Submitted:
27 July 2023
Posted:
28 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Is it rational to design DNA vaccines for T. brucei?
2.1. Advantages
2.2. Disadvantages
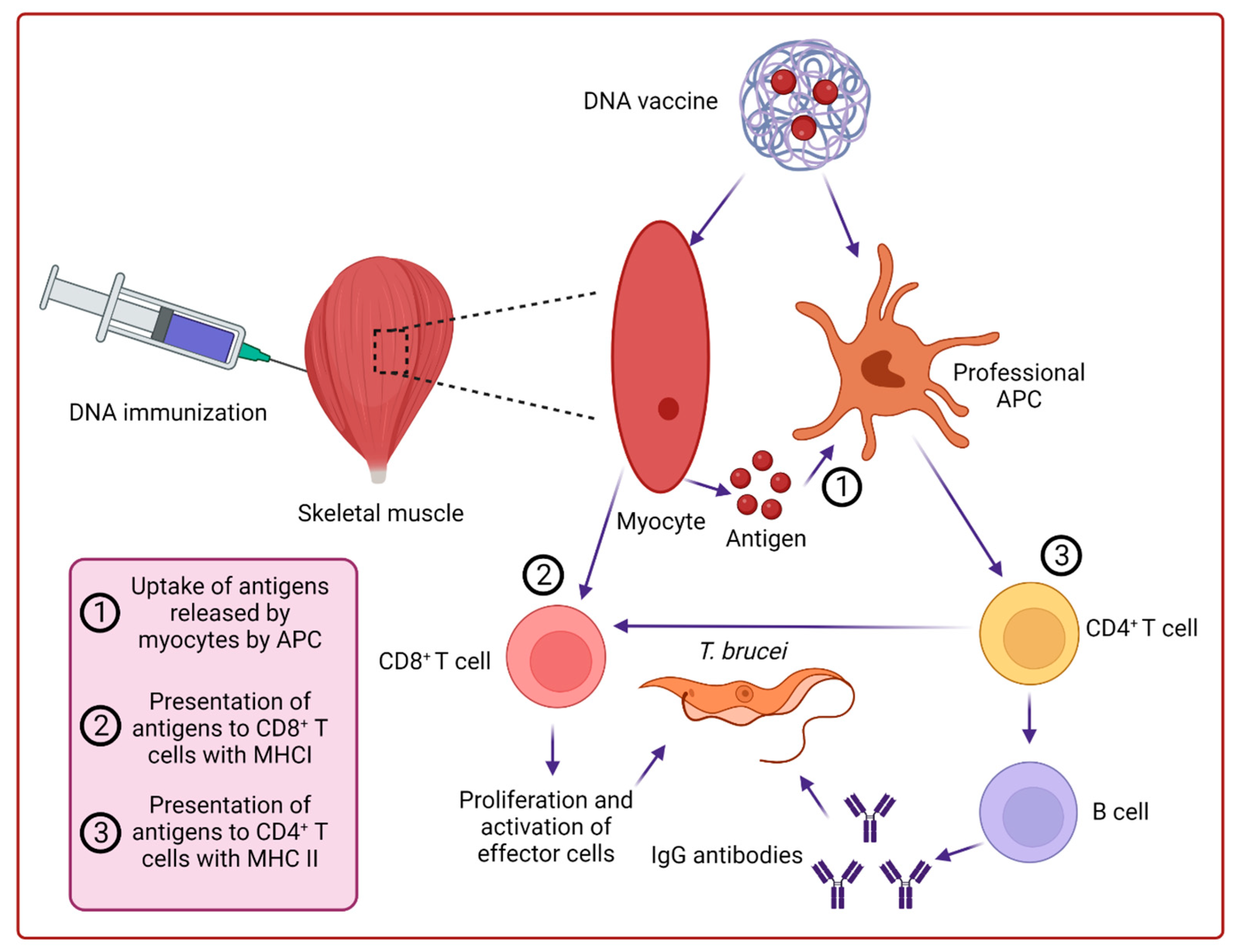
3. How does the DNA vaccine work?
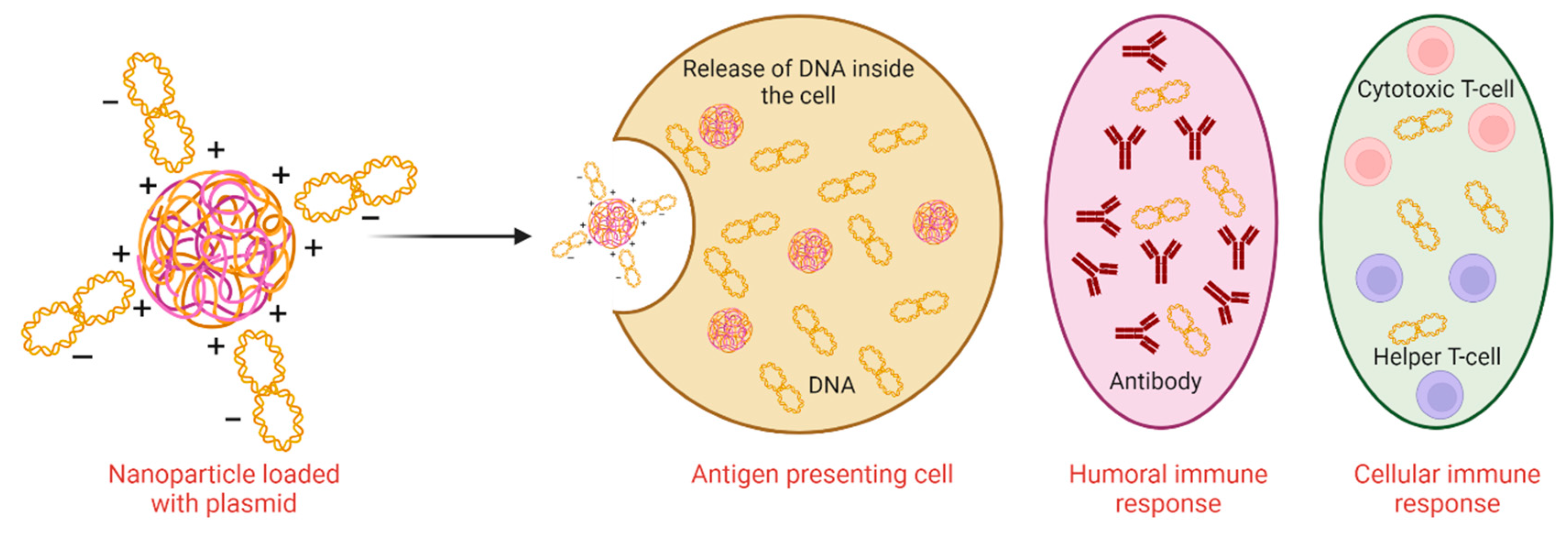
4. Nanoparticulate and microparticulate for the delivery of DNA vaccines
4.1. Polymeric nanoparticulate and microparticulate
4.2. Lipid nano- and micro particulates
4.3. Inorganic nanoparticulate
5. Advancement in the development of anti-Trypanosoma brucei DNA-based vaccines
6. Conclusion and future perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brun, R.; Blum, J.; Chappuis, F.; Burri, C. Human African trypanosomiasis. Lancet 2010, 375, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Casas-Sanchez, A.; Ramaswamy, R.; Perally, S.; Haines, L.R.; Rose, C.; Aguilera-Flores, M.; Portillo, S.; Verbeelen, M.; Hussain, S.; Smithson, L.; et al. The Trypanosoma brucei MISP family of invariant proteins is co-expressed with BARP as triple helical bundle structures on the surface of salivary gland forms, but is dispensable for parasite development within the tsetse vector. PLOS Pathog. 2023, 19, e1011269. [Google Scholar] [CrossRef] [PubMed]
- Büscher, P.; Cecchi, G.; Jamonneau, V.; Priotto, G. Human African trypanosomiasis. Lancet 2017, 390, 2397–2409. [Google Scholar] [CrossRef] [PubMed]
- Steverding, D. The history of African trypanosomiasis. Parasites Vectors 2008, 1, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Simarro, P.P.; Franco, J.R.; Diarra, A.; Jannin, J.G. Epidemiology of human African trypanosomiasis. Clin. Epidemiology 2014, 6, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Legros, D.; Ollivier, G.; Gastellu-Etchegorry, M.; Paquet, C.; Burri, C.; Jannin, J.; Büscher, P. Treatment of human African trypanosomiasis—present situation and needs for research and development. Lancet Infect. Dis. 2002, 2, 437–440. [Google Scholar] [CrossRef]
- Lança, A.S.C.; de Sousa, K.P.; Atouguia, J.; Prazeres, D.M.F.; Monteiro, G.A.; Silva, M.S. Trypanosoma brucei: Immunisation with plasmid DNA encoding invariant surface glycoprotein gene is able to induce partial protection in experimental African trypanosomiasis. Exp. Parasitol. 2011, 127, 18–24. [Google Scholar] [CrossRef]
- Baral, T.N. Immunobiology of African Trypanosomes: Need of Alternative Interventions. J. Biomed. Biotechnol. 2010, 2010, 389153. [Google Scholar] [CrossRef]
- Berriman, M.; Ghedin, E.; Hertz-Fowler, C.; Blandin, G.; Renauld, H.; Bartholomeu, D.C.; Lennard, N.J.; Caler, E.; Hamlin, N.E.; Haas, B.; et al. The Genome of the African Trypanosome Trypanosoma brucei. Science 2005, 309, 416–422. [Google Scholar] [CrossRef]
- Akhoon, B.A.; Slathia, P.S.; Sharma, P.; Gupta, S.K.; Verma, V. In silico identification of novel protective VSG antigens expressed by Trypanosoma brucei and an effort for designing a highly immunogenic DNA vaccine using IL-12 as adjuvant. Microb. Pathog. 2011, 51, 77–87. [Google Scholar] [CrossRef]
- Rhind, S.G.; Shek, P.N. Cytokines in the pathogenesis of human African trypanosomiasis: antagonistic roles of TNF-α and IL-10. Progress in human African trypanosomiasis, sleeping sickness. Springer-Verlag. 1999, 119–135. [CrossRef]
- Kurup, S.P.; Tewari, A.K. Induction of protective immune response in mice by a DNA vaccine encoding Trypanosoma evansi beta tubulin gene. Veter- Parasitol. 2012, 187, 9–16. [Google Scholar] [CrossRef]
- Wilson, P.C.; Andrews, S.F. Tools to therapeutically harness the human antibody response. Nat. Rev. Immunol. 2012, 12, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Smooker, P.M.; Rainczuk, A.; Kennedy, N.; Spithill, T.W. DNA vaccines and their application against parasites – promise, limitations and potential solutions. Biotechnol. Annu. Rev. 2004, 10, 189–236. [Google Scholar] [CrossRef] [PubMed]
- Hasson, S.S.A.A.; Al-Busaidi, J.K.Z.; Sallam, T.A. The past, current and future trends in DNA vaccine immunisations. Asian Pac. J. Trop. Biomed. 2015, 5, 344–353. [Google Scholar] [CrossRef]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef]
- Tang, D.-C.; DeVit, M.; Johnston, S.A. Genetic immunization is a simple method for eliciting an immune response. Nature 1992, 356, 152–154. [Google Scholar] [CrossRef]
- Cui, Z. DNA Vaccine. Adv. Genet. 2005, 54, 257–289. [Google Scholar] [CrossRef]
- Mohajeri, P.; Soltani, S.; Farahani, A.; Dastranj, M.; Momenifar, N.; Emamie, A.D. DNA vaccine: Methods and mechanisms. Adv. Hum. Biol. 2018, 8, 132. [Google Scholar] [CrossRef]
- Gurunathan, S.; Klinman, D.M.; Seder, R.A. DNA Vaccines: Immunology, Application, and Optimization. Annu. Rev. Immunol. 2000, 18, 927–974. [Google Scholar] [CrossRef]
- Farris, E.; Brown, D.M.; E Ramer-Tait, A.; Pannier, A.K. Micro- and nanoparticulates for DNA vaccine delivery. Exp. Biol. Med. 2016, 241, 919–929. [Google Scholar] [CrossRef]
- Saade, F.; Petrovsky, N. Technologies for enhanced efficacy of DNA vaccines. Expert Rev. Vaccines 2012, 11, 189–209. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.A.; He, N.; Li, Z.; Ali, Z.; Zhang, L. Nanoparticles for DNA vaccine delivery. J. Biomed. Nanotechnol. 2014, 10, 2332–2349. [Google Scholar] [CrossRef] [PubMed]
- Kersten, G.; Hirschberg, H. Antigen delivery systems. Expert Rev. Vaccines 2004, 3, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Rodríguez, S.A.; Puel, F.; Briançon, S.; Allémann, E.; Doelker, E.; Fessi, H. Comparative scale-up of three methods for producing ibuprofen-loaded nanoparticles. Eur. J. Pharm. Sci. 2005, 25, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Briones, M.; Ott, G.; O'Hagan, D. Cationic microparticles: A potent delivery system for DNA vaccines. Proc. Natl. Acad. Sci. USA 2000, 97, 811–816. [Google Scholar] [CrossRef]
- Lee, P.-W.; Hsu, S.-H.; Tsai, J.-S.; Chen, F.-R.; Huang, P.-J.; Ke, C.-J.; Liao, Z.-X.; Hsiao, C.-W.; Lin, H.-J.; Sung, H.-W. Multifunctional core-shell polymeric nanoparticles for transdermal DNA delivery and epidermal Langerhans cells tracking. Biomaterials 2010, 31, 2425–2434. [Google Scholar] [CrossRef]
- Liu, W.G.; Zhang, X.; Sun, S.J.; Sun, G.J.; De Yao, K.; Liang, D.C.; Guo, G.; Zhang, J.Y. N-Alkylated Chitosan as a Potential Nonviral Vector for Gene Transfection. Bioconjugate Chem. 2003, 14, 782–789. [Google Scholar] [CrossRef]
- Wilson, K.D.; de Jong, S.D.; Tam, Y.K. Lipid-based delivery of CpG oligonucleotides enhances immunotherapeutic efficacy. Adv. Drug Deliv. Rev. 2009, 61, 233–242. [Google Scholar] [CrossRef]
- Lonez, C.; Bessodes, M.; Scherman, D.; Vandenbranden, M.; Escriou, V.; Ruysschaert, J.-M. Cationic lipid nanocarriers activate Toll-like receptor 2 and NLRP3 inflammasome pathways. Nanomedicine: Nanotechnology, Biol. Med. 2014, 10, 775–782. [Google Scholar] [CrossRef]
- Mukherjee, P.; Bhattacharya, R.; Wang, P.; Wang, L.; Basu, S.; Nagy, J.A.; Atala, A.; Mukhopadhyay, D.; Soker, S. Antiangiogenic Properties of Gold Nanoparticles. Clin. Cancer Res. 2005, 11, 3530–3534. [Google Scholar] [CrossRef]
- Silva, M.S.; Prazeres, D.M.F.; Lança, A.; Atouguia, J.; Monteiro, G.A. Trans-sialidase from Trypanosoma brucei as a potential target for DNA vaccine development against African trypanosomiasis. Parasitol. Res. 2009, 105, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Shoda, L.K.M.; Kegerreis, K.A.; Suarez, C.E.; Roditi, I.; Corral, R.S.; Bertot, G.M.; Norimine, J.; Brown, W.C. DNA from Protozoan ParasitesBabesia bovis, Trypanosoma cruzi, andT. bruceiIs Mitogenic for B Lymphocytes and Stimulates Macrophage Expression of Interleukin-12, Tumor Necrosis Factor Alpha, and Nitric Oxide. Infect. Immun. 2001, 69, 2162–71. [Google Scholar] [CrossRef] [PubMed]
- A Carvalho, J.; Rodgers, J.; Atouguia, J.; Prazeres, D.M.; A Monteiro, G. DNA vaccines: a rational design against parasitic diseases. Expert Rev. Vaccines 2010, 9, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Danazumi, A.U.; Gital, S.I.; Idris, S.; Dibba, L.B.; Balogun, E.O.; Górna, M.W. Immunoinformatic design of a putative multi-epitope vaccine candidate against Trypanosoma brucei gambiense. Comput. Struct. Biotechnol. J. 2022, 20, 5574–5585. [Google Scholar] [CrossRef]
- Li, L.; Saade, F.; Petrovsky, N. The future of human DNA vaccines. J. Biotechnol. 2012, 162, 171–182. [Google Scholar] [CrossRef]
| Candidate antigen | Strain | Extraction site | Host | Effect | Reference |
|---|---|---|---|---|---|
| Metacyclic invariant surface proteins | Trypanosoma brucei brucei strain AnTat 1.1 90:13 | T. brucei-infected tsetse saliva | BALB/c 6–8 weeks old female mice | May act as a transmission blocking vaccine target | [2] |
| DNA encoding trans-sialidase 5′-terminal region | T. brucei brucei GVR 35/1.5 | Genome | BALB-c mice | IgG antibodies were produced and provided 60% protection in challenged mice | [32] |
| DNA encoding invariant surface glycoprotein specific for bloodstream stage | T. b. brucei GVR 35/1.5 | Genome | Balb/C mice | Anti-trypanosoma IgG antibodies were produced suggesting T helper cell type 1 response (Th1) | [7] |
| T. brucei DNA | - | Trypomastigotes were isolated from rats | Cattle macrophages | Promotes B-lymphocyte proliferation | [33] |
| Variant surface glycoprotein | In silico study | Immunogenic | [10] | ||
| Proteins harboring transmembrane helices | May stimulate cellular and humoral immune responses | [35] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





