Submitted:
25 July 2023
Posted:
26 July 2023
You are already at the latest version
Abstract
Keywords:
Summary
References
- Argyropoulos, D. S., Pajer, N., & Crestini, C. (2021). Quantitative 31P NMR Analysis of Lignins and Tannins. Journal of Visualized Experiments, 174. https://doi.org/10.3791/62696. [CrossRef]
- Arshanitsa, A., Ponomarenko, J., Lauberts, M., Jurkjane, V., Jashina, L., Semenischev, A., Akishin, J., & Telysheva, G. (2020). Composition of extracts isolated from black alder bark by microwave assisted water extraction. 87–94. https://doi.org/10.22616/rrd.26.2020.013. [CrossRef]
- Blakeney, A. B., Harris, P. J., Henry, R. J., & Stone, B. A. (1983). A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydrate Research, 113(2), 291–299. https://doi.org/10.1016/0008-6215(83)88244-5. [CrossRef]
- Dizhbite, T., Telysheva, G., Jurkjane, V., & Viesturs, U. (2004). Characterization of the radical scavenging activity of lignins--natural antioxidants. Bioresource Technology, 95(3), 309–317. https://doi.org/10.1016/j.biortech.2004.02.024. [CrossRef]
- Hagerman, A. E. (1995). Acid butanol assay for proanthocyanidins. Tannin Analysis, 45(1983), 24–25.
- Singleton, V. L., Orthofer, R., & Lamuela-Raventós, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent (pp. 152–178). https://doi.org/10.1016/S0076-6879(99)99017-1. [CrossRef]
- Zakis, G. F. (viaf)276849665. (1994). Functional analysis of lignins and their derivatives. Atlanta (Ga.) : TAPPI press. http://lib.ugent.be/catalog/rug01:001647975.
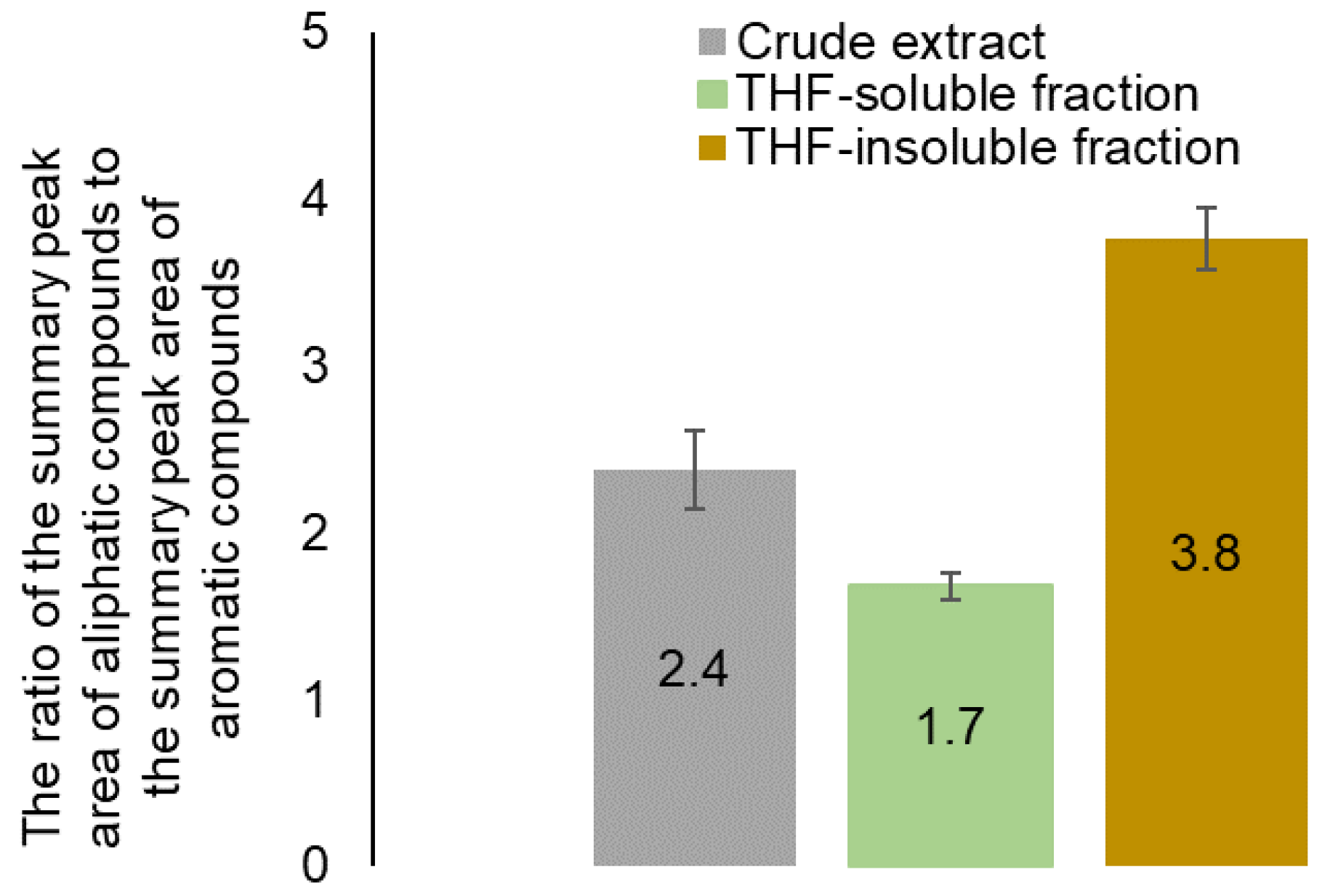
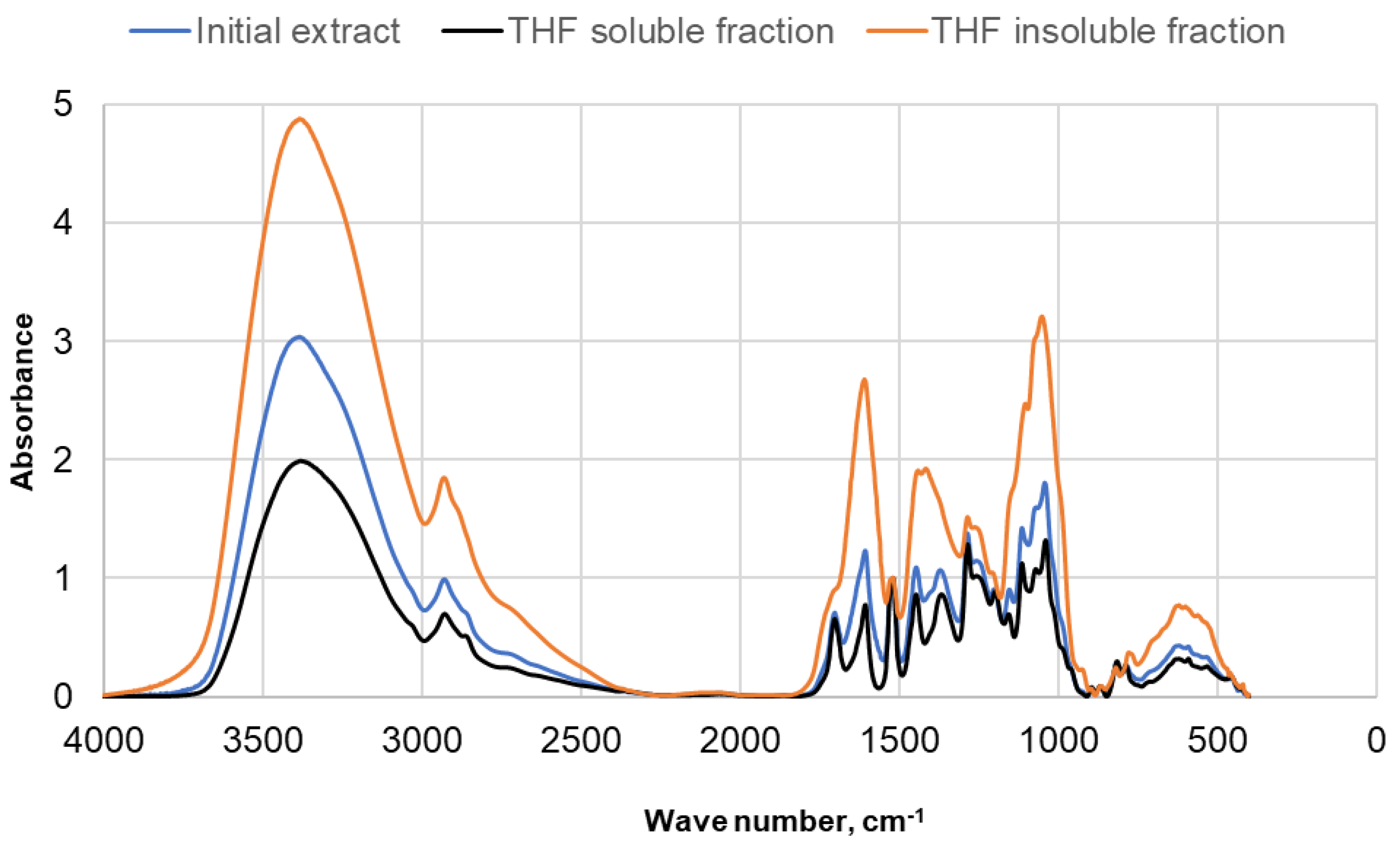
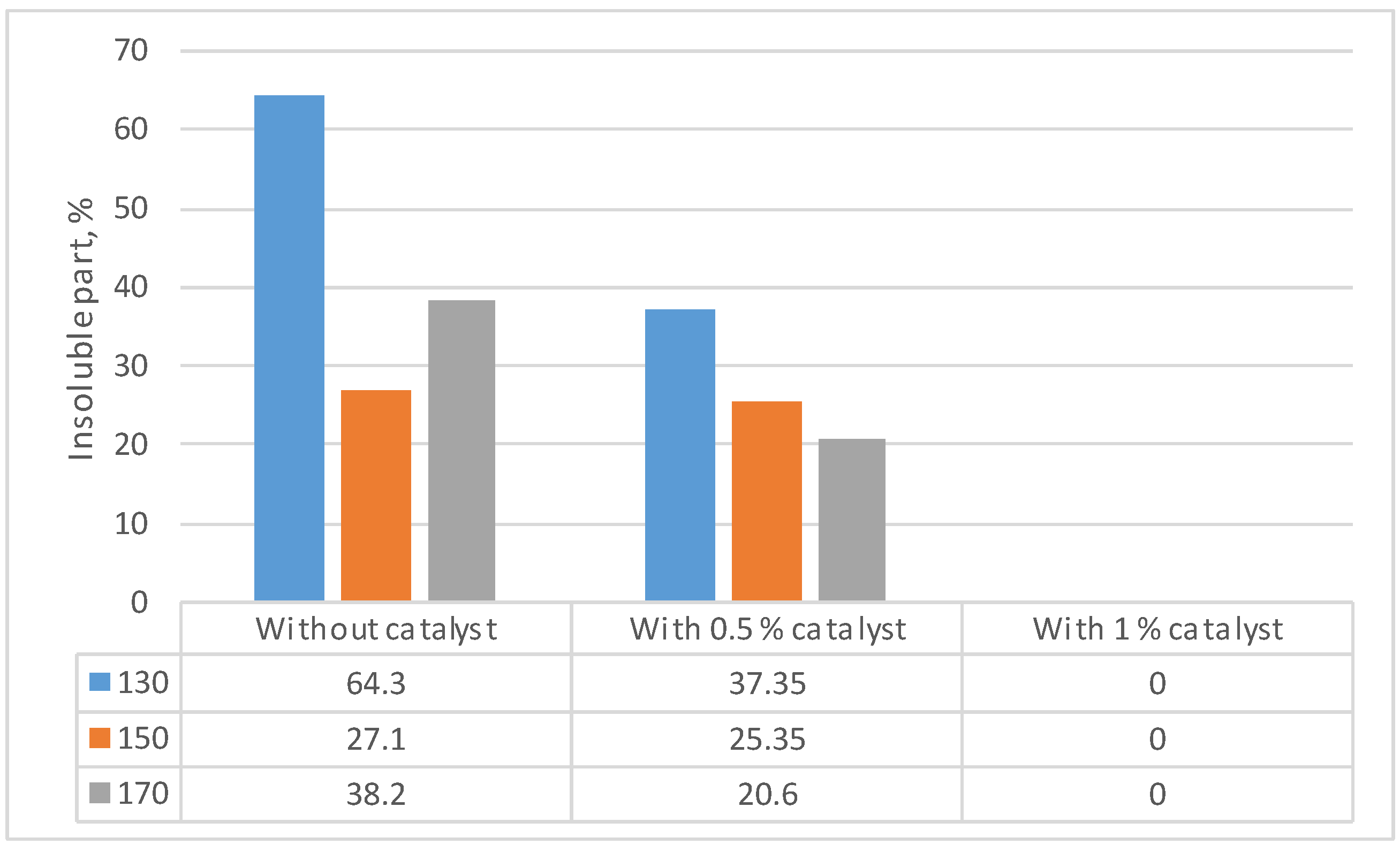
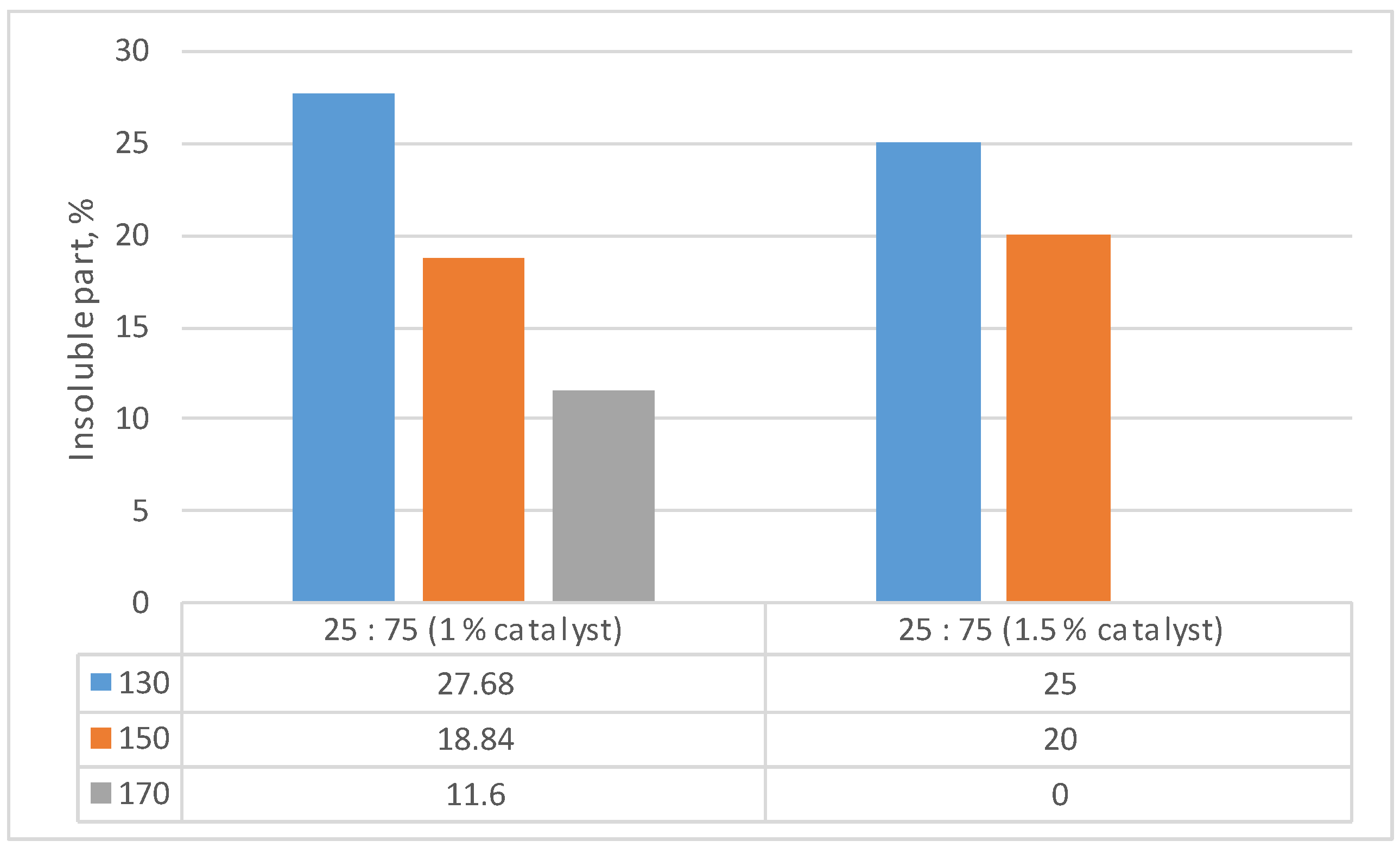
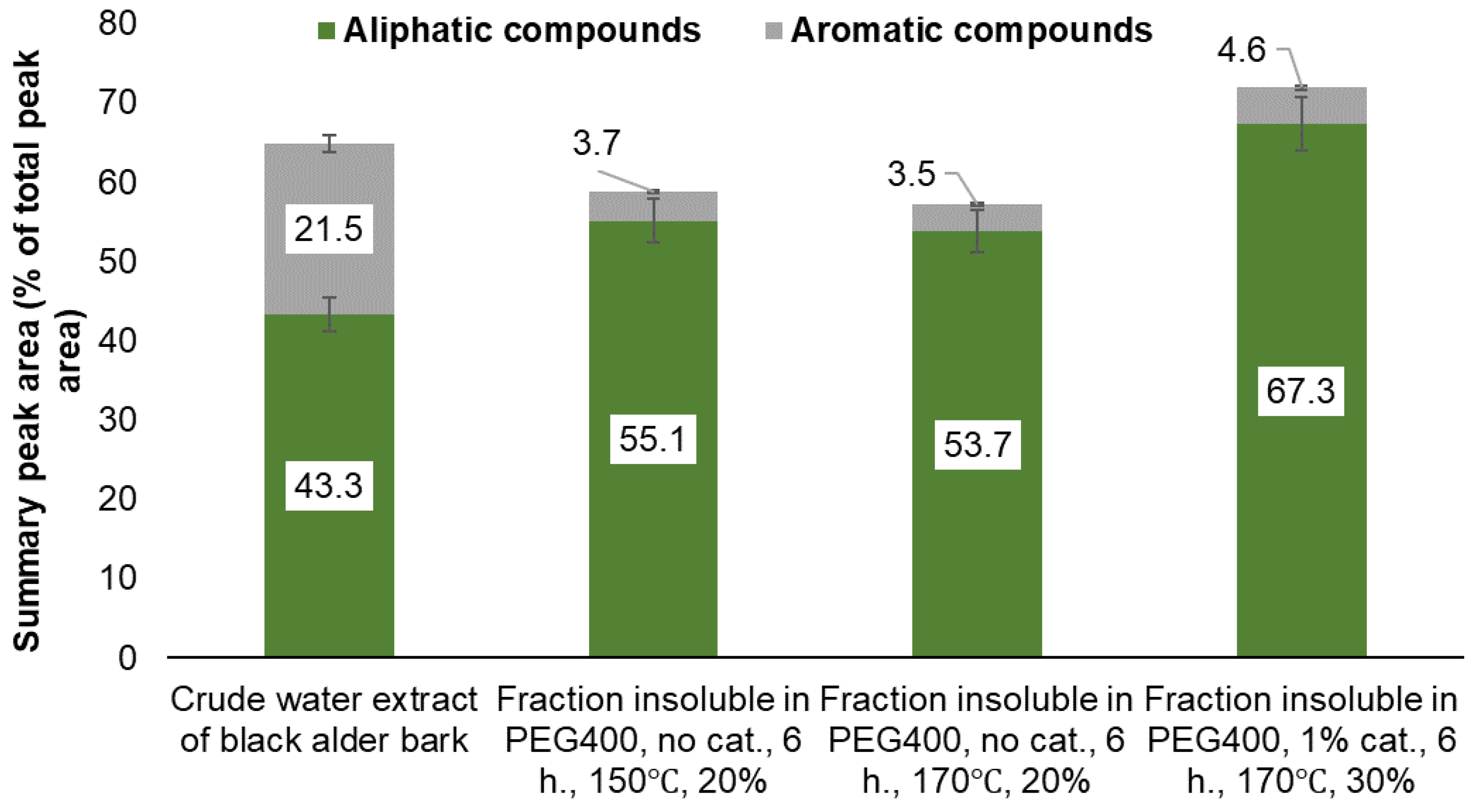
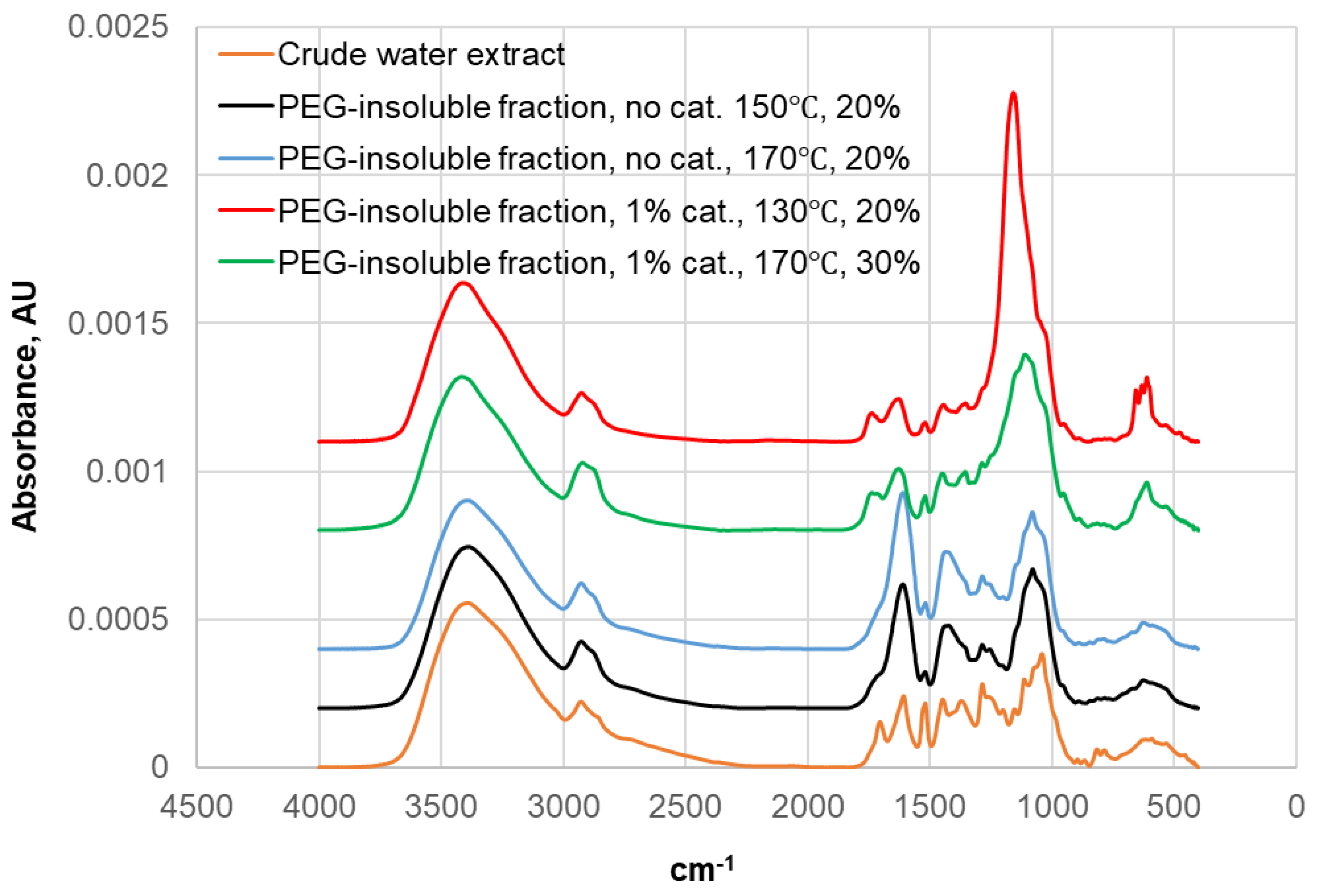
| Identified compound | MW | Retention time, min | Normalized peak area, % from chromatogram | ||
|---|---|---|---|---|---|
| Crude extract | THF-soluble fraction | THF-insoluble fraction | |||
| Carbohydrates derivatives | |||||
| Acetic acid | 60 | 7.417 | 3.97 | 2.73 | 6.64 |
| Formic acid, methyl ester | 60 | 8.538 | 0.55 | 0.36 | 0.25 |
| Propanoic acid | 74 | 9.059 | 0.52 | 0.49 | 0.54 |
| 2-Propenoic acid | 72 | 9.469 | 0.15 | ||
| 2-Propenoic acid, methyl ester | 86 | 9.745 | 0.4 | 0.39 | 0.16 |
| Acetic acid, methyl ester | 74 | 10.025 | 0.74 | 0.55 | 0.85 |
| Propanoic acid, 2-oxo-, methyl ester | 102 | 11.004 | 1.42 | 1.02 | 1.43 |
| Butanoic acid, 2-methyl- | 102 | 12.348 | 0.37 | 0.46 | 0.19 |
| Crotonic acid vinyl ester | 112 | 13.56 | 0.07 | ||
| Propanoic acid, 2-methylpropyl ester | 130 | 14.755 | 0.36 | 0.14 | 0.48 |
| Propanoic acid, 2,2-dimethyl-, methyl ester | 116 | 15.363 | 0.07 | 0.09 | |
| 1,3-Propanediol, 2-ethyl-2-(hydroxymethyl)- | 134 | 26.724 | 0.36 | 2.1 | |
| Methylglyoxal | 72 | 5.912 | 3.67 | 3.53 | 1.98 |
| 2,3-Butanedione | 86 | 6.522 | 0.75 | 1.06 | 1.7 |
| 2-Butanone, 1-hydroxy- | 88 | 6.675 | 0.46 | 0.51 | |
| Acetaldehyde, hydroxy- | 60 | 7.141 | 2.5 | 5.92 | 1.15 |
| Glycolaldehyde dimer | 120 | 7.209 | 1.96 | 1.85 | |
| 2,3-Pentanedione | 100 | 7.706 | 0.24 | 0.25 | 0.37 |
| 2-Propanone, 1-hydroxy- | 74 | 8.292 | 6.91 | 6.34 | 6.66 |
| 2-Butanone, 3-hydroxy- | 88 | 8.897 | 0.17 | 0.18 | 0.2 |
| 2-Butanone, 1-hydroxy-, isomer | 88 | 10.135 | 1.18 | 0.8 | 0.88 |
| Propanal and Butanedial | 58/86 | 11.158 | 0.24 | ||
| 3-Hexanone, 4-ethyl- | 128 | 12.083 | 0.1 | 0.09 | |
| 2-Propanone, 1-(acetyloxy)- | 116 | 12.569 | 1.28 | 1.17 | 0.98 |
| 2-Heptanone, 3-methyl- | 128 | 12.958 | 0.13 | 0.11 | 0.2 |
| 2,5-Hexanedione | 114 | 14.507 | 0.07 | 0.03 | 0.09 |
| 2-Butanone, 1-(acetyloxy)- | 130 | 14.826 | 0.25 | 0.1 | 0.31 |
| 2-Propanone, 1,3-dihydroxy- | 90 | 16.796 | 0.75 | 0.81 | 0.44 |
| 2,3-Pentanedione, 4-methyl- | 114 | 18.221 | 0.22 | 0.13 | |
| Pentanal and Pentanadial | 86/100 | 20.855 | 0.27 | 0.18 | 0.45 |
| 2-Cyclopenten-1-one | 82 | 11.606 | 0.51 | ||
| 2-Cyclopenten-1-one, 2-methyl- | 96 | 12.95 | 0.17 | 0.09 | 0.22 |
| 4-Cyclopentene-1,3-dione | 96 | 13.767 | 0.11 | 0.08 | 0.23 |
| 1,3-Cyclopentanedione | 98 | 14.285 | 0.59 | 0.37 | 0.5 |
| 2-Cyclopenten-1-one, 3-methyl- | 96 | 15.461 | 0.16 | 0.11 | 0.32 |
| 1,2-Cyclopentanedione, 3-methyl- | 112 | 16.808 | 0.17 | 0.13 | 0.44 |
| 2-Cyclopenten-1-one, 3-ethyl-2-hydroxy- | 126 | 18.945 | 0.21 | ||
| Furan, 2-methyl- | 82 | 6.256 | 0.41 | 0.21 | 0.33 |
| 2(3H)-Furanone | 84 | 10.499 | 0.08 | ||
| 3(2H)-Furanone | 84 | 10.835 | 0.14 | 0.13 | 0.09 |
| 2(3H)-Furanone, 5-methyl- | 98 | 11.152 | 0.4 | 0.47 | 0.23 |
| Furfural | 96 | 11.55 | 1.19 | 1.57 | 0.91 |
| 2-Furanmethanol | 98 | 12.421 | 0.36 | 0.29 | 0.53 |
| Acetylfuran | 110 | 13.256 | 0.15 | 0.11 | 0.24 |
| 2-Furancarboxaldehyde, 5-methyl- | 110 | 14.937 | 0.12 | 0.08 | 0.16 |
| 2(3H)-Furanone, dihydro- | 86 | 15.604 | 0.26 | 0.2 | 0.42 |
| 2(5H)-Furanone | 84 | 15.948 | 0.21 | 0.1 | 0.37 |
| 2(5H)-Furanone, 5-methyl- | 98 | 16.15 | 0.08 | 0.1 | |
| 2,5-Furandione, 3-methyl- | 112 | 16.437 | 0.43 | 0.34 | |
| 3(2H)-Furanone, 4-hydroxy-2,5-dimethyl- | 128 | 18.039 | 0.34 | ||
| Methyl 2-furoate | 126 | 18.944 | 0.07 | 0.04 | |
| 5-(Hydroxymethyl)dihydro-2(3H)-furanone | 116 | 20.161 | |||
| 2(3H)-Furanone, 5-acetyldihydro- | 128 | 22.117 | 0.22 | ||
| 2,4(3H,5H)-Furandione, 3-methyl- | 114 | 22.725 | 0.12 | 0.09 | 0.12 |
| Benzofuran, 2,3-dihydro- | 120 | 23.481 | 3.83 | 3.13 | 5.53 |
| 2-Furancarboxaldehyde, 5-(hydroxymethyl)- | 126 | 24.698 | 0.61 | 0.2 | 0.33 |
| 5-Hydroxymethyldihydrofuran-2-one | 116 | 25.188 | 0.22 | 0.3 | |
| 4-Hydroxy-,5,6-dihydro-(2H)-pyran-2-one | 114 | 16.244 | 0.11 | 0.4 | 0.04 |
| 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | 144 | 20.397 | 0.64 | ||
| 1,6-Anhydro--D-glucopyranose | 162 | 31.935 | 1.09 | 1.04 | 0.32 |
| Non-methoxylated aromatic compounds | |||||
| Phenol | 94 | 17.331 | 3.7 | 4.26 | 3.04 |
| Phenol, 2-methyl- | 108 | 18.503 | 0.41 | 0.46 | 0.38 |
| Phenol, 3-and 4-methyl- | 108 | 19.38 | 2.03 | 2.85 | 0.67 |
| Phenol, 2-ethyl- | 122 | 20.302 | 0.06 | 0.09 | |
| Phenol, 3,4-dimethyl- | 122 | 20.493 | 0.13 | 0.19 | 0.15 |
| Phenol, 4-ethyl- | 122 | 21.45 | 1.32 | 1.77 | 0.86 |
| Phenol, 2,6-dimethyl- | 122 | 22.093 | 0.09 | 0.15 | |
| Phenol, 2-ethyl-5-methyl- | 136 | 22.475 | |||
| Benzene, 4-ethyl-1,2-dimethoxy- | 166 | 22.542 | 0.06 | 0.18 | |
| Benzene, (ethenyloxy)- | 120 | 23.131 | 0.07 | 0.08 | |
| Benzene, 2,4-dimethyl-1-(1-methylethyl)- | 148 | 25.205 | 0.13 | ||
| Phenol, 4-(2-propenyl)- | 134 | 25.547 | |||
| Benzaldehyde, 3,4-dimethyl- | 134 | 25.551 | 0.34 | 0.67 | |
| Phenol, 2,4,6-tris(1-methylethyl)- | 220 | 25.699 | 0.49 | 0.94 | |
| 1,4-Benzenedicarboxaldehyde, 2-methyl- | 148 | 25.805 | |||
| Benzenemethanol, .alpha.-ethynyl- | 132 | 25.903 | 0.23 | 0.23 | |
| Phenol, 2-(2-methyl-2-propenyl)- | 148 | 26.384 | 0.31 | 0.55 | |
| 1,4-Benzenediol | 110 | 27.074 | 1.24 | 0.66 | 2.49 |
| 1,4-Benzenediol, 2-methyl- | 124 | 27.925 | 0.18 | ||
| 2-Propyn-1-ol, 3-(4-methylphenyl)- | 146 | 28.327 | 0.45 | 0.7 | |
| 1,4-Benzenediol, 2-methoxy- | 140 | 28.537 | 0.27 | 0.46 | 0.05 |
| 2-Butanone, 4-(4-hydroxyphenyl)- | 164 | 31.533 | 1.07 | 1.97 | 0.15 |
| Guaiacyl derivates | |||||
| Guaiacol | 124 | 17.949 | 1.5 | 2.16 | 0.93 |
| p-Methylguaiacol | 138 | 20.405 | 0.27 | 0.24 | trace |
| p-Ethylguaiacol | 152 | 22.323 | 0.19 | 0.26 | 0.09 |
| p-Vinylguaiacol | 150 | 23.67 | 0.73 | 0.8 | 0.36 |
| Eugenol | 164 | 23.979 | 0.7 | 0.9 | 0.13 |
| trans-isoeugenol | 164 | 26.5 | 0.14 | 0.16 | 0.08 |
| Guaiacylacetone | 180 | 29.777 | 0.18 | 0.15 | 0.13 |
| Acetoguaiacon | 166 | 30.791 | 0.12 | 0.09 | 0.13 |
| Dihydroconifery alcohol | 182 | 32.172 | 0.23 | 0.29 | |
| Syringyl derivates | |||||
| Syringol | 154 | 24.82 | 0.83 | 1.44 | 0.49 |
| Syringol, 4-vinyl- | 180 | 29.38 | 0.39 | 0.28 | 0.17 |
| N-containing compounds | |||||
| 1H-Pyrrole, 1-ethyl- | 95 | 8.508 | 0.07 | ||
| Formamide, N,N-dimethyl- | 73 | 11.042 | |||
| Butanal, O-methyloxime(C5H11NO) or Acetamide, N-methyl- | 101/73 | 14.948 | 0.16 | ||
| Other compounds | |||||
| 1,4-Dioxin, 2,3-dihydro- | 86 | 8.099 | 0.33 | 0.32 | 0.18 |
| 2-Pentene, 4-methyl- | 84 | 8.819 | 0.13 | ||
| 2-Cycloocten-1-one | 124 | 12.083 | 0.14 | ||
| 2-Cyclohexen-1-one | 96 | 14.061 | 0.08 | ||
| Linalool oxide | 170 | 15.87 | 0.08 | ||
| 1,3-Dioxolane-2-methanol, 2,4-dimethyl- | 132 | 19.55 | 0.19 | 0.09 | |
| 2-Naphthalenol | 144 | 30.626 | 0.17 | 0.25 | |
| 1-Tridecanol | 200 | 32.569 | 0.13 | 0.11 | 0.1 |
| 1-Naphthalenol, 2-methyl- | 158 | 32.832 | 0.18 | ||
| 2-Naphthyl methyl ketone | 170 | 33.783 | 0.17 | 0.14 | |
| IC50 mg·L-1 | |
| Crude black alder bark water extract | 7.4 |
| THF-soluble fraction | 5.06 |
| THF-insoluble fraction | 31.07 |
| Irganox1010 | 7.72 |
| Identified compound | MW | Retention time, min | Normalized peak area, % from chromatogram | |||
|---|---|---|---|---|---|---|
| Crude water extract of black alder bark | Fraction insoluble in PEG400, no cat., 6 h., 150℃, 20% |
Fraction insoluble in PEG400, no cat., 6 h., 170℃, 20% |
Fraction insoluble in PEG400, 1% cat., 6 h., 170℃, 30% |
|||
| Carbon dioxide | 44 | 5.035 | 11.72 | 21.13 | 21.89 | 10.82 |
| Water | 18 | 5.198 | 19.61 | 19.37 | 19.72 | 15.75 |
| Carbohydrates derivatives | ||||||
| Formic acid | 46 | 6.637 | 1.2 | 0.35 | 0.36 | 0.42 |
| Acetic acid | 60 | 7.091 | 3.18 | 5.09 | 4.62 | 1.98 |
| (S)-2-Hydroxypropanoic acid | 90 | 7.316 | 0.18 | 0.28 | 0.23 | 0.05 |
| Acetic acid, hydroxy-, methyl ester | 90 | 8.21 | 0.02 | 0.06 | ||
| Formic acid, methyl ester | 60 | 8.322 | 0.45 | 0.22 | 0.17 | 0.2 |
| Propanoic acid | 74 | 8.627 | 0.46 | 0.64 | 0.58 | 0.41 |
| 2-Propenoic acid | 72 | 8.985 | 0.16 | 0.19 | 0.33 | 0.21 |
| Acetic acid, methyl ester | 74 | 9.811 | 0.41 | 0.63 | 0.58 | 0.31 |
| 2-Propenoic acid, methyl ester | 86 | 9.492 | 0.29 | 0.28 | 0.17 | 0.1 |
| 2-Propenoic acid, 2-methyl- | 86 | 10.667 | 0.05 | 0.09 | ||
| Propanoic acid, 2-oxo-, methyl ester | 102 | 10.802 | 0.93 | 0.96 | 0.8 | 0.64 |
| 2-Butenoic acid | 86 | 11.822 | 0.07 | 0.06 | 0.05 | |
| Crotonic acid vinyl ester | 112 | 13.284 | 0.09 | 0.1 | 0.1 | 0.08 |
| Propanoic acid, 2-methylpropyl ester | 130 | 14.583 | 0.1 | 0.11 | 0.07 | 0.07 |
| Propanoic acid, 2-hydroxy-, ethyl ester, (S)- | 118 | 15.542 | 0.06 | |||
| 2-Butene, 1,4-diethoxy- | 144 | 23.224 | 0.42 | 1.71 | 1.22 | 0.78 |
| 1,2-Ethanediol | 62 | 9.492 | 0.15 | 0.25 | 0.49 | 0.23 |
| 1-Butanol, 3-methyl- | 88 | 14.506 | 0.09 | 0.18 | 0.2 | 0.14 |
| 2-Propanol, 1-ethoxy- | 104 | 15.091 | 0.12 | 0.13 | 0.23 | |
| 6-Heptene-2,4-diol | 130 | 15.85 | 0.04 | 0.07 | 0.25 | |
| 2-Heptanol, 5-methyl- | 130 | 16.445 | 1.2 | |||
| Glycerin (1,2,3-Propanetriol) | 92 | 18.403 | 0.6 | 0.41 | 0.4 | 0.23 |
| 4-Heptanol, 2-methyl- | 130 | 22.33 | 0.27 | 0.4 | 0.37 | |
| Methylglyoxal | 72 | 5.698 | 2.84 | 3.57 | 3.3 | 2.41 |
| 2,3-Butanedione | 86 | 6.321 | 0.51 | 0.89 | 0.92 | 0.45 |
| 2-Butanone, 1-hydroxy- | 88 | 6.47 | 0.25 | 0.37 | 0.33 | 0.15 |
| Acetaldehyde, hydroxy- | 60 | 6.952 | 5.82 | 4.08 | 4.12 | 2.95 |
| 2,3-Pentanedione | 100 | 7.526 | 0.19 | 0.38 | 0.33 | 0.31 |
| 2-Propanone, 1-hydroxy- | 74 | 8.047 | 3.89 | 4.8 | 4.18 | 1.3 |
| 2-Butanone, 3-hydroxy- | 88 | 8.687 | 0.09 | 0.17 | 0.06 | |
| 2-Butanone, 1-hydroxy-, isomer | 88 | 9.904 | 0.61 | 0.62 | 0.61 | 0.25 |
| Propanal and Butanedial | 58/86 | 10.983 | 0.11 | 0.68 | 1.02 | 0.35 |
| 3-Hexanone, 4-ethyl- | 128 | 11.867 | 0.06 | 0.1 | 0.09 | 0.07 |
| 2-Propanone, 1-(acetyloxy)- | 116 | 12.38 | 0.65 | 1.06 | 0.71 | 0.33 |
| 2-Heptanone, 3-methyl- | 128 | 12.753 | 0.13 | 0.15 | 0.16 | 0.09 |
| 3-Hexen-2-one, 5-methyl- | 112 | 14.042 | 0.06 | 0.05 | 0.06 | |
| 2-Butanone, 1-(acetyloxy)- | 130 | 14.662 | 0.17 | 0.19 | 0.12 | 0.22 |
| 2-Propanone, 1,3-dihydroxy- | 90 | 15.099 | 0.27 | |||
| Glycolaldehyde dimer | 120 | 16.293 | 0.91 | |||
| 2-Butanone, 4-hydroxy-3-methyl- | 102 | 18.517 | 0.55 | 0.23 | ||
| 2,3-Pentanedione, 4-methyl- | 114 | 17.693 | 0.3 | 0.16 | 0.17 | |
| Propanal, 2,3-dihydroxy- | 90 | 19.243 | 0.36 | 0.22 | 0.15 | 0.06 |
| Pentanal and Pentanadial | 86/100 | 20.559 | 0.22 | 0.76 | 0.96 | 0.09 |
| 3-Pentanone, 2-hydroxy- | 102 | 28.236 | 0.57 | 0.59 | 0.61 | |
| 2-Cyclopenten-1-one | 82 | 11.386 | 0.13 | 0.48 | 0.58 | 0.14 |
| 2-Cyclopenten-1-one, 2-methyl- | 96 | 12.742 | 0.09 | 0.24 | 0.21 | 0.04 |
| 4-Cyclopentene-1,3-dione | 96 | 13.508 | 0.14 | 0.17 | 0.12 | 0.12 |
| 1,2-Cyclopentanedione | 98 | 13.916 | 0.63 | 0.82 | 0.96 | 0.56 |
| 2-Cyclopenten-1-one, 3-methyl- | 96 | 15.203 | 0.2 | 0.27 | 0.27 | 0.1 |
| 1,2-Cyclopentanedione, 3-methyl- | 112 | 16.47 | 0.3 | 0.85 | 0.79 | |
| 2-Cyclopenten-1-one, 2,3-dimethyl- | 110 | 16.566 | 0.07 | 0.1 | 0.11 | 0.15 |
| 2-Cyclopenten-1-one, 3-ethyl- | 110 | 18.042 | 0.05 | 0.06 | ||
| 2-Cyclopenten-1-one, 3-ethyl-2-hydroxy- | 126 | 18.615 | 0.17 | 0.15 | ||
| Furan, 2-methyl- | 82 | 6.059 | 0.39 | 0.4 | 0.49 | 0.36 |
| Furan, 2,5-dihydro-3-methyl- | 84 | 10.101 | 0.05 | 0.07 | ||
| 2(3H)-Furanone | 84 | 10.284 | 0.05 | 0.05 | 0.07 | 0.04 |
| 3(2H)-Furanone | 84 | 10.549 | 0.27 | 0.29 | 0.35 | 0.84 |
| Furfural | 96 | 11.321 | 1.53 | 1.06 | 0.54 | 2.36 |
| Furan, 2-propyl- | 110 | 11.565 | 0.04 | |||
| 2-Furanmethanol | 98 | 12.107 | 0.51 | 0.55 | 0.44 | 0.21 |
| Acetylfuran | 110 | 13.027 | 0.11 | 0.16 | 0.12 | 0.14 |
| 2(3H)-Furanone, dihydro-4-hydroxy- | 102 | 13.651 | 0.26 | 0.29 | 0.33 | 0.38 |
| 2-Furancarboxaldehyde, 5-methyl- | 110 | 14.672 | 0.14 | 0.11 | 0.08 | 0.27 |
| 2(3H)-Furanone, dihydro- | 86 | 15.387 | 0.21 | 0.26 | 0.22 | 0.07 |
| 2(5H)-Furanone | 84 | 15.643 | 0.25 | 0.39 | 0.49 | 0.32 |
| 2,5-Furandione, 3-methyl- | 112 | 16.174 | 0.29 | 2.09 | 2.8 | 1.92 |
| 2(5H)-Furanone, 3-methyl- | 98 | 16.767 | 0.18 | 0.16 | ||
| 3(2H)-Furanone, 4-hydroxy-2,5-dimethyl- | 128 | 17.699 | 0.56 | |||
| Methyl 2-furoate | 126 | 18.6 | 0.13 | 0.11 | ||
| 3-Hydroxydihydro-2(3H)-furanone | 102 | 18.943 | 0.28 | 0.45 | 0.3 | 0.14 |
| 4-Methyl-5H-furan-2-one | 98 | 19.598 | 0.1 | 0.11 | 0.11 | |
| 5-(Hydroxymethyl)dihydro-2(3H)-furanone | 116 | 19.801 | 0.22 | 0.25 | ||
| 2,5-Furandione, dihydro-3-methyl- | 114 | 19.909 | 0.05 | 0.1 | 0.16 | |
| 2(3H)-Furanone, 5-acetyldihydro- | 128 | 21.943 | 0.04 | |||
| Furan, 4-methyl-2-propyl- | 124 | 21.962 | 0.32 | 0.22 | 0.44 | |
| 2,4(3H,5H)-Furandione, 3-methyl- | 114 | 22.375 | 0.16 | 0.43 | ||
| 2(5H)-Furanone, 4-hydroxy-3,5-dimethyl- | 128 | 22.44 | 0.25 | 0.14 | 0.11 | |
| Benzofuran, 2,3-dihydro- | 120 | 23.162 | 1.19 | 0.65 | 0.55 | 0.43 |
| 2-Furancarboxaldehyde, 5-(hydroxymethyl)- | 126 | 24.383 | 0.91 | 0.77 | 0.05 | 1.94 |
| 5-Hydroxymethyldihydrofuran-2-one | 116 | 24.79 | 0.19 | 0.34 | 0.34 | 0.08 |
| 2(3H)-Furanone, dihydro-4-hydroxy- | 102 | 25.095 | 0.11 | 0.09 | 0.09 | |
| Benzofuran, 3-methyl- | 132 | 25.618 | 0.12 | |||
| 4-Hydroxy-,5,6-dihydro-(2H)-pyran-2-one | 114 | 15.928 | 0.38 | 0.18 | 0.17 | 1.27 |
| 4H-Pyran-4-one, 3-hydroxy-2-methyl- | 126 | 18.827 | 0.07 | 0.09 | 0.09 | |
| 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | 144 | 20.022 | 0.23 | 0.11 | 0.08 | 0.15 |
| 4H-Pyran-4-one, 3,5-dihydroxy-2-methyl- | 142 | 20.491 | 0.18 | 0.22 | ||
| 2H-Pyran-3(4H)-one, dihydro- | 100 | 22.533 | 0.05 | 0.16 | 0.19 | |
| 2-Hydroxymethyl-5-hydroxy-2,3-dihydro-(4H)-pyran-4-one | 144 | 25.815 | 0.08 | 0.38 | ||
| Isosorbide (D-Glucitol, 1,4;3,6-dianhydro-) | 146 | 22.171 | 0.05 | 0.07 | ||
| 1,4;3,6-Dianhydro-.alpha.-d-glucopyranose | 144 | 22.976 | 0.28 | 0.34 | ||
| 2-Deoxy-D-galactose | 164 | 26.224 | 1.18 | 0.41 | 0.45 | 1.9 |
| DL-Arabinose | 150 | 27.836 | 0.11 | 0.16 | ||
| 1,6-Anhydro--D-glucopyranose; isomer | 162 | 28.689 | 0.19 | 0.28 | 0.32 | 1.52 |
| -D-Glucopyranoside, methyl- | 194 | 30.048 | 0.13 | 0.11 | 0.68 | |
| -D-Glucopyranoside, methyl 3,6-anhydro- | 176 | 31.054 | 0.49 | |||
| 1,6-Anhydro--D-glucopyranose | 162 | 31.457 | 3.73 | 1.55 | 2.69 | 9.32 |
| 1,6-Anhydro--D-glucofuranose | 162 | 34.05 | 0.18 | 0.93 | ||
| Phenyl and benzyl derivates | ||||||
| Phenol | 94 | 17.019 | 1.42 | 0.44 | 0.47 | 0.34 |
| Phenol, 2-methyl- | 108 | 18.221 | 0.2 | 0.17 | 0.15 | 0.05 |
| Phenol, 3-and 4-methyl- | 108 | 19.078 | 0.84 | 0.27 | 0.25 | 0.51 |
| Phenol, 2,3-dimethyl- | 122 | 20.217 | 0.11 | 0.05 | 0.07 | 0.02 |
| Phenol, 2,6-dimethyl | 122 | 21.098 | 0.07 | 0.07 | ||
| Phenol, 4-ethyl- | 122 | 21.161 | 0.29 | 0.06 | 0.1 | 0.3 |
| Phenol, 2,4-dimethyl- | 122 | 21.807 | 0.06 | |||
| 1,2-Benzenediol(Pyrocatechol) | 110 | 24.314 | 7.16 | 0.55 | 0.56 | 1.4 |
| 1,2-Benzenediol, 3-methyl-(Pyrocatechol, 3-methyl-) | 124 | 25.116 | 0.29 | |||
| Phenol, 4-(2-propenyl)- | 134 | 25.256 | 0.1 | 0.07 | ||
| 1,2-Benzenediol, 4-methyl-(Homopyrocatechol) | 124 | 25.974 | 2.51 | 0.08 | 0.12 | 0.44 |
| 1,4-Benzenediol (Hydroquinone ) | 110 | 26.606 | 0.45 | 0.64 | 0.54 | 0.2 |
| 1,4-Benzenediol, 2,3-dimethyl- | 138 | 26.729 | 0.05 | |||
| Phenol, 2-ethoxy-4-methyl- | 152 | 27.483 | 0.07 | 0.1 | 0.08 | |
| 1,2-Benzenediol, 4-ethyl- | 138 | 27.771 | 1.2 | 0.08 | ||
| 1,2-Benzenediol, 3-methoxy- | 140 | 28.164 | 0.51 | 0.11 | ||
| Benzoic acid, 2,5-dimethyl- | 150 | 30.749 | 0.27 | |||
| 2-Butanone, 4-(4-hydroxyphenyl)- | 164 | 31.257 | 0.49 | 0.32 | ||
| Benzoic acid, 4-(1-methylethyl)- | 164 | 32.131 | 0.65 | |||
| Guaiacyl derivates | ||||||
| Guaiacol | 124 | 17.708 | 1.41 | 0.51 | 0.49 | 0.1 |
| p-Methylguaiacol | 138 | 20.178 | 0.21 | 0.05 | 0.05 | 0.08 |
| p-Ethylguaiacol | 152 | 22.111 | 0.22 | |||
| p-Vinylguaiacol | 150 | 23.436 | 0.54 | 0.22 | 0.24 | 0.17 |
| Eugenol | 164 | 23.77 | 0.11 | |||
| Vanillin | 152 | 26.899 | 0.16 | |||
| -Hydroxypropiovanillone | 196 | 30.538 | 0.06 | |||
| Dihydroconiferyl alcohol | 182 | 31.837 | 0.44 | 0.17 | 0.15 | 0.12 |
| Syringyl derivates | ||||||
| Syringol | 154 | 24.646 | 0.84 | 0.22 | 0.18 | 0.15 |
| Syringol, 4-methyl- | 168 | 26.502 | 0.08 | 0.05 | 0.07 | 0.07 |
| Syringol, 4-ethyl- | 182 | 28.01 | 0.08 | |||
| Syringol, 4-vinyl- | 180 | 29.151 | 0.13 | |||
| Other compounds | ||||||
| 1,4-Dioxin, 2,3-dihydro- | 86 | 7.855 | 0.19 | 0.18 | 0.14 | 0.11 |
| 2-Naphthalenol | 144 | 30.263 | 0.06 | |||
| 2-Naphthyl methyl ketone | 170 | 33.393 | 0.42 | 0.15 | ||
| Ethanol, 2-[2-(2-propenyloxy)ethoxy]- | 146 | 22.919 | 0.55 | 0.72 | ||
| Triethylene glycol | 150 | 23.002 | 1.65 | |||
| Ethanol, 2-[2-(2-methoxyethoxy)ethoxy]- | 164 | 27.619 | 0.1 | |||
| Ethanol, 2-[2-(2-ethoxyethoxy)ethoxy]- | 178 | 28.603 | 0.23 | |||
| 1,3-Dioxan-5-ol (Glycerol formal) | 104 | 28.851 | 0.56 | 0.34 | 0.29 | |
| Ethanol, 2,2'-[oxybis(2,1-ethanediyloxy)]bis- | 194 | 28.945 | 0.91 | 0.99 | 2.72 | |
| Ethanol, 2-(2-butoxyethoxy)- | 162 | 31.358 | 1.34 | |||
| Ethanol, 2-[2-(2-ethoxyethoxy)ethoxy]- | 178 | 33.844 | 0.32 | |||
| Hexagol (3,6,9,12,15-Pentaoxaheptadecane-1,17-diol) | 282 | 34.292 | 2.3 | 2.27 | 4.62 | |
| Heptaethylene glycol | 326 | 39.649 | 4.64 | 4.46 | 9.17 | |
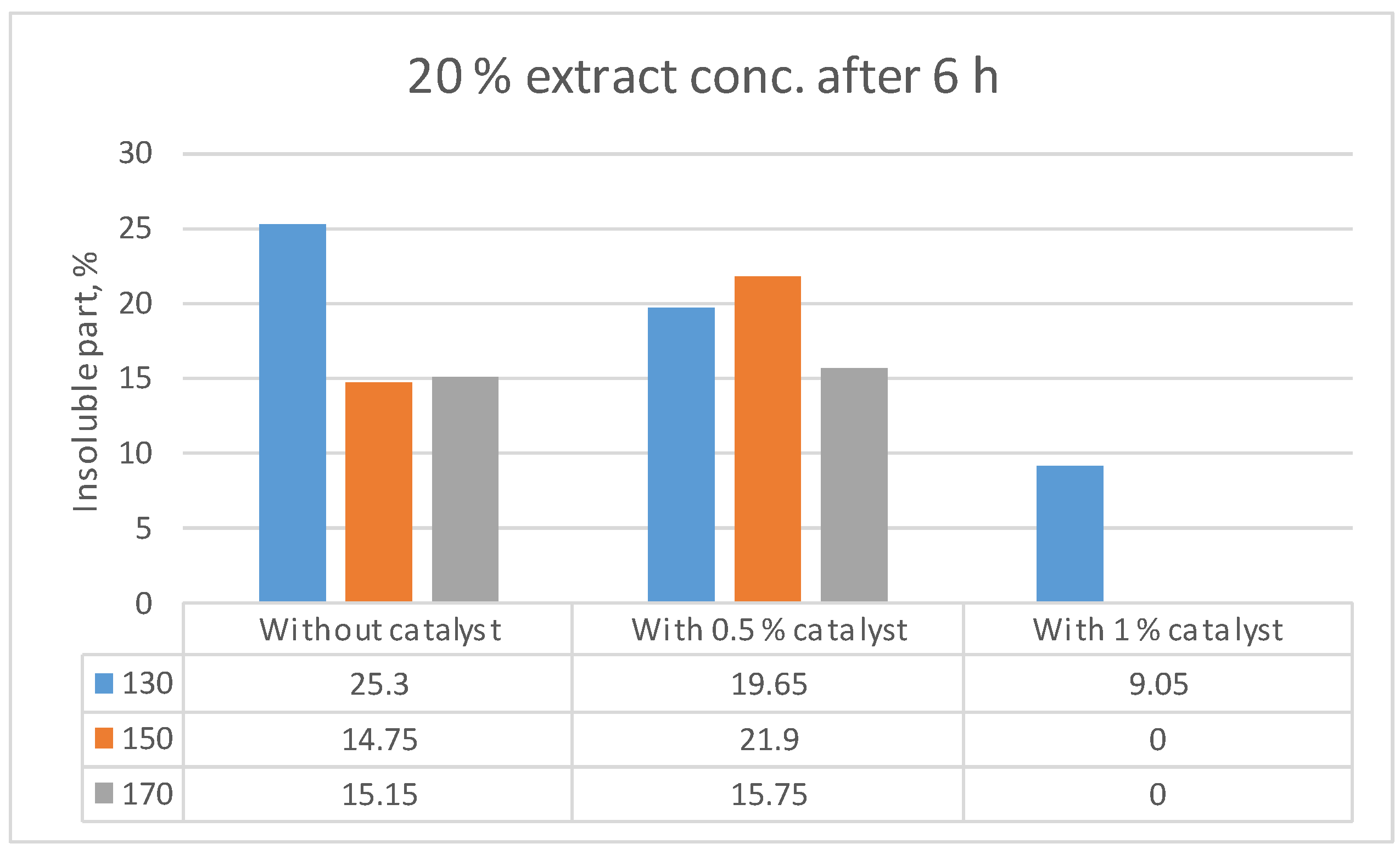
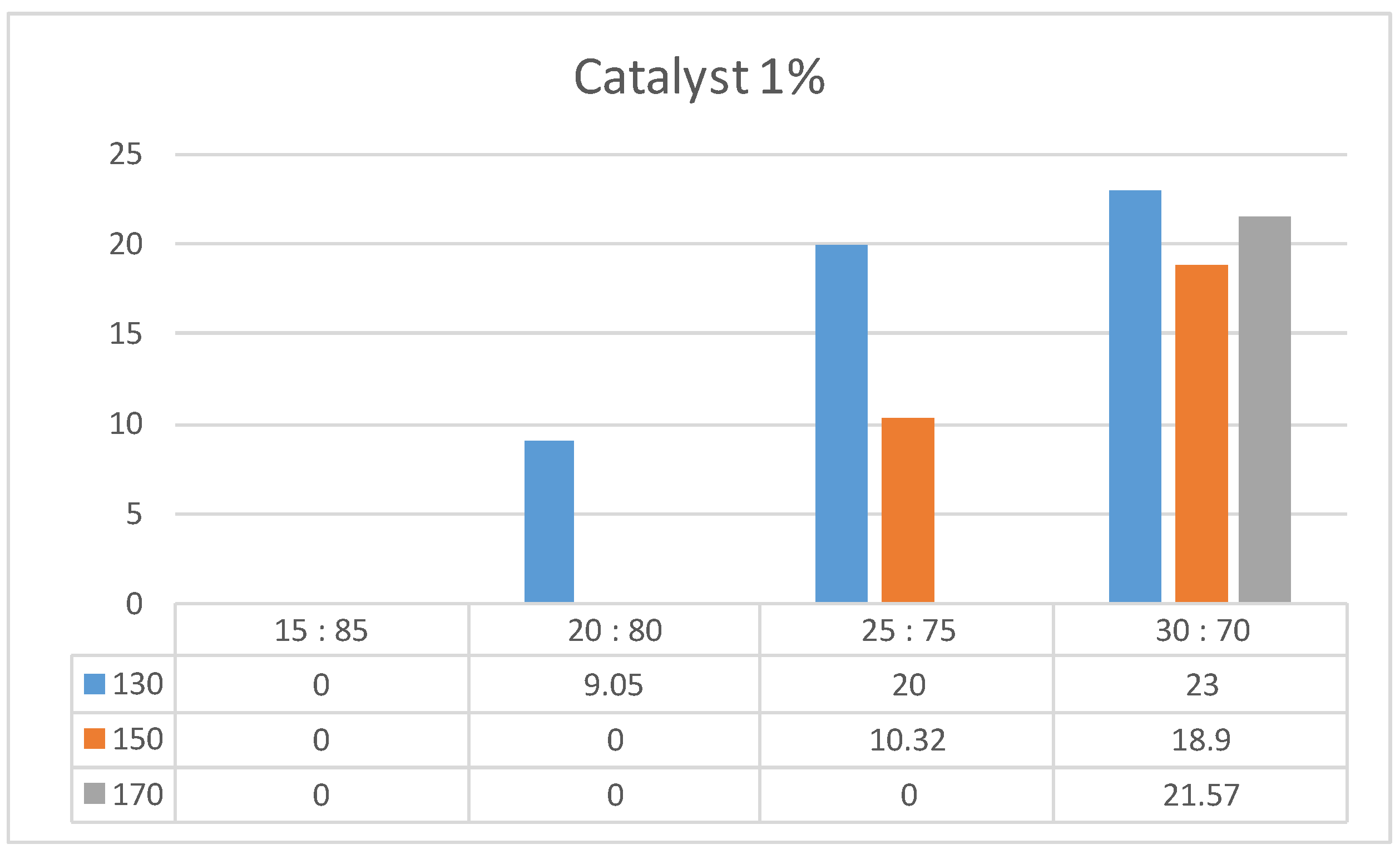
| Liquefaction temperature (°C) | Extract-to-PEG400 ratio | Catalyst, % | Concentration of dissolved extract in PEG solution, % | Percentage of dissolved portion of the introduced extract | Ratio of absorbance at 1100 cm-1 to 1515 cm-1 |
|---|---|---|---|---|---|
| Crude black alder bar water extract | 1.7 | ||||
| 150 | 20:80 | 0 | 17.1 | 85.3 | 3.8 |
| 170 | 20:80 | 0 | 17.0 | 85.0 | 2.9 |
| 130 | 20:80 | 1 | 18.2 | 91.0 | 18.0 |
| 170 | 30:70 | 1 | 23.5 | 78.3 | 5.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





