1. Introduction
The lower body is an essential component in performance in most sports, however, the calf is the most injured part of the lower body [
1,
2] and Achilles Tendon rupture seems to keep increasing despite enhancement of knowledge and research [
3]. Prevention and rehabilitation recommend progressively increasing the training load and working with longer contractions to lead to better tendinous adaptations [
4].
Along with rehabilitation recommendations, incline running (IR) seems to increase the duration of triceps surae contraction by modifying its amplitude, creating longer contraction. In addition, IR leads to a lower reduction in the ankle range of motion. At the same time, as the passive eccentric and active concentric phases of the muscle are amplified during IR, along with the increases in the slope, muscles work is increased [
5,
6]. Finally, during IR, as the intensity of the contraction increase, the electromyographic activity (EMGA) also increases a phenomenon which may explain the modulation of the EMGA in incline running [
7]. This increase in EMGA is produced by an increased motor drive and more motor unit recruitment, which may create greater muscle fiber tension and a better rehabilitation program. Nevertheless, IR is not easily accessible at an early stage of rehabilitation, and it is well established that early mobilisation of the injured member is crucial for a quick and successful rehabilitation program [
8,
9]. It is necessary to find a way to allowed injured athletes to run in incline slope as soon as possible.
Among the methods/types of equipment that are commonly used among physiotherapists and rehabilitation trainers during a rehabilitation program for the lower limbs are Body Weight Support (BWS) treadmills, which are capable of reducing the active body weight of the patients during a rehabilitation program, and thus they help to unload lower extremities while the patient is walking or running on a treadmill. This type of training is mainly used in non-healthy populations, like Spatic Paretic [
10] or Post Stroke patients [
11,
12,
13], or in patients with Spinal Cord Injury [
14,
15] or even with Parkinson's disease [
16]. However, BWS seems to favor rehabilitation, even in athletic populations. Indeed, even if walking and running are essential stimuli during athletes' rehabilitation programs, they can also traumatize joints and muscles because injured athletes show greater vertical impact and impact loading [
17], with the latter being one of the principal components that may affect the success or not of a lower limb rehabilitation program. Thus, BWS reduces the ground forces the injured athlete produces during running and avoids exacerbating the injury. [
18]. However, until now, the data about the effectiveness of BWS have been controversial. It seems that BWS does not reduce the total rehabilitation time significantly but allows patients to run 2 weeks before the control group for Achille rehabilitation [
19]. Also, for anterior cruciate ligament reconstruction rehabilitation, using BWS is significantly more efficient than standard rehabilitation at 12 weeks follow-up but not at 24 weeks [
20]. Moreover, after a 6-week speed training program, no differences were found between standard training and BWS training; only a decrease in injury was found for BWS training (66% against 8%) [
21]. Furthermore, during a BWS running training program, EMGA decreased [
15,
22]. With body support ranging between 10% and 40% of the patient’s body weight, a decreased EMGA of the triceps surae is observed [
22], reductions which seem to be more pronounced in Gastrocnemius Medialis (GM) and Lateralis (GL) than Soleus (SOL) [
23], which may lead to different effectiveness of BWS.
According to the above, it seems that BWS and IR, separately, can significantly affect the success of a rehabilitation program for the lower extremities, with each one providing different and necessary physiological stimuli to the injured athlete. However, neither can provide all the needed physiological stimuli. So, is it possible to combine the benefit of BWS and IR on triceps surae? In theory, simultaneous use of BWS and IR during a rehabilitation program could allow benefiting from the longer muscle contraction (and a better tendinous adaptation) and early access to running. Those two factors combined may lead to a faster time to return to sport.
However, at least to our knowledge, this has never been investigated until now. Thus, the aim of the present study was to investigate the interaction between BWS and IR on the EMGA of the Triceps Surae and compare it to flat running. It was hypothesized that running with a positive slope and 30% of BWS could result in EMGA comparable to level running.
2. Materials and Methods
2.1. Experimental Design
All participants performed all four experimental conditions on different days, with 7 days rest intervals between them. No familiarization session was included, kinematics familiarization time is 4 min while walking [
24], so we introduce running on the treadmill in the warm-up. The order of the four training sessions was randomized: 1) level running and without BWS (IR0BWS0), 2) incline running at 7% and 0% of BWS (IR7BWS0), 3) incline running at 7% and 15% of BWS (IR7BWS15), 4) incline running at 7% and 30% of BWS (IR7BWS30). During each trial, the electromyographic activity of the Vastus Lateralis (VL), Gastrocnemius Lateralis (GL), Medialis (GM), and Soleus (SOL) was evaluated. At the same time, before and after 2 minutes, jumping performance and low-frequency fatigue of the right quadriceps muscles were assessed. All procedures were in accordance with the Declaration of Helsinki and approved by the local university ethics committee (CERUBFC-2021-11-23-041), while all participants signed a written informed consent before entering the research procedure.
2.2. Subjects
Eighteen healthy men participate in this study (age: 20.3 ± 1.2 yr old, body weight: 70.2 ± 4.8 kg, size: 179.6 ± 5.4 cm). Participants were recruited from the Sports Department of the University of Dijon. Each participant practices an average of 6h of sport/week for a year. For inclusion, the participant needed to be clear of any lower limb injury within 6 months before the experiment. Subjects were asked to have similar activity the day before each session.
2.3. Procedures and Running Trials
Each training session begins with a 4 minutes warm-up at the preferred pace on a bicycle (CMVC20; Laroq, La-Roque, PACA, France) followed by a 3’run on the Harness Base Body Weight Support Treadmill (Airwalk ap; H/P/Cosmos, Nussdorf, BE, Germany) at 8 km/h and 0% incline with the BWS Treadmill harness on them. Then, each participant was equipped on the right leg with an EMG captor device. The running sessions lasted 10’, and the spatiotemporal gait parameters (contact time, flight time, step frequency) were taken during a 30s intervals at 1’, 5’, and 9 minutes (Optojump “Old Bare”; Microgate, Bolzano, Italy). A Borg CR10 scale was used to evaluate the rate of perceived exertion (RPE) at the 5th, 7th, and 10th minute of each running trial. All participants performed all running trials, (IR0BWS0, IR7BWS0, IR7BWS15, IR7BWS30) with random order. Before each trial, participants’ body weight was evaluated. We select two BWS protocols, a 15% and 30% body weight reduction. Indeed, the literature recommend to limit from 15% to 30% of BWS for rehabilitation program [
25,
26], in addition, 40% and more BWS lead to trunk electromyographic (EMG) modification [
27] and running pattern modification. IR was set at 7% of slope; internal work is increased at more than 5% [
28], and EMGA of the Triceps Surae increased at 7% of slope [
7]. Furthermore, it is recommended not to run beyond 7 to 10% slope without modifying the running pattern [
29,
30].
2.4. Evaluations of Jumping Performance
Before and 2 minutes after each running trial, participants performed 2 Counter Movement Jump (CMJ) on two force platform (Kforce Plates, Kinvent, Montpellier, France, 600kg weighting plateform, 320x160x30mm, sampling frequency 2,4 Ghz) with 30 seconds rest between trials. Analysis was made with the best jump height.
2.5. Evaluation of Low-Frequency Fatigue
Low Frequency Fatigue (LFF) was assessed by the recommendation of Myocène® and, respectively, as demonstrated in a recent study [
31]. The right leg is set in a force sensor (recording rate at 4 kHz). Evoked forces were assessed with muscle electrical stimulation (width of 400µs, three series of stimuli; 1- a single pulse, 2- low frequency train at 20Hz, and 3- high-frequency train 120Hz) and applied with three electrodes (MyoPro-1-electrodes, Myocene, Liège). Within 2 min, 16 sets of pulse were performed with 5s interval in-between, the stimulation intensity increased each set by 1 mA (From 25 mA to 40 mA). Anodes (5x5cm) were placed over the vastus lateralis and medialis. In addition, cathode (5x10cm) was placed on the proximal portion of the rectus femoris (transversely). The Myocène® system integrates an algorithm calculating instantaneously the LFF. Calculus were made at each set, and the median values of all ratios were given by the software, therefore, use in our analysis.
2.6. Elecromyographic Activity
The EMGA of Vastus Lateralis (VL), Gastrocnemius Lateralis (GL), Gastrocnemius Medialis (GM), and Soleus (SOL) were taken on the right leg. The participant's skin was prepared following Seniam recommendations (i.e., shave the skin, clean with alcohol, and waiting for dry skin). Surface electrodes were placed as recommended by Barbero and al. (Atlas of Muscle Innervation Zones, Understanding Surface Electromyography, and Its Applications). Muscle activities were recorded with BioNomadix 2CH Wireless EMG transmitter system from Biopac system.inc. (BN-EMG2-T) and rectangular surface Ag/AgCl electrodes (3M Health Care).
Each participant realized a 30s run before the experimental run at 10km/h and 0% IR for normalization. All EMGA datas were analyzed with the mean root mean square (mRMS; length 200ms, 20 samples, 1 point overlapping). The mRMS of the experimental run were divided into 3 intervals of 200s (total of 10’) and were evaluated in percentage of the 30s normalization run. Datas were analyzed with Acqknowledge 4.2. Each recording was collected at 2 kHz, and bandlimited from 5.0Hz to 500 Hz.
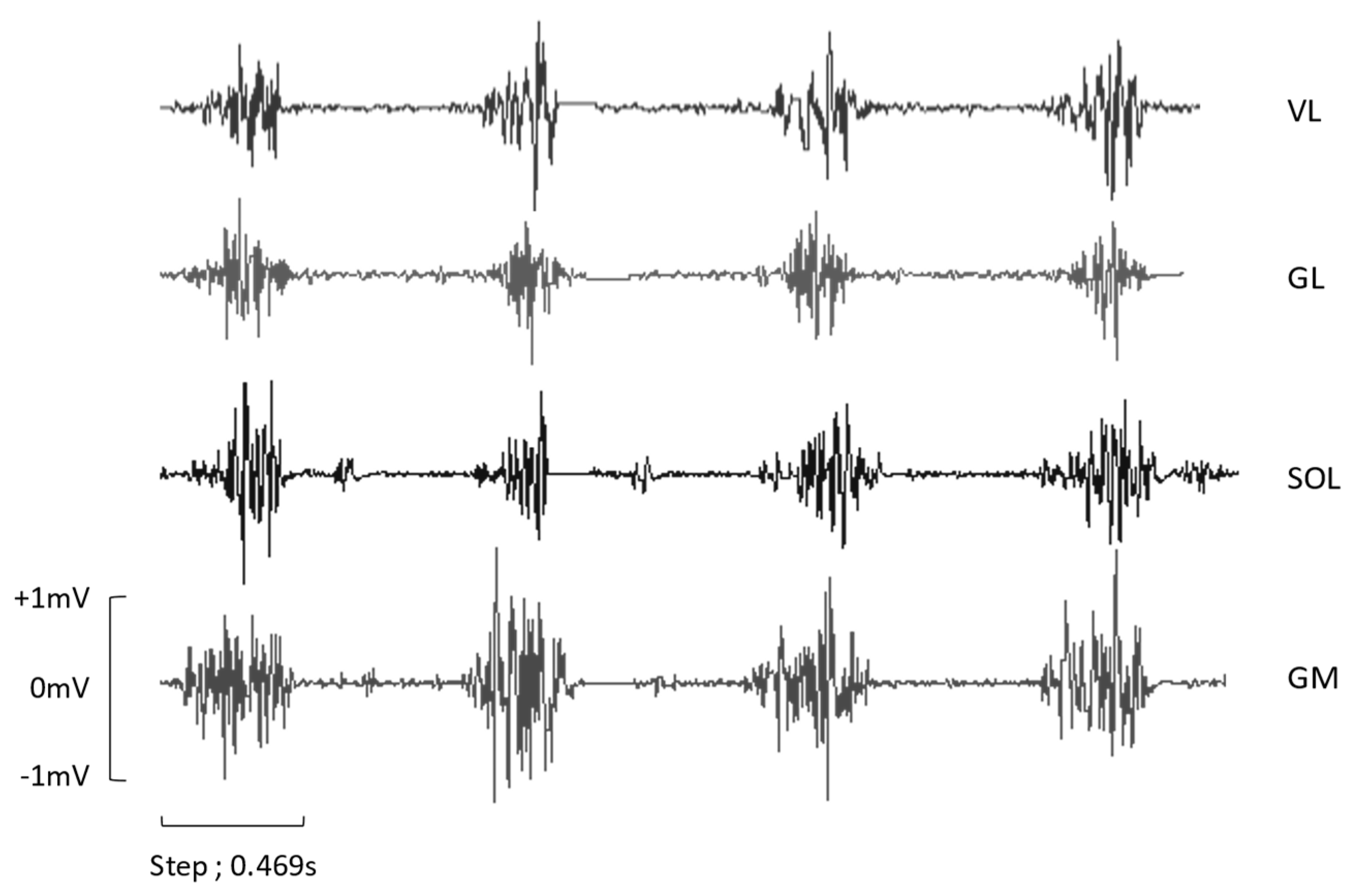
Figure 1.
ROW EMG of four steps from a participant during IR0BWS0. VL: Vastus Lateralis, GL: Gastrocnemius Lateralis, SOL: Soleus, GM: Gastrocnemius Lateralis.
Figure 1.
ROW EMG of four steps from a participant during IR0BWS0. VL: Vastus Lateralis, GL: Gastrocnemius Lateralis, SOL: Soleus, GM: Gastrocnemius Lateralis.
2.7. Statistical Analysis
All data are presented as mean and standard deviation (±SD). All data followed a normal distribution (Shapiro-Wilk test), and sphericity was respected except for RPE and mRMS GL; Greenhouse-Geisser correction was then applied. Two-way repeated analysis of variance (ANOVA; Bonferroni Post Hoc) was used to investigate the difference (Condition, Time, Condition x Time). Variable Time is composed of three moments of 200s: from onset to 200s (3,3 min), from 200s to 400s (6,6 min), and from 400s to this end (10’). Also, group-sized effects were calculated with Cohen’s d (0,2 – 0,5 small; 0,5 – 0,8 medium; >0,8 large effect). Statistical analyses were performed with JASP (JASP Team, 2021 - Version 0.16). P≤0.05 was used as a level of significance.
3. Results
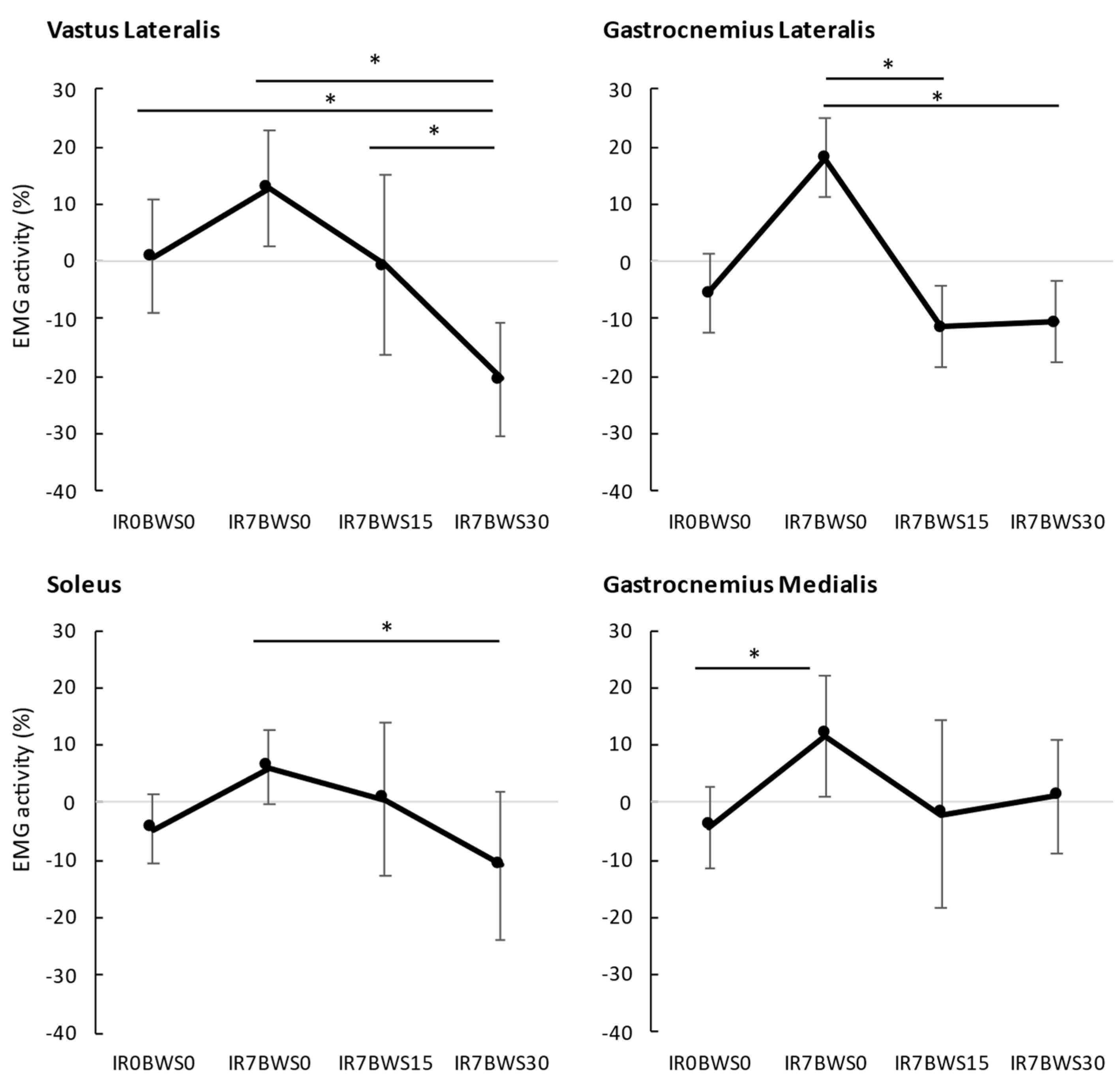
Muscles Activity. The mean EMG of each condition is represented in
Figure 2, and the percentage of EMG compared to the normalized run is shown in
Table 1.
For GL, the ANOVA has shown significative differences between Conditions F(dlc = 1.573, dlt = 12.580, p = 0.015, ηp2 = 0.346), represented in
Figure 2. A large size effect was found between IR0BWS0 and IR7BWS0 (p = 0.085, d = -0.882). Significant differences in Time were found between each three moments F(dlc = 1.690, dlt = 13.523, p < 0.001, ηp2 = 0.781). EMGA keeps decreasing with time; EMGA from the last 200s is inferior to the second one (p < 0.022), and both are inferior to the first 200s (p < 0.001). No interaction was found F(dlc = 6, dlt = 48, p = 0.446, ηp2 = 0.110).
For SOL, significant differences between Conditions were found F(dlc = 3, dlt = 21, p = 0.008, ηp2 = 0.423), presented in
Figure 2. A group effect size was shown between IR0BWS0 and IR7BWS0 (p = 0.103, Large, d = 0.916) and between IR7BWS0 and IR7BWS15 (p = 0.303, Medium, d = 0.734). Significant differences in Time were found F(dlc = 2, dlt = 14, p < 0.001, ηp2 = 0.893); as GL, EMGA from the last 200s is inferior to the second one (p < 0.007), and both are inferior to the first 200s (p < 0.001). No interaction was found between Condition x Time F(dlc = 6, dlt = 42, p = 0.538, ηp2 = 0.852).
For GM, the ANOVA has shown significant differences between Condition F(dlc = 3, dlt = 24, p = 0.017, ηp2 = 0.339) shown in
Figure 2. A group sized effect was found between IR7BWS0 and IR7BWS15 (p = 0.07, Large, d = 0.849). Significant differences in Time have been shown F(dlc = 1.129, dlt = 9.030, p = 0.013, ηp2 = 0.528); EMGA of the first 200s is significantly superior at the 2nd and 3rd moment of 200s (p < 0.001). However, no significant interaction was found F(dlc = 1.401, dlt = 11.210, p = 0.453, ηp2 = 0.085).
For VL, significant differences between Conditions were found F(dlc = 3, dlt = 15, p < 0.001, ηp2 = 0.745) presented in
Figure 2. The ANOVA did not reveal any significant differences for Time F(dlc = 2, dlt = 10, p = 0.183, ηp2 = 0.288) and the interaction Conditon x Time F(dlc = 6, dlt = 30, p = 0.626, ηp2 = 0.128).
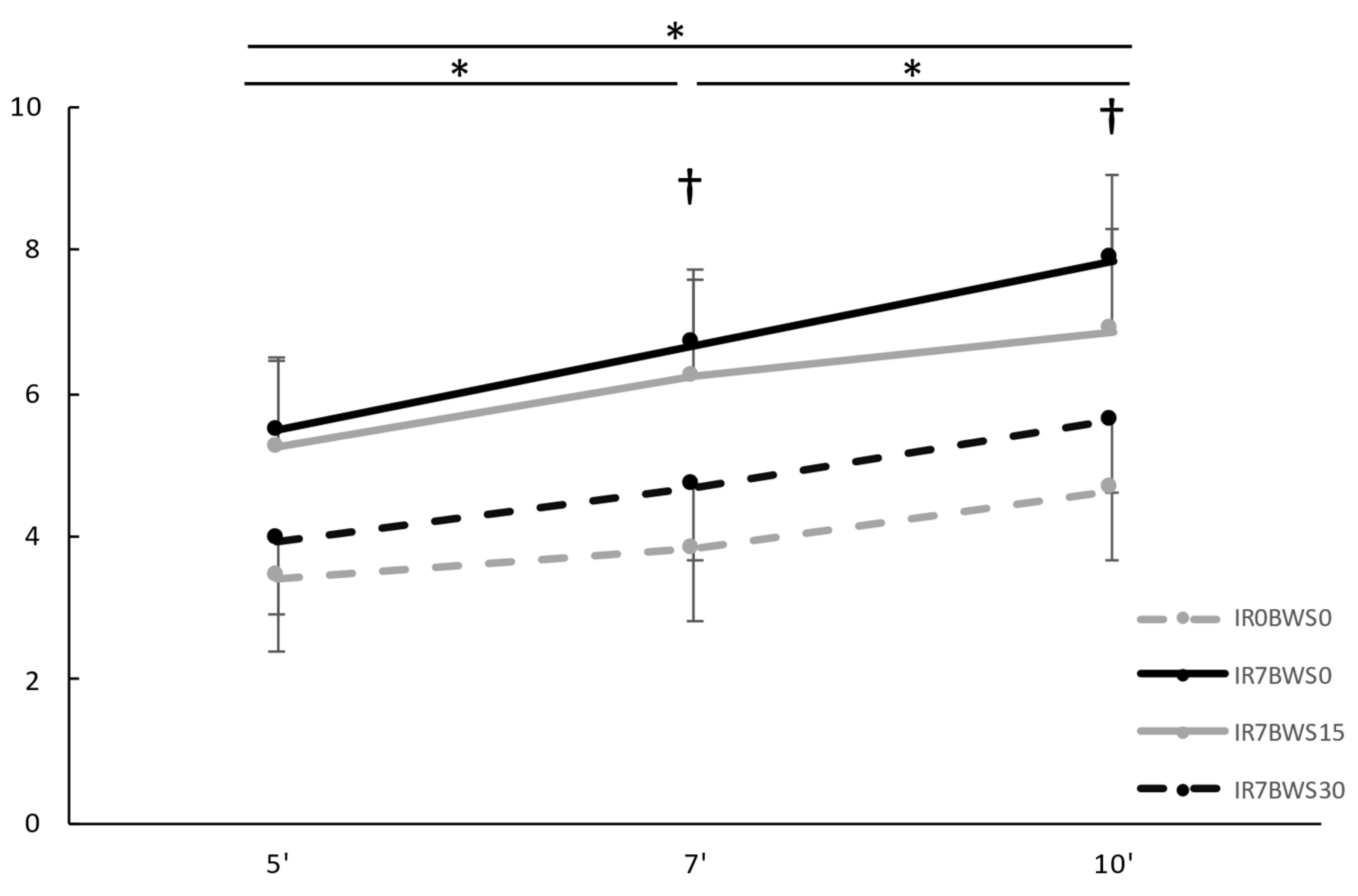
Rating of Perceived Exertion. All results are shown in
Figure 3. Our result show significant differences between Condition F(dlc = 3, dlt = 33, p < 0.001, ηp2 = 0.723); but not between IR0BWS0 and IR7BWS30 (p = 0.079). Significant differences in Time F(dlc = 1.113, dlt = 22, p < 0.001, ηp2 = 0.749), and interaction Condition x Time F(dlc = 3,259, dlt = 66, p = 0.003, ηp2 = 0.330) was found. The interaction Condition x Time has shown no significant differences between IR0BWS0 and IR7BWS30 through time (At 5’ p = 1.00; at 7’ p = 0.625 and at 10’ p = 0.625).
Jumping performance. Running at 10 km/h for 10 minutes did not affect high jump performance for all Conditions F(dlc = 3, dlt = 33, p = 0.246, ηp2 = 0.117).
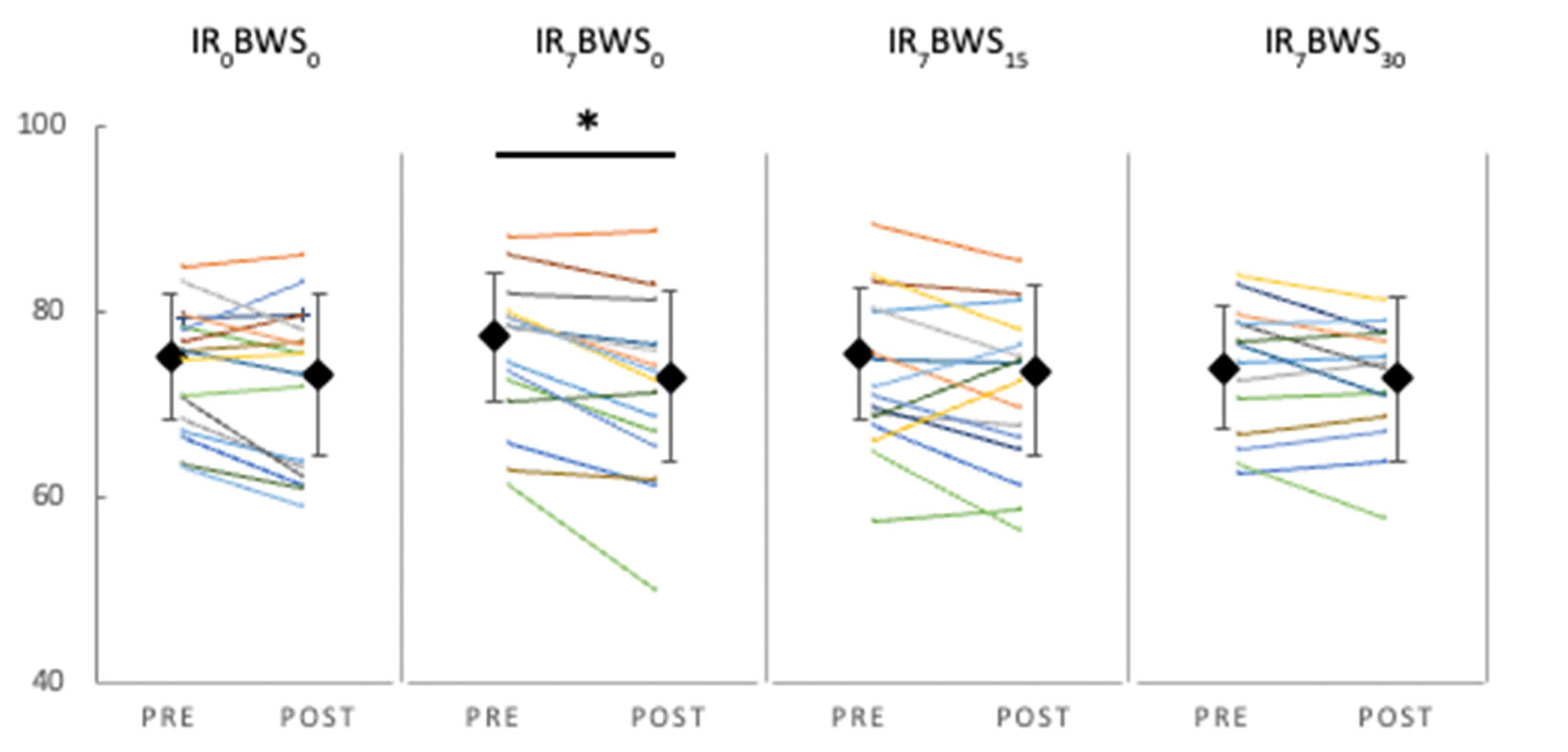
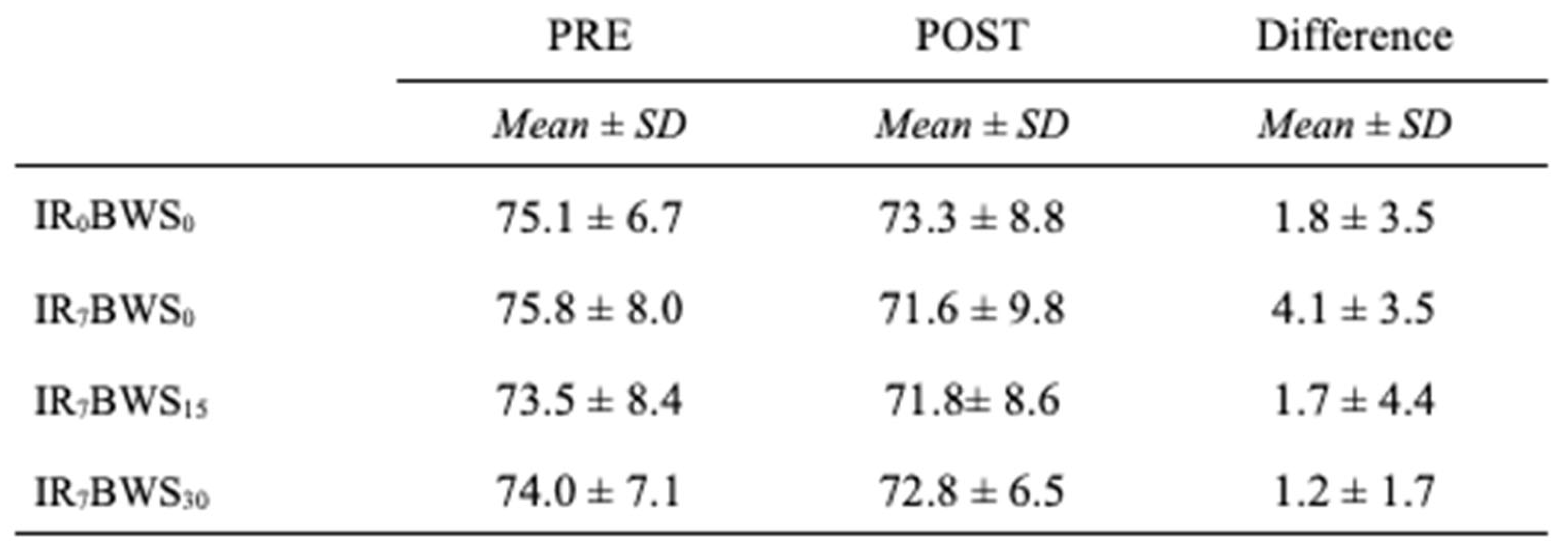
LFF. Post Values are significantly inferior to the Pre values F(dlc = 1, dlt = 8, p < 0.018, ηp2 = 0.523). Also, ANOVA revealed a significant decrease only in the LFF ratio for IR7BWS0 (p = 0.011). Results are shown in
Figure 4 and
Table 2.
.
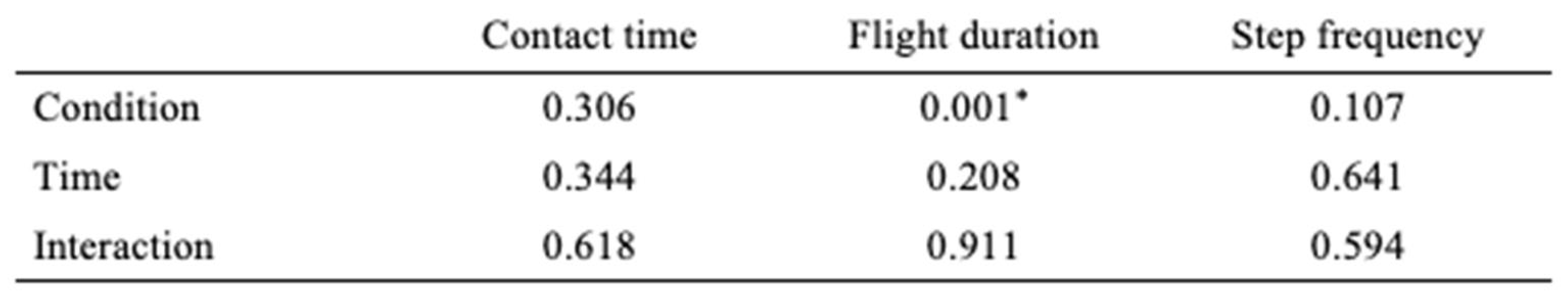
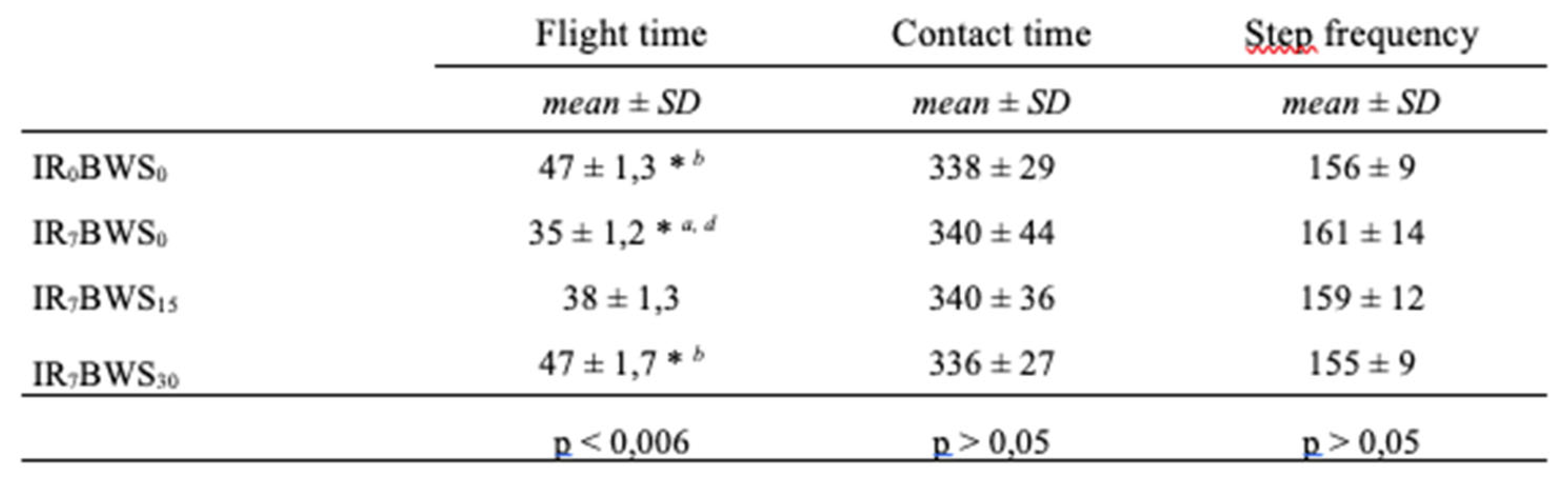
Gait spatiotemporal parameters. Our results did not demonstrate differences (p > 0,05) in gait spatiotemporal parameters (Condition, Time, or interaction of both) except in flight time. Results are presented in
Table 3 and
Table 4.
4. Discussion
The main finding of the present study was that running with a slope of 7% and 30% of BWS could result in EMG activity comparable to that observed during level running, verifying our hypothesis. Furthermore, as was hypothesized, BWS can decrease running-induced muscle fatigue (LFF).
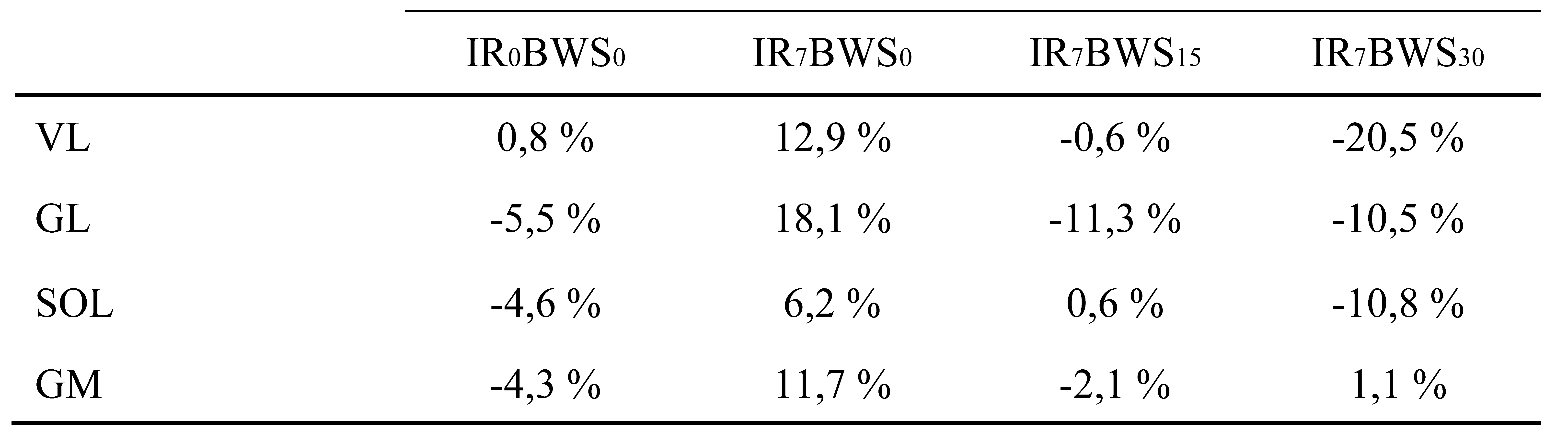
Our results correspond to previous studies that confirm an increase in EMG for GL, SOL, and GM for 7% of incline running [
7] and in mechanical power [
32]. As shown in
Figure 1, EMG of SOL seems less impacted by the slope and BWS15% than the GL and the GM. Indeed, SOL EMG increased by 10.8% from LR to IR7%, but GL and GM increased by 23.6% and 16%, respectively. On the other hand, with BWS15%, SOL EMG decreased by 4.8%, GL, and GM by 29.4%, and 13.8%. This is mainly due to muscle insertion; SOL is a monoarticular muscle that only participates in plantarflexion, and the gastrocnemius is a bi-articular muscle used for knee flexion. Another explanation of this phenomenon can be the different roles of the gastrocnemius and soleus. A previous study has shown that, in the single-leg stance period, SOL is mainly responsible for forward trunk progression and support and the gastrocnemius of the leg swing [
33]. However, this study was focused on walking locomotion, it could be interesting to elucidate the behavior of those two muscles while running.
After that, EMG of VL and triceps surae is decreased with 15% of BWS compared to incline running and has an almost similar muscular activity to level running; VL (-0.6%), SOL (0.6%), GM (-2.1%). Only GL (-11.3%) appears more affected by 15% of body weight unload. Moreover, 30% of BWS is effective to have EMG activity lower than for level running: VL (-20.5%), GL (-10.5%), SOL (-10.8%), except for GM (1.1%). However, EMG activity of gastrocnemius muscle did not decrease while unloading from 15% to 30%. Conversely, VL EMG significantly decreased from BWS15% to BWS30%. We suppose that as IR from 5% to 10% modifying running pattern (increase GM/GL EMG and mechanical work), adding more BWS could also change the running pattern. During the single leg stance from BWS15% to BWS30%, it could be possible that the percentage of work from the Gastrocnemius increases, but as the weight decreases, EMG does not change. Those results are similar to Sainton et al. [
34], who find a significant decrease in muscle activity push-off phase at 20% of BWS but not 40% for both gastrocnemii. Our results show decreases in EMG activity over time, our warm-up protocol can not be sufficient, or the time to equip the subject is too long, so tendon adaptation was done during the 10 minutes run.
The statistical analyses show a better decreased EMG for the VL than the triceps surae muscles when BWS is active. According to previous studies, the force produced by triceps surae is mostly created by the tendon's length change and not the fascicle length [
35], even with a positive or negative slope [
36]. Therefore, the main difference in our results can be explained by muscle behavior, and we can hypothesize that the subject's unloading body weight would affect the VL's EMG activity and the stretching/shortening of the Achilles Tendon. However, the muscular activity of VL seems less affected by the incline running at 7% than GL and GM (increased by 12.1% compared with 23.6% and 16%). Those results were expected, indeed, EMG activity and mechanical power of VL significantly increased from 9.1% [
37] and 10% of slope [
32,
38,
39].
Although running-induced muscle fatigue was assessed on the quadriceps, our results showed that a10-minute run at 7% of IR can create LFF compared to LR and BWS conditions. As the slope increases, the muscle's mechanical work increases, as does the LFF. Likewise, using BWS is efficient in decreasing muscle fatigue in IR. Thus, we supposed that muscle fatigue is not created by repetitive contractions but by the ground force reaction in IR conditions.
The statistical analysis showed two modulations in RPE; increasing the slope increases the RPE, and BWS leads to a lower RPE. As the unloading subject causes less ground force reaction, a decrease in RPE with BWS was expected. Those results agree with the previous study on BWS [
40,
41] and assert its utility in rehabilitation. Indeed, lower RPE may lead to a longer time of sustained exercise and, at last, increase muscle contraction time.
Furthermore, our study shows that 30% of BWS and 7% of IR are the right parameters to have similar perceived difficulty to level running (respectively, RPE is 5.6 and 4.6). Nevertheless, 15% of BWS does not seem sufficient to reduce RPE compared to flat running, but it can reduce perceived difficulty through time (at 7’ and 10’, RPE of 15% of BWS is lower than without).
According to our data, spatiotemporal parameters such as contact time and step frequency are not statistically affected by BWS and IR, even if IR suggests an increase in step frequency. We supposed that because BWS [
34,
42,
43] and IR [
5,
44,
45] have the opposite effect on step frequency, no significant differences could be found, however, flight time increases with more BWS. This is probably due by a strategy of the runner; as BWS increases, the participant lets himself be supported by the mechanical system. This explanation can also justify the decreases in RPE and the modification of the EMG activity.
5. Conclusions
Our study encourages practitioners to use body weight support to decrease total ground force reaction supported by the less electromyographic activity of triceps surae with incline running for amplifying the passive eccentric and active concentric phases of the calf muscles.
This method may have two assets; progressive loading through BWS and optimizing time contraction with incline running. All of this without increasing difficulty and have more considerable access for all kinds of populations and injury. This method can also be used in strengthening training programs without creating a decrease in a simple performance like jumping.
Author Contributions
Conceptualization, T.T., and C.P.; methodology, T.T., N.B and C. P.; software, T.T ; validation, T.T., and C.P.; formal analysis, T.T.; investigation, T.T.; resources, T.T.; data curation, T. T.; writing—original draft preparation, T.T., N.A. and C.P. .; writing—review and editing, C.P., S.M., N.B. and C.C.; visualization, C.P., S.M., N.B., N.A and C.C.; supervision, C.P.; project administration, C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding.
Institutional Review Board Statement
All procedures were in accordance with the Declaration of Helsinki and approved by the local university ethics committee (CERUBFC-2021-11-23-041).
Informed Consent Statement
Informed and written consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank the participants at the Centre d'Expertise de la Performance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Green, B.; Pizzari, T. Calf muscle strain injuries in sport: a systematic review of risk factors for injury. Br. J. Sports Med. 2017, 51, 1189–1194. [Google Scholar] [CrossRef]
- Campbell, J.T. Posterior Calf Injury. Foot Ankle Clin. 2009, 14, 761–771. [Google Scholar] [CrossRef]
- Lantto, I.; Heikkinen, J.; Flinkkilä, T.; Ohtonen, P.; Leppilahti, J. Epidemiology of Achilles tendon ruptures: Increasing incidence over a 33-year period. Scand. J. Med. Sci. Sports 2014, 25, e133–e138. [Google Scholar] [CrossRef]
- Kubo, K.; Kanehisa, H.; Fukunaga, T. Effects of different duration isometric contractions on tendon elasticity in human quadriceps muscles. J. Physiol. 2001, 536, 649–655. [Google Scholar] [CrossRef]
- Padulo, J.; Annino, G.; Migliaccio, G.M.; D'Ottavio, S.; Tihanyi, J. Kinematics of Running at Different Slopes and Speeds. J. Strength Cond. Res. 2012, 26, 1331–1339. [Google Scholar] [CrossRef]
- Abe, D.; Fukuoka, Y.; Muraki, S.; Yasukouchi, A.; Sakaguchi, Y.; Niihata, S. Effects of Load and Gradient on Energy Cost of Running. J. Physiol. Anthr. 2011, 30, 153–160. [Google Scholar] [CrossRef]
- Padulo, J.; Powell, D.; Milia, R.; Ardigò, L.P. A Paradigm of Uphill Running. PLoS ONE 2013, 8, e69006. [Google Scholar] [CrossRef]
- Järvinen, T.A.; Järvinen, T.L.; Kääriäinen, M.; Äärimaa, V.; Vaittinen, S.; Kalimo, H.; Järvinen, M. Muscle injuries: optimising recovery. Best Pract. Res. Clin. Rheumatol. 2007, 21, 317–331. [Google Scholar] [CrossRef]
- Järvinen, M.J.; Lehto, M.U.K. The Effects of Early Mobilisation and Immobilisation on the Healing Process Following Muscle Injuries. Sports Med. 1993, 15, 78–89. [Google Scholar] [CrossRef]
- Barbeau, H.; Wainberg, M.; Finch, L. Description and application of a system for locomotor rehabilitation. Med Biol. Eng. Comput. 1987, 25, 341–344. [Google Scholar] [CrossRef]
- Mehrholz, J.; Thomas, S.; Elsner, B. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst. Rev. 2017, 2017, CD002840. [Google Scholar] [CrossRef] [PubMed]
- Cherni, Y.; Begon, M.; Chababe, H.; Moissenet, F. Use of electromyography to optimize Lokomat ® settings for subject-specific gait rehabilitation in post-stroke hemiparetic patients: A proof-of-concept study. Neurophysiol. Clin. 2017, 47, 293–299. [Google Scholar] [CrossRef]
- Gama, G.L.; Trigueiro, L.C.d.L.; Simão, C.R.; de Sousa, A.V.C.; Silva, E.M.G.d.S.e.; Galvão. R.V.P.; Lindquist, A.R.R. Effects of Treadmill Inclination on Hemiparetic Gait. Am. J. Phys. Med. Rehabilitation 2015, 94, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Meyns, P.; A A Van de Crommert, H.W.; Rijken, H.; van Kuppevelt, D.H.J.M.; Duysens, J. Locomotor training with body weight support in SCI: EMG improvement is more optimally expressed at a low testing speed. Spinal Cord 2014, 52, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Fenuta, A.M.; Hicks, A.L. ; Hbsc Muscle activation during body weight-supported locomotion while using the ZeroG. J. Rehabilitation Res. Dev. 2014, 51, 51–58. [Google Scholar] [CrossRef]
- Atan, T.; Taşkiran, Ö. Ö; Tokçaer, A.B.; Karataş, G.K.; Çalişkan, A.K.; Karaoğlan, B. Effects of different percentages of body weight-supported treadmill training in Parkinson’s disease: a double-blind randomized controlled trial. Turk. J. Med Sci. 2019, 49, 999–1007. [Google Scholar] [CrossRef]
- Hreljac, A. Etiology, Prevention, and Early Intervention of Overuse Injuries in Runners: a Biomechanical Perspective. Phys. Med. Rehabilitation Clin. North Am. 2005, 16, 651–667. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, A.M.; Kram, R. Effects of Velocity and Weight Support on Ground Reaction Forces and Metabolic Power during Running. J. Appl. Biomech. 2008, 24, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Granot, A. Use of an Anti-gravity Treadmill in the Rehabilitation of the Operated Achilles Tendon: A Pilot Study. J. Foot Ankle Surg. 2011, 50, 558–561. [Google Scholar] [CrossRef]
- Luo, Y.; Shen, W.; Jiang, Z.; Sha, J. Treadmill training with partial body-weight support after anterior cruciate ligament reconstruction: a randomized controlled trial. J. Phys. Ther. Sci. 2016, 28, 3325–3329. [Google Scholar] [CrossRef]
- Johnson, A.W.; Eastman, C.S.; Feland, J.B.; Mitchell, U.H.; Mortensen, B.B.; Eggett, D. Effect of High-Speed Treadmill Training With a Body Weight Support System in a Sport Acceleration Program With Female Soccer Players. J. Strength Cond. Res. 2013, 27, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Liebenberg, J.; Scharf, J.; Forrest, D.; Dufek, J.S.; Masumoto, K.; Mercer, J.A. Determination of muscle activity during running at reduced body weight. J. Sports Sci. 2011, 29, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Mun, K.-R.; Bin Lim, S.; Guo, Z.; Yu, H. Biomechanical effects of body weight support with a novel robotic walker for over-ground gait rehabilitation. Med Biol. Eng. Comput. 2016, 55, 315–326. [Google Scholar] [CrossRef]
- Matsas, A.; Taylor, N.; McBurney, H. Knee joint kinematics from familiarised treadmill walking can be generalised to overground walking in young unimpaired subjects. Gait Posture 2000, 11, 46–53. [Google Scholar] [CrossRef]
- Thomson, A.; Whiteley, R.; Hansen, C.; Welzel, J.; Racinais, S.; Wilson, M.G. Effect of speed and gradient on plantar force when running on an AlterG® treadmill. BMC Sports Sci. Med. Rehabilitation 2021, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.G.; Wolf, A. Assessment of the effects of body weight unloading on overground gait biomechanical parameters. Clin. Biomech. 2015, 30, 454–461. [Google Scholar] [CrossRef]
- Swinnen, E.; Baeyens, J.-P.; Pintens, S.; Van Nieuwenhoven, J.; Ilsbroukx, S.; Clijsen, R.; Buyl, R.; Goossens, M.; Meeusen, R.; Kerckhofs, E. Trunk muscle activity during walking in persons with multiple sclerosis: The influence of body weight support1. NeuroRehabilitation 2014, 34, 323–335. [Google Scholar] [CrossRef]
- Minetti, A.E.; Ardigò, L.P.; Saibene, F. Mechanical Determinants of the Minimum Energy Cost of Gradient Running in Humans. J. Exp. Biol. 1994, 195, 211–225. [Google Scholar] [CrossRef]
- Telhan, G.; Franz, J.R.; Dicharry, J.; Wilder, R.P.; Riley, P.O.; Kerrigan, D.C. Lower Limb Joint Kinetics During Moderately Sloped Running. J. Athl. Train. 2010, 45, 16–21. [Google Scholar] [CrossRef]
- Vernillo, G.; Martinez, A.; Baggaley, M.; Khassetarash, A.; Giandolini, M.; Horvais, N.; Edwards, W.B.; Millet, G.Y. Biomechanics of graded running: Part I - Stride parameters, external forces, muscle activations. Scand. J. Med. Sci. Sports 2020, 30, 1632–1641. [Google Scholar] [CrossRef]
- Ridard, J.; Rozand, V.; Millet, G.Y.; Lapole, T. On-field low-frequency fatigue measurement after repeated drop jumps. Front. Physiol. 2022, 13, 1039616. [Google Scholar] [CrossRef]
- Nuckols, R.W.; Takahashi, K.Z.; Farris, D.J.; Mizrachi, S.; Riemer, R.; Sawicki, G.S. Mechanics of walking and running up and downhill: A joint-level perspective to guide design of lower-limb exoskeletons. PLOS ONE 2020, 15, e0231996. [Google Scholar] [CrossRef]
- Neptune, R.R.; Kautz, S.A.; Zajac, F.E. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J. Biomech. 2001, 34, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Sainton, P.; Nicol, C.; Cabri, J.; Barthelemy-Montfort, J.; Berton, E.; Chavet, P. Influence of short-term unweighing and reloading on running kinetics and muscle activity. Eur. J. Appl. Physiol. 2015, 115, 1135–1145. [Google Scholar] [CrossRef]
- Fukunaga, T.; Kubo, K.; Kawakami, Y.; Fukashiro, S.; Kanehisa, H.; Maganaris, C.N. In vivo behaviour of human muscle tendon during walking. Proc. R. Soc. B: Boil. Sci. 2001, 268, 229–233. [Google Scholar] [CrossRef]
- Lichtwark, G.A.; Wilson, A.M. Interactions between the human gastrocnemius muscle and the Achilles tendon during incline, level and decline locomotion. J. Exp. Biol. 2006, 209, 4379–4388. [Google Scholar] [CrossRef] [PubMed]
- Yokozawa, T.; Fujii, N.; Ae, M. Muscle activities of the lower limb during level and uphill running. J. Biomech. 2007, 40, 3467–3475. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.J.; Belliveau, R.A. Sources of mechanical power for uphill running in humans. J. Exp. Biol. 2005, 208, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Wall-Scheffler, C.M.; Chumanov, E.; Steudel-Numbers, K.; Heiderscheit, B. Electromyography activity across gait and incline: The impact of muscular activity on human morphology. Am. J. Phys. Anthr. 2010, 143, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Ruckstuhl, H.; Schlabs, T.; Rosales-Velderrain, A.; Hargens, A.R. Oxygen Consumption During Walking and Running Under Fractional Weight Bearing Conditions. Aviat. Space, Environ. Med. 2010, 81, 550–554. [Google Scholar] [CrossRef]
- Hoffman, M.D.; Donaghe, H.E. Physiological Responses to Body Weight–Supported Treadmill Exercise in Healthy Adults. Arch. Phys. Med. Rehabilitation 2011, 92, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Barnes, K.R.; Janecke, J.N. Physiological and Biomechanical Responses of Highly Trained Distance Runners to Lower-Body Positive Pressure Treadmill Running. Sports Med. - Open 2017, 3, 41–41. [Google Scholar] [CrossRef] [PubMed]
- Raffalt, P.C.; Hovgaard-Hansen, L.; Jensen, B.R. Running on a Lower-Body Positive Pressure Treadmill: VO2max, Respiratory Response, and Vertical Ground Reaction Force. Res. Q. Exerc. Sport 2013, 84, 213–222. [Google Scholar] [CrossRef]
- Dewolf, A.H.; Peñailillo, L.E.; Willems, P.A. The rebound of the body during uphill and downhill running at different speeds. J. Exp. Biol. 2016, 219, jeb–142976. [Google Scholar] [CrossRef]
- Okudaira, M.; Willwacher, S.; Kawama, R.; Ota, K.; Tanigawa, S. Sprinting kinematics and inter-limb coordination patterns at varying slope inclinations. J. Sports Sci. 2021, 39, 2444–2453. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).