1. Introduction
Particulate matter (PM) is suspended dust with each particle having a diameter of less than 10 μm (PM10) and can be inhaled and can accumulate in lung alveoli causing considerable respiratory health risks [
1]. Exposure to PM causes aggravated respiratory symptoms, induces pulmonary inflammation, and exacerbates allergic responses owing to its stimulatory effect on oxidative stress and its toxicity, leading to a rise in the prevalence of bronchial asthma, chronic respiratory disorder, and lung cancer [
2,
3,
4,
5]. In Asia, PM contains seasonal yellow dust, fine dust from China, and domestic air pollutants generated from combustion sources, such as gasoline and diesel engines, coal combustion, and biomass boiling. In particular, diesel exhaust particles (DEPs) consist of hundreds of different chemicals (primarily of carbon, polycyclic aromatic hydrocarbon, ash, metallic abrasion particles, sulfates, nitrates, and other trace elements) and lead to the highest toxicity in the human respiratory system [
6,
7].
Siraitia grosvenorii Swingle fruit (also known as Luo Han Guo or monk fruit, of the Cucurbitaceae family) is a traditional remedial plant used in Korea and China to treat respiratory diseases such as cold, cough, laryngitis, bronchitis, and sore throat as well as stomach ailments and is also used as low-calorie sweeteners for drinks [
8].
S. grosvenorii extract (SGE) showed antiglycation, antioxidative, anti-inflammatory, and neuroprotective properties [
9,
10,
11]. Previous studies reported SGE alleviated symptoms of allergic illnesses such as asthma and atopic dermatitis [
12,
13]. Mogroside is one of the main ingredients of
S. grosvenorii fruit, and it decreased airway hyperresponsiveness and airway inflammation by preventing nuclear factor-kappa B (NF-κB) activation induced by ovalbumin in the Kunming asthmatic mouse model [
14]. Our recently published study showed that
S. grosvenorii ethanolic extract ameliorated airway inflammation in lipopolysaccharide-stimulated bronchial epithelial cells and in a lipopolysaccharide- and cigarette smoke- induced chronic obstructive pulmonary disease (COPD) mice [
15]. Thus, this current study aimed to examine the effect of SGE on airway inflammation in a fine dust-exposed chronic respiratory disorder mouse model and the signaling mechanisms underlying its effects.
2. Materials and Methods
2.1. Extraction of SGE
The
S. grosvenorii extract, Mogron®, was given to us by Suheung Co., Ltd. (Cheongju, Korea). Its preparation has been described previously [
15].
2.2. Mouse model
Seven-week-old male BALB/c mice, bought from Orient Bio Inc. (Seongnam, Korea), were kept at 21°C ± 2°C and humidity of 60% ± 10%. The approval of studies was carried out by the Committee for Animal Welfare at Daejeon University (ethical approval code DJUARB2022-041), and experiment was performed in accordance with the Guide for the Care and Use of Laboratory Animals. After acclimatization, mice were randomized to five groups (n = 8): (1) normal, (2) PM10D-control (CTL), (3) PM10D-dexamethasone 3 mg/kg, (4) PM10D-SGE 100 mg/kg, or (5) PM10D-SGE 50 mg/kg. The dose of SGE was determined based on our previous studies using a COPD mouse model [
15]. PM10 and DEP were dissolved in 99% saline and 1% aluminum hydroxide-based gel adjuvants. Chronic inflammation was induced by intranasal administration of a fine dust mixture (PM10D) that included 3 mg/mL PM10 (ERMCZ120, MilliporeSigma, Burlington, MA, USA) and 0.6 mg/mL DEP (NIST2975, MilliporeSigma) to mice on days 4, 7, and 10 as reported previously [
16]. The vehicle solution was treated intranasally to the normal mice, and the fine dust mixture was treated intranasally to the remaining mice. SGE (50 or 100 mg/kg) or dexamethasone (3 mg/kg) as a positive control were orally administered every day for 12 days. The experimental setup is expressed in
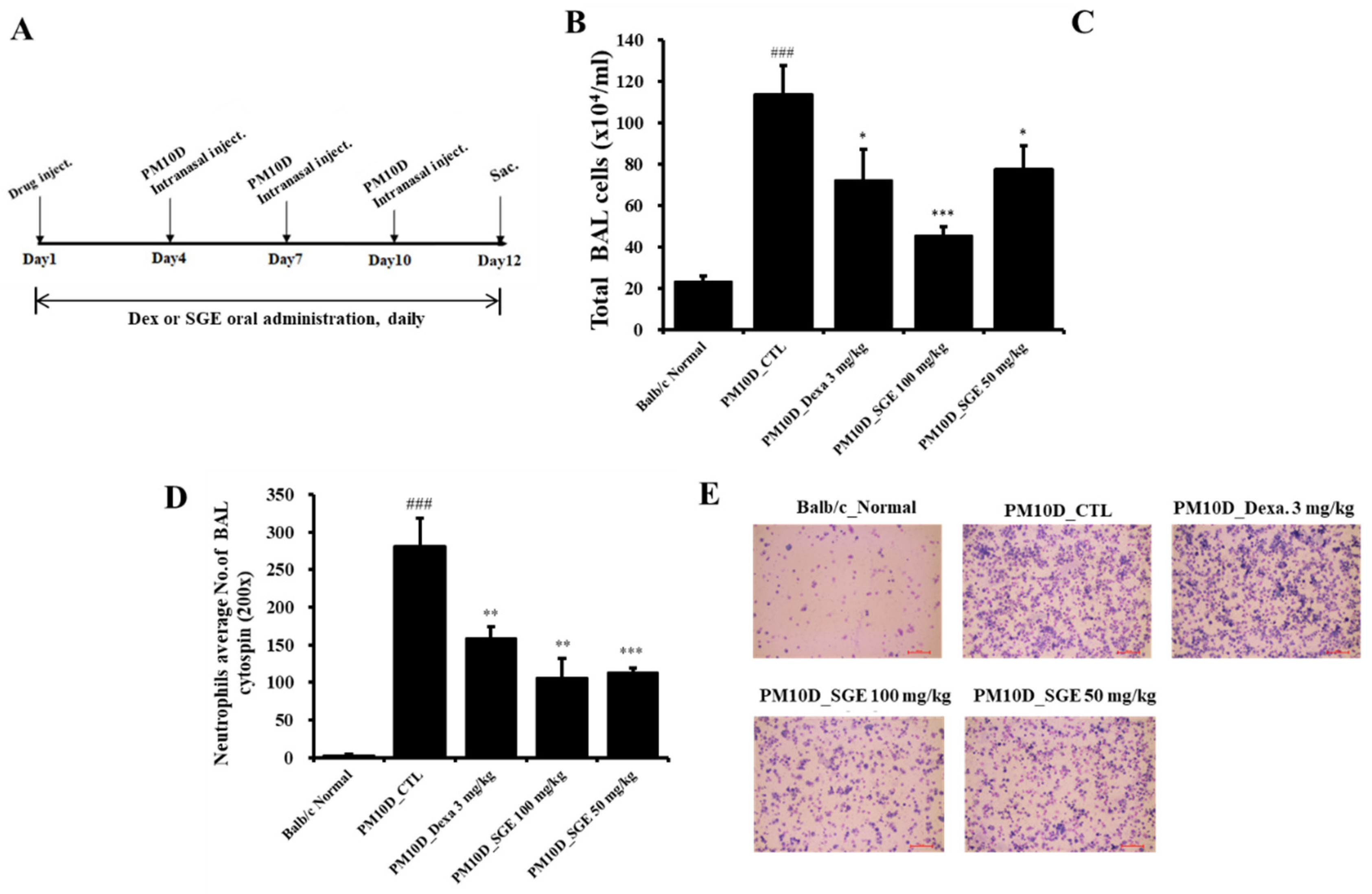
Figure 1A.
2.3. Collection of mouse lung cells from bronchoalveolar lavage fluid
Bronchoalveolar lavage fluid (BALF) was collected on day 12 by cannulating the trachea of mice and adding Dulbecco’s Modified Eagle Medium into the lung, and the medium was then collected. The number of lung cells collected from the BALF was counted with a hemocytometer, and the cells were prepared by cytospin centrifugation (Hanil, Gimpo, Korea) and stained with Diff-Quik stain solution. Differential cell counts were assessed using a cytospin slide. The lung cells were separated as a single-cell suspension and treated in phosphate-buffered saline (PBS) containing 1 mg/mL collagenase IV (MilliporeSigma) for 40 min at 37°C. Then, the cell suspension was filtered and centrifuged at 492 x g for 20 min, and pellets were collected.
2.4. Flow cytometry analysis
Collected cells from lung and BALF were incubated for 30 min with antibodies against CD8a (53-6.7, rat IgG2a), CD4 (RM4-5, rat IgG2a), CD69 (H1.2F3, hamster IgG1), CD62L (MEL-14, rat IgG2a), CD44 (IM7, rat IgG2b), CD21/CD35 (7G6, rat IgG2b), B220 (RA3-6B2, rat IgG2a), Gr-1 (RB6-8C5, rat IgG2b), SiglecF (1RNM44N, rat IgG2a), and CD11b (M1/70, rat IgG2b). All antibodies were obtained from BD Biosciences (Franklin Lakes, NJ, USA), except for anti-SiglecF (Thermo Fisher Scientific, Waltham, MA, USA). The cells were treated with phycoerythrin- or fluorescein isothiocyanate-labeled secondary antibodies for 35 min, washed, and fixed with 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA) for 15 min. After washing, the cells were analyzed on a BD flow cytometer FACSCalibur (BD Biosciences).
2.5. Measurement of cytokines
BALF was centrifuged at 492 x g for 10 min, and interleukin (IL)-1a, IL-17, macrophage inflammatory protein (MIP)-2, chemokine (C-X-C motif) ligand (CXCL)-1, and tumor necrosis factor (TNF)-a levels were determined in the supernatant by enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA). The optical density of the supernatant was determined at 450 nm with a SpectraMax microplate analyzer (Molecular Devices, San Jose, CA, USA).
2.6. Histopathologic examination of lung tissue
Mouse lung was perfused with 1 mL 10% (v/v) neutral-buffered formalin fixation solution through the trachea; then the lung was eliminated and submerged in formalin buffer for 22 h. The tissue was paraffinized, cut to 5-μm thickness, and stained with Masson’s trichrome or hematoxylin and eosin solution for the observation of for inflammatory cell penetration and collagen fiber formation (Sigma-Aldrich).
2.7. Quantitative reverse transcription-polymerase chain reaction
Total RNA from lung tissue was isolated with total RNA prep kit (HiGene, BIOFACT, Daejeon, Korea). To quantify mRNA expression of genes, quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed using a Real-Time PCR instrument (Applied Biosystems 7500 Fast, Thermo Fisher Scientific) with a SYBR Green master mixture (Applied Biosystems) and primer. The primer sequences are recorded in
Table 1. Gene transcript was expressed as ΔΔCt, normalized to the b-actin gene.
2.8. Immunoblot
Proteins from lung tissues were extracted in protein extraction buffer (PRO-PREP, Intron Biotechnology, Seongnam, Korea), separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to membrane by a Trans-Blot Turbo transfer system (Bio-Rad Laboratories, Hercules, CA, USA). The membrane was blocked using blocking solution (EzBlock Chemi, ATTO, Tokyo, Japan) for 40 min and treated with antibodies raised against β-actin, phosphorylated (NF-κB, IκB, ERK, p38, or JNK), NF-κB, ERK, IκB, p38, or JNK (Cell Signaling Technology, Danvers, MA, USA). The membrane was incubated with anti-rabbit secondary antibodies (Cell Signaling Technology) for 40 min. The signal was visualized with enhanced chemiluminescence (Thermo Scientific). Images of the membranes were evaluated by ImageJ software.
2.9. Statistical Analysis
Results are presented as the mean ± standard error of the mean. Statistical testing among groups was performed by one-way analysis of variance and Duncan’s multiple comparison test. The statistical analysis was performed using GraphPad Prism 7.0 software. p < 0.05 was considered to have a significant difference. #p < 0.05, ##p < 0.01, and ###p < 0.001 are compared with the normal group, and *p < 0.05, **p < 0.01, and ***p < 0.001 are compared with the PM10D Control group.
3. Results
3.1. Effect of SGE on neutrophil infiltration in PM10D-exposed mice
PM10D exposure to mice for 12 days increased the total cell number in BALF. The total cell number in BALF decreased after oral administration of SGE or dexamethasone, but the total number of lung cells did not (
Figure 1B). In particular, the number of neutrophils in BALF by cytospin was increased after PM10D exposure compared with normal mice, and neutrophil infiltration was significantly decreased after SGE administration (
Figure 1C,D).
3.2. Effect of SGE on white blood cells in Blood
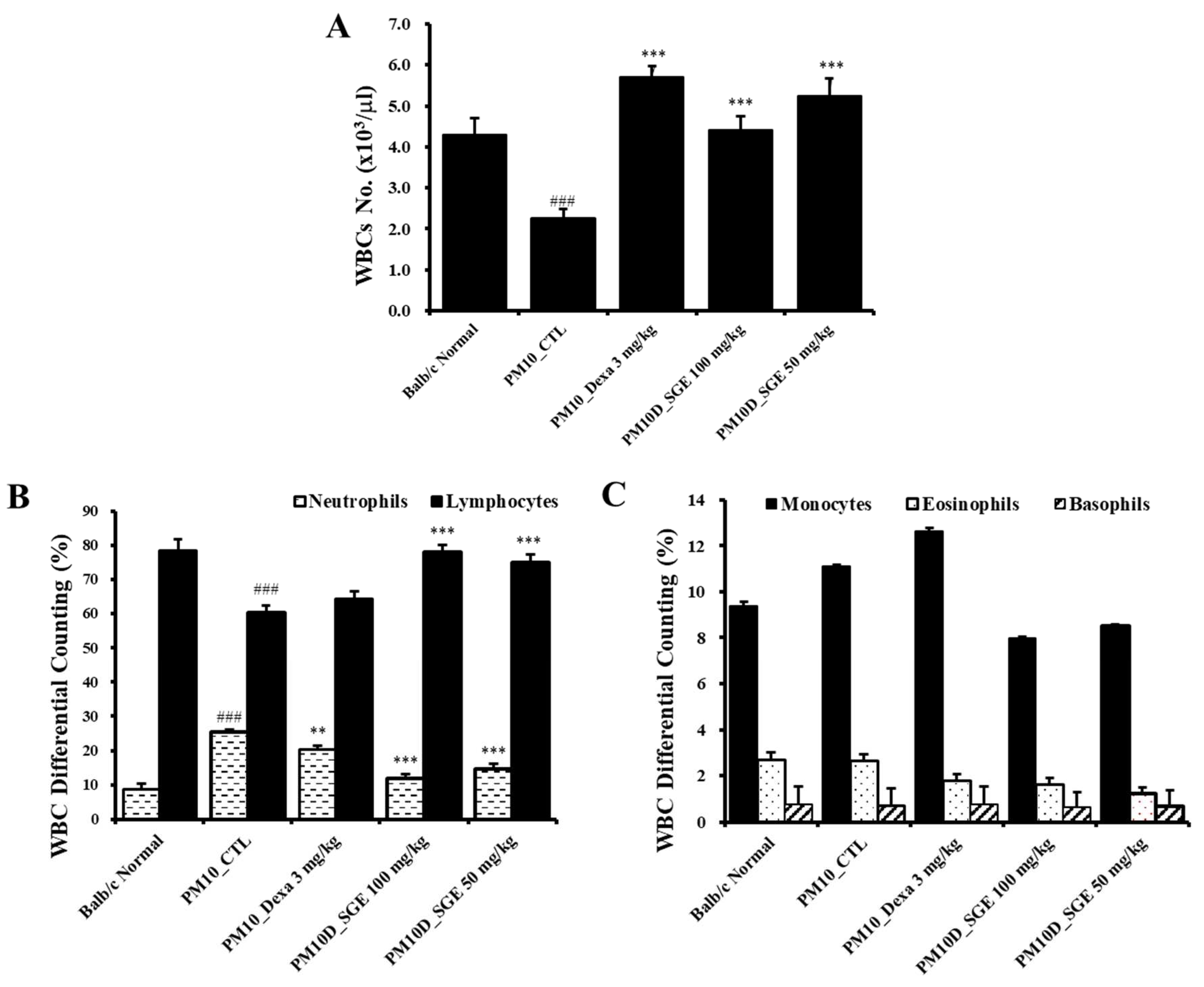
Changes in the cell types in Blood, particularly neutrophils, offer significant information about the development of airway inflammation. Hematological examination of white blood cells (WBCs) in Blood indicated that the total count of WBC was decreased after PM10D exposure compared with controls, and the decreased levels recovered after SGE administration (
Figure 2A). The number of neutrophils was increased after PM10D exposure compared with normals and was significantly decreased after SGE administration (
Figure 2B). The number of lymphocytes was reduced after PM10D exposure compared with normals and was increased after SGE administration (
Figure 2B). Monocytes, eosinophils, and basophils did not show significant differences among the groups (
Figure 2C).
3.3. Effect of SGE on the release of inflammatory mediators in BALF
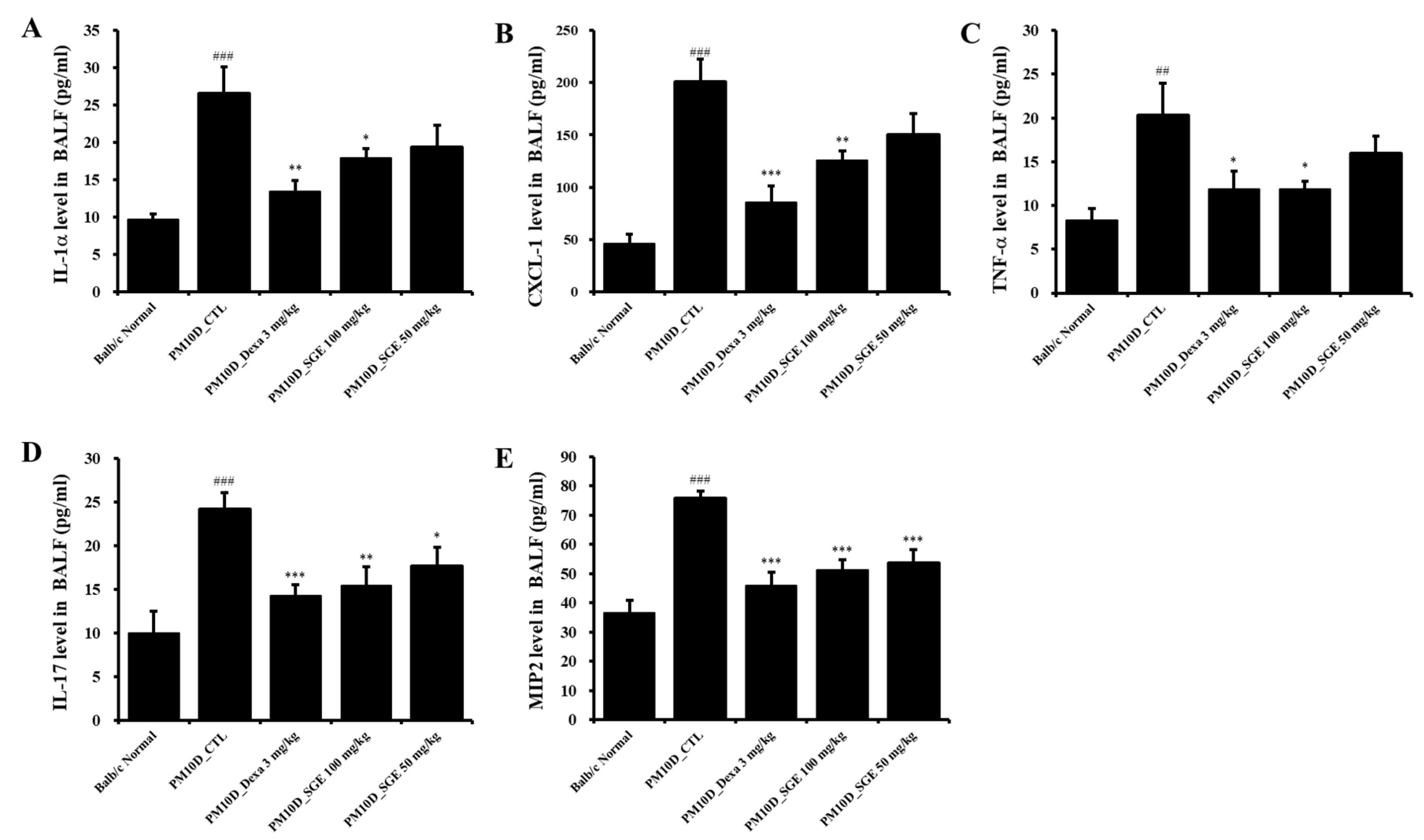
Inflammatory cytokines and chemokines produced by the inflammatory response contribute to the pathology and severity of airway inflammation. IL-1a, CXCL-1, TNF- a, IL-17, and MIP-2 levels in BALF were increased after PM10D exposure compared with normals and were significantly decreased by administration of SGE or dexamethasone (
Figure 3A–E).
3.4. Effect of SGE on lung histopathology
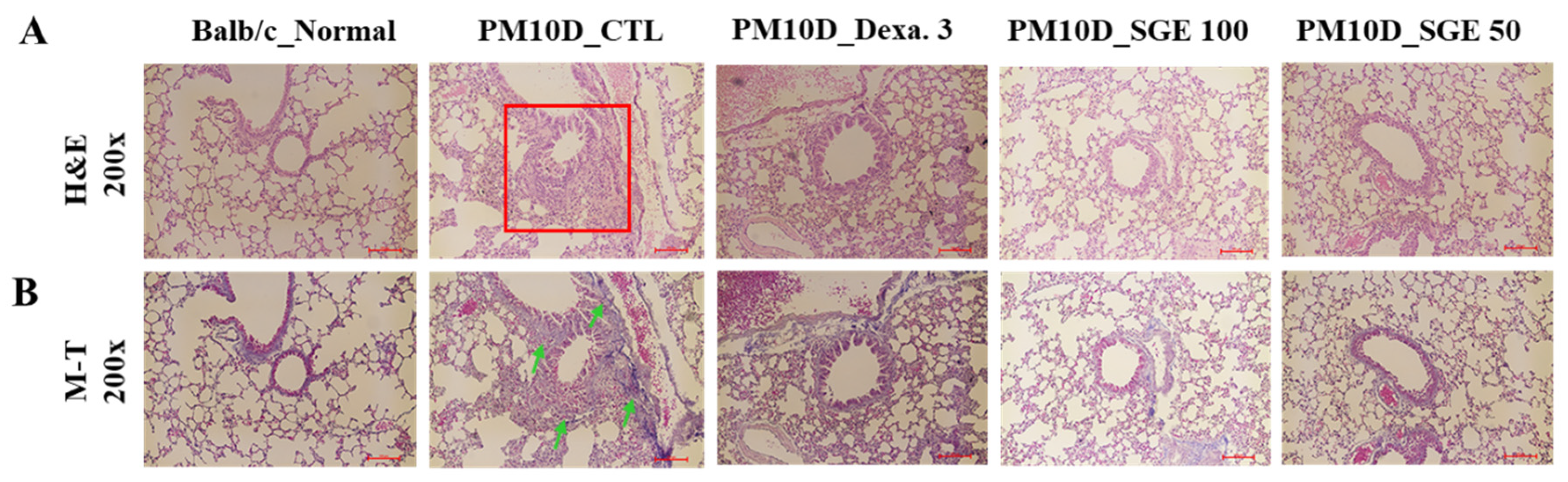
To examine the effect of SGE on lung histopathology in PM10D-induced airway inflammation, lung tissue was dyed with Masson’s trichrome or hematoxylin and eosin stain solutions. We observed thickening of the airway wall with infiltration of inflammatory cells around the airway and collagen fibrosis in the lungs of PM10D-exposed mice. In contrast, lung sections from mice treated with SGE or dexamethasone had reduced inflammatory cell infiltration and fibrosis (
Figure 4). These results show that SGE constrains histopathological changes to airway inflammation in the lungs of PM10D-exposed mice.
3.5. Effect of SGE on the expression of inflammatory mediators in lung tissue
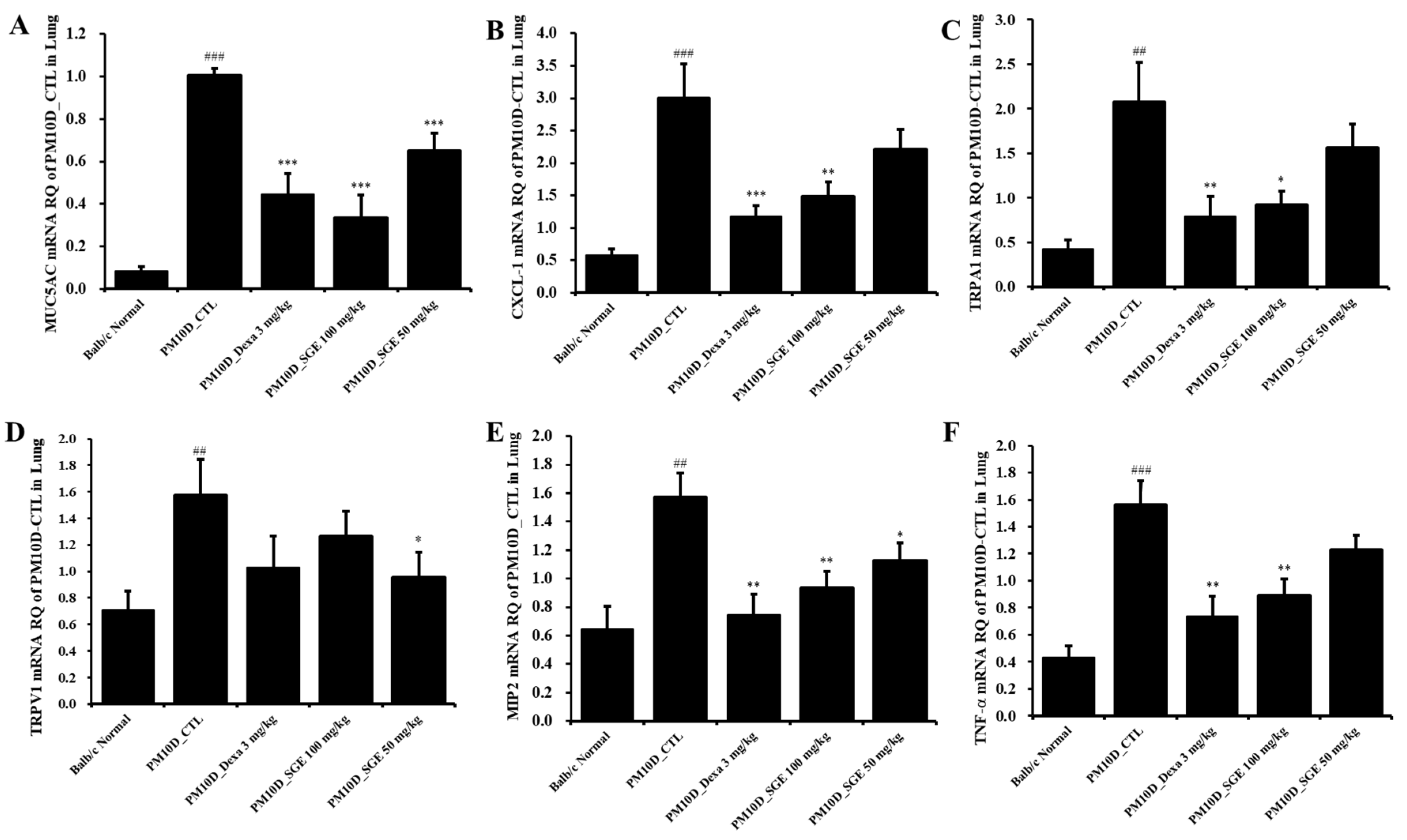
As shown in
Figure 5, the mRNA expression of mucin 5AC (MUC5AC), CXCL-1, transient receptor potential (TRP) vanilloid 1 (TRPV1), TRP ankyrin 1 (TRPA1), MIP2, and TNF- in lung tissue were increased after PM10D exposure compared with normals and were significantly decreased after administration of SGE or dexamethasone (
Figure 5).
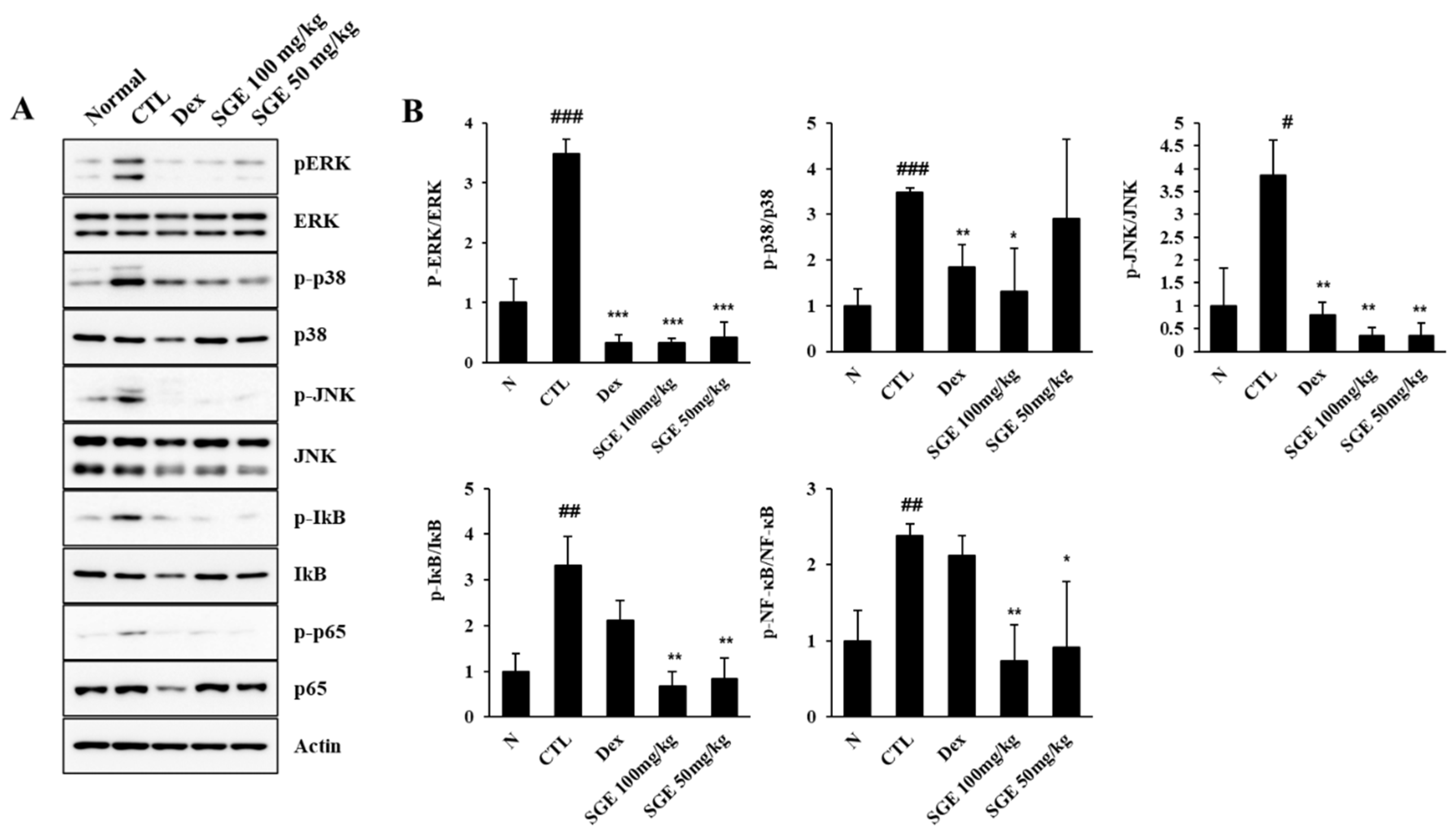
3.6. Effect of SGE on the mitogen-activated protein kinase/NF-κB signaling pathway in lung tissue
To identify the potential active signaling pathways controlling the inhibitory effect of SGE on airway inflammation in PM10D-exposed mice, we analyzed the activation of signaling molecules in the mitogen-activated protein kinase (MAPK)/NF-κB pathway (
Figure 6). The phosphorylation of JNK, p38, and ERK was increased after PM10D exposure and was decreased after SGE or dexamethasone administration. The phosphorylation of IκB and NF-κB-p65 was also upregulated after PM10D exposure and was reduced after SGE administration. These results indicate that the anti-inflammatory activity of SGE on airway inflammation is related with the MAPK/NF-κB (ERK, p38, and JNK) signaling pathway.
3.7. Effect of SGE on immune cell numbers in lung tissue and BALF
We investigated the effects of SGE on the change in immune cell numbers after PM10D exposure by flow cytometry analysis of BALF and lung tissue. Neutrophil numbers in BALF and lung were increased after PM10D exposure and were decreased after SGE administration (
Table 2). The absolute number of activated T lymphocytes (CD4+, CD8+, and CD62L-/CD44high+) and neutrophils (Gr-1+/SiglecF-) in BALF was increased after PM10D exposure and was reduced after administration of SGE or dexamethasone. Additionally, the absolute number of activated T cells (CD4+CD69+ and CD62L-/CD44high+), neutrophils (Gr-1+/SiglecF-), and myeloid cells (GR-1+CD11b+) in the lung was increased after PM10D exposure and was significantly decreased after administration of SGE or dexamethasone. These results indicate that SGE suppressed the airway immune response and neutrophilic airway inflammation observed after PM10D exposure.
4. Discussion
In this study, we observed the inhibitory effect of SGE on airway inflammation in a PM10D-induced chronic inflammatory disease mice model. SGE reduced the release of the inflammatory mediators IL-1a, TNF- a, CXCL-1, IL-17, and MIP2 in BALF. In addition, SGE decreased the mRNA expression of TRPA1, TRPV1, MUC5AC, and inflammatory cytokines (CXCL-1, MIP2, and TNF- a) and also reduced histopathological changes such as inflammatory cell infiltration and collagen fibrosis in the lungs of PM10D mice. SGE effectively decreased the neutrophils number in the WBC count, lung, and BALF. SGE administration in PM10D-exposed mice reduced the absolute cell number of CD8+ T cells, CD62L-/CD44high+ activated T cells, CD4+ T cells, and Gr-1+/SiglecF- neutrophils in BALF and decreased the cell number of activated CD4+CD69+ and CD62L-/CD44high+ T cells, Gr-1+/SiglecF- neutrophils, and GR-1+CD11b+ myeloid-derived suppressor cells in lung tissue.
Neutrophilic airway inflammation is crucial in the early and progressive stages of pulmonary illness, and IL-1R signaling by IL-1 a in the early stage drives neutrophilic inflammation and subsequent structural changes in the lungs and bronchi [
17]. Thus, the increased levels of IL-1 a in BALF of PM10D-exposed mice that we observed correlate with neutrophilic inflammation.
CD8+ and CD4+ T cells, the predominant activated T cell subtypes, lead to airway neutrophilic inflammation through the secretion of proinflammatory cytokines [
18]. CD4+ T cells cause the aggravation of chronic airway inflammation by producing IL-17 [
19]. Gr-1 is an indicator for granulocytes that is associated with the differentiation and maturation of granulocytes, and CD11b is an indicator of myeloid cells of the macrophage lineage [
20,
21]. Thus, the population of Gr-1+CD11b+ cells with Gr-1+/SiglecF- cells may constitute a substantial portion of neutrophils after PM10D exposure [
22,
23].
CXCL-1 and MIP2 (CXCL-2) are major neutrophils chemoattractants that were produced in the lung in an airway inflammation model induced by DEP exposure; an increase in the pulmonary expression of these C-X-C chemokines exacerbated airway inflammation [
23]. MUC5AC, a key constituent of respiratory mucin secreted in the bronchial epithelia, contributes to airway mucus hypersecretion in respiratory illnesses such as asthma and COPD [
24,
25]. Both TRPV1 and TRPA1, members of the TRP channel superfamily, play a key part in lung inflammation through the release of neuropeptides, such as substance P, and inflammatory factors, such as leukotriene, IL-1 a, and TNF- a, which cause primary airway inflammation [
26]. Activation of these TRP channels by exposure to environmental lung-toxic irritants such as PM, DEP, and cigarette smoke, causes cough by stimulation of nociceptive C-fibers in the airways of humans and animals, and TRP channel-induced neurogenic inflammation could lead to the progression of airway inflammatory illnesses such as lung fibrosis, COPD, and allergic asthma [
22,
27,
28,
29].
Exposure to environmental hazards such as PM, DEP, and cigarette smoke stimulate proinflammatory mediators such as IL-1 and TNF- a that activate the NF-kB transcription factor or MAPK signaling molecules. This process leads to lung inflammation with neutrophil recruitment to the lung via pulmonary expression of cytokines and neutrophil chemokines such as CXCL-1 and MIP2 [
30,
31,
32]. Our results show that SGE alleviates neutrophilic airway inflammation by preventing NF-kB and ERK/p38/JNK MAPK signaling.
A limitation of our studies is that we did not test the effects of SGE in both sexes of mice. Recently, studies have reported that in human, females are more susceptible than males to airway inflammation caused by environmental air pollution owing to sex hormone differences [
33]. Thus, the effect of SGE in female mice should be tested in a further study. Nevertheless, our studies show that SGE could be a promising candidate to prevent respiratory illness by ameliorating airway inflammation caused by PM10D.
5. Conclusions
This study showed that SGE ameliorates neutrophilic airway inflammation and lung injury by downregulation of NF-kB and MAPK signaling pathways in a PM10D-induced respiratory disease murine model. These results suggest that SGE would be a promising candidate for treatment of respiratory disorders.
Author Contributions
Conceptualization, Y.-Y.S. and D.-S.K.; methodology, S.-H.K.; validation, Y.-Y.S.; investigation, M.-S.K., H.J.Y., S.-H.K., and W.-K.Y.; resources, D.-S.K., G.D.P., K.S.K. and W.J.H; data curation, Y.-Y.S.; writing—original draft preparation, Y.-Y.S.; writing—review and editing, Y.-Y.S.; visualization, Y.-Y.S.; supervision, D.-S.K.; funding acquisition, Y.-Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the INNOPOLIS Foundation of Korea, grant number 2022-DD-RD-0097.
Institutional Review Board Statement
The approval of studies was carried out by the Committee for Animal Welfare at Daejeon University (ethical approval code DJUARB2022-041), and experiment was performed in accordance with the Guide for the Care and Use of Laboratory Animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kyung, S.Y.; Jeong, S.H. Particulate-Matter Related Respiratory Diseases. Tuberc. Respir. Dis. 2020, 83, 116–121. [Google Scholar] [CrossRef]
- Dockery, D.W.; Pope, C.A.; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; et al. An association between air pollution mortality in six US cities, N. Engl. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef]
- Abbey, D.E.; Burchette, R.J.; Knutsen, S.F.; McDonnell, W.F.; Lebowitz, M.D.; Enright, P.L. Long-term particulate and other air pollutants and lung function in nonsmokers. Am. J. Respir. Crit. Care. Med. 1998, 158, 289–298. [Google Scholar] [CrossRef]
- Pope, C.A. , Burnett, R.T., Thun, M.J., Calle, E.E., Krewski, D., Ito, K., et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002, 287, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, G.; Tian, L.; Guo, Q.; Pan, X. Short-term exposure to air pollution and morbidity of COPD and asthma in East Asian area: a systematic review and meta-analysis. Environ. Res. 2016, 148, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, H.E. Diesel exhaust particles. Inhal. Toxicol. 2007, 19 (Suppl. S1), 241–244. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M. Adenophora stricta root extract protects lung injury from exposure to Particulate Matter 2.5 in mice. Antioxidants 2022, 11, 1376. [Google Scholar] [CrossRef] [PubMed]
- Kinghora, A.D.; Soejarto, D.D.; Inglett, G.E. Sweetening agents of plant origin. Crit. Rev. Plant Sci. 1986, 4, 79–120. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Qi, X.; Zou, J.; Sun, Z. Antiglycation and antioxidant activities of mogroside extract from Siraitia grosvenorii (Swingle) fruits. J. Food Sci. Technol. 2018, 5, 1880–1888. [Google Scholar] [CrossRef]
- Chen, G.; Liu, C.; Meng, G.; Zhang, C.; Chen, F.; Tang, S.; Hong, H.; Zhang, C. Neuroprotective effect of mogrol against Aβ1–42-induced memory impairment neuroinflammation and apoptosis in mice. J. Pharm. Pharmacol. 2019, 71, 869–877. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Romeih, E.; Huang, Z.; Enomoto, T.; Huang, L.; Li, L. Bioactive properties of probiotic set-yogurt sup-plemented with Siraitia grosvenorii fruit extract. Food chemistry 2020, 303, 125400. [Google Scholar] [CrossRef]
- Sung, Y.Y.; Kim, S.H.; Yuk, H.J.; Yang, W.K.; Lee, Y.M.; Son, E.; Kim, D.S. Siraitia grosvenorii residual extract attenuates ovalbumin-induced lung inflammation by down-regulating IL-4, IL-5, IL-13, IL-17, and MUC5AC expression in mice. Phytomedicine 2019, 61, 152835. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.Y.; Yuk, H.J.; Yang, W.K.; Kim, S.H.; Kim, D.S. Siraitia grosvenorii Residual Extract Attenuates Atopic Dermatitis by Regulating Immune Dysfunction and Skin Barrier Abnormality. Nutrients 2020, 12, 3638. [Google Scholar] [CrossRef] [PubMed]
- Song, J.L.; Qian, B.; Pan, C.; Lv, F.; Wang, H.; Gao, Y.; Zhou, Y. Protective activity of mogroside V against ovalbumin-induced experimental allergic asthma in Kunming mice. J. Food Biochem. 2019, 43, e12973. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, D.S.; Yuk, H.J.; Kim, S.H.; Yang, W.K.; Park, G.D.; Kim, K.S.; Ham, W.J.; Sung, Y.Y. Siraitia grosvenorii extract attenuates airway inflammation in a murine model of chronic obstructive pulmonary disease induced by cigarette smoke and lipopolysaccharide. Nutrients 2023, 15, 468. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Yang, W.K.; Park, Y.R.; Park, Y.C.; Park, I.J.; Lee, G.J.; Kang, H.S.; Kim, B.K.; Kim, S.H. Opuntia ficus-indica Alleviates Particulate Matter 10 Plus Diesel Exhaust Particles (PM10D)-Induced Airway Inflammation by Suppressing the Expression of Inflammatory Cytokines and Chemokines. Plants (Basel) 2022, 11, 520. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, S.T.; Dittrich, A.S.; Garratt, L.W.; Turkovic, L.; Frey, D.L.; Stick, S.M.; Mall, M.A.; Kicic, A. ; AREST; CF Interleukin-1 is associated with inflammation and structural lung disease in young children with cystic fibrosis. J. Cyst. Fibros. 2018, 17, 715–722. [Google Scholar] [CrossRef]
- Schaller, M.A.; Lundy, S.K.; Huffnagle, G.B. ; Lukacs NWCD8+ T cell contributions to allergen induced pulmonary inflammation airway hyperreactivity. Eur J. Immunol. 2005, 35, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Voo, K.S.; Liu, B.; Chen, C.Y.; Uygungil, B.; Spoede, W.; Bernstein, J.A.; Huston, D.P.; Liu, Y.J. A novel subset of CD4+ Th2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J. Exp. Med. 2010, 207, 2479–2491. [Google Scholar] [CrossRef]
- Fleming, T.J.; Fleming, M.L.; Malek, T.R. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J. Immunol. 1993, 151, 2399–2408. [Google Scholar] [CrossRef]
- Yachie, A.; Toma, T.; Miyawaki, T.; Taniguchi, N. Expression of surface CD11b antigen and eosinophil activation. Nippon. Rinsho. 1993, 51, 593–597. [Google Scholar] [PubMed]
- Suzuki, E.; Maverakis, E.; Sarin, R.; Bouchareychas, L.; Kuchroo, V.K.; Nestle, F.O.; Adamopoulos, I.E. T Cell-independent mechanisms associated with neutrophil extracellular trap formation and selective autophagy in IL-17A-mediated epidermal hyperplasia. J. Immunol. 2016, 197, 4403–4412. [Google Scholar] [CrossRef]
- Kim, J.; Natarajan, S.; Vaickus, L.J.; Bouchard, J.C.; Beal, D.; Cruikshank, W.W.; Remick, D.G. Diesel exhaust particulates exacerbate asthma-like inflammation by increasing CXC chemokines. Am. J. Pathol. 2011, 179, 2730–2739. [Google Scholar] [CrossRef]
- Bonser, L.R.; Erle, D.J. Airway mucus and asthma: The role of MUC5AC and MUC5B. J.Clin. Med. 2017, 6, 112. [Google Scholar] [CrossRef]
- Li, J.; Ye, Z. The Potential Role and Regulatory Mechanisms of MUC5AC in Chronic Obstructive Pulmonary Disease. Molecules 2020, 25, 4437. [Google Scholar] [CrossRef] [PubMed]
- Xu, M. , Zhang, Y., Wang, M., Zhang, H., Chen, Y., Adcock, I.M., Chung, K.F., Mo, J., Zhang, Y., Li, F. TRPV1 and TRPA1 in Lung Inflammation and Airway Hyperresponsiveness Induced by Fine Particulate Matter (PM2.5). Oxid. Med. Cell. Longev. 2019, 2019, 7450151. [Google Scholar] [CrossRef]
- Grace, M.S.; Baxter, M.; Dubuis, E.; Birrell, M.A.; Belvisi, M.G. Transient receptor potential (TRP) channels in the airway: role in airway disease. Br. J. Pharmacol. 2014, 171, 2593–607. [Google Scholar] [CrossRef]
- Dietrich, A.; Steinritz, D.; Gudermann, T. Transient receptor potential (TRP) channels as molecular targets in lung toxicology and associated diseases. Cell Calcium 2017, 67, 123–137. [Google Scholar] [CrossRef]
- Bonvini, S.J.; Belvisi, M.G. Cough and airway disease: the role of ion channels. Pulm. Pharmacol. Ther. 2017, 47, 21–28. [Google Scholar] [CrossRef]
- Batra, S.; Balamayooran, G.; Sahoo, M.K. Nuclear Factor-κB: a key regulator in health and disease of lungs. Arch. Immunol. Ther. Exp (Warsz). 2011, 59, 335–351. [Google Scholar] [CrossRef]
- Jones, M.R.; Simms, B.T.; Lupa, M.M.; Kogan, M.S.; Mizgerd, J.P. Lung NF-κB activation and neutrophil recruitment require IL-1 and TNF receptor signaling during pneumococcal pneumonia. J. Immunol. 2005, 175, 7530–7535. [Google Scholar] [CrossRef]
- Park, S.M. Adenophora stricta root extract protects lung injury from exposure to Particulate Matter 2.5 in mice. Antioxidants 2022, 11, 1376. [Google Scholar] [CrossRef] [PubMed]
- Cabello, N.; Mishra, V.; Sinha, U.; DiAngelo, S.L.; Chroneos, Z.C.; Ekpa, N.A.; Cooper, T.K.; Caruso, C.R.; Silveyra, P. Sex differences in the expression of lung inflammatory mediators in response to ozone. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L1150–L1163. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Experimental setup and effect of Siraitia grosvenorii extract (SGE) on total and immune cell numbers in a particulate matter 10 plus diesel exhaust particles (PM10D)-induced airway inflammation model. (A) Experimental setup. Number of (B) total bronchoalveolar lavage fluid (BALF) cells, (C) total lung cells, and (D) neutrophils in (E) BALF cytospin (magnification: 200×). N = 8. ###p < 0.001 vs. Normal mice. *p < 0.05, **p < 0.01, and ***p < 0.001 vs. PM10D control (CTL) mice.
Figure 1.
Experimental setup and effect of Siraitia grosvenorii extract (SGE) on total and immune cell numbers in a particulate matter 10 plus diesel exhaust particles (PM10D)-induced airway inflammation model. (A) Experimental setup. Number of (B) total bronchoalveolar lavage fluid (BALF) cells, (C) total lung cells, and (D) neutrophils in (E) BALF cytospin (magnification: 200×). N = 8. ###p < 0.001 vs. Normal mice. *p < 0.05, **p < 0.01, and ***p < 0.001 vs. PM10D control (CTL) mice.
Figure 2.
E Effect of Siraitia grosvenorii extract (SGE) on (A) the number of white blood cells (WBC) and (B, C) WBC differential cells counting. N = 8. ###p < 0.001 vs. Normal mice. **p < 0.01, and ***p < 0.001 vs. PM10D control (CTL) mice.
Figure 2.
E Effect of Siraitia grosvenorii extract (SGE) on (A) the number of white blood cells (WBC) and (B, C) WBC differential cells counting. N = 8. ###p < 0.001 vs. Normal mice. **p < 0.01, and ***p < 0.001 vs. PM10D control (CTL) mice.
Figure 3.
Effect of Siraitia grosvenorii extract (SGE) on the release of cytokines and chemokines in bronchoalveolar lavage fluid (BALF) of a particulate matter 10 plus diesel exhaust particles (PM10D)-induced airway inflammation model. BALF production of (A) IL-1a, (B) CXCL-1, (C) TNF-a, (D) IL-17, and (E) MIP2 (n = 8). ##p < 0.01, and ###p < 0.001 vs. normal mice. *p < 0.05, **p < 0.01, and ***p < 0.001 vs. PM10D control (CTL) mice.
Figure 3.
Effect of Siraitia grosvenorii extract (SGE) on the release of cytokines and chemokines in bronchoalveolar lavage fluid (BALF) of a particulate matter 10 plus diesel exhaust particles (PM10D)-induced airway inflammation model. BALF production of (A) IL-1a, (B) CXCL-1, (C) TNF-a, (D) IL-17, and (E) MIP2 (n = 8). ##p < 0.01, and ###p < 0.001 vs. normal mice. *p < 0.05, **p < 0.01, and ***p < 0.001 vs. PM10D control (CTL) mice.
Figure 4.
Lung histopathology. (A) Hematoxylin and eosin (H&E) staining and (B) Masson’s trichrome (M-T) staining of the lung tissue of mice with particulate matter 10 plus diesel exhaust particles (PM10D)-induced airway inflammation (magnification: 200×).
Figure 4.
Lung histopathology. (A) Hematoxylin and eosin (H&E) staining and (B) Masson’s trichrome (M-T) staining of the lung tissue of mice with particulate matter 10 plus diesel exhaust particles (PM10D)-induced airway inflammation (magnification: 200×).
Figure 5.
Effect of Siraitia grosvenorii extract (SGE) on the mRNA expression of airway inflammation-related genes in the lung tissue of mice with particulate matter 10 plus diesel exhaust particles (PM10D)-induced airway inflammation. The mRNA expression levels of (A) MUC5AC, (B) CXCL-1, (C) TRPA1, (D) TRPV1, (E) MIP2, and (F) TNF-a (n=8). ##p < 0.01, and ###p < 0.001 vs. normal mice. *p < 0.05, **p < 0.01, and ***p < 0.001 vs. PM10D control (CTL) mice.
Figure 5.
Effect of Siraitia grosvenorii extract (SGE) on the mRNA expression of airway inflammation-related genes in the lung tissue of mice with particulate matter 10 plus diesel exhaust particles (PM10D)-induced airway inflammation. The mRNA expression levels of (A) MUC5AC, (B) CXCL-1, (C) TRPA1, (D) TRPV1, (E) MIP2, and (F) TNF-a (n=8). ##p < 0.01, and ###p < 0.001 vs. normal mice. *p < 0.05, **p < 0.01, and ***p < 0.001 vs. PM10D control (CTL) mice.
Figure 6.
Effect of Siraitia grosvenorii extract (SGE) on particulate matter 10 plus diesel exhaust particles (PM10D)-induced mitogen-activated protein kinase (MAPK)/nuclear factor-kappa B (NF-κB) signaling in the lung tissue of mice with PM10D-induced airway inflammation. (A) Protein expression of pERK, ERK, p-p38, p38, p-JNK, JNK, p-p65, p65, and b-actin, and (B) quantitative analysis of protein bands was performed with ImageJ (n = 8). #p < 0.05, ##p < 0.01, and ###p < 0.001 vs. normal mice. *p < 0.05, **p < 0.01, and ***p < 0.001 vs. PM10D control (CTL) mice.
Figure 6.
Effect of Siraitia grosvenorii extract (SGE) on particulate matter 10 plus diesel exhaust particles (PM10D)-induced mitogen-activated protein kinase (MAPK)/nuclear factor-kappa B (NF-κB) signaling in the lung tissue of mice with PM10D-induced airway inflammation. (A) Protein expression of pERK, ERK, p-p38, p38, p-JNK, JNK, p-p65, p65, and b-actin, and (B) quantitative analysis of protein bands was performed with ImageJ (n = 8). #p < 0.05, ##p < 0.01, and ###p < 0.001 vs. normal mice. *p < 0.05, **p < 0.01, and ***p < 0.001 vs. PM10D control (CTL) mice.
Table 1.
Primer sequences used for quantitative reverse transcription-polymerase chain reaction.
Table 1.
Primer sequences used for quantitative reverse transcription-polymerase chain reaction.
| Gene |
Primer direction |
Oligonucleotide sequence (5′-3′) |
| b-actin |
F |
TGGAATCCTGTGGCATCCAT |
| R |
TAAAACGCAGCTCGTAACAG |
| TNF-α |
F |
CCTGTAGCCCACGTCGTAGC |
| R |
TTGACCTCAGCGCTGAGTTG |
| MIP2 |
F |
ATGCCTGAAGACCCTGCCAAG |
| R |
GGTCAGTTAGCCTTGCCTTTG |
| CXCL-1 |
F |
CCGAAGTCATAGCCACAC |
| R |
GTGCCATCAGAGCAGTCT |
| MUC5AC |
F |
AGAATATCTTTCAGGACCCCT |
| R |
ACACCAGTGCTGAGCATACTT |
| TRPV1 |
F |
CATCTTCACCACGGCTGCTTAC |
| R |
CAGACAGGATCTCTCCAGTGAC |
| TRPA1 |
F |
TGAGATCGACCGGAGT |
| R |
TGCTGAAGGCATCTTG |
Table 2.
Effect of Siraitia grosvenorii extract (SGE) on immune cell subtypes in the bronchoalveolar lavage fluid and lung tissue of a particulate matter 10 plus diesel exhaust particles (PM10D)-induced airway inflammation model using flow cytometry analysis.
Table 2.
Effect of Siraitia grosvenorii extract (SGE) on immune cell subtypes in the bronchoalveolar lavage fluid and lung tissue of a particulate matter 10 plus diesel exhaust particles (PM10D)-induced airway inflammation model using flow cytometry analysis.
| Cell types |
Absolute no. (mean ± standard error of the mean) |
| Balb/c Normal |
PM10D-CTL |
PM10D-Dexa 3 mg/kg |
PM10D-SGE 100 mg/kg |
PM10D-SGE 50 mg/kg |
| BALF |
|
|
|
|
|
| Lymphocytes (×104 cells) |
2.73±0.68 |
7.45±1.76#
|
4.20±1.02 |
3.39±0.65*
|
4.90±1.94 |
| Neutrophils (×104 cells) |
5.41±1.14 |
53.09±6.44###
|
21.19±5.56**
|
11.53±2.39***
|
23.18±5.37**
|
| Eosinophils (×104 cells) |
11.74±3.89 |
48.90±12.93##
|
44.74±16.68 |
28.97±3.16 |
47.26±9.15 |
| CD4+ (×104 cells) |
0.55±0.25 |
31.56±8.11##
|
10.51±2.32**
|
7.71±1.00**
|
15.90±4.20 |
| CD8+ (×104 cells) |
0.10±0.06 |
17.01±2.15###
|
3.98±1.01***
|
6.69±2.30**
|
10.39±3.02 |
| CD62L-/CD44high+(×104cells) |
1.64±0.39 |
89.75±15.92###
|
55.28±15.35 |
25.55±3.39**
|
56.39±10.65 |
| Gr-1+SiglecF- (× 104 cells) |
1.09±0.53 |
53.18±9.61###
|
14.35±4.32**
|
7.63±2.02***
|
20.40±4.99**
|
| Lung |
|
|
|
|
|
| Lymphocytes (× 104 cells) |
14.46±1.96 |
24.06±4.26#
|
33.31±2.46 |
29.20±9.50 |
37.82±12.24 |
| Neutrophils (× 104 cells) |
24.15±5.70 |
81.34±17.00##
|
50.86±2.19 |
40.57±10.67*
|
51.09±10.56 |
| Eosinophils (× 104 cells) |
5.96±0.73 |
12.20±2.48#
|
12.53±0.55 |
12.55±4.09 |
12.70±3.01 |
| CD4+ (× 104 cells) |
15.94±3.51 |
34.79±5.47##
|
33.93±1.28 |
30.73±9.48 |
32.90±7.49 |
| CD8+ (× 104 cells) |
6.68±1.49 |
21.77±4.24##
|
17.45±0.79 |
14.79±3.55 |
15.70±4.68 |
| CD4+CD69+ (× 104 cells) |
1.38±0.44 |
4.06±0.67##
|
2.41±0.34*
|
2.13±0.59*
|
2.66±0.59 |
| CD62L-/CD44high+(× 104 cells) |
3.88±0.76 |
17.88±1.47###
|
9.00±1.01***
|
9.68±2.79**
|
11.82±3.51 |
| CD21+/CD35+B220+ (× 104 cells) |
4.33±2.15 |
21.08±4.84##
|
8.09±2.14*
|
10.40±3.27 |
14.78±5.39 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).