1. Introduction
The prognostic impact of tumor-infiltrating lymphocyte (TIL) populations in cervical cancer is still debated, probably due to the fact that prognostic studies on TILs in cervical cancer cases are limited [
1]. This is surprising because the cytotoxic activities of TILs isolated from gynecologic malignant tumors (including uterine cervical cancers) against various fresh tumor cells were reported more than 30 years ago [
2]. In the following years, major effort was put on the isolation and characterization of TILs from cervical carcinomas [
3]. In order to define the anticancer-directed immune response
in situ, the group of Höhn
et al. characterized CD4(+) and CD8(+) T cells from peripheral blood lymphocytes, freshly harvested tumor tissue, and TIL from a patient with cervical cancer [
4]. The group of Santin
et al. found that cervical cancer TILs contain higher numbers of type 1 cytokine expressors and DR+ T cells compared with lymphocytes from tumor draining lymph nodes and peripheral blood [
5].
Additional research approaches include the immune concept of human papillomaviruses and related antigens in local cancer milieu of human cervical neoplasia [
6]. In that study, the presence of HPV-specific cytotoxic T lymphocytes (CTL) observed in the majority of cervical cancer patients provides an avenue for the further investigation of their functional role in modulating this malignancy. TILs develop as an expression of host immune system recognition and defense against malignant cells [
6]. Cancer cells can overcome immune surveillance by either downregulating the proliferation of HPV-specific CTL or altering the effector composition of immune cells against HPV infection. TILs in the tumor microenvironment may be functionally inhibited and lose the ability to clonally proliferate as a result of decreased expression of IL-2Rα [
6].
A more recent study on TIL characterization showed that tumor-infiltrating lymphocytes in cervical cancers contain a higher proportion of FoxP3(+) T lymphocytes [
7]. In addition, our own studies showed that high CCL22(+) infiltrating cells particularly M2-like macrophages, are associated with a poor outcome of cervical cancer patients [
8]. CCL22 expression is positively correlated with FoxP3 expression [
8], could polarize TAMs toward M2a macrophages [
9] and may represent a novel prognostic marker and therapeutic target for the treatment of cervical cancer.
Because the link between cervical cancer histopathological subtypes, prognosis and the level of TILs is still unclear, the aim of this study was to quantify the density of tumor-infiltrating immune cells, assessed as mononuclear cells located in the stroma, in a panel of 250 sporadic cervical cancers and investigate its correlation with cervical cancer subtype and patient survival.
2. Materials and Methods
2.1. Tissue sample
For this study, we included formalin fixed paraffin embedded cervical cancer samples of 250 patients (without distant metastasis), that underwent surgery in the years of 1993 until 2002 at the Department of Gynecology and Obstetrics, Ludwig Maximilians University Munich, Germany. This happened without any preselection. Only patients with tumors corresponding to squamous cell carcinoma and adenocarcinoma histological subtypes participated in the cohort. The clinical und follow-up data such as patient age, OS, lymph node status, tumor size, presence of metastases, histopathological grading, tumor subtype and FIGO (Fédération Internationale de Gynécologie et d'Obstétrique) stage, were retrieved from the Munich Cancer Registry.
2.2. Ethical approval
The tissue samples used in this study were left over material after all diagnostics had been completed and were retrieved from the archive of Gynecology and Obstetrics, Ludwig-Maximilian-University, Munich, Germany. All patients gave informed consent for additional research before undergoing surgery. The procedures were in accordance with the Helsinki declaration from 1975. All information and data of the patients were fully anonymized and encoded for further statistical analysis. This study was approved by the Ethics Committee of the Ludwig Maximilian University Munich, Germany.
2.3. Quantification of TILs
Tumor infiltrating inflammatory cells were quantified by an experienced gynaeco-pathologist (M-C.C). We adapted the scoring method of Klintrup et al. [
10] developed originally for the quantification of inflammatory cell reaction in colorectal cancer at the invasive margin, therefore representing immune cells around the tumor, and classified in 3 categories:
Score 1 = low and patchy increase of inflammatory cells at the invasive margin
Score 2 = increased inflammatory cells forming a band-like infiltration at the invasive margin
Score 3 = prominent inflammatory reaction forming a cup-like zone at the invasive margin
2.4. Correlation analyses
TIL levels were correlated with various staining’s of the same samples including glucocorticoid receptor, E6, LCoR, RIP140, nuclear p53, H3K9ac and H3K4me3, which had recently been published [
11,
12,
13].
2.5. Statistical analysis
For statistical analysis the IBM Statistical Package for the Social Sciences (IBM SPSS Statistic v24.0 Inc., Chicago, IL, USA) was used. Survival times were compared by Kaplan-Meier analysis. The Cox Mantel log-rank test was used for the differences in overall survival. Non-parametric tests, such as Kruskal-Wallis or Mann-Whitney-U tests, were performed for comparisons of different groups. A p-value < 0.05 was considered to be significant. The p-value and the number of patients analyzed in each group are given for each chart.
3. Results
3.1. Quantification of tumor infiltrating inflammatory cells
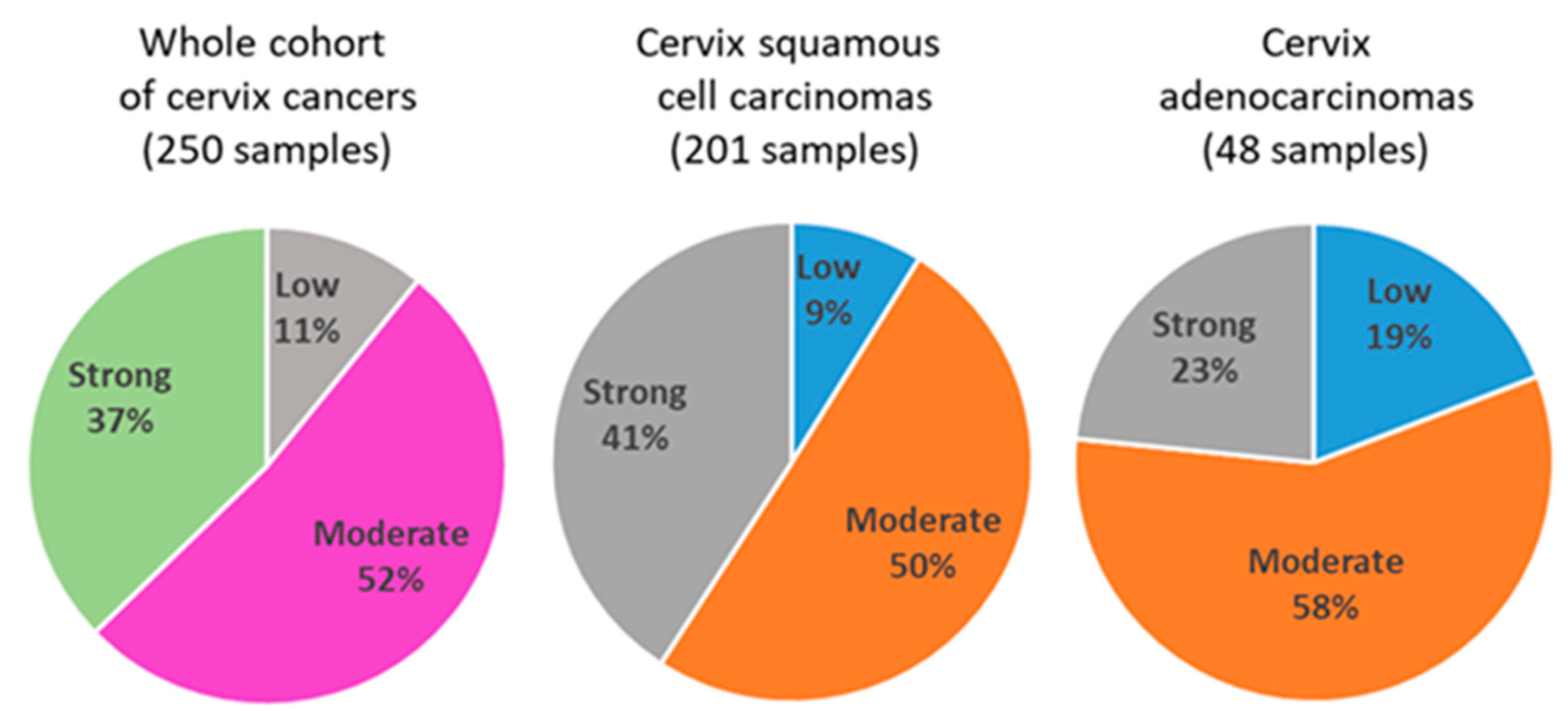
Quantification of tumor infiltrating inflammatory cells in the whole cohort (250 samples) revealed 26 cases (11%) with low level of inflammatory cells, 124 cases (52%) with moderate levels of inflammatory cells and 89 cases (37%) with strong infiltration of immune cells (
Figure 1). In squamous cell carcinoma samples (201 samples), we identified 17 cases (9%) with low, 96 cases (50%) with moderate and 78 cases (41%) with strong infiltration of immune cells. In adenocarcinoma samples, we found 9 cases (19%) with low, 27 cases (58%) with moderate and 11 cases (23%) with strong infiltration of immune cells.
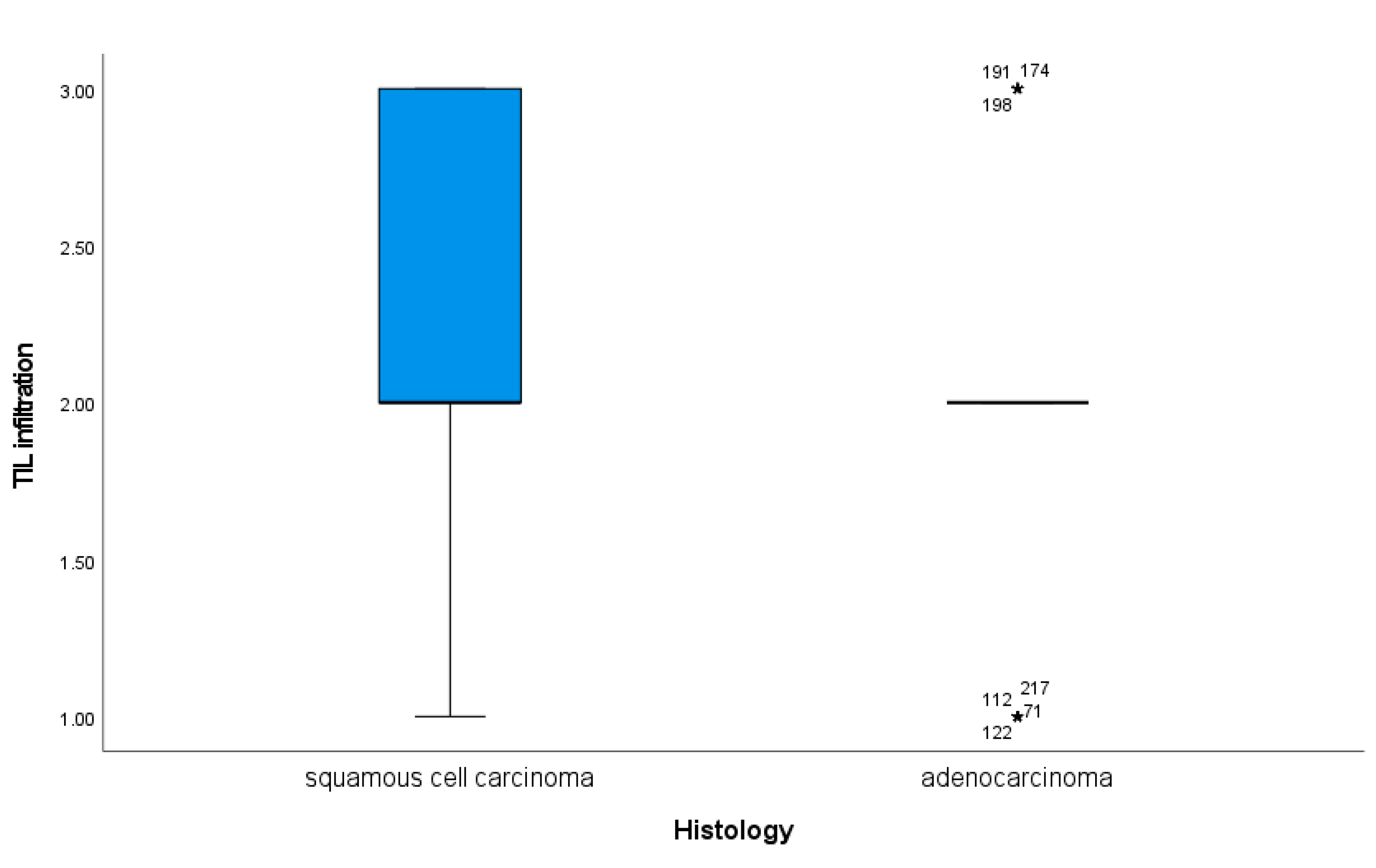
Infiltration of immune cells appeared significantly different according to the histological subtype of cervical cancer. Indeed, in cervix squamous cell carcinomas, we identified a significantly higher TIL levels as compared to cervical adenocarcinoma (
Figure 2, p = 0.01).
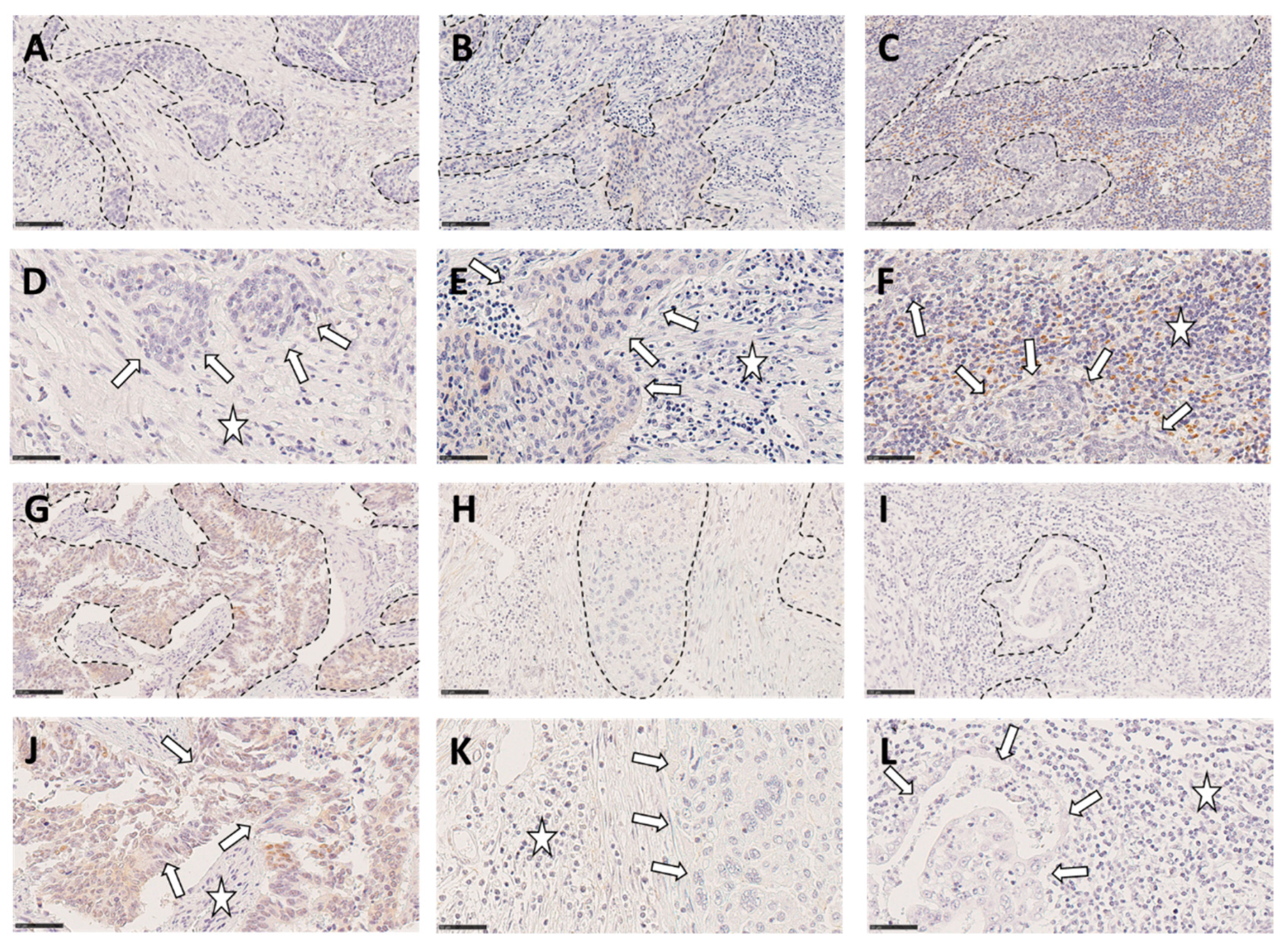
Examples for low, moderate and strong TIL infiltration in squamous cell carcinomas (upper row) and adenocarcinomas (lower row), respectively of cervical cancer tissue is shown in
Figure 3.
3.2. Correlation analyses of TIL levels with tumor properties and marker protein
We then analyzed the correlation of TIL levels with tumor properties and protein staining results obtained from former studies of our group. Results for squamous cell carcinomas are presented in
Table 1. In squamous cell carcinomas, TIL infiltration shows a negative correlation to age, FIGO stage and to the nuclear histone protein modification H3K4me3 [
14].
Correlation between TIL and cervical cancer prognostic markers in the adenocarcinoma subtype are shown in
Table 2. TIL shows a positive correlation to p16 expression [
15], a negative correlation to MDM2 expression [
15] and a positive correlation to the glucocorticoid receptor [
13].
3.3. Survival analyses according to histology
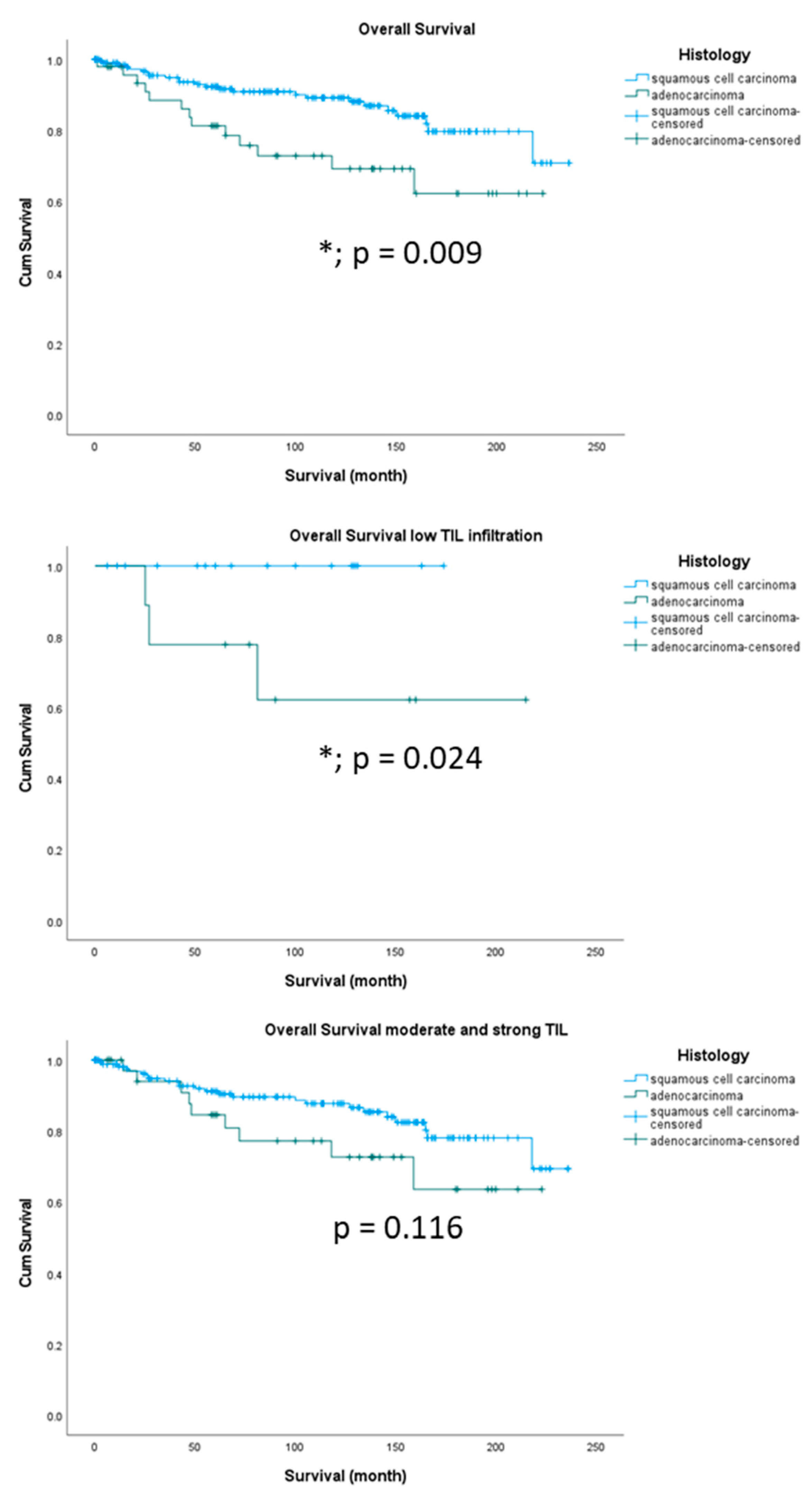
TIL levels were significantly enhanced in squamous cell carcinomas versus adenocarcinomas as shown in
Figure 2. Survival analyses of our patient cohort showed that patients with squamous cell carcinomas had a significantly better overall survival than patients with adenocarcinomas (
Figure 4A, p = 0.009) which is in concordance with the literature [
16]. Subgroup analyses including TIL revealed that this difference is determined by the group of patients with low TIL infiltration (
Figure 4B, p = 0.024). Patients with moderate and strong TIL showed no significant overall survival differences in squamous cell carcinomas or adenocarcinomas (
Figure 4C, p = 0.116). Survival analyses of our patient cohort showed that patients with squamous cell carcinomas had no significantly different disease-free survival compared to patients with adenocarcinomas (
Supplementary Figure S1, p = 0.088)
3.4. Survival analyses according to TIL in different histological subtypes of cervical cancer
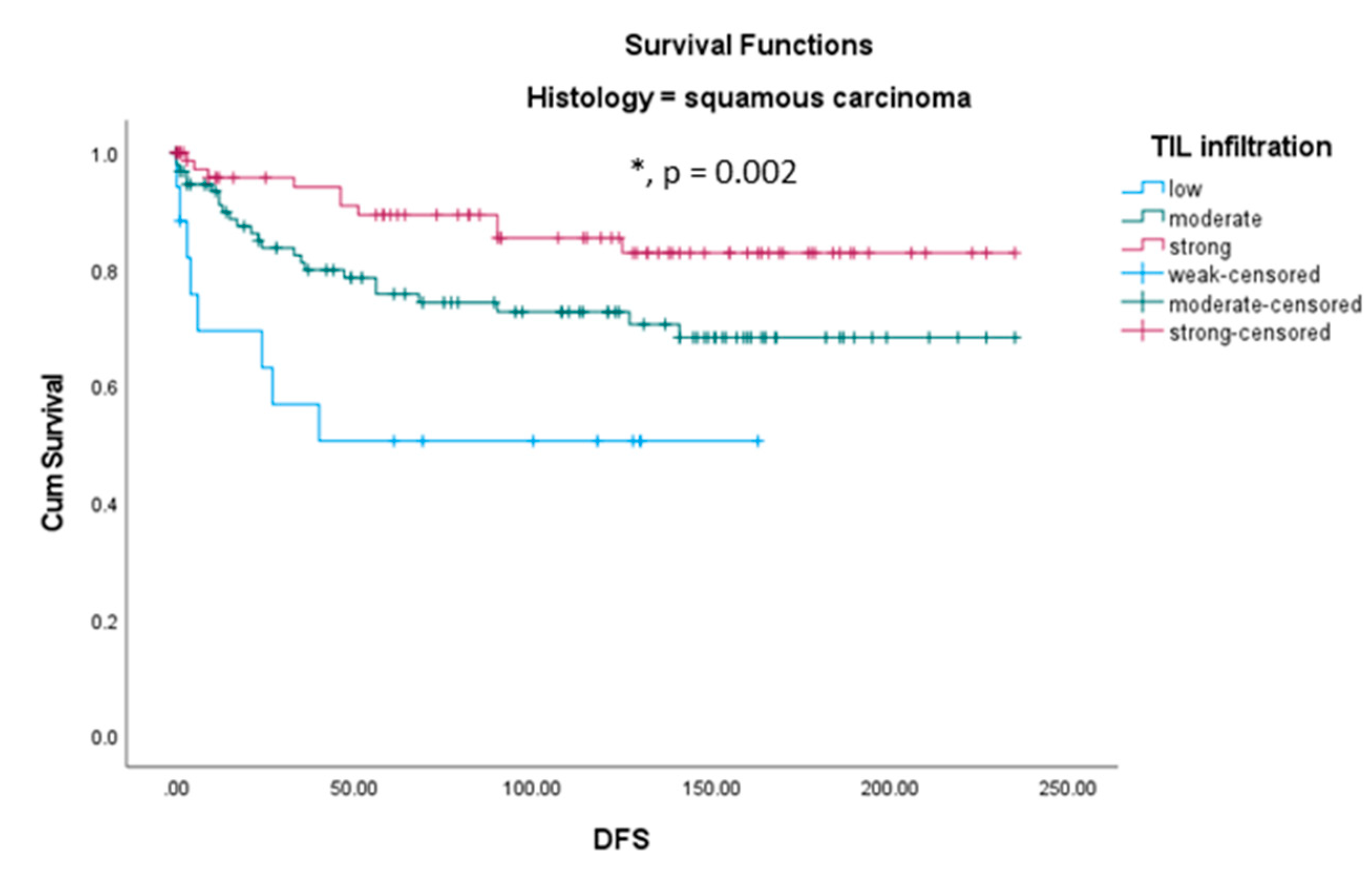
Within our cohort, we analyzed the influence of TIL infiltration on overall survival (OS) in the different histological subgroups. Neither in squamous cell carcinomas nor in adenocarcinomas, TIL level was a prognosticator for overall survival (data not shown). In opposite, immune infiltration was a strong positive prognosticator for disease free survival (DFS) in patients with squamous cell carcinomas. Patients with a squamous carcinoma showing low TIL infiltration showed the shortest DFS time while patients with strong TIL infiltration showed the longest DFS time and patients with moderate TIL infiltration were between both groups (
Figure 6). In patients with adenocarcinomas, TIL level was without prognostic value.
Due to the discrepancy between short DFS in patients with scc and low TIL and long OS (
Figure S1) we compared both groups. There was no significant difference for age, grading, pT, pN or FIGO-stage (Data not shown). DSF and OS were similar in scc with moderate and strong immune infiltration (
Figure S2).
Table 4.
Mean DFS according to TIL infiltration (low, moderate and strong) in squamous cell carcinomas. Estimate = mean survival in month.
Table 4.
Mean DFS according to TIL infiltration (low, moderate and strong) in squamous cell carcinomas. Estimate = mean survival in month.
| Means for Survival Time |
|
|
|
|
|
| Histology |
TIL |
Mean |
|
|
|
| |
|
Estimate |
Std. Error |
95% Confidence Interval |
|
| |
|
|
|
Lower Bound |
Upper Bound |
| squamous cell carcinoma |
low |
88.798 |
18.835 |
51.882 |
125.714 |
| |
moderate |
174.022 |
10.521 |
153.401 |
194.643 |
| |
strong |
204.295 |
8.942 |
186.768 |
221.821 |
| |
Overall |
181.431 |
7.160 |
167.398 |
195.465 |
Multivariate Cox regression including tumor parameter, age and TIL infiltration identified TIL, tumor size (pT) and grading as independent prognostic factors for DFS in squamous cell carcinomas (
Table 5).
4. Discussion
Within this study, we analyzed the density of tumor-infiltrating immune cells using a quantification method adapted from Klintrup criteria [
17,
18], in a cohort of 250 cervical cancer cases in relation to the histological subtype and patient survival. TIL were significantly enhanced in squamous cell carcinomas versus adenocarcinomas. Survival analyses of our patient cohort showed that patients with squamous cell carcinomas had a significantly better overall survival than patients with adenocarcinomas, this difference being observed only in the group of patients with low TIL. This is in line with with Chen et al, who describe a shorter overall survival for patients with less intraepithelial CD8+ lymphocyte counts[
19]. Interestingly, immune infiltration was an independent positive prognosticator for DFS in patients with squamous cell carcinomas.
Because survival rates are different in squamous cell carcinomas compared to adenocarcinomas of the cervix, we performed correlation analyses separately for both histological subtypes. In the group of squamous cell carcinomas, tumor infiltration by immune cells was negatively correlated to age, FIGO stage and to the histone protein modification H3K4me3, the latter was analyzed in a recent study of our group [
6]. H3K4 methylation is a modification that occurs at the fourth lysine residue of the N-terminus of histone H3. It can be mono-, di- and trimethylated, which makes the analysis of its effects on the genome even more complex [
20,
21]. H3K4me3 is generally associated with transcriptional activation and has been proposed as a predictive factor of poor prognosis in several types of cancer, such as liver and cervical cancer [
14,
22]. In our former analyses, high expression of H3K4me3 was associated with reduced overall and recurrence free survival, this is accordance with our negative correlation results with TIL infiltration. Within this study, we found that squamous carcinoma patients with strong TIL infiltration showed the longest DFS time.
In the group of adenocarcinoma patients, tumor infiltration by immune cells showed a positive correlation to p16 [
15] and to the glucocorticoid receptor (GR) [
13] and a negative correlation to MDM2 [
15]. The cell cycle regulation protein p16 is expressed at high levels in HPV infected epithelial cells, which is why it acts as a marker for the diagnosis of an HPV associated carcinoma [
23,
24]. The positive association of high TIL rates and p16 expression was already described in a variety of carcinomas including oropharyngeal and hypopharyngeal [
25,
26], breast [
27], oropharynx squamous cell carcinomas [
28] and others but not in cervical cancer cases and not related to histopathology. MDM2 promotes the ubiquitination and degradation of p53 [
29]. On the one hand p53 is regulated by MDM2 oncoprotein through a negative feedback mechanism in non-carcinoma tissue. On the other hand, there is an association between MDM2 and p53 polymorphisms and the advancement of cervical carcinoma [
30]. Again, our findings are in agreement with another study on head and neck squamous cell carcinomas, showing that proliferative lymphocytes are vulnerable to MDM2 inhibition [
31]. This finding might explain that high expression of MDM2 is associated with low TIL rates in the adenocarcinoma subtype of cervical cancer. Finally, we detected a positive correlation of TIL and GR in adenocarcinoma cases. Although this relationship was not described before, triple-negative breast cancers with expression of glucocorticoid receptor in immune cells showed a better prognosis [
32]. Our former study showed the same result, an advanced GR expression went along with significant better overall-survival compared to low GR expression in cervical cancer cells [
13].
It has been long known that squamous cell carcinomas had a significantly better overall survival than patients with adenocarcinomas [
33,
34]. This was also conformed with our collection of patients with cervical cancer. Inclusion of TIL revealed that this effect is determined only in the group of low TIL infiltration. In addition, in that group, no patient with squamous cell carcinoma and low TIL infiltration deceased. Although this concerns only a small group of patients (17 out of 250; 6.8%), this subgroup can be reassured about their overall survival rate.
In contrast, in squamous cell carcinoma cases, patients with low peritumoral inflammation showed a very short disease-free survival time. Immune infiltration was an independent positive prognosticator for disease free survival (DFS) in patients with squamous cell carcinomas. Squamous carcinoma patients with low TIL infiltration showed the shortest DFS time whereas squamous carcinoma patients with strong TIL infiltration showed the longest DFS time. On the first view, this seems to be contradictory to the overall survival rate of patients with low TIL. In general, recurrence is not protective and this is also true within our study group. We see a strong correlation of recurrence and fatality rate in the whole cohort of squamous carcinoma cases (Correlation Coefficient = 0.451, p < 0.001). Therefore, only the group of patients with low TIL seems to be not affected by worse outcome in combination with early recurrence. In other tumor entities like oral squamous carcinomas (OSCC), TIL in the nonrecurrent group were significantly higher than those in the recurrent group [
35]. In addition, a high ratio of TILs was associated with an overall survival improvement in OSCC patients. This is in opposite to our findings on cervical cancer. On the other hand, low PD-L1 expression in TIL predicted local recurrence in oral squamous cell carcinomas [
36]. Although we did not investigate PD-L1 on TILs, this could also be an explanation for our findings.
This study has some limitations, considering its retrospective nature, and the way TILs was assessed. For instance, we herein only performed a global analysis of TILs, and since these cells may be immunogenic or immune-suppressive, more precise methods based on immunohistochemical detection of the different lymphocyte subtypes (including cytotoxic and regulatory T cells, or B/plasma cells) would have been more informative. These points will be addressed in further studies, which are also needed to determine the prognostic value of checkpoint molecule expression on different TIL populations.
5. Conclusions
TIL infiltration is an independent positive prognosticator for disease free survival (DFS) in patients with squamous cell carcinomas. Squamous carcinoma patients with low TIL in-filtration showed the shortest DFS time whereas squamous carcinoma patients with strong TIL infiltration showed the longest DFS time and patients with moderate TIL in-filtration were between both groups. Neither in squamous cell carcinomas nor in adenocarcinomas TIL level was a prognosticator for overall survival.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org., Figure S1: Survival analyses in combination with TIL infiltration.
Author Contributions
Conceptualization, V.C. and U.J.; methodology, F.G.; software, M.K.; validation, H.H., C.D. and A.V.; formal analysis, H.H.; investigation, M.C.C., F.B.M.; resources, M.K.; data curation, V.C.; writing—original draft preparation, U.J. and M.W.; writing—review and editing, V.C.; visualization, M.C.C.; supervision, U.J.; project administration, U.J. and C.D.; funding acquisition, C.D. and F.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Medical Faculty of the University Augsburg, Doctoral Funding Program for Fabian Garrido and supported by the Medical Faculty of the UKA.
Institutional Review Board Statement
All procedures involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki declaration of 1964 and its later amendments or comparable ethical standards. The study was approved by the local ethics committee of the Ludwig-Maximilians University of Munich (reference number 259-16, 2016).
Informed Consent Statement
When the current study was performed, all diagnostic procedures were completed, and the patients’ data were anonymized. The ethical principles adopted in the Declaration of Helsinki 1975 have been respected. As per the declaration of our ethics committee, no written informed consent of the participants or permission to publish is needed given the circumstances described above. Researchers were blinded from patient data during experimental and statistical analysis.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical issues.
Acknowledgments
We would like to thank Christina Kuhn (MTA) for excellent technical assistance. The authors would like to thank Jutta Engel, M.P.H. and Max Wiedemann (The Munich Cancer Registry of the Tumorzentrum München [TZM—Munich Tumor Center]) for the follow-up data.
Conflicts of Interest
C.D. is funded by Roche, AstraZeneca, TEVA, Mentor, MCI Healthcare. All other authors declare no conflict of interest.
References
- Kazemi, M.H.; Sadri, M.; Najafi, A.; Rahimi, A.; Baghernejadan, Z.; Khorramdelazad, H.; Falak, R. Tumor-infiltrating lymphocytes for treatment of solid tumors: It takes two to tango? Front Immunol 2022, 13, 1018962. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Yahata, G.; Takeuchi, S.; Seidoh, T.; Tanaka, K. A correlation between the expression of CD 8 antigen and specific cytotoxicity of tumor-infiltrating lymphocytes. Jpn J Cancer Res 1989, 80, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Hilders, C.G.; Ras, L.; van Eendenburg, J.D.; Nooyen, Y.; Fleuren, G.J. Isolation and characterization of tumor-infiltrating lymphocytes from cervical carcinoma. International journal of cancer 1994, 57, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Hohn, H.; Pilch, H.; Gunzel, S.; Neukirch, C.; Freitag, K.; Necker, A.; Maeurer, M.J. Human papillomavirus type 33 E7 peptides presented by HLA-DR*0402 to tumor-infiltrating T cells in cervical cancer. Journal of virology 2000, 74, 6632–6636. [Google Scholar] [CrossRef] [PubMed]
- Santin, A.D.; Ravaggi, A.; Bellone, S.; Pecorelli, S.; Cannon, M.; Parham, G.P.; Hermonat, P.L. Tumor-infiltrating lymphocytes contain higher numbers of type 1 cytokine expressors and DR+ T cells compared with lymphocytes from tumor draining lymph nodes and peripheral blood in patients with cancer of the uterine cervix. Gynecologic oncology 2001, 81, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Sheu, B.C.; Chang, W.C.; Lin, H.H.; Chow, S.N.; Huang, S.C. Immune concept of human papillomaviruses and related antigens in local cancer milieu of human cervical neoplasia. The journal of obstetrics and gynaecology research 2007, 33, 103–113. [Google Scholar] [CrossRef]
- Wu, M.Y.; Kuo, T.Y.; Ho, H.N. Tumor-infiltrating lymphocytes contain a higher proportion of FOXP3(+) T lymphocytes in cervical cancer. Journal of the Formosan Medical Association = Taiwan yi zhi 2011, 110, 580–586. [Google Scholar] [CrossRef]
- Wang, Q.; Schmoeckel, E.; Kost, B.P.; Kuhn, C.; Vattai, A.; Vilsmaier, T.; Mahner, S.; Mayr, D.; Jeschke, U.; Heidegger, H.H. Higher CCL22+ Cell Infiltration is Associated with Poor Prognosis in Cervical Cancer Patients. Cancers 2019, 11. [Google Scholar] [CrossRef]
- Wang, Q.; Sudan, K.; Schmoeckel, E.; Kost, B.P.; Kuhn, C.; Vattai, A.; Vilsmaier, T.; Mahner, S.; Jeschke, U.; Heidegger, H.H. CCL22-Polarized TAMs to M2a Macrophages in Cervical Cancer In Vitro Model. Cells 2022, 11. [Google Scholar] [CrossRef]
- Klintrup, K.; Makinen, J.M.; Kauppila, S.; Vare, P.O.; Melkko, J.; Tuominen, H.; Tuppurainen, K.; Makela, J.; Karttunen, T.J.; Makinen, M.J. Inflammation and prognosis in colorectal cancer. Eur J Cancer 2005, 41, 2645–2654. [Google Scholar] [CrossRef]
- Tang, D.; Wu, D.; Hirao, A.; Lahti, J.M.; Liu, L.; Mazza, B.; Kidd, V.J.; Mak, T.W.; Ingram, A.J. ERK activation mediates cell cycle arrest and apoptosis after DNA damage independently of p53. The Journal of biological chemistry 2002, 277, 12710–12717. [Google Scholar] [CrossRef] [PubMed]
- Freier, C.P.; Stiasny, A.; Kuhn, C.; Mayr, D.; Alexiou, C.; Janko, C.; Wiest, I.; Jeschke, U.; Kost, B. Immunohistochemical Evaluation of the Role of p53 Mutation in Cervical Cancer: Ser-20 p53-Mutant Correlates with Better Prognosis. Anticancer Res 2016, 36, 3131–3137. [Google Scholar] [PubMed]
- Kost, B.P.; Beyer, S.; Schroder, L.; Zhou, J.; Mayr, D.; Kuhn, C.; Schulze, S.; Hofmann, S.; Mahner, S.; Jeschke, U.; et al. Glucocorticoid receptor in cervical cancer: an immunhistochemical analysis. Arch Gynecol Obstet 2019, 299, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Beyer, S.; Zhu, J.; Mayr, D.; Kuhn, C.; Schulze, S.; Hofmann, S.; Dannecker, C.; Jeschke, U.; Kost, B.P. Histone H3 Acetyl K9 and Histone H3 Tri Methyl K4 as Prognostic Markers for Patients with Cervical Cancer. Int J Mol Sci 2017, 18. [Google Scholar] [CrossRef]
- Stiasny, A.; Freier, C.P.; Kuhn, C.; Schulze, S.; Mayr, D.; Alexiou, C.; Janko, C.; Wiest, I.; Dannecker, C.; Jeschke, U.; et al. The involvement of E6, p53, p16, MDM2 and Gal-3 in the clinical outcome of patients with cervical cancer. Oncol Lett 2017, 14, 4467–4476. [Google Scholar] [CrossRef]
- Silcocks, P.B.; Thornton-Jones, H.; Murphy, M. Squamous and adenocarcinoma of the uterine cervix: a comparison using routine data. Br J Cancer 1987, 55, 321–325. [Google Scholar] [CrossRef]
- Vayrynen, J.P.; Kantola, T.; Vayrynen, S.A.; Klintrup, K.; Bloigu, R.; Karhu, T.; Makela, J.; Herzig, K.H.; Karttunen, T.J.; Tuomisto, A.; et al. The relationships between serum cytokine levels and tumor infiltrating immune cells and their clinical significance in colorectal cancer. International journal of cancer 2016, 139, 112–121. [Google Scholar] [CrossRef]
- Vayrynen, J.P.; Sajanti, S.A.; Klintrup, K.; Makela, J.; Herzig, K.H.; Karttunen, T.J.; Tuomisto, A.; Makinen, M.J. Characteristics and significance of colorectal cancer associated lymphoid reaction. International journal of cancer 2014, 134, 2126–2135. [Google Scholar] [CrossRef]
- Chen, T.H.; Fukuhara, K.; Mandai, M.; Matsumura, N.; Kariya, M.; Takakura, K.; Fujii, S. Increased cyclooxygenase-2 expression is correlated with suppressed antitumor immunity in cervical adenocarcinomas. Int J Gynecol Cancer 2006, 16, 772–779. [Google Scholar] [CrossRef]
- Takahashi, Y.H.; Lee, J.S.; Swanson, S.K.; Saraf, A.; Florens, L.; Washburn, M.P.; Trievel, R.C.; Shilatifard, A. Regulation of H3K4 trimethylation via Cps40 (Spp1) of COMPASS is monoubiquitination independent: implication for a Phe/Tyr switch by the catalytic domain of Set1. Mol Cell Biol 2009, 29, 3478–3486. [Google Scholar] [CrossRef]
- Takahashi, Y.H.; Shilatifard, A. Structural basis for H3K4 trimethylation by yeast Set1/COMPASS. Adv Enzyme Regul 2010, 50, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shen, L.; Chen, K.N. Association between H3K4 methylation and cancer prognosis: A meta-analysis. Thorac Cancer 2018, 9, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Balasubramanian, A.; Yu, M.; Kiviat, N.; Ridder, R.; Reichert, A.; Herkert, M.; von Knebel Doeberitz, M.; Koutsky, L.A. Evaluation of a new p16(INK4A) ELISA test and a high-risk HPV DNA test for cervical cancer screening: results from proof-of-concept study. International journal of cancer. Journal international du cancer 2007, 120, 2435–2438. [Google Scholar] [CrossRef] [PubMed]
- Melkane, A.E.; Mirghani, H.; Auperin, A.; Saulnier, P.; Lacroix, L.; Vielh, P.; Casiraghi, O.; Griscelli, F.; Temam, S. HPV-related oropharyngeal squamous cell carcinomas: a comparison between three diagnostic approaches. American journal of otolaryngology 2014, 35, 25–32. [Google Scholar] [CrossRef]
- Atipas, K.; Laokulrath, N.; Petsuksiri, J.; Ratanaprasert, N.; Pongsapich, W. CD8+ T Cells and PD-L1 Expression as Prognostic Indicators in a Low Prevalence of HPV-Associated Oropharyngeal Squamous Cell Carcinoma. Current oncology 2023, 30, 1450–1460. [Google Scholar] [CrossRef]
- Ji, M.; Lin, L.; Huang, Q.; Hu, C.; Zhang, M. HPV16 status might correlate to increasing tumor-infiltrating lymphocytes in hypopharyngeal cancer. Acta oto-laryngologica 2023, 1–8. [Google Scholar] [CrossRef]
- Calderon, G.; Castaneda, C.A.; Castillo, M.; Sanchez, J.; Bernabe, L.; Suarez, N.; Tello, K.; Torres, E.; Cotrina, J.M.; Dunstan, J.; et al. Human Papillomavirus, Cytomegalovirus Infection and P16 Staining in Breast Tumors from Peruvian Women. Asian Pacific journal of cancer prevention : APJCP 2022, 23, 1571–1576. [Google Scholar] [CrossRef]
- Ljokjel, B.; Haave, H.; Lybak, S.; Vintermyr, O.K.; Helgeland, L.; Aarstad, H.J. Tumor Infiltration Levels of CD3, Foxp3 (+) Lymphocytes and CD68 Macrophages at Diagnosis Predict 5-Year Disease-Specific Survival in Patients with Oropharynx Squamous Cell Carcinoma. Cancers 2022, 14. [Google Scholar] [CrossRef]
- Assmann, G.; Sotlar, K. [HPV-associated squamous cell carcinogenesis]. Pathologe 2011, 32, 391–398. [Google Scholar] [CrossRef]
- Adams, A.K.; Wise-Draper, T.M.; Wells, S.I. Human papillomavirus induced transformation in cervical and head and neck cancers. Cancers 2014, 6, 1793–1820. [Google Scholar] [CrossRef]
- Shao, S.; Scholtz, L.U.; Gendreizig, S.; Martinez-Ruiz, L.; Florido, J.; Escames, G.; Schurmann, M.; Hain, C.; Hose, L.; Mentz, A.; et al. Primary head and neck cancer cell cultures are susceptible to proliferation of Epstein-Barr virus infected lymphocytes. BMC cancer 2023, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, J.S.; Patil, S.; Rajarajan, S.; Ce, A.; Nair, M.; Alexander, A.; Ramesh, R.; Bs, S.; Sridhar, T. Triple-negative breast cancers with expression of glucocorticoid receptor in immune cells show better prognosis. Annals of oncology : official journal of the European Society for Medical Oncology 2021, 32. [Google Scholar] [CrossRef]
- Chen, R.J.; Lin, Y.H.; Chen, C.A.; Huang, S.C.; Chow, S.N.; Hsieh, C.Y. Influence of histologic type and age on survival rates for invasive cervical carcinoma in Taiwan. Gynecologic oncology 1999, 73, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, K.; Hrafnkelsson, J.; Geirsson, G.; Gudmundsson, J.; Salvarsdottir, A. Screening as a prognostic factor in cervical cancer: analysis of survival and prognostic factors based on Icelandic population data, 1964-1988. Gynecologic oncology 1991, 43, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Yamasaki, S.; Shintani, T.; Matsui, K.; Obayashi, F.; Koizumi, K.; Tani, R.; Yanamoto, S.; Okamoto, T. Tumor-Infiltrating CD45RO(+) Memory Cells Are Associated with Favorable Prognosis in Oral Squamous Cell Carcinoma Patients. Cancers 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, N.; Nambiar, A.; Dhar, S.; Thankappan, K.; Koyakutty, M.; Balasubramanian, D.; Das, M.; Iyer, S. Low PDL1 Expression in Tumour Infiltrating Lymphocytes Predicts Local Recurrence in Oral Squamous Cell Carcinoma. Indian journal of surgical oncology 2021, 12, 408–414. [Google Scholar] [CrossRef]
Figure 1.
Quantification of tumor infiltrating inflammatory cells in cervical cancer tissue in the whole cohort, in squamous cell carcinoma and in adenocarcinoma tissues.
Figure 1.
Quantification of tumor infiltrating inflammatory cells in cervical cancer tissue in the whole cohort, in squamous cell carcinoma and in adenocarcinoma tissues.
Figure 2.
Box-plot of tumor infiltrating inflammatory cells in cervical cancer tissue in squamous cell carcinoma and adenocarcinoma cases. Boxes present the 50th percentile and error bars the 5th to 95th percentile with the median in the middle. Asterisks with case numbers represent outliers.
Figure 2.
Box-plot of tumor infiltrating inflammatory cells in cervical cancer tissue in squamous cell carcinoma and adenocarcinoma cases. Boxes present the 50th percentile and error bars the 5th to 95th percentile with the median in the middle. Asterisks with case numbers represent outliers.
Figure 3.
Illustration of low (A, D, G, J), moderate (B, E, H, K) or marked (C, F, I, L) inflammation in 3 squamous cell carcinomas (A-F) and 3 adenocarcinomas (G-L). Tumors nests are surrounded by dotted line; (A-C and G-I Scale bar: 100µm (Magnification x200). For panels D-F and J-L, arrows and stars indicate neoplastic cells and infiltrating inflammatory cells area, respectively. Scale bar: 50µm (Magnification x400).
Figure 3.
Illustration of low (A, D, G, J), moderate (B, E, H, K) or marked (C, F, I, L) inflammation in 3 squamous cell carcinomas (A-F) and 3 adenocarcinomas (G-L). Tumors nests are surrounded by dotted line; (A-C and G-I Scale bar: 100µm (Magnification x200). For panels D-F and J-L, arrows and stars indicate neoplastic cells and infiltrating inflammatory cells area, respectively. Scale bar: 50µm (Magnification x400).
Figure 4.
Kaplan-Meier survival analyses for overall survival in different histological subtypes of cervical cancer (A) and according the level of immune infiltration on overall survival (OS). OS differences between histological subtype is determined by the level of TIL infiltration. Only patients with low TIL infiltration showed significant differences (B), whereas patients with moderate and strong TIL had no OS differences according to histological subtype (C).
Figure 4.
Kaplan-Meier survival analyses for overall survival in different histological subtypes of cervical cancer (A) and according the level of immune infiltration on overall survival (OS). OS differences between histological subtype is determined by the level of TIL infiltration. Only patients with low TIL infiltration showed significant differences (B), whereas patients with moderate and strong TIL had no OS differences according to histological subtype (C).
Figure 6.
Kaplan-Meier survival analyses for disease free survival (DFS) according to immune infiltration in squamous cell cervical carcinoma cases.
Figure 6.
Kaplan-Meier survival analyses for disease free survival (DFS) according to immune infiltration in squamous cell cervical carcinoma cases.
Table 1.
The table shows correlation coefficient, significance (Sig.) and number of cases (N) in squamous cell carcinomas.
Table 1.
The table shows correlation coefficient, significance (Sig.) and number of cases (N) in squamous cell carcinomas.
| Peritumoral inflammation |
Correlation Coefficient |
1.000 |
| |
Sig. (2-tailed) |
. |
| |
N |
191 |
| Age |
Correlation Coefficient |
-.177 |
| |
Sig. (2-tailed) |
.015 |
| |
N |
187 |
| FIGO |
Correlation Coefficient |
-.184 |
| |
Sig. (2-tailed) |
.011 |
| |
N |
191 |
| H3K4me3 (nuclear) |
Correlation Coefficient |
-.293 |
| |
Sig. (2-tailed) |
<.001 |
| |
N |
191 |
Table 2.
Correlation between TIL and cervical cancer prognostic markers in the adenocarcinoma subtype. The table shows correlation coefficient, significance (Sig.) and number of cases (N) in adenocarcinoma tissue.
Table 2.
Correlation between TIL and cervical cancer prognostic markers in the adenocarcinoma subtype. The table shows correlation coefficient, significance (Sig.) and number of cases (N) in adenocarcinoma tissue.
| Immune infiltration |
Correlation Coefficient |
1.000 |
| |
Sig. (2-tailed) |
. |
| |
N |
47 |
| p16 (cytoplasmic) |
Correlation Coefficient |
.322 |
| |
Sig. (2-tailed) |
.031 |
| |
N |
45 |
| MDM2 (nuclear) |
Correlation Coefficient |
-.422 |
| |
Sig. (2-tailed) |
.003 |
| |
N |
47 |
| Glucocorticoid receptor (nuclear) |
Correlation Coefficient |
.389 |
| |
Sig. (2-tailed) |
.007 |
| |
N |
47 |
Table 3.
Mean survival time according to histology. Estimate = mean survival in month.
Table 3.
Mean survival time according to histology. Estimate = mean survival in month.
| Histology |
Mean |
|
|
|
| |
Estimate |
Std. Error |
95% Confidence Interval |
| |
|
|
Lower Bound |
Upper Bound |
| squamous cell carcinoma |
205.785 |
5.629 |
194.752 |
216.818 |
| Adenocarcinoma |
165.761 |
13.145 |
139.997 |
191.524 |
| Overall |
199.287 |
5.420 |
188.664 |
209.909 |
Table 5.
Multivariate Cox regression analysis of immune infiltration and tumor parameter in relation to DFS in squamous cell carcinomas. pN = lymph node involvement, pM = distant metastasis, pT = tumor size.
Table 5.
Multivariate Cox regression analysis of immune infiltration and tumor parameter in relation to DFS in squamous cell carcinomas. pN = lymph node involvement, pM = distant metastasis, pT = tumor size.
| |
Coefficient |
Significance |
Hazard ratio |
95% Confidence Interval |
| |
|
|
|
Lower |
Upper |
| Immune infiltration |
-.551 |
.015 |
.576 |
.370 |
.898 |
| pN |
.349 |
.258 |
1.418 |
.774 |
2.599 |
| pM |
-.521 |
.138 |
.594 |
.298 |
1.183 |
| age |
.007 |
.524 |
1.007 |
.986 |
1.029 |
| pT |
.238 |
.005 |
1.268 |
1.073 |
1.499 |
| FIGO |
.003 |
.914 |
1.003 |
.943 |
1.068 |
| Grading |
.488 |
.046 |
1.628 |
1.010 |
2.626 |
| Histology |
.348 |
.289 |
1.417 |
.744 |
2.698 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).