Submitted:
26 July 2023
Posted:
27 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Study site and species
2.2. Nitrogen addition experiment
2.3. Measurements of vegetative and flower trait
2.4. Measurements of pollinator visitation
2.5. Measurements of seed traits
2.6. Data analysis
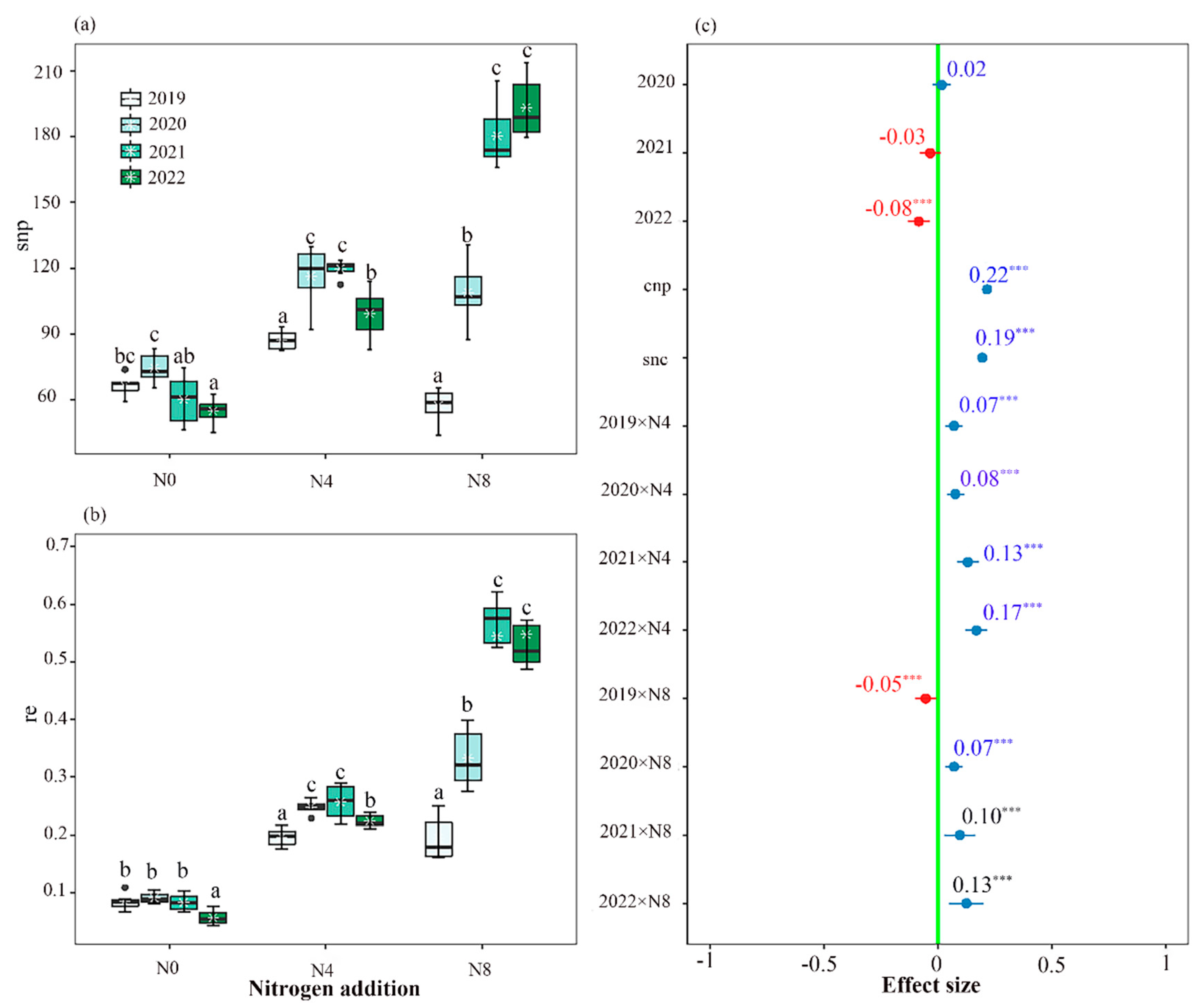
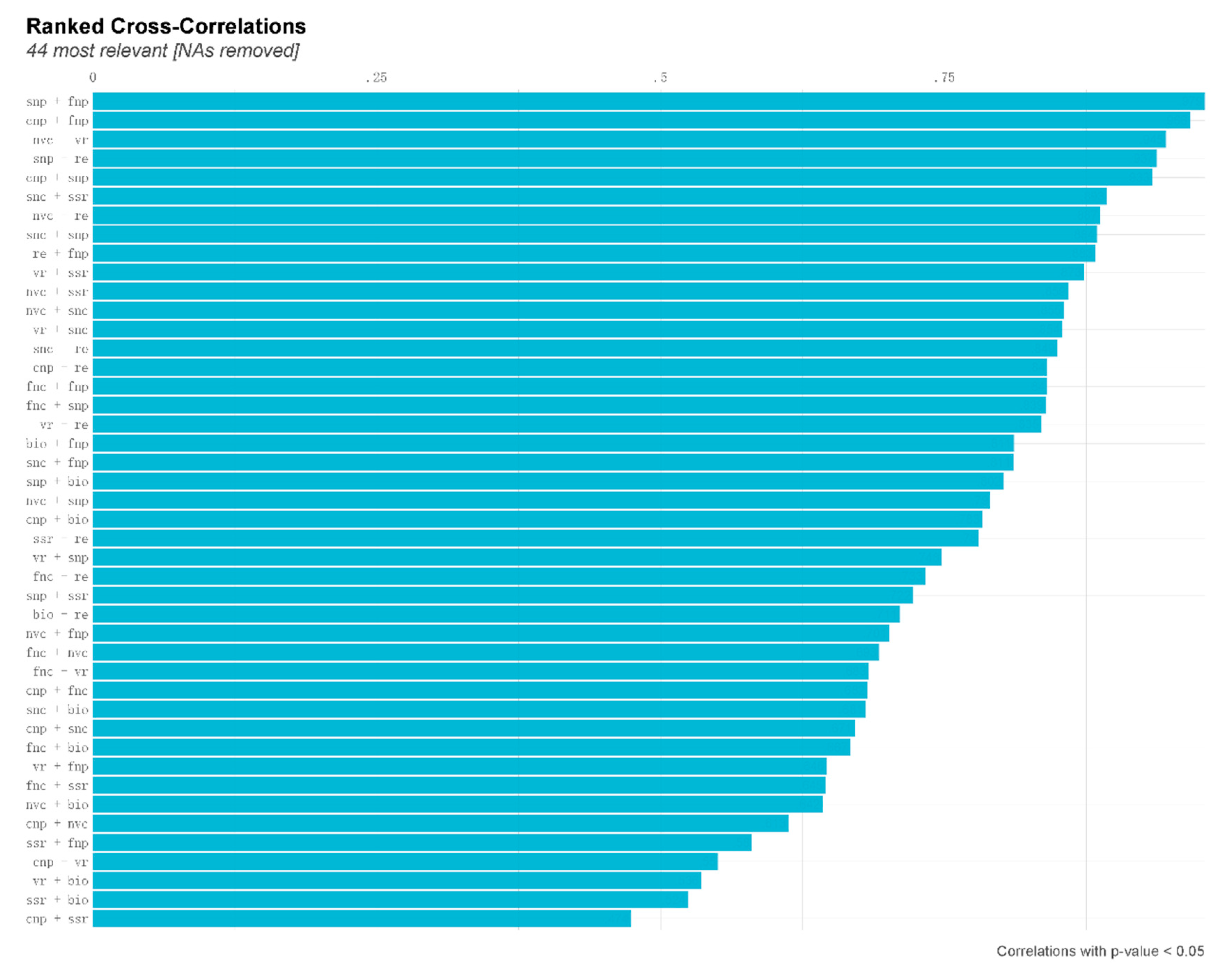
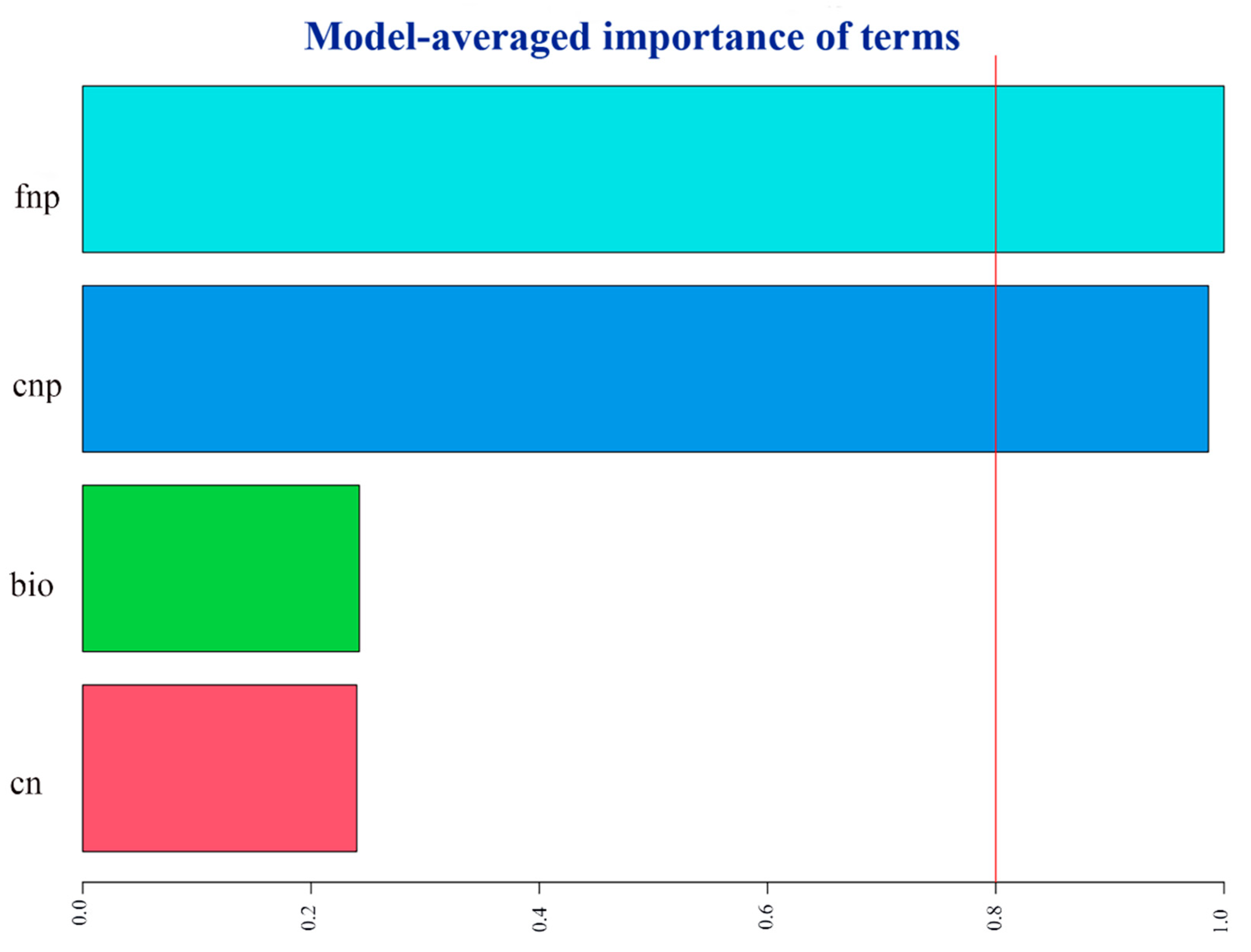
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedman, J. The Evolution of Annual and Perennial Plant Life Histories: Ecological Correlates and Genetic Mechanisms. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 461–481. [Google Scholar] [CrossRef]
- Herrera, C.M. Population-level estimates of interannual variability in seed production: what do they actually tell us? Oikos 1998, 82, 612–616. [Google Scholar] [CrossRef]
- Pearse, I.S.; LaMontagne, J.M.; Koenig, W.D. Inter-annual variation in seed production has increased over time (1900–2014). Proc. R. Soc. B: Boil. Sci. 2017, 284, 20171666. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Yang, Y.; Patch, H.M.; Grozinger, C.M.; Mu, J. Soil moisture affects plant–pollinator interactions in an annual flowering plant. Philos. Trans. R. Soc. B: Biol. Sci. 2022, 377, 20210423. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhou, X.; Luo, Y.; Yang, Y.; Fang, C.; Chen, J.; Li, B. Minor stimulation of soil carbon storage by nitrogen addition: A meta-analysis. Agric. Ecosyst. Environ. 2011, 140, 234–244. [Google Scholar] [CrossRef]
- Deng, Q.; Hui, D.; Dennis, S.; Reddy, K.C.; Xu, X. Responses of terrestrial ecosystem phosphorus cycling to nitrogen addition: A meta-analysis. Glob. Ecol. Biogeogr. 2017, 26, 713–728. [Google Scholar] [CrossRef]
- Grechi, I.; Vivin, P.; Hilbert, G.; Milin, S.; Robert, T.; Gaudillère, J.P. Effect of light and nitrogen supply on internal C: N balance and control of root-to-shoot biomass allocation in grapevine. Environ. Exp. Bot. 2007, 59, 139–149 101016/jenvexpbot200511002. [Google Scholar] [CrossRef]
- Metay, A.; Magnier, J.; Guilpart, N.; Christophe, A. Nitrogen supply controls vegetative growth, biomass and nitrogen allocation for grapevine (cv. Shiraz) grown in pots. Funct. Plant Biol. 2015, 42, 105–114. [Google Scholar] [CrossRef]
- Vaudo, A.D.; Erickson, E.; Patch, H.M.; Grozinger, C.M.; Mu, J. Impacts of soil nutrition on floral traits, pollinator attraction, and fitness in cucumbers (Cucumis sativus L.). Sci. Rep. 2022, 12, 1–12. [Google Scholar] [CrossRef]
- Wang, R.; Lou, Y.; Deng, D.; Che, P.; Zhao, C.; Yang, Y.; Mu, J. Summer grazing change fitness in a Tibetan lotus. Glob. Ecol. Conserv. 2023, 44. [Google Scholar] [CrossRef]
- Bobbink, R.; Lamers, L.P.M. Effects of increased nitrogen deposition. In Air Pollution and Plant Life. Chichester, UK, 2002; pp. 201–235.
- Drewniak, B.; Gonzalez-Meler, M.A. Earth System Model Needs for Including the Interactive Representation of Nitrogen Deposition and Drought Effects on Forested Ecosystems. Forests 2017, 8, 267. [Google Scholar] [CrossRef]
- Burkle, L.A.; Irwin, R.E. Beyond biomass: measuring the effects of community-level nitrogen enrichment on floral traits, pollinator visitation and plant reproduction. J. Ecol. 2010, 98, 705–717. [Google Scholar] [CrossRef]
- Hoover, S.E.R.; Ladley, J.J.; Shchepetkina, A.A.; Tisch, M.; Gieseg, S.P.; Tylianakis, J.M. Warming, CO2, and nitrogen deposition interactively affect a plant-pollinator mutualism. Ecol. Lett. 2012, 15, 227–234. [Google Scholar] [CrossRef]
- Ceulemans, T.; Hulsmans, E.; Ende, W.V.; Honnay, O. Nutrient enrichment is associated with altered nectar and pollen chemical composition in Succisa pratensis Moench and increased larval mortality of its pollinator Bombus terrestris L. PLOS ONE 2017, 12, e0175160. [Google Scholar] [CrossRef]
- David, T.I.; Storkey, J.; Stevens, C.J. Understanding how changing soil nitrogen affects plant–pollinator interactions. Arthropod-Plant Interactions 2019, 13, 671–684. [Google Scholar] [CrossRef]
- Bleeker, A.; Hicks, W.K.; Dentener, F.; Galloway, J.N.; Erisman, J.W. Nitrogen Deposition as a Threat to the World’s Protected Areas Under the Convention on Biological Diversity (CBD). 2014, 295–303. [CrossRef]
- Liu, X.; Zhang, Y.; Han, W.; Tang, A.; Shen, J.; Cui, Z.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Christie, P.; et al. Enhanced nitrogen deposition over China. Nature 2013, 494, 459–462. [Google Scholar] [CrossRef]
- Bowman, W.D.; Cleveland, C.C.; Halada, .; Hreško, J.; Baron, J.S. Negative impact of nitrogen deposition on soil buffering capacity. Nat. Geosci. 2008, 1, 767–770. [CrossRef]
- Lü, C.; Tian, H. Spatial and temporal patterns of nitrogen deposition in China: Synthesis of observational data. J. Geophys. Res. Atmos. 2007, 112. [Google Scholar] [CrossRef]
- Mu, J.; Peng, Y.; Xi, X.; Wu, X.; Griffin, J.N.; Niklas, K.J.; Sun, S. Domesticated honeybees evolutionarily reduce flower nectar volume in a Tibetan lotus. Ecology 2014, 95, 3161–3172. [Google Scholar] [CrossRef]
- Su, R.; Dai, W.; Yang, Y.; Wang, X.; Gao, R.; He, M.; Zhao, C.; Mu, J. Introduced honey bees increase host plant abundance but decrease native bumble bee species richness and abundance. Ecosphere 2022, 13. [Google Scholar] [CrossRef]
- Xia, J.; Wan, S. Independent effects of warming and nitrogen addition on plant phenology in the Inner Mongolian steppe. Ann. Bot. 2013, 111, 1207–1217. [Google Scholar] [CrossRef]
- Real, L.A.; Rathcke, B.J. Individual Variation in Nectar Production and Its Effect on Fitness in Kalmia Latifolia. Ecology 1991, 72, 149–155. [Google Scholar] [CrossRef]
- Vaudo, A.D.; Patch, H.M.; Mortensen, D.A.; Grozinger, C.M.; Tooker, J.F. Bumble bees exhibit daily behavioral patterns in pollen foraging. Arthropod-Plant Interactions 2014, 8, 273–283. [Google Scholar] [CrossRef]
- Arroyo, M.T.K.; Armesto, J.J.; Primack, R.B. Community studies in pollination ecology in the high temperate Andes of central Chile II. effect of temperature on visitation rates and pollination possibilities. Plant Syst. Evol. 1985, 149, 187–203. [Google Scholar] [CrossRef]
- Bates, D.M.; Maechler, M. Lme 4: Linear mixed-effects models using S4 classes. R package version. 2011, 0.999375-42 [Computer software]. Available at http://cran.r-project.org/web/packages/lme4.
- Chen, J.; Luo, Y.; Van, Groenigen, K.J.; Hungate, B.A.; Cao, J.; Zhou, X.; Wang, R.W. A keystone microbial enzyme for nitrogen control of soil carbon storage. Sci. Adv. 2018, 4(8), eaaq1689. [CrossRef]
- Terrer, C.; Vicca, S.; Hungate, B.A.; Phillips, R.P.; Prentice, I.C. Mycorrhizal association as a primary control of the CO 2 fertilization effect. Science 2016, 353, 72–74. [Google Scholar] [CrossRef]
- Niu, S.; Wu, M.; Han, Y.; Xia, J.; Zhang, Z.; Yang, H.; Wan, S. Nitrogen effects on net ecosystem carbon exchange in a temperate steppe. Glob. Chang. Biol. 2010, 16, 144–155. [Google Scholar] [CrossRef]
- Yan, Z.; Eziz, A.; Tian, D.; Li, X.; Hou, X.; Peng, H.; Han, W.; Guo, Y.; Fang, J. Biomass Allocation in Response to Nitrogen and Phosphorus Availability: Insight From Experimental Manipulations of Arabidopsis thaliana. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Leith, I.D.; Hicks, W.K.; Fowler, D.; Woodin, S.J. Differential responses of UK upland plants to nitrogen deposition. New Phytol. 1999, 141, 277–289. [Google Scholar] [CrossRef]
- Verma, P.; Sagar, R.; Verma, H.; Verma, P.; Singh, D.K. Changes in species composition, diversity and biomass of herbaceous plant traits due to N amendment in a dry tropical environment of India. J. Plant Ecol. 2014, 8, 321–332. [Google Scholar] [CrossRef]
- Mu, J.; Peng, Y.; Xi, X.; Wu, X.; Li, G.; Niklas, K.J.; Sun, S. Artificial asymmetric warming reduces nectar yield in a Tibetan alpine species of Asteraceae. Ann. Bot. 2015, 116, 899–906. [Google Scholar] [CrossRef]
- Burkle, L.A.; Irwin, R.E. The importance of interannual variation and bottom–up nitrogen enrichment for plant–pollinator networks. Oikos 2009, 118, 1816–1829. [Google Scholar] [CrossRef]
- Shuel, R.W. SOME ASPECTS OF THE RELATION BETWEEN NECTAR SECRETION AND NITROGEN, PHOSPHORUS, AND POTASSIUM NUTRITION. Can. J. Plant Sci. 1957, 37, 220–236. [Google Scholar] [CrossRef]
- Gardener, M.C.; Gillman, M.P. The effects of soil fertilizer on amino acids in the floral nectar of corncockle, Agrostemma githago (Caryophyllaceae). Oikos 2001, 92, 101–106. [Google Scholar] [CrossRef]
- Gijbels, P.; Ende, W.V.D.; Honnay, O. Landscape scale variation in nectar amino acid and sugar composition in a Lepidoptera pollinated orchid species and its relation with fruit set. J. Ecol. 2013, 102, 136–144. [Google Scholar] [CrossRef]
- Gijbels, P.; Ceulemans, T.; Van den Ende, W.; Honnay, O. Experimental fertilization increases amino acid content in floral nectar, fruit set and degree of selfing in the orchid Gymnadenia conopsea. Oecologia 2015, 179, 785–795. [Google Scholar] [CrossRef]
- Búrquez, A.; Corbet, S.A. Do flowers reabsorb nectar? Funct. Ecol. 1991,5, 369–379. [CrossRef]
- Castellanos, M.C.; Wilson, P.; Thomson, J.D. Dynamic nectar replenishment in flowers of Penstemon (Scrophulariaceae). Am. J. Bot. 2002, 89, 111–118. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Irwin, R.E.; Flanagan, R.J.; Karron, J.D. Ecology and evolution of plant–pollinator interactions. Ann. Bot. 2009, 103, 1355–1363. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Ren, Z.-X.; Lázaro, A.; Wang, H.; Bernhardt, P.; Li, H.-D.; Li, D.-Z. Floral traits influence pollen vectors’ choices in higher elevation communities in the Himalaya-Hengduan Mountains. BMC Ecol. 2016, 16, 1–8. [Google Scholar] [CrossRef]
- Sponsler, D.; Iverson, A.; Steffan-Dewenter, I. Pollinator competition and the structure of floral resources. Ecography 2023, e06651, 1–16. [Google Scholar] [CrossRef]
- Wright, G.A.; Nicolson, S.W.; Shafir, S. Nutritional Physiology and Ecology of Honey Bees. Annu. Rev. Èntomol. 2018, 63, 327–344. [Google Scholar] [CrossRef]
- Waller, G.D. Evaluating responses of honeybees to sugar solutions using an artificial-flower feeder. Ann. Entomol. Soc. Am. 1972, 65, 857–862. [Google Scholar] [CrossRef]
- Crane, E. Bee products. Bee World 1972, 53: 38–39.
- Burkle, L.A.; Runyon, J.B. Floral volatiles structure plant–pollinator interactions in a diverse community across the growing season. Funct. Ecol. 2019, 33, 2116–2129. [Google Scholar] [CrossRef]
- Mu, J.; Wu, Q.; Yang, Y.; Huang, M.; Grozinger, C.M. Plant reproductive strategies vary under low and high pollinator densities. Oikos 2018, 127, 1081–1094. [Google Scholar] [CrossRef]
- Vico, G.; Manzoni, S.; Nkurunziza, L.; Murphy, K.; Weih, M. Trade-offs between seed output and life span – a quantitative comparison of traits between annual and perennial congeneric species. New Phytol. 2015, 209, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Delph, L.F.; Meagher, T.R. Sexual Dimorphism Masks Life History Trade-Offs in the Dioecious Plant Silene Latifolia. Ecology 1995, 76, 775–785. [Google Scholar] [CrossRef]
- Poulton, J.L.; Bryla, D.; Koide, R.T.; Stephenson, A.G. Mycorrhizal infection and high soil phosphorus improve vegetative growth and the female and male functions in tomato. New Phytol. 2002, 154, 255–264. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




