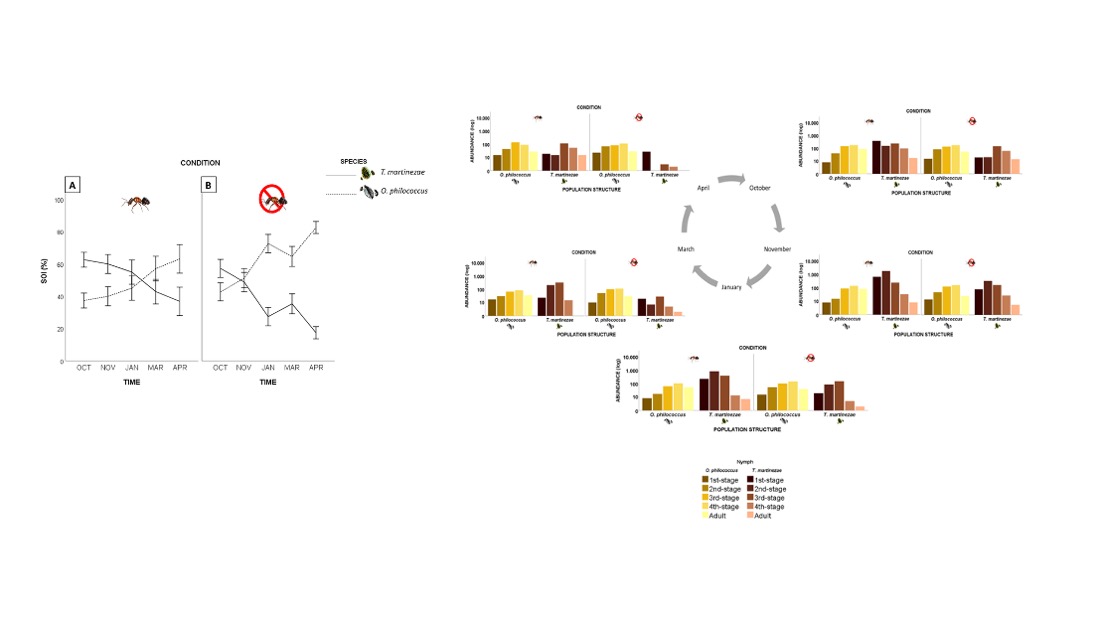
3. Results
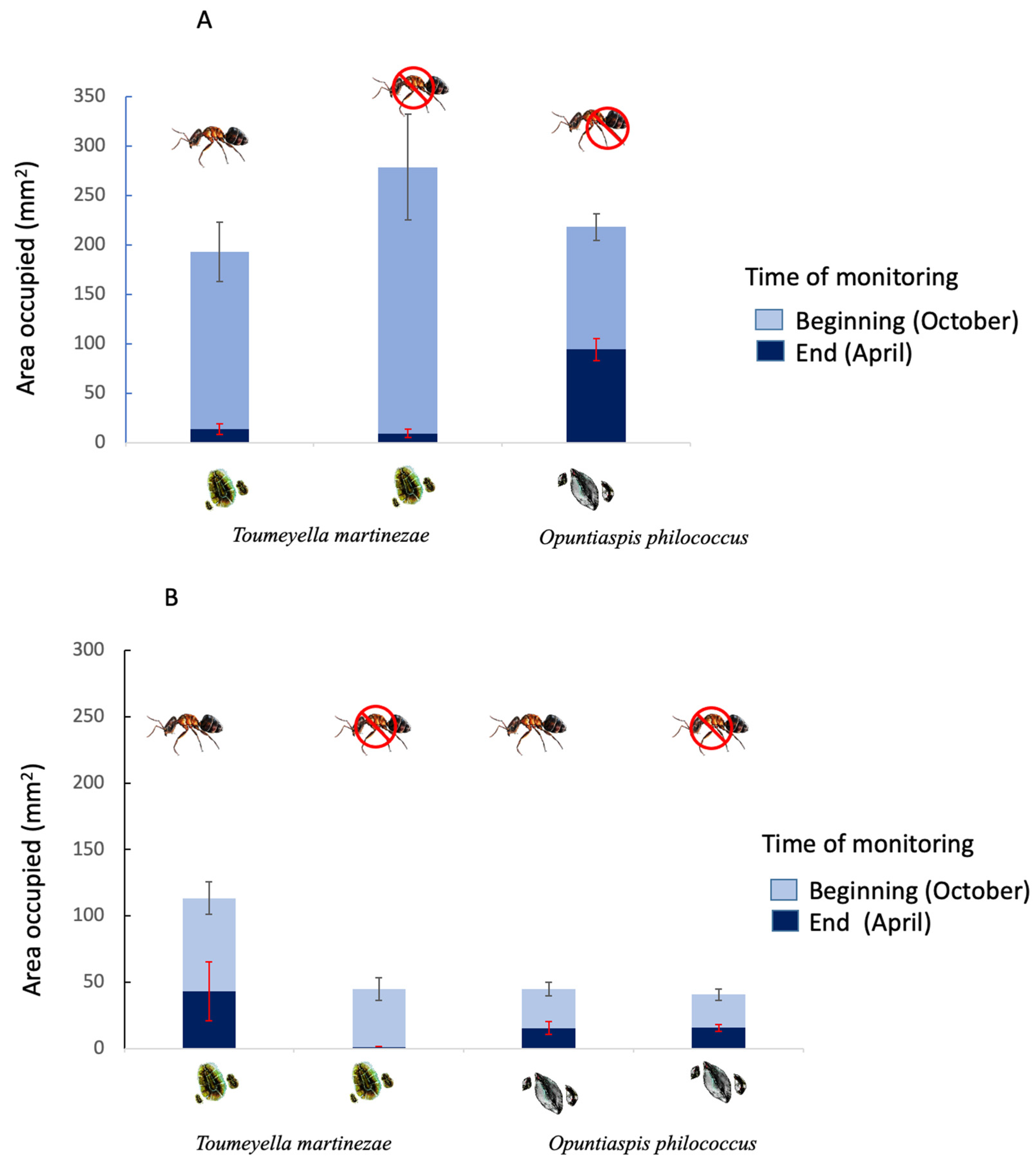
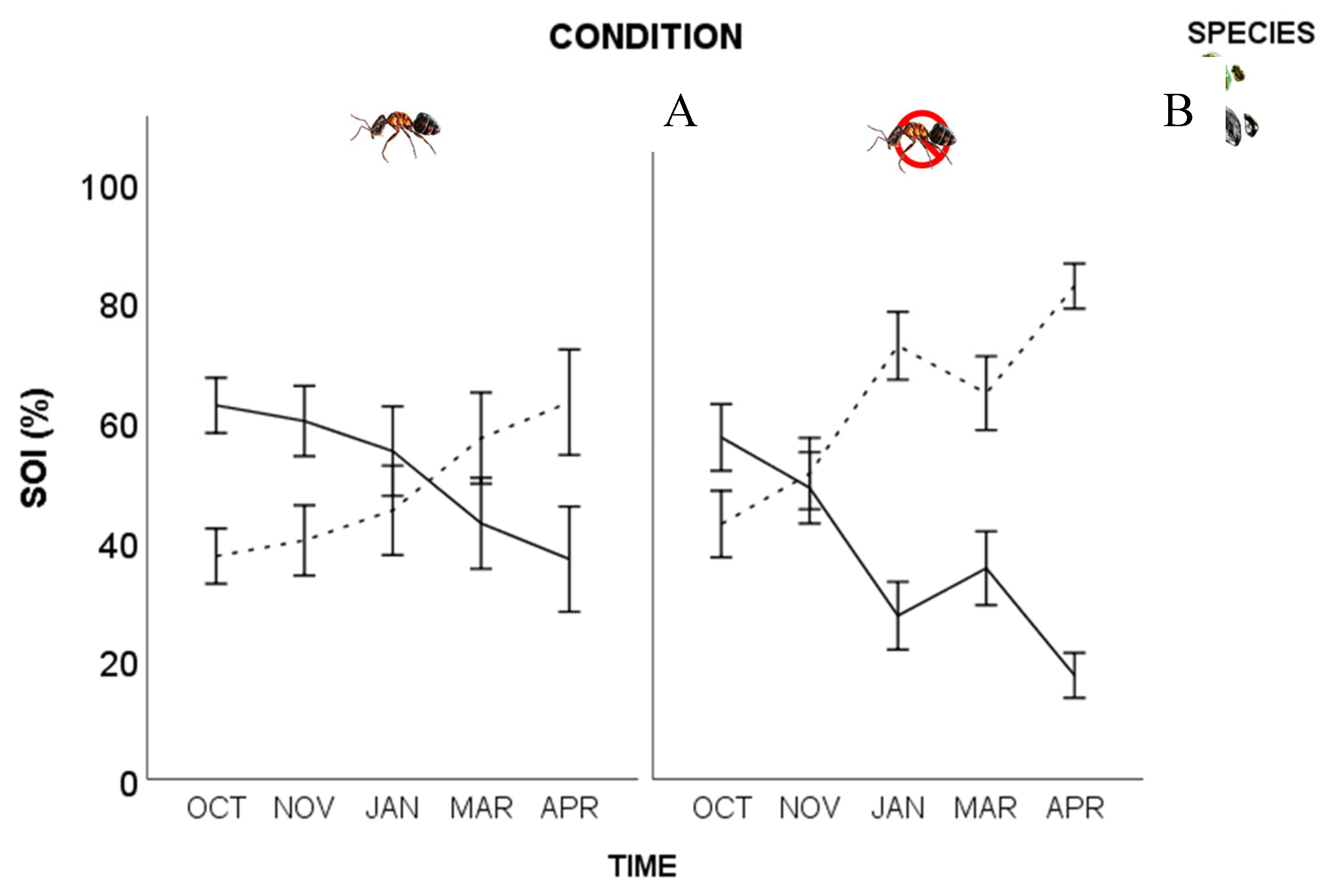
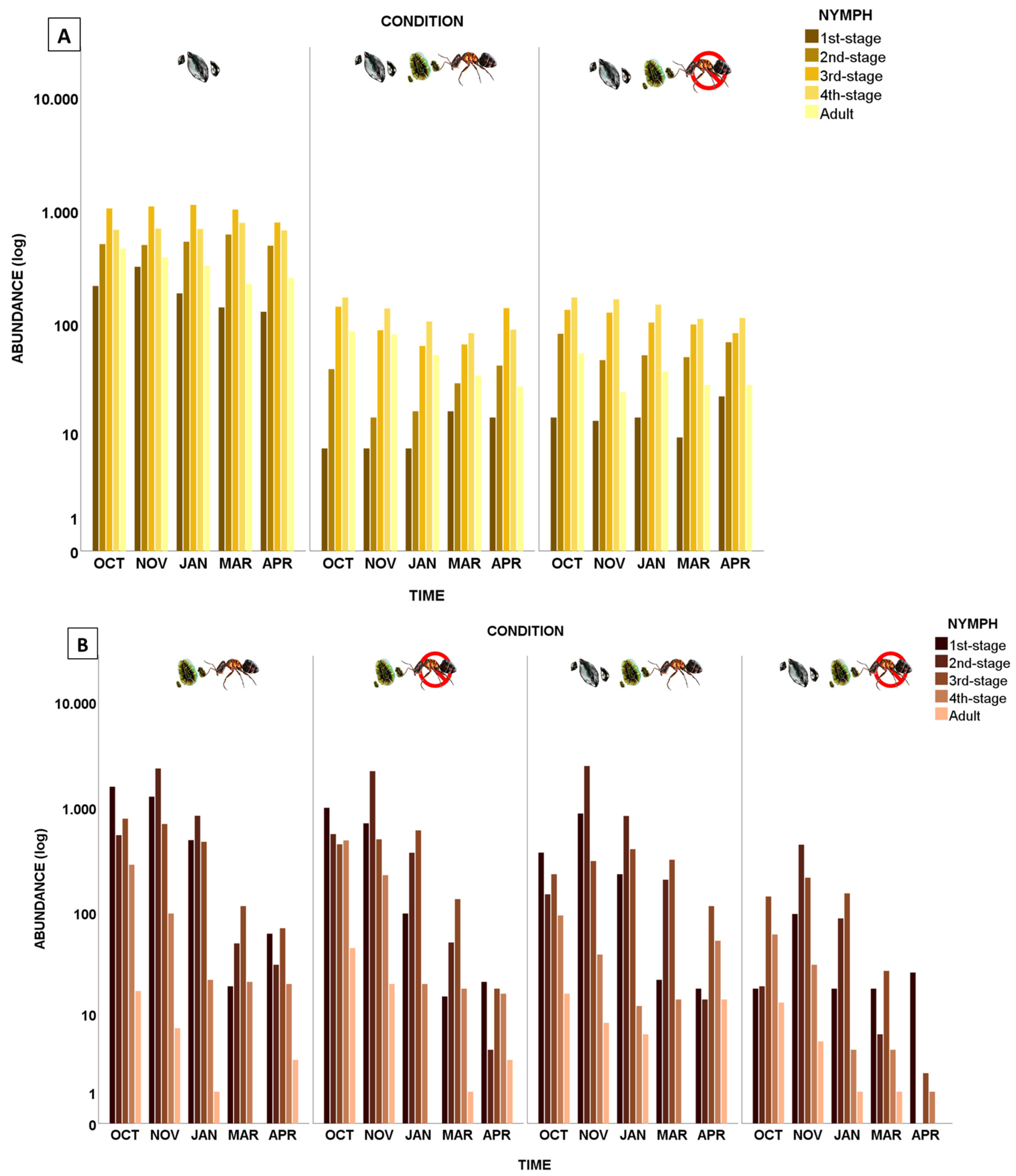
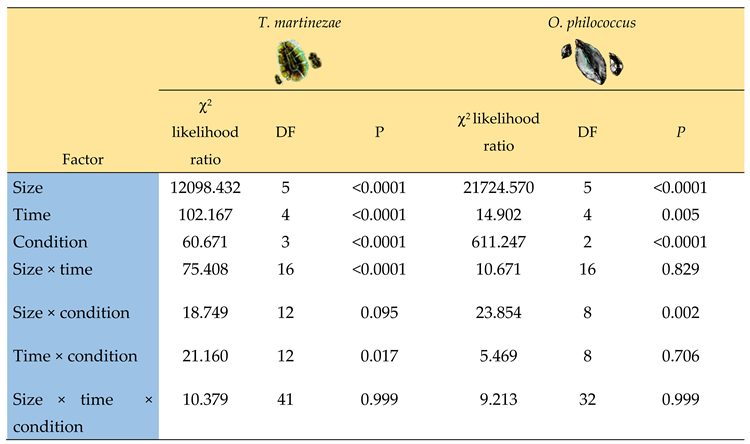
3.1. Competition for space
3.2. Population structure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Connell, J. H. On the prevalence and relative importance of interspecific competition: evidence from field experiments. Am. Nat 1983, 122, 661–696. [Google Scholar] [CrossRef]
- Denno, R. F.; McClure, M. S.; Ott, J. R. Interpecific interactions in phytophagous insects: competition reexamined and resurrected. Ann. Rev. Entomol. 1995, 40, 297–331. [Google Scholar] [CrossRef]
- Bell, G. The co-distribution of species in relation to the neutral theory of community ecology. Ecology 2005, 86, 1757–1770. [Google Scholar] [CrossRef]
- Kaplan, I.; Denno, R. F. Interspecific interactions in phytophagous insects revisited: a quantitative assessment of competition theory. Ecol. Lett. 2007, 10, 977–994. [Google Scholar] [CrossRef]
- Mooney, K.; Jones, P.; Agrawal, A. Coexisting congeners: demography, competition, and interactions with cardenolides for two milkweed-feeding aphids. Oikos 2008, 117, 450–458. [Google Scholar] [CrossRef]
- Smith, R. A.; Mooney, K. A.; Agrawal, A. Coexistence of three specialist aphids on common milkweed, Asclepia syriaca. Ecology 2008, 89, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Inbar, M.; Doostdar, H.; Leibee, G. L.; Mayer, R. T. The role of plant rapidly induced responses in asymmetric interspecific interactions among insect herbivores. J. Chem. Ecol. 1999, 25, 1961–1979. [Google Scholar] [CrossRef]
- Denno, R. F.; Peterson, M. A.; Gratton, C.; Cheng, J.; Langellotto, G. A.; Huberty, A. F.; Finke, D. L. Feeding-induced changes in plant quality mediate interspecific competition between sap-feeding herbivores. Ecology 2000, 81, 1814–1827. [Google Scholar] [CrossRef]
- Denno, R.F.; Kaplan, I. Plant-mediated interactions in herbivorous insects: mechanisms, symmetry, and challenging the paradigms of competition past. In Ecological Communities: Plant Mediation in Indirect Interaction Webs; Ohgushi, T.; Craig, T.P.; Price, P.W. Cambridge University Press, Cambridge, UK, 2007; pp. 19–50.
- Yamamichi, M.; Gibbs, T.; Levine, J. M. Integrating eco-evolutionary dynamics and modern coexistence theory. Ecol. Lett. 2022, 25, 2091–2106. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A. A. A scale-dependent framework for trade-offs, syndromes, and specialization in organismal biology. Ecology 2020, 101, e02924. Available online: https://www.cabdirect.org/cabdirect/abstract/19610501730. [CrossRef] [PubMed]
- Ito, Y. Ecological studies on population increase and habitat segregation among barley aphids. Bull. Natn. Inst. Agric. Sci. 1960, C11, 45–130. [Google Scholar]
- Denno, R. F.; Roderick, G. K. Density-Related dispersal in planthoppers : Effects of interspecific crowding. Ecology 1992, 73, 1323–1334. [Google Scholar] [CrossRef]
- McClure, M. S.; Price, P. W. Competition among sympatric Erythroneura leafhoppers (Homoptera: Cicadellidae) on America sycamore. Ecology 1975, 56, 1388–1397. [Google Scholar] [CrossRef]
- DeBach, P.; Hendrickson, R. M.; Rose, M. Competitive displacement: extinction of the yellow scale, Aonidiella citrina (Coq.) (Homoptera: Diaspididae), by its ecological homologue, the California red scale, Aonidiella aurantii (Mask.) in Southern California. Hilgardia 1978, 46, 1–35. [Google Scholar] [CrossRef]
- McClure, M. S. Competition between exotic species: scale insects on Hemlock. Ecology 1980, 61, 1391–1401. [Google Scholar] [CrossRef]
- McClure, M. S. Biology, population trends, and damage of Pineus boerneri and P. coloradensis (Homoptera: Adelgidae) on Red Pine. Environ. Entomol. 1989, 18, 1066–1073. [Google Scholar] [CrossRef]
- Gullan, P. J.; Martin, J. H. Sternorrhyncha: (jumping plant-lice, whiteflies, aphids, and scale insects). In Encyclopedia of Insects, 2nd ed.; Resh, V.H., Cardé R., T., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2009; pp. 957–967. [Google Scholar] [CrossRef]
- McClure, M. S. Effects of voltinism, interspecific competition and parasitism on the population dynamics of the hemlock scales, Fiorinia externa and Tsugaspidiotus tsugae (Homoptera: Diaspididae). Ecol. Entomol. 1981, 6, 47–54. [Google Scholar] [CrossRef]
- Gullan, P. J.; Kosztarab, M. Adaptations in scale insects. Annu. Rev. Entomol. 1997, 42, 23–50. [Google Scholar] [CrossRef]
- Forrest, J. R. Complex responses of insect phenology to climate change. Curr. Opin. Insect Sci. 2016, 17, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Zeuss, D.; Brunzel, S.; Brandl, R. Environmental drivers of voltinism and body size in insect assemblages across Europe. Glob. Ecol. Biogeogr. 2017, 26, 154–165. [Google Scholar] [CrossRef]
- Just, M. G.; Frank, S. D. Thermal tolerance of gloomy scale (Hemiptera: Diaspididae) in the Eastern United States. Environ. Entomol. 2020, 49, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, Y.; Ezoe, H.; Yamauchi, A. Evolutionarily stable seasonal timing of univoltine and bivoltine insects. In Insect life-cycle polymorphism, Series Entomologica, Danks, H. V., Ed.; Springer: New York, NY, USA, 1994, 43, pp. 69–89. [Google Scholar] [CrossRef]
- Arias, S.; Gama, S.; Guzman, L. Flora del Valle de Tehiacán-Cuicatlán. Fascículo 14. Cactaceae A.L. Juss. Universidad Nacional Autónoma de México. DF Ciudad de México, México, 1997; pp. 146. Available online: http://www.ibiologia.unam.mx/BIBLIO68/fulltext/fasiculosfloras/fas14.pdf.
- Callejas-Chavero, A.; Vargas-Mendoza, C. F.; Flores-Martínez, A.; Martínez-Hernández, D.; Moncada-Orellana, A.; Dias-Quiñones, J. Y. Herbivory in cacti: Fitness effects of two herbivories, one tending-ant on Myrtillocactus geometrizans (Cactaceae). In Evolutionary Ecology of Plant-Herbivore Interaction; Nuñez-Farfán, J.; Valverde, P., Eds; Springer: New York, NY, USA; 2020 pp. 109–134. [CrossRef]
- Callejas-Chavero, A.; Martínez, D. G.; Nicolás, D. E. Diversidad de artrópodos asociados a Myrtillocactus geometrizans (MART) en tres poblaciones del Valle de México. In Memorias del XLVIII Congreso Nacional de Entomología; Equihua, M.A., Estrada, V.E, Acuña, S.J., Chaires, G.P., Eds.; Sociedad Mexicana de Entomología: Ciudad de México, México; 2013; pp. 685–689. Available online: http://www.entomologia.socmexent.org/revista/2013/EC/685-689.pdf.
- Beardsley Jr, J. W.; Gonzalez, R. H. The biology and ecology of armored scales. Annu. Rev. Entomol. 1975, 20, 47–73. Available online: https://www.antwiki.net/wiki/images/4/40/Diaspididae_armored_scales_annurev1975.pdf. [CrossRef] [PubMed]
- Kondo, T.; González, H. A new species of Toumeyella Cockerell (Hemiptera: Coccidae) on Myrtillocactus geometrizans (Cactaceae) from Mexico with a checklist of known species of Toumeyella in the world. Insecta Mundi 2014, 0396, 1–10. Available online: https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1899&context=insectamundi.
- Martínez, D.G. Efecto de Liometopum apiculatum (Hymenoptera: Formicidae) sobre la tasa de parasitoidismo de Toumeyella martinezae (Hemiptera: Coccidae) asociados a Myrtillocactus geometrizans (Cactaceae) en un Matorral Xerófilo de Huichapan, Hidalgo. Bachelor’s thesis, Instituto Politécnico Nacional. Ciudad de México, México, 2015.
- Martínez-Hernández, D. G.; Arriola-Padilla, V. J.; Callejas-Chavero, A.; González-Hernández, H.; Gijón-Hernández, A. Biología de la escama blanda Toumeyella martinezae Kondo y Gonzalez (Hemiptera: Coccidae) del Garambullo (Myrtillocactus geometrizans) (Mart. ex Pfeiff) Console) en Huichapan, Hidalgo. Entomol. Mex. 2015, 2, 172–179. Available online: http://www.entomologia.socmexent.org/revista/2015/BHN/PAG172-179.pdf.
- González-Villa, H. Efecto del parasitoidismo y mutualismo sobre la demografía de Toumeyella martinezae (Hemiptera: Coccidae) asociada a Myrtillocactus geometrizans (Cactaceae) en un matorral xerófilo de Huichapan, Hidalgo. Master Degree, Instituto Politécnico Nacional. Ciudad de México, México, 2021.
- Islas-Estrada, S. A. Ciclo de vida de Opuntiaspis philococcus (Cockerell, 1893) y sus enemigos naturales, asociados a Myrtillocactus geometrizans en Hidalgo, México. Bachelor’s thesis, Instituto Politécnico Nacional. Ciudad de México, México, 2020.
- Velasco-Corona, C.; Corona-Vargas, M. del C.; Peña-Martínez, R. Liometopum apiculatum (Formicidae: Dolichoderinae) y su relación trofobiótica con Hemiptera Sternorrhyncha en Tlaxco, Tlaxcala, México. Acta Zool. Mex. 2007, 23, 31–42. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0065-17372007000200003.
- Bravo-Hollis, H. Las cactáceas de México (Vol. 1) Universidad Nacional Autónoma de México: Ciudad de México, México,. 1978, 1, pp. 743.
- Bustamante, E.; Búrquez, A. Fenología y biología reproductiva de las cactáceas columnares. Cact. Suc. Mex. 2005, 50, 68–80. Available online: http://web.ecologia.unam.mx/cactsucmex/CACTACEAS2005_3.pdf.
- Hernández, M.; Terrazas, T.; Delgado, A.; Luna, M. Los estomas de Myrtillocactus geometrizans (Mart. ex. Pfeiff.) Console (Cactaceae): Variación en su área de distribución. Rev. Fitotec. Mex. 2007, 30, 235–240. Available online: https://www.redalyc.org/pdf/610/61003004.pdf. [CrossRef]
- Del Toro, I.; Pacheco, J. A.; Mackay, W. P. Revision of the ant genus Liometopum (Hymenoptera: Formicidae). Sociobiology 2009, 53, 299–369. Available online: https://www.researchgate.net/publication/260866074_Revision_of_the_Ant_Genus_Liometopum_Hymenoptera_Formicidae.
- Cruz-Labana, J. D.; Tarango-Arámbula, L. A.; Alcántara-Carbajal, J. L.; Pimentel-López, J.; Ugalde-Lezama, S.; Ramírez-Valverde, G.; Méndez-Gallegos, S. J. Habitat use by the escamolera ant (Liometopum apiculatum Mayr) in Central Mexico. Agrociencia 2014, 48, 569–582. Available online: http://www.colpos.mx/agrocien/Bimestral/2014/ago-sep/art-1.pdf.
- González, R.H. Estrategias de control de la Escama de San José. Rev. Frutíc. 1980, 1, 11–14. [Google Scholar]
- Hamon, A. B. Opuntiaspis philococcus (Cockerell) (Homoptera: Coccoidea: Diaspididae). Entomology Circular 1980, 214, 1–2. [Google Scholar]
- Rodríguez, W. D.; Navarrete-Heredia, J. L.; Vásquez-Bolaños, M.; Rodríguez-Macías, R.; Briceño-Félix, G. A.; Coronado Blanco, J. M.; Ruíz-Cancino, E. Insects associated with the genus Agave spp. (Asparagaceae) in Mexico. Zootaxa 2019, 4612, 451–493. [Google Scholar] [CrossRef] [PubMed]
- Motic Images 2000, version 1.3; Motic China Group Co LTD) and statistical data analyses were processed using Microcal Origin 8.5 Pro package software. Available online: https://imaris.oxinst.com/packages?gad=1&gclid=Cj0KCQjwho-lBhC_ARIsAMpgMoePWZaj3hwhbqh1qml-wRthy575_wd8lWYBEXiv_bMBupO_1An5sU4aArSiEALw_wcB (accessed on 6 Agust 2018).
- Weigelt, A.; Jolliffe, P. Indices of plant competition. J. Ecol. 2003, 91, 707–720. [Google Scholar] [CrossRef]
- Ghosh, P. K.; Manna, M. C.; Bandyopadhyay, K. K.; Ajay, A. K.; Tripathi, R. H.; Wanjari, R. H.; Hati, K. M.; Misra, A. K.; Acharya, C. L.; Subba Rao, A. Interspecific interaction and nutrient use in soybean/sorghum intercropping system. Agron. J. 2006, 98, 1097–1108. [Google Scholar] [CrossRef]
- Grace, J. B. On the measurement of plant competition intensity. Ecology 1995, 76, 305–308. [Google Scholar] [CrossRef]
- Yates, F.; Dutton, S. J. A simple procedure for the analysis of experiments on mixtures of varieties or species of agronomic interest. J. Agric. Sci. 1988, 110, 13–24. [Google Scholar] [CrossRef]
- Pekas, A.; Aguilar, A.; Tena, A.; Garcia-Marí, F. Influence of host size on parasitism by Aphytis chrysomphali and A. melinus (Hymenoptera: Aphelinidae) in Mediterranean populations of California red scale Aonidiella aurantii (Hemiptera: Diaspididae). Biol. Control. 2010, 55, 132–140. [Google Scholar] [CrossRef]
- IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.
- Stadler, B.; Dixon, A. F. G. Ecology and evolution of aphid-ant interactions. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 345–372. [Google Scholar] [CrossRef]
- Mehrparvar, M.; Zytynska, S. E.; Balog, A.; Weisser, W. W. Coexistence through mutualist-dependent reversal of competitive hierarchies. Ecol. Evol. 2018, 8, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J. A.; López-Gallego, E.; La-Spina, M. The impact of ant mutualistic and antagonistic interactions on the population dynamics of sap-sucking hemipterans in pear orchards. Pest Manag. Sci. 2020, 76, 1422–1434. [Google Scholar] [CrossRef] [PubMed]
- Hill, M. G.; Mauchline, N. A.; Hall, A. J.; Stannard, K. A. Life table parameters of two armoured scale insect (Hemiptera: Diaspididae) species on resistant and susceptible kiwifruit (Actinidia spp.) germplasm. N. Z. J. Crop Hortic. 2009, 37, 335–343. [Google Scholar] [CrossRef]
- Martínez, D. G. Efecto de las interacciones que establecen los herbívoros Toumeyella martinezae y Opuntiaspis philococcus sobre la calidad y reproducción de Myrtillocactus geometrizans Master Degree, Instituto Politécnico Nacional. Ciudad de México, México, 20217. Available online: http://tesis.ipn.mx/handle/123456789/28371.
- Bale, J. S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; Good, J.E.G.; Harrington, R.; Hartley, S.; Jones, T.H.; Lindroth, R.L.; Press, M.C.; Symrnioudis, I.; Watt, A.D.; Whittaker, J.B. Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Glob. Chang. Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Schmitt, R. J. , & Holbrook, S. J. Seasonally fluctuating resources and temporal variability of interspecific competition. Oecologia 1986, 69, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Backe, K. M.; Frank, S. D. Chronology of gloomy scale (Hemiptera: Diaspididae) infestations on urban trees. Environ. Entomol. 2019, 48, 1113–1120. [Google Scholar] [CrossRef]
- Nishikawa, K. C. Competition and the evolution of aggressive behavior in two species of terrestrial salamanders. Evolution 1985, 39, 1282–1294. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B. D.; Grime, J. P. An experimental test of plant strategy theory. Ecology 1992, 73, 15–29. [Google Scholar] [CrossRef]
- Aerts, R. Interspecific competition in natural plant communities: mechanisms, trade-offs and plant-soil feedbacks. J. Exp. Biol. 1999, 50, 29–37. [Google Scholar] [CrossRef]
- Bronstein, J. L. The costs of mutualism. Am. Zool. 2001, 41, 825–839. [Google Scholar] [CrossRef]
- Lieutier, F.; Paine, T. D. Responses of mediterranean forest phytophagous insects to climate change. In Insects and diseases of mediterranean forest systems; Paine T.D.; Lieutier F. Eds.; Springer: New York, NY, USA, 2016; pp. 801–858. [Google Scholar] [CrossRef]
- Stadler, B.; Dixon, A. F. G. Costs of ant attendance for aphids. J. Anim. Ecol. 1998, 67, 454–459. [Google Scholar] [CrossRef]
- Jones, E. I.; Bronstein, J. L.; Ferrière, R. The fundamental role of competition in the ecology and evolution of mutualisms. Ann. N. Y. Acad. Sci. 2012, 1256, 66–88. [Google Scholar] [CrossRef] [PubMed]
- Ntiri, E. S.; Calatayud, P.-A.; Van den Berg, J.; Le Ru, B. P. Density dependence and temporal plasticity of competitive interactions during utilisation of resources by a community of lepidopteran stemborer species. Entomol. Exp. Appl. 2017, 162, 272–283. [Google Scholar] [CrossRef]
- Moll, J. D.; Brown, J. S. Competition and coexistence with multiple life-history stages. Am. Nat. 2008, 171, 839–843. [Google Scholar] [CrossRef]
- Leisnham, P. T.; Juliano, S. A. Spatial and temporal patterns of coexistence between competing Aedes mosquitoes in urban Florida. Oecologia 2009, 160, 343–352. [Google Scholar] [CrossRef]
- Chamberlain, S. A.; Bronstein, J. L.; Rudgers, J. A. How context dependent are species interactions? Ecol. Lett. 2014, 17, 881–890. [Google Scholar] [CrossRef]
- Soliveres, S.; Lehmann, A.; Boch, S.; Altermatt, F.; Carrara, F.; Crowther, T. W.; Delgado-Baquerizo, M.; Kempel, A.; Maynard, D. S.; Rillig, M. C.; Singh, B. K.; Trivedi, P.; Allan, E. Intransitive competition is common across five major taxonomic groups and is driven by productivity, competitive rank and functional traits. J. Ecol. 2018, 106, 852–864. [Google Scholar] [CrossRef]
- Siepielski, A. M.; McPeek, M. A. On the evidence for species coexistence: a critique of the coexistence program. Ecology 2010, 91, 3153–3164. [Google Scholar] [CrossRef]
- Sutherland, W. J.; Freckleton, R. P.; Godfray, H. C. J.; Beissinger, S. R.; Benton, T.; Cameron, D. D. . Wiegand, T. Identification of 100 fundamental ecological questions. J. Ecol. 2013, 101, 58–67. [Google Scholar] [CrossRef]
- Ghilarov, A. M. The paradox of the plankton reconsidered; or, why do species coexist? Oikos 1984, 43, 46–52. [Google Scholar] [CrossRef]
- Abrams, P. A. Ecological vs evolutionary consequences of competition. Oikos 1990, 57, 147–151. [Google Scholar] [CrossRef]
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




