Submitted:
21 July 2023
Posted:
26 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Dry rot of Potato Tuber
2.1. The causal agent causing dry rot
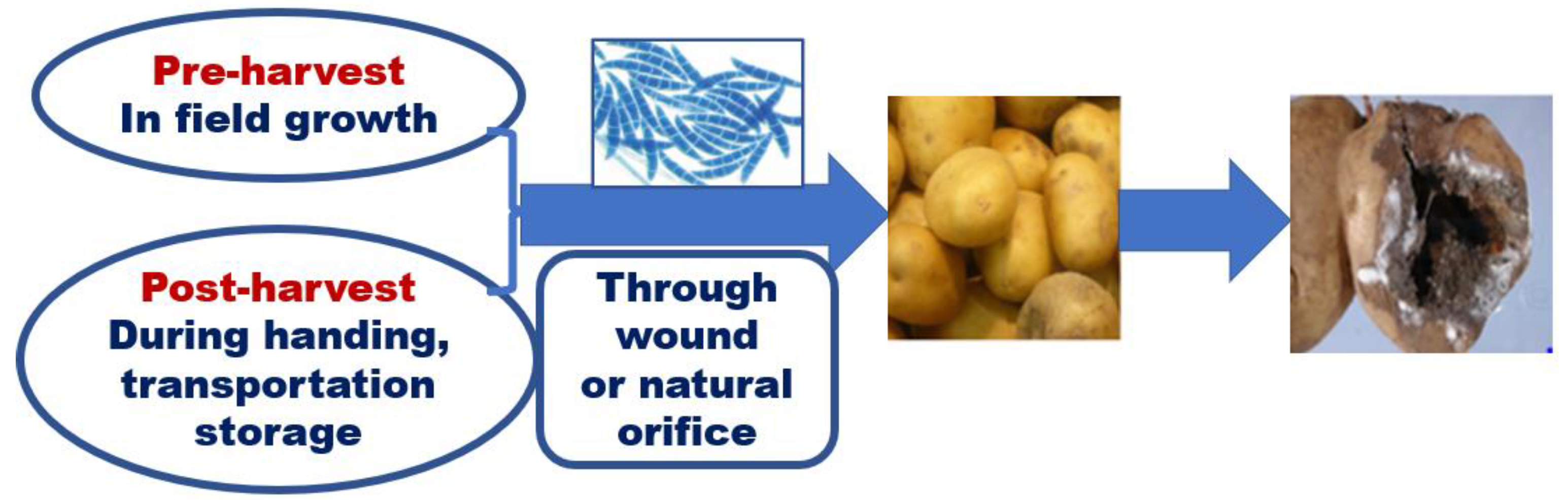
2.2. Pathogen infection and the symptom of potato dry rot
| Fusarium species | region | reference |
|---|---|---|
| F. sambucinum | North Amercian and some regions of Europe | [16,17,18] |
|
F. coeruleum F. graminearum F. solani and F. oxysporum F. sulphureum and F. solani F. sambucinum F. oxysporum F. verticillioides F. incarnatum F. avenaceum, F. oxysporum, F.sporotrichiodes F. solani, F. trichothecioides, F. solani var. coeruleum F. sambucinum F. semitectum, F. solani F. sambucinum F. culmorum, F. gibbosum, F. macroceras, F. solani var. coeruleum, F. acuminatum, F. equiseti and F. redolens F. sambucinem F. avenaceum F. solani var. coeruleum, F. oxysporum, F. acuminatum F. sambucinem F. avenaceum F. graminearum F. solani F. sulphureum |
United Kingdom and Great Britain North Dakota South Africa Michigan Iran Egypt Heilongjiang Province and Inner Mongolia Autonomous Region North of China Shanxi Province Northwest of China, Gansu Province, Ningxia Hui Autonomous Region |
[19,20,21] [22] [23,24] [25] [26] [27] [27] [7,8,10,11,12,27] |
|
F. tricinctum, F. avenaceum, F. oxysporum, F. solani, F. acuminatum, F. equiseti F. solani, F. moniliform, F. redolens |
Northwest of China, Qinghai Province South of China Zhejiang Province |
[6] [6] |
2.3. Mycotoxin accumulation associated with Fusarium dry rot
2.4. Dry rot of control
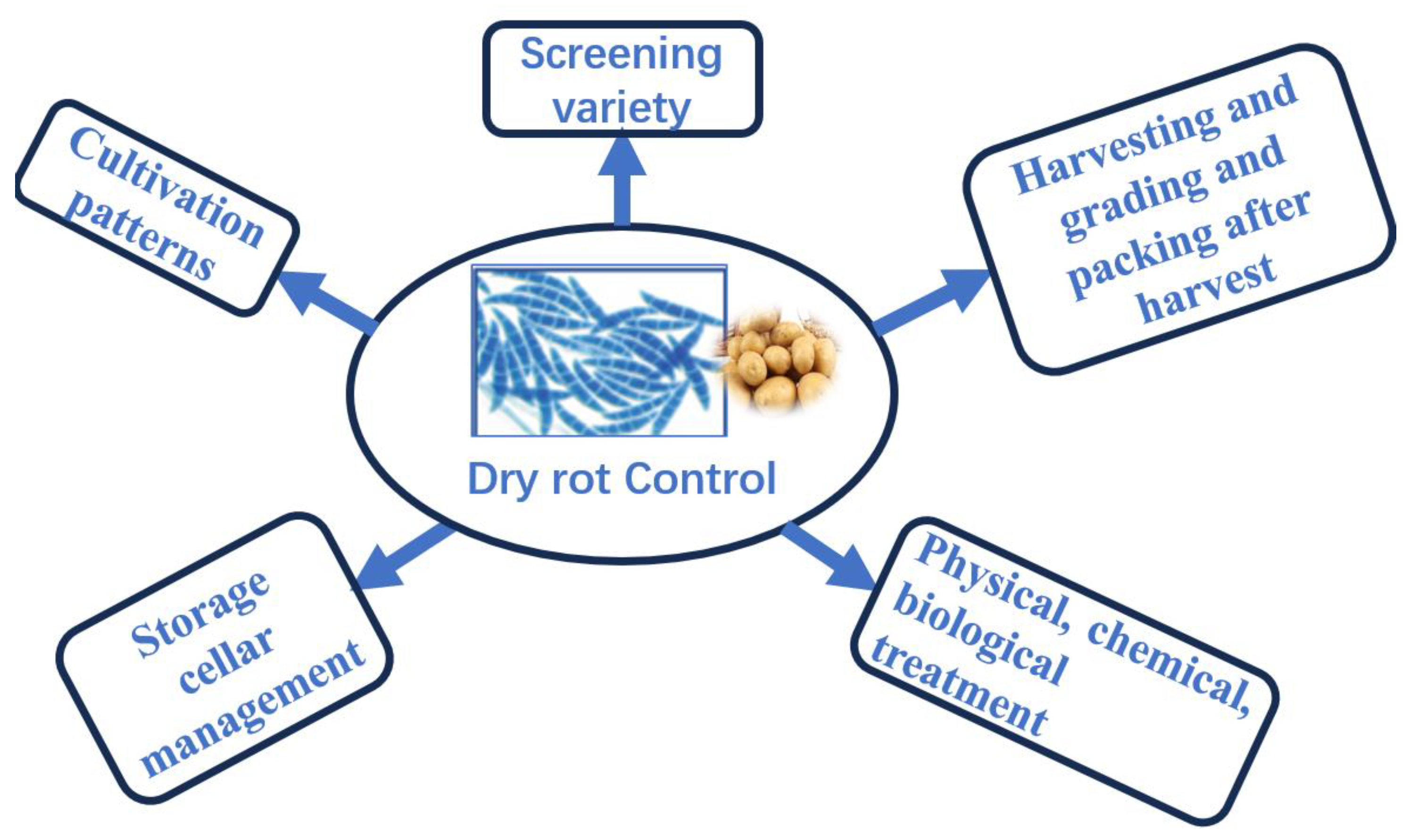
2.4.1. Varieties Screening
2.4.2. Cultivation patterns
2.4.3. Harvesting and grading and packing after harvest
2.4.4. Storage cellar management
2.4.5. Physical, chemical, and biological treatment
2.4.5.1. Physical treatment
2.4.5.2. Chemical treatment
2.4.5.3. Biological treatment
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Sun, Z.Q.; Zou, Y.P.; Li, W.H.; He, F.Y.; Huang, X.Y.; Lin, C.L.; Cai, Q.N.; Wisniewski, M.; Wu, X.H. Pre- and postharvest measures used to control decay and mycotoxigenic fungi in potato (Solanum tuberosum L.) during storage. Critical Reviews in Food Science and Nutrition 2022, 62, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, T.; Song, Y.; Feng, L.; Kear, P.J.; Riseh, R.S.; Sitohy, M.; Datla, R.; Ren, M. Salicylic acid fights against Fusarium wilt by inhibiting target of rapamycin signaling pathway in Fusarium oxysporum. Journal of Advanced Research 2022a, 39, 1–13. [Google Scholar] [CrossRef]
- Li, Y.; Xia, X.; Zhao, Q.; Dong, P. The biocontrol of potato dry rot by microorganisms and bioactive substances: A review. Physiological and Molecular Plant Pathology 2022b, 122, 101919. [Google Scholar] [CrossRef]
- Xue, H.L.; Bi, Y.; Wei, J.M.; Tang, Y.M.; Zhao, Y.; Wang, Y. A new method for the simultaneous analysis of types A and B trichothecenes by ultrahigh-performance liquid chromatography coupled with tandem mass spectrometry in potato tubers inoculated with Fusarium sulphureum. Journal of Agricultural and Food Chemistry. 2013, 61, 9333–9338. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhang, W.; Kang, Y.; Shi, M.; Yang, X.; Yu, H.; Zhang, R.; Liu, Y.; Qin, S. 2021. Physiological and dynamic transcriptome analysis of two potato varieties reveal response of lignin and MAPK signal to dry rot caused by Fusarium sulphureum. Scientia Horticulturae. 2021, 289, 110470. [Google Scholar] [CrossRef]
- Wang, W.Z.; Min, F.X.; Yang, S.; Wei, Q.; Guo, M.; Gao, Y.F.; Hu, L.S.; Sheng, W.M. Research progress on potato dry rot disease in China and its control measures. China Vegetables 2020, 4, 22–29. [Google Scholar]
- Xue, H.L.; Bi, Y.; Zong, Y.Y.; Alejandro, C.U.; Wang, H.J.; Pu, L.M.; Wang, Y.; Li, Y.C. Effects of elicitors on trichothecene accumulation and Tri genes expression in potato tubers inoculated with Fusarium sulphureum. European Journal of Plant Pathology, 2017, 148, 673–685. [Google Scholar]
- Li, Y.C.; Bi, Y.; Ge, Y.H.; Sun, X.J. Wang, Y. Antifungal activity of sodium silicate on Fusarium sulphureum and its effect on dry rot of potato tubers. Journal of Food Science 2009a, 74, 213–218. [Google Scholar] [CrossRef]
- Yaganza, E. S.; Tweddell, R.J.; Arul, J. Postharvest application of organic and inorganic salts to control potato (Solanum tuberosum L.) storage soft rot: plant tissue-salt physicochemical interactions. Journal of Agricultural and Food Chemistry, 2014, 62, 9223–9231. [Google Scholar] [CrossRef]
- Li, Y.C.; Sun, X.J.; Bi, Y.; Ge, Y.H.; Wang, Y. Antifungal activity of chitosan on Fusarium sulphureum in relation to dry rot of potato tuber. Agricultural Sciences in China 2009b, 8, 597–604. [Google Scholar] [CrossRef]
- Li, X.D.; Xue, H.L. Antifungal activity of the essential oil of Zanthoxylum bungeanum and its major constituent on Fusarium sulphureum and dry rot of potato tubers. Phytoparasitica, 2014, 42, 509–517. [Google Scholar]
- Wei, J.; Bi, Y.; Xue, H. Antifungal activity of cinnamaldehyde against Fusarium sambucinum involves inhibition of ergosterol biosynthesis. Journal of Applied Microbiology, 2020, 129, 256–65. [Google Scholar] [CrossRef]
- Al-Mughrabi, K. I.; Vikram, A.; Peters, R.D.; Howard, R.J.; L. Grant, T.; Barasubiye, K.; Lynch, R.; Poirier, K. A.; Drake, I. K. Efficacy of Pseudomonas syringae in the management of critical reviews in food science and nutrition potato tuber diseases in storage. Biological Control 2013, 64, 315–322. [Google Scholar] [CrossRef]
- Aydin, M. H. Evaluation of some Trichoderma species in biological control of potato dry rot caused by Fusarium sambucinum fuckel isolates. Applied Ecology and Environmental Research, 2019, 17, 533–546. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Kumar, R.; Sharma, S.; Sagar, V.; Aggarwal, R.; Naga, K.C.; Lal, M. K.; Chourasia, K.N.; Kumar, D.; Kumar, M. Potato dry rot disease: current status, pathogenomics and management. Biotechnology 2020, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ray, H.; Hammerschmidt, R. Responses of potato tuber to infection by Fusarium sambucinum. Physiological and Molecular Plant Pathology. 1998, 53, 82–91. [Google Scholar] [CrossRef]
- Wharton, P.S.; Tumbalam, P.; Kirk, W.W. First report of potato tuber sprout rot caused by Fusarium sambucinum in Michigan. Plant disease, 2006, 90, 1460. [Google Scholar] [CrossRef]
- Heltoft, P.; Molteberg, E.L.; Nastad, R.; Hermansen, A. Effect of maturity level and potato cultivar on development of Fusarium dry rot in Norway. Potato Research, 2015, 58, 205–219. [Google Scholar] [CrossRef]
- Oosterhaven, K.; Chambel Leitao, A.; Gorris, L.G.M.; Smid, E.J. Comparative study on the action of S-(+)-carvone, in situ, on the potato storage fungi Fusarium solani var. coeruleum and F. sulphureum. Journal of Applied Bacteriology, 1996, 80, 535–539. [Google Scholar] [CrossRef]
- Heltoft, P.; Brurberg, M.B.; Skogen, M.; Le, V.H.; Razzaghian, J.; Hermansen, A. Fusarium spp. causing dry rot on potatoes in Norway and development of a real-time PCR method for detection of Fusarium coeruleum. Potato Research, 2016, 59, 67–80. [Google Scholar] [CrossRef]
- Carnegie, S.F.; Cameron, A.M. Occurrence of Polyscytalum pustulans, Phoma foveata and Fusarium solani var. coeruleum in field soils in Scotland. Plant Pathology, 1990, 39, 517–523. [Google Scholar] [CrossRef]
- Ali, S.; Rivera, V.V.; Secor, G.A. First Report of Fusarium graminearum causing dry rot of potato in North Dakota. Plant Disease, 2005, 89, 105. [Google Scholar] [CrossRef]
- Villarino, M.; Cal, A. D.; Melgarejo, P.; Larena, I. Development of a multiplex PCR for the identification of Fusarium solani and F. oxysporum in a single step. Journal of Plant Diseases and Protection, 2021, 128, 1275–1290. [Google Scholar] [CrossRef]
- Theron, D.J.; Holz, G. Effect of temperature on dry rot development of potato tubers inoculated with different Fusarium spp. Potato Research, 1990, 33, 109–117. [Google Scholar] [CrossRef]
- Esfahani, M. N. Present status of Fusarium dry rot of potato tubers in Isfahan. Indian Phytopathology, 2012, 59, 2. [Google Scholar]
- Gherbawy, Y.A.; Hussein, M.A.; El-dawy, E.G.A. Identification of Fusarium spp. associated with potato tubers in upper Egypt by morphological and molecular characters. Asian Journal Biochemistry Genetics Molecular Biology, 2019, 2, 1–14. [Google Scholar] [CrossRef]
- Du, M.; Ren, X.; Sun, Q.; Wang, Y.; Zhang, R. Characterization of Fusarium spp. causing potato dry rot in China and susceptibility evaluation of Chinese potato germplasm to the pathogen. Potato Research, 2012, 55, 175–184. [Google Scholar] [CrossRef]
- Xue, H. L.; Bi, Y.; Tang, Y.M.; Zhao, Y.; Wang, Y. Effect of cultivars, Fusarium strains and storage temperature on trichothecenes production in inoculated potato tubers. Food Chemistry, 2014, 151, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.M.; Bi, Y.; Li, Y.C.; Kou, Z.H.; Bao, G.H.; Liu, C.K.; Wang, Y.; Wang, D. Changes of cell wall degrading enzymes in potato tuber tissue slices infected by Fusarium sulphureum. Scientia Agricultura Sinica 2012, 45, 127–134. [Google Scholar]
- Chai, Y.; Xu, Y.Q.; Fu, Y.; Li, X.Y.; He, F.M.; Han, Y.Q.; Feng, Z.; Li, F.L. Characteristics of cell wall degradation enzyme produced by main pathogenic Fusarium spp. in potato dry rot. Crops 2018, 4, 154–160. [Google Scholar]
- Bao, G.H.; Bi, Y.; Li, Y.C.; Kou, Z.H.; Hu, L.G.; Ge, Y.H.; Wang, Y.; Wang, D. Overproduction of reactive oxygen species involved in the pathogenicity of Fusarium in potato tubers. Physiological and Molecular Plant Pathology 2014, 86, 35–42. [Google Scholar] [CrossRef]
- Zhang, X.F.; Wu, C.Y.; Hua, H.H.; Cai, Q.N.; Wu, X.H. Characterization of the first Alternavirus identified in Fusarium avenaceum, the causal agent of potato dry rot. Viruses, 2023, 15, 145. [Google Scholar] [CrossRef]
- Vatankhah, M.; Saberi Riseh, R.; Moradzadeh Eskandari, M.; Sedaghati, E.; Alaie, H.; Afzali, H. Biological control of Fusarium dry rot of potato using some probiotic bacteria. Journal of Agricultural and Science Technology 2019, 21, 1301–1312. [Google Scholar]
- Schultz, B.; Ellner, F. M.; Gossmann, M.; Buettner, C. Investigation into virulence and mycotoxin formation of the dry rot causing pathogen Fusarium sambucinum on potatoes. Mycotoxin Research, 2008, 23, 78–84. [Google Scholar] [CrossRef]
- Li, L.; Xue, H.L.; Bi, Y.; Zhang, R.; Carelle, J.K.; Liu, Q.L.; Nan, M.N.; Pu, L.P.; Dov, P. Ozone treatment inhibits dry rot development and diacetoxyscirpenol accumulation in inoculated potato tuber by influencing growth of Fusarium sulphureum and ergosterol biosynthesis. Postharvest Biology and Technology, 2022, 185, 111796. [Google Scholar] [CrossRef]
- Song, H.H.; Lee, H.S.; Jeong, J.H.; Park, H.S.; Lee, C. Diversity in beauvericin and enniatins H, I, and MK1688 by Fusarium oxysporum isolated from potato. International Journal of Food Microbiology 2006, 122, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Stefańczyk, E.; Sobkowiak, S.; Brylińska, M.; Śliwka, J. Diversity of Fusarium spp. associated with dry rot of potato tubers in Poland, European Journal of Plant Pathology, 2016, 145, 871–884. [Google Scholar]
- Golinski, P.; Vesonder, R.F.; Latus-Zietkiewicz, D.; Perkowski, J. Formation of fusarenone X, nivalenol, zearalenone, alpha-trans-zearalenol, beta-trans-zearalenol, and fusarin C by Fusarium crookwellense. Applied and Environmental Microbiology, 1998, 54, 2147–2148. [Google Scholar] [CrossRef]
- Latus-Zietkiewicz, D.; Perkowski, J.; Tanaka, T.; Yamamoto, S.; Kawamura, O.; Sugiura, Y.; Ueno, Y. Formation of trichothecenes and zearalenone by Fusarium isolated from potato tubers. Microbiology Aliments Nutrition 1990, 8. [Google Scholar]
- Sydenham, E.W.; Marasas, W.F.O.; Thiel, P.G.; Shephard, G.S.; Nieuwenhuis, J.J. Production of mycotoxins by selected Fusarium graminearum and F. crookwellense isolates. Food Additives and Contaminant 1991, 8, 31–41. [Google Scholar] [CrossRef]
- El-Hassan, K.I.; El-Saman, M.G.; Mosa, A.A.; Mostafa, M.H. Variation among Fusarium spp. the causal of potato tuber dry rot in their pathogenicity and mycotoxins production. Egyptian Journal of Phytopathology, 2007, 35, 53–68. [Google Scholar]
- Kim, J.C.; Lee, Y.W. Sambutoxin, a new mycotoxin produced by toxic Fusarium isolates obtained from rotted potato tuber. Applied and Environmental Microbiology, 1994, 60, 4380–4386. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Lee, Y.W.; Tamura, H.; Yoshizawa, T. Sambutoxin: a new mycotoxin isolated from Fusarium sambucinum. Tetrahedron Letter 1995a, 36, 1047–1050. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, Y.W.; Yu, S.H. Sambutoxin-producing isolates of Fusarium species and occurrence of sambutoxin in rotten potato tubers. Applied and Environmental Microbiology 1995b, 61, 3750–3751. [Google Scholar] [CrossRef]
- Venter, S.L.; Steyn, P.J.; Hester, S. F. Production of fusaric acid by Fusarium oxysporum. Potato Research, 1996, 39, 79–83. [Google Scholar] [CrossRef]
- Venter, S.L.; Steyn, P.J. Correlation between fusaric acid production and virulence of isolates of Fusarium oxysporum that causes potato dry rot in South Africa. Potato Research, 1998, 41, 289–294. [Google Scholar] [CrossRef]
- Zhang, R.; Xue, H.L.; Li, L.; Bi, Y.; Zong, Y.Y.; Jimdjio, K.C. Study on the adsorption effect of diatomite on neosolaniol (NEO) in muskmelon fruits inoculated with Fusarium sulphureum. International Journal of Food Engineering 2021. [Google Scholar] [CrossRef]
- Tang, Y.M.; Xue, H.L.; Bi, Y.; Li, Y.C.; Wang, Y.; Zhao, Y.; Shen, K.P. A method of analysis for T-2 toxin and neosolaniol by UPLC-MS/MS in apple fruit inoculated with Trichothecium roseum. Food Additives & Contaminants- Part A Chemistry, Analysis, Control, Exposure and Risk Assessment 2015, 32, 480–487. [Google Scholar]
- Pierron, A.; Neves, M.; Puel, S.; Lippi, Y.; Soler, L.; Miller, J.D.; Oswald, I.P. Intestinal toxicity of the new type A trichothecenes, NX and 3ANX. Chemosphere 2022, 288, 132415. [Google Scholar] [CrossRef]
- Ellner, F.M. Mycotoxins in potato tubers infected by Fusarium sambucinum. Mycotoxin Research, 2002, 18, 57–61. [Google Scholar] [CrossRef]
- Desjardins, A.E.; Plattner, R.D. Trichothecene toxin production by strains of Gibberella pulicaris (Fusarium sambucinum) in liquid culture and in potato tubers. Journal of Agricultural and Food Chemistry 1989, 37, 388–392. [Google Scholar] [CrossRef]
- Jelen, H.H.; Mirocha, C.J.; Wasowicz, E.; Kaminski, E. Production of volatile sesquiterpenes by Fusarium sambucinum strains with different abilities to synthesize trichothecenes. Applied and Environmental Microbiology 1995, 61, 3815–3820. [Google Scholar] [CrossRef]
- El-Banna, A.A.; Scott, P.M.; Lau, P.Y.; Sakuma, T.; Platt, H.W.; Campbell, V. Formation of trichothecenes by Fusarium solani var. coeruleum and Fusarium sambucinum in potatoes. Applied and Environmental Microbiology, 1984, 47, 1169–1171. [Google Scholar] [CrossRef] [PubMed]
- Lenc, L.; Lukanowski, A.; Sadowski, Cz. The use of PCR amplification in determining the toxigenic potential of Fusarium sambucinum and F. solani isolated from potato tubers with symptoms of dry rot. Phytopathologia Polonica, 2008, 2008, 48. [Google Scholar]
- Vesonder, R.F.; Golinski, P.; Plattner, R.; Zietkiewicz, D.L. ; Mycotoxin formation by different geographic isolates of Fusarium crookwellense. Mycopathologia 1991, 113, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.F.; Thrane, U. Fast methods for screening of trichothecenes in fungal cultures using gas chromatography–tandem mass spectrometry. Journal of Chromatography A, 2001, 929, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.A.; Schwarz, P.B.; Gillespie, J.; Rivera-Varas, V.V.; Secor, G.A. Trichothecene mycotoxins associated with potato dry rot caused by Fusarium graminearum. Phytopathology, 2010, 100, 290–296. [Google Scholar] [CrossRef]
- Burlakoti, R.R.; Estrada, R.; Rivera, V.V.; Boddeda, A.; Secor, G.A.; Adhikari, T.B. Real-time PCR quantification and mycotoxin production of Fusarium graminearum in wheat inoculated with isolates collected from potato, sugar beet, and wheat. Phytopathology, 2007, 97, 835–841. [Google Scholar] [CrossRef]
- Burlakoti, R.R.; Ali, S.; Secor, G.A.; Neate, S.M.; McMullen, M.P.; Adhikari, T.B. Genetic relationships among populations of Gibberella zeae from barley, wheat, potato, and sugar beet in the Upper Midwest of the United States. Phytopathology, 2008, 98, 969–976. [Google Scholar] [CrossRef]
- Latus-Zi˛etkiewicz, D.; Perkowski, J.; Chełkowski, J. Fusarium species as pathogens of potato tubers during storage and their ability to produce mycotoxins. Mycotoxin Research. 1987, 3, 99–104. [Google Scholar] [CrossRef]
- Shams, M.; Mitterbauer, R.; Corradini, R.; Wiesenberger, G.; Dall’Asta, C.; Schuhmacher, R.; Krska, R.; Adam, G.; Berthiller, F. Isolation and characterization of a new less-toxic derivative of the Fusarium mycotoxin diacetoxyscirpenol after thermal treatment. Journal of Agricultural and Food Chemistry, 2011, 59, 9709–9714. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, K.; Akhtar, M.H. Potato production, usage, and Nutrition-A Review. Critical Reviews in Food Science and Nutrition. 2016, 56, 711–721. [Google Scholar]
- Trabelsi, B.M.; Abdallah, R.A.B.; Ammar, N.; Kthiri, Z.; Hamada, W. Bio-suppression of Fusarium wilt disease in potato using nonpathogenic potatoassociated fungi. Journal of Plant Pathology & Microbioogy 2016, 7, 347–354. [Google Scholar]
- Esfahani, M.N. Susceptibility assessment of potato cultivars to Fusarium dry rot species. Potato Research, 2005, 48, 215–226. [Google Scholar] [CrossRef]
- Yilma, S.; Vales, M.I.; Charlton, B.A.; Hane, D.C.; James, S.R.; Shock, C.C.; Mosley, A.R.; Culp, D.; Feibert, E.; Leroux, L.; Karaagac, E.; Knowles, N.R.; Pavek, M.J.; Stark, J.C.; Novy, R.G.; Whitworth, J.L.; Pavek, J.J.; Corsini, D.L.; Brandt, T.L.; Olsen, N.; Brown, C.R. Owyhee Russet: A variety with high yields of U.S. No. 1 tubers, excellent processing quality, and moderate resistance to Fusarium dry rot (Fusarium solani var. coeruleum). American Journal of Potato Research, 2012, 89, 175–183. [Google Scholar] [CrossRef]
- Valluru, R.; Christ, B.J.; Haynes, K.G.; Vinyard, B.T. Inheritance and stability of resistance to Fusarium tuber rot in tetraploid potatoes. American Journal of Potato Research, 2006, 83, 335–341. [Google Scholar] [CrossRef]
- Burkhart, C.R.; Christ, B.J.; Haynes, K.G. Non-additive genetic variance governs resistance to Fusarium dry rot in a diploid hybrid potato population. American Journal of Potato Research, 2007, 84, 199–204. [Google Scholar] [CrossRef]
- Mejdoub-Trabelsi, B.; Jabnoun-Khiareddine, H.; Daami-Remadi, M. Effect of Fusarium species and temperature of storage on the susceptibility ranking of potato cultivars to tuber dry rot biocontrol of soilborne fungal diseases of vegetable crops. Pest Technology, 2012, 6, 41–46. [Google Scholar]
- Chen, D.; Nahar, K.; Bizimungu, B. A simple and efficient inoculation method for Fusarium dry rot evaluations in potatoes. American Journal of Potato Research 2020, 3. [Google Scholar] [CrossRef]
- Nadakuduti, S.S.; Starker, C.G.; Voytas, D.F.; Buell, C.R.; Douches, D.S. Genome editing in potato with CRISPR/Cas9. Methods in Molecular Biology (Clifton, N.J.) 2019, 1917, 183–201. [Google Scholar]
- Veillet, F.; Perrot, L.; Chauvin, L.; Kermarrec, M.P.; Guyon-Debast, A.; Chauvin, J.E.; Nogue, F.; Mazier, M. Transgene-free genome editing in tomato and potato plants using agrobacterium-mediated delivery of a CRISPR/Cas9 cytidine base editor. International Journal of Molecular Sciences, 2019, 20, 402. [Google Scholar] [CrossRef] [PubMed]
- Bojanowski, A.; T. J. Avis, S. Pelletier, R. J. Tweddell. Management of potato dry rot. Postharvest Biology and Technology, 2013, 84, 99–109. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Kumar, R.; Sharma, S. Continuous and emerging challenges of silver scurf disease in potato. International Journal of Pest Management, 2021, 68, 89–101. [Google Scholar] [CrossRef]
- Qin, J.H.; Bian, C.S.; Duan, S.G.; Wang, W.X.; Li, G.C.; Jin, L.P. Effects of different rotation cropping systems on potato yield, rhizosphere microbial community and soil biochemical properties. Frontiers in Plant Science, 2022, 13, 999730. [Google Scholar] [CrossRef] [PubMed]
- Kwambai, T. K.; Griffin, D.; Nyongesa, M.; Byrne, S.; Gorman, M.; Struik, P.C. Dormancy and physiological age of seed tubers from a diverse set of potato cultivars grown at different altitudes and in different seasons in Kenya. Potato Research, 2023, 23, 1–25. [Google Scholar] [CrossRef]
- Zhou, H.L.; Lei, Y.; Wang, P.; Liu, M.Y.; Hu, X.X. Development of SYBR Green real-time PCR and nested RT-PCR for the detection of Potato Mop-top Virus (PMTV) and viral surveys in Progeny tubers derived from PMTV infected Potato tubers. Molecular and Cellular Probes 2019, 47, 101438. [Google Scholar] [CrossRef]
- Knowles, N.R.; Plissey, E.S. Maintaining tuber health during harvest, storage, and post-storage handling. In: Johnson DA (ed) Potato health management. St. Paul Minnesota, APS Press. 2008, 79–99. [Google Scholar]
- Han, Y.; Yang, R.R.; Wang, Q.H.; Wang, B.; Prusky, D. Sodium silicate promotes wound healing by inducing the deposition of suberin polyphenolic and lignin in potato tubers. Frontiers in Plant Science 2022, 13, 942022. [Google Scholar] [CrossRef]
- Han, Y.; Yang, RR.; Zhang, X.J.; Wang, Q.H.; Wang, B.; Zheng, X.Y.; Li, Y.C.; Prusky, D.; Bi, Y. Brassinosteroid accelerates wound healing of potato tubers by activation of reactive oxygen metabolism and phenylpropanoid metabolism. Foods 2022, 11, 906. [Google Scholar] [CrossRef]
- Jiang, H.; Li, X.; Ma, L.; Ren, YY.; Bi, Y.; Prusky, D. Transcriptome sequencing and differential expression analysis of natural and BTH-treated wound healing in potato tubers (Solanum tuberosum L.). BMC Genomics, 2022, 23, 1–20. [Google Scholar] [CrossRef]
- Su, Q.H.; Kondo, N.; Li, M.Z.; Sun, H.; Al Riza, D.F.; Habaragamuwa, H. Potato quality grading based on machine vision and 3D shape analysis. Computers and Electronics in Agriculture, 2018, 152, 261–268. [Google Scholar] [CrossRef]
- Pinhero, R.G.; Coffin, R.; Yada, R.Y. Post-harvest storage of potatoes. In Advances in potato chemistry and technology; Elsevier, 2009; pp. 339–370. [Google Scholar]
- Romanazzi, G.; Smilanick, J.L.; Feliziani, E.; Droby, S. Integrated management of postharvest gray mold on fruit crops. Postharvest Biology and Technology, 2016, 113, 69–76. [Google Scholar] [CrossRef]
- Jakubowski, T. Use of UV-C radiation for reducing storage losses of potato tubers. Bangladesh Journal of Botany 2018, 47, 533–537. [Google Scholar] [CrossRef]
- Ranganna, B.; Kushalappa, A.C.; Raghavan, G.S.V. Ultraviolet irradiance to control dry rot and soft rot of potato in storage. Canadian Journal of Plant Pathology 1997, 19, 30–35. [Google Scholar] [CrossRef]
- Yu, B.; Nan, Y.Y.; Kong, N.N.; Dang, R.M.; Bai, Z.L.; Liu, J. Effect of short-wave Ultraviolet irradiation on dry rot and relevant enzymes activities of post-harvest potato tubers. Journal of Nuclear Agricultural Sciences 2017, 31, 1730–1736. [Google Scholar]
- Jakubowski, T.; Krolczyk, J.B. Method for the reduction of natural losses of potato tubers during their long-term storage. Sustainability, 2020, 12, 1048. [Google Scholar] [CrossRef]
- Chudinova, E.M.; Kokaeva, Y.L.; Elansky, S.N.; Kutuzova, I.A.; Pertsev, A.S.; Pobendinskaya, M.A. The occurrence of thiabendazole-resistant isolates of Helminthosporium solani on potato seed tubers in Russia. Journal of Plant Diseases and Protection, 2020, 127, 421–423. [Google Scholar] [CrossRef]
- Gachango, E.; Hanson, L.E.; Rojas, A. Fusarium spp. causing dry rot of seed potato tubers in Michigan and their sensitivity to fungicides. Plant Disease, 2012, 96, 1767–1774. [Google Scholar] [CrossRef]
- Malyuga1, A.A.; Chulikova, N.S.; Ilyin, M.M.; Khalikov, S.S. Fludioxonil-based preparations for protecting potatoes from diseases and their effectiveness. Russian Agricultural Sciences 2022, 48, S74–S83. [Google Scholar] [CrossRef]
- Raigond, P.; Sagar, V.; Mishra, T. Chitosan: a safe alternative to synthetic fungicides to manage dry rot in stored potatoes. Potato Research 2019, 62, 393–409. [Google Scholar] [CrossRef]
- Xue, H.; Bi, Y.; Prusky, D. The mechanism of induced resistance against Fusarium dry rot in potato tubers by the T-2 toxin. Postharvest Biology and Technology, 2019, 153, 69–78. [Google Scholar] [CrossRef]
- Ma, L.; Jiang, H.; Ren, Y.Y.; Yang, J.W.; Han, Y.; Si, H.J.; Prusky, D.; Bi, Y.; Wang, Y. Overexpression of StCDPK23 promotes wound healing of potato tubers by regulating StRbohs. Plant Physiology and Biochemistry 2022, 185, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Hay, W.T.; Fanta, G.F.; Rich, J.O. Antifungal activity of a fatty ammonium chloride amylose inclusion complex against Fusarium sambucinum; control of dry rot on multiple potato varieties. American Journal of Potato Research, 2019, 96, 79–85. [Google Scholar] [CrossRef]
- Mahmoud, G.A.; El-Tobgy, K.M.K.; Abo-El-Seoud, M.A. Utilisation of biocides for controlling pest attacks on potato tubers. Arch Phytopathology Plant Protection, 2010, 43, 251–258. [Google Scholar] [CrossRef]
- Velluti, A.; Sanchis, V.; Ramos, A. J.; Turon, C.; Marin, S. Impact of essential oils on growth rate, zearalenone and deoxynivalenol production by Fusarium graminearum under different temperature and water activity conditions in maize grain. Journal of Applied Microbiology. 2004, 96, 716–724. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran. D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain & Oil Science and Technology, 2019, 2, 49–55. [Google Scholar]
- Othmane, M.; Djazouli, Z.; Zebib, B. Aqueous extract of algerian nettle (Urtica dioïca L.) as possible alternative pathway to control some plant diseases. Iranian Journal of Science and Technology: Science, Transaction A, 2021, 45, 463–468. [Google Scholar]
- Boivin, M.; Nathalie, B.; Simon, B.; Isabel, D. Black spruce extracts reveal antimicrobial and sprout suppressive potentials to prevent potato (Solanum tuberosum L.) losses during storage. Journal of Agriculture and Food Science 2021, 5, 100187. [Google Scholar] [CrossRef]
- Mvuemba, H.; Green, S.; Tsopmo, A.; Avis, T. Antimicrobial efficacy of cinnamon, ginger, horseradish and nutmeg extracts against spoilage pathogens. Phytoprotection, 2009, 90, 65–70. [Google Scholar] [CrossRef]
- Li, M.; Tian, S.L.; Shen, J.; Wang, X.Z.; Cheng, J.X.; Li, S.Q.; Ge, X.; Tian, J.C. Effects of chlorine dioxide on morphology and ultrastructure of Fusarium sulphureum and its virulence to potato tubers. International Journal of Agricultural and Biological Engineering 2017, 10, 242–250. [Google Scholar]
- Liu, Q.L.; Zhang, R.; Xue, H.L.; Bi, Y.; Li, L.; Zhang, Q.Q.; Carelle, J.K.; Nan, M.N. Prusky, D. Ozone controls potato dry rot development and diacetoxyscirpenol accumulation by targeting the cell membrane and affecting the growth of Fusarium sulphureus. Physiological and Molecular Plant Pathology, 2022, 118, 101785. [Google Scholar] [CrossRef]
- Liu, Z.G.; Yang, X.; Xue, H.L.; Bi, Y.; Zhang, Q.Q.; Liu, Q.L.; Chen, J.Y.; Nan, M.N.; Prusky, D. Reactive oxygen species metabolism and diacetoxyscirpenol biosynthesis modulation in potato tuber inoculated with ozone-treated Fusarium sulphureum. Journal of Food Processing and Preservation 2023. [Google Scholar] [CrossRef]
- Schisler, D.A.; Slininger, P.J. ; Selection and performance of bacterial strains for biologically controlling Fusarium dry rot of potatoes incited by Gibberella pulicaris. Plant Disease, 1994, 78, 251–255. [Google Scholar] [CrossRef]
- Schisler, D.A.; Slininger, P.J.; Bothast, R.J. ; Effects of antagonist cell concentration and two-strain mixtures on biological control of Fusarium dry rot of potatoes. Phytopathology, 1997, 87, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Çakar, G.; Tozlu, E. The biological control of Fusarium oxysporum, the causal agent of potato rot. Gesunde Pflanzen 2022, 74, 305–315. [Google Scholar] [CrossRef]
- Daami-Remadi, M.; Hibar, K.; Jabnoun-Khiareddine, H.; Ayed, F.; El Mahjoub, M. 2006. Effect of two Trichoderma species on severity of potato tuber dry rot caused by Tunisian Fusarium complex. International Journal of Agricultural Research, 2006, 1, 432–441. [Google Scholar]
- El-Kot, G.A.N. Biological control of black scurf and dry rot of potato. Egypt Journal of Phytopathology, 2008, 36, 45–56. [Google Scholar]
- Paul, N.C.; Park, S.; Liu, H.F.; Lee, J.G.; Han, G.H.; Kim, H.; Sang, H. Fungi associated with postharvest diseases of sweet potato storage roots and in vitro antagonistic assay of Trichoderma harzianum against the diseases. Journal of Fungi 2021, 7, 927. [Google Scholar] [CrossRef]
- Wharton, P.S.; Kirk, W.W. Evaluation of biological seed treatments in combination with management practices for the control of Fusarium dry rot of potato. Biocontrol Control 2014, 73, 23–30. [Google Scholar] [CrossRef]
- Hussain, T.; Khan, A.A.; Mohamed, H.I. Metabolites composition of Bacillus subtilis Hussaint-Amu determined by LC-MS and their effect on Fusarium dry rot of potato seed tuber. Phyton-International Journal of Experimental Botany, 2023, 92, 783–799. [Google Scholar] [CrossRef]
- Yu, X.Y.; Bi, Y.; Yan, L.; Liu, X.; Wang, Y.; Shen, K.P.; Li, Y.C. Activation of phenylpropanoid pathway and PR of potato tuber against Fusarium sulphureum by fungal elicitor from Trichothecium roseum. World Journal of Microbiology and Biotechnology, 2016, 32, 142. [Google Scholar] [CrossRef]
- Recep, K.; Fikrettin, S.; Erkol, D.; Cafer, E. Biological control of the potato dry rot caused by Fusarium species using PGPR strains. Biological Control, 2009, 50, 194–198. [Google Scholar] [CrossRef]
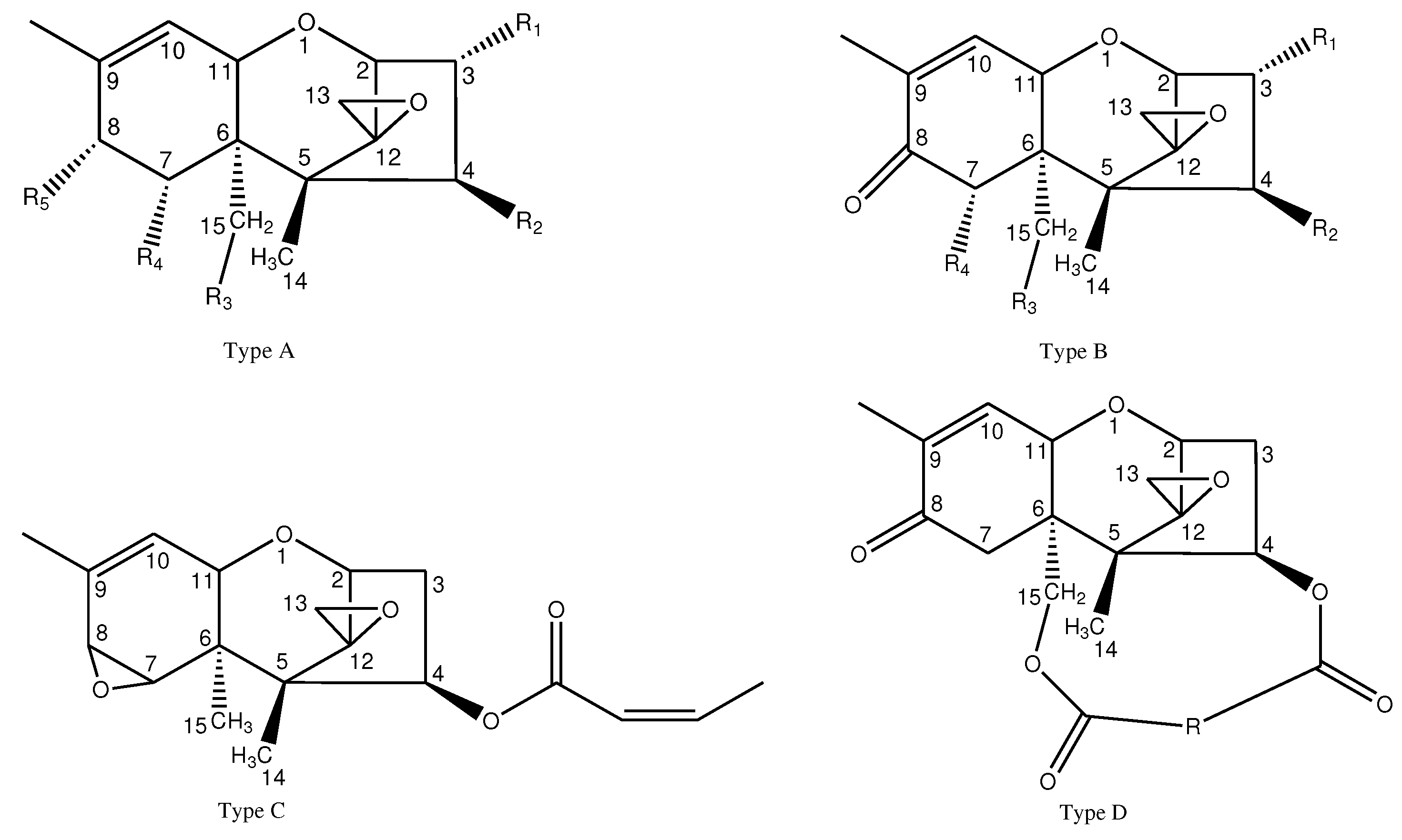
| Fusarium species | non-trichothecenes | reference |
|---|---|---|
|
F. oxysporum F. sambucinum F. solani F. oxysporum F. crookwellense F. equiseti |
BEA, ENN ZEA, FUS FUM |
[36] [37,38,39,40] [41] |
| F. oxysporum | ||
|
F. sambucinum F. semitectum F. oxysporum F. solan F. sambucinum F. oxysporum |
SAM FA |
[41,42,43,44] [1,41,45,46] |
| Fusarium species | trichothecenes | reference | |
|---|---|---|---|
| F. sambucinum | trichothecene | [37,38,39] | |
|
F. solani F. oxysporum F. sulphureum, F. solani F. sambucinum F. sambucinum |
3-ADON, T-2, FUS, DAS 4,15-DAS, DAS DAS, MAS, NEO, T-2, HT-2 4,15-DAS, 15-MAS, 4-MASc DON, NIV, HT-2 |
[4] [50] [51] [52] [53] |
|
|
F. sambucinum F. solani F. crookwellense F. graminearum F. culmorum F. equiseti F. oxysporum |
T-2 MAS, DAS NIV, FX NIV, DAS FX DON, NIV, FX, 3-ADON, 15-ADON DON, NIV, FX NIV, T-2, 3,15-ADON, 15-SCRP NIV, FX, DON, 3-ADON, 15-ADON DON, 3-ADON, 15-ADON NIV, FX, DON, 3-ADON NIV, FX, 4,15-MAS, DAS, SCR DON, 3-ADON NIV, FX, DON, 3-ADON NIV, FX, 4,15-MAS, DAS, SCR T-2 T-2 |
[41] [54] [38] [55] [56] [57] [40] [59] [56] [59] [56] [60] [56] [56] [41] [41] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





