Submitted:
26 July 2023
Posted:
27 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
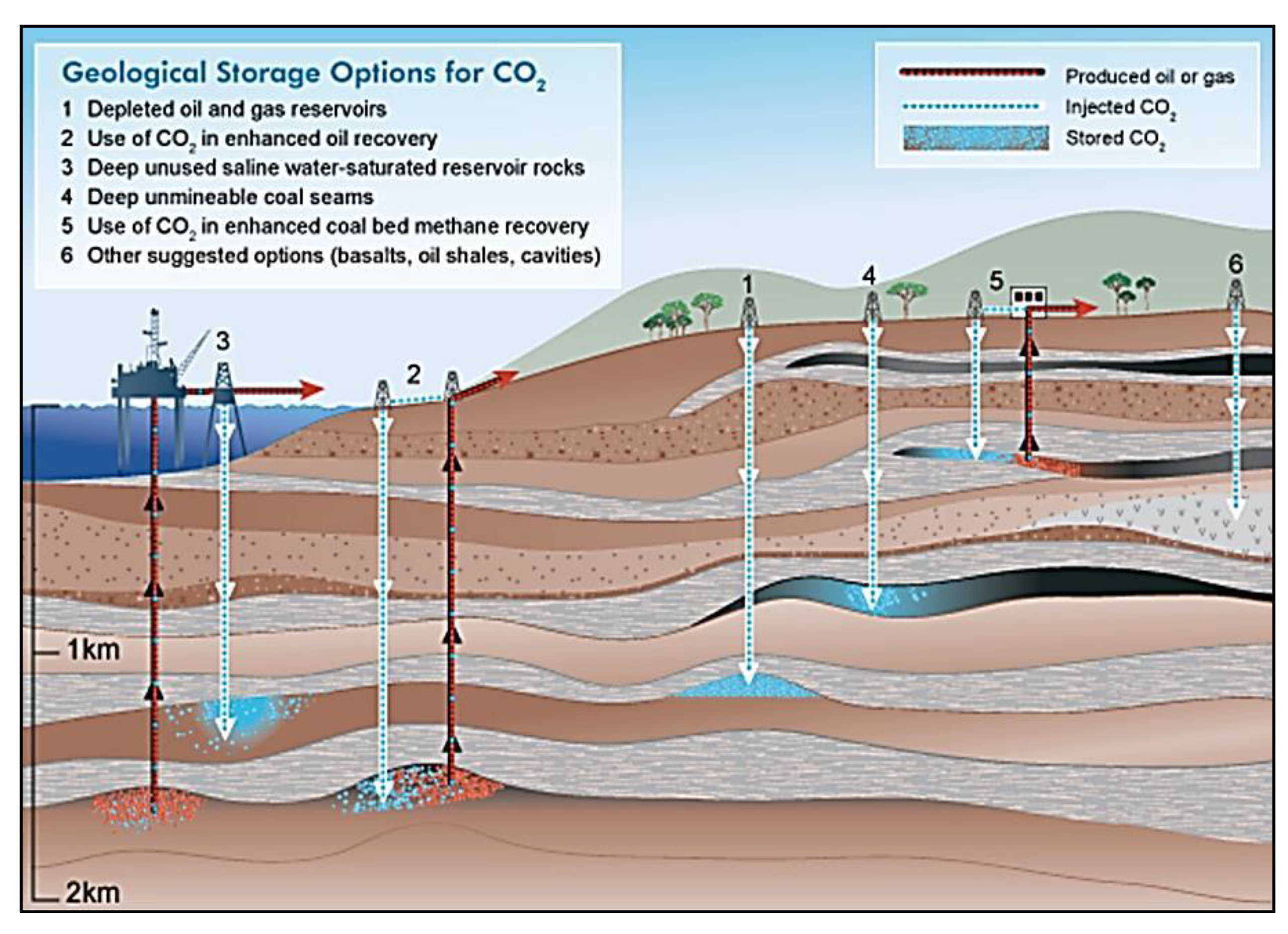
2. CO2 Sequestration Methods
2.1. Storage in Subsurface Reservoir Formations
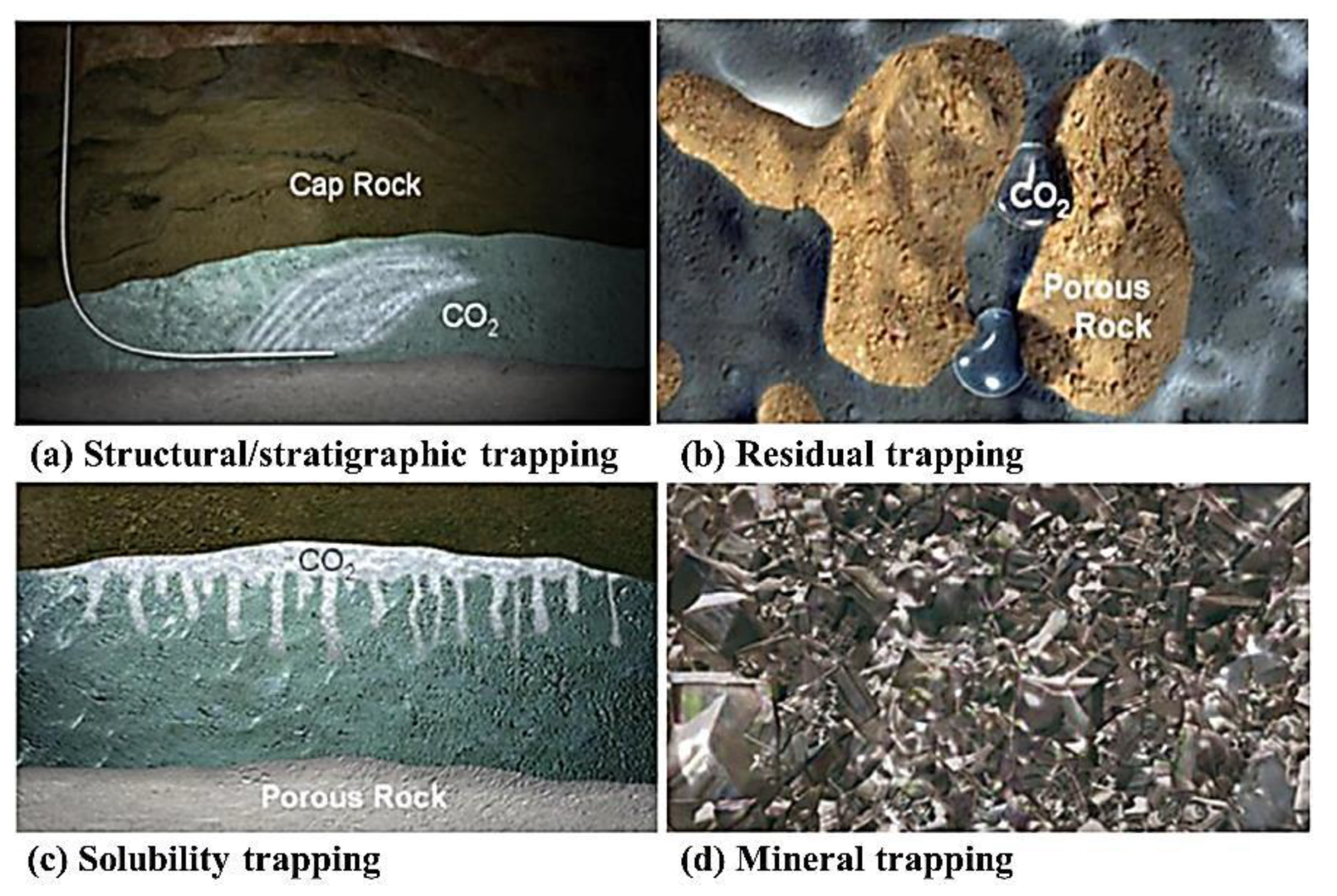
2.2. Brine Aquifers
- Structural/stratigraphic
- Residual
- Solubility
- Mineral trapping.
2.3. Drained Hydrocarbon Reservoir Formations
- Drained hydrocarbon reservoirs have been the subject of substantial research both before and during the hydrocarbon exploring period, including research about their capacity for storage
- Both onshore and offshore infrastructural facilities, existing infrastructure, including CO2 injection wells and transportation, may be used with little modification for the storage process (Sigman et al. 2021)
- If this was not the case, CO2 gas injection to enhance oil recovery would have been less attractive and ends many years ago. Suitable hydrocarbon field’s data as analogue may be utilise in illustrating the efficacy of cap-rock across geologic timeframe to strengthen oil and gas reservoirs (Heinemann et al. 2012).
- Additional site characterization involves investigating potential leakage risks, such as the condition of the cap rock and any abandoned wells with integrity problems.
- Additional evaluations of surface processing plants' fugitive and discharging emissions
- Leakage rates may be estimated from specific locations and the normality of the reservoir's behaviour can be determined by increased monitoring and field surveillance.
- Using numerical evidence, (Tenasaka 2011) proved that this was possible within the normal range of CO2 injection. In the San Joaquin basin, scientists injected around 2.0HCPV (hydrocarbon pore volume) of CO2 to prove that there was a greater possibility to extract more oil, almost 67% of the originally present oil (OOIP) was recovered. In addition, (Tenasaka 2011) demonstrated that there was a greater recovery of oil from his numerical methodology
- Using a better and innovative CO2 flooding design and well management can positively influence more oil recovery from the reservoir
- Increasing the mobility-ratio by raising water’s viscosity (Thomas 2008). Minimising miscibility pressure using miscibility-enhancing agents, Kuuskraa (2008).
2.4. In-accessible Coal Seams
- The homogeneity Reservoir
- Threshold of fractures and fault planes
- Upper depth limit
- Coal geomorphology
- Permeability adequacy
- Even in constrained reservoirs, continuous CO2 injection is feasible.
- Injection may be performed notwithstanding a decrease in injectivity.
- Expected Significantly Enhanced CBM Production
- The injected carbon dioxide stays in the reservoir, boosting sweep efficiency, (Lakeman 2016).
2.5. Subsurface Basalt Formations
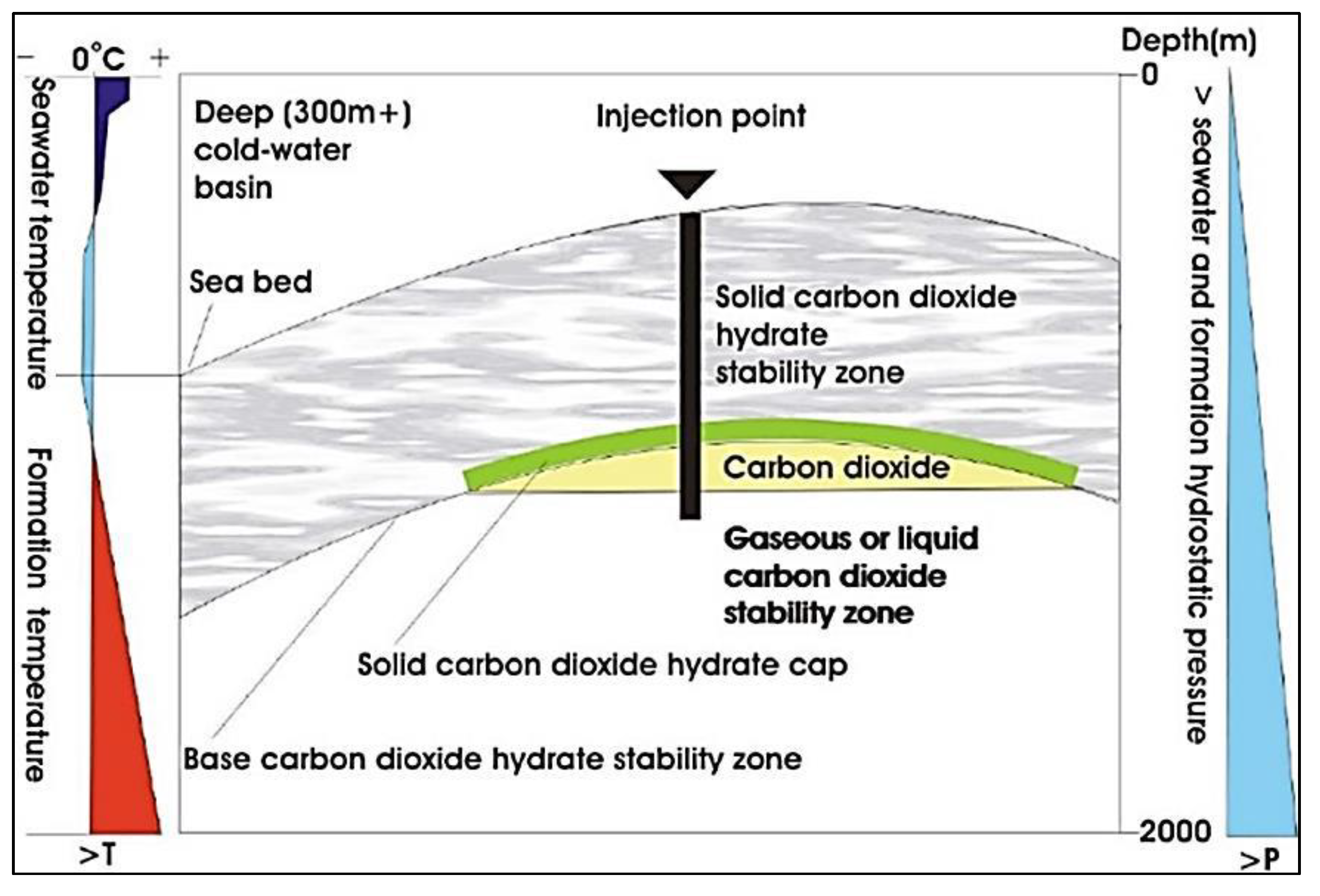
- Because it offers sufficient depth, denser CO2 liquid may sink, which delays the release of CO2 back into the atmosphere
- It makes it possible to form stable carbonates in a shorter amount of time than would normally be required by geologic processes
- It prevents acidic basement fluids from rising via an impervious sediment layer
- It can be converted into a stable hydrate
- It is essential to remember that a small quantity of CO2 leaking does not inevitably damage the sea bottom ecosystems.
2.6. CO2 Sequestration in Hydrate deep Formations
2.7. Enhanced Geothermal Systems Based on CO2
2.8. Carbonation of Mineral
- The potential for less terrain change
- Mineral carbonation in terms of mineral and CO2 dissolution.
- Material stratum diffusion
- Managing mineral impurities throughout the sequestration process
2.9. CO2 Sequestration in Ocean Floor Sediments
3. Conclusion
Conflicts of Interest
Acknowledgements
Abbreviations
References
- Anon (n.d.) Communication Supporting the Research on CO2 Storage at the Ketzin Pilot Site, Germany – A Status Report after Ten Years of Public Outreach | Elsevier Enhanced Reader. https://reader.elsevier.com/reader/sd/pii/S1876610214008947?token=10B64E5A1D23D16D86B29D14466B1CE4BCAF556D3264EC284914F038F0B4716FC9FDBC1381E41B0FEBCC90BC579D72B8&originRegion=eu-west-1&originCreation=20220825114237 Accessed 25 August 2022.
- Armitage PJ, Worden RH, Faulkner DR, Aplin AC, Butcher AR, Espie AA. (2013) Mercia Mudstone Formation caprock to carbon capture and storage sites: Petrology and petrophysical characteristics. J Geol Soc London 2013;170:119–32. [CrossRef]
- Assima GP, Larachi F, Molson J, Beaudoin G. (2014) Impact of temperature and oxygen availability on the dynamics of ambient CO2 mineral sequestration by nickel mining residues. Chem Eng J 2014;240:394–403. [CrossRef]
- BGS. (2017) Man-made (anthropogenic) greenhouse gases. Accessed on September 23, 2022 at https://www.bgs.ac.uk/discovering-geology/climate-change/CCS/Anthropogenic.html.
- Bobicki ER, Liu Q, Xu Z, Zeng H. (2012) Carbon capture and storage using alkaline industrial wastes. Prog Energy Combust Sci 2012;38:302–20. [CrossRef]
- Bruni J, Canepa M, Chiodini G, Cioni R, Cipolli F, Longinelli A, et al. (2002) Irreversible water–rock mass transfer accompanying the generation of the neutral, Mg–HCO3 and high-pH, Ca–OH spring waters of the Genova province, Italy. Appl Geochemistry 2002;17:455–74.
- Buttinelli, M., Procesi, M., Cantucci, B., Quattrocchi, F. and Boschi, E. (2011) The geo-database of caprock quality and deep saline aquifers distribution for geological storage of CO2 in Italy. Energy 36 (5), Elsevier Ltd2968–2983. [CrossRef]
- Calabrò A, Deiana P, Fiorini P, Girardi G, Stendardo S. (2008) Possible optimal configurations for the ZECOMIX high efficiency zero emission hydrogen and power plant. Energy 2008;33:952–62. [CrossRef]
- Cantucci, B., Montegrossi, G., Vaselli, O., Tassi, F., Quattrocchi, F. and Perkins, E.H. (2009) Geochemical modeling of CO2 storage in deep reservoirs: The Weyburn Project (Canada) case study. Chemical Geology 265 (1–2), 181–197. [CrossRef]
- Circone S., Stern LA., Kirby SH., Durham WB., Chakoumakos BC., Rawn CJ., et al. (2003) CO2 hydrate: Synthesis, composition, structure, dissociation behavior, and a comparison to structure I CH4 hydrate. J Phys Chem B 2003;107:5529–39.
- CO2CRC. (2015) CO2 dispersion. Coop Res Cent Greenh Gas Technol.
- CO2CRC. (2009) CO2 storage demonstration projects around the world: active projects.
- CO2CRC. (2021) Injection & storage 2015. Accessed June 20, 2022 at http://old.co2crc.com.au/aboutccs/storage.
- Ekpo Johnson, E., Scherwath, M., Moran, K., Dosso, S.E. and Rohr, K.M. (2023) Fault Slip Tendency Analysis for a Deep-Sea Basalt CO2 Injection in the Cascadia Basin. GeoHazards 4 (2), 121–135. [CrossRef]
- EPA. (2021) Class II Oil and Gas Related Injection Wells. United States Environ Prot Agency n.d. https://www.epa.gov/uic/class-ii-oil-and-gas-related-injection-wells Opened on September 15.
- Fleury, M., Pironon, J., Le Nindre, Y.M., Bildstein, O., Berne, P., Lagneau, V., et al. (2010) Evaluating Sealing Efficiency of Caprocks for CO2 Storage: An Overview of the Geocarbone-Integrity Program and Results. Oil Gas Sci Technol – Rev IFP 2010;65:435–44.
- Frerichs J, Rakoczy J, Ostertag-Henning C, Krüger M. (2014) Viability and Adaptation Potential of Indigenous Microorganisms from Natural Gas Field Fluids in High Pressure Incubations with Supercritical CO2. Environ Sci Technol 2014;48:1306–14. [CrossRef]
- GCCSI. (2011) Accelerating the Uptake of CCS: Industrial Use of Captured Carbon Dioxide.
- GCCSI. (2017) Alberta Carbon Trunk Line (“ACTL”) with North West Sturgeon Refinery CO2 Stream. Glob CCS Inst 2017. https://co2re.co/FacilityData.
- GCCSI. (2010) CO2 for use in enhanced oil recovery (EOR). Glob CCS Inst.
- GCCSI. Frio Brine Pilot Project 2010. https://www.globalccsinstitute.com/archive/hub/publications/158508/strategic-plan-implementation-report.pdf.
- Gao, R.S. Gao, R.S., Sun, A.Y. and Nicot, J.P. (2016) Identification of a representative dataset for long-term monitoring at the Weyburn CO2-injection enhanced oil recovery site, Saskatchewan, Canada. International Journal of Greenhouse Gas Control 54, Elsevier Ltd454–465.
- Ghavipour, Mohammad, Ghavipour, Mina, Chitsazan, M., Najibi, S.H. and Ghidary, S.S. (2013) Experimental study of natural gas hydrates and a novel use of neural network to predict hydrate formation conditions. Chemical Engineering Research and Design 91 (2), 264–273. [CrossRef]
- Gilliland E., Ripepi N., Karmis M., Conrad M. (2012) An examination of MVA techniques applicable for CCUS in thin, stacked coals of the central appalachian basin. 29th Annu. Int. Pittsburgh Coal Conf. 2012, PCC 2012, vol. 3, 2012, p. 1931–8.
- Gilliland ES., Ripepi N., Conrad M., Miller MJ., Karmis M. (2013) Selection of monitoring techniques for a carbon storage and enhanced coalbed methane recovery pilot test in the Central Appalachian Basin. Int J Coal Geol 2013;118:105–12. [CrossRef]
- Gunter WD, Bachu S, Benson S. (2004) The role of hydrogeological and geochemical trapping in sedimentary basins for secure geological storage of carbon dioxide. Geol Soc London, Spec Publ 2004;233:129–45. [CrossRef]
- Han, Y. and Winston Ho, W.S. (2020) Recent advances in polymeric facilitated transport membranes for carbon dioxide separation and hydrogen purification. Journal of Polymer Science. [CrossRef]
- Hanak DP, Anthony EJ, Manovic V. (2015) A review of developments in pilot-plant testing and modelling of calcium looping process for CO2 capture from power generation systems. Energy Environ Sci 2015;8:2199–249. [CrossRef]
- Heinemann, N., Stewart, R.J., Wilkinson, M., Pickup, G.E. and Haszeldine, R.S. (2016) Hydrodynamics in subsurface CO2 storage: Tilted contacts and increased storage security. International Journal of Greenhouse Gas Control 54, Elsevier Ltd322–329. [CrossRef]
- Hofmann M, Schellnhuber HJ. (2010) Ocean acidification: A millennial challenge. Energy Environ Sci 2010;3:1883–96. [CrossRef]
- IEAGHG. (2011) Effects of Impurities on Geological Storage of CO2. Cheltenham, UK.
- IEAGHG. (2009) CO2 storage in depleted gas fields. Oxford.
- IEAGHG. (2013) Induced seismicity and its implications for CO2 storage. Cheltenham.
- IEAGHG. (2009) Long term integrity of CO2 storage – well abandonment..
- Iglauer S, Pentland CH, Busch A. (2014) CO2 wettability of seal and reservoir rocks and the implications for carbon geo-sequestration. Water Resour Res 2014;51:729–74. [CrossRef]
- Igunnu ET, Chen GZ. (2014) Produced water treatment technologies. Int J Low-Carbon Technol 2014;9:157. [CrossRef]
- International Energy Agency IEA. (2015) Storing CO2 through Enhanced Oil Recovery, Combining EOR with CO2 storage (EOR) for profit.
- IPCC. (2005). Special Report on Carbon Dioxide Capture and Storage. Cambridge: 2005.
- Jaramillo P, Griffin WM, Matthews HS. (2008) Comparative Analysis of the Production Costs and Life-Cycle GHG Emissions of FT Liquid Fuels from Coal and Natural Gas. Environ Sci Technol 2008;42:7559–65. [CrossRef]
- Javaheri M, Jessen K. (2011) Residual Trapping in Simultaneous Injection of CO2 and Brine in Saline Aquifers. SPE West. North Am. Reg. Meet., Society of Petroleum Engineers; 2011, p. 603. [CrossRef]
- Jemai K, Kvamme B, Vafaei MT. (2014) Theoretical studies of CO2 hydrates formation and dissociation in cold aquifers using retrasocodebright simulator. WSEAS Trans Heat Mass Transf 2014;9:150–68.
- Khabibullin T, Falcone G, Teodoriu C. (2011) Drilling through gas-hydrate sediments: Managing wellbore-stability risks. SPE Drill Complet 2011;26:287–94. [CrossRef]
- Kim Y, Jang H, Kim J, Lee J. (2017) Prediction of storage efficiency on CO2 sequestration in deep saline aquifers using artificial neural network. Appl Energy 2017;185, Part:916–28. [CrossRef]
- Kneafsey TJ, Pruess K. (2010) Laboratory Flow Experiments for Visualizing Carbon Dioxide-Induced, Density-Driven Brine Convection. Transp Porous Media 2010;82:123–39.
- Krooss BM., Van Bergen F., Gensterblum Y., Siemons N., Pagnier HJM., David P. (2002) High-pressure methane and carbon dioxide adsorption on dry and moisture-equilibrated Pennsylvanian coals. Int J Coal Geol 2002;51:69–92. [CrossRef]
- Kuuskraa V, Ferguson R. (2008) Storing CO2 with enhanced oil recovery. Washington, D.C.: 2008. [CrossRef]
- Lakeman B. (2016) Alberta Research Council Enhanced Coalbed Methane Recovery Project in Alberta, Canada.
- Le Gallo Y, Couillens P, Manai T. (2002) CO2 Sequestration in Depleted Oil or Gas Reservoirs. Int. Conf. Heal. Saf. Environ. Oil Gas Explor. Prod., Society of Petroleum Engineers; 2002, p. 1390–2. [CrossRef]
- Lim M, Han G-C, Ahn J-W, You K-S. (2010) Environmental Remediation and Conversion of Carbon Dioxide (CO2) into Useful Green Products by Accelerated Carbonation Technology. Int J Environ Res Public Health 2010;7:203–28. [CrossRef]
- Luo T, Zhou L, Jiao Z, Bai Y, Wang S. (2014) The Ordos Basin: A Premier Basin for Integrating geological CO2 Storage with Enhanced oil Recovery Projects in China. Energy Procedia 2014;63:7772–9. [CrossRef]
- MacDowell N, Florin N, Buchard A, Hallett J, Galindo A, Jackson G, et al. (2013) An overview of CO2 capture technologies. Energy Environ Sci 2010;3:1645–69. [CrossRef]
- Marston P. (2013) Bridging the gap: An analysis and comparison of legal and regulatory frameworks for CO2-EOR and CO2-CCS.
- Masuda Y, Yamanaka Y, Sasai Y, Magi M, Ohsumi T. (2009) Site selection in CO2 ocean sequestration: Dependence of CO2 injection rate on eddy activity distribution. Int J Greenh Gas Control 2009;3:67–76. [CrossRef]
- Matter JM., Broecker WS., Gislason SR., Gunnlaugsson E., Oelkers EH., Stute M., et al. (2011) The CarbFix Pilot Project - Storing carbon dioxide in basalt. Energy Procedia, vol. 4, 2011, p. 5579–85.
- Matter JM., Stute M., Snæbjörnsdottir SÓ., Oelkers EH., Gislason SR., Aradottir ES., et al. (2016) Rapid carbon mineralization for permanent disposal of anthropogenic carbon dioxide emissions. Science (80-) 2016;352:1312–4.
- McGrail BP., Schaef HT., Ho AM., Chien Y-J., Dooley JJ., Davidson CL. (2006) Potential for carbon dioxide sequestration in flood basalts. J Geophys Res Solid Earth 2006;111. [CrossRef]
- NASA - National Aeronautics and Space Administration. (2018) A blanket around the earth 2018. Accessed on September 23 at https://climate.nasa.gov/causes/.
- NASA - National Aeronautics and Space Administration. (2021) Carbon Dioxide 2021. https://climate.nasa.gov/vital-signs/carbon-dioxide/.
- Olajire AA. (2013) A review of mineral carbonation technology in sequestration of CO2. J Pet Sci Eng . [CrossRef]
- Oldenburg CM. (2003) Carbon sequestration in natural gas reservoirs: Enhanced gas recovery and natural gas storage.
- Perera MSA, Gamage RP, Rathnaweera TD, Ranathunga AS, Koay A, Choi X. A (2016) Review of CO2-Enhanced Oil Recovery with a Simulated Sensitivity Analysis. Energies 2016;9.
- Plaksina T, White C. (2016) Modeling coupled convection and carbon dioxide injection for improved heat harvesting in geopressured geothermal reservoirs. Geotherm Energy 2016;4:2. [CrossRef]
- Pollyea RM., Fairley JP., Podgorney RK., Mcling TL. (2014) Physical constraints on geologic CO2 sequestration in low-volume basalt formations. Bull Geol Soc Am 2014;126:344–51. [CrossRef]
- Porter, R.T.J., Fairweather, M., Pourkashanian, M. and Woolley, R.M. (2015) The range and level of impurities in CO2 streams from different carbon capture sources. International Journal of Greenhouse Gas Control 36, Elsevier Ltd161–174. [CrossRef]
- Preston C., Whittaker S., Rostron B., Chalaturnyk R., White D., Hawkes C., et al. IEA GHG Weyburn-Midale CO2 monitoring and storage project-moving forward with the Final Phase. Energy Procedia, vol. 1, 2009, p. 1743–50. [CrossRef]
- Procesi M, Cantucci B, Buttinelli M, Armezzani G, Quattrocchi F, Boschi E. (2013) Strategic use of the underground in an energy mix plan: Synergies among CO2, CH4 geological storage and geothermal energy. Latium Region case study (Central Italy). Appl Energy 2013;110:104–31. [CrossRef]
- Pruess K. (2006) Enhanced geothermal systems (EGS) using CO2 as working fluid—A novel approach for generating renewable energy with simultaneous sequestration of carbon. Geothermics 2006;35:351–67. [CrossRef]
- Pruess K. (2008) On production behavior of enhanced geothermal systems with CO2 as working fluid. Energy Convers Manag 2008;49:1446–54. [CrossRef]
- Quattrocchi, F., Boschi, E., Spena, A., Buttinelli, M., Cantucci, B. and Procesi, M. (2013) Synergic and conflicting issues in planning underground use to produce energy in densely populated countries, as Italy. Geological storage of CO2, natural gas, geothermics and nuclear waste disposal. Applied Energy 101, Elsevier Ltd393–412. [CrossRef]
- Quattrocchi, F., Galli, G., Gasparini, A., Magno, L., Pizzino, L., Sciarra and Voltattorni, N. (2011) Very slightly anomalous leakage of CO2, CH4 and radon along the main activated faults of the strong l’Aquila earthquake (Magnitude 6.3, Italy). Implications for risk assessment monitoring tools & public acceptance of CO2 and CH4 underground storage. Energy Procedia Elsevier Ltd4067–4075. [CrossRef]
- Rehder, G., Leifer, I., Brewer, P.G., Friederich, G. and Peltzer, E.T. (2009) Controls on methane bubble dissolution inside and outside the hydrate stability field from open ocean field experiments and numerical modeling. Marine Chemistry 114 (1–2), 19–30. [CrossRef]
- Rochelle, C.A., Camps, A.P., Long, D., Milodowski, A., Bateman, K., Gunn, D., Jackson, P., Lovell, M.A. and Rees, J. (2009) Can CO2 hydrate assist in the underground storage of carbon dioxide? Geological Society Special Publication 319, 171–183.
- Ruffine L, Donval JP, Charlou JL, Cremière A, Zehnder BH. (2010) Experimental study of gas hydrate formation and destabilisation using a novel high-pressure apparatus. Mar Pet Geol 2010;27:1157–65. [CrossRef]
- Rutqvist J. (2012) The Geomechanics of CO2 Storage in Deep Sedimentary Formations. Geotech Geol Eng 2012;30:525–51. [CrossRef]
- Seifritz W. (1990) CO2 disposal by means of silicates. Nature 1990;345:486–486. [CrossRef]
- SIS. (2021) Enhanced Oil Recovery (EOR). Schlumberger Inf Solut Ltd 2021. Accessed on August 21st 2021 at https://www.slb.com/technical-challenges/enhanced-oil-recovery.
- Shi, Y., Lu, Y., Rong, Y., Bai, Z., Bai, H., Li, M. and Zhang, Q. (2023) Geochemical reaction of compressed CO2 energy storage using saline aquifer. Alexandria Engineering Journal 64, . [CrossRef]
- Shukla, R., Ranjith, P., Haque, A. and Choi, X. (2010) A review of studies on CO2 sequestration and caprock integrity. Fuel Elsevier Ltd2651–2664. [CrossRef]
- Sigman, D.M., Fripiat, F., Studer, A.S., Kemeny, P.C., Martínez-García, A., Hain, M.P., Ai, X., Wang, X., Ren, H. and Haug, G.H. (2021) The Southern Ocean during the ice ages: A review of the Antarctic surface isolation hypothesis, with comparison to the North Pacific. Quaternary Science Reviews.
- Song J, Zhang D. (2013) Comprehensive review of caprock-sealing mechanisms for geologic carbon sequestration. Environ Sci Technol 2013; 47:9–22. [CrossRef]
- Sundal A, Hellevang H, Miri R, Dypvik H, Nystuen JP, Aagaard P. (2014) Variations in mineralization potential for CO2 related to sedimentary facies and burial depth – a comparative study from the North Sea. Energy Procedia 2014;63:5063–70. [CrossRef]
- Talaghat MR, Esmaeilzadeh F, Fathikaljahi J. (2009) Experimental and theoretical investigation of simple gas hydrate formation with or without presence of kinetic inhibitors in a flow mini-loop apparatus. Fluid Phase Equilib 2009;279:28–40. [CrossRef]
- Tenasaka I. (2011) Global CCS Institute Bridging the Commercial Gap For Carbon Capture and Storage July 2011. Maryland, USA.
- Thomas, S. (2008) Enhanced Oil Recovery - An Overview. Oil Gas Sci Technol - Rev IFP 2008;63:9–19. [CrossRef]
- Trémosa J, Castillo C, Vong CQ, Kervévan C, Lassin A, Audigane P. (2014) Long-term assessment of geochemical reactivity of CO2 storage in highly saline aquifers: Application to Ketzin, In Salah and Snøhvit storage sites. Int J Greenh Gas Control 2014;20:2–26. [CrossRef]
- Van Pham TH, Aagaard P, Hellevang H. (2012) On the potential for CO2 mineral storage in continental flood basalts - PHREEQC batch- and 1D diffusion-reaction simulations. Geochem Trans 2012. [CrossRef]
- Wei, N., Li, X., Jiao, Z., Stauffer, P.H., Liu, S., Ellett, K. and Middleton, R.S. (2022) A Hierarchical Framework for CO2 Storage Capacity in Deep Saline Aquifer Formations. Frontiers in Earth Science. [CrossRef]
- Wdowin M, Tarkowski R, Manecki M. (2013) Petrographic-mineralogical and textural changes in reservoir and sealing rocks (Zaosie anticline) as a result of a long-term experiment in CO2-brine-rock interactions. Gospod Surowcami Miner - Miner Resour Manag 2013;29:137. [CrossRef]
- White D. (2009) Monitoring CO2 storage during EOR at the Weyburn-Midale field. Lead Edge 2009;28:838–42. [CrossRef]
- Xu Y, Ishizaka J, Aoki S. (1999) Simulations of the distribution of sequestered CO2 in the North Pacific using a regional general circulation model. Energy Convers Manag 1999;40:683–91. [CrossRef]
- Yamasaki A. (2003) An overview of CO2 mitigation options for global warming - Emphasizing CO2 sequestration options. J Chem Eng Japan 2003;36:361–75. [CrossRef]
- Zaluski, W., El-Kaseeh, G., Lee, S.Y., Piercey, M. and Duguid, A. (2016) Monitoring technology ranking methodology for CO2-EOR sites using the Weyburn-Midale Field as a case study. International Journal of Greenhouse Gas Control 54, Elsevier Ltd466–478. [CrossRef]
- Zangeneh, H., Jamshidi, S. and Soltanieh, M. (2013) Coupled optimization of enhanced gas recovery and carbon dioxide sequestration in natural gas reservoirs: Case study in a real gas field in the south of Iran. International Journal of Greenhouse Gas Control 17, 515–522. [CrossRef]
- Zero CO2. (2015) CCS-International legislation. Zero Emiss Resour Organ 2015. http://www.zeroco2.no/introduction/ccs-international-legislation (Opened September 3, 2021).
- Zhao X, Liao X, Wang W, Chen C, Rui Z, Wang H. (2014) The CO2 storage capacity evaluation: Methodology and determination of key factors. J Energy Inst 2014;87:297–305. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




