Submitted:
21 July 2023
Posted:
27 July 2023
You are already at the latest version
Abstract
Keywords:
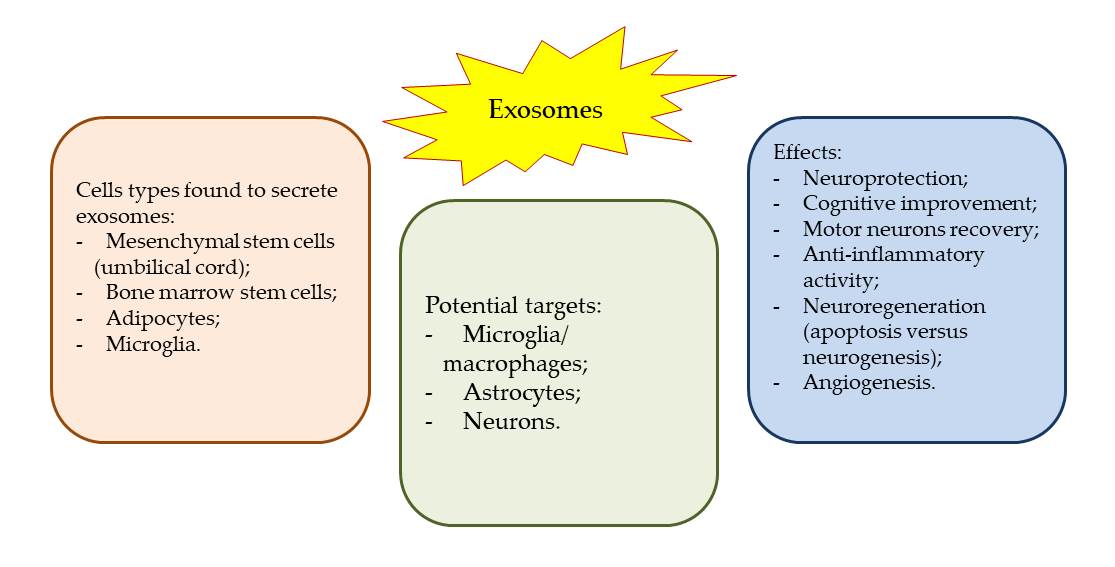
1. Introduction
2. Materials and Methods
2.1. Literature search
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Analysis
2.4. Data Synthesis
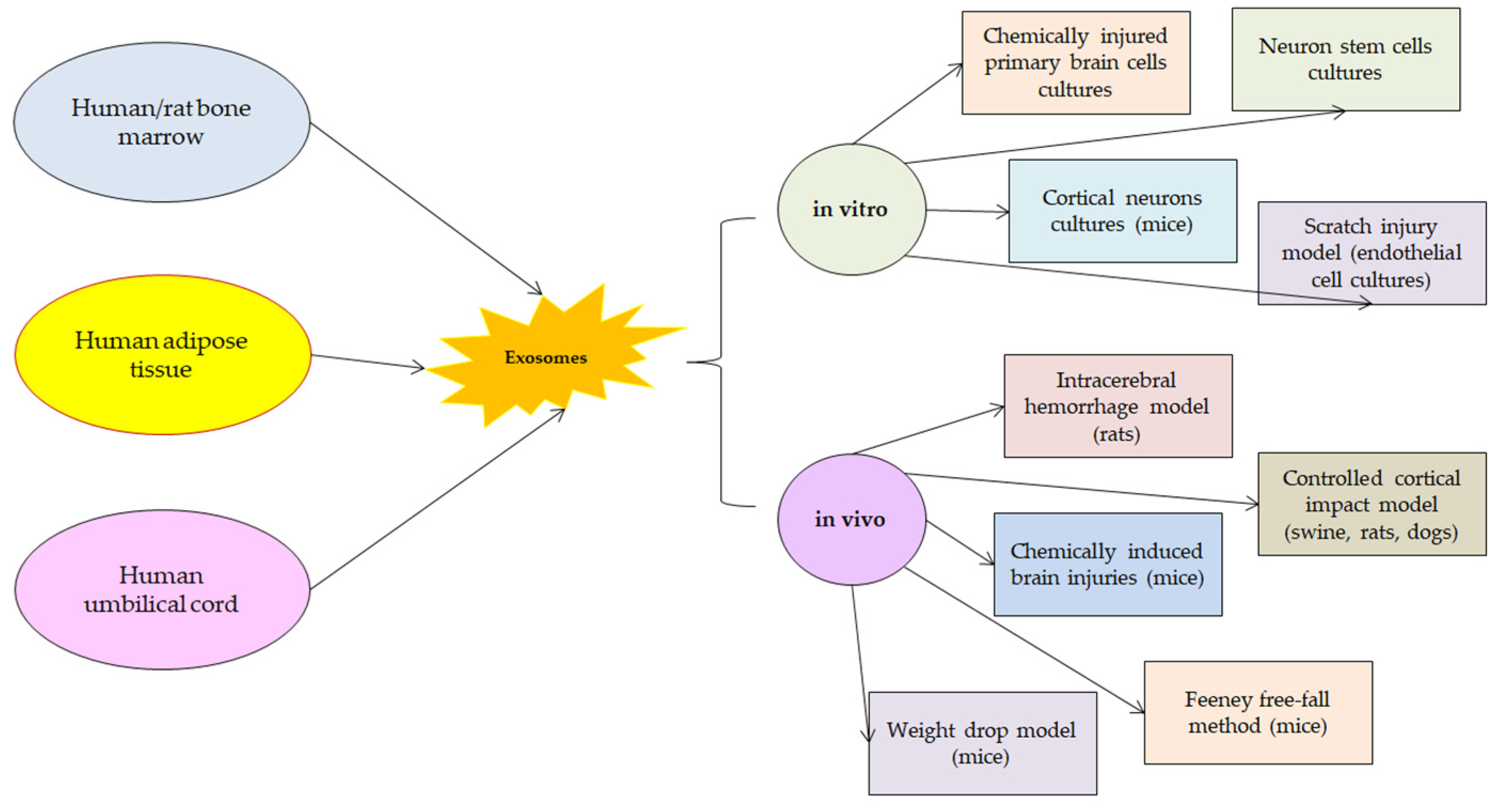
3. Results
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
References
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2018, 9, 19. [Google Scholar] [CrossRef]
- Edgar, J.R. Q&A: What are exosomes, exactly? BMC Biol. 2016, 14, 46. [Google Scholar] [CrossRef]
- Chen, H.; Wang, L.; Zeng, X.; Schwarz, H.; Nanda, H.S.; Peng, X.; Zhou, Y. Exosomes, a New Star for Targeted Delivery. Front. Cell Dev Biol, 2021, 9, 751079. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Invest. 2016, 126, 1208–1215. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol 2018, 19, 213–228. [Google Scholar] [CrossRef]
- McAndrews, K.M.; Kalluri, R. Mechanisms associated with biogenesis of exosomes in cancer. Mol. Cancer, 2019, 18, 52. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol, 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular vesicle heterogeneity: subpopulations, isolation techniques, and diverse functions in cancer progression. Front. Immunol 2018, 9, 738. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef]
- Moore, M.; Sandsmark, D.K. Clinical Updates in Mild Traumatic Brain Injury (Concussion). Neuroimaging Clin N Am. 2023, 33(2), 271–278. [Google Scholar] [CrossRef]
- Mavroudis, I.; Kazis, D.; Chowdhury, R.; Petridis, F.; Costa, V.; Balmus, I.M.; Ciobica, A.; Luca, A.C.; Radu, I.; Dobrin, R.P.; Baloyannis, S. Post-Concussion Syndrome and Chronic Traumatic Encephalopathy: Narrative Review on the Neuropathology, Neuroimaging and Fluid Biomarkers. Diagnostics (Basel). 2022, 12(3), 740. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, S.; Ge, X.; Yin, Z.; Li, M.; Guo, M.; Hu, T.; Han, Z.; Kong, X.; Li, D.; Zhao, J.; Wang, L.; Liu, Q.; Chen, F.; Lei, P. Mesenchymal stromal cell treatment attenuates repetitive mild traumatic brain injury-induced persistent cognitive deficits via suppressing ferroptosis. J Neuroinflammation. 2022, 19(1), 185. [Google Scholar] [CrossRef] [PubMed]
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; Gangoda, L.; Mathivanan, S. ExoCarta: A web-based compendium of exosomal cargo. J. Mol. Biol, 2016; 428, 688–692. [Google Scholar] [CrossRef]
- Pathan, M.; Fonseka, P.; Chitti, S.V.; Kang, T.; Sanwlani, R.; Van Deun, J.; Hendrix, A.; Mathivanan, S. Vesiclepedia 2019: A compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res 2019, 47, D516–D519. [Google Scholar] [CrossRef] [PubMed]
- van Balkom, B.W.; Eisele, A.S.; Pegtel, D.M.; Bervoets, S.; Verhaar, M.C. Quantitative and qualitative analysis of small RNAs in human endothelial cells and exosomes provides insights into localized RNA processing, degradation and sorting. J. Extracell. Vesicles 2015, 4, 26760. [Google Scholar] [CrossRef] [PubMed]
- Lasda, E.; Parker, R. Circular RNAs co-precipitate with extracellular vesicles: A possible mechanism for circRNA clearance. PLOS ONE 2016, 11, e0148407. [Google Scholar] [CrossRef]
- Chevillet, J.R.; Kang, Q.; Ruf, I.K.; Briggs, H.A.; Vojtech, L.N.; Hughes, S.M.; Cheng, H.H.; Arroyo, J.D.; Meredith, E.K.; Gallichotte, E.N.; Pogosova-Agadjanyan, E.L.; Morrissey, C.; Stirewalt, D.L.; Hladik, F.; Yu, E.Y.; Higano, C.S.; Tewari, M. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc. Natl. Acad. Sci. 2014, 111, 14888–14893. [Google Scholar] [CrossRef]
- Wen, S.W.; Lima, L.G.; Lobb, R.J.; Norris, E.L.; Hastie, M.L.; Krumeich, S.; Möller, A. Breast cancer-derived exosomes reflect the cell-of-origin phenotype. Proteomics 2019, 19, e1800180. [Google Scholar] [CrossRef]
- Xian, P.; Hei, Y.; Wang, R.; Wang, T.; Yang, J.; Li, J.; Di, Z.; Liu, Z.; Baskys, A.; Liu, W.; Wu, S.; Long, Q. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics. 2019, 9(20), 5956–5975. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Ma, B.; Li, N.; Wang, S.; Sun, Z.; Xue, C.; Han, Q.; Wei, J.; Zhao, RC. MSC-derived exosomes promote recovery from traumatic brain injury via microglia/macrophages in rat. Aging (Albany NY). 2020, 12(18), 18274–18296. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Wang, P.; Zhong, L.; Wang, S.; Feng, Q.; Wei, X.; Zhou, L. Hypoxia-pretreated mesenchymal stem cell-derived exosomes-loaded low-temperature extrusion 3D-printed implants for neural regeneration after traumatic brain injury in canines. Front Bioeng Biotechnol. 2022, 10, 1025138. [Google Scholar] [CrossRef]
- Li, D.; Huang, S.; Yin, Z.; Zhu, J.; Ge, X.; Han, Z.; Tan, J.; Zhang, S.; Zhao, J.; Chen, F.; Wang, H.; Lei, P. Increases in miR-124-3p in Microglial Exosomes Confer Neuroprotective Effects by Targeting FIP200-Mediated Neuronal Autophagy Following Traumatic Brain Injury. Neurochem Res. 2019, 44(8), 1903–1923. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Chopp, M.; Zhang, ZG.; Mahmood, A.; Xiong, Y. Mesenchymal Stem Cell-Derived Exosomes Improve Functional Recovery in Rats After Traumatic Brain Injury: A Dose-Response and Therapeutic Window Study. Neurorehabil Neural Repair. 2020, 34(7), 616–626. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Garg, S.; Ghosh, S. Cell-Derived Exosome Therapy: A Novel Approach to Treat Post-traumatic Brain Injury Mediated Neural Injury. ACS Chem Neurosci. 2020, 11(14), 2045–2047. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.M.; Dennahy, I.S.; Bhatti, U.F.; Halaweish, I.; Xiong, Y.; Chang, P.; Nikolian, V.C.; Chtraklin, K.; Brown, J.; Zhang, Y.; Zhang, Z.G.; Chopp, M.; Buller, B.; Alam, H.B. Mesenchymal Stem Cell-Derived Exosomes Provide Neuroprotection and Improve Long-Term Neurologic Outcomes in a Swine Model of Traumatic Brain Injury and Hemorrhagic Shock. J Neurotrauma. 2019, 36(1), 54–60. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Seyfried, D.; Meng, Y.; Yang, D.; Schultz, L.; Chopp, M.; Seyfried, D. Multipotent mesenchymal stromal cell-derived exosomes improve functional recovery after experimental intracerebral hemorrhage in the rat. J Neurosurg. 2018, 131(1), 290–300. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, C.; Zhang, Y.; Chen, S.; Ding, J.; Chen, Z.; Wu, K.; Wu, X.; Zhou, T.; Zeng, M.; Wei, D.; Sun, J.; Fan, H.; Zhou, L. Hyaluronan-based hydrogel integrating exosomes for traumatic brain injury repair by promoting angiogenesis and neurogenesis. Carbohydr Polym. 2023, 306, 120578. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, Y.; Zhang, R.; Wen, L.; Wu, K.; Li, Y.; Yao, Y.; Duan, R.; Jia, Y. Bone Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Recovery Following Spinal Cord Injury via Improvement of the Integrity of the Blood-Spinal Cord Barrier. Front Neurosci. 2019, 13, 209. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, Y.; Bai, W.; Sun, L.; Tian, M. Human umbilical cord mesenchymal stem cell-derived exosome suppresses programmed cell death in traumatic brain injury via PINK1/Parkin-mediated mitophagy. CNS Neurosci Ther. 2023. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Luo, W.; Jiang, W.; Li, H.; Xu, J.; Liu, X.; Wang, B.; Wang, J.; Chen, G. Human umbilical cord mesenchymal stem cell-derived exosomes promote neurological function recovery in rat after traumatic brain injury by inhibiting the activation of microglia and astrocyte. Regen Ther. 2022, 21, 282–287. [Google Scholar] [CrossRef]
- Williams, A.M.; Higgins, G.A.; Bhatti, U.F.; Biesterveld, B.E.; Dekker, S.E.; Kathawate, R.G.; Tian, Y.; Wu, Z.; Kemp, M.T.; Wakam, G.K.; Liu, B.; Li, Y.; Buller, B.; Alam, H.B. Early treatment with exosomes following traumatic brain injury and hemorrhagic shock in a swine model promotes transcriptional changes associated with neuroprotection. J Trauma Acute Care Surg. 2020, 89(3), 536–543. [Google Scholar] [CrossRef]
- Williams, A.M.; Wu, Z.; Bhatti, U.F.; Biesterveld, B.E.; Kemp, M.T.; Wakam, G.K.; Vercruysse, C.A.; Chtraklin, K.; Siddiqui, A.Z.; Pickell, Z.; Dekker, S.E.; Tian, Y.; Liu, B.; Li, Y.; Buller, B.; Alam, H.B. Early single-dose exosome treatment improves neurologic outcomes in a 7-day swine model of traumatic brain injury and hemorrhagic shock. J Trauma Acute Care Surg. 2020, 89(2), 388–396. [Google Scholar] [CrossRef]
- Ge, X.; Guo, M.; Hu, T.; Li, W.; Huang, S.; Yin, Z.; Li, Y.; Chen, F.; Zhu, L.; Kang, C.; Jiang, R.; Lei, P.; Zhang, J. Increased Microglial Exosomal miR-124-3p Alleviates Neurodegeneration and Improves Cognitive Outcome after rmTBI. Mol Ther. 2020, 28(2), 503–522. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ge, X.; Yu, J.; Han, Z.; Yin, Z.; Li, Y.; Chen, F.; Wang, H.; Zhang, J.; Lei, P. Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J. 2018, 32(1), 512–528. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, Y.; Wang, D.; Yan, W.; Zhang, S.; Li, D.; Han, Z.; Chen, F.; Lei, P. MiR-124-3p attenuates brain microvascular endothelial cell injury in vitro by promoting autophagy. Histol Histopathol. 2022, 37(2), 159–168. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y. G.; Wang, J.L.; Zhang, Y.X.; Li, L.; Reza, A.M.M.T.; Gurunathan, S. Biogenesis, Composition and Potential Therapeutic Applications of Mesenchymal Stem Cells Derived Exosomes in Various Diseases. Int J Nanomed. 2023, 18, 3177–3210. [Google Scholar] [CrossRef] [PubMed]
- Yari, H.; Mikhailova, M.V.; Mardasi, M.; Jafarzadehgharehziaaddin, M.; Shahrokh, S.; Thangavelu, L.; Ahmadi, H.; Shomali, N.; Yaghoubi, Y.; Zamani, M.; Akbari, M.; Alesaeidi, S. Emerging role of mesenchymal stromal cells (MSCs)-derived exosome in neurodegeneration-associated conditions: a groundbreaking cell-free approach. Stem Cell Res Ther. 2022, 13(1), 423. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Wang, S.; Zhao, R.C. Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomarker Res. 2019, 7, 8. [Google Scholar] [CrossRef]
- Reza-Zaldivar, E.E. , Hernández-Sapiéns, M.A., Minjarez, B., Gutiérrez-Mercado, Y.K., Márquez-Aguirre, A.L., Canales-Aguirre, A.A. Potential Effects of MSC-Derived Exosomes in Neuroplasticity in Alzheimer's Disease. Front Cel Neurosci. 2018, 12, 317. [Google Scholar] [CrossRef]
- Fayazi, N.; Sheykhhasan, M.; Soleimani Asl, S.; Najafi, R. Stem Cell-Derived Exosomes: a New Strategy of Neurodegenerative Disease Treatment. Mol. Neurobiol. 2021, 58(7), 3494–3514. [Google Scholar] [CrossRef]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10(8), 1959. [Google Scholar] [CrossRef]
- Rarinca, V.; Nicoara, M.; Ciobica, A.; Mavroudis, I. A short editorial view on the relevance of exosomes in some neuropsychiatric manifestations - model studies, Academy of Romanian Scientists Annals, Series on Biological Sciences. 2022, Academy of Romanian Scientists Volume 11, No. 2, 124-126. [CrossRef]
| Study | Objective | Models and administration | Key Findings |
|---|---|---|---|
| [19] | The therapeutic effects of MSC-Exo on astrocytic alterations induced by inflammation | In vitro: in cell cultures, hippocampal astrocytic inflammation was obtained by administering lipopolysaccharide to primary cultures;In vivo: in mice, pilocarpine administration produced status epilepticus.Treatment with MSC-Exo was performed in both cases. | The studied exosomes were incorporated into hippocampal astrocytes and attenuated reactive astrogliosis and inflammatory responses. They also ameliorated learning and memory impairments in mice with inflammation-induced astrocytic activation. |
| [20] | The effects of MSC-Exo on functional recovery, neuroinflammation, neuronal apoptosis, and neurogenesis | In vivo: weight-drop TBI rat model Human adipose MSC-Exo were administered intracerebroventricularly by microinjection | The studied exosomes promoted functional recovery, suppressed neuroinflammation, reduced neuronal apoptosis, and increased neurogenesis. They specifically entered microglia/macrophages and suppressed their activation during brain injury. |
| [21] | The potential of MSC-derived exosomes to regenerate the brain after TBI | In vivo: TBI canine model (Beagle dogs receiving small shocks using an electronic cortical contusion impactor through a hole in the skull).Hypoxia-treated human umbilical cord mesenchymal stem cells-derived exosomes were used for a 3D-printed collagen/silk fibroin/exosomes scaffold. | Exosomal treatment significantly promoted neuroregeneration and angiogenesis and inhibited nerve cell apoptosis and proinflammatory factor expression. Neuroregenerative properties and good biocompatibility were observed for hypoxia-induced human umbilical cord-derived MSC exosomes. |
| [23] | The effects of MSC-Exo in regarding the doses and times of administration | In vivo: unilateral moderate cortical concussion rat models.Single intravenous administration of exosomes at different time points after brain injury. | Exosome treatment improved sensorimotor and cognitive function, reduced hippocampal neuronal cell loss, promoted angiogenesis and neurogenesis, and reduced neuroinflammation.Earlier treatment administration (1-day post-TBI) provided a significantly greater improvement in functional and histological outcomes, as compared to delayed treatments. |
| [25] | The effects of MSC-Exo from bone marrow when administered in large animal models of TBI and hemorrhagic shock | In vivo: swine models that received TBI and hemorrhagic shock by a computer-controlled cortical impact device. MSC-Exo were separated from a single human donor of bone marrow and administered intravenously at 9h, 1, 5, 9, 13 days post-injury. | A single dose of MSC-Exo could attenuate the effects of the brain injury and provide better functional neurological recovery.They speculate that in large animal models the effects of MSC-Exo could be the result of central nervous system and peripheral immune response modulation. |
| [26] | The effects of multipotent MSC-Exo on functional recovery, neurovascular remodeling, and neurogenesis | In vivo: Wistar rat model of intracerebral hemorrhageMSC-Exo were administered in the tail at 24h after the injury | MSC-Exo promoted significant improvement of functional neurological recovery (spatial learning and motor functions). It was speculated that MSC-Exo could modulate endogenous angiogenesis and neurogenesis. |
| [27] | The efficiency of hyaluronan-collagen hydrogel incorporated bone marrow MSC-Exo in TBI treatment | In vitro: neuronal stem cells cultures | MSC-Exo incorporated in hydrogel could induce angiogenesis and neurogenesis. Axonal regeneration, remyelination, and synapse formation were observed. Brain structural remodeling could also be obtained by MSC-Exo treatment suggesting that neurological functional recovery after TBI could be achieved. |
| [28] | The effects of bone marrow MSC-Exo treatment in spinal cord injury. The potential mechanisms through which MSC-Exo protect the blood spinal cord barrier. | In vivo: rat model of spinal cord injury carried out by contusive injury with a spinal cord impactorMSC-Exo were isolated from rat bone marrow and administered intravenous 1-day post-injury. | It was showed that MSC-Exo could promote regeneration and neuronal survival reduced brain cell death, enhanced neuronal survival and regeneration, and improved motor functions.NF-κB p65 signaling in pericytes was identified as a possible action pathway of MSC-Exo. |
| [29] | The protective effects of human umbilical cord MSC-Exo in both in vivo and in vitro TBI models | In vitro: mice cortical neurons cultures exposed to mechanical stretch injury.In vivo: mice models of TBI (controlled cortical impact).Human umbilical cord MSC-Exo were obtained from expanded cultures and administered in 5 different doses in animal models, and in 3 doses in cultured neurons. | Improvement of neurological function, and decrease of cerebral edema were found to contribute to brain regeneration after TBI lesion. MSC-Exo suppressed apoptosis, pyroptosis, and ferroptosis and modulated PINK1/Parkin pathway-mediated mitophagy. |
| [30] | The mechanism through which umbilical cord MSC-Exo promote repair after TBI injury | In vivo: rat model of Feeney free-fall method for brain injuryMSC-Exo were isolated from 3rd-5th generation of umbilical cord stem cells cultures | MSC-Exo treatment promoted functional recovery and reduced neuronal apoptosis after TBI injury. Also, microglia and astrocytes were inhibited. |
| [31] | The molecular mechanisms that are contributing to brain repair after TBI injury and treatment with MSC-Exo | In vivo: swine model of severe TBI and hemorrhagic shock Bone marrow derived MSC-Exo were administered intravenously 1 hour after the injury. | Genes expression associated with neurogenesis, neuronal development, synaptogenesis, and neuroplasticity were significantly increased. Genes expression associated with stroke, neuroinflammation, neuroepithelial cell proliferation, and non-neuronal cell proliferation were significantly reduced. |
| [32] | The impact of early single-dose exosome treatment in a 7-day survival model | In vivo: swine model of severe TBI and hemorrhagic shock Bone marrow derived MSC-Exo were administered intravenously 1 hour after the injury. | It was showed that one dose of exosomes extract could improve survival of a large TBI model by decreasing brain lesion size, inhibiting inflammation and apoptosis, and promoting neural plasticity, when administered early. |
| [33] | The role of miRNAs in regulating post-traumatic neurodegeneration | In vivo: mice TBI models (close impact injury) | miR-124-3p modulated neurodegeneration and inhibited β-amyloid abnormalities. miR-124-3p could pass through the blood-brain barrier. miR-124-3p improved the cognitive outcome after repetitive mild TBI. |
| [34] | To explore the regulatory mechanism of microglial exosomes on neuronal inflammation in TBI by investigating the impact of microglial exosomal miRNAs on injured neurons | In vivo: mice model of controlled cortical impact In vitro: scratch injury model of cultured neurons | miR-124-3p inhibited neuronal inflammation and contributed to neurite outgrowth. |
| [35] | The effects of miR-124-3p on brain microvascular endothelial cells function and their molecular mechanisms | In vitro: specific cell scratch wound model for endothelial cell injury.Overexpression of miR-124-3p in endothelial cells was performed by treatment with Lipofectamine3000. | miR-124-3p overexpression prevented apoptosis and reduced blool-brain barrier leakage.miR-124-3p could be implicated in mTOR signaling and autophagy modulation in endothelial cells. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





