You are currently viewing a beta version of our website. If you spot anything unusual, kindly let us know.
Preprint
Article
Selection and Characterization of Phosphate Solubilizing Fungi and Their Effects on Coffee Plantations
Altmetrics
Downloads
151
Views
52
Comments
0
A peer-reviewed article of this preprint also exists.
Abstract
The use of phosphate-solubilizing fungi in coffee cultivation is an alternative with which to reduce the use of fertilizers. The objective of this study was to analyze the mechanisms involved in the phosphorus solubilization of the fungal strains and to evaluate the effect of a phosphate-solubilizing strain on the coffee plants. For this, phosphorus-solubilizing fungal strains were selected for evaluation of their solubilization potential and phosphatase activity. Coffee plants were inoculated in the field with a phosphate solubilizing strain, and soil and foliar soluble phosphorus, as well as coffee bean yield, were quantified. Of the 151 strains analyzed, Aspergillus niger, Penicillium waksmanii, and Penicillium brevicompactum showed the highest solubilization. Aspergillus niger and P. waksmanii presented the highest soluble phosphorus values; however, P. brevicompactum showed the highest phosphatase activity. The P. brevicompactum strain inoculated on the coffee plants did not favor foliar phosphorus content but increased the soil soluble phosphorus content in two of the coffee plantations. The plants inoculated with the phosphate solubilizing strain showed an increase in coffee bean weight on all plantations, although this increase was only significant in two of the three selected coffee plantations.
Keywords:
Subject: Biology and Life Sciences - Biology and Biotechnology
1. Introduction
Cultivated coffee is considered the world's leading agricultural commodity, with a market generating more than 90 billion dollars annually. Around 8% of the world's population (approximately 500 million people) are involved in the coffee market, from sowing to final consumption. Coffee is produced in 14 states of Mexico, of which Chiapas, Veracruz, Puebla, and Oaxaca account for 90% of production. In 2019, Chiapas contributed 40.9% of the national production, followed by Veracruz with 24.2%, Puebla with 16.0%, and Guerrero with 9.4% [1]. The state of Veracruz is the second most important coffee-producing region in Mexico. The coffee is produced in 842 communities of 82 municipalities. Sixty percent of the coffee is grown above 750 m asl in elevation. The regions with the highest production are Coatepec, Cordoba, Huatusco, Misantla, and Atzalan. Currently, 21,089 producers in the central region of Veracruz produce this crop over a total area of 58,712 ha, representing 7.3% of the area dedicated to coffee growing nationally. The rural development district of Coatepec cultivates 28,873 hectares in 21 municipalities. Jilotepec belongs to this district and has a planted area of 1,776 hectares [2].
Coffee growing is considered a fundamental strategic activity, because it allows the integration of productive chains, as well as the generation of foreign currency and employment, and is the means of subsistence for many small producers and indigenous groups [3,4]. In addition, it is of great ecological importance since more than 90% of the area cultivated with this crop is under shade, which contributes to biodiversity conservation and provides vital environmental services to society [5,6]. In Mexico, the soils where coffee is produced are generally of volcanic origin and are characterized by an acidic pH of 4.5-5.2, as well as low availability of essential macronutrients such as phosphorus (P) [7]. This is mainly because this element is associated with other ionic elements such as calcium, iron, and aluminum, present in forms that cannot be assimilated by the plants [8]. The amount of available P depends on the modification of the dynamic equilibrium that maintains the dissolution of insoluble inorganic compounds and on the decomposition of organic matter [9]. Phosphorus is important in coffee cultivation since it plays key roles in many plant processes such as energy metabolism, the synthesis of nucleic acids and membranes, photosynthesis, respiration, nitrogen fixation, and enzyme regulation [10]. During the early stages of coffee plant development, this element is responsible for vigorous plant growth, participates in the formation of effective root systems, and acts as a promoter of flowering and fruit development. During the reproductive stage, phosphorus is essential for the formation, growth, and multiplication mechanisms of the flower organs. Phosphorus deficiency in the soil can be observed in coffee plants through the yellowing of leaves and a lack of fruit ripening. In severe cases, the leaves near the ripening fruit fall off completely. To remedy a lack of P, coffee producers apply large amounts of phosphate fertilizers [1], although 75-90% of the phosphate added precipitates through the formation of metal cation complexes [11]. In addition, excessive use of fertilizers leads to eutrophication, water toxicity, groundwater contamination, air pollution, soil and ecosystem degradation, biological imbalances, and reduced biodiversity, so it is necessary to seek another option to release P from inorganic and organic pools of total soil P. One alternative is the application of bioinoculants, which are preparations of microorganisms for inoculation with the aim of partially or completely replacing inorganic fertilization.
In this context, phosphate solubilizing fungi (PSF) are of great importance since they are a functional group of microorganisms that play a fundamental role in the P cycle. Thanks to the activity of these fungi, plants can take advantage of the large reserves of insoluble phosphorus fixed to soil minerals [12]. Some fungi and bacteria can solubilize P from unavailable forms in the rhizosphere. The mechanism of mineral phosphate solubilization by the strains of PSF is associated with the release of low molecular weight organic acids [13], which, through their hydroxyl and carboxyl groups, act to chelate the cations bound to the phosphate, thereby converting it into soluble forms. Phosphate solubilizing fungi can produce extracellular enzymes, i.e. a group of phosphatase enzymes that can mineralize organic P into inorganic P such that the P becomes available for the plants. There are several soil phosphatases, the most common of which are phosphomonoesterases, phosphodiesterases, and phytases. Phosphomonoesterases act on phosphate monoesters and, according to their optimum pH, are categorized into acid and alkaline phosphomonoesterases. These microorganisms have a high potential for promoting plant growth by increasing soil fertility [14]. In agricultural soils, the use of microbial inoculants (biofertilizers) that present P-solubilizing activities is considered an environmentally friendly alternative to further applications of chemical-based P fertilizers [11]. The coffee rhizosphere is associated with many beneficial organisms, including growth-promoting microorganisms that may contribute to fulfilling the nutritional requirements of the plant. The objective of this study was to evaluate the PSF and to obtain further information about the mechanisms involved in the solubilization of P, as well as to evaluate the effect of inoculation of an PSF strain on coffee plants under field conditions.
2. Results
2.1. Selection of strains with phosphate solubilizing capacity
Of 151 soil fungal strains tested, 121 (79.5%) formed solubilization halos (Figure 1). Considering the established scales of solubilization, 55 strains (45.8%) were found to be in scale I, 40 strains (33.3%) in scale II, and 26 strains (20.8%) in scale III. Scale I (halos 1-4 mm in thickness) was represented by 24 genera including Trichoderma, Oidiodendron, Penicillium, Chaetomium, and Humicola; scale II (halos of 5-9 mm in thickness) was represented by 12 genera including Penicillium, Aspergillus, Fusarium, Cylindrocarpon, and Talaromyces; and scale III (halos of 9-12 mm in thickness) was represented by 13 genera, most prominently Penicillium, Aspergillus, Fusarium, and Eupenicillium (Table 1).
According to the maximum values obtained for the SI, and based on the scale used by Silva Filho y Vidor [15], six of the strains evaluated had low solubilizing activity. The species in this group are Aspergillus sclerotiorum, Aspergillus sydowii, Aspergillus sp. 1Y, Humicola sp. 2gh205, Merimbla sp. 3gh18, and Penicillium glabrum. A further 15 species presented medium solubilization: Scopulariopsis brevicaulis, Aspergillus candidus, Fusarium sp. 25, Fusarium sp. 3Y, Eupenicillium euglaucum, Eupenicillium ludwigii, Talaromyces flavus var. flavus, Cladosporium cladosporioides, Acremonium roseolum, Epicoccum nigrum, Penicillium arenicola, Penicillium sp. 90, Penicillium olsonii, Penicillium verruculosum, and Penicillium miczynskii.
All species presented a clear halo zone around their colonies, and Aspergillus niger, Penicillium waksmanii and Penicillium brevicompactum gh103 each presented a maximum solubilization index. The highest phosphate solubilization rate was observed for the strains P. waksmanii (3.88) and P. brevicompactum (3.61). However, the A. niger index was 2.6 and its clear zone became visible on the first day, while the clear zone of the other fungal isolates became visible on the third day.
2.2. Phosphate Solubilization and pH in Sundara
Significant differences were detected in the soluble phosphorus values among the extracts of the PSF strains evaluated (F=50.31 p= 0.0005). The available phosphorus content in the extracts of the treatments corresponding to the PSF strains was significantly higher than that of the control treatment (no PSF) (p=0.05). The available phosphorus concentration varied over the period of evaluation; however, a higher content was observed on day 21 for all strains. The soluble phosphorus values of the extracts of A. niger and P. waksmanii strains (98.22 mg/L and 95.77 mg/L, respectively) were significantly (p>0.05) higher than those of P. brevicompactum (74.63 mg/L) (Figure 2).
The pH of the extracts where the PSF strains (A. niger, P. brevicompactum and P. waksmanii) were inoculated decreased significantly with respect to the pH of the control treatment. The pH of the extracts of the PSF strains was variable between days of incubation. A. niger pH decreased from 7 to 1.9; in P. brevicompactum from low pH 7 to 2.9 and in P. waksmanii from pH 7 to 3.0.
2.3. Acid phosphatases
Regarding phosphatase enzyme activity, significant differences in this enzyme were observed among the strains evaluated (F=63.86, p=0.001). Production of acid phosphatase in the P. brevicompactum strain was significantly higher than that detected for the rest of the strains tested (A. niger and P. waksmanii) and the control treatment (no PSF).
The highest acid phosphatase production was observed in the extract of P. brevicompactum strain on days 15 and 18 (189.75 and 176.94 AEU mg/protein, respectively). In the A. niger strain, this was detected on days 15 and 21 (59.44 and 69.59 AEU mg/protein, respectively), while in P. waksmanii, the highest acid phosphatase activity was found on day 21 (38.81 AEU mg/protein) (Figure 3).
Three bands were detected in the results of the acid phosphatase isoenzyme patterns: one monomorphic (1) and two polymorphic (2 and 3). Three different isoenzyme patterns were also observed: the first shows two bands (2 and 3) for the A. niger strain. The second pattern exhibits two bands (1 and 3) for the P. waksmanii strain. The third pattern exhibited one band (3) for the P. brevicompactum strain (Figure 4).
2.4. Coffee plant foliar phosphorus
At the San Isidro coffee plantation, the initial foliar phosphorus content in the plants was 1112.96 mg/kg. Sixty days after establishing the experiment, the foliar phosphorus content in the plants inoculated with the PSF strain (P. brevicompactum) was 1528.12 mg/kg, while in the non-inoculated plants, this value was 1695.31 mg/kg. At 120 days, there was a decrease in the foliar phosphorus content of both the PSF-inoculated and non-inoculated plants (933.27 and 1366.18 mg/kg, respectively). At 180 days, there was an increase in leaf phosphorus content in both inoculated (1456.95 mg/kg) and non-inoculated (1557.29 mg/kg) plants. In all cases, these differences were not significant (p=0.05) (Figure 5A).
In the coffee plantation “Los Bambus”, the initial foliar phosphorus content in the plants was on average 1113 and 1118 mg/kg in the two plots. None of the samplings (at 60, 120, and 180 days) revealed significant differences between inoculated and non-inoculated plants. At 60 days, the average leaf phosphorus content in the plants inoculated with the PSF strain (P. brevicompactum) was 1353 mg/kg, while in non-inoculated plants this value was 1406 mg/kg. At 120 days, there was a slight decrease in the leaf phosphorus content of inoculated (1215 mg/kg) and non-inoculated (1311 mg/kg) P. brevicompactum plants. At 180 days, a significant increase in leaf phosphorus content was observed in both plants inoculated with the P. brevicompactum strain (2164 mg/kg) and non-inoculated plants (2315 mg/kg) (Figure 5B).
In the coffee plantation “La Barranca”, the average initial foliar phosphorus content in the plants was 998 and 992 mg/kg in the two plots. No significant differences were detected in leaf phosphorus content between plants from two plots (inoculated and non-inoculated) at 60, 120, and 180 days (p<0.05).
At 60 days, the leaf phosphorus content of plants inoculated with the PSF strain (P. brevicompactum) averaged 1287 mg/kg, while that of the non-inoculated plants averaged 1342 mg/kg. At 120 days, there was a slight decrease in the foliar phosphorus content of both inoculated (981 mg/kg) and non-inoculated (1007 mg/kg) P. brevicompactum plants. At 180 days, a significant increase in leaf phosphorus content was observed in both plots of plants inoculated with P. brevicompactum strain (1824 mg/Kg) and non-inoculated plants (2115 mg/Kg) (Figure 5C).
2.5. Soil phosphorus available to the coffee plants
In the coffee plantation “San Isidro”, the initial soil soluble phosphorus content was 4.6 mg/kg. Sixty days after inoculation with the phosphate solubilizing fungus P. brevicompactum, an increased soil soluble phosphorus content of 5.64 mg/kg was observed, while in the soil of the uninoculated plants, this value was 4.74 mg/kg. However, these increases were not significant (F= 0.017, p=0.89). At 120 days, a decrease in soluble phosphorus content was detected in the soil of both inoculated and non-inoculated (3.82 and 2.30 mg/kg respectively) P. brevicompactum plants, being significantly higher in the inoculated plants. By 180 days, available phosphorus had increased significantly, and the content of this element was higher in plants inoculated with PSF (4.98 mg/kg) (Figure 6A).
In the coffee plantation “Los Bambus”, the soil presented 2.93 mg/kg of initial soluble phosphorus. Sixty days after inoculation with the PSF P. brevicompactum, an increase in soluble phosphorus content to 5.08 mg/kg was observed, while the soil of uninoculated plants increased to 3.27 mg/kg. At 120 days, a decrease in soluble phosphorus content was detected in the soil of both inoculated and uninoculated plants (2.65 and 1.79 mg/kg, respectively). At 180 days, the soluble phosphorus content in the soil of the inoculated plants was 3.32 mg/kg while that of the non-inoculated plants was 2.31 mg/kg. In all three samplings, the measured soluble phosphorus content was significantly higher in the P. brevicompactum inoculated plant treatments than in the non-inoculated plants (p>0.05) (Figure 6B).
In the coffee plantation “La Barranca”, the available phosphorus content in the soil was 0.93 mg/kg. In the first sampling at 60 days after inoculation with the phosphate solubilizing fungus P. brevicompactum, an increase in the soluble phosphorus content of the soil to 1.22 mg/kg was observed, while the soil of the uninoculated plants increased to 1.14 mg/kg. At 120 days, the soluble phosphorus content decreased in both the soil of P. brevicompactum inoculated plants and the soil of non-inoculated plants (0.96 and 0.89 mg/kg, respectively). At 180 days, the soluble phosphorus content in the inoculated and non-inoculated plants was 1.32 mg/Kg and 1.56 mg/Kg, respectively) (Figure 20). In all samplings, the differences in available phosphorus between plants inoculated with P. brevicompactum fungus and non-inoculated plants were not significant (p<0.05) (Figure 6C).
2.6. Production of mature cherries in the coffee plants
In the coffee plantation “San Isidro”, 270 days after inoculation of the coffee plants with the P. brevicompactum strain, significant differences in bean yield were observed between inoculated and non-inoculated plants (F=9.95, p=0.003). The coffee bean weight of plants inoculated with the PSF P. brevicompactum was 485.13 g, which was significantly higher than that of the uninoculated plants (277.6 g), a difference in mean weight of 207.53 g (F=9.95, p=0.003).
In the coffee plantation “Los Bambus”, the bean weight of the coffee cherry of the inoculated plants was 53.78 g while that of the non-inoculated plants was 50.65 g. This increase was only 3.13 g and the difference was not significant (F=0.05, p=0.81).
In the coffee plantation “La Barranca”, a significant increase in bean yield was detected in the inoculated plants relative to the non-inoculated plants (F=10.02, p=0.0037). The mature coffee cherry bean weight of PSF inoculated plants was 555.73 g, while non-inoculated plants were 173.86 g (Figure 7), a difference of 381.87 g. (F=0.05, p=0.81).
3. Discussion
For this study, a high percentage of the total number of strains evaluated formed solubilization halos (79.4%). This result is higher than the percentage detected by Posada et al., [16], who report only 9.73% of the strains isolated from coffee plantations in Mexico, and slightly higher than that reported by Arias et al., [17] (71.5%) from the rhizosphere of coffee plants in Mexico (Coffea arabica var. Costa Rica). The results of this study confirm that shaded coffee plantations and tropical montane cloud forest harbor a high number of soil fungi with phosphate solubilizing capacity. Due to the presence of different tree species native to the cloud forest and the high diversity of plant species in the shaded coffee plantations, these sites have a considerable accumulation of plant debris that, once decomposed, increase the humus content of the upper soil horizons. This could explain the high number of solubilizing species, given that the presence of a high population of solubilizing micromycetes has been positively related to soil organic matter content [18]. Another contributing factor could be the metabolic activity of the plant roots through exudates [19]. The high population of PSF could also be related to the presence of certain nutrients, pH, moisture content, organic matter, and some soil enzyme activities [20]. Djuuna et. al., [21] detected a correlation between the number of PSF and the level of soil P availability and moisture content, indicating an increase in soil P availability with a greater abundance of PSF in the soil. Some strains of filamentous fungi have been studied in research involving species of the genera Alternaria [22,23], Aspergillus [24], Eupenicillium [25], Fusarium [26], Cladosporium [27], Gongronella [28] Helminthosporium [29], Mortierella [30] Rhizopus [22], Talaromyces [28,31], Trichoderma [32], prominent among which are the Aspergillus y Penicillium [32,33].
In this study, the following 21 genera presented a positive response to the solubilization of Ca3(PO4)2: Anungitopsis, Arthrographis, Aspergillus, Beauveria, Gliocephalotrichum, Nigrospora, Chrysosporium, Cordana, Cylindrocarpon, Eladia, Geomyces, Merimbla, Nectria, Oidiodendron, Penicillium, Phialomyces, Phialophora, Pseudogliomastix, Sagenomella, Sporotrix, and Umbelopsis. Several studies indicate that the genera Aspergillus and Penicillium have a high capacity for solubilization of phosphates of Ca, Al, and Fe [34,35,36,37,38,39].
The results obtained here corroborate the high phosphate solubilizing capacity of the Penicillium and Aspergillus species. Of the 25 Penicillium species evaluated, 23 formed solubilization halos, and all strains corresponding to the genera Eupenicillium and Talaromyces, which are sexual or perfect stages of Penicillium, were positive. Species of the Penicillium genus are the most studied as phosphate solubilizers, and have produced the strains that are currently used as biofertilizers; e.g., the product Fosfosol© marketed in Colombia [40,41] and mainly aimed at rice cultivation, with the active ingredient Penicillium janthinellum. In Canada, JumpStart®, produced from a strain of Penicillium bilaiae and tested on wheat, has been marketed since 1990. As with Penicillium, most of the Aspergillus strains tested had positive responses to solubilization. In this case, five of the six strains formed halos, with four of these reaching scale III.
In this study, the range of solubilization rates (RSR) was 3.5 to 3.8. These values are higher than those reported by Morales et al. [42], who report a maximum RSR of 1.3 for strains of Penicillium albidum, P. thomii, P. restrictum, P. frequentans, Gliocladium roseum, and Penicillium sp. Elías et al. [43] report an RSR of 2.87 for Aspergillus sp., Hernandez et al. [44] report an RSR of 3 for Paecilomyces lilacinus, and Verma and Ekka [45] report 2.25 for Penicillium purpureogenum. Other studies have reported phosphate solubilization rate values of up to 5.3 RSR for Trichosporon beigelii [46]. Romero-Fernandez et al. [47] report ranges of 2.06-6.85, the latter value for a Penicillium strain. In a study of strains isolated from the rhizosphere of Coffea arabica var. Costa Rica, Arias et al. [17] present RSR values in a range of 1.13-6.5. The use of revealing tests using halos in solid culture media and RSR data is a tool with which to detect the phosphate solubilizing capacity of strains rapidly and easily; however, the formation of halos in solid media should not be considered the only test for evaluating solubilization capacities. Quantitative tests are also necessary since Arias et al. [17] did not find a significant relationship between RSR in a solid medium and solubilized phosphorus content in a liquid medium.
In the quantitative evaluation, the absence of soluble P in the controls clearly demonstrates that all three strains tested are phosphate solubilizers and that the manipulation of the cultures was appropriate. The A. niger strains solubilized a high amount of insoluble phosphorus at 98.22 mg/L, followed by P. waksmanii at 95.77 mg/L and P. brevicompactum at 74.63 mg/L. The values presented here exceed those highlighted in other studies [17,37,42,44,48,49,50,51]. However, they were lower than the values presented for the Aspergillus sp. strain (167.7 mg/L) [37].
Several authors argue that fungal species use the production of organic acids as a solubilization mechanism, reflected in a reduced pH [52,53,54]. The data presented here showed that Ca3(PO4)2 can be solubilized by lowering the pH of 1.9 The PSF have been shown to play a crucial role in phosphorus solubilization due to the production of organic acids. Aspergillus niger and some Penicillium sp. have been reported for solubilization of rock phosphate, phosphate mobilization [35,55]. Acidification of the growth medium through the production of organic acids can be explained by the utilization of glucose as a carbon source during growth. Similar results were extrapolated from previous studies [56]. Organic acids secreted by fungi dissolve mineral phosphate because of the anion exchange of PO43- for the acid anion [57].
Seven different organic acids (salicylic, ascorbic, citric, formic, lactic, oxalic, and malic acid) were detected during in vitro phosphate solubilization. Solubilization by acidification depends on the nature and strength of the acid produced by the fungus since there are those such as tri- or di-carboxylic acids that are more efficient solubilizers than mono-basic and aromatic acids [44,58,59]. Salicylic acid was the organic acid most produced by all the PSF. Its concentration was 1823.8 mg/l as produced by Aspergillus japonicus. Salicylic acid was the most quantitatively produced organic acid for all PSF isolates which has an important role in defense mechanisms against biotic and a biotic stress [54].
Some research has reported that enzymes, such as acid phosphatase and phytases, are involved in P solubilization [58,60,61,62,63]. These enzymes cleave the ester bond of the insoluble forms of P, leaving orthophosphate ions that can be assimilated by plants [64]. Previous reports indicate that the genera Aspergillus and Penicillium both actively participate in the mineralization of soil organic P through the production of phosphatase enzymes [65].
The results presented here evidence the production of acid phosphatase by the solubilizing fungi of the two strains of the genus Penicillium and the strain of A. niger. The highest production of acid phosphatases was detected in the P. brevicompactum strain. These results support those obtained by Gomez [66], who found that some Penicillium species may have a higher capacity to produce acid phosphatases than Aspergillus species. Similar reports were obtained by Valenzuela et al. [67] who evaluated 180 fungal strains and found that Penicillium strains showed a higher activity of this enzyme than was produced by Aspergillus strains.
This study shows that the A. niger strain was superior to the other strains in terms of its ability to solubilize tricalcium phosphate. Other studies report that A. niger produces oxalic acid [68] which could explain its higher P solubilization capacity compared to the other microorganisms. Solubilization in the P. brevicompactum strain may be mostly achieved through the production of phosphatase enzymes since the results show that this strain presented the highest content of these.
Microorganisms harbored in the rhizosphere of plants play several key roles in improving plant productivity, nutrient cycling, and soil fertility. Overall, in this field study conducted on three coffee plantations in Jilotepec, Veracruz, inoculation with the phosphate solubilizing fungus strain P. brevicompactum increased productivity in the coffee plants. The phosphate solubilizing potential of the P. brevicompactum strain had already been assessed on coffee (Coffea arabica var. Garnica) plants by Perea et al. [69]; however, that study was conducted under controlled conditions. In this study, the inoculation of PSF (both alone and together with mycorrhizae) favored the development and availability of phosphorus in the soil, as well as foliar phosphorus content. However, given that the study was carried out under controlled conditions and with 8-month-old seedlings, it was not possible to determine the effect of PSF on coffee bean production.
About the soil soluble phosphorus, this parameter increased after two months of inoculation with P. brevicompactum. In the second sampling (fourth month of inoculation), however, it had significantly reduced although the values remained higher in the plants inoculated with P. brevicompactum.
In the first sampling, the foliar phosphorus content increased but, by the second sampling, it had reduced significantly. Contrary to the results for soluble soil phosphorus, foliar phosphorus content was higher in the uninoculated plants. The subsequent decrease in both soil and leaf phosphorus content may have been because the solubilized phosphorus was being used by the plant in coffee cherry production. In a study in North Bengal in India, Chakraborty et al. [70] evaluated the ability of some phosphate-solubilizing fungi to promote plant growth in soybean seedlings and found a reduction of phosphorus in the soil. In that study, the concentration of phosphorus was significantly increased in the roots. It is therefore recommended that further research be conducted to monitor the phosphorus in the plant from the roots to the leaves.
Several studies have documented the successful use of bioinoculants and they have been used as an alternative to traditional fertilization. In a study in Australia [39] used bioinoculants based on Penicillium bilaiae and Penicillium radicum and showed beneficial effects on wheat crops. In Canada, a biofertilizer based on the fungus Penicillium bilaiae was registered in 1990 for use on wheat and tested initially on a few hectares with good results and, in 2002, approximately one million hectares sown with principal crops in Canada were using this biofertilizer [71].
In Colombia, a biofertilizer with phosphate-solubilizing microorganisms, the active ingredient of which is Penicillium janthinellum, has also been marketed. This product was used in rice cultivation, producing high increases in the production of this cereal [41]. The results obtained in this study are promising for the development of a bioinoculant of the fungal strain P. brevicompactum; however, further research is required to establish appropriate doses and re-inoculation schedules. The vigorous microbial activities in soil optimize nutrient cycling and maximize the efficiency of their use in agronomy [72]. A recent extensive review [73] of greenhouse and field trials has shown a marked improvement in the growth responses of various crops to inoculations of phosphate-solubilizing microorganisms.
Most studies have been conducted with bacteria; however, although they are the main decomposers of organic matter and dictate soil carbon and other elements, the role of fungi is the subject of relatively little study. Many fungi can solubilize insoluble phosphates or facilitate P acquisition by plants and therefore form an important part of commercial microbial products, with Aspergillus, Penicillium, and Trichoderma being the most efficient [74].
The use of bioinoculants, together with the rationalized use of phosphate fertilizers, is important and it is necessary to adjust fertilization levels (especially of P and N) to reduce the negative impact on the environment. Chemical-based agriculture has had a negative impact on beneficial microbial communities, significantly reducing microbial biodiversity. There is therefore a need to adopt ecological farming practices for sustainable agriculture. Indigenous or native microorganisms are considered an important tool with which to overcome problems associated with the overuse of chemical fertilizers and pesticides.
Although research has been conducted using phosphate-solubilizing bacteria and fungi in vitro on plant growth promotion, particularly in coffee (Coffea arabica L), there are few reports regarding their impact on growth under field conditions. This study is the first worldwide to evaluate the potential of this group of microorganisms on coffee bean production.
4. Materials and Methods
Determination of Solubilization Index
The strains were subjected to a screening test for their phosphate solubilization potential. Sundara medium containing tricalcium phosphate was prepared and poured into sterilized Petri plates. After 72 hours of incubation at 25 °C, the formation of a halo around the fungal growth on the medium indicated phosphate solubilization. The degree of phosphate solubilization was determined by measuring the clear zone (in mm) around the colonies. The solubilization index (SI) was calculated as the ratio of the total diameter (colony + halo zone) to the colony diameter [75]. For the selection of strains with a higher SI, the scale established by Silva Filho and Vidor [15] was used. In this scale, the solubilizing capacity is considered low where the SI is lower than 2, medium if it is higher than 2 and lower than 3, and high if it is higher than 3.
Phosphate Solubilization Efficiency of Fungal Isolates
For this test, three strains were selected from the high solubilization range. These strains were inoculated into a Sundara liquid medium [76] supplemented with 0.5 g-1 tricalcium phosphate as a source of insoluble phosphorus. The strains were inoculated with 5 mm diameter mycelium discs with 8 days of growth on solid PDA, in triplicate. Negative controls were established using discs of medium with no inoculum. The cultures were incubated in darkness at 25 °C for 21 days. In each sampling, the pH of each sample was recorded, and the biomass achieved for each fungus was determined. To obtain the fungal extracts, the samples were filtered with Whatman® 42 filter paper and the soluble phosphorus was quantified. The pH was measured in the potentiometer. Soluble phosphorus was measured with the ascorbic acid method Clesceri et al. [77] while absorbance was measured in a spectrophotometer at 880 nm. The data were compared with a standard curve for phosphorus and expressed in mg/ml.
Phosphatase activity assay
Acid phosphatase activity was monitored according to Tabatabai and Bremner [78]. In total, 900 μl of enzyme extract, 90 μl of 1M acetate buffer (pH 5), and 10 μl of 15 mM p-nitrophenyl phosphate were mixed and incubated for 1 h at 37 °C. The results were expressed in μmoles of p-NP released min-1 U g-1.
Characterization of isoenzymatic patterns
The electrophoretic profiles were analyzed using polyacrylamide gels with the following modifications: 4% polyacrylamide stacking gel and 10% separating gel. Electrophoresis was run in a vertical electrophoresis chamber with 19 mM Tris-Glycine buffer of pH 8.3 at 25 mA and 4 ºC for 2 h. Each well contained 20 μg of protein. The acid phosphatase activity was measured in 500 mM Citrate buffer at pH 5.5 with β naphthyl sodium phosphate and fast black K salt as a substrate. The gel was incubated at 37 °C in darkness with agitation (125 rpm) for 4 h.
Inoculation of an PSF strain in coffee plants under field conditions
The coffee plantations where the solubilizing fungi were inoculated are located in Jilotepec, Veracruz. For this study, three shade coffee plantations were selected, with coffee plants of Coffea arabica var. Costa Rica (Figure 8).
The geographical characteristics, elevation, precipitation, temperature, and soil type of the sites are listed in Table 3.
Within each of the three plantations of approximately 2 ha, plots of 500 m2 were delimited (Plot 1/Plot 2). In each plot, 25 coffee plants (Coffea arabica L.) of the Costa Rica variety were marked in sampled in “cinco de oros” [79]. In each plantation, plot 1 was used for inoculation of the PFS strain, while plot 2 represented the control treatment (uninoculated).
Strain RA103 (Penicillium brevicompactum) was propagated on potato dextrose agar media and incubated for 1 week at 25 °C. Spores were then scraped from the medium and suspended in a solution containing 10 mL of Inex A to create a solution of 1x1010 spores/ml.
In plot 1 of each plantation, 10 mL of fungal inoculant (Penicillium brevicompactum) was applied to each of the 25 plants using a syringe. These applications were made at the base of each plant near the stem and on the roots.
Variable measurement
At the beginning of the experiment (July 2021), and every 2 months (3 samplings) thereafter, soil samples (500 g) were taken to measure soluble phosphorus content and leaf samples (10 leaves per plant) were collected to measure foliar phosphorus. In addition, during March and April 2022, coffee bean production was measured.
The collected soil was dried at ambient temperature and passed through sieves (DAIGGER ATM 25SS8F, 800-621-7193) to remove stones. The collected coffee leaves were dried in an oven (BINDER 09-08078) at 60 °C for approximately 8 hours and ground in a mill before measurement of leaf phosphorus.
Leaf phosphorus was measured following the technique of McKean [80]. For this, 0.25 g of dried leaves were weighed and placed in porcelain crucibles inside a muffle furnace at 500 °C for 2 h. The resulting ashes were dissolved in 25 ml of 0.3 M HCl in test tubes. The extracts were filtered through Whatman filter paper (42). After filtration, 18 ml of the combined reagent was added to 2 ml of each sample and allowed to stand for 20 min. The samples were then measured in a spectrophotometer at 660 nm.
The soluble phosphorus was quantified using the technique of Bray and Kurtz [81]. For this, 1.2 g of soil was weighed and 10 mL of the extractant solution (0.03 N ammonium fluoride, hydrochloric acid, and distilled water) was added. The extracts were transferred to Whatman filter paper. Subsequently, a 1 mL aliquot of extracted sample was taken, to which 5 mL of combined reagent (ascorbic acid in stock solution) was added and made up to 50 mL with distilled water. The samples were measured in a spectrophotometer (JENWAY, model 6305, Bibby Scientific, United King) at 882 nm, using distilled water as a blank. To measure the effect of PSF inoculation on coffee bean production, ripe fruits were cut in both plots 270 days after inoculation and their weight (gr) was recorded on an analytical balance.
Physico-chemical analysis of the soil
Physicochemical analyses of the rhizospheric soil of the coffee plantation were conducted according to NOM 021-RECNAT-2000 (Table 4). Organic matter (OM) and organic carbon (OC) were quantified following the modified Walkley-Black method, pH by the electrometric method, cation exchange capacity (CEC) with 1N ammonium acetate (pH 7.0), total nitrogen by micro-Kjeldahl, and retained phosphorus by the Blakemore method. These analyses were conducted at the Soil, Plant, and Water Analysis Laboratory of the Instituto de Ecologia, A.C.
Statistical analysis of data
Data were analyzed using one-way ANOVA and Fisher's LSD means tests to determine significant differences between groups at p< 0.05. All statistical analyses were performed in Statistica version 7.0 [82].
Author Contributions
Conceptualization, R.M.A.M and Y.C.E; methodology, R.M.A.M., Y.P.R and K.Y.G.G.; validation, R.M.A.M and Y.C.E.; writing original draft preparation R.M.A.M., Y.P.R and G.H.A.; writing review and editing, G.H.A and R.M.A.M; supervision, R.M.A.M., Y.C.E and G.H.A.; funding acquisition, R.M.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a project COVEICYDET 131627: "Development of a fungal bioinoculant that solubilizes soil phosphorus and promotes the growth and productivity of coffee plants (Coffea arabica var. Costa Rica) in Jilotepec, Veracruz".
Acknowledgments
The authors thank the owners of the farms for allowing access to take soil samples, to Alberto Donaldo Torres Salas and Alondra Juarez for their help in taking the samples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- SAGARPA. Café, la bebida que despierta a México. Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación: https://www.gob.mx/agricultura/articulos/cafe-la-bebida-que-despierta-a-mexico?idiom=es (Accessed 01/06/2023).
- SIAP. Avance De Siembras Y Cosechas Resumen Nacional Por Estado Otoño-Invierno Riego Temporal. Servicio de Información Agroalimentaria y Pesquera: http://www.cenacafe.org.mx/plataformas.html (Accessed 05/05/2023).
- Vandermeer, J.H. The coffee Agroecosystem in the Neotropics: Combining Ecological and Economic Goals. Trop. Subtrop. Agroecosystems. 2003, 159–194. [Google Scholar]
- Giovannucci, D.; Juárez, C.R. Análisis Prospectivo de Política Cafetalera. México, Proyecto Evaluación Alianza para el campo 2005. SAGARPA 2006.
- Moguel, Y.; Toledo, V. El café en México. Ecología, cultura indígena y sustentabilidad. Ciencias 1996, 43, 40–51. [Google Scholar]
- Arcila, J. Crecimiento y desarrollo de la planta de café. In Sistemas de producción de café en Colombia; Ospina, H. y Marín, S., Ed.; Cenicafe: Colombia, 2007; pp. 21–60. [Google Scholar]
- Geissert, D.; Ibañez, A. Calidad y ambiente fisicoquímico de los suelos. In Agroecosistemas cafetaleros del estado de Veracruz biodiversidad, manejo y conservación; Manson, R.H., Hernández, V., Gallina, O.S., Mehltreter, K. Eds. Instituto de Ecología, México 2008; pp. 15-44.
- Kour, D.; Rana, K.L.; Kaur, T.; Yadav, N.; Yadav, A.N.; Kumar, M.; Kumar, V.; Dhaliwal, H.S.; Saxena, A.K. Biodiversity, current developments and potential biotechnological applications of phosphorus solubilizing and mobilizing microbes: A review. Pedosphere 2021, 31, (1),43–75. [Google Scholar] [CrossRef]
- Navarro, G.; Navarro, S. En Química agrícola: el suelo y los elementos químicos esenciales para la vida vegetal, 2nd ed.; Ediciones Mundi Prensa; España, 2003.
- Yadav, B.K.; Verma, A. Phosphate solibilization and mobilization in soil through microorganisms under arid ecosystems. In The Functioning of Ecosystems; Ali, M., Ed.; The Functioning of Ecosystems. Croatia, 2012; pp. 99–108. [Google Scholar]
- Sharma, S.; Sayyed, R.; Trivedi, M.; Gobi, T. Review Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus 2013, 2, 587. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, M. La solubilización de fosfatos como estrategia microbiana para promover el crecimiento vegetal. Cien. Tecnol. Agropecuaria 2014, 15, 101–113. [Google Scholar] [CrossRef]
- Arcand, M.M.; Schneider, K.D. Plant-and microbial-based mechanisms to improve the agronomic effectiveness of phosphate rock: a review. An. Acad. Bras. 2006, 78, 791–807. [Google Scholar] [CrossRef]
- Souza, R.D.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef]
- Silva, G.N.; Vidor, C. Solubilização de fosfatos por microrganismos na presença de fontes de carbono. Rev. Bras. Cienc. Solo 2000, 24, 311–329. [Google Scholar] [CrossRef]
- Posada, R.H.; De Prager, M.S.; Sieverding, E.; Dorantes, K.A.; Heredia, G.P. Relaciones entre los hongos filamentosos y solubilizadores de fosfatos con algunas variables edáficas y el manejo de cafetales. Rev. Biol. Trop. 2012, 60, 1075–1096. [Google Scholar] [CrossRef]
- Arias, R.M; Juárez, A.; Heredia, G.; De la Cruz, Y. Capacidad fosfato solubilizadora de hongos rizosféricos provenientes de cafetales de Jilotepec, Veracruz. AyT BUAP 2022, 7(27), 69–86. [Google Scholar] [CrossRef]
- Vera, D.H.; Herando, P.; Herando, V. Aislamiento de hongos solibilizadores de fosfatos de la rizósfera de Arazá (Eugenia stipitata, Myrtaceae). Acta Biol. Colomb. 2002, 7, 33–4. [Google Scholar]
- Oliveros, A.; Macías, F.; Fernández, C.; Marín, D.; Molinillo, J. Exudados de la raíz y su relevancia actual en las interacciones alelopáticas. Quim. Nova 2009, 32, 198–213. [Google Scholar] [CrossRef]
- Ponmurugan, P.; Gopi, C. Distribution pattern and screening of phosphate solubilizing bacteria isolated from different food and forage crops. J. Agron. 2006, 5, 600–604. [Google Scholar] [CrossRef]
- Djuuna, A.; Prabawardani, S.; Massora, M. Population distribution of phosphate-solubilizing microorganisms in agricultural soil. Microbes Environ. 2022, 37. [Google Scholar] [CrossRef] [PubMed]
- Ceci, A.; Pinzari, F.; Russo, F.; Maggi, O.; Persiani, A. Saprotrophic soil fungi to improve phosphorus solubilisation and release: In vitro abilities of several species. Ambio 2018, 47, 30–40. [Google Scholar] [CrossRef]
- Nahidan, S.; Ahadi, N.; Abduolrahimi, S. Efficiency of some fungal species in phosphate solubilization and potassium and iron release from phlogopite and muscovite. Applied Soil Research 2023, 11, 112–124. [Google Scholar]
- Jyothi, V.; Basaiah, T. Isolation and Characterization of Phosphofungi–Aspergillus niger from Rhizosphere Soil to supplement Phospho-biofertilizer. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Vyas, P.; Rahi, P.; Chauhan, A.; Gulati, A. Phosphate solubilization potencial ans stress tolerance of Eupenicillium parvum from tea soil. Mycol Res. 2007, 111, 931–938. [Google Scholar] [CrossRef]
- Krishna, P.; Sukla, L. Isolation of phosphate solubilizing fungus from rice fields of Odisha for agro-ecological sustainable agriculture. Environ. Qual. Manag. 2022, 32, 19–27. [Google Scholar] [CrossRef]
- Răut, I.; Călin, M.; Capră, L.; Gurban, A.; Doni, M.; Radu, N.; Jecu, L. Cladosporium sp. isolate as fungal plant growth promoting agent. Agron. 2021, 11. [Google Scholar] [CrossRef]
- Doilom, M.; Guo, J.; Phookamsak, R.; Mortimer, P.; Karunarathna, S.; Dong, W. , Liao, C.; Yan, K.; Pem, D.; Suwannarach, N.; Promputtha, I.; Lumyong, S.; Xu, J. Screening of phosphate-solubilizing fungi from air and soil in Yunnan, China: four novel species in Aspergillus, Gongronella, Penicillium, and Talaromyces. Front. Microbiol. 2020, 11, 585215. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Das, S.J. Phosphate solubilizing fungi from Mangroves of Bhitarkanika, Orissa. Hayati J. Biosci. 2008, 15, 90–92. [Google Scholar] [CrossRef]
- Sang, Y.; Jin, L.; Zhu, R.; Yu, X.; Hu, S.; Wang, B.; Ruan, H.; Jin, F.; Lee, H. Phosphorus-Solubilizing Capacity of Mortierella Species Isolated from Rhizosphere Soil of a Poplar Plantation. Microorganisms 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Sano, A.; Hossain, M.; Sakagami, J. Isolation and molecular characterization of phosphate solubilizing filamentous fungi from subtropical soils in Okinawa. Appl. Ecol. Environ. Res. 2019, 17. [Google Scholar] [CrossRef]
- Yasser, M.; Mousa, A.; Massoud, O.; Nasr, S. Solubilization of inorganic phosphate by phosphate solubilizing fungi isolated from Egyptian Soils. J. Biol. Earth Sci. 2014, 4. [Google Scholar]
- Ahmad, A.; Moin, S.; Liaqat, I.; Saleem, S.; Muhammad, F.; Mujahid, T.; Zafar, U. Isolation, Solubilization of Inorganic Phosphate, and Production of Organic Acids by Individual and Co-inoculated Microorganisms. Geomicrobiol. J. 2022, 1–11. [Google Scholar] [CrossRef]
- Fenice, M.; Selbman, L.; Federici, F.; Vassilev, N. Application of encapsulated Penicillium variable P16 in solubilization of rock phosphate. Bioresour. Technol. 2000, 73, 157–162. [Google Scholar] [CrossRef]
- Pandey, A.; Das, N.; Kumar, B.; Rinu, K.; Trivedi, P. Phosphate solubilization by Penicillium spp. isolated from soil samples of Indian Himalayan region. World j. Microbiol. Biotechnol. 2008, 24, 97–102. [Google Scholar] [CrossRef]
- Morales, A.; Alvear. M.; Valenzuela, E.; Castillo, C.E.; Borie, F. Screening, evaluation and selection ofphosphate-solubilising fungi as potential biofertiliser. J. Soil Sci. Plant Nutr. 2011, 11, 89–103. [Google Scholar] [CrossRef]
- Rathore, P.; Phanse, N.; Patel, B. Screening for microorganisms possessing phosphate solubilizing potential. Indian J. Res. 2014, 3, 172–174. [Google Scholar] [CrossRef]
- Gizaw, B.; Tsegay, Z.; Tefera, G.; Aynalem, E.; Wassie, M.; Abatneh, E. Phosphate solubilizing fungi isolated and characterized from Teff rhizosphere soil collected from North Showa zone, Ethiopia. Afr. J. Microbiol. Res. 2017, 11, 687–696. [Google Scholar] [CrossRef]
- Wakelin, S.; Warren, R.; Harvey, P.; Ryder, M. Phosphate solubilization by Penicillium spp. closely associated with wheat roots. Biol. Fertil. Soils 2004, 40, 36–43. [Google Scholar] [CrossRef]
- Ñústez, C.E.; Acevedo, J.C. Evaluación del uso de Penicillium janthinellum Biourge sobre la eficiencia de la fertilización fosfórica en el cultivo de la papa (Solanum tuberosum L. var. Diacol Capiro). Agron. Colomb. 2005, 23, 290–298. [Google Scholar]
- Moreno, N.; Moreno, L.; Uribe, D. Biofertilizantes para la agricultura en Colombia. En Biofertilizantes en Iberoamérica: una visión técnica, científica y empresarial; Izaguirre-Mayoral, M.L., Labanderay, C., Sanjuán, J. Eds.; Editorial Universitaria: Cuba 2007; pp. 38-45.
- Morales, A.; Alvear, M.; Valenzuela, E.; Castillo, C.E.; Borie, F. Screening, evaluation and selection ofphosphate-solubilising fungi as potential biofertiliser. J. Soil Sci. Plant Nutr. 2011, 11, 89–103. [Google Scholar] [CrossRef]
- Elías, F.; Woyessa, D.; Muleta, D. Phosphate solubilization potential of Rhizosphere fungi isolated from plants in Jimma Zone, Southwest Ethiopia. Int. J. Microbiol. 2016. [Google Scholar] [CrossRef]
- Hernández, T.; Carrión, G.; Heredia, G. Solubilización in vitro de fosfatos por una cepa de Paecilomyces lilacinus (Thom) Samson. Agrociencia 2011, 45, 881–892. [Google Scholar]
- Verma, A.; Ekka, A. Isolation, screening and assessment of phosphate solubiling efficiency of some fungal isolates of Raipur, Chhattisgarh. IOSR J. Environ. Sci., Toxicol. Food Technol. 2015, 1, 29–39. [Google Scholar]
- Hajjam, Y.; Cherkaoui, S. The influence of phosphate solubilizing microorganisms on symbiotic nitrogen fixation: Perspectives for sustainable agriculture. J. Mater. Environ. Sci. 2017, 8, 801–808. [Google Scholar]
- Romero, J.; Arias, R.; Bañuelos, J.; De la Cruz, Y. (2019). Efecto de la inoculación de hongos solubilizadores de fósforo y micorrízicos arbusculares sobre el crecimiento de plantas de jitomate (Lycopersicon esculentum Mill). Rev. Mex. Cienc. Agri. 2019, 10, 1747–1757. [Google Scholar] [CrossRef]
- Saxena, J.; Saini, A.; Ravi, I.; Chandra, S.; Garg, V. Consortium of phosphate solubilizing bacteria and fungi for promotion of growth and yield of chickpea (Cicer arietinum). J. Crop Improv. 2015, 29, 353–369. [Google Scholar] [CrossRef]
- Moreno, A.; Osorio, N.; González, O. In vitro dissolution of acidulated rock phosphate by phosphate solubilizing microorganisms. Acta Biol. Colomb. 2015, 20, 65–71. [Google Scholar] [CrossRef]
- Lima-Rivera, D.; López-Lima, D.; Desgarennes, D.; Velázquez-Rodríguez, A.; Carrión, G. Phosphate solubilization by fungi with nematicidal potential. J. Soil Sci. Plant Nutr. 2016, 16, 507–524. [Google Scholar] [CrossRef]
- Selvi, K.; Paul, J.; Vijaya, V.; Saraswathi, K. Analyzing the Efficacy of Phosphate Solubilizing Microorganisms. Enrichment Culture Techniques. Biochem. Mol. Biol. 2017, 3, 7–1. [Google Scholar] [CrossRef]
- Illmer, P.; Barbato, A.; Schinner, F. Solubilization of hardly-soluble AlPO4 with P-solubilizing microorganisms. Soil Biol. Biochem. 1995, 27, 265–270. [Google Scholar] [CrossRef]
- Whitelaw, M.; Harden, T.; Helyar, K. Phosphate solubilization in solution culture by the soil fungus Penicillium radicum. Soil biol. Biochem. 1999, 31, 655–665. [Google Scholar] [CrossRef]
- Nasr, S.; Mousa, A.; Marzouk, M.; Yasser, M. Quantitative and Qualitative Analysis of Organic Acids Produced by Phosphate Solubilizing Fungi. Egypt. J. Bot. 2021, 61, 167–176. [Google Scholar] [CrossRef]
- Richa, G.; Khosla, B.; Reddy, M.S. Improvement of maize plant growth by phosphate solubilizing fungi in rock phosphate amended soils. World J. Agric. Sci. 2007, 3, 481–484. [Google Scholar]
- Rodríguez, H.; Gonzalez, T.; Goire, I.; Bashan, Y. Gluconic acid production and phosphate solubilization by the plant growth-promoting bacterium Azospirillum spp. Naturwissenschaften 2004, 91, 552–555. [Google Scholar] [CrossRef]
- Gyaneshwar, P.; Naresh, G.; Parekh, L.; Poole, P. Role of soil microorganisms in improving P nutrition of plants. Plant and soil 2002, 245, 83–93. [Google Scholar] [CrossRef]
- Gómez, Y.; Zabala, M. Determinación de la capacidad solubilizadora del P en hongos aislados de la rizósfera del mani (Arachis hypogaea L.). Saber Universidad de Oriente 2001, 13, 8–13. [Google Scholar]
- Chung-Chao, C.; Yu-Lin, K.; Chen-Ching, C.; Wei-Liang, C. Solubilization of inorganic phosphates and plant growth promotion by Aspergillus niger. Biol. Fertil. Soils 2007, 43, 575–584. [Google Scholar] [CrossRef]
- Vitorino, L.C.; Silva, F.G.; Soares, M.A.; Souchie, E.L.; Costa, A.C.; Lima, W.C. Solubilization of calcium and iron phosphate and in vitro production of Indoleacetic acid by Endophytic isolates of Hyptis marrubioides Epling (Lamiaceae). Int. Res. J. Biotechnol. 2012, 3, 47–54. [Google Scholar]
- Narsian, V.; Patel, H.H. Aspergillus aculeatus as a rock phosphate solubilizer. Soil Biol. Biochem. 2000, 32, 559–565. [Google Scholar] [CrossRef]
- Reddy, M.S.; Kumar, S.; Babita, K.; Reddy, M.S. Biosolubilization of poorly soluble rock phosphates by Aspergiullus tubigensis and Aspergillus niger. Bioresour. Technol. 2002, 84, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Barroso, C.B.; Nahas, E. The status of soil phosphate fractions and the ability of fungi to dissolve hardly soluble phosphates. Appl. Soil Ecol. 2005, 29, 73–83. [Google Scholar] [CrossRef]
- Sayer, J.A.; Raggett, S.L.; Gadd, G.M. Solubilization of insoluble metal compounds by soil fungi: development of a screening method for solubilizing ability and metal tolerance. Mycol. Res. 1995, 99, 987–993. [Google Scholar] [CrossRef]
- Anand, K.; Kumari, B.; Mallick, M. Phosphate solubilizing microbes: an effective and alternative approach as biofertilizers. Int. J. Pharm. Sci. 2016, 8, 37–40. [Google Scholar]
- Gómez, Y. Actividad de las fosfatasas ácidas y alcalinas (extracelulares e intracelulares) en hongos de la rizosfera de Arachis hypogaea (Papiloneaceae). Rev. Biol. Trop. 2004, 52, 287–295. [Google Scholar] [CrossRef]
- Valenzuela, E.; Toro, V.; Martínez, O.; Pinochet, D. Determinación de fosfatasas en hongos de praderas. Bol. Micol. 2005, 20. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, L.; Yuan, S.; Liu, W.; Zhang, C.; Tian, D.; Ye, X. The Production of Oxalate by Aspergillus niger under Different Lead Concentrations. Agronomy 2023, 13. [Google Scholar] [CrossRef]
- Perea, Y.; Arias, R.; Medel, R.; Trejo, D.; Heredia, G.; Rodríguez, Y. Effects of native arbuscular mycorrhizal and phosphate-solubilizing fungi on coffee plants. Agrofor. Syst. 2018, 93, 961–972. [Google Scholar] [CrossRef]
- Chakraborty, B.; Chakraborty, U.; Saha, A.; Sunar, K.; Dey, P. Evaluation of phosphate solubilizers from soils of North Bengal and their diversity analysis. World J. Agric. Sci. 2010, 6, 195–200. [Google Scholar]
- Patiño, C.O.; Sanclemente, O.E. Los microorganismos solubilizadores de fósforo (MSF): una alternativa biotecnológica para una agricultura sostenible. Entramado 2014, 10, 288–297. [Google Scholar]
- Sashidhar, B.; Podile, A.R. Mineral phosphate solubilization by rhizosphere bacteria and scope for manipulation of the direct oxidation pathway involving glucose dehydrogenase. J. Appl. Microbiol. 2010, 109, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Emami-Karvani, Z.; Chitsaz-Esfahani, Z. Phosphorus Solubilization: Mechanisms, Recent Advancement and Future Challenge. In: Yadav, A. Eds. Soil Microbiomes for Sustainable Agriculture. Sustainable Development and Biodiversity, 2021, vol 27, pp. 85-131. [CrossRef]
- Vassileva, M.; Mocali, S.; Canfora, L.; Malusá, E.; García del Moral, L.; Martos, V.; Peregrin, E.; Vassilev, N. Safety level of microorganism-bearing products applied in soil-plant systems. Front. Plant Sci. 2022, 13, 862875. [Google Scholar] [CrossRef] [PubMed]
- Edi-Premono, M.; Moawad, A.; Vleck, L.G. Effect of phosphate-solubilizing Pseudomonas putida on the growth of maize and its survival in the rhizosphere. Indones. J. Crop Sci. 1996, 11, 13. [Google Scholar] [CrossRef]
- Sundara, V.B.; Sinha, M.K. Phosphate dissolving organisms in the soil and rizosphere. Indian j. Sci. 1963, 33, 272–278. [Google Scholar]
- Clesceri, S.; Greenberg, A.; Trusell, R. En Métodos normalizados para el análisis de aguas potables y residuales, 17th ed.; Díaz Santos Ed; España 1992; pp. 4201-4202.
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Senasica. Manual Técnico Operativo. Centro Nacional de Referencia Fitosanitaria, Área de Vigilancia Epidemiológica Fitosanitaria. Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria: https://prod.senasica.gob.mx/SIRVEF/ContenidoPublico/Accion%20operativa/Manual%20operativo/Manual%20operativo%20VEF%202019.pdf (Accessed 01/05/2021).
- McKean, S.J. Manual de análisis de suelos y tejido vegetal: Una guía teórica y práctica de metodologías. CIAT 1993, (129), 1–99. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic and available forms of phosphorus in soil. Soil Sci. 1945, 59, 39–45. [Google Scholar] [CrossRef]
- StatSoft Inc. Statistica to Windows v.7.0. Data analysis software system. Tulsa 2007. 2007.
Figure 1.
Fungi showing phosphate solubilization leading to formation of clear zone in Sundara´s medium A: Aspergillus candidus; B: Aspergillus sclerotiorum; C) Acremonium roseolu;, D) Aspergillus sp. 1Y; E) Cladosporium cladosporioides; F) Eupenicillium euglaucum; G) Penicillium glabrum, H) Eupenicillium ludwigii, I) Fusarium sp. 25, J) Penicillium arenicola, K) P. glabrum; L) Penicillium waksmanii; M) Sagenomella diversispora; M) Paecilomyces marquandii; N) Aspergillus niger, O) Beauveria bassiana, P) Penicillium brevicompactum.
Figure 1.
Fungi showing phosphate solubilization leading to formation of clear zone in Sundara´s medium A: Aspergillus candidus; B: Aspergillus sclerotiorum; C) Acremonium roseolu;, D) Aspergillus sp. 1Y; E) Cladosporium cladosporioides; F) Eupenicillium euglaucum; G) Penicillium glabrum, H) Eupenicillium ludwigii, I) Fusarium sp. 25, J) Penicillium arenicola, K) P. glabrum; L) Penicillium waksmanii; M) Sagenomella diversispora; M) Paecilomyces marquandii; N) Aspergillus niger, O) Beauveria bassiana, P) Penicillium brevicompactum.
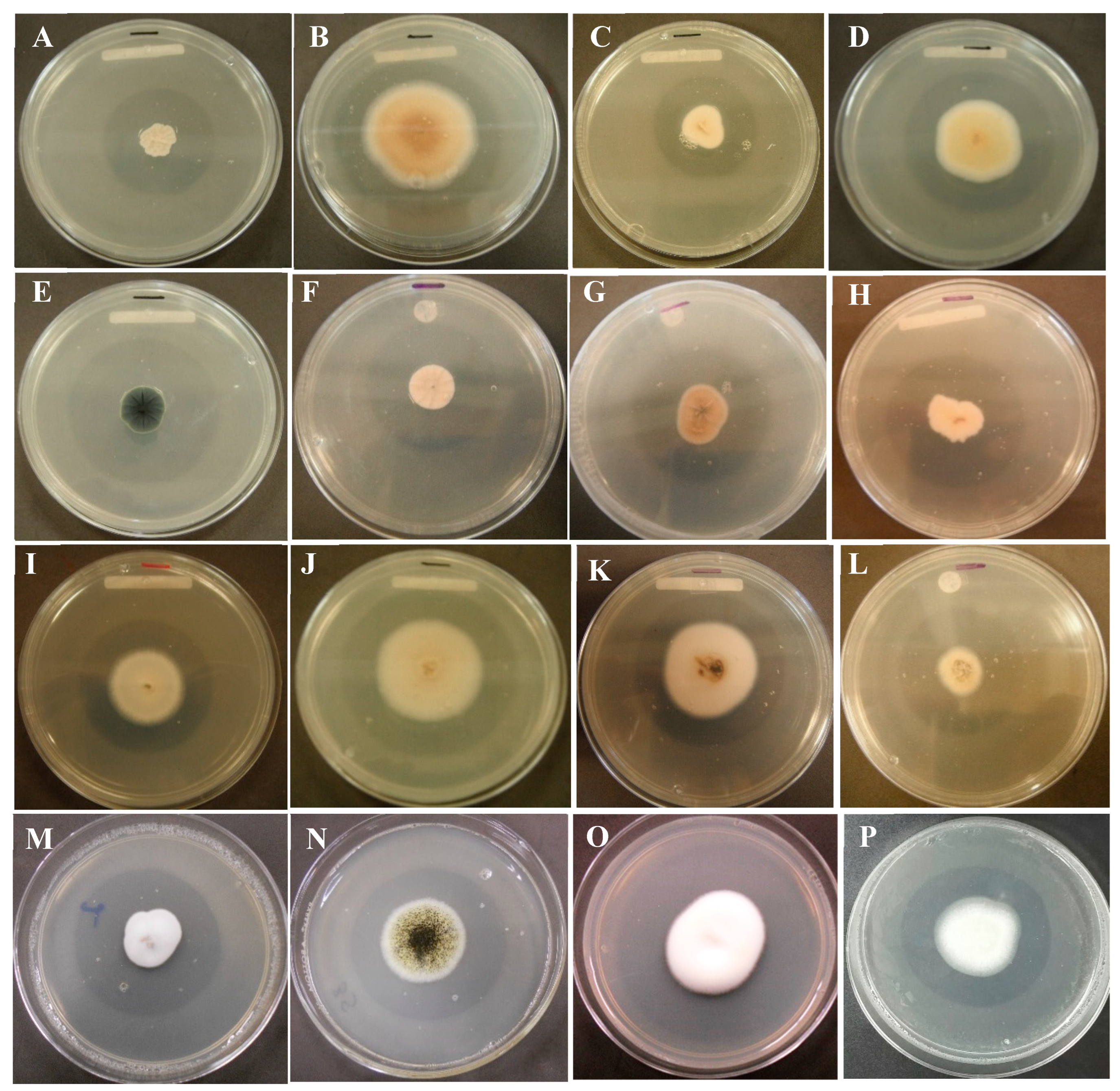
Figure 2.
Figure 2. Soluble phosphorus and pH from extracts of three PSF strains (A. niger AN, P. brevicompactum PB, P. waksamnii PW) and the control (C), over 21 days of incubation (measured at 9, 12, 15, 18 and 21 days). Data are the average of three replicates ± standard error. Identical letters in columns indicate no significant difference (p≤0.05).
Figure 2.
Figure 2. Soluble phosphorus and pH from extracts of three PSF strains (A. niger AN, P. brevicompactum PB, P. waksamnii PW) and the control (C), over 21 days of incubation (measured at 9, 12, 15, 18 and 21 days). Data are the average of three replicates ± standard error. Identical letters in columns indicate no significant difference (p≤0.05).
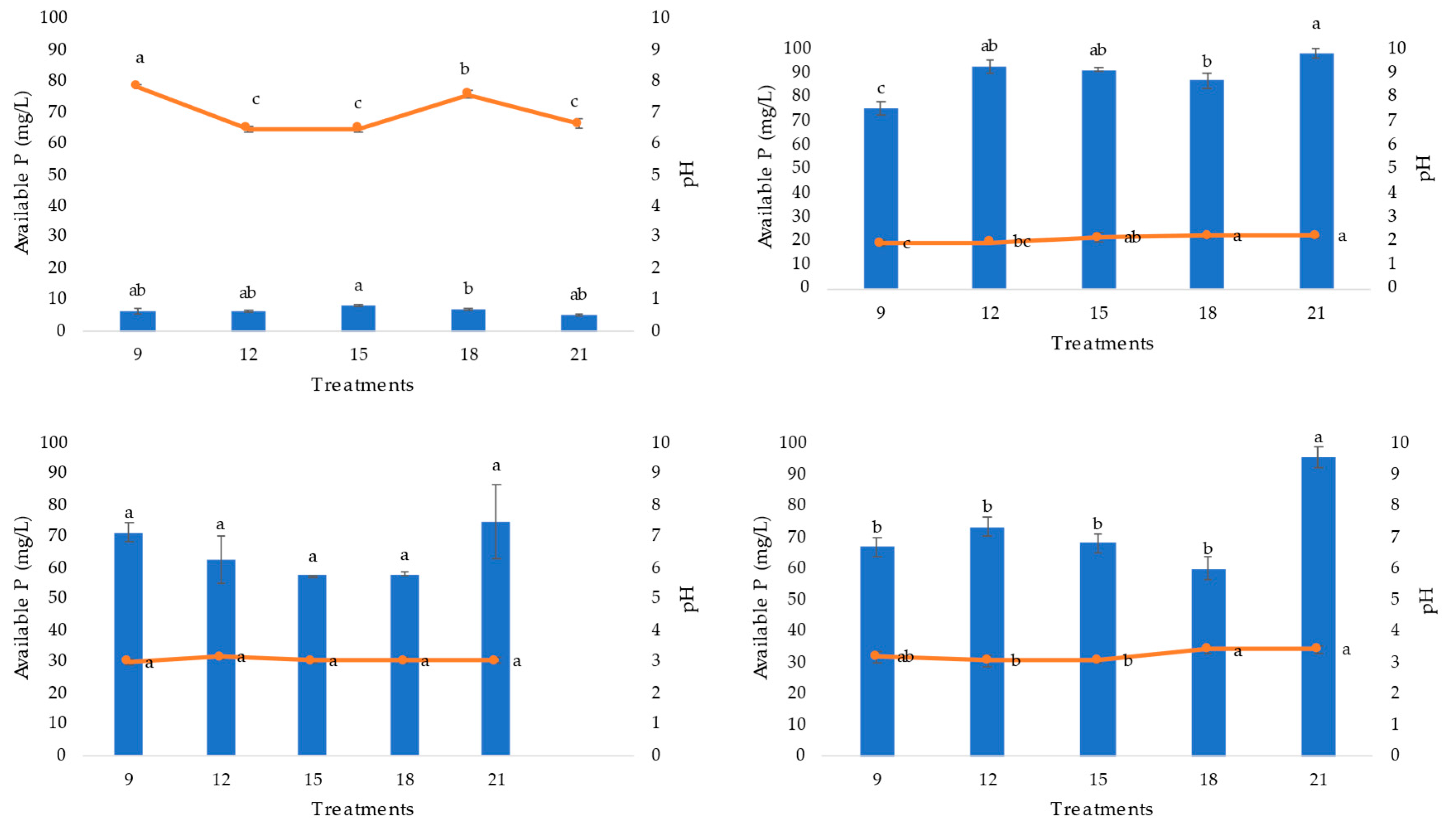
Figure 3.
Acid phosphatase extracts of the PSF strains (A. niger AN, P. brevicompactum PB, and P. waksamnii PW) and the control (C), over 21 days of incubation (9, 12, 15, 18, and 21). Data are the average of three replicates ±standard error. Identical letters in the columns indicate no significant differences p≤0.05.
Figure 3.
Acid phosphatase extracts of the PSF strains (A. niger AN, P. brevicompactum PB, and P. waksamnii PW) and the control (C), over 21 days of incubation (9, 12, 15, 18, and 21). Data are the average of three replicates ±standard error. Identical letters in the columns indicate no significant differences p≤0.05.
Figure 4.
Isoenzymatic patterns of in vitro acid phosphatases of the strains (A. niger AN, P. brevicompactum PB, P. waksamnii PW).
Figure 4.
Isoenzymatic patterns of in vitro acid phosphatases of the strains (A. niger AN, P. brevicompactum PB, P. waksamnii PW).

Figure 5.
Soil soluble phosphorus content of coffee (Coffea arabica var. Costa Rica) plants from “San Isidro (A), "Los Bambus" (B), “La Barranca” (C) coffee plantation, in Jilotepec. PSF: Plants inoculated with the phosphorus solubilizing fungus (P. brevicompactum); N-PSF: Uninoculated plants at 60, 120 and 180 days. Data are the average of fifteen replicates ±standard error. Identical letters in the columns indicate no significant differences between plantations (p≤0.05).
Figure 5.
Soil soluble phosphorus content of coffee (Coffea arabica var. Costa Rica) plants from “San Isidro (A), "Los Bambus" (B), “La Barranca” (C) coffee plantation, in Jilotepec. PSF: Plants inoculated with the phosphorus solubilizing fungus (P. brevicompactum); N-PSF: Uninoculated plants at 60, 120 and 180 days. Data are the average of fifteen replicates ±standard error. Identical letters in the columns indicate no significant differences between plantations (p≤0.05).
Figure 6.
Soil soluble phosphorus content of coffee (Coffea arabica var. Costa Rica) plants in the coffee plantation “San Isidro (A), “Los Bambus” (B) and "La Barranca" (C), in Jilotepec. PSF: Plants inoculated with P. brevicompactum; N-PSF: Uninoculated plants at 60, 120 and 180 days. Data are the average of fifteen replicates ±standard error. Identical letters in the columns indicate no significant differences between plantations (p≤0.05).
Figure 6.
Soil soluble phosphorus content of coffee (Coffea arabica var. Costa Rica) plants in the coffee plantation “San Isidro (A), “Los Bambus” (B) and "La Barranca" (C), in Jilotepec. PSF: Plants inoculated with P. brevicompactum; N-PSF: Uninoculated plants at 60, 120 and 180 days. Data are the average of fifteen replicates ±standard error. Identical letters in the columns indicate no significant differences between plantations (p≤0.05).
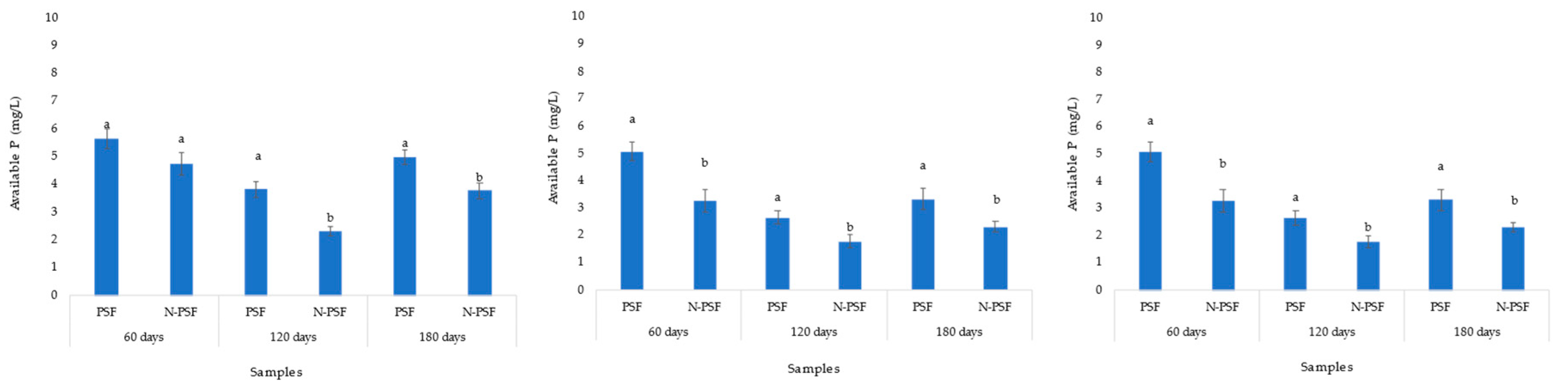
Figure 7.
Production of cherry coffee beans from plants of Coffea arabica var. Costa Rica of three coffee plantations. PSF: Plants inoculated with the phosphorus-solubilizing fungus P. brevicompactum; N-PSF: Plants without the phosphorus-solubilizing fungus. Data are the average of fifteen replicates ±standard error. Different letters between columns indicate significant differences (p≤0.05).
Figure 7.
Production of cherry coffee beans from plants of Coffea arabica var. Costa Rica of three coffee plantations. PSF: Plants inoculated with the phosphorus-solubilizing fungus P. brevicompactum; N-PSF: Plants without the phosphorus-solubilizing fungus. Data are the average of fifteen replicates ±standard error. Different letters between columns indicate significant differences (p≤0.05).
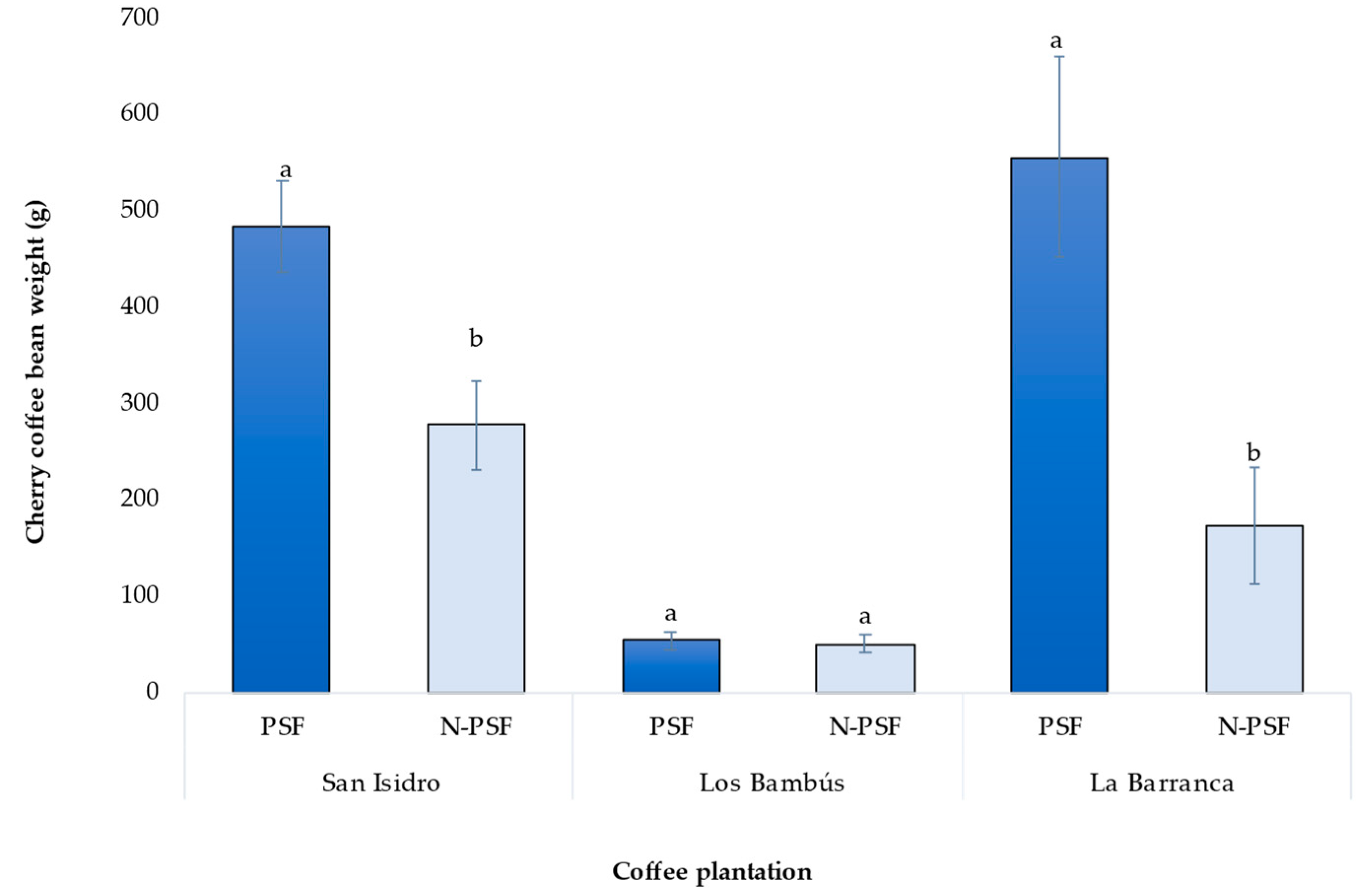
Figure 8.
Selected coffee plantations of central Veracruz state, Mexico: A) “San Isidro”; B) “Los Bambus” and C) “La Barranca”.
Figure 8.
Selected coffee plantations of central Veracruz state, Mexico: A) “San Isidro”; B) “Los Bambus” and C) “La Barranca”.

Table 1.
fungal strains in scale III.
| Fungal strain | Solubilization index | ||||||||
| Aspergillus niger | 2.60 | 1.91 | 1.90 | ||||||
| Acremonium roseolum | 1.72 | 2.34 | 2.44 | 2.43 | 2.46 | 2.54 | 2.47 | 2.45 | |
| Aspergillus candidus | 2.35 | 2.64 | 2.53 | 2.95 | 2.97 | 2.88 | 2.88 | 2.00 | |
| Aspergillus sclerotiorum | 2.44 | 1.20 | 1.30 | 1.36 | 1.48 | 1.50 | 1.48 | 1.41 | |
| Aspergillus sydowii | 1.80 | 1.75 | 1.66 | 1.63 | 1.59 | 1.39 | 1.32 | 1.14 | |
| Aspergillus sp. 1Y | 1.37 | 1.43 | 1.61 | 1.69 | 1.81 | 1.77 | 1.70 | 1.62 | |
| Cladosporium cladosporioides | 1.59 | 2.27 | 2.72 | 2.61 | 2.60 | 2.58 | 2.36 | 2.54 | |
| Epicoccum nigrum | 1.87 | 2.22 | 2.12 | 2.03 | 1.97 | 1.82 | 1.74 | 1.59 | |
| Eupenicillium euglaucum | 2.00 | 2.39 | 2.51 | 2.58 | 2.73 | 2.82 | 2.82 | 2.86 | |
| Eupenicillium ludwigii | 3.53 | 3.08 | 2.61 | 2.64 | 2.65 | 2.65 | 3.05 | 2.91 | |
| Fusarium sp. C25 | 2.80 | 2.76 | 2.68 | 2.67 | 2.67 | 2.48 | 2.34 | 2.19 | |
| Fusarium sp. 3Y | 2.50 | 2.42 | 2.44 | 2.34 | 2.27 | 2.05 | 1.96 | ||
| Humicola sp. 2gh205 | 2.14 | 2.01 | 1.79 | 1.76 | 1.72 | 1.69 | 1.72 | 1.55 | |
| Merimbla sp. 3gh18 | 1.76 | 1.75 | 1.76 | 1.72 | 1.63 | 1.59 | 1.54 | 1.77 | |
| Penicillium arenicola | 1.42 | 1.74 | 1.77 | 2.02 | 1.68 | 1.63 | 1.41 | 1.31 | |
| Penicillium brevicompactum | 2.47 | 2.96 | 3.37 | 3.61 | 3.36 | 3.11 | 2.94 | 2.64 | |
| Penicillium glabrum | 1.47 | 1.70 | 1.72 | 1.62 | 1.64 | 1.53 | 1.39 | 1.35 | |
| Penicillium miczynskii | 2.26 | 2.09 | 1.94 | 2.17 | 2.70 | 1.96 | 1.97 | 1.72 | |
| Penicillium olsonii | 1.82 | 1.98 | 2.00 | 1.93 | 1.87 | 1.77 | 1.68 | 1.67 | |
| Penicillium verrucosum | 1.43 | 1.93 | 1.99 | 2.07 | 1.86 | 1.61 | 1.47 | 1.34 | |
| Penicillium waksmanii | 1.76 | 2.80 | 3.20 | 3.60 | 3.67 | 3.88 | 3.83 | 3.79 | |
| Penicillium sp. 90 | 2.13 | 2.20 | 2.12 | 2.16 | 2.05 | 2.12 | 2.05 | 1.85 | |
| Sagenomella diversispora | 2.83 | 2.93 | 3.23 | 3.10 | 3.15 | 2.95 | 2.83 | 2.63 | |
| Scopulariopsis brevicaulis | 1.98 | 2.77 | 2.95 | 2.76 | 2.61 | 2.66 | 2.30 | 2.16 | |
| Talaromyces flavus var. flavus | 3.52 | 2.66 | 2.02 | 2.00 | 2.12 | 2.14 | 2.30 | 2.21 | |
| Trichocladium asperum | 2.43 | 2.87 | 2.85 | 3.00 | 3.34 | 3.15 | 3.33 | 3.00 | |
Table 3.
Geographic location, elevation, and characteristics of the study sites.
| Sites | Annual mean precipitation | Latitude | Longitude | Elevation (masl) | Mean temperature | Management type | Soil type |
|---|---|---|---|---|---|---|---|
| San Isidro | 241 | 19°36’42.74’’ | 96°56’16.01’’ | 1370 | 22 | Traditional polyculture | Andosol |
| Los Bambús | 275.9 | 19°36’38.07’’ | 96°55’40.57’’ | 1414 | 25 | Traditional polyculture | Andosol |
| La Barranca | 298.2 | 19°36’12.15’’ | 96°54’44.91’’ | 1484 | 25 | Traditional polyculture | Andosol |
Table 4.
Physico-chemical characteristics of the coffee plantations evaluated.
| Coffee plantations | |||
|---|---|---|---|
| “San Isidro” | “Los Bambus” | “La Barranca” | |
| pH | 6.11 | 6.69 | 5.43 |
| Retained P | 87.35 | 89.8 | 81.63 |
| Organic material | 12.46 | 3.93 | 4.72 |
| Organic carbon | 7.23 | 2.28 | 2.74 |
| Cation exchange capacity (CEC) | 27.09 | 20.88 | 21.51 |
| field capacity (FC) | 31.72 | 22.69 | 21.62 |
| Bulk density | 0.893 | 1.016 | 0.994 |
| Clay | 29.8 | 45.8 | 49.8 |
| Silt | 30.56 | 22.56 | 28.56 |
| Sand | 39.64 | 31.64 | 21.64 |
| Texture | Clay loam | Clay | Clay |
| Fe | 69.38 | 111.63 | 163.03 |
| C | 8.5 | 2.9 | 3.5 |
| N | 0.72 | 0.27 | 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Submitted:
24 July 2023
Posted:
26 July 2023
You are already at the latest version
Alerts
A peer-reviewed article of this preprint also exists.
Submitted:
24 July 2023
Posted:
26 July 2023
You are already at the latest version
Alerts
Abstract
The use of phosphate-solubilizing fungi in coffee cultivation is an alternative with which to reduce the use of fertilizers. The objective of this study was to analyze the mechanisms involved in the phosphorus solubilization of the fungal strains and to evaluate the effect of a phosphate-solubilizing strain on the coffee plants. For this, phosphorus-solubilizing fungal strains were selected for evaluation of their solubilization potential and phosphatase activity. Coffee plants were inoculated in the field with a phosphate solubilizing strain, and soil and foliar soluble phosphorus, as well as coffee bean yield, were quantified. Of the 151 strains analyzed, Aspergillus niger, Penicillium waksmanii, and Penicillium brevicompactum showed the highest solubilization. Aspergillus niger and P. waksmanii presented the highest soluble phosphorus values; however, P. brevicompactum showed the highest phosphatase activity. The P. brevicompactum strain inoculated on the coffee plants did not favor foliar phosphorus content but increased the soil soluble phosphorus content in two of the coffee plantations. The plants inoculated with the phosphate solubilizing strain showed an increase in coffee bean weight on all plantations, although this increase was only significant in two of the three selected coffee plantations.
Keywords:
Subject: Biology and Life Sciences - Biology and Biotechnology
1. Introduction
Cultivated coffee is considered the world's leading agricultural commodity, with a market generating more than 90 billion dollars annually. Around 8% of the world's population (approximately 500 million people) are involved in the coffee market, from sowing to final consumption. Coffee is produced in 14 states of Mexico, of which Chiapas, Veracruz, Puebla, and Oaxaca account for 90% of production. In 2019, Chiapas contributed 40.9% of the national production, followed by Veracruz with 24.2%, Puebla with 16.0%, and Guerrero with 9.4% [1]. The state of Veracruz is the second most important coffee-producing region in Mexico. The coffee is produced in 842 communities of 82 municipalities. Sixty percent of the coffee is grown above 750 m asl in elevation. The regions with the highest production are Coatepec, Cordoba, Huatusco, Misantla, and Atzalan. Currently, 21,089 producers in the central region of Veracruz produce this crop over a total area of 58,712 ha, representing 7.3% of the area dedicated to coffee growing nationally. The rural development district of Coatepec cultivates 28,873 hectares in 21 municipalities. Jilotepec belongs to this district and has a planted area of 1,776 hectares [2].
Coffee growing is considered a fundamental strategic activity, because it allows the integration of productive chains, as well as the generation of foreign currency and employment, and is the means of subsistence for many small producers and indigenous groups [3,4]. In addition, it is of great ecological importance since more than 90% of the area cultivated with this crop is under shade, which contributes to biodiversity conservation and provides vital environmental services to society [5,6]. In Mexico, the soils where coffee is produced are generally of volcanic origin and are characterized by an acidic pH of 4.5-5.2, as well as low availability of essential macronutrients such as phosphorus (P) [7]. This is mainly because this element is associated with other ionic elements such as calcium, iron, and aluminum, present in forms that cannot be assimilated by the plants [8]. The amount of available P depends on the modification of the dynamic equilibrium that maintains the dissolution of insoluble inorganic compounds and on the decomposition of organic matter [9]. Phosphorus is important in coffee cultivation since it plays key roles in many plant processes such as energy metabolism, the synthesis of nucleic acids and membranes, photosynthesis, respiration, nitrogen fixation, and enzyme regulation [10]. During the early stages of coffee plant development, this element is responsible for vigorous plant growth, participates in the formation of effective root systems, and acts as a promoter of flowering and fruit development. During the reproductive stage, phosphorus is essential for the formation, growth, and multiplication mechanisms of the flower organs. Phosphorus deficiency in the soil can be observed in coffee plants through the yellowing of leaves and a lack of fruit ripening. In severe cases, the leaves near the ripening fruit fall off completely. To remedy a lack of P, coffee producers apply large amounts of phosphate fertilizers [1], although 75-90% of the phosphate added precipitates through the formation of metal cation complexes [11]. In addition, excessive use of fertilizers leads to eutrophication, water toxicity, groundwater contamination, air pollution, soil and ecosystem degradation, biological imbalances, and reduced biodiversity, so it is necessary to seek another option to release P from inorganic and organic pools of total soil P. One alternative is the application of bioinoculants, which are preparations of microorganisms for inoculation with the aim of partially or completely replacing inorganic fertilization.
In this context, phosphate solubilizing fungi (PSF) are of great importance since they are a functional group of microorganisms that play a fundamental role in the P cycle. Thanks to the activity of these fungi, plants can take advantage of the large reserves of insoluble phosphorus fixed to soil minerals [12]. Some fungi and bacteria can solubilize P from unavailable forms in the rhizosphere. The mechanism of mineral phosphate solubilization by the strains of PSF is associated with the release of low molecular weight organic acids [13], which, through their hydroxyl and carboxyl groups, act to chelate the cations bound to the phosphate, thereby converting it into soluble forms. Phosphate solubilizing fungi can produce extracellular enzymes, i.e. a group of phosphatase enzymes that can mineralize organic P into inorganic P such that the P becomes available for the plants. There are several soil phosphatases, the most common of which are phosphomonoesterases, phosphodiesterases, and phytases. Phosphomonoesterases act on phosphate monoesters and, according to their optimum pH, are categorized into acid and alkaline phosphomonoesterases. These microorganisms have a high potential for promoting plant growth by increasing soil fertility [14]. In agricultural soils, the use of microbial inoculants (biofertilizers) that present P-solubilizing activities is considered an environmentally friendly alternative to further applications of chemical-based P fertilizers [11]. The coffee rhizosphere is associated with many beneficial organisms, including growth-promoting microorganisms that may contribute to fulfilling the nutritional requirements of the plant. The objective of this study was to evaluate the PSF and to obtain further information about the mechanisms involved in the solubilization of P, as well as to evaluate the effect of inoculation of an PSF strain on coffee plants under field conditions.
2. Results
2.1. Selection of strains with phosphate solubilizing capacity
Of 151 soil fungal strains tested, 121 (79.5%) formed solubilization halos (Figure 1). Considering the established scales of solubilization, 55 strains (45.8%) were found to be in scale I, 40 strains (33.3%) in scale II, and 26 strains (20.8%) in scale III. Scale I (halos 1-4 mm in thickness) was represented by 24 genera including Trichoderma, Oidiodendron, Penicillium, Chaetomium, and Humicola; scale II (halos of 5-9 mm in thickness) was represented by 12 genera including Penicillium, Aspergillus, Fusarium, Cylindrocarpon, and Talaromyces; and scale III (halos of 9-12 mm in thickness) was represented by 13 genera, most prominently Penicillium, Aspergillus, Fusarium, and Eupenicillium (Table 1).
According to the maximum values obtained for the SI, and based on the scale used by Silva Filho y Vidor [15], six of the strains evaluated had low solubilizing activity. The species in this group are Aspergillus sclerotiorum, Aspergillus sydowii, Aspergillus sp. 1Y, Humicola sp. 2gh205, Merimbla sp. 3gh18, and Penicillium glabrum. A further 15 species presented medium solubilization: Scopulariopsis brevicaulis, Aspergillus candidus, Fusarium sp. 25, Fusarium sp. 3Y, Eupenicillium euglaucum, Eupenicillium ludwigii, Talaromyces flavus var. flavus, Cladosporium cladosporioides, Acremonium roseolum, Epicoccum nigrum, Penicillium arenicola, Penicillium sp. 90, Penicillium olsonii, Penicillium verruculosum, and Penicillium miczynskii.
All species presented a clear halo zone around their colonies, and Aspergillus niger, Penicillium waksmanii and Penicillium brevicompactum gh103 each presented a maximum solubilization index. The highest phosphate solubilization rate was observed for the strains P. waksmanii (3.88) and P. brevicompactum (3.61). However, the A. niger index was 2.6 and its clear zone became visible on the first day, while the clear zone of the other fungal isolates became visible on the third day.
2.2. Phosphate Solubilization and pH in Sundara
Significant differences were detected in the soluble phosphorus values among the extracts of the PSF strains evaluated (F=50.31 p= 0.0005). The available phosphorus content in the extracts of the treatments corresponding to the PSF strains was significantly higher than that of the control treatment (no PSF) (p=0.05). The available phosphorus concentration varied over the period of evaluation; however, a higher content was observed on day 21 for all strains. The soluble phosphorus values of the extracts of A. niger and P. waksmanii strains (98.22 mg/L and 95.77 mg/L, respectively) were significantly (p>0.05) higher than those of P. brevicompactum (74.63 mg/L) (Figure 2).
The pH of the extracts where the PSF strains (A. niger, P. brevicompactum and P. waksmanii) were inoculated decreased significantly with respect to the pH of the control treatment. The pH of the extracts of the PSF strains was variable between days of incubation. A. niger pH decreased from 7 to 1.9; in P. brevicompactum from low pH 7 to 2.9 and in P. waksmanii from pH 7 to 3.0.
2.3. Acid phosphatases
Regarding phosphatase enzyme activity, significant differences in this enzyme were observed among the strains evaluated (F=63.86, p=0.001). Production of acid phosphatase in the P. brevicompactum strain was significantly higher than that detected for the rest of the strains tested (A. niger and P. waksmanii) and the control treatment (no PSF).
The highest acid phosphatase production was observed in the extract of P. brevicompactum strain on days 15 and 18 (189.75 and 176.94 AEU mg/protein, respectively). In the A. niger strain, this was detected on days 15 and 21 (59.44 and 69.59 AEU mg/protein, respectively), while in P. waksmanii, the highest acid phosphatase activity was found on day 21 (38.81 AEU mg/protein) (Figure 3).
Three bands were detected in the results of the acid phosphatase isoenzyme patterns: one monomorphic (1) and two polymorphic (2 and 3). Three different isoenzyme patterns were also observed: the first shows two bands (2 and 3) for the A. niger strain. The second pattern exhibits two bands (1 and 3) for the P. waksmanii strain. The third pattern exhibited one band (3) for the P. brevicompactum strain (Figure 4).
2.4. Coffee plant foliar phosphorus
At the San Isidro coffee plantation, the initial foliar phosphorus content in the plants was 1112.96 mg/kg. Sixty days after establishing the experiment, the foliar phosphorus content in the plants inoculated with the PSF strain (P. brevicompactum) was 1528.12 mg/kg, while in the non-inoculated plants, this value was 1695.31 mg/kg. At 120 days, there was a decrease in the foliar phosphorus content of both the PSF-inoculated and non-inoculated plants (933.27 and 1366.18 mg/kg, respectively). At 180 days, there was an increase in leaf phosphorus content in both inoculated (1456.95 mg/kg) and non-inoculated (1557.29 mg/kg) plants. In all cases, these differences were not significant (p=0.05) (Figure 5A).
In the coffee plantation “Los Bambus”, the initial foliar phosphorus content in the plants was on average 1113 and 1118 mg/kg in the two plots. None of the samplings (at 60, 120, and 180 days) revealed significant differences between inoculated and non-inoculated plants. At 60 days, the average leaf phosphorus content in the plants inoculated with the PSF strain (P. brevicompactum) was 1353 mg/kg, while in non-inoculated plants this value was 1406 mg/kg. At 120 days, there was a slight decrease in the leaf phosphorus content of inoculated (1215 mg/kg) and non-inoculated (1311 mg/kg) P. brevicompactum plants. At 180 days, a significant increase in leaf phosphorus content was observed in both plants inoculated with the P. brevicompactum strain (2164 mg/kg) and non-inoculated plants (2315 mg/kg) (Figure 5B).
In the coffee plantation “La Barranca”, the average initial foliar phosphorus content in the plants was 998 and 992 mg/kg in the two plots. No significant differences were detected in leaf phosphorus content between plants from two plots (inoculated and non-inoculated) at 60, 120, and 180 days (p<0.05).
At 60 days, the leaf phosphorus content of plants inoculated with the PSF strain (P. brevicompactum) averaged 1287 mg/kg, while that of the non-inoculated plants averaged 1342 mg/kg. At 120 days, there was a slight decrease in the foliar phosphorus content of both inoculated (981 mg/kg) and non-inoculated (1007 mg/kg) P. brevicompactum plants. At 180 days, a significant increase in leaf phosphorus content was observed in both plots of plants inoculated with P. brevicompactum strain (1824 mg/Kg) and non-inoculated plants (2115 mg/Kg) (Figure 5C).
2.5. Soil phosphorus available to the coffee plants
In the coffee plantation “San Isidro”, the initial soil soluble phosphorus content was 4.6 mg/kg. Sixty days after inoculation with the phosphate solubilizing fungus P. brevicompactum, an increased soil soluble phosphorus content of 5.64 mg/kg was observed, while in the soil of the uninoculated plants, this value was 4.74 mg/kg. However, these increases were not significant (F= 0.017, p=0.89). At 120 days, a decrease in soluble phosphorus content was detected in the soil of both inoculated and non-inoculated (3.82 and 2.30 mg/kg respectively) P. brevicompactum plants, being significantly higher in the inoculated plants. By 180 days, available phosphorus had increased significantly, and the content of this element was higher in plants inoculated with PSF (4.98 mg/kg) (Figure 6A).
In the coffee plantation “Los Bambus”, the soil presented 2.93 mg/kg of initial soluble phosphorus. Sixty days after inoculation with the PSF P. brevicompactum, an increase in soluble phosphorus content to 5.08 mg/kg was observed, while the soil of uninoculated plants increased to 3.27 mg/kg. At 120 days, a decrease in soluble phosphorus content was detected in the soil of both inoculated and uninoculated plants (2.65 and 1.79 mg/kg, respectively). At 180 days, the soluble phosphorus content in the soil of the inoculated plants was 3.32 mg/kg while that of the non-inoculated plants was 2.31 mg/kg. In all three samplings, the measured soluble phosphorus content was significantly higher in the P. brevicompactum inoculated plant treatments than in the non-inoculated plants (p>0.05) (Figure 6B).
In the coffee plantation “La Barranca”, the available phosphorus content in the soil was 0.93 mg/kg. In the first sampling at 60 days after inoculation with the phosphate solubilizing fungus P. brevicompactum, an increase in the soluble phosphorus content of the soil to 1.22 mg/kg was observed, while the soil of the uninoculated plants increased to 1.14 mg/kg. At 120 days, the soluble phosphorus content decreased in both the soil of P. brevicompactum inoculated plants and the soil of non-inoculated plants (0.96 and 0.89 mg/kg, respectively). At 180 days, the soluble phosphorus content in the inoculated and non-inoculated plants was 1.32 mg/Kg and 1.56 mg/Kg, respectively) (Figure 20). In all samplings, the differences in available phosphorus between plants inoculated with P. brevicompactum fungus and non-inoculated plants were not significant (p<0.05) (Figure 6C).
2.6. Production of mature cherries in the coffee plants
In the coffee plantation “San Isidro”, 270 days after inoculation of the coffee plants with the P. brevicompactum strain, significant differences in bean yield were observed between inoculated and non-inoculated plants (F=9.95, p=0.003). The coffee bean weight of plants inoculated with the PSF P. brevicompactum was 485.13 g, which was significantly higher than that of the uninoculated plants (277.6 g), a difference in mean weight of 207.53 g (F=9.95, p=0.003).
In the coffee plantation “Los Bambus”, the bean weight of the coffee cherry of the inoculated plants was 53.78 g while that of the non-inoculated plants was 50.65 g. This increase was only 3.13 g and the difference was not significant (F=0.05, p=0.81).
In the coffee plantation “La Barranca”, a significant increase in bean yield was detected in the inoculated plants relative to the non-inoculated plants (F=10.02, p=0.0037). The mature coffee cherry bean weight of PSF inoculated plants was 555.73 g, while non-inoculated plants were 173.86 g (Figure 7), a difference of 381.87 g. (F=0.05, p=0.81).
3. Discussion
For this study, a high percentage of the total number of strains evaluated formed solubilization halos (79.4%). This result is higher than the percentage detected by Posada et al., [16], who report only 9.73% of the strains isolated from coffee plantations in Mexico, and slightly higher than that reported by Arias et al., [17] (71.5%) from the rhizosphere of coffee plants in Mexico (Coffea arabica var. Costa Rica). The results of this study confirm that shaded coffee plantations and tropical montane cloud forest harbor a high number of soil fungi with phosphate solubilizing capacity. Due to the presence of different tree species native to the cloud forest and the high diversity of plant species in the shaded coffee plantations, these sites have a considerable accumulation of plant debris that, once decomposed, increase the humus content of the upper soil horizons. This could explain the high number of solubilizing species, given that the presence of a high population of solubilizing micromycetes has been positively related to soil organic matter content [18]. Another contributing factor could be the metabolic activity of the plant roots through exudates [19]. The high population of PSF could also be related to the presence of certain nutrients, pH, moisture content, organic matter, and some soil enzyme activities [20]. Djuuna et. al., [21] detected a correlation between the number of PSF and the level of soil P availability and moisture content, indicating an increase in soil P availability with a greater abundance of PSF in the soil. Some strains of filamentous fungi have been studied in research involving species of the genera Alternaria [22,23], Aspergillus [24], Eupenicillium [25], Fusarium [26], Cladosporium [27], Gongronella [28] Helminthosporium [29], Mortierella [30] Rhizopus [22], Talaromyces [28,31], Trichoderma [32], prominent among which are the Aspergillus y Penicillium [32,33].
In this study, the following 21 genera presented a positive response to the solubilization of Ca3(PO4)2: Anungitopsis, Arthrographis, Aspergillus, Beauveria, Gliocephalotrichum, Nigrospora, Chrysosporium, Cordana, Cylindrocarpon, Eladia, Geomyces, Merimbla, Nectria, Oidiodendron, Penicillium, Phialomyces, Phialophora, Pseudogliomastix, Sagenomella, Sporotrix, and Umbelopsis. Several studies indicate that the genera Aspergillus and Penicillium have a high capacity for solubilization of phosphates of Ca, Al, and Fe [34,35,36,37,38,39].
The results obtained here corroborate the high phosphate solubilizing capacity of the Penicillium and Aspergillus species. Of the 25 Penicillium species evaluated, 23 formed solubilization halos, and all strains corresponding to the genera Eupenicillium and Talaromyces, which are sexual or perfect stages of Penicillium, were positive. Species of the Penicillium genus are the most studied as phosphate solubilizers, and have produced the strains that are currently used as biofertilizers; e.g., the product Fosfosol© marketed in Colombia [40,41] and mainly aimed at rice cultivation, with the active ingredient Penicillium janthinellum. In Canada, JumpStart®, produced from a strain of Penicillium bilaiae and tested on wheat, has been marketed since 1990. As with Penicillium, most of the Aspergillus strains tested had positive responses to solubilization. In this case, five of the six strains formed halos, with four of these reaching scale III.
In this study, the range of solubilization rates (RSR) was 3.5 to 3.8. These values are higher than those reported by Morales et al. [42], who report a maximum RSR of 1.3 for strains of Penicillium albidum, P. thomii, P. restrictum, P. frequentans, Gliocladium roseum, and Penicillium sp. Elías et al. [43] report an RSR of 2.87 for Aspergillus sp., Hernandez et al. [44] report an RSR of 3 for Paecilomyces lilacinus, and Verma and Ekka [45] report 2.25 for Penicillium purpureogenum. Other studies have reported phosphate solubilization rate values of up to 5.3 RSR for Trichosporon beigelii [46]. Romero-Fernandez et al. [47] report ranges of 2.06-6.85, the latter value for a Penicillium strain. In a study of strains isolated from the rhizosphere of Coffea arabica var. Costa Rica, Arias et al. [17] present RSR values in a range of 1.13-6.5. The use of revealing tests using halos in solid culture media and RSR data is a tool with which to detect the phosphate solubilizing capacity of strains rapidly and easily; however, the formation of halos in solid media should not be considered the only test for evaluating solubilization capacities. Quantitative tests are also necessary since Arias et al. [17] did not find a significant relationship between RSR in a solid medium and solubilized phosphorus content in a liquid medium.
In the quantitative evaluation, the absence of soluble P in the controls clearly demonstrates that all three strains tested are phosphate solubilizers and that the manipulation of the cultures was appropriate. The A. niger strains solubilized a high amount of insoluble phosphorus at 98.22 mg/L, followed by P. waksmanii at 95.77 mg/L and P. brevicompactum at 74.63 mg/L. The values presented here exceed those highlighted in other studies [17,37,42,44,48,49,50,51]. However, they were lower than the values presented for the Aspergillus sp. strain (167.7 mg/L) [37].
Several authors argue that fungal species use the production of organic acids as a solubilization mechanism, reflected in a reduced pH [52,53,54]. The data presented here showed that Ca3(PO4)2 can be solubilized by lowering the pH of 1.9 The PSF have been shown to play a crucial role in phosphorus solubilization due to the production of organic acids. Aspergillus niger and some Penicillium sp. have been reported for solubilization of rock phosphate, phosphate mobilization [35,55]. Acidification of the growth medium through the production of organic acids can be explained by the utilization of glucose as a carbon source during growth. Similar results were extrapolated from previous studies [56]. Organic acids secreted by fungi dissolve mineral phosphate because of the anion exchange of PO43- for the acid anion [57].
Seven different organic acids (salicylic, ascorbic, citric, formic, lactic, oxalic, and malic acid) were detected during in vitro phosphate solubilization. Solubilization by acidification depends on the nature and strength of the acid produced by the fungus since there are those such as tri- or di-carboxylic acids that are more efficient solubilizers than mono-basic and aromatic acids [44,58,59]. Salicylic acid was the organic acid most produced by all the PSF. Its concentration was 1823.8 mg/l as produced by Aspergillus japonicus. Salicylic acid was the most quantitatively produced organic acid for all PSF isolates which has an important role in defense mechanisms against biotic and a biotic stress [54].
Some research has reported that enzymes, such as acid phosphatase and phytases, are involved in P solubilization [58,60,61,62,63]. These enzymes cleave the ester bond of the insoluble forms of P, leaving orthophosphate ions that can be assimilated by plants [64]. Previous reports indicate that the genera Aspergillus and Penicillium both actively participate in the mineralization of soil organic P through the production of phosphatase enzymes [65].
The results presented here evidence the production of acid phosphatase by the solubilizing fungi of the two strains of the genus Penicillium and the strain of A. niger. The highest production of acid phosphatases was detected in the P. brevicompactum strain. These results support those obtained by Gomez [66], who found that some Penicillium species may have a higher capacity to produce acid phosphatases than Aspergillus species. Similar reports were obtained by Valenzuela et al. [67] who evaluated 180 fungal strains and found that Penicillium strains showed a higher activity of this enzyme than was produced by Aspergillus strains.
This study shows that the A. niger strain was superior to the other strains in terms of its ability to solubilize tricalcium phosphate. Other studies report that A. niger produces oxalic acid [68] which could explain its higher P solubilization capacity compared to the other microorganisms. Solubilization in the P. brevicompactum strain may be mostly achieved through the production of phosphatase enzymes since the results show that this strain presented the highest content of these.
Microorganisms harbored in the rhizosphere of plants play several key roles in improving plant productivity, nutrient cycling, and soil fertility. Overall, in this field study conducted on three coffee plantations in Jilotepec, Veracruz, inoculation with the phosphate solubilizing fungus strain P. brevicompactum increased productivity in the coffee plants. The phosphate solubilizing potential of the P. brevicompactum strain had already been assessed on coffee (Coffea arabica var. Garnica) plants by Perea et al. [69]; however, that study was conducted under controlled conditions. In this study, the inoculation of PSF (both alone and together with mycorrhizae) favored the development and availability of phosphorus in the soil, as well as foliar phosphorus content. However, given that the study was carried out under controlled conditions and with 8-month-old seedlings, it was not possible to determine the effect of PSF on coffee bean production.
About the soil soluble phosphorus, this parameter increased after two months of inoculation with P. brevicompactum. In the second sampling (fourth month of inoculation), however, it had significantly reduced although the values remained higher in the plants inoculated with P. brevicompactum.
In the first sampling, the foliar phosphorus content increased but, by the second sampling, it had reduced significantly. Contrary to the results for soluble soil phosphorus, foliar phosphorus content was higher in the uninoculated plants. The subsequent decrease in both soil and leaf phosphorus content may have been because the solubilized phosphorus was being used by the plant in coffee cherry production. In a study in North Bengal in India, Chakraborty et al. [70] evaluated the ability of some phosphate-solubilizing fungi to promote plant growth in soybean seedlings and found a reduction of phosphorus in the soil. In that study, the concentration of phosphorus was significantly increased in the roots. It is therefore recommended that further research be conducted to monitor the phosphorus in the plant from the roots to the leaves.
Several studies have documented the successful use of bioinoculants and they have been used as an alternative to traditional fertilization. In a study in Australia [39] used bioinoculants based on Penicillium bilaiae and Penicillium radicum and showed beneficial effects on wheat crops. In Canada, a biofertilizer based on the fungus Penicillium bilaiae was registered in 1990 for use on wheat and tested initially on a few hectares with good results and, in 2002, approximately one million hectares sown with principal crops in Canada were using this biofertilizer [71].
In Colombia, a biofertilizer with phosphate-solubilizing microorganisms, the active ingredient of which is Penicillium janthinellum, has also been marketed. This product was used in rice cultivation, producing high increases in the production of this cereal [41]. The results obtained in this study are promising for the development of a bioinoculant of the fungal strain P. brevicompactum; however, further research is required to establish appropriate doses and re-inoculation schedules. The vigorous microbial activities in soil optimize nutrient cycling and maximize the efficiency of their use in agronomy [72]. A recent extensive review [73] of greenhouse and field trials has shown a marked improvement in the growth responses of various crops to inoculations of phosphate-solubilizing microorganisms.
Most studies have been conducted with bacteria; however, although they are the main decomposers of organic matter and dictate soil carbon and other elements, the role of fungi is the subject of relatively little study. Many fungi can solubilize insoluble phosphates or facilitate P acquisition by plants and therefore form an important part of commercial microbial products, with Aspergillus, Penicillium, and Trichoderma being the most efficient [74].
The use of bioinoculants, together with the rationalized use of phosphate fertilizers, is important and it is necessary to adjust fertilization levels (especially of P and N) to reduce the negative impact on the environment. Chemical-based agriculture has had a negative impact on beneficial microbial communities, significantly reducing microbial biodiversity. There is therefore a need to adopt ecological farming practices for sustainable agriculture. Indigenous or native microorganisms are considered an important tool with which to overcome problems associated with the overuse of chemical fertilizers and pesticides.
Although research has been conducted using phosphate-solubilizing bacteria and fungi in vitro on plant growth promotion, particularly in coffee (Coffea arabica L), there are few reports regarding their impact on growth under field conditions. This study is the first worldwide to evaluate the potential of this group of microorganisms on coffee bean production.
4. Materials and Methods
Determination of Solubilization Index
The strains were subjected to a screening test for their phosphate solubilization potential. Sundara medium containing tricalcium phosphate was prepared and poured into sterilized Petri plates. After 72 hours of incubation at 25 °C, the formation of a halo around the fungal growth on the medium indicated phosphate solubilization. The degree of phosphate solubilization was determined by measuring the clear zone (in mm) around the colonies. The solubilization index (SI) was calculated as the ratio of the total diameter (colony + halo zone) to the colony diameter [75]. For the selection of strains with a higher SI, the scale established by Silva Filho and Vidor [15] was used. In this scale, the solubilizing capacity is considered low where the SI is lower than 2, medium if it is higher than 2 and lower than 3, and high if it is higher than 3.
Phosphate Solubilization Efficiency of Fungal Isolates
For this test, three strains were selected from the high solubilization range. These strains were inoculated into a Sundara liquid medium [76] supplemented with 0.5 g-1 tricalcium phosphate as a source of insoluble phosphorus. The strains were inoculated with 5 mm diameter mycelium discs with 8 days of growth on solid PDA, in triplicate. Negative controls were established using discs of medium with no inoculum. The cultures were incubated in darkness at 25 °C for 21 days. In each sampling, the pH of each sample was recorded, and the biomass achieved for each fungus was determined. To obtain the fungal extracts, the samples were filtered with Whatman® 42 filter paper and the soluble phosphorus was quantified. The pH was measured in the potentiometer. Soluble phosphorus was measured with the ascorbic acid method Clesceri et al. [77] while absorbance was measured in a spectrophotometer at 880 nm. The data were compared with a standard curve for phosphorus and expressed in mg/ml.
Phosphatase activity assay
Acid phosphatase activity was monitored according to Tabatabai and Bremner [78]. In total, 900 μl of enzyme extract, 90 μl of 1M acetate buffer (pH 5), and 10 μl of 15 mM p-nitrophenyl phosphate were mixed and incubated for 1 h at 37 °C. The results were expressed in μmoles of p-NP released min-1 U g-1.
Characterization of isoenzymatic patterns
The electrophoretic profiles were analyzed using polyacrylamide gels with the following modifications: 4% polyacrylamide stacking gel and 10% separating gel. Electrophoresis was run in a vertical electrophoresis chamber with 19 mM Tris-Glycine buffer of pH 8.3 at 25 mA and 4 ºC for 2 h. Each well contained 20 μg of protein. The acid phosphatase activity was measured in 500 mM Citrate buffer at pH 5.5 with β naphthyl sodium phosphate and fast black K salt as a substrate. The gel was incubated at 37 °C in darkness with agitation (125 rpm) for 4 h.
Inoculation of an PSF strain in coffee plants under field conditions
The coffee plantations where the solubilizing fungi were inoculated are located in Jilotepec, Veracruz. For this study, three shade coffee plantations were selected, with coffee plants of Coffea arabica var. Costa Rica (Figure 8).
The geographical characteristics, elevation, precipitation, temperature, and soil type of the sites are listed in Table 3.
Within each of the three plantations of approximately 2 ha, plots of 500 m2 were delimited (Plot 1/Plot 2). In each plot, 25 coffee plants (Coffea arabica L.) of the Costa Rica variety were marked in sampled in “cinco de oros” [79]. In each plantation, plot 1 was used for inoculation of the PFS strain, while plot 2 represented the control treatment (uninoculated).
Strain RA103 (Penicillium brevicompactum) was propagated on potato dextrose agar media and incubated for 1 week at 25 °C. Spores were then scraped from the medium and suspended in a solution containing 10 mL of Inex A to create a solution of 1x1010 spores/ml.
In plot 1 of each plantation, 10 mL of fungal inoculant (Penicillium brevicompactum) was applied to each of the 25 plants using a syringe. These applications were made at the base of each plant near the stem and on the roots.
Variable measurement
At the beginning of the experiment (July 2021), and every 2 months (3 samplings) thereafter, soil samples (500 g) were taken to measure soluble phosphorus content and leaf samples (10 leaves per plant) were collected to measure foliar phosphorus. In addition, during March and April 2022, coffee bean production was measured.
The collected soil was dried at ambient temperature and passed through sieves (DAIGGER ATM 25SS8F, 800-621-7193) to remove stones. The collected coffee leaves were dried in an oven (BINDER 09-08078) at 60 °C for approximately 8 hours and ground in a mill before measurement of leaf phosphorus.
Leaf phosphorus was measured following the technique of McKean [80]. For this, 0.25 g of dried leaves were weighed and placed in porcelain crucibles inside a muffle furnace at 500 °C for 2 h. The resulting ashes were dissolved in 25 ml of 0.3 M HCl in test tubes. The extracts were filtered through Whatman filter paper (42). After filtration, 18 ml of the combined reagent was added to 2 ml of each sample and allowed to stand for 20 min. The samples were then measured in a spectrophotometer at 660 nm.
The soluble phosphorus was quantified using the technique of Bray and Kurtz [81]. For this, 1.2 g of soil was weighed and 10 mL of the extractant solution (0.03 N ammonium fluoride, hydrochloric acid, and distilled water) was added. The extracts were transferred to Whatman filter paper. Subsequently, a 1 mL aliquot of extracted sample was taken, to which 5 mL of combined reagent (ascorbic acid in stock solution) was added and made up to 50 mL with distilled water. The samples were measured in a spectrophotometer (JENWAY, model 6305, Bibby Scientific, United King) at 882 nm, using distilled water as a blank. To measure the effect of PSF inoculation on coffee bean production, ripe fruits were cut in both plots 270 days after inoculation and their weight (gr) was recorded on an analytical balance.
Physico-chemical analysis of the soil
Physicochemical analyses of the rhizospheric soil of the coffee plantation were conducted according to NOM 021-RECNAT-2000 (Table 4). Organic matter (OM) and organic carbon (OC) were quantified following the modified Walkley-Black method, pH by the electrometric method, cation exchange capacity (CEC) with 1N ammonium acetate (pH 7.0), total nitrogen by micro-Kjeldahl, and retained phosphorus by the Blakemore method. These analyses were conducted at the Soil, Plant, and Water Analysis Laboratory of the Instituto de Ecologia, A.C.
Statistical analysis of data
Data were analyzed using one-way ANOVA and Fisher's LSD means tests to determine significant differences between groups at p< 0.05. All statistical analyses were performed in Statistica version 7.0 [82].
Author Contributions
Conceptualization, R.M.A.M and Y.C.E; methodology, R.M.A.M., Y.P.R and K.Y.G.G.; validation, R.M.A.M and Y.C.E.; writing original draft preparation R.M.A.M., Y.P.R and G.H.A.; writing review and editing, G.H.A and R.M.A.M; supervision, R.M.A.M., Y.C.E and G.H.A.; funding acquisition, R.M.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a project COVEICYDET 131627: "Development of a fungal bioinoculant that solubilizes soil phosphorus and promotes the growth and productivity of coffee plants (Coffea arabica var. Costa Rica) in Jilotepec, Veracruz".
Acknowledgments
The authors thank the owners of the farms for allowing access to take soil samples, to Alberto Donaldo Torres Salas and Alondra Juarez for their help in taking the samples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- SAGARPA. Café, la bebida que despierta a México. Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación: https://www.gob.mx/agricultura/articulos/cafe-la-bebida-que-despierta-a-mexico?idiom=es (Accessed 01/06/2023).
- SIAP. Avance De Siembras Y Cosechas Resumen Nacional Por Estado Otoño-Invierno Riego Temporal. Servicio de Información Agroalimentaria y Pesquera: http://www.cenacafe.org.mx/plataformas.html (Accessed 05/05/2023).
- Vandermeer, J.H. The coffee Agroecosystem in the Neotropics: Combining Ecological and Economic Goals. Trop. Subtrop. Agroecosystems. 2003, 159–194. [Google Scholar]
- Giovannucci, D.; Juárez, C.R. Análisis Prospectivo de Política Cafetalera. México, Proyecto Evaluación Alianza para el campo 2005. SAGARPA 2006.
- Moguel, Y.; Toledo, V. El café en México. Ecología, cultura indígena y sustentabilidad. Ciencias 1996, 43, 40–51. [Google Scholar]
- Arcila, J. Crecimiento y desarrollo de la planta de café. In Sistemas de producción de café en Colombia; Ospina, H. y Marín, S., Ed.; Cenicafe: Colombia, 2007; pp. 21–60. [Google Scholar]
- Geissert, D.; Ibañez, A. Calidad y ambiente fisicoquímico de los suelos. In Agroecosistemas cafetaleros del estado de Veracruz biodiversidad, manejo y conservación; Manson, R.H., Hernández, V., Gallina, O.S., Mehltreter, K. Eds. Instituto de Ecología, México 2008; pp. 15-44.
- Kour, D.; Rana, K.L.; Kaur, T.; Yadav, N.; Yadav, A.N.; Kumar, M.; Kumar, V.; Dhaliwal, H.S.; Saxena, A.K. Biodiversity, current developments and potential biotechnological applications of phosphorus solubilizing and mobilizing microbes: A review. Pedosphere 2021, 31, (1),43–75. [Google Scholar] [CrossRef]
- Navarro, G.; Navarro, S. En Química agrícola: el suelo y los elementos químicos esenciales para la vida vegetal, 2nd ed.; Ediciones Mundi Prensa; España, 2003.
- Yadav, B.K.; Verma, A. Phosphate solibilization and mobilization in soil through microorganisms under arid ecosystems. In The Functioning of Ecosystems; Ali, M., Ed.; The Functioning of Ecosystems. Croatia, 2012; pp. 99–108. [Google Scholar]
- Sharma, S.; Sayyed, R.; Trivedi, M.; Gobi, T. Review Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus 2013, 2, 587. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, M. La solubilización de fosfatos como estrategia microbiana para promover el crecimiento vegetal. Cien. Tecnol. Agropecuaria 2014, 15, 101–113. [Google Scholar] [CrossRef]
- Arcand, M.M.; Schneider, K.D. Plant-and microbial-based mechanisms to improve the agronomic effectiveness of phosphate rock: a review. An. Acad. Bras. 2006, 78, 791–807. [Google Scholar] [CrossRef]
- Souza, R.D.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef]
- Silva, G.N.; Vidor, C. Solubilização de fosfatos por microrganismos na presença de fontes de carbono. Rev. Bras. Cienc. Solo 2000, 24, 311–329. [Google Scholar] [CrossRef]
- Posada, R.H.; De Prager, M.S.; Sieverding, E.; Dorantes, K.A.; Heredia, G.P. Relaciones entre los hongos filamentosos y solubilizadores de fosfatos con algunas variables edáficas y el manejo de cafetales. Rev. Biol. Trop. 2012, 60, 1075–1096. [Google Scholar] [CrossRef]
- Arias, R.M; Juárez, A.; Heredia, G.; De la Cruz, Y. Capacidad fosfato solubilizadora de hongos rizosféricos provenientes de cafetales de Jilotepec, Veracruz. AyT BUAP 2022, 7(27), 69–86. [Google Scholar] [CrossRef]
- Vera, D.H.; Herando, P.; Herando, V. Aislamiento de hongos solibilizadores de fosfatos de la rizósfera de Arazá (Eugenia stipitata, Myrtaceae). Acta Biol. Colomb. 2002, 7, 33–4. [Google Scholar]
- Oliveros, A.; Macías, F.; Fernández, C.; Marín, D.; Molinillo, J. Exudados de la raíz y su relevancia actual en las interacciones alelopáticas. Quim. Nova 2009, 32, 198–213. [Google Scholar] [CrossRef]
- Ponmurugan, P.; Gopi, C. Distribution pattern and screening of phosphate solubilizing bacteria isolated from different food and forage crops. J. Agron. 2006, 5, 600–604. [Google Scholar] [CrossRef]
- Djuuna, A.; Prabawardani, S.; Massora, M. Population distribution of phosphate-solubilizing microorganisms in agricultural soil. Microbes Environ. 2022, 37. [Google Scholar] [CrossRef] [PubMed]
- Ceci, A.; Pinzari, F.; Russo, F.; Maggi, O.; Persiani, A. Saprotrophic soil fungi to improve phosphorus solubilisation and release: In vitro abilities of several species. Ambio 2018, 47, 30–40. [Google Scholar] [CrossRef]
- Nahidan, S.; Ahadi, N.; Abduolrahimi, S. Efficiency of some fungal species in phosphate solubilization and potassium and iron release from phlogopite and muscovite. Applied Soil Research 2023, 11, 112–124. [Google Scholar]
- Jyothi, V.; Basaiah, T. Isolation and Characterization of Phosphofungi–Aspergillus niger from Rhizosphere Soil to supplement Phospho-biofertilizer. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Vyas, P.; Rahi, P.; Chauhan, A.; Gulati, A. Phosphate solubilization potencial ans stress tolerance of Eupenicillium parvum from tea soil. Mycol Res. 2007, 111, 931–938. [Google Scholar] [CrossRef]
- Krishna, P.; Sukla, L. Isolation of phosphate solubilizing fungus from rice fields of Odisha for agro-ecological sustainable agriculture. Environ. Qual. Manag. 2022, 32, 19–27. [Google Scholar] [CrossRef]
- Răut, I.; Călin, M.; Capră, L.; Gurban, A.; Doni, M.; Radu, N.; Jecu, L. Cladosporium sp. isolate as fungal plant growth promoting agent. Agron. 2021, 11. [Google Scholar] [CrossRef]
- Doilom, M.; Guo, J.; Phookamsak, R.; Mortimer, P.; Karunarathna, S.; Dong, W. , Liao, C.; Yan, K.; Pem, D.; Suwannarach, N.; Promputtha, I.; Lumyong, S.; Xu, J. Screening of phosphate-solubilizing fungi from air and soil in Yunnan, China: four novel species in Aspergillus, Gongronella, Penicillium, and Talaromyces. Front. Microbiol. 2020, 11, 585215. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Das, S.J. Phosphate solubilizing fungi from Mangroves of Bhitarkanika, Orissa. Hayati J. Biosci. 2008, 15, 90–92. [Google Scholar] [CrossRef]
- Sang, Y.; Jin, L.; Zhu, R.; Yu, X.; Hu, S.; Wang, B.; Ruan, H.; Jin, F.; Lee, H. Phosphorus-Solubilizing Capacity of Mortierella Species Isolated from Rhizosphere Soil of a Poplar Plantation. Microorganisms 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Sano, A.; Hossain, M.; Sakagami, J. Isolation and molecular characterization of phosphate solubilizing filamentous fungi from subtropical soils in Okinawa. Appl. Ecol. Environ. Res. 2019, 17. [Google Scholar] [CrossRef]
- Yasser, M.; Mousa, A.; Massoud, O.; Nasr, S. Solubilization of inorganic phosphate by phosphate solubilizing fungi isolated from Egyptian Soils. J. Biol. Earth Sci. 2014, 4. [Google Scholar]
- Ahmad, A.; Moin, S.; Liaqat, I.; Saleem, S.; Muhammad, F.; Mujahid, T.; Zafar, U. Isolation, Solubilization of Inorganic Phosphate, and Production of Organic Acids by Individual and Co-inoculated Microorganisms. Geomicrobiol. J. 2022, 1–11. [Google Scholar] [CrossRef]
- Fenice, M.; Selbman, L.; Federici, F.; Vassilev, N. Application of encapsulated Penicillium variable P16 in solubilization of rock phosphate. Bioresour. Technol. 2000, 73, 157–162. [Google Scholar] [CrossRef]
- Pandey, A.; Das, N.; Kumar, B.; Rinu, K.; Trivedi, P. Phosphate solubilization by Penicillium spp. isolated from soil samples of Indian Himalayan region. World j. Microbiol. Biotechnol. 2008, 24, 97–102. [Google Scholar] [CrossRef]
- Morales, A.; Alvear. M.; Valenzuela, E.; Castillo, C.E.; Borie, F. Screening, evaluation and selection ofphosphate-solubilising fungi as potential biofertiliser. J. Soil Sci. Plant Nutr. 2011, 11, 89–103. [Google Scholar] [CrossRef]
- Rathore, P.; Phanse, N.; Patel, B. Screening for microorganisms possessing phosphate solubilizing potential. Indian J. Res. 2014, 3, 172–174. [Google Scholar] [CrossRef]
- Gizaw, B.; Tsegay, Z.; Tefera, G.; Aynalem, E.; Wassie, M.; Abatneh, E. Phosphate solubilizing fungi isolated and characterized from Teff rhizosphere soil collected from North Showa zone, Ethiopia. Afr. J. Microbiol. Res. 2017, 11, 687–696. [Google Scholar] [CrossRef]
- Wakelin, S.; Warren, R.; Harvey, P.; Ryder, M. Phosphate solubilization by Penicillium spp. closely associated with wheat roots. Biol. Fertil. Soils 2004, 40, 36–43. [Google Scholar] [CrossRef]
- Ñústez, C.E.; Acevedo, J.C. Evaluación del uso de Penicillium janthinellum Biourge sobre la eficiencia de la fertilización fosfórica en el cultivo de la papa (Solanum tuberosum L. var. Diacol Capiro). Agron. Colomb. 2005, 23, 290–298. [Google Scholar]
- Moreno, N.; Moreno, L.; Uribe, D. Biofertilizantes para la agricultura en Colombia. En Biofertilizantes en Iberoamérica: una visión técnica, científica y empresarial; Izaguirre-Mayoral, M.L., Labanderay, C., Sanjuán, J. Eds.; Editorial Universitaria: Cuba 2007; pp. 38-45.
- Morales, A.; Alvear, M.; Valenzuela, E.; Castillo, C.E.; Borie, F. Screening, evaluation and selection ofphosphate-solubilising fungi as potential biofertiliser. J. Soil Sci. Plant Nutr. 2011, 11, 89–103. [Google Scholar] [CrossRef]
- Elías, F.; Woyessa, D.; Muleta, D. Phosphate solubilization potential of Rhizosphere fungi isolated from plants in Jimma Zone, Southwest Ethiopia. Int. J. Microbiol. 2016. [Google Scholar] [CrossRef]
- Hernández, T.; Carrión, G.; Heredia, G. Solubilización in vitro de fosfatos por una cepa de Paecilomyces lilacinus (Thom) Samson. Agrociencia 2011, 45, 881–892. [Google Scholar]
- Verma, A.; Ekka, A. Isolation, screening and assessment of phosphate solubiling efficiency of some fungal isolates of Raipur, Chhattisgarh. IOSR J. Environ. Sci., Toxicol. Food Technol. 2015, 1, 29–39. [Google Scholar]
- Hajjam, Y.; Cherkaoui, S. The influence of phosphate solubilizing microorganisms on symbiotic nitrogen fixation: Perspectives for sustainable agriculture. J. Mater. Environ. Sci. 2017, 8, 801–808. [Google Scholar]
- Romero, J.; Arias, R.; Bañuelos, J.; De la Cruz, Y. (2019). Efecto de la inoculación de hongos solubilizadores de fósforo y micorrízicos arbusculares sobre el crecimiento de plantas de jitomate (Lycopersicon esculentum Mill). Rev. Mex. Cienc. Agri. 2019, 10, 1747–1757. [Google Scholar] [CrossRef]
- Saxena, J.; Saini, A.; Ravi, I.; Chandra, S.; Garg, V. Consortium of phosphate solubilizing bacteria and fungi for promotion of growth and yield of chickpea (Cicer arietinum). J. Crop Improv. 2015, 29, 353–369. [Google Scholar] [CrossRef]
- Moreno, A.; Osorio, N.; González, O. In vitro dissolution of acidulated rock phosphate by phosphate solubilizing microorganisms. Acta Biol. Colomb. 2015, 20, 65–71. [Google Scholar] [CrossRef]
- Lima-Rivera, D.; López-Lima, D.; Desgarennes, D.; Velázquez-Rodríguez, A.; Carrión, G. Phosphate solubilization by fungi with nematicidal potential. J. Soil Sci. Plant Nutr. 2016, 16, 507–524. [Google Scholar] [CrossRef]
- Selvi, K.; Paul, J.; Vijaya, V.; Saraswathi, K. Analyzing the Efficacy of Phosphate Solubilizing Microorganisms. Enrichment Culture Techniques. Biochem. Mol. Biol. 2017, 3, 7–1. [Google Scholar] [CrossRef]
- Illmer, P.; Barbato, A.; Schinner, F. Solubilization of hardly-soluble AlPO4 with P-solubilizing microorganisms. Soil Biol. Biochem. 1995, 27, 265–270. [Google Scholar] [CrossRef]
- Whitelaw, M.; Harden, T.; Helyar, K. Phosphate solubilization in solution culture by the soil fungus Penicillium radicum. Soil biol. Biochem. 1999, 31, 655–665. [Google Scholar] [CrossRef]
- Nasr, S.; Mousa, A.; Marzouk, M.; Yasser, M. Quantitative and Qualitative Analysis of Organic Acids Produced by Phosphate Solubilizing Fungi. Egypt. J. Bot. 2021, 61, 167–176. [Google Scholar] [CrossRef]
- Richa, G.; Khosla, B.; Reddy, M.S. Improvement of maize plant growth by phosphate solubilizing fungi in rock phosphate amended soils. World J. Agric. Sci. 2007, 3, 481–484. [Google Scholar]
- Rodríguez, H.; Gonzalez, T.; Goire, I.; Bashan, Y. Gluconic acid production and phosphate solubilization by the plant growth-promoting bacterium Azospirillum spp. Naturwissenschaften 2004, 91, 552–555. [Google Scholar] [CrossRef]
- Gyaneshwar, P.; Naresh, G.; Parekh, L.; Poole, P. Role of soil microorganisms in improving P nutrition of plants. Plant and soil 2002, 245, 83–93. [Google Scholar] [CrossRef]
- Gómez, Y.; Zabala, M. Determinación de la capacidad solubilizadora del P en hongos aislados de la rizósfera del mani (Arachis hypogaea L.). Saber Universidad de Oriente 2001, 13, 8–13. [Google Scholar]
- Chung-Chao, C.; Yu-Lin, K.; Chen-Ching, C.; Wei-Liang, C. Solubilization of inorganic phosphates and plant growth promotion by Aspergillus niger. Biol. Fertil. Soils 2007, 43, 575–584. [Google Scholar] [CrossRef]
- Vitorino, L.C.; Silva, F.G.; Soares, M.A.; Souchie, E.L.; Costa, A.C.; Lima, W.C. Solubilization of calcium and iron phosphate and in vitro production of Indoleacetic acid by Endophytic isolates of Hyptis marrubioides Epling (Lamiaceae). Int. Res. J. Biotechnol. 2012, 3, 47–54. [Google Scholar]
- Narsian, V.; Patel, H.H. Aspergillus aculeatus as a rock phosphate solubilizer. Soil Biol. Biochem. 2000, 32, 559–565. [Google Scholar] [CrossRef]
- Reddy, M.S.; Kumar, S.; Babita, K.; Reddy, M.S. Biosolubilization of poorly soluble rock phosphates by Aspergiullus tubigensis and Aspergillus niger. Bioresour. Technol. 2002, 84, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Barroso, C.B.; Nahas, E. The status of soil phosphate fractions and the ability of fungi to dissolve hardly soluble phosphates. Appl. Soil Ecol. 2005, 29, 73–83. [Google Scholar] [CrossRef]
- Sayer, J.A.; Raggett, S.L.; Gadd, G.M. Solubilization of insoluble metal compounds by soil fungi: development of a screening method for solubilizing ability and metal tolerance. Mycol. Res. 1995, 99, 987–993. [Google Scholar] [CrossRef]
- Anand, K.; Kumari, B.; Mallick, M. Phosphate solubilizing microbes: an effective and alternative approach as biofertilizers. Int. J. Pharm. Sci. 2016, 8, 37–40. [Google Scholar]
- Gómez, Y. Actividad de las fosfatasas ácidas y alcalinas (extracelulares e intracelulares) en hongos de la rizosfera de Arachis hypogaea (Papiloneaceae). Rev. Biol. Trop. 2004, 52, 287–295. [Google Scholar] [CrossRef]
- Valenzuela, E.; Toro, V.; Martínez, O.; Pinochet, D. Determinación de fosfatasas en hongos de praderas. Bol. Micol. 2005, 20. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, L.; Yuan, S.; Liu, W.; Zhang, C.; Tian, D.; Ye, X. The Production of Oxalate by Aspergillus niger under Different Lead Concentrations. Agronomy 2023, 13. [Google Scholar] [CrossRef]
- Perea, Y.; Arias, R.; Medel, R.; Trejo, D.; Heredia, G.; Rodríguez, Y. Effects of native arbuscular mycorrhizal and phosphate-solubilizing fungi on coffee plants. Agrofor. Syst. 2018, 93, 961–972. [Google Scholar] [CrossRef]
- Chakraborty, B.; Chakraborty, U.; Saha, A.; Sunar, K.; Dey, P. Evaluation of phosphate solubilizers from soils of North Bengal and their diversity analysis. World J. Agric. Sci. 2010, 6, 195–200. [Google Scholar]
- Patiño, C.O.; Sanclemente, O.E. Los microorganismos solubilizadores de fósforo (MSF): una alternativa biotecnológica para una agricultura sostenible. Entramado 2014, 10, 288–297. [Google Scholar]
- Sashidhar, B.; Podile, A.R. Mineral phosphate solubilization by rhizosphere bacteria and scope for manipulation of the direct oxidation pathway involving glucose dehydrogenase. J. Appl. Microbiol. 2010, 109, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Emami-Karvani, Z.; Chitsaz-Esfahani, Z. Phosphorus Solubilization: Mechanisms, Recent Advancement and Future Challenge. In: Yadav, A. Eds. Soil Microbiomes for Sustainable Agriculture. Sustainable Development and Biodiversity, 2021, vol 27, pp. 85-131. [CrossRef]
- Vassileva, M.; Mocali, S.; Canfora, L.; Malusá, E.; García del Moral, L.; Martos, V.; Peregrin, E.; Vassilev, N. Safety level of microorganism-bearing products applied in soil-plant systems. Front. Plant Sci. 2022, 13, 862875. [Google Scholar] [CrossRef] [PubMed]
- Edi-Premono, M.; Moawad, A.; Vleck, L.G. Effect of phosphate-solubilizing Pseudomonas putida on the growth of maize and its survival in the rhizosphere. Indones. J. Crop Sci. 1996, 11, 13. [Google Scholar] [CrossRef]
- Sundara, V.B.; Sinha, M.K. Phosphate dissolving organisms in the soil and rizosphere. Indian j. Sci. 1963, 33, 272–278. [Google Scholar]
- Clesceri, S.; Greenberg, A.; Trusell, R. En Métodos normalizados para el análisis de aguas potables y residuales, 17th ed.; Díaz Santos Ed; España 1992; pp. 4201-4202.
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Senasica. Manual Técnico Operativo. Centro Nacional de Referencia Fitosanitaria, Área de Vigilancia Epidemiológica Fitosanitaria. Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria: https://prod.senasica.gob.mx/SIRVEF/ContenidoPublico/Accion%20operativa/Manual%20operativo/Manual%20operativo%20VEF%202019.pdf (Accessed 01/05/2021).
- McKean, S.J. Manual de análisis de suelos y tejido vegetal: Una guía teórica y práctica de metodologías. CIAT 1993, (129), 1–99. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic and available forms of phosphorus in soil. Soil Sci. 1945, 59, 39–45. [Google Scholar] [CrossRef]
- StatSoft Inc. Statistica to Windows v.7.0. Data analysis software system. Tulsa 2007. 2007.
Figure 1.
Fungi showing phosphate solubilization leading to formation of clear zone in Sundara´s medium A: Aspergillus candidus; B: Aspergillus sclerotiorum; C) Acremonium roseolu;, D) Aspergillus sp. 1Y; E) Cladosporium cladosporioides; F) Eupenicillium euglaucum; G) Penicillium glabrum, H) Eupenicillium ludwigii, I) Fusarium sp. 25, J) Penicillium arenicola, K) P. glabrum; L) Penicillium waksmanii; M) Sagenomella diversispora; M) Paecilomyces marquandii; N) Aspergillus niger, O) Beauveria bassiana, P) Penicillium brevicompactum.
Figure 1.
Fungi showing phosphate solubilization leading to formation of clear zone in Sundara´s medium A: Aspergillus candidus; B: Aspergillus sclerotiorum; C) Acremonium roseolu;, D) Aspergillus sp. 1Y; E) Cladosporium cladosporioides; F) Eupenicillium euglaucum; G) Penicillium glabrum, H) Eupenicillium ludwigii, I) Fusarium sp. 25, J) Penicillium arenicola, K) P. glabrum; L) Penicillium waksmanii; M) Sagenomella diversispora; M) Paecilomyces marquandii; N) Aspergillus niger, O) Beauveria bassiana, P) Penicillium brevicompactum.

Figure 2.
Figure 2. Soluble phosphorus and pH from extracts of three PSF strains (A. niger AN, P. brevicompactum PB, P. waksamnii PW) and the control (C), over 21 days of incubation (measured at 9, 12, 15, 18 and 21 days). Data are the average of three replicates ± standard error. Identical letters in columns indicate no significant difference (p≤0.05).
Figure 2.
Figure 2. Soluble phosphorus and pH from extracts of three PSF strains (A. niger AN, P. brevicompactum PB, P. waksamnii PW) and the control (C), over 21 days of incubation (measured at 9, 12, 15, 18 and 21 days). Data are the average of three replicates ± standard error. Identical letters in columns indicate no significant difference (p≤0.05).

Figure 3.
Acid phosphatase extracts of the PSF strains (A. niger AN, P. brevicompactum PB, and P. waksamnii PW) and the control (C), over 21 days of incubation (9, 12, 15, 18, and 21). Data are the average of three replicates ±standard error. Identical letters in the columns indicate no significant differences p≤0.05.
Figure 3.
Acid phosphatase extracts of the PSF strains (A. niger AN, P. brevicompactum PB, and P. waksamnii PW) and the control (C), over 21 days of incubation (9, 12, 15, 18, and 21). Data are the average of three replicates ±standard error. Identical letters in the columns indicate no significant differences p≤0.05.

Figure 4.
Isoenzymatic patterns of in vitro acid phosphatases of the strains (A. niger AN, P. brevicompactum PB, P. waksamnii PW).
Figure 4.
Isoenzymatic patterns of in vitro acid phosphatases of the strains (A. niger AN, P. brevicompactum PB, P. waksamnii PW).

Figure 5.
Soil soluble phosphorus content of coffee (Coffea arabica var. Costa Rica) plants from “San Isidro (A), "Los Bambus" (B), “La Barranca” (C) coffee plantation, in Jilotepec. PSF: Plants inoculated with the phosphorus solubilizing fungus (P. brevicompactum); N-PSF: Uninoculated plants at 60, 120 and 180 days. Data are the average of fifteen replicates ±standard error. Identical letters in the columns indicate no significant differences between plantations (p≤0.05).
Figure 5.
Soil soluble phosphorus content of coffee (Coffea arabica var. Costa Rica) plants from “San Isidro (A), "Los Bambus" (B), “La Barranca” (C) coffee plantation, in Jilotepec. PSF: Plants inoculated with the phosphorus solubilizing fungus (P. brevicompactum); N-PSF: Uninoculated plants at 60, 120 and 180 days. Data are the average of fifteen replicates ±standard error. Identical letters in the columns indicate no significant differences between plantations (p≤0.05).

Figure 6.
Soil soluble phosphorus content of coffee (Coffea arabica var. Costa Rica) plants in the coffee plantation “San Isidro (A), “Los Bambus” (B) and "La Barranca" (C), in Jilotepec. PSF: Plants inoculated with P. brevicompactum; N-PSF: Uninoculated plants at 60, 120 and 180 days. Data are the average of fifteen replicates ±standard error. Identical letters in the columns indicate no significant differences between plantations (p≤0.05).
Figure 6.
Soil soluble phosphorus content of coffee (Coffea arabica var. Costa Rica) plants in the coffee plantation “San Isidro (A), “Los Bambus” (B) and "La Barranca" (C), in Jilotepec. PSF: Plants inoculated with P. brevicompactum; N-PSF: Uninoculated plants at 60, 120 and 180 days. Data are the average of fifteen replicates ±standard error. Identical letters in the columns indicate no significant differences between plantations (p≤0.05).

Figure 7.
Production of cherry coffee beans from plants of Coffea arabica var. Costa Rica of three coffee plantations. PSF: Plants inoculated with the phosphorus-solubilizing fungus P. brevicompactum; N-PSF: Plants without the phosphorus-solubilizing fungus. Data are the average of fifteen replicates ±standard error. Different letters between columns indicate significant differences (p≤0.05).
Figure 7.
Production of cherry coffee beans from plants of Coffea arabica var. Costa Rica of three coffee plantations. PSF: Plants inoculated with the phosphorus-solubilizing fungus P. brevicompactum; N-PSF: Plants without the phosphorus-solubilizing fungus. Data are the average of fifteen replicates ±standard error. Different letters between columns indicate significant differences (p≤0.05).

Figure 8.
Selected coffee plantations of central Veracruz state, Mexico: A) “San Isidro”; B) “Los Bambus” and C) “La Barranca”.
Figure 8.
Selected coffee plantations of central Veracruz state, Mexico: A) “San Isidro”; B) “Los Bambus” and C) “La Barranca”.

Table 1.
fungal strains in scale III.
| Fungal strain | Solubilization index | ||||||||
| Aspergillus niger | 2.60 | 1.91 | 1.90 | ||||||
| Acremonium roseolum | 1.72 | 2.34 | 2.44 | 2.43 | 2.46 | 2.54 | 2.47 | 2.45 | |
| Aspergillus candidus | 2.35 | 2.64 | 2.53 | 2.95 | 2.97 | 2.88 | 2.88 | 2.00 | |
| Aspergillus sclerotiorum | 2.44 | 1.20 | 1.30 | 1.36 | 1.48 | 1.50 | 1.48 | 1.41 | |
| Aspergillus sydowii | 1.80 | 1.75 | 1.66 | 1.63 | 1.59 | 1.39 | 1.32 | 1.14 | |
| Aspergillus sp. 1Y | 1.37 | 1.43 | 1.61 | 1.69 | 1.81 | 1.77 | 1.70 | 1.62 | |
| Cladosporium cladosporioides | 1.59 | 2.27 | 2.72 | 2.61 | 2.60 | 2.58 | 2.36 | 2.54 | |
| Epicoccum nigrum | 1.87 | 2.22 | 2.12 | 2.03 | 1.97 | 1.82 | 1.74 | 1.59 | |
| Eupenicillium euglaucum | 2.00 | 2.39 | 2.51 | 2.58 | 2.73 | 2.82 | 2.82 | 2.86 | |
| Eupenicillium ludwigii | 3.53 | 3.08 | 2.61 | 2.64 | 2.65 | 2.65 | 3.05 | 2.91 | |
| Fusarium sp. C25 | 2.80 | 2.76 | 2.68 | 2.67 | 2.67 | 2.48 | 2.34 | 2.19 | |
| Fusarium sp. 3Y | 2.50 | 2.42 | 2.44 | 2.34 | 2.27 | 2.05 | 1.96 | ||
| Humicola sp. 2gh205 | 2.14 | 2.01 | 1.79 | 1.76 | 1.72 | 1.69 | 1.72 | 1.55 | |
| Merimbla sp. 3gh18 | 1.76 | 1.75 | 1.76 | 1.72 | 1.63 | 1.59 | 1.54 | 1.77 | |
| Penicillium arenicola | 1.42 | 1.74 | 1.77 | 2.02 | 1.68 | 1.63 | 1.41 | 1.31 | |
| Penicillium brevicompactum | 2.47 | 2.96 | 3.37 | 3.61 | 3.36 | 3.11 | 2.94 | 2.64 | |
| Penicillium glabrum | 1.47 | 1.70 | 1.72 | 1.62 | 1.64 | 1.53 | 1.39 | 1.35 | |
| Penicillium miczynskii | 2.26 | 2.09 | 1.94 | 2.17 | 2.70 | 1.96 | 1.97 | 1.72 | |
| Penicillium olsonii | 1.82 | 1.98 | 2.00 | 1.93 | 1.87 | 1.77 | 1.68 | 1.67 | |
| Penicillium verrucosum | 1.43 | 1.93 | 1.99 | 2.07 | 1.86 | 1.61 | 1.47 | 1.34 | |
| Penicillium waksmanii | 1.76 | 2.80 | 3.20 | 3.60 | 3.67 | 3.88 | 3.83 | 3.79 | |
| Penicillium sp. 90 | 2.13 | 2.20 | 2.12 | 2.16 | 2.05 | 2.12 | 2.05 | 1.85 | |
| Sagenomella diversispora | 2.83 | 2.93 | 3.23 | 3.10 | 3.15 | 2.95 | 2.83 | 2.63 | |
| Scopulariopsis brevicaulis | 1.98 | 2.77 | 2.95 | 2.76 | 2.61 | 2.66 | 2.30 | 2.16 | |
| Talaromyces flavus var. flavus | 3.52 | 2.66 | 2.02 | 2.00 | 2.12 | 2.14 | 2.30 | 2.21 | |
| Trichocladium asperum | 2.43 | 2.87 | 2.85 | 3.00 | 3.34 | 3.15 | 3.33 | 3.00 | |
Table 3.
Geographic location, elevation, and characteristics of the study sites.
| Sites | Annual mean precipitation | Latitude | Longitude | Elevation (masl) | Mean temperature | Management type | Soil type |
|---|---|---|---|---|---|---|---|
| San Isidro | 241 | 19°36’42.74’’ | 96°56’16.01’’ | 1370 | 22 | Traditional polyculture | Andosol |
| Los Bambús | 275.9 | 19°36’38.07’’ | 96°55’40.57’’ | 1414 | 25 | Traditional polyculture | Andosol |
| La Barranca | 298.2 | 19°36’12.15’’ | 96°54’44.91’’ | 1484 | 25 | Traditional polyculture | Andosol |
Table 4.
Physico-chemical characteristics of the coffee plantations evaluated.
| Coffee plantations | |||
|---|---|---|---|
| “San Isidro” | “Los Bambus” | “La Barranca” | |
| pH | 6.11 | 6.69 | 5.43 |
| Retained P | 87.35 | 89.8 | 81.63 |
| Organic material | 12.46 | 3.93 | 4.72 |
| Organic carbon | 7.23 | 2.28 | 2.74 |
| Cation exchange capacity (CEC) | 27.09 | 20.88 | 21.51 |
| field capacity (FC) | 31.72 | 22.69 | 21.62 |
| Bulk density | 0.893 | 1.016 | 0.994 |
| Clay | 29.8 | 45.8 | 49.8 |
| Silt | 30.56 | 22.56 | 28.56 |
| Sand | 39.64 | 31.64 | 21.64 |
| Texture | Clay loam | Clay | Clay |
| Fe | 69.38 | 111.63 | 163.03 |
| C | 8.5 | 2.9 | 3.5 |
| N | 0.72 | 0.27 | 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated





