Submitted:
26 July 2023
Posted:
27 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Epidemiological Background of Bacterial Isolates
2.2. Susceptability to Acidic pH
2.3. Susceptability to PAA
2.3. Statistical Analysis
3. Results and Discussion
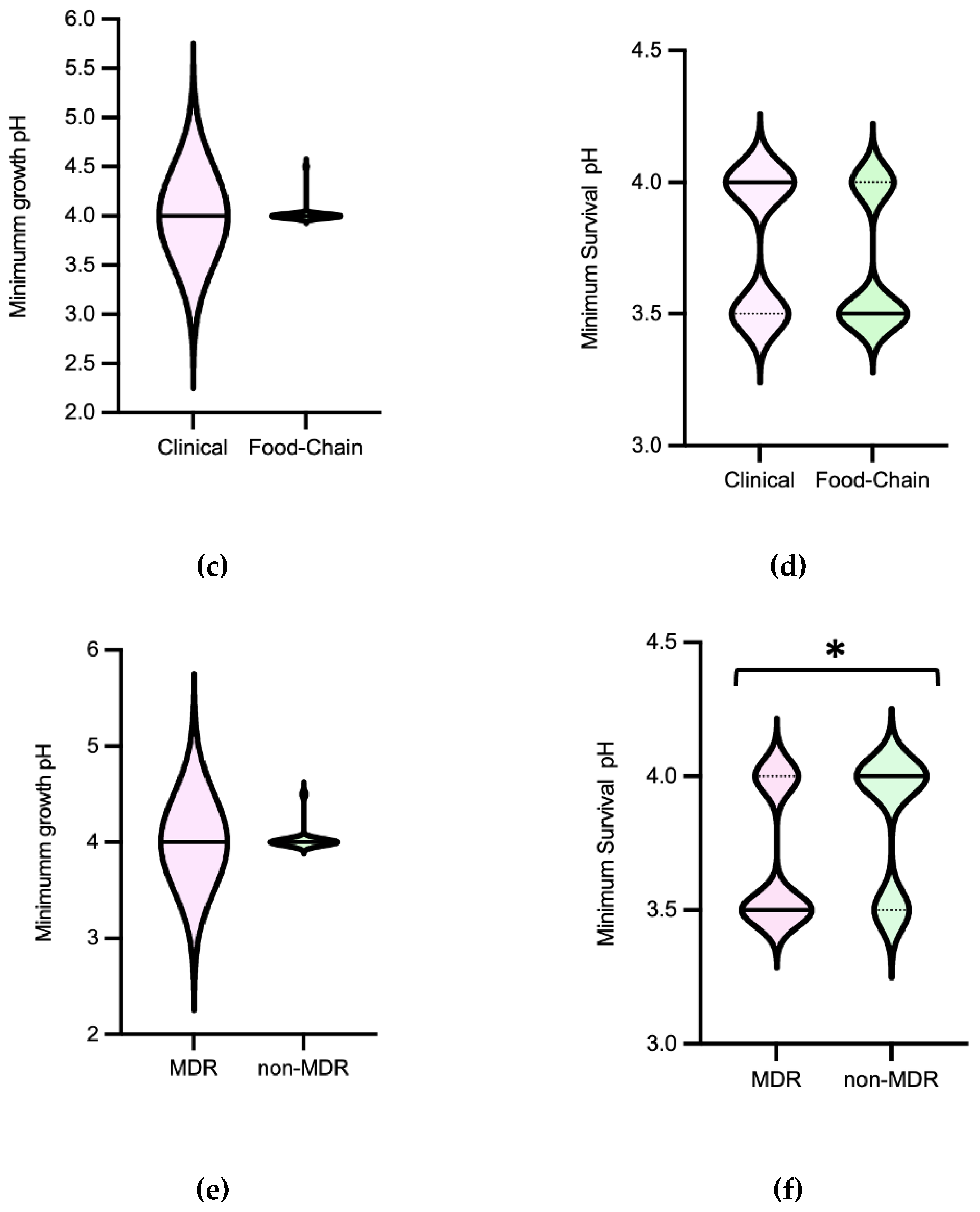
3.1. Susceptability to Acidic pH
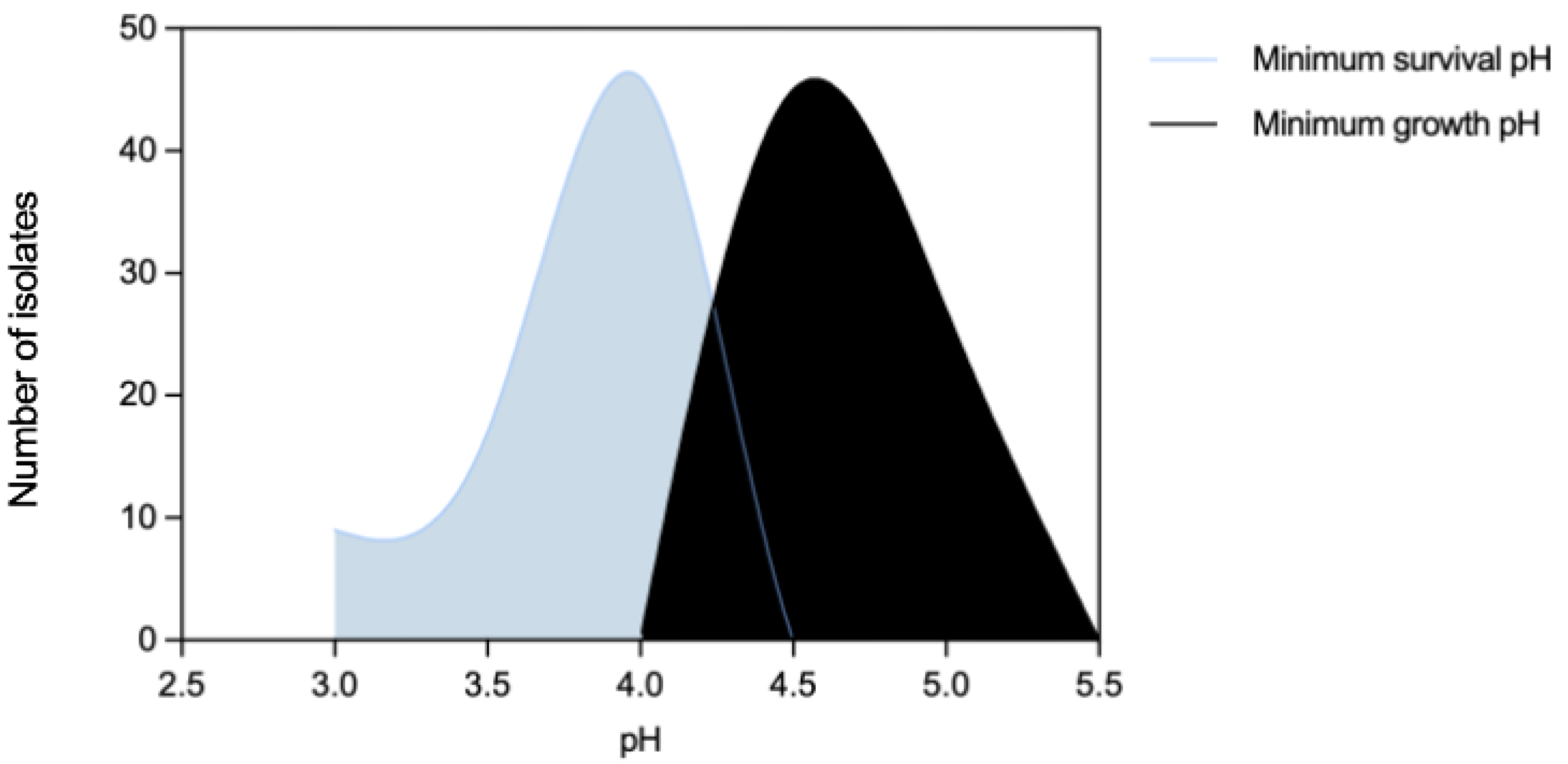
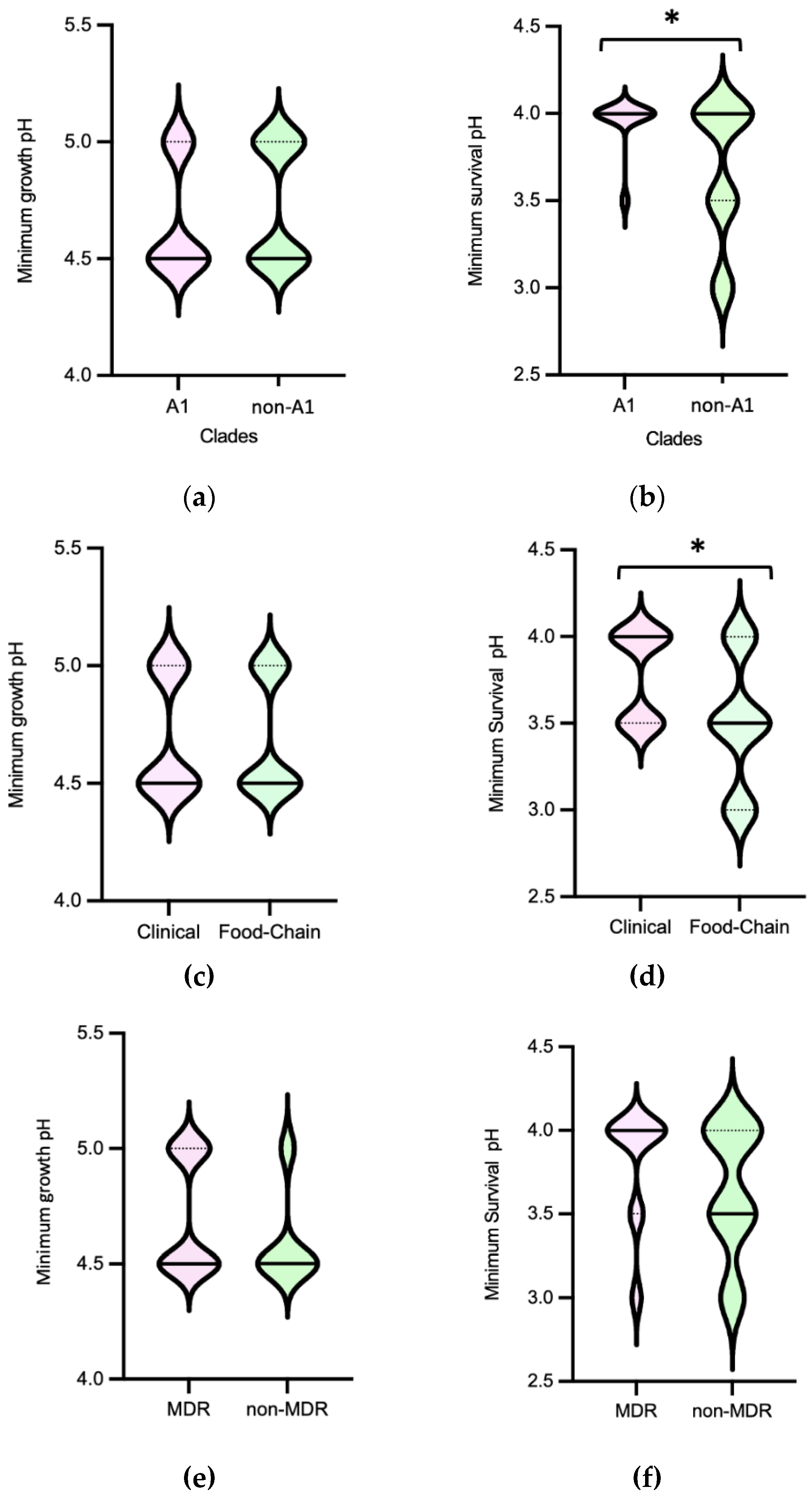
3.1.1. Enterococcus Faecium
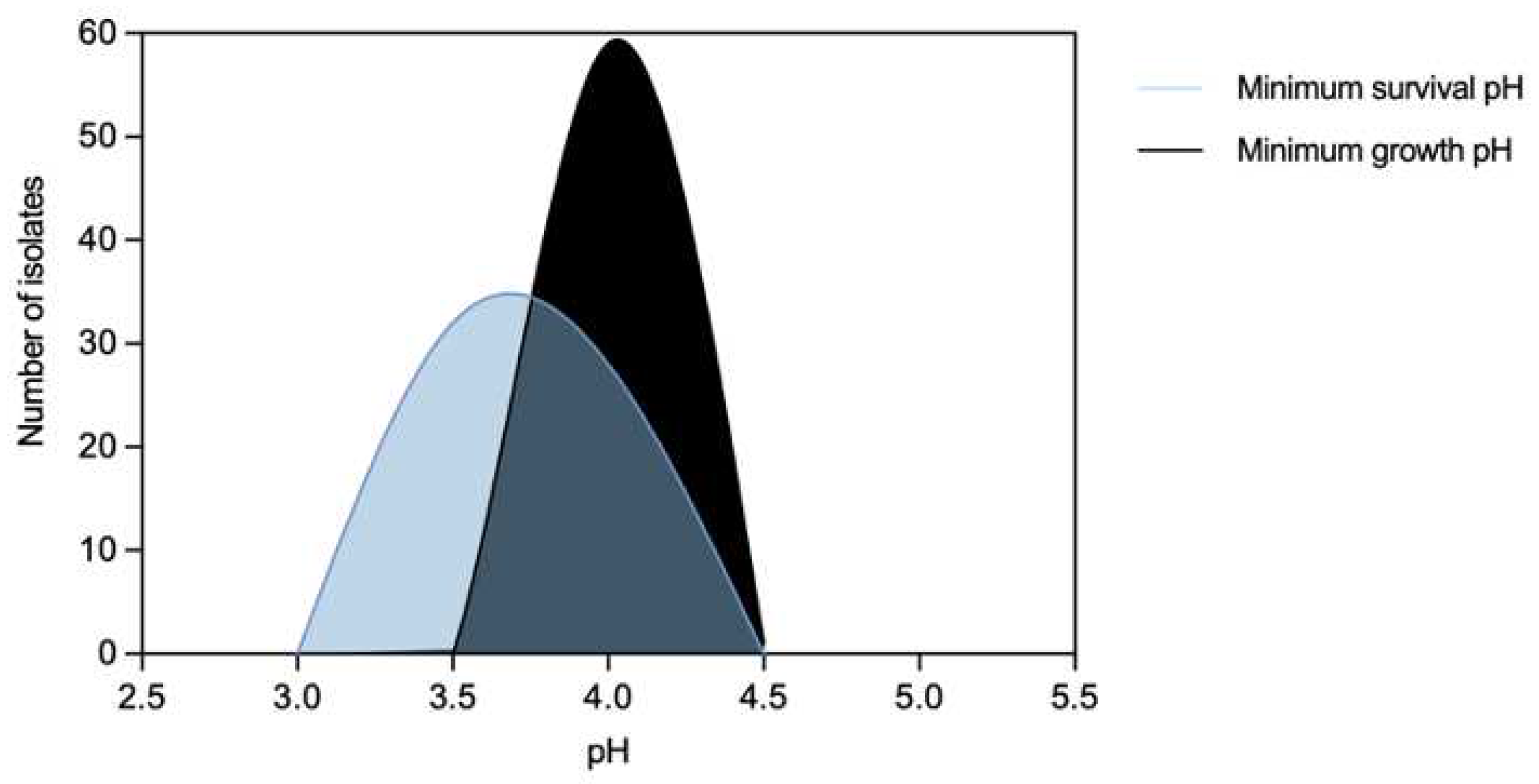
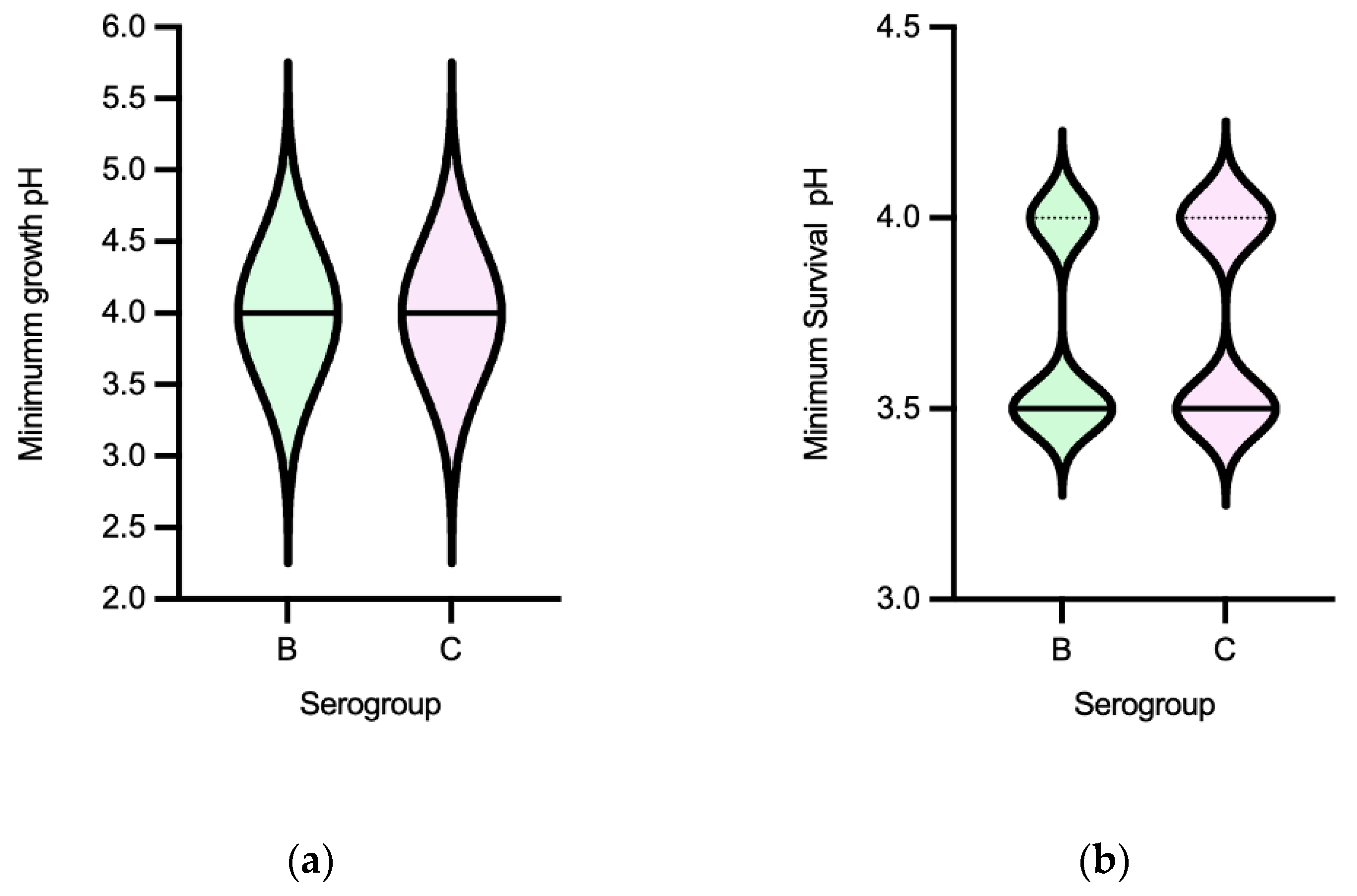
3.1.2. Non-Typhoidal Salmonella
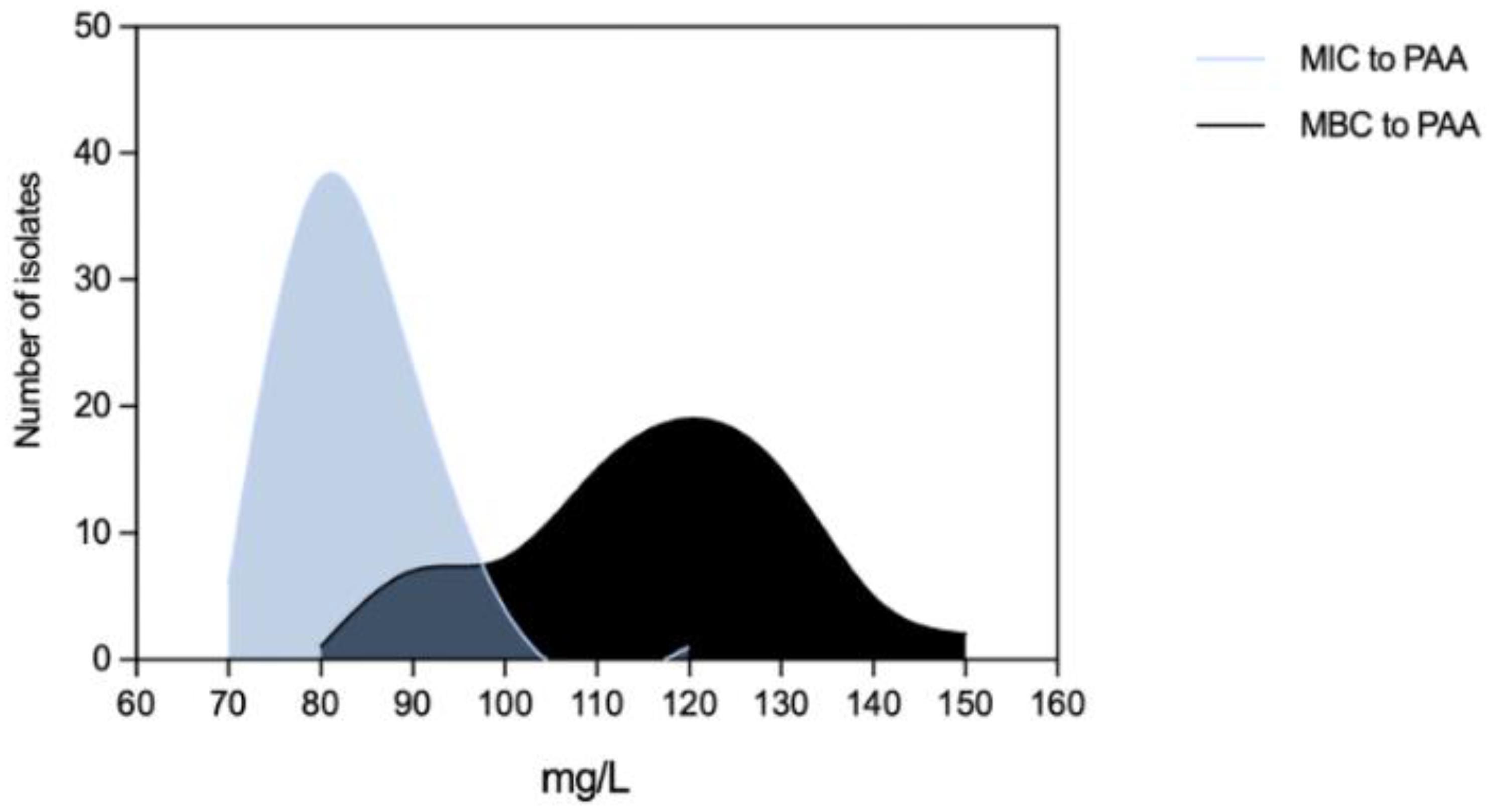
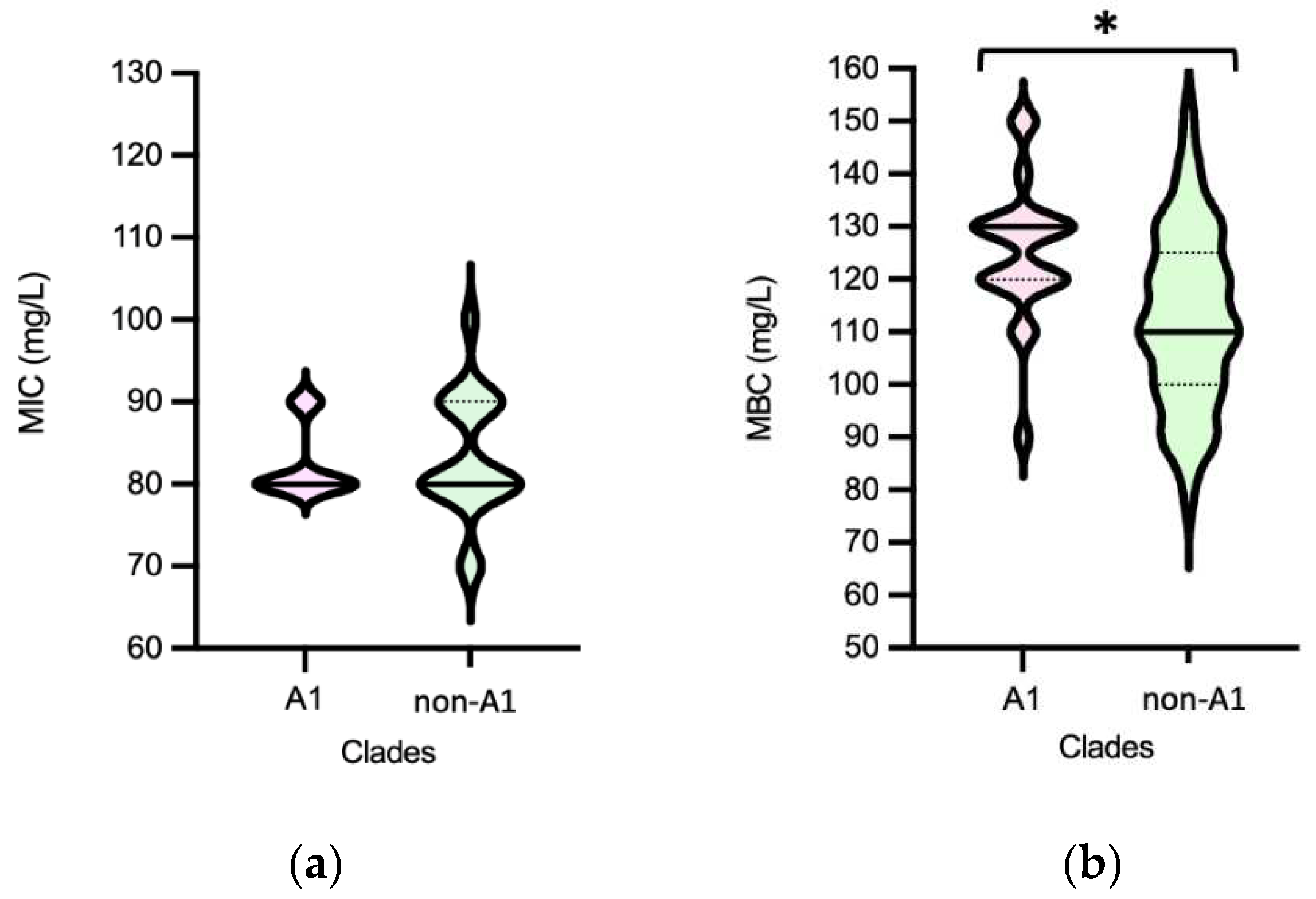
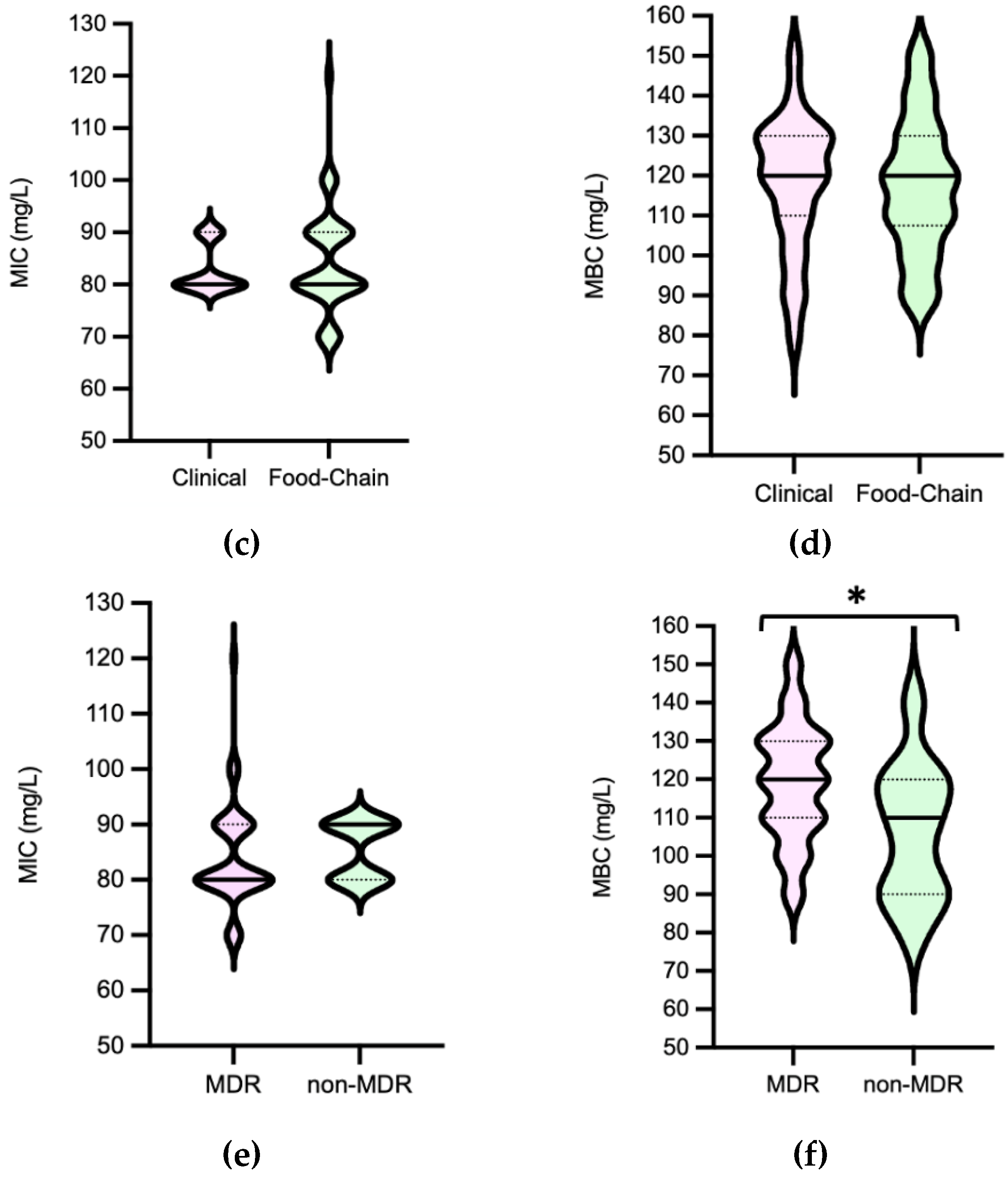
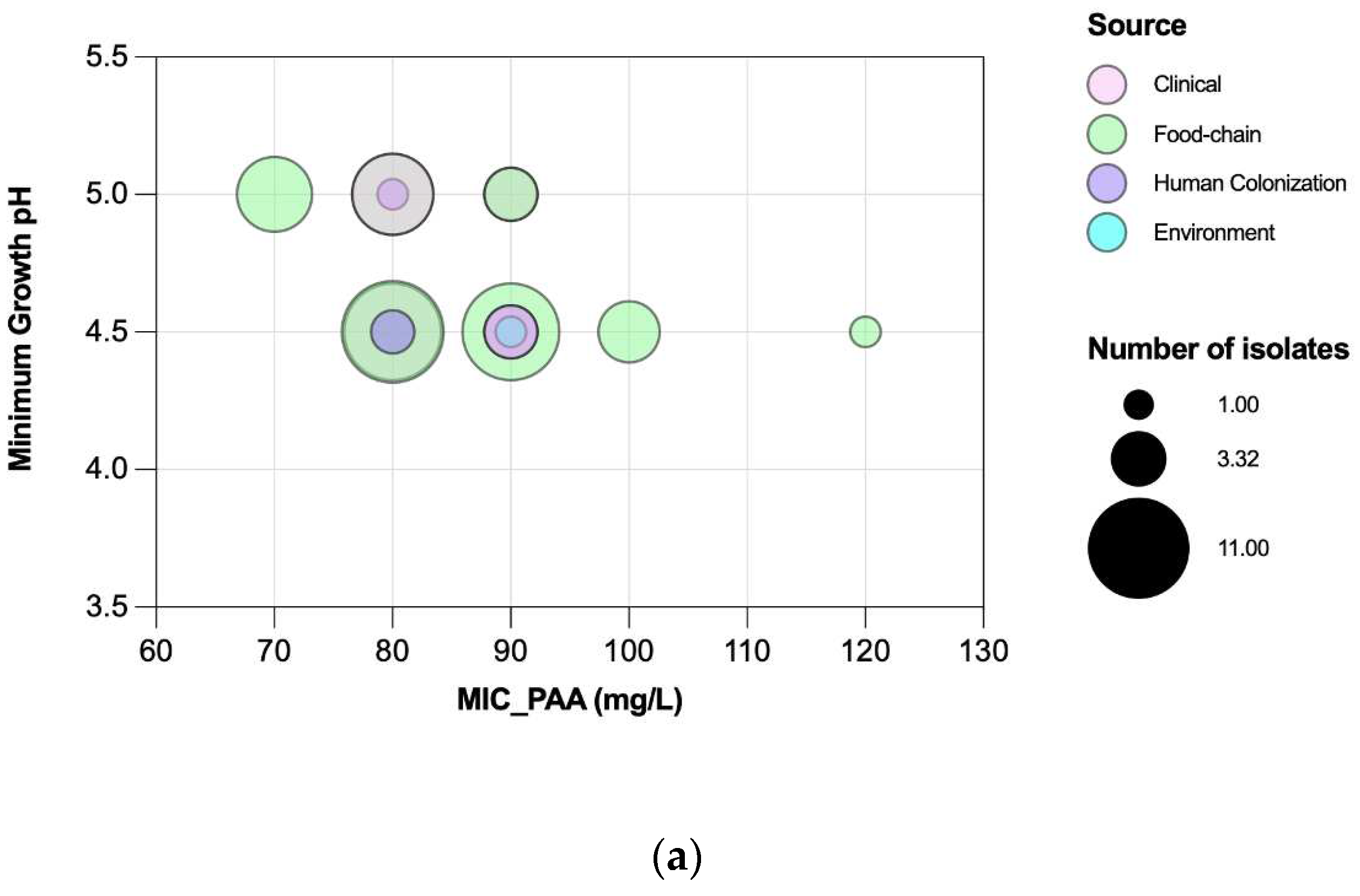
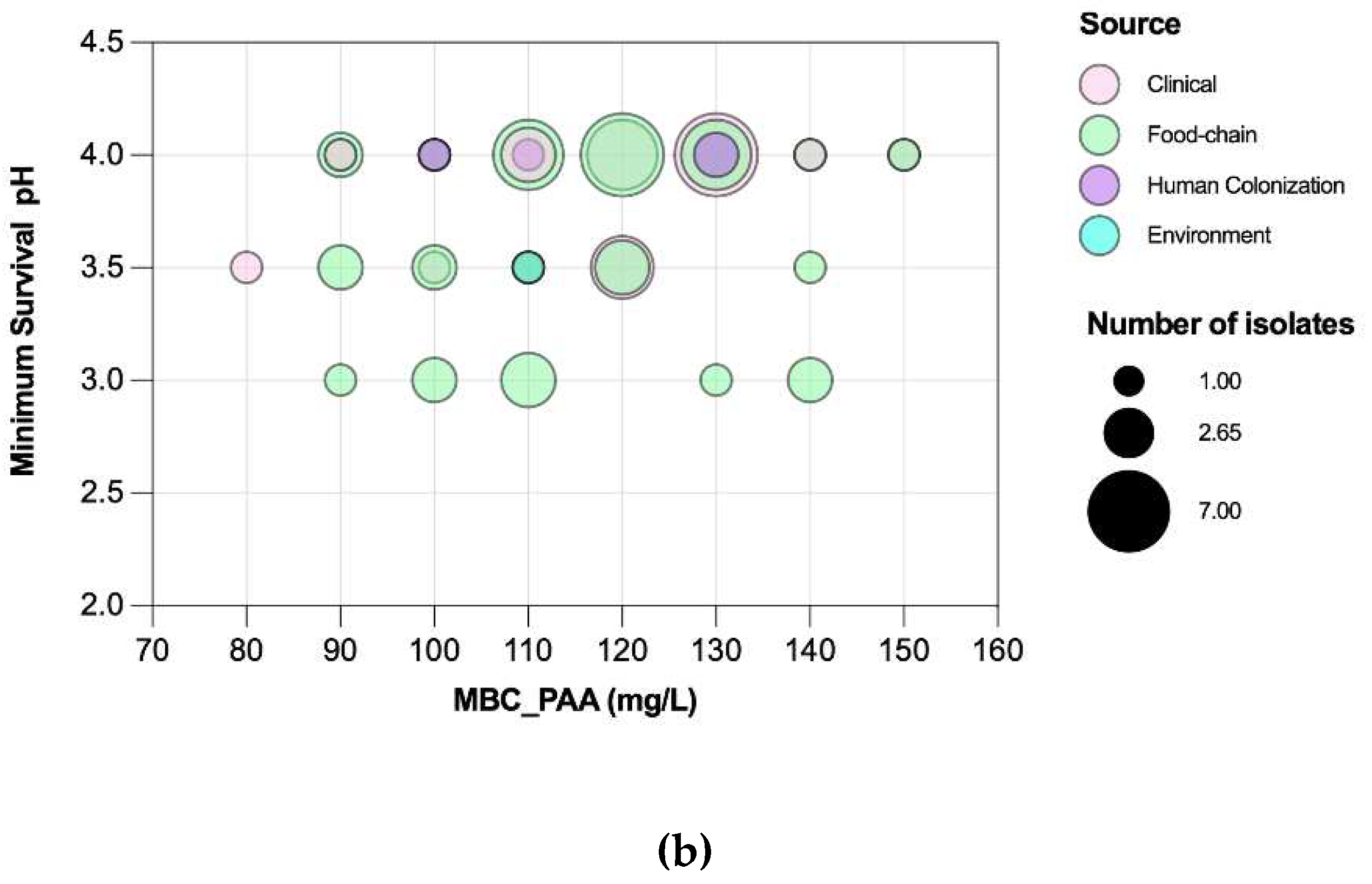
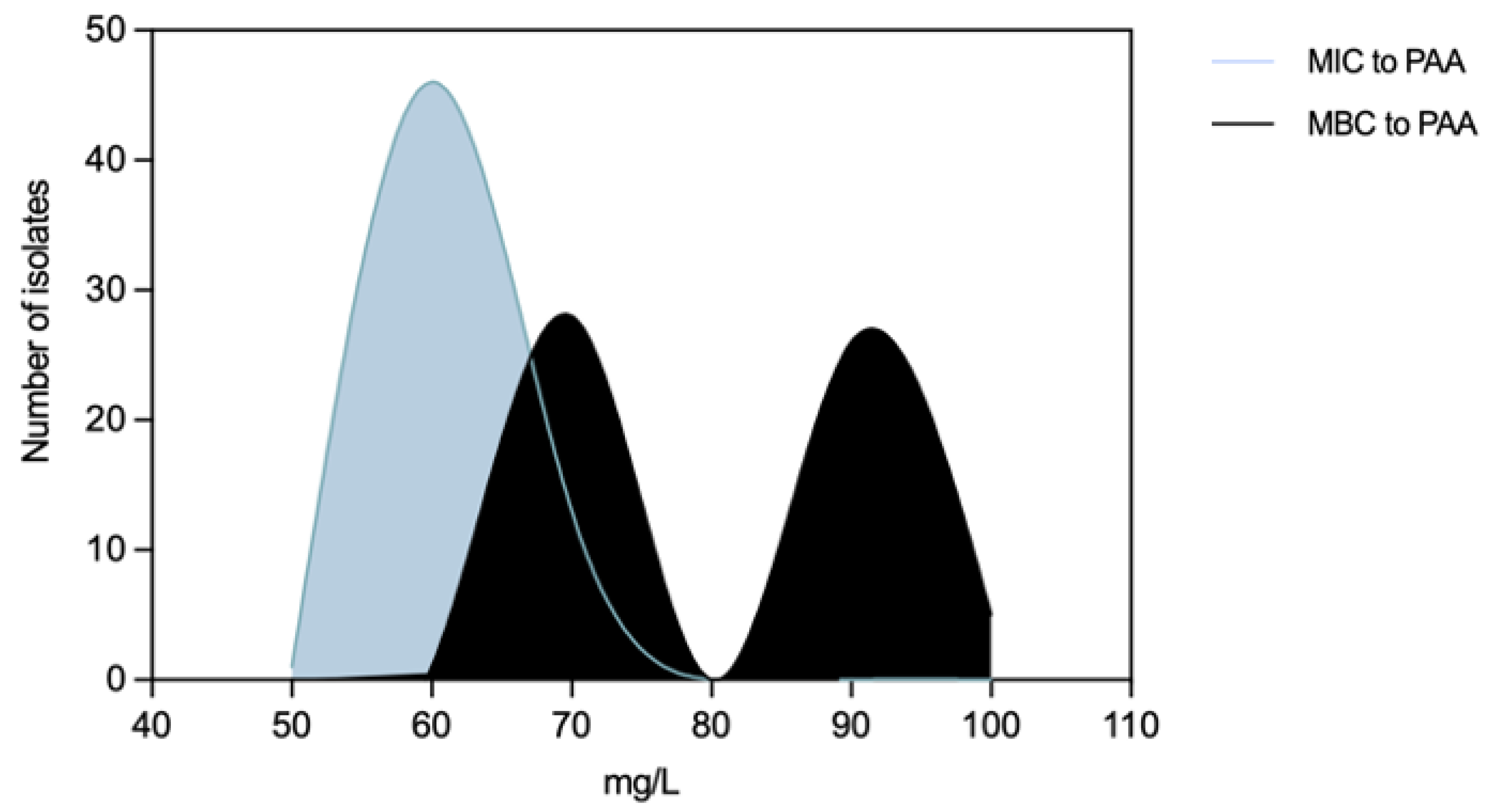
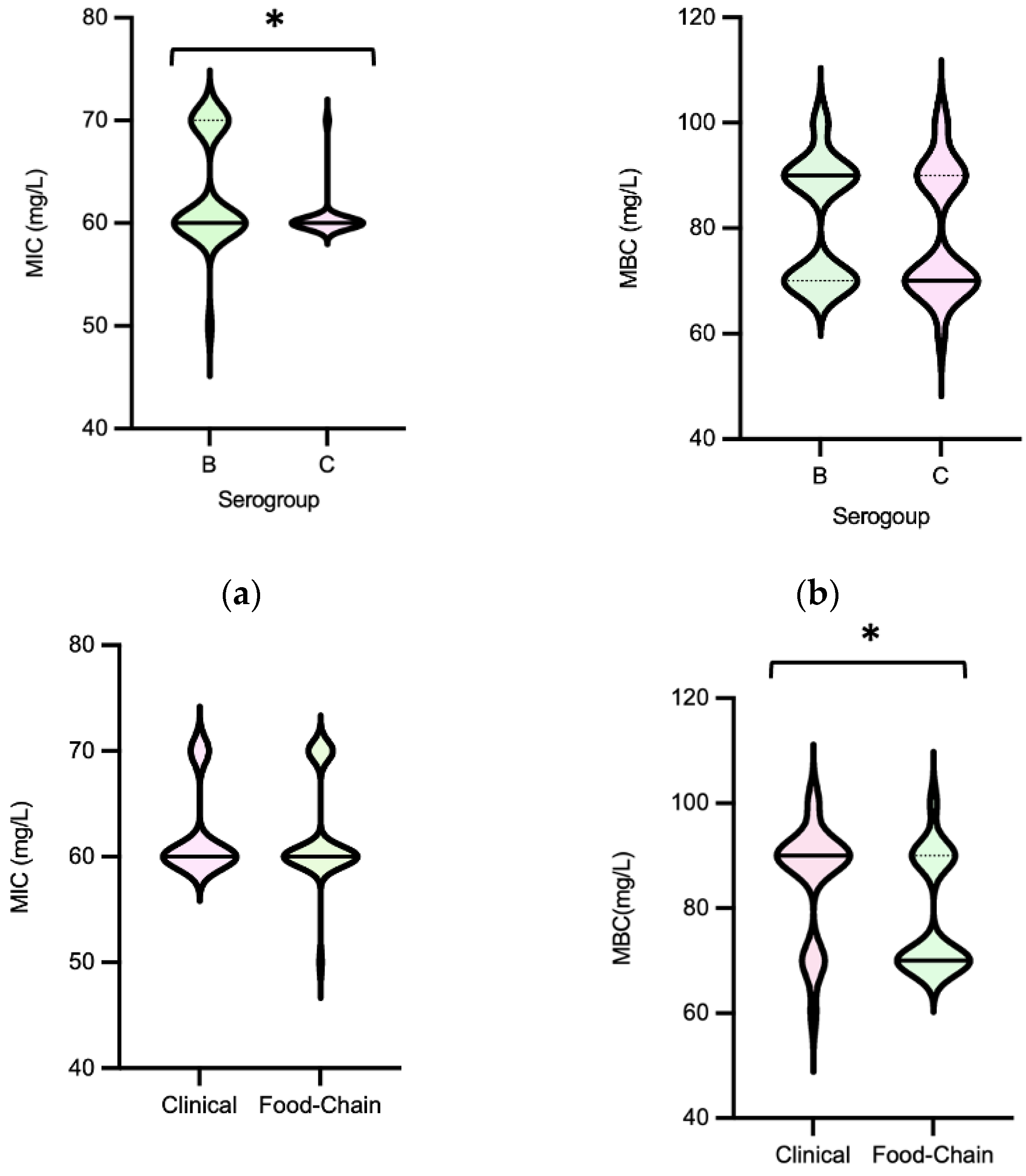
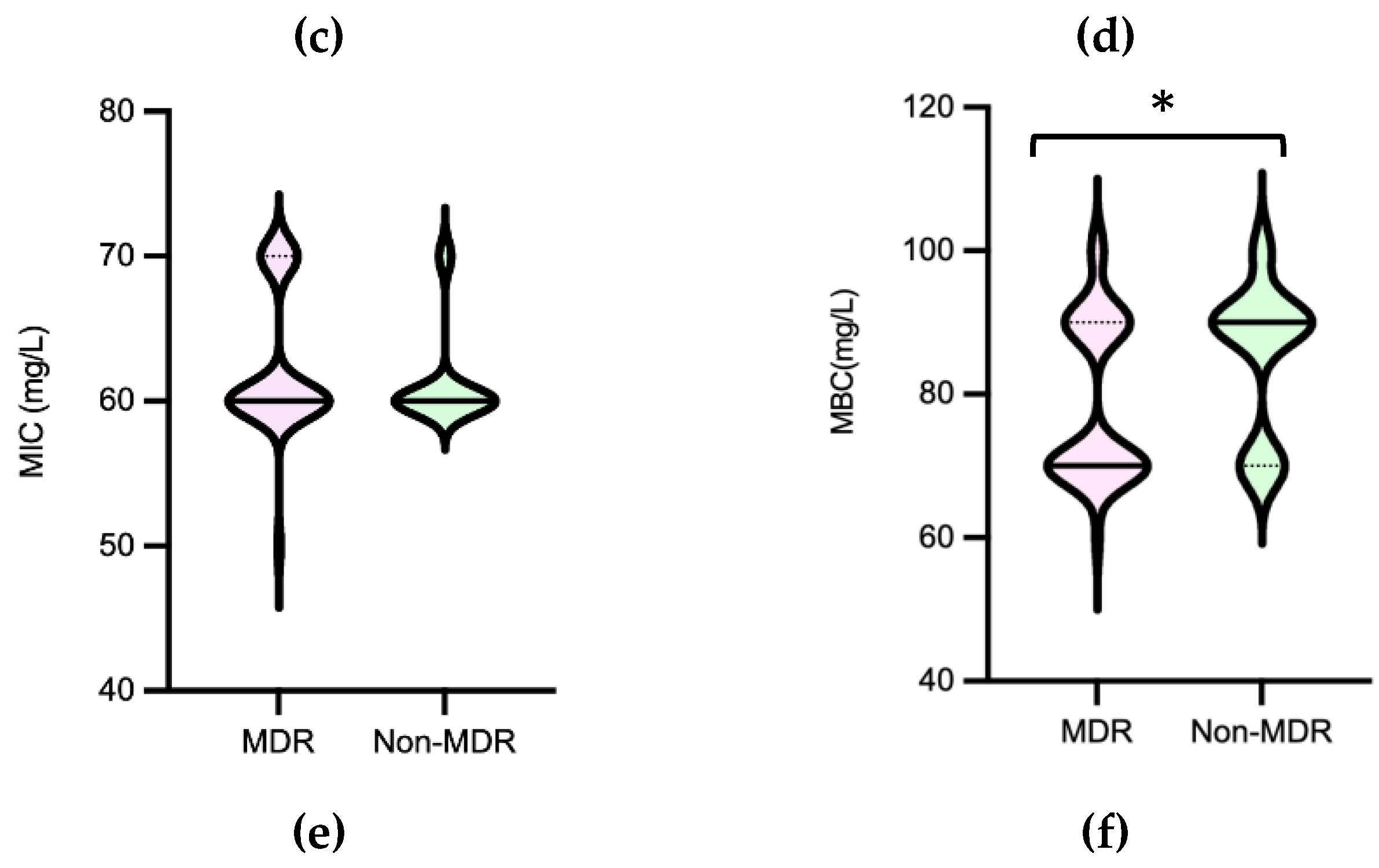
3.2. Susceptability to PAA
3.2.1. Enterococcus Faecium
3.2.2. Non-Typhoidal Salmonella
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lund, P.A.; De Biase, D.; Liran, O.; Scheler, O.; Mira, N.P.; Cetecioglu, Z.; Fernández, E.N.; Bover-Cid, S.; Hall, R.; Sauer, M.; et al. Understanding How Microorganisms Respond to Acid pH Is Central to Their Control and Successful Exploitation. Front. Microbiol. 2020, 11, 556140. [Google Scholar] [CrossRef]
- Ricke, S. Perspectives on the Use of Organic Acids and Short Chain Fatty Acids as Antimicrobials. Poult. Sci. 2003, 82, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Wessels, S.; Ingmer, H. Modes of Action of Three Disinfectant Active Substances: A Review. Regul. Toxicol. Pharmacol. 2013, 67, 456–467. [Google Scholar] [CrossRef] [PubMed]
- European Union Regulation (EC) No 1831/2003 of the European Parliament and of the council of 22 September 2003 on additives for use in animal nutrition, Off J Eur Union. 2003;L 268 Oct 18.
- Saleem, K.; Saima; Rahman, A. ; Pasha, T.N.; Mahmud, A.; Hayat, Z. Effects of Dietary Organic Acids on Performance, Cecal Microbiota, and Gut Morphology in Broilers. Trop. Anim. Health Prod. 2020, 52, 3589–3596. [Google Scholar] [CrossRef]
- Gadde, U.; Kim, W.H.; Oh, S.T.; Lillehoj, H.S. Alternatives to Antibiotics for Maximizing Growth Performance and Feed Efficiency in Poultry: A Review. Anim. Health Res. Rev. 2017, 18, 26–45. [Google Scholar] [CrossRef] [PubMed]
- European Chemicals Agency (ECHA); Biocidal Products Committee (BPC) Regulation (EU) No 528/2012 Concerning the Making Available on the Market and Use of Biocidal Products – Evaluation of Active Substances, Assessment Report of Peracetic Acid (Product Type 1-6). 2015.
- U.S. Food and Drug Administration Code of Federal Regulation. Title 21—Food and Drugs—Chemicals Used in Washing or to Assist in the Peeling of Fruits and Vegetables 2012.
- U.S. Department of Agriculture, Food Safety and Inspection Service Safe and Suitable Ingredients Used in the Production of Meat, Poultry, and Egg Products 2021.
- Petri, E.; Virto, R.; Mottura, M.; Parra, J. Comparison of Peracetic Acid and Chlorine Effectiveness during Fresh-Cut Vegetable Processing at Industrial Scale. J. Food Prot. 2021, 84, 1592–1602. [Google Scholar] [CrossRef] [PubMed]
- Huyghebaert, G.; Ducatelle, R.; Immerseel, F.V. An Update on Alternatives to Antimicrobial Growth Promoters for Broilers. Vet. J. 2011, 187, 182–188. [Google Scholar] [CrossRef]
- Guan, N.; Liu, L. Microbial Response to Acid Stress: Mechanisms and Applications. Appl. Microbiol. Biotechnol. 2020, 104, 51–65. [Google Scholar] [CrossRef]
- Cherrington, C.A.; Hinton, M.; Chopra, I. Effect of Short-Chain Organic Acids on Macromolecular Synthesis in Escherichia coli. J. Appl. Bacteriol. 1990, 68, 69–74. [Google Scholar] [CrossRef]
- Trček, J.; Mira, N.P.; Jarboe, L.R. Adaptation and Tolerance of Bacteria against Acetic Acid. Appl. Microbiol. Biotechnol. 2015, 99, 6215–6229. [Google Scholar] [CrossRef]
- Mani-López, E.; García, H.S.; López-Malo, A. Organic Acids as Antimicrobials to Control Salmonella in Meat and Poultry Products. Food Res. Int. 2012, 45, 713–721. [Google Scholar] [CrossRef]
- Shi, C.; Li, C.; Wang, Y.; Guo, J.; Barry, S.; Zhang, Y.; Marmier, N. Review of Advanced Oxidation Processes Based on Peracetic Acid for Organic Pollutants. Water 2022, 14, 2309. [Google Scholar] [CrossRef]
- Kitis, M. Disinfection of Wastewater with Peracetic Acid: A Review. Environ. Int. 2004, 30, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, W.P.; Carlos, T.D.; Cavallini, G.S.; Pereira, D.H. Peracetic Acid: Structural Elucidation for Applications in Wastewater Treatment. Water Res. 2020, 168, 115143. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, H.; Lin, Z.; Xu, P. Mechanisms of Acid Tolerance in Bacteria and Prospects in Biotechnology and Bioremediation. Biotechnol. Adv. 2015, 33, 1484–1492. [Google Scholar] [CrossRef]
- Lund, P.; Tramonti, A.; De Biase, D. Coping with Low pH: Molecular Strategies in Neutralophilic Bacteria. FEMS Microbiol. Rev. 2014, 38, 1091–1125. [Google Scholar] [CrossRef]
- Gaca, A.O.; Lemos, J.A. Adaptation to Adversity: The Intermingling of Stress Tolerance and Pathogenesis in Enterococci. Microbiol. Mol. Biol. Rev. 2019, 83, e00008–19. [Google Scholar] [CrossRef]
- Álvarez-Ordóñez, A.; Prieto, M.; Bernardo, A.; Hill, C.; López, M. The Acid Tolerance Response of Salmonella spp.: An Adaptive Strategy to Survive in Stressful Environments Prevailing in Foods and the Host. Food Res. Int. 2012, 45, 482–492. [Google Scholar] [CrossRef]
- Spector, M.P.; Kenyon, W.J. Resistance and Survival Strategies of Salmonella Enterica to Environmental Stresses. Food Res. Int. 2012, 45, 455–481. [Google Scholar] [CrossRef]
- Mourão, J.; Rebelo, A.; Ribeiro, S.; Peixe, L.; Novais, C.; Antunes, P. Atypical Non-H2S-Producing Monophasic Salmonella Typhimurium ST3478 Strains from Chicken Meat at Processing Stage Are Adapted to Diverse Stresses. Pathogens 2020, 9, 701. [Google Scholar] [CrossRef]
- Rebelo, A.; Duarte, B.; Ferreira, C.; Mourão, J.; Ribeiro, S.; Freitas, A.R.; Coque, T.M.; Willems, R.; Corander, J.; Peixe, L.; et al. Enterococcus spp. from Chicken Meat Collected 20 Years Apart Overcome Multiple Stresses Occurring in the Poultry Production Chain: Antibiotics, Copper and Acids. Int. J. Food Microbiol. 2023, 384, 109981. [Google Scholar] [CrossRef]
- Alonso-Hernando, A.; Capita, R.; Prieto, M.; Alonso-Calleja, C. Adaptation and Cross-Adaptation of Listeria monocytogenes and Salmonella enterica to Poultry Decontaminants. J. Microbiol. 2009, 47, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Hernando, A.; Capita, R.; Prieto, M.; Alonso-Calleja, C. Comparison of Antibiotic Resistance Patterns in Listeria monocytogenes and Salmonella enterica Strains Pre-Exposed and Exposed to Poultry Decontaminants. Food Control 2009, 20, 1108–1111. [Google Scholar] [CrossRef]
- Gantzhorn, M.R.; Pedersen, K.; Olsen, J.E.; Thomsen, L.E. Biocide and Antibiotic Susceptibility of Salmonella Isolates Obtained before and after Cleaning at Six Danish Pig Slaughterhouses. Int. J. Food Microbiol. 2014, 181, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Morandi, S.; Brasca, M.; Alfieri, P.; Lodi, R.; Tamburini, A. Influence of pH and Temperature on the Growth of Enterococcus faecium and Enterococcus faecalis. Le Lait 2005, 85, 181–192. [Google Scholar] [CrossRef]
- Shi, Y.; Zhai, M.; Li, J.; Li, B. Evaluation of Safety and Probiotic Properties of a Strain of Enterococcus faecium Isolated from Chicken Bile. J. Food Sci. Technol. 2020, 57, 578–587. [Google Scholar] [CrossRef]
- Fernández, A.; Cebrián, G.; Álvarez-Ordóñez, A.; Prieto, M.; Bernardo, A.; López, M. Influence of Acid and Low-Temperature Adaptation on Pulsed Electric Fields Resistance of Enterococcus faecium in Media of Different pH. Innov. Food Sci. Emerg. Technol. 2018, 45, 382–389. [Google Scholar] [CrossRef]
- Vassos, D.; Bezirtzoglou, E.; Voidarou, C.; Alexopoulos, A.; Maipa, V. Biochemical and Antimicrobial Profile of Enterococcus faecium and E. faecalis Isolated from Traditional Dairy Products and Infant Intestinal Microbiota. Microb. Ecol. Health Dis. 2009, 21, 241–250. [Google Scholar] [CrossRef]
- Suchomel, M.; Lenhardt, A.; Kampf, G.; Grisold, A. Enterococcus Hirae, Enterococcus faecium and Enterococcus faecalis Show Different Sensitivities to Typical Biocidal Agents Used for Disinfection. J. Hosp. Infect. 2019, 103, 435–440. [Google Scholar] [CrossRef]
- Rebelo, A.; Mourão, J.; Freitas, A.R.; Duarte, B.; Silveira, E.; Sanchez-Valenzuela, A.; Almeida, A.; Baquero, F.; Coque, T.M.; Peixe, L.; et al. Diversity of Metal and Antibiotic Resistance Genes in Enterococcus Spp. from the Last Century Reflects Multiple Pollution and Genetic Exchange among Phyla from Overlapping Ecosystems. Sci. Total Environ. 2021, 787, 147548. [Google Scholar] [CrossRef]
- Mourão, J.; Rebelo, A.; Ribeiro, S.; Peixe, L.; Novais, C.; Antunes, P. Tolerance to Arsenic Contaminant among Multidrug-resistant and Copper-tolerant Salmonella Successful Clones Is Associated with Diverse ars Operons and Genetic Contexts. Environ. Microbiol. 2020, 22, 2829–2842. [Google Scholar] [CrossRef]
- Arredondo-Alonso, S.; Top, J.; McNally, A.; Puranen, S.; Pesonen, M.; Pensar, J.; Marttinen, P.; Braat, J.C.; Rogers, M.R.C.; van Schaik, W.; et al. Plasmids Shaped the Recent Emergence of the Major Nosocomial Pathogen Enterococcus faecium. mBio 2020, 11, e03284–19. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). ISO 20776–1:2019: Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices—Part 1: Reference Method for Testing the in Vitro Activity of Antimicrobial Agents against Rapidly Growing Aerobic Bacteria Involved in Infectious Diseases; International Organization for Standardization (ISO): Geneva, Switzerland, 2019. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline; CLSI Document M26-A; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 1999. [Google Scholar]
- Dean, J.A. , Lange, N.A. Lange’s Handbook of Chemistry; 13. ed.; McGraw-Hill: New York, 1985; ISBN 978-0-07-016192-4. [Google Scholar]
- Mohammed, S.; Çon, A.H. Isolation and Characterization of Potential Probiotic Lactic Acid Bacteria from Traditional Cheese. LWT 2021, 152, 112319. [Google Scholar] [CrossRef]
- Park, S.-H.; Choi, M.-R.; Park, J.-W.; Park, K.-H.; Chung, M.-S.; Ryu, S.; Kang, D.-H. Use of Organic Acids to Inactivate Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes on Organic Fresh Apples and Lettuce. J. Food Sci. 2011, 76, M293–M298. [Google Scholar] [CrossRef]
- Thomas, C.; Schönknecht, A.; Püning, C.; Alter, T.; Martin, A.; Bandick, N. Effect of Peracetic Acid Solutions and Lactic Acid on Microorganisms in On-Line Reprocessing Systems for Chicken Slaughter Plants. J. Food Prot. 2020, 83, 615–620. [Google Scholar] [CrossRef]
- Lin, J.; Lee, I.S.; Frey, J.; Slonczewski, J.L.; Foster, J.W. Comparative Analysis of Extreme Acid Survival in Salmonella Typhimurium, Shigella flexneri, and Escherichia coli. J. Bacteriol. 1995, 177, 4097–4104. [Google Scholar] [CrossRef]
- Koutsoumanis, K.P.; Kendall, P.A.; Sofos, J.N. Modeling the Boundaries of Growth of Salmonella Typhimurium in Broth as a Function of Temperature, Water Activity, and pH. J. Food Prot. 2004, 67, 53–59. [Google Scholar] [CrossRef]
- Álvarez-Ordóñez, A.; Fernández, A.; Bernardo, A.; López, M. Acid Tolerance in Salmonella Typhimurium Induced by Culturing in the Presence of Organic Acids at Different Growth Temperatures. Food Microbiol. 2010, 27, 44–49. [Google Scholar] [CrossRef]
- Li, K.; Chiu, Y.-C.; Jiang, W.; Jones, L.; Etienne, X.; Shen, C. Comparing the Efficacy of Two Triple-Wash Procedures With Sodium Hypochlorite, a Lactic–Citric Acid Blend, and a Mix of Peroxyacetic Acid and Hydrogen Peroxide to Inactivate Salmonella, Listeria monocytogenes, and Surrogate Enterococcus faecium on Cucumbers and Tomatoes. Front. Sustain. Food Syst. 2020, 4, 19. [Google Scholar] [CrossRef]
- Zhu, M.-J.; Shen, X.; Sheng, L.; Mendoza, M.; Hanrahan, I. Validation of Enterococcus faecium NRRL B-2354 as a Surrogate for Listeria monocytogenes on Fresh Apples during Pilot Spray-Bar Peroxyacetic Acid Intervention. Food Control 2021, 119, 107472. [Google Scholar] [CrossRef]
- Turolla, A.; Sabatino, R.; Fontaneto, D.; Eckert, E.M.; Colinas, N.; Corno, G.; Citterio, B.; Biavasco, F.; Antonelli, M.; Mauro, A.; et al. Defence Strategies and Antibiotic Resistance Gene Abundance in Enterococci under Stress by Exposure to Low Doses of Peracetic Acid. Chemosphere 2017, 185, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Humayoun, S.B.; Hiott, L.M.; Gupta, S.K.; Barrett, J.B.; Woodley, T.A.; Johnston, J.J.; Jackson, C.R.; Frye, J.G. An Assay for Determining the Susceptibility of Salmonella Isolates to Commercial and Household Biocides. PloS One 2018, 13, e0209072. [Google Scholar] [CrossRef]
- Micciche, A.C.; Feye, K.M.; Rubinelli, P.M.; Lee, J.A.; Knueven, C.J.; Ricke, S.C. Comparison of Acid Sanitizers on Salmonella Typhimurium Inoculated Commercial Poultry Processing Reuse Water. Front. Sustain. Food Syst. 2019, 2, 90. [Google Scholar] [CrossRef]
- Alonso-Hernando, A.; Alonso-Calleja, C.; Capita, R. Effects of Exposure to Poultry Chemical Decontaminants on the Membrane Fluidity of Listeria monocytogenes and Salmonella enterica Strains. Int. J. Food Microbiol. 2010, 137, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Jolivet-Gougeon, A.; Sauvager, F.; Bonnaure-Mallet, M.; Colwell, R.R.; Cormier, M. Virulence of Viable but Nonculturable S. Typhimurium LT2 after Peracetic Acid Treatment. Int. J. Food Microbiol. 2006, 112, 147–152. [Google Scholar] [CrossRef]
- Bauermeister, L.J.; Bowers, J.W.J.; Townsend, J.C.; McKee, S.R. The Microbial and Quality Properties of Poultry Carcasses Treated with Peracetic Acid as an Antimicrobial Treatment. Poult. Sci. 2008, 87, 2390–2398. [Google Scholar] [CrossRef] [PubMed]
- Mathew, E.N.; Muyyarikkandy, M.S.; Bedell, C.; Amalaradjou, M.A. Efficacy of Chlorine, Chlorine Dioxide, and Peroxyacetic Acid in Reducing Salmonella Contamination in Wash Water and on Mangoes Under Simulated Mango Packinghouse Washing Operations. Front. Sustain. Food Syst. 2018, 2, 18. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




