Submitted:
26 July 2023
Posted:
27 July 2023
You are already at the latest version
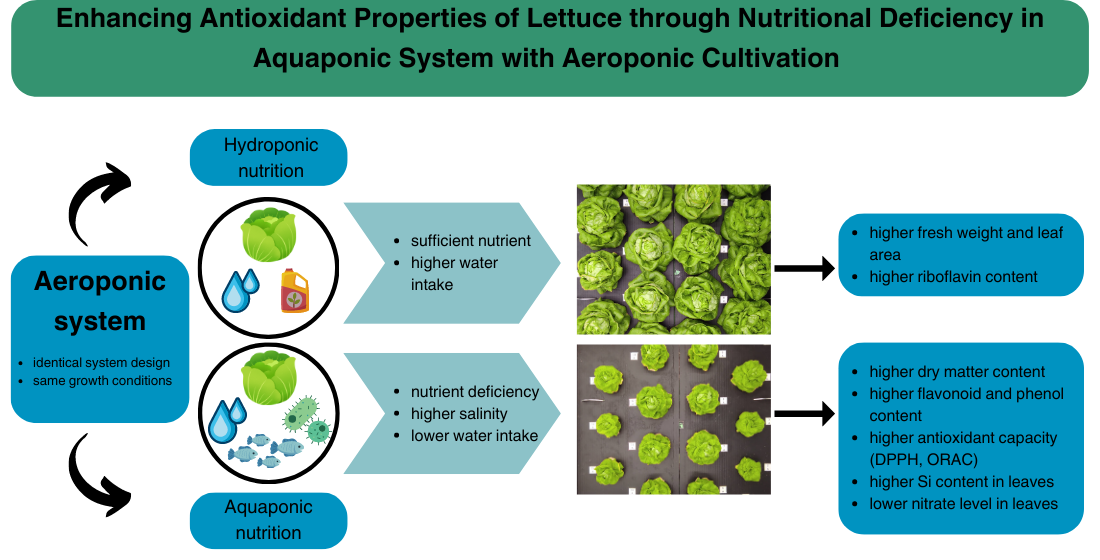
Abstract
Keywords:
1. Introduction
2. Material and Methods
2.1. Experimental Setup
2.2. Lettuce Growth Parameters
2.3. Analysis of nutrient concentrations in water
2.4. Bioactive and nutritionally important compounds in lettuce leaves
2.4.1. Total flavonoid content
2.4.2. Evaluation of antioxidant capacity
2.4.3. Determination of vitamin content
2.4.4. Determination of inorganic anions and low molecular mass organic acids
2.4.5. Mineral content
2.5. Sensory analysis
2.6. Statistical analysis
3. Results
3.1. Growth parameters of lettuce
3.2. Leaf area
3.3. Water quality
3.4. Bioactive and nutritionally important compounds
3.4.1. Total flavonoid content and antioxidant capacity
3.4.2. Vitamin content
3.4.3. Mineral content in lettuce biomass
3.5. Anions and Organic Acids
3.6. Sensory analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lakhiar, I.A.; Gao, J.; Syed, T.N.; Chandio, F.A.; Buttar, N.A. Modern Plant Cultivation Technologies in Agriculture under Controlled Environment: A Review on Aeroponics. J Plant Interact 2018, 13, 338–352. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Good Agricultural Practices for Greenhouse Vegetable Crops: Principles for Mediterranean Climate Areas.; Rome, 2013, pp. 303–355. ISBN 9789251076491.
- Gopinath, P.; Vethamoni, P.I.; Gomathi, M. Chemical Science Review and Letters Aeroponics Soilless Cultivation System for Vegetable Crops. Chem Sci Rev Lett 2017, 6. [Google Scholar]
- NASA Environmental and Agricultural Resources, Progressive Plant Growing Has Business Blooming. NASA Spinoff 2006, 64–77.
- Santi, A.A. Controlling Nutrition Level in Aeroponic Supply System Using Proportional-Integral Method. Industrial. Industrial Manufacture 2021.
- Barak, P.; Smith, J.D.; Krueger, A.R.; Peterson, L.A. Measurement of Short-Term Nutrient Uptake Rates in Cranberry by Aeroponics. Plant Cell Environ 1996, 19, 237–242. [Google Scholar] [CrossRef]
- Chang, D.C.; Park, C.S.; Kim, S.Y.; Kim, S.J.; Lee, Y.B. Physiological Growth Responses by Nutrient Interruption in Aeroponically Grown Potatoes. American Journal of Potato Research 2008, 85, 315–323. [Google Scholar] [CrossRef]
- Johnstone, P.R.; Nichols, M.A.; Fisher, K.J.; Reid, J. Nutritional Studies with Processing Tomato Grown in Aeroponics. Acta Hortic 2001, 143–152. [Google Scholar] [CrossRef]
- Broadley, M.R.; Escobar-Gutiérrez, A.J.; Burns, A. Nitrogen-Limited Growth of Lettuce Is Associated with Lower Stomatal Conductance; 2001; Vol. 152, pp. 97–106.
- Mensinga, T.T.; Speijers, G.J.A.; Meulenbelt, J. Health Implications of Exposure to Environmental Nitrogenous Compounds. Toxicol Rev 2003, 22, 41–51. [Google Scholar] [CrossRef]
- Yoneyama, K.; Xie, X.; Kim, H. Il; Kisugi, T.; Nomura, T.; Sekimoto, H.; Yokota, T.; Yoneyama, K. How Do Nitrogen and Phosphorus Deficiencies Affect Strigolactone Production and Exudation? Planta 2012, 235, 1197–1207. [Google Scholar] [CrossRef]
- Roosta, H.R. Effects of Foliar Spray of K on Mint, Radish, Parsley and Coriander Plants in Aquaponic System. J Plant Nutr 2014, 37, 2236–2254. [Google Scholar] [CrossRef]
- Petrazzini, L.L.; Souza, G.A.; Rodas, C.L.; Emrich, E.B.; Carvalho, J.G.; Souza, R.J. Nutritional Deficiency in Crisphead Lettuce Grown in Hydroponics. Hortic Bras 2014, 32, 310–313. [Google Scholar] [CrossRef]
- Sakamoto, M.; Komatsu, Y.; Suzuki, T. Nutrient Deficiency Affects the Growth and Nitrate Concentration of Hydroponic Radish. Horticulturae 2021, 7, 525. [Google Scholar] [CrossRef]
- Jie, H.; Kong, L.S. Growth and Photosynthetic Responses of Three Aeroponically Grown Lettuce Cultivars ( Lactuca Sativa L. ) to Different Rootzone Temperatures and Growth Irradiances under Tropical Aerial Conditions. J Hortic Sci Biotechnol 1998, 73, 173–180. [Google Scholar]
- Chiipanthenga, M. Potential of Aeroponics System in the Production of Quality Potato (Solanum Tuberosum l. ) Seed in Developing Countries. Afr J Biotechnol 2012, 11, 3993–3999. [Google Scholar]
- Osvald, J.; Petrovic, N.; Demsar, J. Sugar and Organic Acid Content of Tomato Fruits (Lycopersicon Lycopersicum Mill. ) Grown on Aeroponics at Different Plant Density. Acta Aliment 2001, 30, 53–61. [Google Scholar]
- Fascella, G.; Zizzo, G.V. Preliminary Results of Aeroponic Cultivation of Anthurium Andreanum for Cut Flower Production. Acta Hortic 2007, 233–240. [Google Scholar] [CrossRef]
- Hayden, A.L.; Brigham, L.A.; Giacomelli, G.A. Aeroponic Cultivation of Ginger (Zingiber Officinale) Rhizomes. Acta Hortic 2004, 397–402. [Google Scholar] [CrossRef]
- Pasch, J.; Appelbaum, S.; Palm, H.W.; Knaus, U. Growth of Basil (Ocimum Basilicum) in Aeroponics, DRF, and Raft Systems with Effluents of African Catfish (Clarias Gariepinus) in Decoupled Aquaponics (s. s.). AgriEngineering 2021, 3, 559–574. [Google Scholar] [CrossRef]
- Tsoumalakou, E.; Mente, E.; Kormas, K.A.; Katsoulas, N.; Vlahos, N.; Kapsis, P.; Levizou, E. Precise Monitoring of Lettuce Functional Responses to Minimal Nutrient Supplementation Identifies Aquaponic System’s Nutrient Limitations and Their Time-Course. Agriculture 2022, 12, 1278. [Google Scholar] [CrossRef]
- Yep, B.; Zheng, Y. Aquaponic Trends and Challenges – A Review. J Clean Prod 2019, 228, 1586–1599. [Google Scholar] [CrossRef]
- Goddek, S.; Vermeulen, T. Comparison of Lactuca Sativa Growth Performance in Conventional and RAS-Based Hydroponic Systems. Aquaculture International 2018, 26, 1377–1386. [Google Scholar] [CrossRef]
- Lennard, W.; Ward, J. A Comparison of Plant Growth Rates between an NFT Hydroponic System and an NFT Aquaponic System. Horticulturae 2019, 5, 27. [Google Scholar] [CrossRef]
- Ayipio, E.; Wells, D.E.; McQuilling, A.; Wilson, A.E. Comparisons between Aquaponic and Conventional Hydroponic Crop Yields: A Meta-Analysis. Sustainability 2019, 11, 6511. [Google Scholar] [CrossRef]
- Singh, H.; Bruce, D. Electrical Conductivity and PH Guide for Hydroponics. Oklahoma Cooperative Extension Service 2016.
- Matić, P.; Sabljić, M.; Jakobek, L. Validation of Spectrophotometric Methods for the Determination of Total Polyphenol and Total Flavonoid Content. J AOAC Int 2017, 100, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent 1999, 299, 152–178.
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT - Food Science and Technology 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Langhansova, L.; Pumprova, K.; Haisel, D.; Ekrt, L.; Pavicic, A.; Zajíčková, M.; Vanek, T.; Dvorakova, M. European Ferns as Rich Sources of Antioxidants in the Human Diet. Food Chem 2021, 356, 129637. [Google Scholar] [CrossRef]
- Cao, G.; Prior, R.L. [5] Measurement of Oxygen Radical Absorbance Capacity in Biological Samples 1999, 299, 50–62.
- Sami, R.; Li, Y.; Qi, B.; Wang, S.; Zhang, Q.; Han, F.; Ma, Y.; Jing, J.; Jiang, L. HPLC Analysis of Water-Soluble Vitamins (B2, B3, B6, B12, and C) and Fat-Soluble Vitamins (E, K, D, A, and β -Carotene) of Okra (Abelmoschus Esculentus). J Chem 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Mozumder, N.H.M.R.; Akhter, Most.J.; Khatun, A.A.; Rokibuzzaman, M.; Akhtaruzzaman, M. Estimation of Water-Soluble Vitamin B-Complex in Selected Leafy and Non-Leafy Vegetables by HPLC Method. Oriental Journal Of Chemistry. 2019, 35, 1344–1351.
- Tapan, S.; Kausik, Ch.; Basundhara, P. Simultaneous Estimation of Water Soluble Vitamin by High Performance Liquid Chromatography (HPLC) Method in Five Wild Edible Plants Consumed by the Tribal People of North-Eastern Region in India. J Pharmacogn Phytochem 2019, 8, 2393–2398. [Google Scholar]
- Vondráčková, S.; Száková, J.; Drábek, O.; Tejnecký, V.; Hejcman, M.; Müllerová, V.; Tlustoš, P. Aluminium Uptake and Translocation in Al Hyperaccumulator Rumex Obtusifolius Is Affected by Low-Molecular-Weight Organic Acids Content and Soil PH. PLoS One 2015, 10, e0123351. [Google Scholar] [CrossRef]
- Goddek, S.; Delaide, B.; Mankasingh, U.; Ragnarsdottir, K.; Jijakli, H.; Thorarinsdottir, R. Challenges of Sustainable and Commercial Aquaponics. Sustainability 2015, 7, 4199–4224. [Google Scholar] [CrossRef]
- König, B.; Janker, J.; Reinhardt, T.; Villarroel, M.; Junge, R. Analysis of Aquaponics as an Emerging Technological Innovation System. J Clean Prod 2018, 180, 232–243. [Google Scholar] [CrossRef]
- Lakhiar, I.; Gao, J.; Syed, T.; Chandio, F.A.; Tunio, M.; Ahmad, F.; Solangi, K. Overview of the Aeroponic Agriculture – An Emerging Technology for Global Food Security. International Journal of Agricultural and Biological Engineering 2020, 13, 1–10. [Google Scholar] [CrossRef]
- Gruda, N.S. Gruda, N.S. Does Soilless Culture Have an Influence on Product Quality of Vegetables? Production and Quality of Vegetables View Project Rooftop Urban Agriculture View Project Do Soilless Culture Systems Have an Influence on Product Quality of Vegetables. 2009, 82, 141–147. [Google Scholar]
- Colla, G.; Kim, H.-J.; Kyriacou, M.C.; Rouphael, Y. Nitrate in Fruits and Vegetables. Sci Hortic 2018, 237, 221–238. [Google Scholar] [CrossRef]
- Brkić, D.; Bošnir, J.; Bevardi, M.; Bošković, A.G.; Miloš, S.; Lasić, D.; Krivohlavek, A.; Racz, A.; Mojsović – Ćuić, A.; Trstenjak, N.U. Nitrate in Leafy Green Vegetables and Estimated Intake. African Journal of Traditional, Complementary and Alternative Medicines 2017, 14, 31–41. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The Critical Role of Potassium in Plant Stress Response. Int J Mol Sci 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Malhotra, H.; Vandana; Sharma, S.; Pandey, R. Phosphorus Nutrition: Plant Growth in Response to Deficiency and Excess. In Plant Nutrients and Abiotic Stress Tolerance; Springer Singapore: Singapore, 2018; pp. 171–190.
- Eyal, R. Micro-Elements in Agriculture. Practical Hydroponics and Greenhouses 2016, 35–44. [Google Scholar]
- Tavakkoli, E.; Rengasamy, P.; McDonald, G.K. The Response of Barley to Salinity Stress Differs between Hydroponic and Soil Systems. Functional Plant Biology 2010, 37, 621. [Google Scholar] [CrossRef]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating Physiological Responses of Plants to Salinity Stress. Ann Bot 2017, 119, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Senge, M.; Dai, Y. Effects of Salinity Stress on Growth, Yield, Fruit Quality and Water Use Efficiency of Tomato under Hydroponics System. Reviews in Agricultural Science 2016, 4, 46–55. [Google Scholar] [CrossRef]
- Cakmak, I.; Hengeler, C.; Marschner, H. Partitioning of Shoot and Root Dry Matter and Carbohydrates in Bean Plants Suffering from Phosphorus, Potassium and Magnesium Deficiency. J Exp Bot 1994, 45, 1245–1250. [Google Scholar] [CrossRef]
- Rüdiger, H. Molecular Physiology of Plant Sulfur Metabolism. Planta 1997, 202, 138–148. [Google Scholar]
- Britto, D.T.; Kronzucker, H.J. NH4+ Toxicity in Higher Plants: A Critical Review. J Plant Physiol 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Cruz, C.; Bio, A.F.M.; Domínguez-Valdivia, M.D.; Aparicio-Tejo, P.M.; Lamsfus, C.; Martins-Loução, M.A. How Does Glutamine Synthetase Activity Determine Plant Tolerance to Ammonium? Planta 2006, 223, 1068–1080. [Google Scholar] [CrossRef]
- Monsees, H.; Kloas, W.; Wuertz, S. Decoupled Systems on Trial: Eliminating Bottlenecks to Improve Aquaponic Processes. PLoS One 2017, 12, e0183056. [Google Scholar] [CrossRef]
- Tyson, R. V.; Treadwell, D.D.; Simonne, E.H. Opportunities and Challenges to Sustainability in Aquaponic Systems. Horttechnology 2011, 21, 6–13. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E.; Rahmat, A.; Rahman, Z.A. The Relationship between Phenolics and Flavonoids Production with Total Non-Structural Carbohydrate and Photosynthetic Rate in Labisia Pumila Benth. under High CO2 and Nitrogen Fertilization. Molecules 2010, 16, 162–174. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J Nutr Sci 2016, 5, e47. [Google Scholar] [CrossRef]
- Williamson, G.; Manach, C. Bioavailability and Bioefficacy of Polyphenols in Humans. II. Review of 93 Intervention Studies. Am J Clin Nutr 2005, 81, 243S–255S. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of Antioxidant Activity. J Funct Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- USDA Database for the Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods. Nutrient Data Laboratory (U.S.) 2010, Release 2.
- Schmautz, Z.; Loeu, F.; Liebisch, F.; Graber, A.; Mathis, A.; Griessler Bulc, T.; Junge, R. Tomato Productivity and Quality in Aquaponics: Comparison of Three Hydroponic Methods. Water (Basel) 2016, 8, 533. [Google Scholar] [CrossRef]
- Lei, C.; Engeseth, N.J. Comparison of Growth Characteristics, Functional Qualities, and Texture of Hydroponically Grown and Soil-Grown Lettuce. LWT 2021, 150, 111931. [Google Scholar] [CrossRef]
- Stefanelli, D.; Winkler, S.; Jones, R. Reduced Nitrogen Availability during Growth Improves Quality in Red Oak Lettuce Leaves by Minimizing Nitrate Content and Increasing Antioxidant Capacity and Leaf Mineral Content. Agricultural Sciences 2011, 02, 477–486. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under Stressful Environments: An Overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, Q. i. n.; Liu, Q.; Zhang, W.; Ding, R. Exogenous Silicon (Si) Increases Antioxidant Enzyme Activity and Reduces Lipid Peroxidation in Roots of Salt-Stressed Barley (Hordeum VulgareL. ). J Plant Physiol 2003, 160, 1157–1164. [Google Scholar] [CrossRef]
- Fanasca, S.; Colla, G.; Maiani, G.; Venneria, E.; Rouphael, Y.; Azzini, E.; Saccardo, F. Changes in Antioxidant Content of Tomato Fruits in Response to Cultivar and Nutrient Solution Composition. J Agric Food Chem 2006, 54, 4319–4325. [Google Scholar] [CrossRef]
- Guntzer, F.; Keller, C.; Meunier, J.-D. Benefits of Plant Silicon for Crops: A Review. Agron Sustain Dev 2012, 32, 201–213. [Google Scholar] [CrossRef]
- Luyckx, M.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Silicon and Plants: Current Knowledge and Technological Perspectives. Front Plant Sci 2017, 8, 411. [Google Scholar] [CrossRef]
- Lee, S.K.; Sohn, E.Y.; Hamayun, M.; Yoon, J.Y.; Lee, I.J. Effect of Silicon on Growth and Salinity Stress of Soybean Plant Grown under Hydroponic System. Agroforestry Systems 2010, 80, 333–340. [Google Scholar] [CrossRef]
- Schmautz, Z.; Graber, A.; Jaenicke, S.; Goesmann, A.; Junge, R.; Smits, T.H.M. Microbial Diversity in Different Compartments of an Aquaponics System. Arch Microbiol 2017, 199, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Statement on Possible Public Health Risks for Infants and Young Children from the Presence of Nitrates in Leafy Vegetables. EFSA Journal 2010, 8.
- Santamaria, P. Nitrate in Vegetables: Toxicity, Content, Intake and EC Regulation. J Sci Food Agric 2006, 86, 10–17. [Google Scholar] [CrossRef]
- Schlaeppi, K.; Bulgarelli, D. The Plant Microbiome at Work. Molecular Plant-Microbe Interactions® 2015, 28, 212–217. [Google Scholar] [CrossRef]


| Attribute | Definition | Scale |
|---|---|---|
| Sample acceptability | An individual's appraisal of the sample's appearance | 0 = unacceptable |
| 100 = fully acceptable | ||
| Edge browning | Extent of browning observed on the leaf edges | 0 = no browning |
| 100 = extensive browning | ||
| Freshness | Perception of vibrancy and brightness, indicative of vitality | 0 = withered |
| 100 = fresh | ||
| Fragrance intensity | Strength of aroma following gentle leaf rubbing and sniffing | 0 = no fragrance |
| 100 = highly franrant | ||
| Crispiness | Degree of crunch experienced during the initial bite | 0 = not crispy |
| 100 = highly crispy | ||
| Taste intensity | Perception of flavor strength after five chewing actions | 0 = tasteless |
| 100 = distinctive taste | ||
| Taste acceptability | Pleasantness of flavor after ten chewing actions | 0 = unpleasant taste |
| 100 = highly pleasant taste | ||
| Bitterness | Detection of bitter, sharp, or pungent taste after ten chews | 0 = no bitterness |
| 100 = highly bitter | ||
| Overall acceptability | Subjective appraisal of the sample's overall acceptability | 0 = unacceptable |
| 100 = fully acceptable |
| Lettuce growth parameters | Hydroponic system | Aquaponic system |
|---|---|---|
| Head fresh weight (g) | 215 ± 46.9a,1 | 36.5 ± 16.3b |
| Head dry matter content (%) | 5.49 ± 0.46b | 8.65 ± 0.80a |
| Root fresh weight (g) | 33.2 ± 8.59a | 24.7 ± 10.3b |
| Root–to–shoot ratio | 0.16 ± 0.03b | 0.71 ± 0.16a |
| Number of leaves | 49 ± 5.23a | 27 ± 8.14b |
| Water consumption (L ∙ kg–1) | 28.9 | 15.0 |
| Hydroponic system cm2 |
Aquaponic system cm2 |
|
|---|---|---|
| 1st week | 7.53 ± 1.73a,1 | 6.93 ± 1.76a |
| 2nd week | 30.5 ± 5.77a | 26.5 ± 6.51a |
| 3rd week | 153 ± 27.6a | 91.4 ± 26.9b |
| 4th week | 308 ± 45.8a | 119 ± 35.6b |
| 5th week | 369 ± 55.7a | 137 ± 40.5b |
| Hydroponic system mg ∙ L–1 |
Aquaponic system mg ∙ L–1 |
|
|---|---|---|
| F– | 0.05 ± 0.01a,1 | 0.06 ± 0.12a |
| Cl– | 20.9 ± 0.29b | 136 ± 5.22a |
| NO2– | 0.19 ± 0.21b | 0.34 ± 0.36a |
| NO3– | 541 ± 6.44a | 37.1 ± 34.6b |
| NH4+ | 44.8 ± 1.23a | 3.48 ± 2.29b |
| Hydroponic system mg ∙ L–1 |
Aquaponic system mg ∙ L–1 |
|
|---|---|---|
| B | 0.22 ± 0.01a,1 | 0.17 ± 0.03a |
| Ca | 76.5 ± 1.46a | 69.7 ± 3.24a |
| Cu | 0.23 ± 0.02a | 0.07 ± 0.01b |
| Fe | 1.94 ± 0.09 | – |
| K | 148 ± 3.58a | 29.6 ± 3.58b |
| Mg | 26.7 ± 1.39b | 36.4 ± 1.23a |
| Mn | 1.35 ± 0.01a | 0.02 ± 0.01b |
| Na | 6.69 ± 0.02b | 83.8 ± 3.99a |
| P | 32.2 ± 1.09a | 14.3 ± 1.48b |
| S | 38.8 ± 1.12b | 63.7 ± 3.05a |
| Si | – | 2.36 ± 0.32 |
| Zn | 0.33 ± 0.01 | – |
| Hydroponic system DW mg ∙ g–1 |
Aquaponic system DW mg ∙ g–1 |
Hydroponic system FW mg ∙ 100g–1 |
Aquaponic system FW mg ∙ 100g–1 |
|
|---|---|---|---|---|
| Flavonoid content1 | 8.84 ± 1.65b,2 | 16.8 ± 2.32a | 48.6 ± 10.5b | 140 ± 30.5a |
| Phenol content | 11.1 ± 2.2b | 24.9 ± 5.07a | 60.6 ± 13.9b | 209 ± 59.9a |
| Hydroponic system DW |
Aquaponic system DW |
Hydroponic system FW |
Aquaponic system FW |
|
|---|---|---|---|---|
| μg ∙ mL–1 | μg ∙ mL–1 | mg ∙ mL–1 | mg ∙ mL–1 | |
| IC50 DPPH | 638 ± 76.36a,1 | 271 ± 58.8b | 11.7 ± 1.6a | 3.3 ± 1.0b |
| Hydroponic system DW μM TE ∙ g–1 |
Aquaponic system DW μM TE ∙ g–1 |
Hydroponic system FW μM TE ∙ 100g–1 |
Aquaponic system FW μM TE ∙ 100g–1 |
|
|---|---|---|---|---|
| ORAC | 221 ± 36.6b,1 | 572 ± 126.96a | 1219 ± 247b | 4793 ± 1382a |
| Hydroponic system DW mg ∙ g–1 |
Aquaponic system DW mg ∙ g–1 |
Hydroponic system FW mg ∙ 100g–1 |
Aquaponic system FW mg ∙ 100g–1 |
|
|---|---|---|---|---|
| Vit C | 1.96 ± 0.41a,1 | 1.67 ± 0.49a | 11.1 ± 2.4a | 13.9 ± 4.6a |
| Vit B2 | 15.5 ± 4.92b | 6.33 ± 1.79a | 86.5 ± 30.2b | 51.7 ± 13.7a |
| Hydroponic system DW mg ∙ g–1 |
Aquaponic system DW mg ∙ g–1 |
|
|---|---|---|
| Ca | 10.7 ± 1.07a,1 | 12.4 ± 1.47a |
| K | 37.4 ± 7.44a | 20.6 ± 1.27b |
| Mg | 3.78 ± 0.42b | 5.36 ± 0.55a |
| Na | 0.46 ± 0.28b | 6.67 ± 0.7a |
| P | 7.67 ± 0.72a | 4.48 ± 0.64b |
| S | 2.38 ± 0.15a | 1.71 ± 0.24b |
| Hydroponic system DW μg ∙ g–1 |
Aquaponic system DW μg ∙ g–1 |
|
|---|---|---|
| Al | 6.47 ± 3.75b,1 | 9.79 ± 2.75a |
| B | 24.4 ± 2.35a | 28.4 ± 3.73a |
| Co | 0.19 ± 0.14a | 0.18 ± 0.12a |
| Cr | 6.32 ± 0.52a | 6.7 ± 1.12a |
| Cu | 13.6 ± 1.24a | 4.18 ± 0.68b |
| Fe | 63.7 ± 8.09a | 24.9 ± 4.1b |
| Mn | 232 ± 23.6a | 35.5 ± 4.25b |
| Ni | 7.73 ± 0.39a | 6.24 ± 0.63b |
| Se | 8.49 ± 7.66a | 8.98 ± 6.56a |
| Si | 28.4 ± 9.85b | 89.3 ± 17.9a |
| Zn | 216 ± 49.3a | 13.8 ± 11.1b |
| Hydroponic system DW mg ∙ kg–1 |
Aquaponic system DW mg ∙ kg–1 |
|
|---|---|---|
| F– | 270 ± 128a,1 | 227 ± 52.2a |
| Cl– | 1680 ± 637b | 5978 ± 1129a |
| NO3– | 9569 ± 3055a | 74.5 ± 94.7b |
| SO42– | 815 ± 107b | 1172 ± 150a |
| PO43– | 8203 ± 3368a | 4641 ± 859b |
| Quinate | 198 ± 26.9a | 251 ± 207a |
| Lactate | 356 ± 101a | 259 ± 82.5a |
| Acetate | 311 ± 73.7a | 248 ± 76.9a |
| Propionate | 380 ± 155 | – |
| Formate | 145 ± 50.1 | – |
| Malate | 41376 ± 6052a | 43473 ± 6409a |
| Tartrate | 4278 ± 365b | 5188 ± 660a |
| Oxalate | 154 ± 34.5a | 182 ± 61.9a |
| Citrate | 4575 ± 1448b | 8925 ± 809a |
| Isocitrate | – | 26.4 ± 1.91 |
| Descriptor | LSM Hydroponic system |
LSM Aquaponic system |
SEM | P-value |
|---|---|---|---|---|
| Acceptability of appearance | 83.9 | 65.9 | 6.67 | 0.077 |
| Browning of edges | 20.5 | 31.3 | 7.64 | 0.343 |
| Freshness | 88.6 | 88.7 | 4.54 | 0.983 |
| Fragnance intensity | 57.8 | 45.6 | 6.29 | 0.204 |
| Crispiness | 63.2 | 56.8 | 6.22 | 0.485 |
| Intensity of taste | 51.4 | 51.9 | 6.42 | 0.954 |
| Acceptability of taste | 83.9 | 75.4 | 4.22 | 0.053 |
| Bitterness | 20.6 | 32 | 7.49 | 0.029 |
| Overall sample acceptability | 84.4 | 66.3 | 6.05 | 0.041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





