1. Introduction
Goat meat is commonly produced in many parts of the world, especially in Asia and Africa which accounts for 95% of the total world goat meat production, with goat meat production increasing 23.12% globally from 2006 to 2016 (FAOSTAT, 2019). "However, the global goat meat industry is challenged by rising input costs, making it imperative to continuously enhance goat production and improve meat quality standards to remain competitive in the global market and meet the evolving demands of the health-conscious consumer" (Pophiwa et al., 2020). Adeyemi et al. (2016) stated one of the effective strategies to improve the fatty acid profile of meat by increasing the antioxidant profile of ruminant diets using a dietary mix of 20% palm oil and 80% canola oil to align with the evolving demands and preferences of consumers. The impact of nutrition on meat quality and nutritional content is widely acknowledged and has been well documented. Changes in the diet and breeding strategies can significantly alter biological characteristics of muscle tissue, affecting its quality and nutritional value (Juárez et al., 2021). This relationship between nutrition and meat quality has been demonstrated in several studies (Renand et al., 2001). After COVID-19 pandemic, the search for low-cost, long-term alternatives to requirements animal feed has emerged as a critical research highest priority and strategic aim (Ding et al., 2021).
Over the past decade, there has been extensive advertising worldwide about the beneficial effects of feeding microalgae, leading to the proliferation of algae production enterprises at both small and large levels of production (Abouelezz, 2017; Altmann et al., 2019). Supplementing the diets of small ruminant farming with alternative antioxidant sources, such as Spirulina Platensis (SP), holds a great promise in elevating the standard of goat meat and its nutritional composition.
Spirulina Platensis (SP) is a blue-green alga that offers a range of health beneficial chemicals due to its rich chemical composition. It is widely used as a dietary supplement for livestock as it contains a high percentage of protein (60-70%) and total lipids content (5-6%) along with substantial amounts of vitamins, minerals, chlorophyll, carotenoids, carbohydrates, sterols and pigments such as phycocyanin and allophycocyanin (AlFadhly etal, 2022, Kistanova et al., 2009; Small, 2011; Wan et al., 2016). SP has proven to be a remarkable discovery with a significant biological and economic implications. It has a board range of applications across various industries including food, pharmaceuticals, biofuels, cosmetics, and agriculture (AlFadhly et al., 2022). Habib (2008) and Nawal (2022) showed that spirulina is a rich in polyunsaturated fatty acids (PUFAs) and other health-promoting fatty acids including gamma-linoleic acid, Eicosapentaenoic acid (EPA) and Docosahexaenoic acid (DHA). Furthermore, spirulina has been found to possess numerous health benefits including antiviral, antioxidant, hepatoprotective, anti-allergenic, carotenoids and immunomodulatory properties (Holman et al., 2012; Khan et al., 2005; Kumar et al., 2019). Additionally, spirulina is commonly recommended as a dietary protein supplement that can improve growth rate and meat quality in chickens, pigs, ruminants, and rabbits (Al-Yahyaey et al., 2022; Holman & Malau-Aduli, 2013; Peiretti & Meineri, 2011).
Meat quality is an important factor influencing consumer eating quality parameters and acceptance of the product (Pophiwa et al., 2020). Whilst goat meat is widely consumed globally, its consumption is smaller compared to other red meats such as beef and sheep meat (MLA, 2022). Pophiwa et al. (2020) reported that poor goat meat quality is mostly caused by the use of inappropriate animals, poor nutrition, and inadequate pre- and post-slaughter handling practices. In Oman, goat meat is the preferred red meat of choice (Mahgoub et al., 2005), with consumers eating it on average 14 times a week (MLA, 2022). However, poor nutrition and a lack of managed breeding are the primary reasons for low levels of production in goats under traditional systems in Oman where animals are grown and finished mostly on low-quality rangeland pastures (Kadim et al., 2004). The production of Omani goat meat and meat quality requires research to determine the response to Spirulina supplementation to fill this gap. Therefore, this study aims to evaluate the effect of feeding SP diets on the carcass characteristics, fatty acid profile and meat quality of two Omani goat breeds, Jabbali and Sahrawi, reared under an intensive farming.
2. Materials and Methods
2.1. Ethics Statement
The study was performed at the Livestock Research Center, Directorate General of Agriculture and Livestock Research, Ministry of Agriculture, Fisheries and Water Re-sources, Muscat, Sultanate of Oman. Ethics was approved by the University of Sydney Animal Ethics Committee (AEC) (Project number: 2019/1597, approved 19th August 2019) according to the New South Wales (NSW) Animal Research Act 1985 and its associated Regulations, the Australian Code for the care and use of animals for scientific purposes 8th Edition 2013 and the Australian Code for the Responsible Conduct of Research 2007.
2.2. Animals management and diets
Thirty-six eleven month old bucks (average body weight: 16.44 ± 0.33 kg) of two main domestic breeds of goats in Oman, Jabbali (n=18) and Sahrawi (n=18), were randomized according to a true experimental design to one of three feeding treatments including Control (CON), Treatment 1 (T1) and Treatment 2 (T2) in a 2x3 factorial arrangement (n=6 per group) for 70 days. The CON group was fed a conventional concentrate ration (14% crude protein, and 11.97% energy MJ/kg DM). Animals in T1 and T2 were fed the control ration with an addition of 2g and 4g/head/day of Spirulina platensis (SP), respectively. The basal diet was formulated to meet the goats’ nutrient requirements to achieve a body weight gain at a rate of 0.3 kg/day. All animals were fed twice a day at 8:00 am and 3:00 pm. Goats were fed commercial Spirulina pellets (DXN Inter-national Australia Pty. Ltd.) daily at 8:00 am. All animals were individually housed in randomly allocated pens throughout the experiment, including two weeks of acclimatization, during the winter season (November–February 2019-2020). To provide roughage together with free access to freshwater during the experimental period, Rhodes grass (Chloris Guyana) were fed ad libitum. Feed intake was calculated as the difference between the feed offered and feed residual amounts, and the feed residual was obtained and recorded in the morning of the next day.
Table 1 presents the nutritional and dry matter composition of ingredients in the experimental diets. The proximate compositions of the experimental diet were determined using the methods described by the Association of Official Analytical Chemists AOAC (2000).
2.3. Slaughtering procedure
At the end of the feeding trial, three male bucks were randomly selected from each of the three treatment groups of each breed and slaughtered at the Muscat Municipal Abattoir according to the Ministerial Resolution No. 255/2020 issuing the executive regulations of the Animal Welfare Law-Oman. During processing, individual carcass components including the head, skin, feet, fat (omental, mesenteric and kidney), full and empty alimentary tract, liver, spleen, heart, lung, and trachea were weighed and recorded. The weights of the full and empty reticulorumen were used to calculate the difference between full and empty body weights. The weight of the gut content was deducted from the slaughter weight to determine the Empty Body Weight (EBW). The hot carcass weight (HCW) was recorded within an hour of the slaughter before the carcass was refrigerated to a temperature of 1-4°C for 24 hours.
2.4. Carcass measurements
Following a 24-hour chilling period, several external carcass measurements were collected. Leg length, gigot width, maximum shoulder width, depth from scapula to sternum, and width behind shoulders were all measured. For each carcass, all measurements were taken in cm using the methodology stated by Moxham and Brownlie (1976). Carcasses were separated vertically through the vertebrae using a control band saw. The carcass's shoulder was cut at the caudal side of the 7th rib and then between the last and second-to-last lumbar vertebrae. A cut was made between the 12th and 13th ribs to separate the rack and the loin. The m. longissimus dorsi (LD) was taken from the loin region, its cross-sectional surface removed of fat, and it was then tested to determine its quality traits. Meanwhile, the m. semitendinosus (SM) was extracted from the leg and weighed to determine yield characteristics.
2.5. Meat quality evaluation
LD and SM were used to determine meat quality indicators such as ultimate pH, sarcomere length, expressed juice (drip loss), Warner-Bratzler shear force, cooking loss, and fresh colour parameters (L*, a* and b*), where L*, a* and b* tests relative lightness, relative redness and relative yellowness, respectively (Hernández, 2016). The ultimate pH and chilled muscle samples were measured according to (Kadim, 2004). The lengths of sarcomeres were calculated by laser diffraction using a procedure described by (Cross, 1981). The filter paper method used to test the expressed juice as a total wetted area less meat area (cm2) relative to sample weight (g) as described by Kadim et al. (2008). For about 60 minutes, the fresh cut surface of the meat was exposed to ambient air temperature to allow for light reflection for colour measurements (L*, a*, and b*). The Minolta Chroma Metre CR-300 (Minolta Co., Ltd., Japan) was used to obtain these measurements, which have a colour measuring diameter of 1.1 cm.
2.6. Fatty acid analysis by Gas Chromatograph (GC)
The fatty acid composition of (LD) was analyzed and extracted using the AOAC (2000) method. A 2 g subcutaneous fat sample was well mixed with 1N KOH, and 5 mg of Tricosanoic acid (C23), an internal standard, was used. The fatty acid samples were analyzed and separated using the method described by Hernández (2016). The data was gathered using full-scan mass spectra with a scan range of 35-500 atomic mass units (amu). Each injected sample was 1 μl in volume, with a split ratio of 20:1. The oven temperature was set at 50°C with no hold time, then raised at a rate of 40°C per minute up to 250°C, where it was kept for 10 minutes. For identification of unknown substances, the spectra generated throughout this method were compared to existing mass spectrum libraries, notably NIST 2011 v.2.3 and Wiley's 9th edition. Supelco 37 component FAME mixture (catalogue number 47885-U) was used for further verification. Total saturated fatty acids (SFA), polyunsaturated fatty acids (PUFA), and desirable fatty acids (DFA) were calculated by adding the amounts of each fatty acid type, allowing for a complete assessment of their levels. In addition, the PUFA/SFA ratio was calculated. Using tricosanoic acid (C23) as an internal reference is a useful approach for quantifying fatty acids. It also enables an accurate measurement of fatty acids.
2.7. Statistical analysis
The impact of feeding Spirulina on carcass characteristics, fatty acid profile and meat quality were analyzed using the general linear model (GLM) procedure of Statistical Analysis Software Model (SAS, 2002). The results were presented as least square mean along with their standard errors (LSM±SE). Differences were considered statistically significant at
P<0.05. Differences between mean values of dietary treatments were obtained by Duncan’s Multiple Range Test (Duncan, 1955). All figures were drawn using Origin software according to the following statistical model:
where Y
ijk is the phenotypic traits; μ is the overall mean; A
i is the effect of the i
th breeds; B
j is the effect of the j
th treatments; Further, AB
ij is the interaction between i
th breeds and j
th treatments, and e
ijk is the effect of the random error.
3. Results
3.1. Animal performance and carcass characteristics
No breed differences were detected in most growth performance and carcass characteristics traits such as final body weight (kg), carcass weight (kg), dressing-out%, carcass dimensions (cm) and carcass cut weights (kg) (
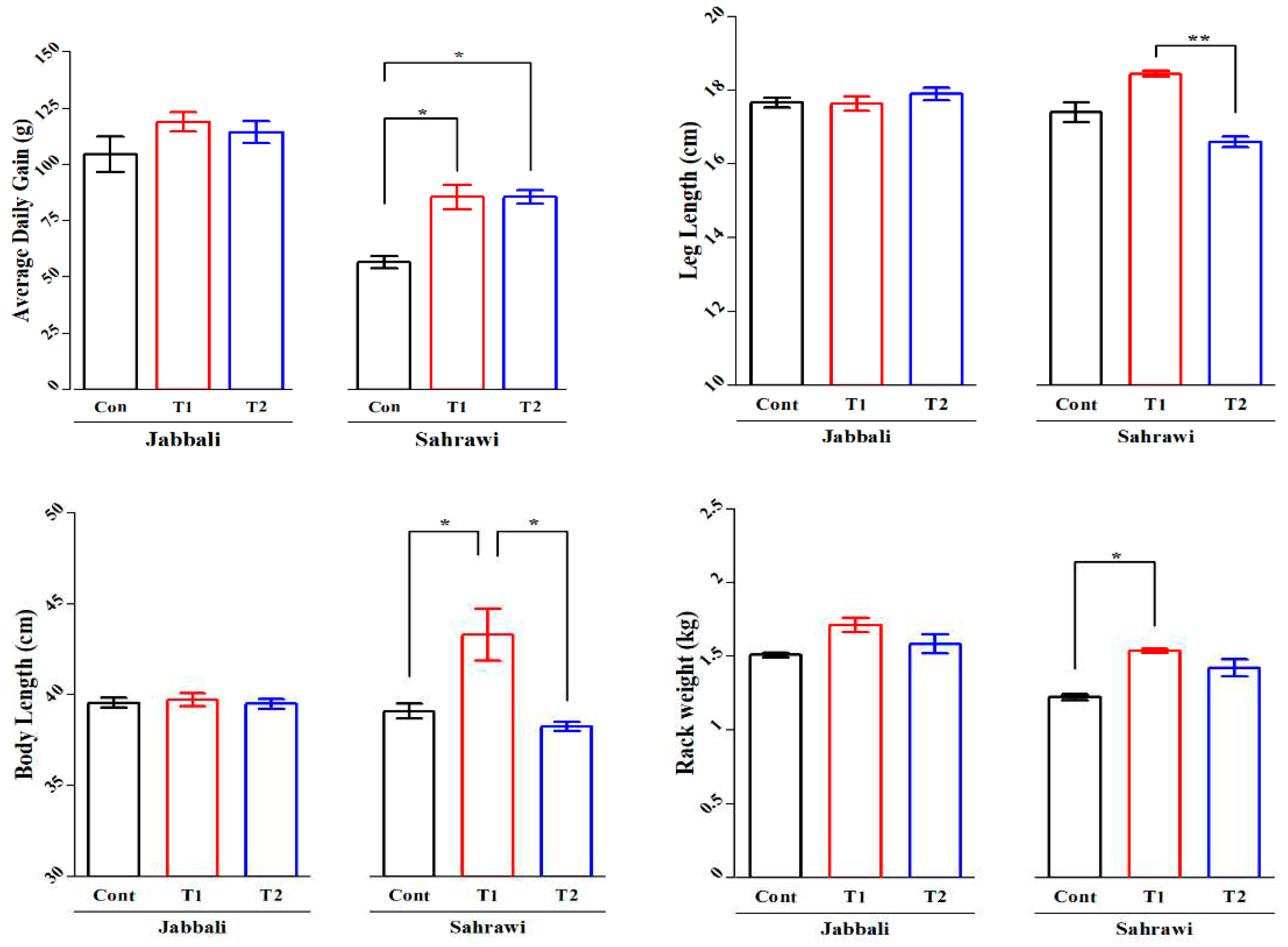
Table 2). The ADG of Sahrawi bucks was statistically significant (
P<0.05) in both supplemented groups (85.5±10.97 g) compared to the control group (56.5±5.38) (
Figure 1a). The body length of Sahrawi bucks in T1 group (43.30±2.87 cm) showed significant (
P<0.05) differences compared to the control and T2 group (39.10±0.81 cm and 38.27±0.52 cm, respectively) (
Figure 1b). Similarly, the leg length of Sahrawi bucks in T1 group (18.45±1.49 cm) showed significant (
P<0.05) differences compared to the T2 group (16.60±0.29 cm) (
Figure 1c). Despite of GLM test showing no significant difference (
P>0.05) between treatments in rack weight (kg), the Duncan separation test showed a significant difference between T1 (1.54±0.03) and the control group (1.22±0.04) in Sahrawi goats (
Figure 1d). All these trends were not observed in Jabbali goats.
There were no breed differences (
P>0.05) for all non-carcass components (
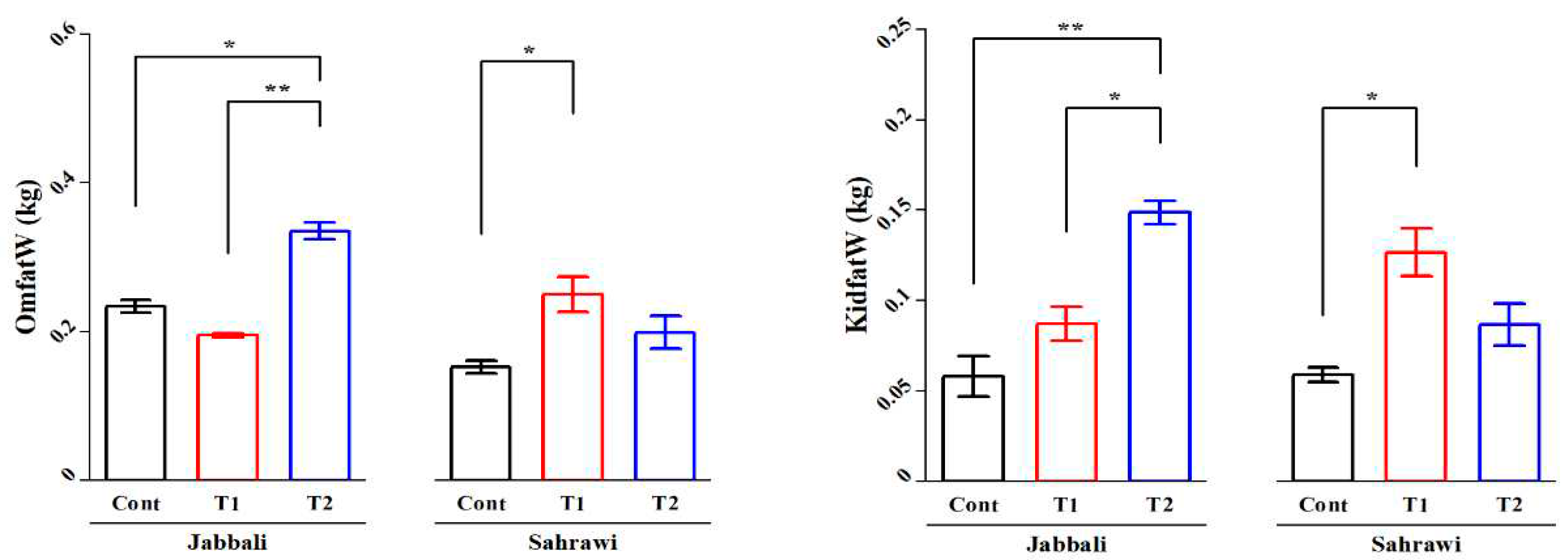
Table 3). The Duncan separation test showed that the weights of omental and kidney fat were significantly increased by Spirulina supplementation compared to the control group in both breeds (
Figure 2a,b). The average weights of the heart (0.36±0.27 g) and kidney (0.29±0.21 g) were significantly higher in control groups compared to both SP supplementation groups (0.09±0.0 and 0.06±0.01) of the Jabbali breed, respectively.
3.2. Meat quality
There were no effects of SP supplementation on all meat quality parameters in either LD or SM muscles (
P>0.05;
Table 4 and
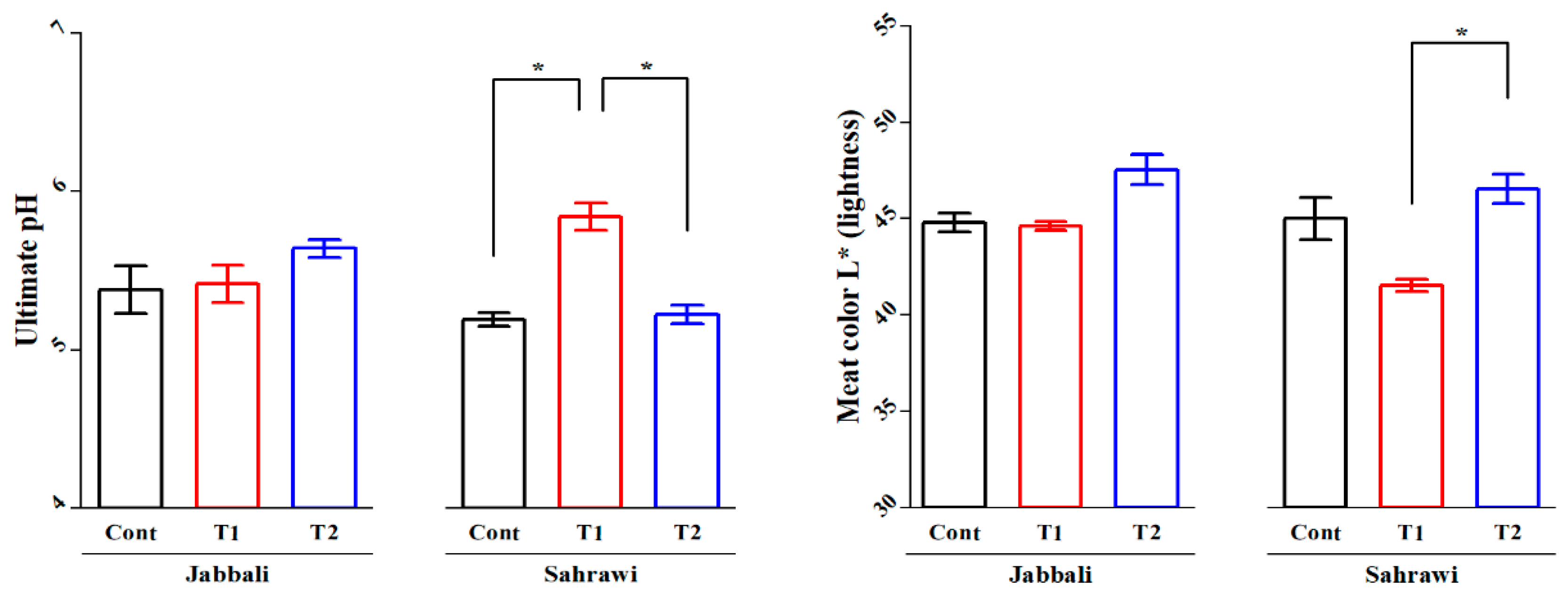
Table 5). However, the Duncan separation test showed that the T1 group (5.84±0.17) had significantly higher average ultimate pH values compared to the control (5.19±0.09) and T2 (5.22±0.09) groups in Sahrawi bucks (
Figure 3a). Furthermore, the T2 group (46.55±1.49) showed highly significant average meat color L* (lightness) values compared to the T1 group (41.52±0.62) in the same breed for the LD muscle (
Figure 3b).
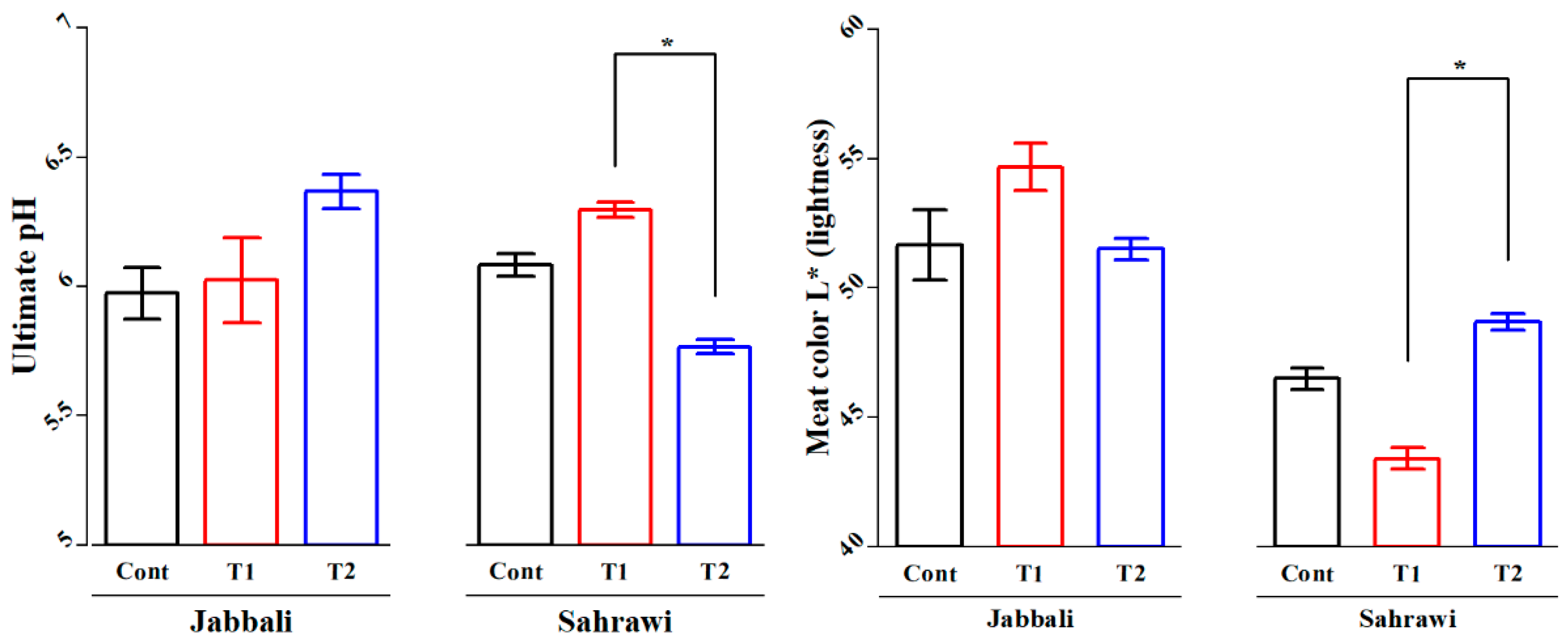
For semitendinosus muscles, the Duncan separation test showed that the T1 group (6.30±0.06) had significantly higher average ultimate Ph values compared to the T2 group (5.73±0.08) in Sahrawi buck (
Figure 4a). Furthermore, T2 group (48.69±0.65) showed highly significant average meat color L* (lightness) values compared to the T1 group (43.41±0.82) in the same breed (
Figure 4b).
3.3. Fatty acid profile
The fatty acid profiles of the LD muscle from Jabbali and Sahrawi bucks fed SP are presented in
Table 6. Some saturated fatty acids (C24:0) and monosaturated fatty acids (C16:1 and C20:3n6) of the LD in Jabali bucks showed significant differences to diets supplemented with SP. However, most of saturated fatty acids except (C16:0 and C20:0) and monosaturated fatty acids except (C20:3n6) of the LD in Sahrawi bucks showed significant differences to diets supplemented with SP (
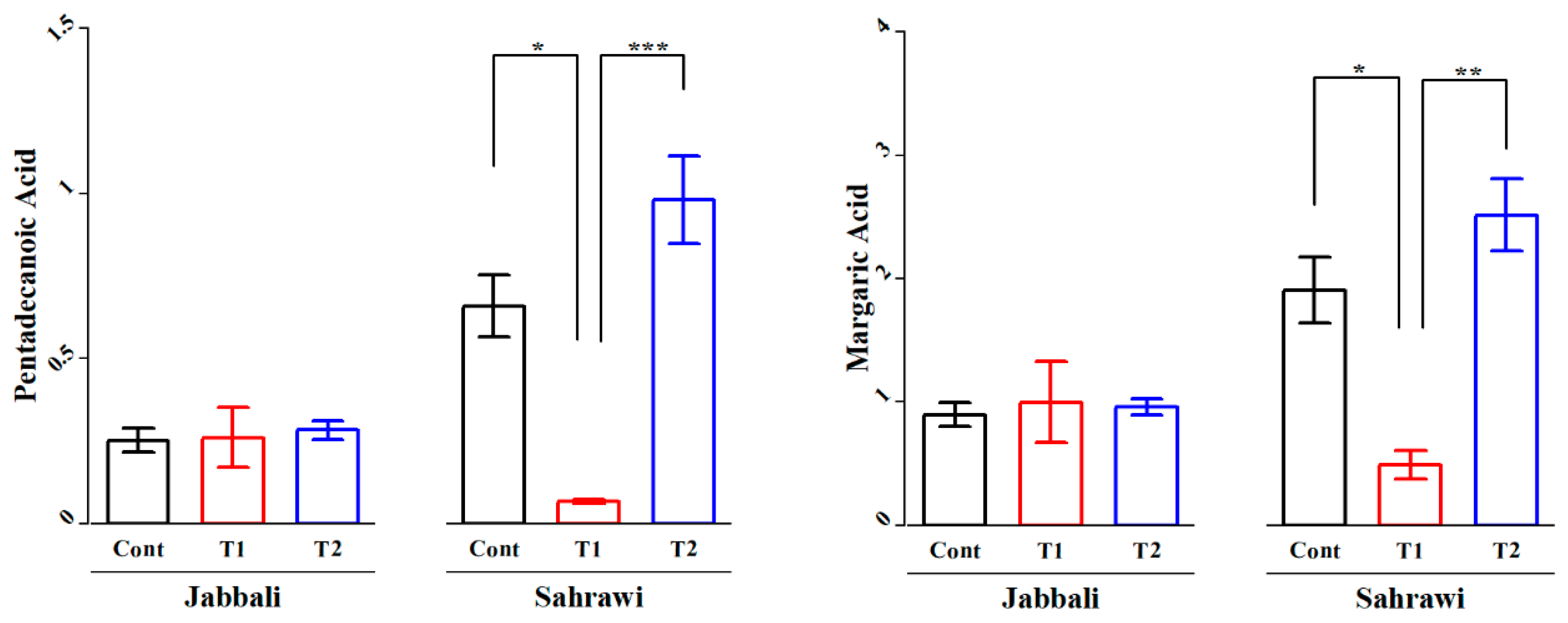
P<0.05). In addition, the LD of Sahrawi goats fed diets supplemented with SP of T1 group (0.07±0.01 and 0.49±0.23) had significantly decreased (
P<0.05) amounts of pentadecanoic and margaric acids compared with the T2 (0.98±0.26 and 2.52±0.59) and control group (0.66±0.19 and 1.91±0.54), respectively (
Figure 5a,b).
4. Discussion
This study revealed that supplemented of Spirulina in animal feed improved growth performance and meat quality in two Omani goat breeds. This improvement could be attributed to an increase in carcass measurements, such as body length, leg length and the rack weight which is confirmed in our research. Average daily gain and carcass traits (body length, leg length and the rack weight) of Sahrawi bucks were significantly higher in the T1 group than in the CON group. However, the outcomes largely depend on several factors such as the chemical composition, incorporation rate, microalgae species, and environmental conditions during growth (Madeira et al. 2017).
Several explanations might be suggested to explain this increase: 1) According to Panjaitan et al. (2010), SP may function as a growth stimulus for gut pathogens, resulting in improved nutritional absorption. 2) As stated by Salmeán et al. (2015), SP is high in all necessary amino acids and can contain up to 70% protein by dry weight. According to Hwangbo et al. (2009), protein is essential for animal growth and performance. As a result, several studies, such as those by Holman and Malau-Aduli (2013) and Madeira et al. (2017), have suggested that adding SP in the diet might improve both animal performance and meat quality. 3) SP supplementation providing the goats with a vitamin mineral premix that are often not available in traditional feed diets. The concentration of vitamins and minerals in an animal's food might affect its performance and carcass quality (Dikeman, 2007). Moury et al. (2018) showed that vitamin supplementation is often unnecessary when Spirulina is added to animal diet. 4) In the research by Al-Yahyaey et al. (2022), goats fed SP had a higher feed conversion ratio (FCR). For example, adding SP to the concentrate diet resulted in a considerably higher FCR in the T1 group (7.70 ± 0.52) than in the CON group (9.88 ± 2.49). In the Jabbali and Sahrawi goat breeds, the T2 group had a significant (P<0.05) effect, with an FCR of (9.79 ± 0.61) whereas the CON group had a less FCR of (15.15 ± 1.67). The current study's increased growth performance in goats given an SP-supplemented diet might be attributed to SP's beneficial nutritional profile. It is high in minerals, vitamins, essential amino acids, fatty acids, and other nutrients that may increase the growth.
Tovar et al. (2002) stated that higher animal performance might be attributed to the high nutritional level of SP and its ability to result in the production of extracellular enzymes by the gut microbiota. Finally, the SP supplementation elicited the desired results within this study with a slight discrepancy between both breeds, possibly due to the variation in genetic composition for muscle growth across breeds.
No differences between breeds were observed in terms of both hot and cold carcass weights. Similarly, Kalbe et al. (2019) reported that there was no significant effect on hot and cold carcass weights with adding 7% and 5% of microalgae supplementation in a piglet diet and fattening diet, respectively. Additionally, the supplementation of SP in the diet in this study did not have a significant impact on dressing percentage and carcass characteristics for either breed. However, the Sahrawi breed showed the only significant difference regarding the effect of SP on body length in T1, possibly due to the non-diet related ability of this breed to climb mountains and possessing an appropriate phenotype.
The author additionally, the weights of the full and empty digestive tract were higher in both SP groups compared to control group, although these differences were not significant in both breeds. This study also showed increased fat deposits due to the incorporation of SP into the diet with a significant increase in the Jabbali breeds’ omental and kidney fat (P>0.05). Mahgoub and Lu (1998) also observed a similar trend in internal visceral fat in various breeds of goats. The ultimate pH of muscle is one of the most critical criteria determining the quality of meat (Jia et al., 2022; Kadim et al., 2010). In the present study, the ultimate pH values observed in goat carcasses exceeded the normal range reported for SM 5.5-5.8 (Warner, 2015), but were found to fall within the acceptable range for LD across the breeds 5.6-5.8 (Pratiwi et al. 2007). The elevated pH readings in goat meat indicates goats are more susceptible to stress in general (Dhanda et al., 2003; Webb, 2014). The optimal pH measures can vary between species due to fiber type composition and physiology
The colour values of LD were not affected by goats consuming the SP diet for both breeds. The SP levels had no effect on the colour characteristics of the SM muscle in the meat, except for L*, which was found to be somewhat higher in the T2 group of the Sahrawi breed compared to the control group. This may be due to the antioxidant activity of the SP diet which contained high carotenoids possibly deposited in the muscle (Abd El-Hakim et al., 2018; Soni et al., 2019). Colour of the meat is an essential quality characteristic since it is the first criterion that customers consider when selecting fresh meat (Cimmino et al., 2018) so having no impact from feeding SP in diets is beneficial. According to Webb et al., (2005), several factors impact meat quality, including breed, age, sex, weight, and nutrition. Although various strategies involving the use of microalgae as a dietary ingredient have shown no effect on certain meat quality parameters such as colour, pH value, and cooking or drip loss, Sardi et al. (2006) found that this supplementation significantly increased DHA concentrations in the meat. Previous literature has indicated that goat breeds and supplementation type can impact on fatty acid profiles of goat meat (Lopes et al., 2014). In this study, no significant differences (P>0.05) were detected in saturated fatty acids (SFA) among the treatments for the two breeds. The LD muscle fat composition and lipid oxidation decreased in the supplemented groups compared to the control group (P<0.05). Furthermore, despite the reported increase in subcutaneous and intermuscular adipose tissue deposition in the C130 versus the C260, internal fat depot accumulation remained similar. However, the muscles of Sahrawi goats fed diets supplemented with SP significantly decreased in the amounts of pentadecanoic and margaric acid compared to control group. Additionally, the content of fatty acid decreased in treatment groups, which was attributed to diet effect. According to Geay et al. (2001), alterations in fat content are caused by a balance between dietary energy and nutritional requirements of the goats. The current study indicated that even increasing the daily energy consumption of Jabbali goats did not raise the rate of intramuscular fat in the Longissimus lumborum muscle.
The current study determined that the levels of Polyunsaturated Fatty Acids (PUFA) n-6 in the muscles of Jabbali goats given a microalgae diet were lower due to their diet included less 18:2 n-6 than the control group. This is in contrast to the findings in Sahrawi goats. Furthermore, the addition of n-3 PUFA, particularly DHA present in microalgae, may inhibit the synthesis of longer chain n-6 PUFA since they need the same elongase and desaturase enzymes (Remize et al., 2021). In study findings, the water-holding capacity (WHC) of pig muscle has been correlated to certain fatty acids, particularly PUFA (tek et al., 2015; Jiang et al., 2017). Furthermore, supplementing with n-3 Polyunsaturated Fatty Acids (PUFA) tends to help muscle cells produce a flexible lipid bilayer membrane, which leads to an increase in their water-holding capacity (WHC). Briolay et al. (2013) and Jeromson et al. (2018) observed an association between membrane restructuring, the subsequent effect on membrane protein function, and increased muscle protein synthesis in response to n-3 Polyunsaturated Fatty Acids (PUFA) treatment. This conclusion is consistent with our finding that goats assigned a microalgae-based diet have higher protein content in the Longissimus Dorsi (LD) muscle. Furthermore, previous study has shown that DHA supplementation promotes protein synthesis for growing pig muscle (Wei et al., 2013). Spirulina has a potential dietary antioxidant supplement in the goat industry, which improves goats' growth and muscular development. The dietary supplementation was helpful in producing meat with less fat and more MUFA. Moreover, Spirulina added in the diet influence the lipid oxidation and stability in the content of MUFA increased (Cimmino et al., 2018).
In summary, incorporation of Spirulina into goat diets had little effect on the muscle fatty acid component, in line with the previous reports on antioxidants for ruminant animals (Jiang et al., 2015). The increasing SP inclusion level are related to an increase in the mono-saturated fatty acid (MUFA) of the LD in Sahrawi buck for C14:1, C16:1, C16:1 Cis 9 and C17:1. While the C13:1 and C18:1 Cis(n9) content was not affected by SP diet for both breeds. This study ascertains the influence of dietary supplementation on muscle fatty acids. The impact of SP on Sahrawi goats was found to be more significant than on Jabbali goats. This difference may be attributed to the natural environment where the goats are raised. Jabbali goats are typically raised in the Al-Hajar Mountains, where the rumen microbiota community is not adapted to digest green algae. Therefore, the inclusion of SP in their diet may not have as significant an impact on their growth performance. In contrast, Sahrawi goats are commonly raised in the plains and valleys where the rumen microbiota community is adapted to digest green algae. This may explain why adding SP to their diet resulted in a more significant improvement in their product traits.
5. Conclusions
Incorporated Spirulina into the diet of goats improved average daily gain, body length, leg length and rack weight of Sahrawi goats, but this trend was not observed in Jabbali goats. Two g/head of SP increased the weights of omental and kidney fat in Sahrawi goats, but this increment required double amount of SP (4g/head) to achieve it in Jabbali goats. Two g/head of SP increased Ultimate pH and 4g/head of SP increased meat color (lightness) in both LD and SM muscles of Sahrawi goats. Two g/head of SP increased most of PUFA n-6 and n-6/n-3 ratio, while 4g/head of SP increased most of MUFA2 and PUFA n-3 fatty acids of Sahrawi goats, which was not observed in Jabbali goats. In conclusion, the present results indicated that including Spirulina in goats’ diet can improve daily gain, carcass characteristics, meat quality and fatty acids composition in Omani goats. However, this improvement is dependent on supplementation level and goat breed. A cost-based analysis should also be taken in account based on local prices. Rumen microbiome study using 16S ribosomal RNA (rRNA) to compare the rumen microbiota of Jabbali and Sahrawi goats that feed different levels of SP is needed.
Declaration of Funding
This research did not receive any specific funding.
Data Availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Acknowledgments
The authors are grateful to the Livestock Research Centre, MAFWR (LRC) and the University of Sydney. We would like to thank Eng. Rashid AL Habsi, Director of the LRC, MAFWR, for his assistance during the goat feeding trial. Special thanks to Dr. Sherif Melak, Researcher at Animal Production Research Institute, for his assistance in statistical analysis. Gratitude is also extended to Houda Al-Ruqaishi, who works at Sultan Qaboos University, for her contribution and researcher, technical and labour staff working at LRC for their help and support in conducting the research trial.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abd El-Hakim, Y. M., Mohamed, W. A., & El-Metwally, A. E. (2018). Spirulina Platensis Attenuates Furan Reprotoxicity by Regulating Oxidative Stress, Inflammation, and Apoptosis in Testis of Rats. Ecotoxicology and Environmental Safety, 161, 25-33. [CrossRef]
- Abril, M., Campo, M., Önenç, A., Sañudo, C., Albertı́, P., & Negueruela, A. (2001). Beef Colour Evolution as a Function of Ultimate Ph. Meat science, 58(1), 69-78. [CrossRef]
- Adeyemi, K. D., Shittu, R. M., Sabow, A. B., Ebrahimi, M., & Sazili, A. Q. (2016). Influence of Diet and Postmortem Ageing on Oxidative Stability of Lipids, Myoglobin and Myofibrillar Proteins and Quality Attributes of Gluteus Medius Muscle in Goats. PLoS ONE, 11(5), e0154603. [CrossRef]
- Al-Yahyaey, F., Shaat, I., Hall, E., & Bush, R. (2022). Effect of Spirulina Platensis Supplementation on Growth, Performance and Body Conformation of Two Omani Goat Breeds. Animal Production Science.
- AOAC. (1990). Official Methods of Analysis Association of Official Analytical Chemists. Association of Official Analytical Chemists, 931-940.
- AOAC. (2000). Official Methods of Analysis of Aoac. International 17th Edition.
- Briolay, A., Jaafar, R., Nemoz, G., & Bessueille, L. (2013). Myogenic Differentiation and Lipid-Raft Composition of L6 Skeletal Muscle Cells Are Modulated by Pufas. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1828(2), 602-613. [CrossRef]
- Cimmino, R., Barone, C., Claps, S., Varricchio, E., Rufrano, D., Caroprese, M., Neglia, G. (2018). Effects of Dietary Supplementation with Polyphenols on Meat Quality in Saanen Goat Kids. BMC Veterinary Research, 14(1), 1-11. [CrossRef]
- Čítek, J., Stupka, R., Okrouhlá, M., Vehovský, K., Brzobohatý, L., Šprysl, M., & Stádník, L. (2015). Effects of Dietary Linseed and Corn Supplement on the Fatty Acid Content in the Pork Loin and Backfat Tissue. Czech Journal of Animal Science, 60(7), 319-326.
- Cross, H., West, R., & Dutson, T. (1981). Comparison of Methods for Measuring Sarcomere Length in Beef Semitendinosus Muscle. Meat science, 5(4), 261-266. [CrossRef]
- Dhanda, J., Taylor, D., & Murray, P. (2003). Part 1. Growth, Carcass and Meat Quality Parameters of Male Goats: Effects of Genotype and Liveweight at Slaughter. Small Ruminant Research, 50(1-2), 57-66. [CrossRef]
- Dikeman, M. (2007). Effects of Metabolic Modifiers on Carcass Traits and Meat Quality. Meat Science, 77(1), 121-135. [CrossRef]
- Ding, Y., Jiang, X., Yao, X., Zhang, H., Song, Z., He, X., & Cao, R. (2021). Effects of Feeding Fermented Mulberry Leaf Powder on Growth Performance, Slaughter Performance, and Meat Quality in Chicken Broilers. Animals, 11(11), 3294. [CrossRef]
- Duncan, D. B. (1955). Multiple Range and Multiple F Tests. Biometrics, 11(1), 1-42. [CrossRef]
- FAOSTAT. (2019). Faostat: Food and Agriculture Organization Statistical Database Retrieved from https://www.fao.org/documents/card/en/c/ca6463en.
- Geay, Y., Bauchart, D., Hocquette, J.-F., & Culioli, J. (2001). Effect of Nutritional Factors on Biochemical, Structural and Metabolic Characteristics of Muscles in Ruminants, Consequences on Dietetic Value and Sensorial Qualities of Meat. Reproduction Nutrition Development, 41(1), 1-26. [CrossRef]
- Habib, M. A. B. (2008). Review on Culture, Production and Use of Spirulina as Food for Humans and Feeds for Domestic Animals and Fish: Food and agriculture organization of the united nations.
- Hernández, B., Sáenz, C., Alberdi, C., & Diñeiro, J. (2016). Cielab Color Coordinates Versus Relative Proportions of Myoglobin Redox Forms in the Description of Fresh Meat Appearance. Journal of Food Science and Technology, 53, 4159-4167. [CrossRef]
- Holman, B., Kashani, A., & Malau-Aduli, A. (2012). Growth and Body Conformation Responses of Genetically Divergent Australian Sheep to Spirulina (Arthrospira Platensis) Supplementation. American Journal of Experimental Agriculture, 2, 160-173.
- Holman, B., Kashani, A., & Malau-Aduli, A. (2014). Effects of Spirulina (Arthrospira Platensis) Supplementation Level and Basal Diet on Liveweight, Body Conformation and Growth Traits in Genetically Divergent Australian Dual-Purpose Lambs During Simulated Drought and Typical Pasture Grazing. Small Ruminant Research, 120(1), 6-14. [CrossRef]
- Holman, B., & Malau-Aduli, A. (2013). Spirulina as a Livestock Supplement and Animal Feed. Journal of Animal Physiology and Animal Nutrition, 97(4), 615-623.
- Hwangbo, S., Choi, S. H., Kim, S. W., Son, D. S., Park, H. S., Lee, S. H., & Jo, I. H. (2009). Effects of Crude Protein Levels in Total Mixed Rations on Growth Performance and Meat Quality in Growing Korean Black Goats. Asian-Australasian Journal of Animal Sciences, 22(8), 1133-1139. [CrossRef]
- Jeromson, S., Mackenzie, I., Doherty, M. K., Whitfield, P. D., Bell, G., Dick, J., . . . Philp, A. (2018). Lipid Remodeling and an Altered Membrane-Associated Proteome May Drive the Differential Effects of Epa and Dha Treatment on Skeletal Muscle Glucose Uptake and Protein Accretion. American Journal of Physiology-Endocrinology and Metabolism, 314(6), E605-E619. [CrossRef]
- Jiang, H., Wang, Z., Ma, Y., Qu, Y., Lu, X., Guo, H., & Luo, H. (2015). Effect of Dietary Lycopene Supplementation on Growth Performance, Meat Quality, Fatty Acid Profile and Meat Lipid Oxidation in Lambs in Summer Conditions. Small Ruminant Research, 131, 99-106. [CrossRef]
- Jiang, J., Tang, X., Xue, Y., Lin, G., & Xiong, Y. L. (2017). Dietary Linseed Oil Supplemented with Organic Selenium Improved the Fatty Acid Nutritional Profile, Muscular Selenium Deposition, Water Retention, and Tenderness of Fresh Pork. Meat science, 131, 99-106. [CrossRef]
- Kadim, I., Mahgoub, O., Al-Ajmi, D., Al-Maqbaly, R., Al-Saqri, N., & Ritchie, A. (2004). An Evaluation of the Growth, Carcass and Meat Quality Characteristics of Omani Goat Breeds. Meat Science, 66(1), 203-210. [CrossRef]
- Kadim, I., Mahgoub, O., Al-Marzooqi, W., Al-Ajmi, D., Al-Maqbali, R., & Al-Lawati, S. (2008). The Influence of Seasonal Temperatures on Meat Quality Characteristics of Hot-Boned, M. Psoas Major and Minor, from Goats and Sheep. Meat science, 80(2), 210-215. [CrossRef]
- Kadim, I., Mahgoub, O., AL-MARZOOQI, W., Khalaf, S., AL-SINAWI, S., & AL-AMRI, I. (2010). Effects of Transportation During the Hot Season, Breed and Electrical Stimulation on Histochemical and Meat Quality Characteristics of Goat Longissimus Muscle. Animal Science Journal, 81(3), 352-361. [CrossRef]
- Kalbe, C., Priepke, A., Nürnberg, G., & Dannenberger, D. (2019). Effects of Long-Term Microalgae Supplementation on Muscle Microstructure, Meat Quality and Fatty Acid Composition in Growing Pigs. Journal of Animal Physiology and Animal Nutrition, 103(2), 574-582.
- Khan, Z., Bhadouria, P., & Bisen, P. (2005). Nutritional and Therapeutic Potential of Spirulina. Current Pharmaceutical Biotechnology, 6(5), 373-379. [CrossRef]
- Kistanova, E., Marchev, Y., Nedeva, R., Kacheva, D., Shumkov, K., Georgiev, B., & Shimkus, A. (2009). Effect of the Spirulina Platensis Included in the Main Diet on the Boar Sperm Quality. Biotechnology in Animal Husbandry, 25(5-6-1), 547-557. [CrossRef]
- Lipina, C., & Hundal, H. S. (2017). Lipid Modulation of Skeletal Muscle Mass and Function. Journal of cachexia, sarcopenia and muscle, 8(2), 190-201. [CrossRef]
- Lopes, L., Martins, S., Chizzotti, M., Busato, K., Oliveira, I., Neto, O. M., . . . Ladeira, M. (2014). Meat Quality and Fatty Acid Profile of Brazilian Goats Subjected to Different Nutritional Treatments. Meat Science, 97(4), 602-608. [CrossRef]
- Madeira, M. S., Cardoso, C., Lopes, P. A., Coelho, D., Afonso, C., Bandarra, N. M., & Prates, J. A. (2017). Microalgae as Feed Ingredients for Livestock Production and Meat Quality: A Review. Livestock Science, 205, 111-121. [CrossRef]
- Mahgoub, O., Kadim, I., Al-Saqry, N., & Al-Busaidi, R. (2005). Potential of Omani Jebel Akhdar Goat for Meat Production under Feedlot Conditions. Small Ruminant Research, 56(1-3), 223-230. [CrossRef]
- Mahgoub, O., & Lu, C. (1998). Growth, Body Composition and Carcass Tissue Distribution in Goats of Large and Small Sizes. Small Ruminant Research, 27(3), 267-278. [CrossRef]
- MLA. (2022). Global Snapshot Goatmeat. Retrieved from https://www.mla.com.au/globalassets/mla-corporate/prices--markets/documents/trends--analysis/goat-industry-summary/mla-global-goatmeat-snapshot-march-2021.pdf.
- Moxham, R., & Brownlie, L. (1976). Sheep Carcass Grading and Classification in Australia. Wool Technology and Sheep Breeding, 23(2).
- Panjaitan, T., Quigley, S. P., McLennan, S. R., & Poppi, D. P. (2010). Effect of the Concentration of Spirulina ( Spirulina Platensis ) Algae in the Drinking Water on Water Intake by Cattle and the Proportion of Algae Bypassing the Rumen. Animal production science, 50(5-6), 405-409. [CrossRef]
- Peiretti, P., & Meineri, G. (2011). Effects of Diets with Increasing Levels of Spirulina Platensis on the Carcass Characteristics, Meat Quality and Fatty Acid Composition of Growing Rabbits. Livestock Science, 140(1-3), 218-224. [CrossRef]
- Pophiwa, P., Webb, E. C., & Frylinck, L. (2020). A Review of Factors Affecting Goat Meat Quality and Mitigating Strategies. Small Ruminant Research, 183, 106035. [CrossRef]
- Pratiwi, N. W., Murray, P., & Taylor, D. (2007). Feral Goats in Australia: A Study on the Quality and Nutritive Value of Their Meat. Meat Science, 75(1), 168-177. [CrossRef]
- Remize, M., Brunel, Y., Silva, J. L., Berthon, J.-Y., & Filaire, E. (2021). Microalgae N-3 Pufas Production and Use in Food and Feed Industries. Marine Drugs, 19(2), 113. [CrossRef]
- Renand, G., Picard, B., Touraille, C., Berge, P., & Lepetit, J. (2001). Relationships between Muscle Characteristics and Meat Quality Traits of Young Charolais Bulls. Meat science, 59(1), 49-60. [CrossRef]
- Salmeán, G. G., Castillo, L. H. F., & Chamorro-Cevallos, G. (2015). Nutritional and Toxicological Aspects of Spirulina (Arthrospira). Nutrición hospitalaria: Organo oficial de la Sociedad española de nutrición parenteral y enteral, 32(1), 34-40.
- Sardi, L., Martelli, G., Lambertini, L., Parisini, P., & Mordenti, A. (2006). Effects of a Dietary Supplement of Dha-Rich Marine Algae on Italian Heavy Pig Production Parameters. Livestock science, 103(1-2), 95-103. [CrossRef]
- Small, E. (2011). 37. Spirulina–Food for the Universe. Biodiversity, 12(4), 255-265.
- Soni, R. A., Sudhakar, K., & Rana, R. (2019). Comparative Study on the Growth Performance of Spirulina Platensis on Modifying Culture Media. Energy Reports, 5, 327-336. [CrossRef]
- Tachtsis, B., Camera, D., & Lacham-Kaplan, O. (2018). Potential Roles of N-3 Pufas During Skeletal Muscle Growth and Regeneration. Nutrients, 10(3), 309. [CrossRef]
- Tovar, D., Zambonino, J., Cahu, C., Gatesoupe, F., Vázquez-Juárez, R., & Lésel, R. (2002). Effect of Live Yeast Incorporation in Compound Diet on Digestive Enzyme Activity in Sea Bass (Dicentrarchus Labrax) Larvae. Aquaculture, 204(1-2), 113-123. [CrossRef]
- Wan, D., Wu, Q., & Kuca, K. (2016). Spirulina (pp. 569-583).
- Webb, E. C. (2014). Goat Meat Production, Composition, and Quality. Animal Frontiers, 4(4), 33-37.
- Webb, E. C., Casey, N. H., & Simela, L. (2005). Goat Meat Quality. Small Ruminant Research, 60(1), 153-166. [CrossRef]
- Wei, H.-K., Zhou, Y., Jiang, S., Tao, Y.-X., Sun, H., Peng, J., & Jiang, S. (2013). Feeding a Dha-Enriched Diet Increases Skeletal Muscle Protein Synthesis in Growing Pigs: Association with Increased Skeletal Muscle Insulin Action and Local Mrna Expression of Insulin-Like Growth Factor 1. British Journal of Nutrition, 110(4), 671-680. [CrossRef]
Figure 1.
The effects of Spirulina on a) average daily gain (g), b) body length (cm), c) leg length (cm) and d) rack weight (kg) of Jabbali and Sahrawi Omani goat breeds. The data showed as least square means and standard errors. P<0.05 is consider as significant. The * represent P<0.05 and ** represent p<0.01.
Figure 1.
The effects of Spirulina on a) average daily gain (g), b) body length (cm), c) leg length (cm) and d) rack weight (kg) of Jabbali and Sahrawi Omani goat breeds. The data showed as least square means and standard errors. P<0.05 is consider as significant. The * represent P<0.05 and ** represent p<0.01.
Figure 2.
Spirulina effect on weights of a) omental and b) kidney fat (g) of Omani Jabbali and Sahrawi goat breeds. The data showed as least square means and their standard errors. P<0.05 is consider as significant. The * represent P<0.05 and ** represent p<0.01.
Figure 2.
Spirulina effect on weights of a) omental and b) kidney fat (g) of Omani Jabbali and Sahrawi goat breeds. The data showed as least square means and their standard errors. P<0.05 is consider as significant. The * represent P<0.05 and ** represent p<0.01.
Figure 3.
The SP effects on a) Ultimate Ph and b) meat color (L* lightness) of Longissimus Dorsi in Jabbali and Sahrawi breeds. The data showed as least square means and standard errors. P<0.05 is consider as significant. The * represent P<0.05.
Figure 3.
The SP effects on a) Ultimate Ph and b) meat color (L* lightness) of Longissimus Dorsi in Jabbali and Sahrawi breeds. The data showed as least square means and standard errors. P<0.05 is consider as significant. The * represent P<0.05.
Figure 4.
The SP effects on a) Ultimate Ph and b) meat color (L* lightness) of semitendinosus muscles in Jabbali and Sahrawi breeds. The data showed as least square means and standard errors. P<0.05 is consider as significant. The * represent P<0.05.
Figure 4.
The SP effects on a) Ultimate Ph and b) meat color (L* lightness) of semitendinosus muscles in Jabbali and Sahrawi breeds. The data showed as least square means and standard errors. P<0.05 is consider as significant. The * represent P<0.05.
Figure 5.
The effect of Spirulina platenisis on a) pentadecanoic and b) margaric acids of Longissimus dorsi of goat bucks. The * represent P<0.05, ** represent p<0.01 and *** represent p<0.001.
Figure 5.
The effect of Spirulina platenisis on a) pentadecanoic and b) margaric acids of Longissimus dorsi of goat bucks. The * represent P<0.05, ** represent p<0.01 and *** represent p<0.001.
Table 1.
Nutrient composition (g/100g DM) and dry matter content (g/100g fresh wt.) of Spirulina and basal diet of Rhodes grass hay and Concentrate.
Table 1.
Nutrient composition (g/100g DM) and dry matter content (g/100g fresh wt.) of Spirulina and basal diet of Rhodes grass hay and Concentrate.
| Nutrients (In dry matter) |
Concentrate |
Spirulina |
Rhodes Grass Hay |
| Dry matter % (DM) |
90.0 |
95.1 |
89.70 |
| Crude protein (CP) |
14.0 |
62.48 |
7.22 |
| Crude fiber (CF) |
9.8 |
2.9 |
34.3 |
| Ether extract (EE) |
2.5 |
1.05 |
1.00 |
| Ash |
9.2 |
7.55 |
9.80 |
| Nitrogen free extract (NFE) |
64.5 |
26.02 |
47.7 |
| Neutral detergent fiber (NDF) |
28.60 |
1.92 |
74.00 |
| Acid detergent fiber (ADF) |
11.42 |
0.37 |
46.7 |
| Metabolisable Energy (ME; MJ/kg DM)1
|
11.97 |
11.63 |
8.30 |
Table 2.
The effects of Spirulina on final body weight, hot carcass weight, cold carcass weight and dressing-out%, carcass linear dimensions and major cut weights (least square means±standard errors) of Jabbali and Sahrawi Omani goat breeds.
Table 2.
The effects of Spirulina on final body weight, hot carcass weight, cold carcass weight and dressing-out%, carcass linear dimensions and major cut weights (least square means±standard errors) of Jabbali and Sahrawi Omani goat breeds.
| Parameters |
Jabbali |
Sahrawi |
| CON |
T1 |
T2 |
P value |
CON |
T1 |
T2 |
P value |
| Final Live BW (kg) |
25.33±1.27 |
25.77±0.94 |
26.57±1.09 |
0.458 |
21.03±1.02 |
23.40±0.56 |
22.73±1.47 |
0.160 |
| HCWT (kg) |
11.09±0.35 |
11.60±0.35 |
12.26±0.87 |
0.107 |
9.96±0.78 |
10.84±0.18 |
10.41±0.62 |
0.385 |
| ColdCWT (kg) |
10.88±0.34 |
11.39±0.34 |
12.05±0.87 |
0.137 |
9.70±0.71 |
10.65±0.17 |
10.20±0.61 |
0.423 |
| Dressing-out (%)1
|
43.87±1.22 |
45.20±2.75 |
46.06±1.62 |
0.785 |
47.29±2.38 |
46.34±0.39 |
45.95±2.25 |
0.698 |
| GigWT (cm) |
13.07±0.24 |
12.57±0.58 |
12.70±0.32 |
0.550 |
12.20±0.40 |
12.17±0.32 |
12.20±0.72 |
0.887 |
| WidBsh cm)2
|
17.33±0.94 |
16.10±1.29 |
18.20±0.21 |
0.480 |
15.37±0.43 |
15.40±0.29 |
16.67±0.58 |
0.337 |
| MxSW (cm)3
|
13.10±0.30 |
12.77±0.24 |
12.83±0.03 |
0.474 |
11.57±0.34 |
12.17±0.38 |
12.13±0.50 |
0.648 |
| Leg Length (cm) |
17.67±0.27 |
17.63±0.39 |
17.90±0.35 |
0.598 |
17.40ab±0.53 |
18.45a±1.49 |
16.60b±0.29 |
0.025 |
| Body Length (cm) |
39.57±0.53 |
39.73±0.73 |
39.50±0.55 |
0.272 |
39.10b±0.81 |
43.30a±2.87 |
38.27b±0.52 |
0.045 |
| DepSst (cm)4
|
24.13±0.13 |
24.47±0.74 |
24.40±0.42 |
0.565 |
24.23±0.69 |
23.80±0.65 |
23.45±0.70 |
0.097 |
| Shol Weight (kg) |
4.76±0.25 |
5.01±0.21 |
5.56±0.38 |
0.151 |
4.43±0.43 |
4.63±0.16 |
4.62±0.39 |
0.629 |
| Rack Weight (kg) |
1.51±0.03 |
1.71±0.10 |
1.58±0.13 |
0.271 |
1.22b±0.04 |
1.54a±0.03 |
1.42ab±0.12 |
0.272 |
| Loin Weight (kg) |
1.01ab±0.08 |
1.06a±0.05 |
0.98b±0.06 |
0.019 |
0.83±0.05 |
1.01±0.06 |
0.88±0.08 |
0.421 |
| Leg Weight (kg) |
3.59±0.14 |
3.59±0.09 |
3.90±0.31 |
0.924 |
3.31±0.29 |
3.43±0.03 |
3.28±0.14 |
0.311 |
Table 3.
Effect of Spirulina on Non-carcass component (least square means±standard errors) of Jabbali and Sahrawi Omani goat breeds.
Table 3.
Effect of Spirulina on Non-carcass component (least square means±standard errors) of Jabbali and Sahrawi Omani goat breeds.
| Parameters1
|
Jabbali |
Sahrawi |
| CON |
T1 |
T2 |
P value |
CON |
T1 |
T2 |
P value |
| Head Weight (kg) |
1.63±0.02 |
1.79±0.04 |
1.78±0.12 |
0.170 |
1.48±0.09 |
1.73±0.03 |
1.57±0.08 |
0.338 |
| Feet Weight (g/kg) |
0.78±0.04 |
0.68±0.03 |
0.70±0.04 |
0.316 |
0.56±0.02 |
0.59±0.03 |
0.60±0.04 |
0.599 |
| Rumen full Weight (kg) |
4.90±0.56 |
5.00±0.95 |
5.37±0.27 |
0.786 |
3.67±0.34 |
4.37±0.18 |
4.32±0.68 |
0.926 |
| Rumen empty Weight (kg) |
1.98±0.16 |
2.13±0.15 |
2.25±0.13 |
0.248 |
1.54±0.05 |
1.69±0.17 |
1.86±0.17 |
0.716 |
| Lung Trachea Weight (g/kg) |
0.42±0.15 |
0.26±0.01 |
0.26±0.01 |
0.543 |
0.38±0.09 |
0.27±0.01 |
0.35±0.09 |
0.838 |
| Heart Weight (g/kg) |
0.36a±0.27 |
0.09b±0.01 |
0.09b±0.01 |
0.667 |
0.09±0.00 |
0.09±0.01 |
0.25±0.17 |
0.834 |
| Spleen Weight (g/kg) |
0.05±0.01 |
0.05±0.00 |
0.05±0.00 |
0.977 |
0.05±0.00 |
0.06±0.01 |
0.06±0.01 |
0.216 |
| Liver Weight (g/kg) |
0.45±0.04 |
0.51±0.04 |
0.49±0.03 |
0.317 |
0.35±0.00 |
0.45±0.05 |
0.40±0.04 |
0.815 |
| Omental Fat Weight (g/kg) |
0.23b±0.02 |
0.20b±0.01 |
0.34a±0.02 |
0.151 |
0.15b±0.02 |
0.25a±0.05 |
0.20ab±0.04 |
0.711 |
| Kidney Weight (g/kg) |
0.29a±0.21 |
0.06b±0.00 |
0.06b±0.00 |
0.151 |
0.06±0.01 |
0.07±0.00 |
0.06±0.00 |
0.714 |
| Kidney Fat Weight (g/kg) |
0.06b±0.02 |
0.09ab±0.02 |
0.15a±0.01 |
0.094 |
0.06b±0.01 |
0.13a±0.03 |
0.09ab±0.02 |
0.448 |
| Mesfat Weight (g/kg) |
0.17±0.04 |
0.18±0.02 |
0.19±0.02 |
0.409 |
0.14±0.02 |
0.15±0.07 |
0.13±0.04 |
0.506 |
| Mesenteric fat (g/kg) |
0.17±0.01 |
0.16±0.02 |
0.23±0.03 |
0.173 |
0.10±0.00 |
0.14±0.04 |
0.10±0.01 |
0.526 |
| Skin (kg) |
2.37±0.29 |
2.23±0.10 |
2.23±0.17 |
0.352 |
1.69±0.22 |
1.84±0.03 |
1.77±0.19 |
0.806 |
Table 4.
Least square means ± standard errors of the SP effects on meat quality characteristics of Longissimus Dorsi in Jabbali and Sahrawi breeds.
Table 4.
Least square means ± standard errors of the SP effects on meat quality characteristics of Longissimus Dorsi in Jabbali and Sahrawi breeds.
| Parameters |
Jabbali |
Sahrawi |
| CON |
T1 |
T2 |
P value |
CON |
T1 |
T2 |
P value |
| Ultimate pH |
5.38±0.30 |
5.42±0.23 |
5.64±0.12 |
0.987 |
5.19b±0.09 |
5.84a±0.17 |
5.22b±0.12 |
0.126 |
| Sarcomere length (lm) |
10.00±0.42 |
9.50±0.69 |
9.67±0.67 |
0.707 |
8.17±0.95 |
9.67±0.76 |
8.78±0.46 |
0.183 |
| EJ (drip loss) (g/cm2)1
|
19.67±6.63 |
21.63±3.71 |
22.20±3.27 |
0.078 |
29.43±4.11 |
20.93±1.14 |
27.10±1.08 |
0.293 |
| Cook loss (%) |
41.20±3.31 |
37.73±3.43 |
38.60±4.33 |
0.976 |
41.27±3.54 |
33.63±3.59 |
41.57±2.23 |
0.836 |
| WBV (kg)2
|
5.50±1.71 |
4.13±1.01 |
5.93±0.83 |
0.268 |
4.80±1.30 |
4.90±1.13 |
6.17±0.58 |
0.528 |
| L* (lightness) |
44.79±0.97 |
44.63±0.43 |
47.54±1.56 |
0.195 |
45.00ab±2.21 |
41.52b±0.62 |
46.55a±1.49 |
0.685 |
| a* (redness) |
21.31±1.41 |
22.27±0.88 |
22.58±1.15 |
0.787 |
19.49±2.16 |
23.26±1.09 |
21.14±0.99 |
0.536 |
| b* (yellowness) |
5.65±0.20 |
5.61±1.01 |
5.89±1.46 |
0.625 |
4.95±0.99 |
4.53±0.45 |
6.06±0.91 |
0.567 |
Table 5.
Least square means±standard errors of the SP effects on meat quality characteristics of semitendinosus muscles in Jabbali and Sahrawi breeds.
Table 5.
Least square means±standard errors of the SP effects on meat quality characteristics of semitendinosus muscles in Jabbali and Sahrawi breeds.
| Parameters |
Jabbali |
Sahrawi |
| CON |
T1 |
T2 |
P value |
CON |
T1 |
T2 |
P value |
| Ultimate Ph |
5.97±0.33 |
6.02±0.43 |
6.37±0.13 |
0.881 |
6.08ab±0.24 |
6.30a±0.06 |
5.73b±0.08 |
0.725 |
| Sarcomere length (lm) |
6.39±0.20 |
5.83±0.35 |
5.83±0.10 |
0.611 |
6.72±0.97 |
7.06±0.82 |
6.72±1.06 |
0.889 |
| EJ (drip loss) (g/cm2)1
|
28.97±3.55 |
24.17±6.21 |
26.60±2.04 |
0.850 |
19.97±3.46 |
21.47±3.26 |
27.03±2.44 |
0.370 |
| Cook loss (%) |
48.57±5.66 |
37.40±8.31 |
36.87±7.33 |
0.655 |
45.33±7.51 |
34.73±2.86 |
44.33±3.92 |
0.301 |
| Tender (kg) |
3.57±0.88 |
3.77±1.05 |
2.50±0.20 |
0.546 |
2.87±0.33 |
2.7±0.44 |
4.27±0.35 |
0.748 |
| L* (lightness) |
51.65±2.72 |
54.68±1.81 |
51.50±0.84 |
0.684 |
46.50ab±0.81 |
43.41b±0.82 |
48.69a±0.65 |
0.088 |
| a* (redness) |
19.31±1.20 |
18.02±0.78 |
19.86±0.51 |
0.993 |
20.97±0.41 |
20.81±0.80 |
20.73±0.46 |
0.720 |
| b* (yellowness) |
4.91±0.26 |
4.93±1.21 |
5.56±0.09 |
0.894 |
4.81±0.24 |
4.65±0.45 |
5.49±0.47 |
0.494 |
Table 6.
The effect of Spirulina platenisis on fatty acid profile (g/100 g FA), groups (g/100 g fat), ratios, and indexes of Longissimus dorsi of goat kids.
Table 6.
The effect of Spirulina platenisis on fatty acid profile (g/100 g FA), groups (g/100 g fat), ratios, and indexes of Longissimus dorsi of goat kids.
| Fatty acid |
Jabbali |
Sahrawi |
| CON |
T1 |
T2 |
P value |
CON |
T1 |
T2 |
P value |
| C10:0 |
0.05±0.01 |
0.06±0.02 |
0.08±0.03 |
0.896 |
0.15±0.09 |
0.11±0.08 |
0.14±0.03 |
0.055 |
| C12:0 |
0.06±0.02 |
0.06±0.03 |
0.25±0.22 |
0.980 |
0.24±0.12 |
0.41±0.38 |
0.27±0.07 |
0.025 |
| C13:0 |
0.01±0.00 |
0.01±0.00 |
0.01±0.01 |
0.856 |
0.05±0.01 |
0.40±0.39 |
0.03±0.01 |
0.012 |
| C14:0 |
0.98±0.27 |
1.00±0.63 |
1.47±0.61 |
0.922 |
2.32ab±0.63 |
1.17b±0.85 |
3.52a±0.87 |
0.020 |
| C15:0 |
0.25±0.07 |
0.26±0.18 |
0.28±0.06 |
0.867 |
0.66a±0.19 |
0.07b±0.01 |
0.98a±0.26 |
0.000 |
| C16:0 |
6.93±1.01 |
6.94±3.12 |
11.08±3.89 |
0.952 |
11.23±2.52 |
10.53±6.74 |
16.67±3.24 |
0.121 |
| C17:0 |
0.90±0.20 |
1.00±0.65 |
0.96±0.13 |
0.966 |
1.91a±0.54 |
0.49b±0.23 |
2.52a±0.59 |
0.000 |
| C18:0 |
6.82±0.89 |
7.64±4.02 |
8.69±1.15 |
0.892 |
11.36a±2.87 |
6.11b±2.48 |
16.14a±3.18 |
0.001 |
| C20:0 |
0.23±0.14 |
0.22±0.13 |
0.27±0.12 |
0.660 |
0.28±0.11 |
2.34±2.23 |
0.62±0.03 |
0.241 |
| C24:0 |
0.06±0.02 |
0.09±0.02 |
0.06±0.02 |
0.003 |
0.08±0.02 |
0.11±0.02 |
0.10±0.01 |
0.007 |
| SFA1 |
16.19±2.46 |
17.10±8.71 |
23.04±5.76 |
0.943 |
28.04ab±6.84 |
19.95b±11.68 |
40.57a±8.29 |
0.040 |
| C13:1 |
0.02±0.00 |
0.06±0.04 |
0.02±0.00 |
0.980 |
0.05±0.02 |
0.89±0.88 |
0.06±0.02 |
0.005 |
| C14:1 |
0.08±0.02 |
0.07±0.05 |
0.09±0.02 |
0.692 |
0.21a±0.07 |
0.02b±0.01 |
0.31a±0.08 |
0.000 |
| C16:1 |
0.24a±0.07 |
0.09b±0.03 |
0.27a±0.06 |
0.000 |
0.33b±0.12 |
0.17b±0.07 |
0.70a±0.18 |
0.005 |
| C17:1 |
0.74±0.19 |
0.73±0.43 |
1.00±0.38 |
0.876 |
1.93a±0.56 |
0.24b±0.02 |
1.94a±0.44 |
0.000 |
| C16:1Cis9 |
1.34±0.32 |
1.73±1.10 |
1.31±0.17 |
0.954 |
2.30b±0.63 |
0.75c±0.33 |
4.54a±1.09 |
0.001 |
| C18:1Cisn9 |
14.22±2.16 |
14.57±6.33 |
19.90±4.89 |
0.906 |
25.81±6.40 |
19.42±12.13 |
30.23±5.44 |
0.027 |
| MUFA2 |
16.63±2.75 |
17.25±7.90 |
22.58±4.97 |
0.914 |
30.63±7.61 |
21.47±13.37 |
37.79±7.18 |
0.024 |
| C18:2Cisn6 |
3.60±1.07 |
2.55±0.81 |
2.93±0.88 |
0.348 |
1.83b±0.56 |
4.64a±1.62 |
4.46a±0.76 |
0.000 |
| C20:3n6 |
0.15a±0.04 |
0.06b±0.00 |
0.05b±0.00 |
0.586 |
0.07±0.01 |
0.07±0.01 |
0.17±0.09 |
0.824 |
| C20:4n6 |
0.81±0.06 |
0.88±0.10 |
0.68±0.08 |
0.174 |
0.95b±0.14 |
2.16a±0.77 |
0.78b±0.10 |
0.001 |
| C22:4n6 |
0.11±0.01 |
0.09±0.02 |
0.12±0.02 |
0.757 |
0.18±0.04 |
0.13±0.01 |
0.12±0.02 |
0.001 |
| PUFA n-6 |
4.61±1.06 |
3.58±0.87 |
3.77±0.86 |
0.307 |
3.03b±0.56 |
6.95a±2.25 |
5.45a±0.77 |
0.000 |
| C18:3Cisn3 |
0.09±0.01 |
0.11±0.06 |
0.11±0.02 |
0.974 |
0.13b±0.04 |
0.07b±0.01 |
0.23a±0.04 |
0.019 |
| cis-5,8,11-Eicosatrienoic |
0.18±0.02 |
0.15±0.04 |
0.18±0.02 |
0.993 |
0.27±0.07 |
0.56±0.41 |
0.28±0.06 |
0.008 |
| PUFA n-3 |
0.26±0.02 |
0.25±0.09 |
0.23±0.03 |
0.957 |
0.40±0.09 |
0.62±0.40 |
0.51±0.09 |
0.018 |
| n-6/n-3 ratio |
18.81±5.60 |
21.52±6.21 |
22.07±6.44 |
0.207 |
14.60ab±3.91 |
21.98a±4.39 |
11.77b±1.20 |
0.001 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).