1. Introduction
The glycemic index (GI) is a physiological assessment of a food's carbohydrate content through its effect on postprandial blood glucose concentrations. GI is measured as the incremental blood glucose area (0-2 h) following ingestion of, usually, 50 g of available carbohydrates as compared with 50 g of carbohydrates from a reference (glucose or white bread) [
1]. Taking glucose as a reference, the values of GI could be classified as high (above 70), medium (56-69), and low (≤ 55) [
2]. High-GI foods are considered unhealthy because their frequent consumption may increase the risk of developing overweight, obesity, type 2 diabetes, and cardiovascular disease [
2,
3].
The consumption of high-GI foods induces a rapid and high transient increase in postprandial glucose; conversely, the consumption of low-GI food is associated with health benefits because it induces a lower blood glucose response [
4]. A low-GI diet improves insulin sensitivity in obese children with high baseline insulin [
5]. Thus, the effects exerted by the consumption of high-GI foods might negatively affect the satiety response, stimulating a proportionally high insulin response, inducing a low blood glucose and fatty acid concentration somewhat early in the postprandial period, mimicking a low fuel state [
6]. Nevertheless, it has been informed that foods with high-GI values might increase satiety and decrease hunger at different points in time in the postprandial period [
1,
2,
7].
Honey is a natural substance produced by bees (
Apis mellifera) from nectar, and it is considered one of the last untreated natural food substances. Honey composition is complex, and influenced by several factors, such as geographical origin, botanical source of nectar, environmental and climatic conditions, as well as processing techniques, like pasteurization or sterilization process [
8,
9]. Botanical and geographical origins are considered the most important factors that determine the specific composition and properties of all types of honey [
10]. According to their botanical origin, honey varieties can be classified as Monofloral or Multifloral. Honey has lower GI and energy value than sugar as a result of its complex composition [
10]. A mean GI value of 61 has been established for honey [
11]; although various factors could alter it, such as the carbohydrate composition and the bioactive substances of honey, such as phenolic compounds. Since these factors greatly vary depending on the botanical origin and geographical location, it is essential to conduct studies assessing the effect of those variations on the GI and other related properties.
Mexico is the fifth-largest producer and the third-largest exporter of honey around the World; consequently, the beekeeping industry in Mexico has a high social and economic value [
12,
13]. Despite the relevance of the beekeeping market, the GI and the potential satiety effect of Mexican honey have not been extensively investigated. Larson-Meyer
, et al. [
14] indicated that as part of a meal, honey improves the satiety postprandial response, increasing anorectic hormones such as the peptide tyrosine-tyrosine (PPY), and delaying orectic hormones such as ghrelin. On the contrary, Gourdomichali and Papakonstantinou [
15] did not find an effect on satiety by honey consumption. The objective of this study was to evaluate the GI and the response to the satiety of Mexican kinds of honey from different botanical and geographical origin.
2. Materials and Methods
2.1 Honey Samples
Three different types of Mexican kinds of honey from
Apis mellifera with different geographical and flora origins were used to carry out the study: Multifloral, Avocado (
Persea americana), and Highland honey. The Multifloral honey was harvested in Campeche state (southeastern region of Mexico); the avocado honey was harvested in Michoacan state, in the avocado-producing region; and Highland honey was harvested in Mexico state (highland region of Mexico). Honey samples were purchased from Hermes Honey (Aguascalientes, Ags., 20337, México); a company certificated by the USDA ORGANIC and the Europe United (
https://hermeshoney.com/calidad/). Samples belonged to the 2019 harvest, which was stored at room temperature and protected from light until analysis.
2.2 Fructose and Glucose Content by HPLC
An Agilent 1100 HPLC (Agilent Technologies, Palo Alto, Ca. USA) was used to quantify fructose and glucose. An aliquot of 20 μL was injected into an Agilent Hi-Plex Ca 7.7 x 300 mm, 8 μm column at 85 °C with a flow rate of 0.6 ml/min. Pure water was used as an eluent. Detection was carried out in an Agilent Refractive Index Detector [
16]. Fructose and glucose calibration curves (0-5 mg/ml) were used for quantification. All samples were injected in triplicate.
2.3 Study Design
This protocol was approved by the ethics committee of the Facultad de Salud Pública y Nutricion (FaSPyN) with the identifier CE 2/2018-19; in addition, all experimental procedures were conducted according to the guidelines laid down in the Declaration of Helsinki of 1975, revised in 2013, and all procedures involving human subjects were made under the regulation of the General Law on Health Research. A cross-sectional study was carried out at Centro de Investigación en Nutrición y Salud Pública from the Autonomous University of Nuevo Leon, with residents of Monterrey, Nuevo León, México. Volunteers of 18 to 40 years old were recruited, with previous acceptance to participate by signing informed consent. Inclusion criteria included: normal body mass index (BMI) 18-24.9 kg/m
2 [
17] and absence of chronic non-contagious diseases, which was corroborated by biochemical studies, including blood chemistry and glycosylated hemoglobin (HbA1c). Participants with a special physiological state, such as pregnancy and lactation, and people with a different physical condition that impeded obtaining anthropometric parameters were excluded.
2.4 Glycemic Response
The GI of honey samples was evaluated using the proposed protocol by Jenkins
, et al. [
18] with some modifications. Briefly, participants arrived at the laboratory under fasting conditions (at less 12 h) to be administered with anhydrous dextrose or honey. Capillary blood glucose was taken at 0, 15, 30, 60, 90, and 120 minutes. 0 minutes was considered as the time participant ingested honey or anhydrous dextrose for the first time. The procedure was repeated using seventy grams of honey with 150 ml of water. The amount of honey administered provided 50 g of available carbohydrates. Then, the glucose concentration was plotted to calculate the area under the curve (AUC), considering 80 mg/dL as the baseline value. The GI was calculated as the mean relation of honey AUC divided by the dextrose AUC, expressing results as a percentage according to equation (1).
2.5 Satiety Response
During the GI test, the satiety response was also evaluated. All participants answered the question “how hungry are you right now?, indicating their satiety level by a visual analogous scale (VAS) [
19]. Subjects rated hunger on a 100 mm line, anchored on the left by “not hungry at all” and on the right by “extremely hungry”. Ratings were performed during the development of curve glucose and the test of honey samples at 0, 15, 30, 45, 60, 90, and 120 minutes. The area under the curve of all rating values was determined, and the average values were calculated and plotted.
2.6 Statistical Analyses
Statistical analyses were conducted using the statistical software JMP version 9.0. Results are given as mean ± standard error of the mean. Differences in GI and satiety response among honey varieties were determined by analysis of Tukey's HSD. Statistical significance was selected at a level of P≤0.05.
3. Results and Discussion
3.1 Fructose and Glucose Content
In this study, three different samples of honey, with a different botanical and geographical origins, were analyzed.
Table 1 indicates the values of fructose and glucose, as well as the ratio F/G. Honey samples presented values of fructose ranging from 272.40-395.10 g/kg, while the glucose value ranged from 232.20-355.50 g/kg. The results of fructose and glucose were statistically different between the honey samples (
Table 1). The ratio F/G of honey samples were 1.45, 1.00, and 1.17 to Highland, Multifloral, and Avocado kinds of honey, respectively.
To our knowledge, the content of fructose and glucose has not been reported on a sample of Avocado and Highland honey of Mexican origin. In general, honey contains 35-40 % fructose and 30-35 % glucose; in authentic honey, the ratio of fructose and glucose is between 1 and 1.2 [
20]. Sugar composition is affected by botanical origin, geographical origin, climate, processing, and storage [
9]. Generally, fructose is the carbohydrate present in the greatest proportion, but in some kinds of honey, such as rape (Brassica napus) and dandelion (Taraxacum officinale) honey, the fraction of glucose is higher than the fraction of fructose; therefore, these honey have a rapid crystallization [
9,
21]. The concentration of fructose and glucose is used as an indicator for the classification of Monofloral honey [
21].
The content of fructose and glucose has been determined in Highland honey from Turkey, which was 409.1 and 275.60 g/kg, respectively; while the mean F/G ratio was 1.43 [
22]; results that are similar to those found, in this study, to highland honey sample. In addition, the FAO has established the ratio F/G for flower honey, which should be between 0.9 and 1.4 [
23]. The F/G ratio found in this study for all honey samples agrees with the values reported by FAO [
23] (
Table 1). On the other hand, the value of fructose and glucose found in Multifloral honey sample is similar to those reported by Mondragón-Cortez, Ulloa, Rosas-Ulloa, Rodríguez-Rodríguez and Resendiz Vázquez [
13] who indicated values of 372.80 to 409.10 and 307.10 to 321.0 g/kg for fructose and glucose, respectively, in Multifloral honey from the western region of Mexico.
Honey is an important natural sweetener due to its high simple sugars content (80%); however, it is healthier than sugar because it contains other important substances such as phenolic compounds, which influence human health [
10,
24].
3.2 Anthropometry and Biochemical Data
Twenty-six participants completed this study.
Table 2 shows the baseline conditions of all participants. The mean age of participants was 23.16±3.73 years. The mean values of serum glucose and HbA1c were 84.29±7.00 mg/dL and 5.38±0.23 %, respectively. The cholesterol, triglycerides, HDL, LDL, and VLDL showed mean values of 149.44±19.96, 68.40±29.88, 53.77±12.65, 82.01±17.22 and 13.07±5.13 mg/dL, respectively. The mean weight of participants was 61.12±11.69 kg, while the mean value of height was 1.62±0.007 m; therefore, participants show a mean value of BMI (body mass index) of 23.19±3.07. These results were considered to choose the participants to be included in the study.
3.3 The Glycemic Index (GI)
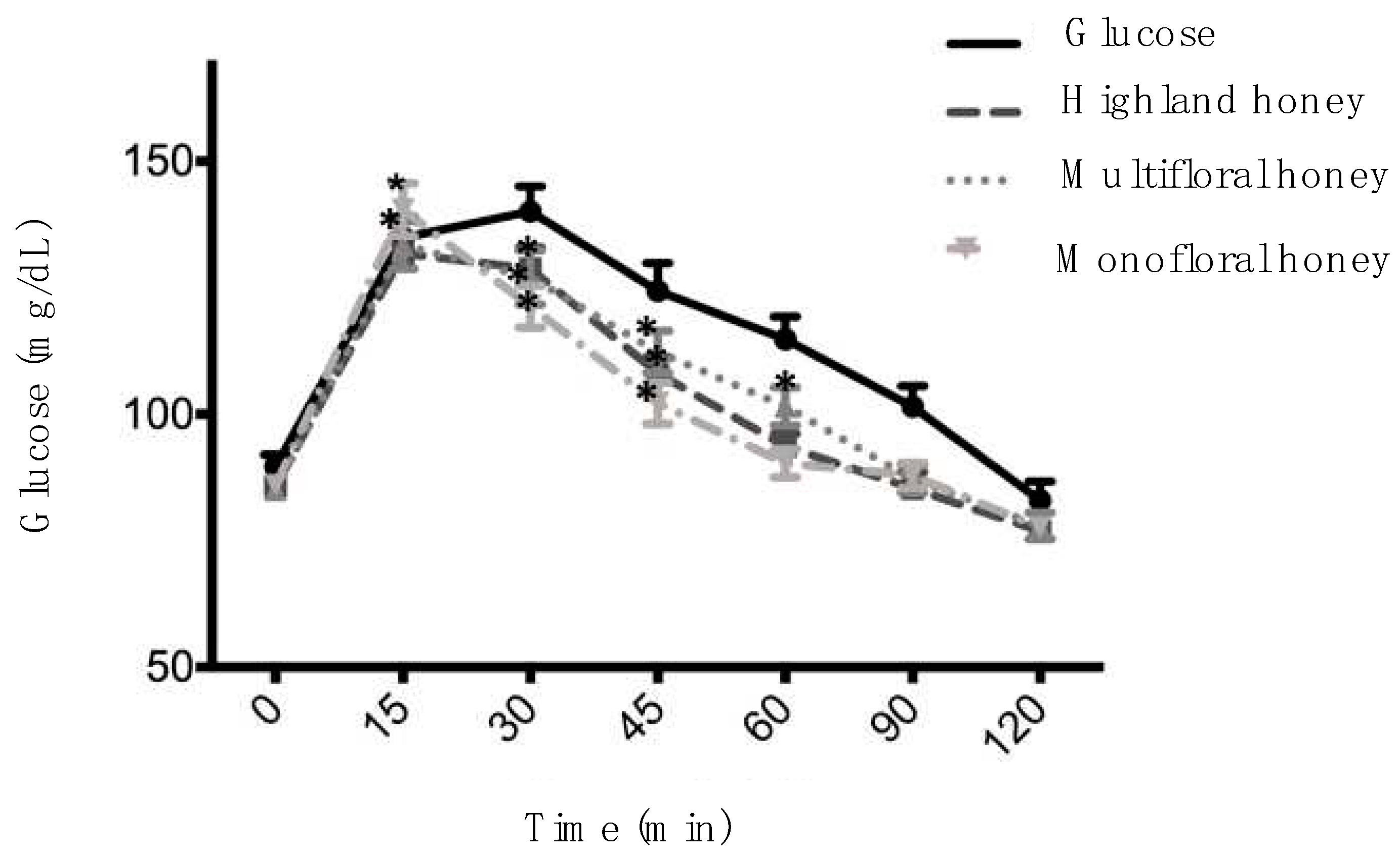
The values of GI and postprandial incremental glucose are shown in
Table 3. The area under the curve (AUC) of honey samples was 155±9.16, 169.06±13.44, and 149.10±12.90 for Highland honey, Multifloral honey, and Avocado honey, respectively. Multifloral honey showed the highest values of AUC, but it was not statistically significant concerning honey samples. However, all values of honey samples were statistically significant compared to the glucose value (224.68±18.48) (
Table 3).
The GI values of honey samples were 69.20±4.07 (Highland honey), 75.24±5.98 (Multifloral honey), and 66.36±5.74 (Avocado honey) (
Table 3), which did not show statistically significant differences (P≤0.05). According to these results, samples of Highland and Avocado honey presented IG medium, while the sample of Multifloral honey showed high GI. Although glucose presented the highest value of postprandial incremental glucose (147.46±4.57 mg/dl), no significant differences were found compared with honey samples (
Table 3).
Figure 1 shows the postprandial glycemic response of honey samples. Sample of Highland and Multifloral honey indicated significant differences up to 45 min for the baseline value. However, the Avocado honey sample showed a significant difference up to 60 min for the baseline value, suggesting a better postprandial glycemic response (
Figure 1).
The GI and response satiety of Mexican kinds of honey has not been extensively investigated. To our knowledge, this is the first report about Highland, Multifloral, and Avocado (Persea americana) kinds of honey of Mexican origin. Honey is naturally sweeter, and its chemical composition can be altered by its botanical origin and geographical region, factors that can affect glycemic response variously (Gourdomichali & Papakonstantinou, 2018). The results of GI obtained in this study are similar to those reported by other authors for different varieties of honey. The result obtained on Highland and Avocado honey (medium GI) is like those obtained on clover, bhekkar, rusberry, and chestnut honey [
25,
26]. On the other hand, the value of Multifloral honey, classified as high GI, is like those found on tupelo, cotton, buckwheat, and heather honey [
15,
25]. However, Vadasery and Ukkuru [
8] reported contrasting values for raw and processed Multifloral honey from India, with GI values of 63 and 63, respectively.
The International Tables of GI and Glycemic load ranks honey as medium GI with an average value of 61 [
11]. The discrepancies observed may be due to different botanical sources and geographical areas, such as soil and climatic conditions, which impact the distribution of nutrients in honey [
11,
15].
Although it is not clear yet, the fructose content of honey, and the presence of antioxidants, may influence GI, because it has been negatively correlated with blood glucose response [
27,
28]. Moreover, the honey's ability to inhibit α-amylase and α-glucosidase has been reported, which also could be influenced by the GI or hypoglycemic effect of honey [
8,
28]. Additionally, some authors have informed that the F/G ratio determines GI in honey; however, other authors have not found a correlation with this factor [
15,
25]. Nevertheless, not only fructose could explain the glucose role in metabolism regulation but also, several fractions of carbohydrates such as sucrose and oligosaccharides [
15,
29]. Deibert, et al. [
30] found that the trisaccharide melezitose significantly increased the GI value of pine honey. Gourdomichali and Papakonstantinou [
15] informed that the sucrose-to-oligosaccharides ratio, sucrose content, fructose content, and fructose-to-glucose ratio significantly affected the glycemic response of different kinds of honey; however, the authors concluded that the sucrose to oligosaccharides ratio showed the strongest influence.
3.4 Satiety Response
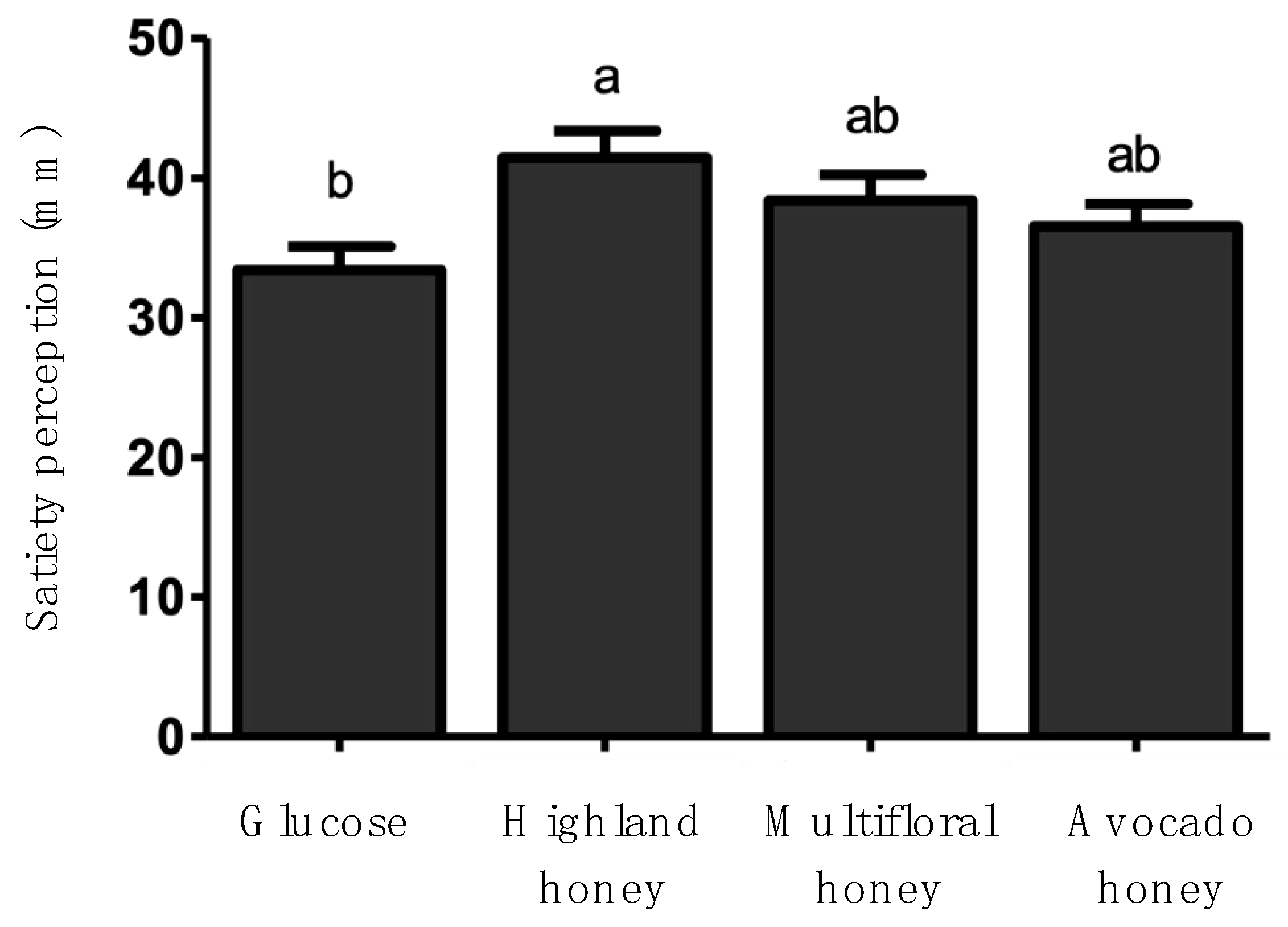
Similarly, to the glycemic response, the satiety response was analyzed through time (
Table 4). Results indicated that Highland honey presented the best satiety response. Participants informed to feel satisfaction from 15 min, after honey consumption, up to 45 min (P≤0.05). The consumption of Avocado honey caused a similar satiety response, but it was up to 30 min (P≤0.05) (
Table 4).
Figure 2, which shows the mean value of satiety response through time, corroborates this finding because it shows that Highland honey presented the best satiety response, which was statistically significant (P≤0.05). On the contrary, Multifloral and Avocado honey presented similar satiety responses to that showed by glucose.
The mechanism by which honey impacts satiety has not been established. Our results indicate that Highland honey showed a significant effect on satiety (
Figure 2), which could be considered as a contribution to the knowledge. It has been hypothesized that high GI foods may decrease satiety due to a low fuel state occurring after consumption of these foods [
2]. However, Flint, et al. [
31] have suggested that insulin, but not glucose, affects satiety; thereby, higher concentrations of insulin after an intake meal are associated with decreased hunger and increased satiety in healthy participants [
2,
31]. Nevertheless, Gourdomichali and Papakonstantinou [
15] showed that honey consumption did not significantly impact insulin levels and satiety in metabolically healthy subjects, but it significantly influenced the glucose response. Interestingly, Larson-Meyer, Willis, Willis, Austin, Hart, Breton and Alexander [
14] showed that consumption of breakfast with honey did not increase the insulin levels, but it delayed postprandial ghrelin response, enhanced PYY response, and diminished the glycemic response compared to sucrose. Recently, Hashim, et al. [
32] have informed that honey could normalize circulating glucose levels due to its fructose content and can prolong gastric emptying, and lowers food intake. Once the honey is consumed, the slow absorption of fructose within the intestinal tracks might delay the interaction between fructose and the intestinal receptor, which might result in satiety. In this study, the sample of Highland honey showed the highest values of fructose, which could be explained by the satiety response.
4. Conclusions
Highland and Avocado honey showed a medium glycemic index, but Multifloral honey exhibited a high glycemic index. Only Highland honey indicated a satiety response. These findings could be explained by the effect of different fractions of carbohydrates present in honey, including its fructose content; however, other components such as phytochemicals might be implicated. Highland and Avocado honey might be consumed as natural sweeteners in replacement of sugar; nevertheless, further studies are needed to clear out the mechanism by which honey could affect satiety response, and long-term studies to determine its effect on control glycemic control.
Author Contributions
Conceptualization, project administration and funding acquisition, H.-S.; Methodology, S.-M., C.-M., A.-M., and P.-d.; formal analysis, R.-J., C.-M., A.-M. and R.-R.; Investigation, P.-d.; writing-original draft preparation, P.-d. and H.-S.; writing-review and editing, C. and d. All authors have read and agreed to the publish version of the manuscript.
Funding
This research was funded by Programa para el Desarrollo Profesional Docente para el Tipo Superior (PRODEP), grant number: UANL-PTC-1026. https://dgesui.ses.sep.gob.mx/programas/programa-para-el-desarrollo-profesional-docente-para-el-tipo-superior-s247-prodep
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki of 1975, revised 2013, and approved by the Ethics Committee of Facultad de Salud Pública y Nutrición (FaSPyN) with the identifier CE 2/2018-19; in addition, all procedures involving human subjects were made under the regulation of the General Law on Health Research.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Therefore, written informed consent has been obtained from the subjects to publish this paper.
Data Availability Statement
The data will be made available upon responsible request.
Acknowledgments
We thank Jorge Llaca-Díaz, from Departamento de Patología Clínica, Hospital Universitario Dr. José Eleuterio González, Monterrey, México, to support bout the biochemical data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Anderson, G.H.; Catherine, N.L.; Woodend, D.M.; Wolever, T.M. Inverse association between the effect of carbohydrates on blood glucose and subsequent short-term food intake in young men. The American Journal of Clinical Nutrition 2002, 76, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.S.H.; Heller, J.M.F.; Hansen, T.T.; Raben, A. Comparison of Low Glycaemic Index and High Glycaemic Index Potatoes in Relation to Satiety: A Single-Blinded, Randomised Crossover Study in Humans. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Manson, J.; Liu, S. Glycemic index, glycemic load, and risk of type 2 diabetes. The American Journal of Clinical Nutrition 2002, 76, 274S–280S. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 2002, 287, 2414–2423. [Google Scholar] [CrossRef]
- Visuthranukul, C.; Sirimongkol, P.; Prachansuwan, A.; Pruksananonda, C.; Chomtho, S. Low-glycemic index diet may improve insulin sensitivity in obese children. Pediatric Research 2015, 78, 567–573. [Google Scholar] [CrossRef]
- Brand-Miller, J.C.; Holt, S.H.; Pawlak, D.B.; McMillan, J. Glycemic index and obesity. The American Journal of Clinical Nutrition 2002, 76, 281S–285S. [Google Scholar] [CrossRef]
- Bornet, F.R.; Jardy-Gennetier, A.E.; Jacquet, N.; Stowell, J. Glycaemic response to foods: impact on satiety and long-term weight regulation. Appetite 2007, 49, 535–553. [Google Scholar] [CrossRef]
- Vadasery, K.; Ukkuru, P. In vitro antidiabetic activity and glycemic index of bee honeys. Indian Journal of Traditional Knowledge 2017, 16, 134–140. [Google Scholar]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability, and authenticity. Food Chemistry 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Bobiş, O.; Dezmirean, D.S.; Moise, A.R. Honey and Diabetes: The Importance of Natural Simple Sugars in Diet for Preventing and Treating Different Type of Diabetes. Oxidative Medicine and Cellular Longevity 2018, 2018, 4757893. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International Tables of Glycemic Index and Glycemic Load Values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef] [PubMed]
- Magaña Magaña, M.A.; Tavera Cortés, M.E.; Salazar Barrientos, L.L.; Sanginés García, J.R. Productivity beekeeping in Mexico and its impact on profitability. Revista Mexicana de Ciencias Agrícolas 2016.
- Mondragón-Cortez, P.; Ulloa, J.; Rosas-Ulloa, P.; Rodríguez-Rodríguez, R.; Resendiz Vázquez, J. Physicochemical characterization of honey from the West region of México. CyTA-Journal of Food 2013, 11, 7–13. [Google Scholar] [CrossRef]
- Larson-Meyer, D.E.; Willis, K.S.; Willis, L.M.; Austin, K.J.; Hart, A.M.; Breton, A.B.; Alexander, B.M. Effect of Honey versus Sucrose on Appetite, Appetite-Regulating Hormones, and Postmeal Thermogenesis. Journal of the American College of Nutrition 2010, 29, 482–493. [Google Scholar] [CrossRef]
- Gourdomichali, T.; Papakonstantinou, E. Short-term effects of six Greek honey varieties on glycemic response: a randomized clinical trial in healthy subjects. European Journal of Clinical Nutrition 2018, 72, 1709–1716. [Google Scholar] [CrossRef]
- Fantoni, E.; Ball, S.; Lloyd, L.; Mapp, K. Honey compositional analysis by HPLC. 2012.
- WHO. A healthy lifestyle - WHO recommendations. (accessed on 03/03/2023).
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Griffiths, C.; Krzeminska, K.; Lawrie, J.A.; Bennett, C.M.; Goff, D.V.; Sarson, D.L.; Bloom, S.R. Slow release dietary carbohydrate improves second meal tolerance. The American Journal of Clinical Nutrition 1982, 35, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Cruz, A.; Manuel Loustaunau-López, V.; Bacardi-Gascón, M. The use of low glycemic and high satiety index food dishes in Mexico: a low cost approach to prevent and control obesity and diabetes. Nutrición Hospitalaria 2006, 21, 353–356. [Google Scholar] [PubMed]
- White, J.W. Honey. In Advances in Food Research, Chichester, C.O., Ed.; Academic Press: 1978; Volume 24, pp. 287-374.
- Escuredo, O.; Dobre, I.; Fernández-González, M.; Seijo, M.C. Contribution of botanical origin and sugar composition of honeys on the crystallization phenomenon. Food Chemistry 2014, 149, 84–90. [Google Scholar] [CrossRef]
- Demir Kanbur, E.; Yuksek, T.; Atamov, V.; Ozcelik, A.E. A comparison of the physicochemical properties of chestnut and highland honey: The case of Senoz Valley in the Rize province of Turkey. Food Chemistry 2021, 345, 128864. [Google Scholar] [CrossRef]
- FAO, I.a.A. Honey. Available online: https://www.fao. 4657. [Google Scholar]
- Ahmad, A.; Azim, M.K.; Mesaik, M.A.; Khan, R.A. Natural honey modulates physiological glycemic response compared to simulated honey and D-glucose. Journal of Food Science 2008, 73, H165–167. [Google Scholar] [CrossRef]
- Ischayek, J.I.; Kern, M. US honeys varying in glucose and fructose content elicit similar glycemic indexes. Journal of the American Dietetic Association 2006, 106, 1260–1262. [Google Scholar] [CrossRef]
- Khan, A.; Ali, D.G. The Effect of Honey-Antioxidants on Blood Glycaemia in Normal Healthy Human Subjects. Pakistan Journal of Life and Social Sciences 2018, 42–47. [Google Scholar]
- Atkinson, F.S.; Brand-Miller, J.C.; Foster-Powell, K.; Buyken, A.E.; Goletzke, J. International tables of glycemic index and glycemic load values 2021: a systematic review. The American Journal of Clinical Nutrition 2021, 114, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Terzo, S.; Mulè, F.; Amato, A. Honey and obesity-related dysfunctions: a summary on health benefits. The Journal of Nutritional Biochemistry 2020, 82, 108401. [Google Scholar] [CrossRef] [PubMed]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S. Fructose might contribute to the hypoglycemic effect of honey. Molecules 2012, 17, 1900–1915. [Google Scholar] [CrossRef] [PubMed]
- Deibert, P.; König, D.; Kloock, B.; Groenefeld, M.; Berg, A. Glycaemic and insulinaemic properties of some German honey varieties. European Journal of Clinical Nutrition 2010, 64, 762–764. [Google Scholar] [CrossRef]
- Flint, A.; Møller, B.K.; Raben, A.; Sloth, B.; Pedersen, D.; Tetens, I.; Holst, J.J.; Astrup, A. Glycemic and insulinemic responses as determinants of appetite in humans. The American Journal of Clinical Nutrition 2006, 84, 1365–1373. [Google Scholar] [CrossRef]
- Hashim, K.-N.; Chin, K.-Y.; Ahmad, F. The mechanism of honey in reversing metabolic syndrome. Molecules 2021, 26, 808. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).