Submitted:
26 July 2023
Posted:
28 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
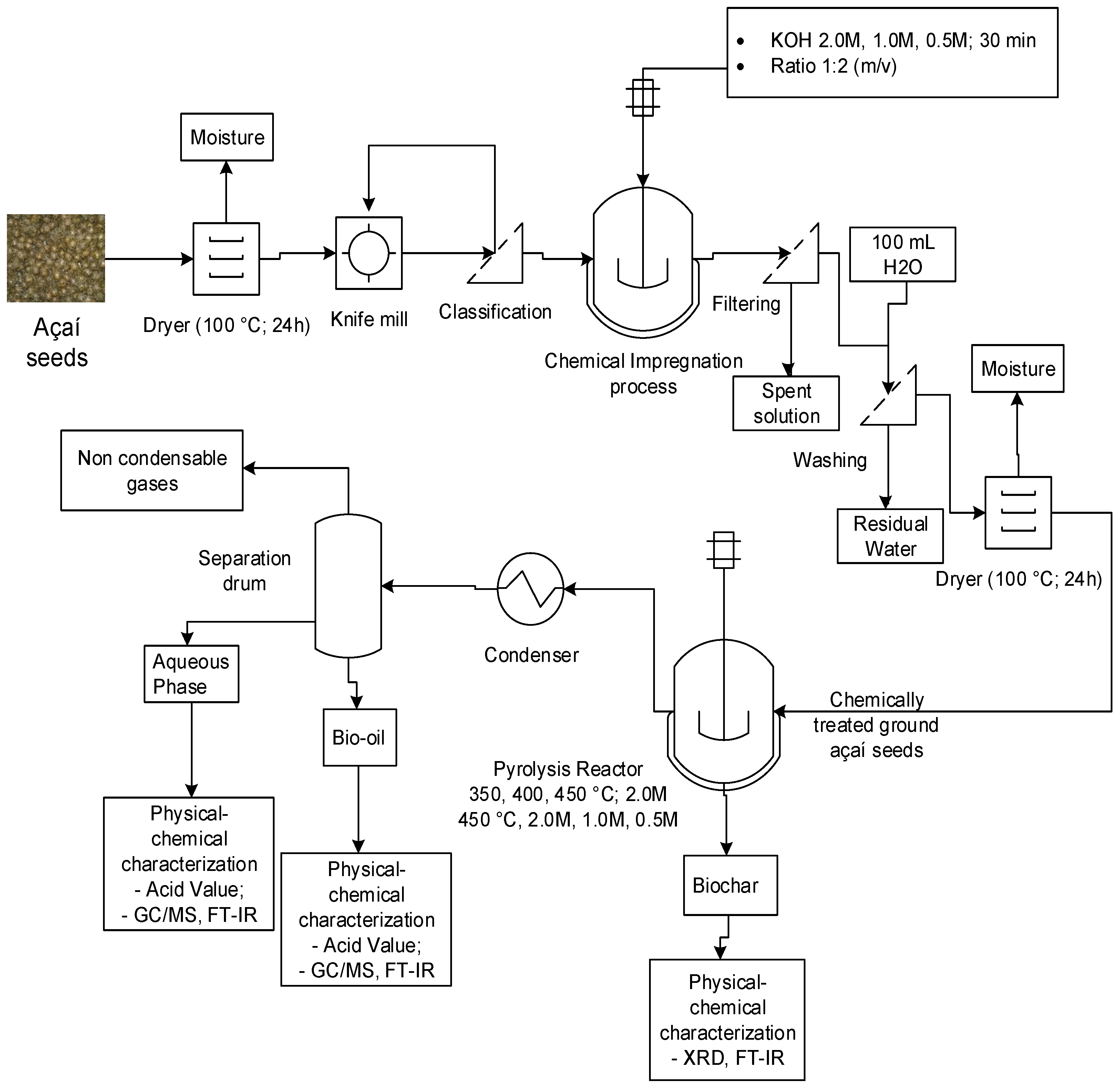
2.1. Methodology
2.2. Materials
2.3. Pre-treatnent of Açaí seeds (Euterpe oleracea, Mart.)
2.3.1. Physical pre-treatment of Açaí seeds (Euterpe oleracea, Mart.)
2.3.2. Chemical activation of Açaí seeds (Euterpe oleracea, Mart.)
2.4. Centesimal and immediate characterization of Açaí seeds
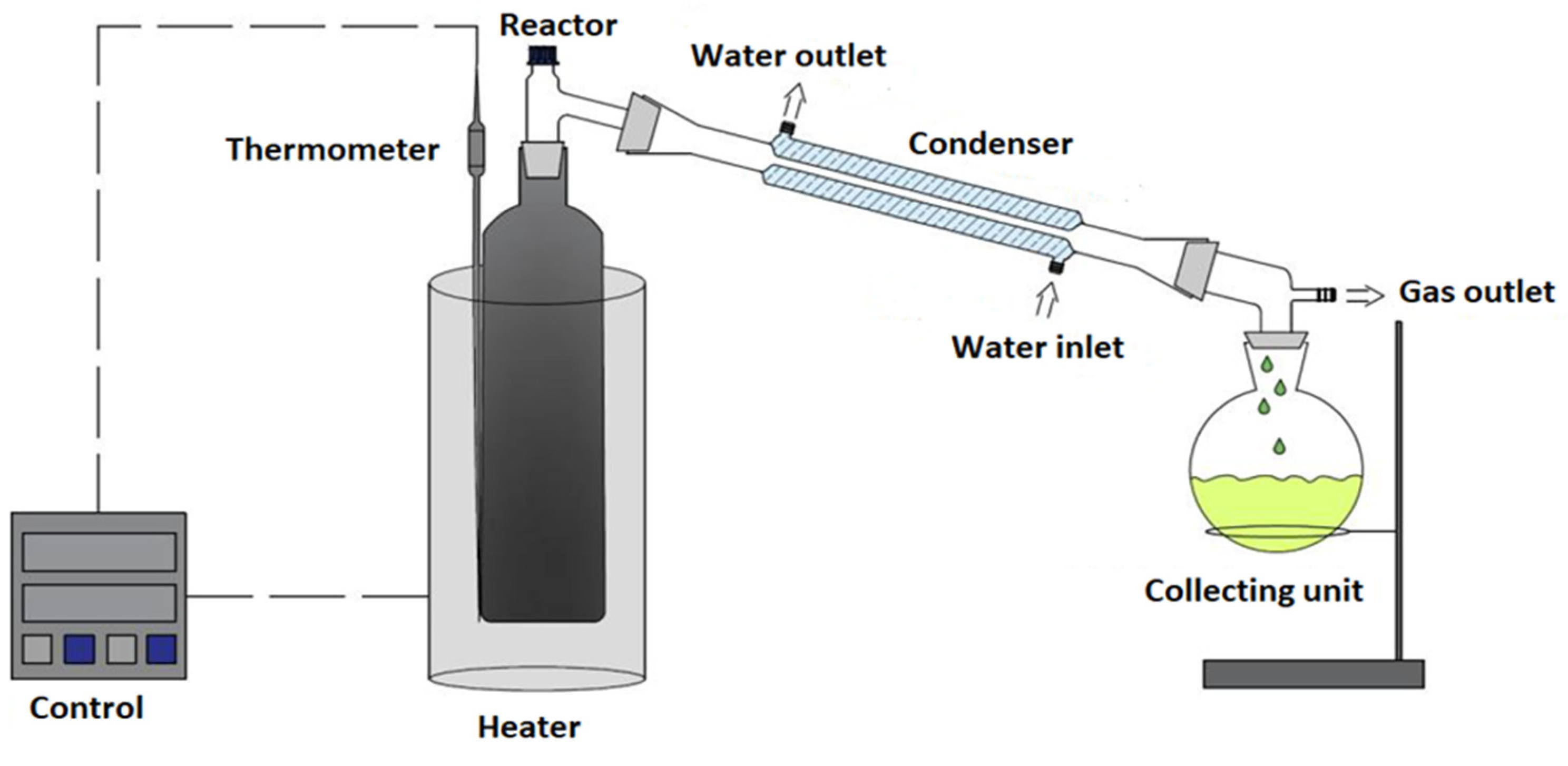
2.5. Experimental apparatus and procedures
2.5.1. Experimental apparatus
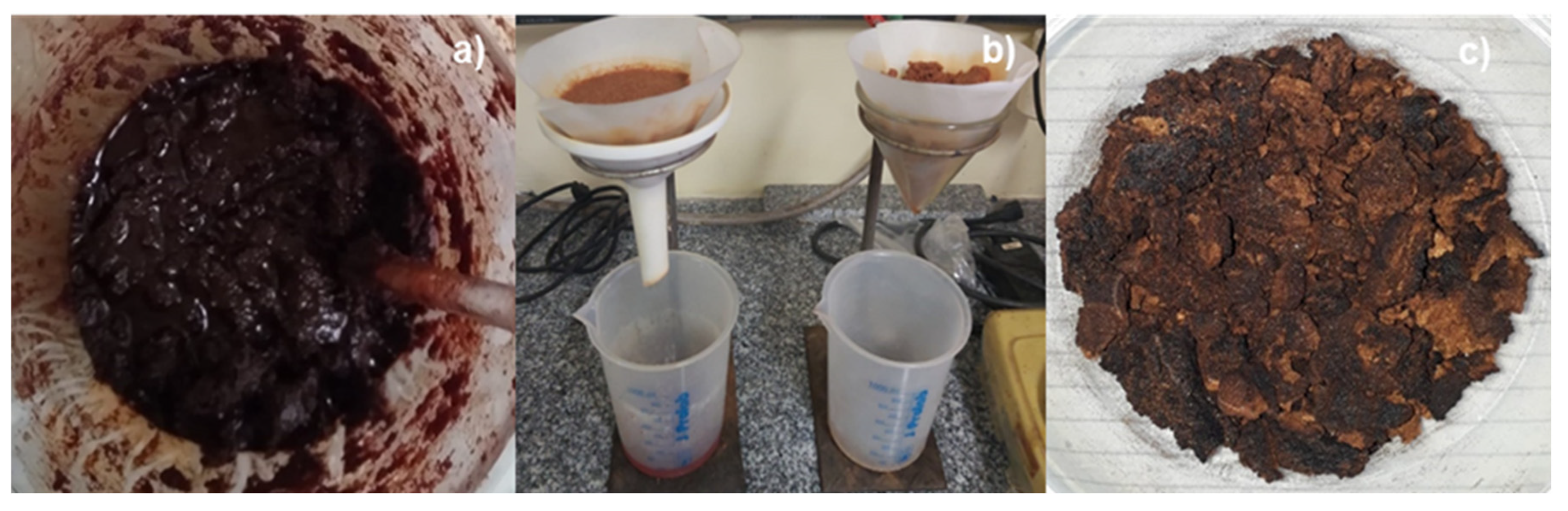
2.5.2. Experimental procedures
2.6. Acidity, antioxidant activity and chemical composition
2.6.1. Acidity of liquid fractions
2.6.2. Antioxidant activity of bio-oils
2.6.2. Chemical composition of bio-oils and aqueous phase
2.7. Characterization of hidrochar
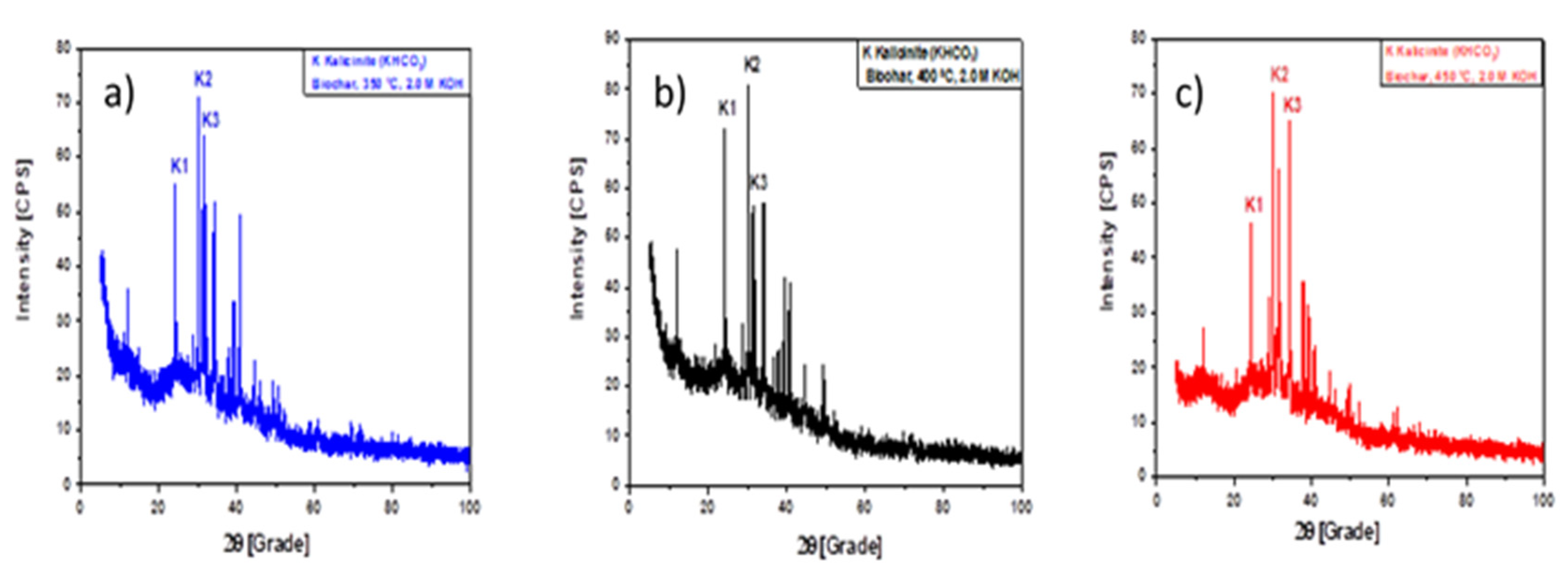
2.7.1. XRD analysis
2.8. Mass balances by pyrolysis of Açaí seeds
3. Results
3.1. Characterization of catalyst
3.1.1. XRD analysis
3.1.1.1. Effect of pyrolysis temperature
3.2. Process Analysis
3.2.1. Effect of process temperature
3.2.1.1. Effect of temperature on the composition of hydrocarbons and oxygenates in bio-oil
3.2.1.2. Effect of temperature on chemical composition of products
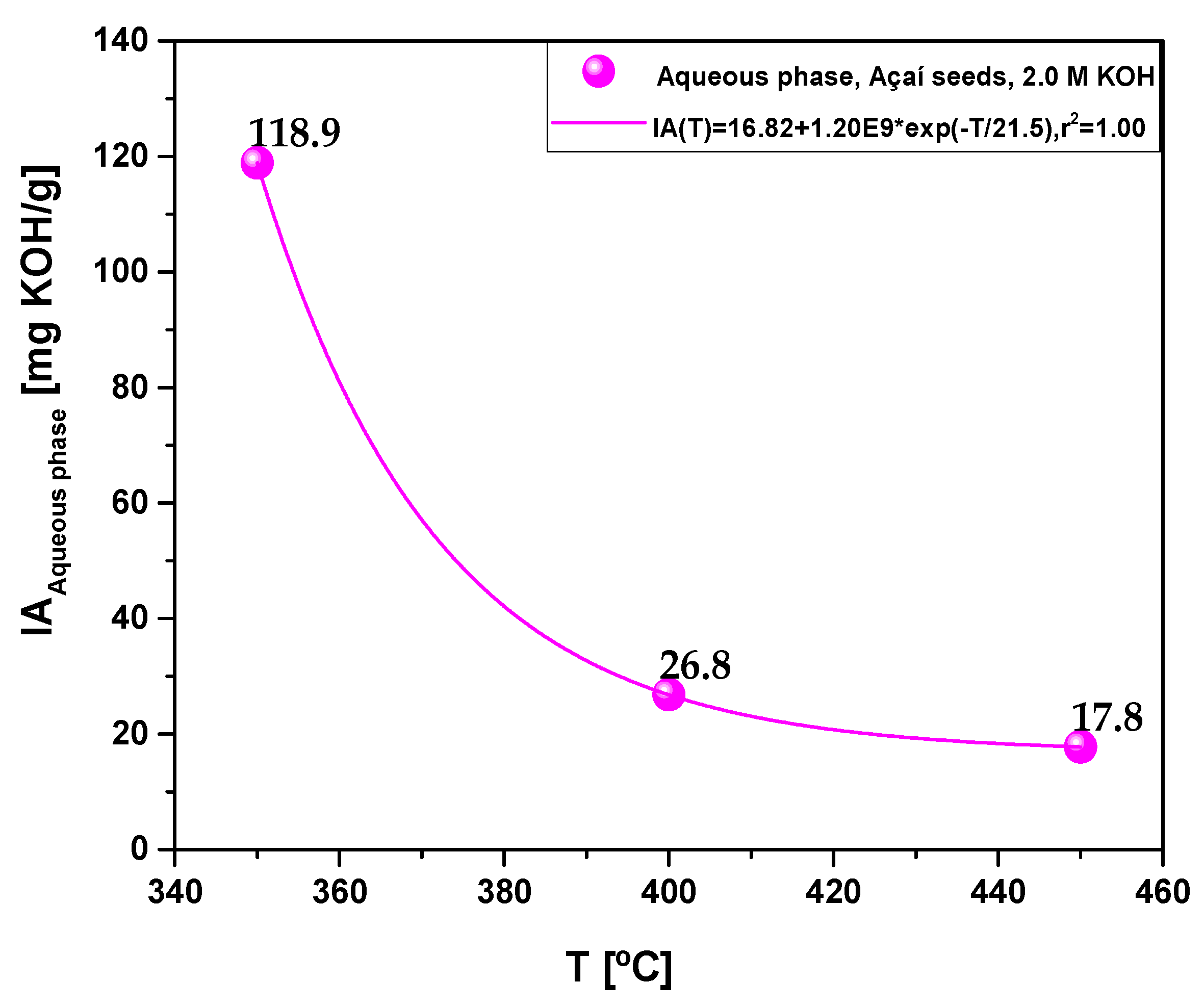
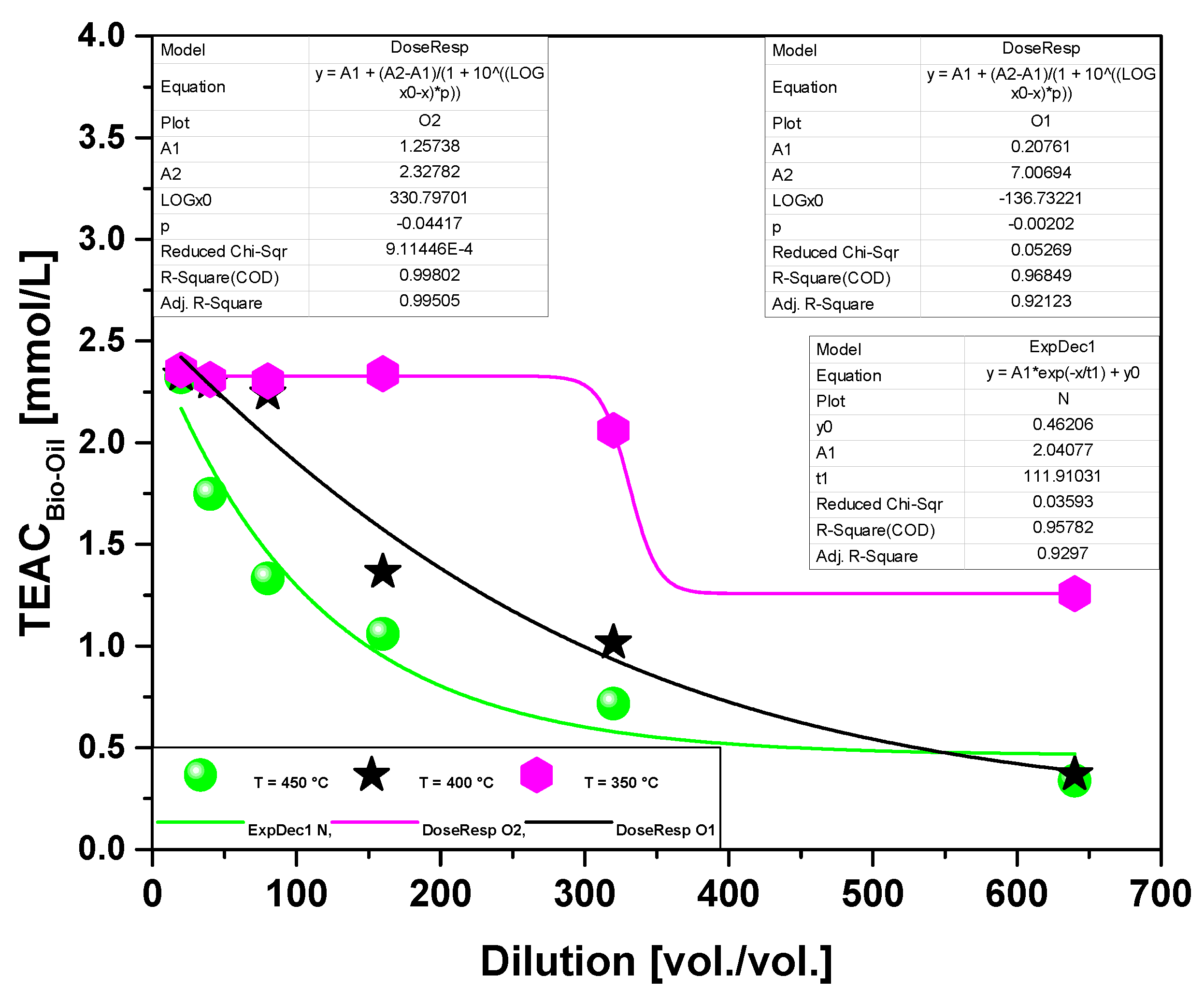
3.2.1.3. Effect of temperature on the antioxidant activity of bio-oil
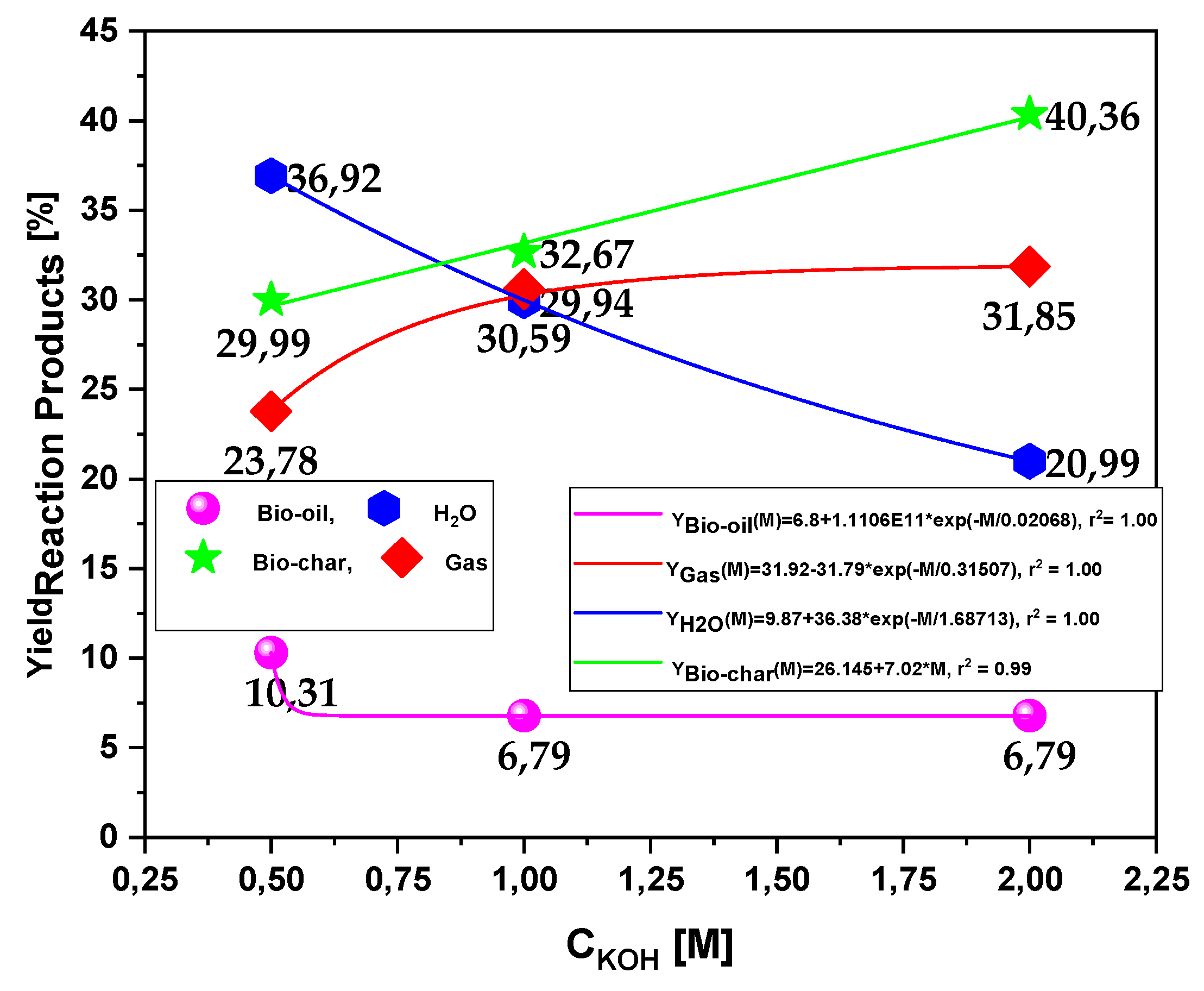
3.2.2. Effect of KOH solution molarity
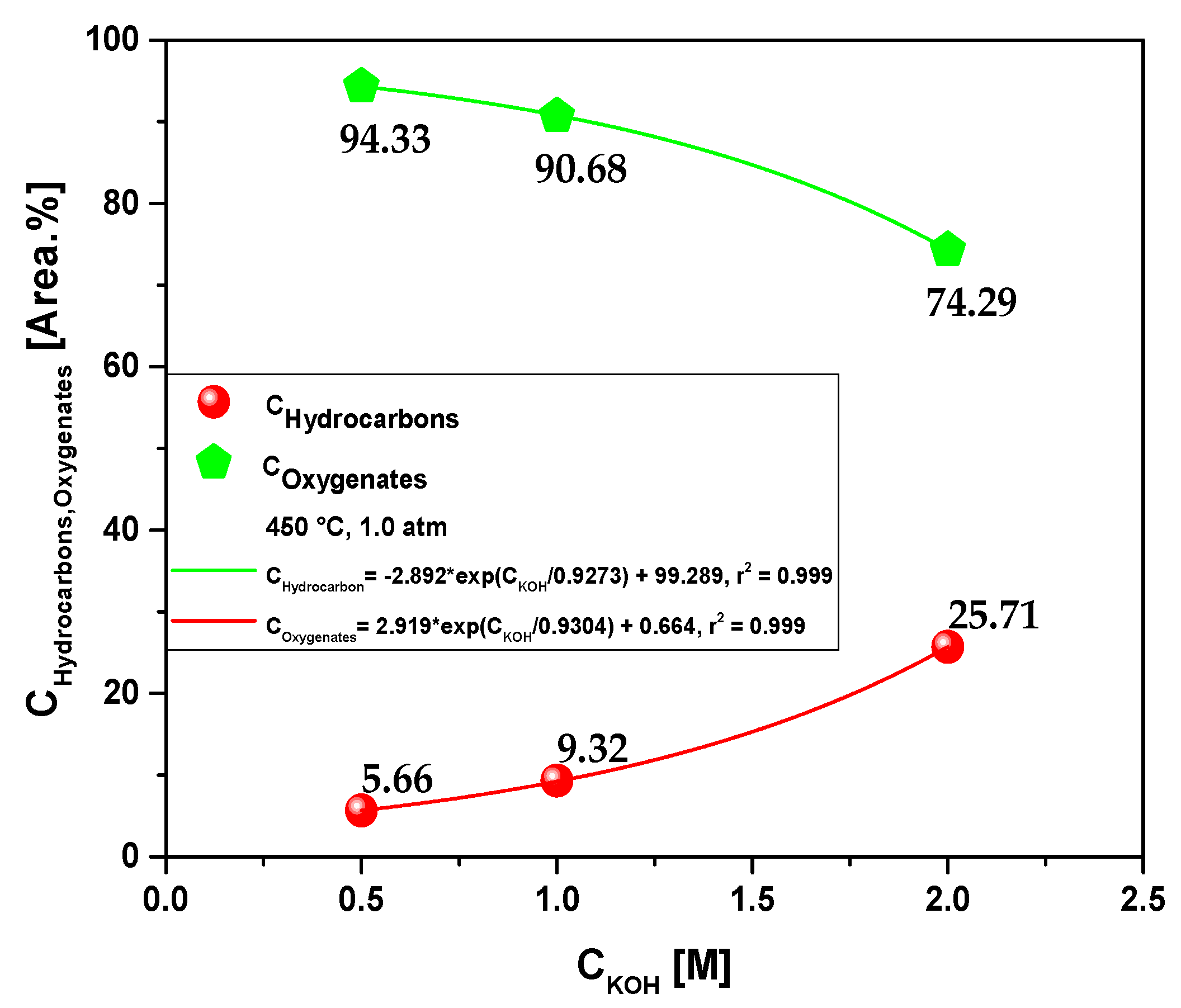
3.2.2.1. Effect of molarity in chemical composition of bio-oil
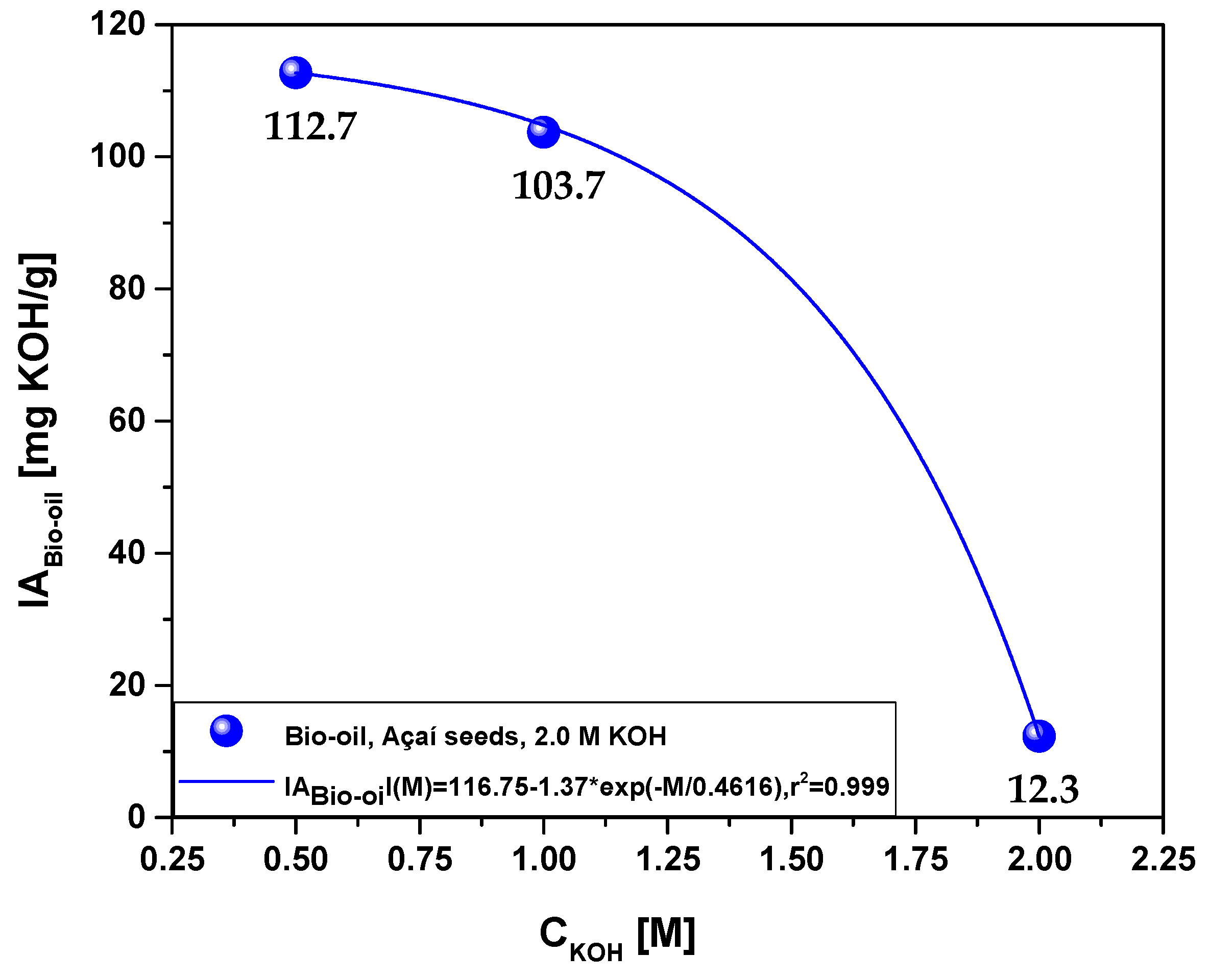
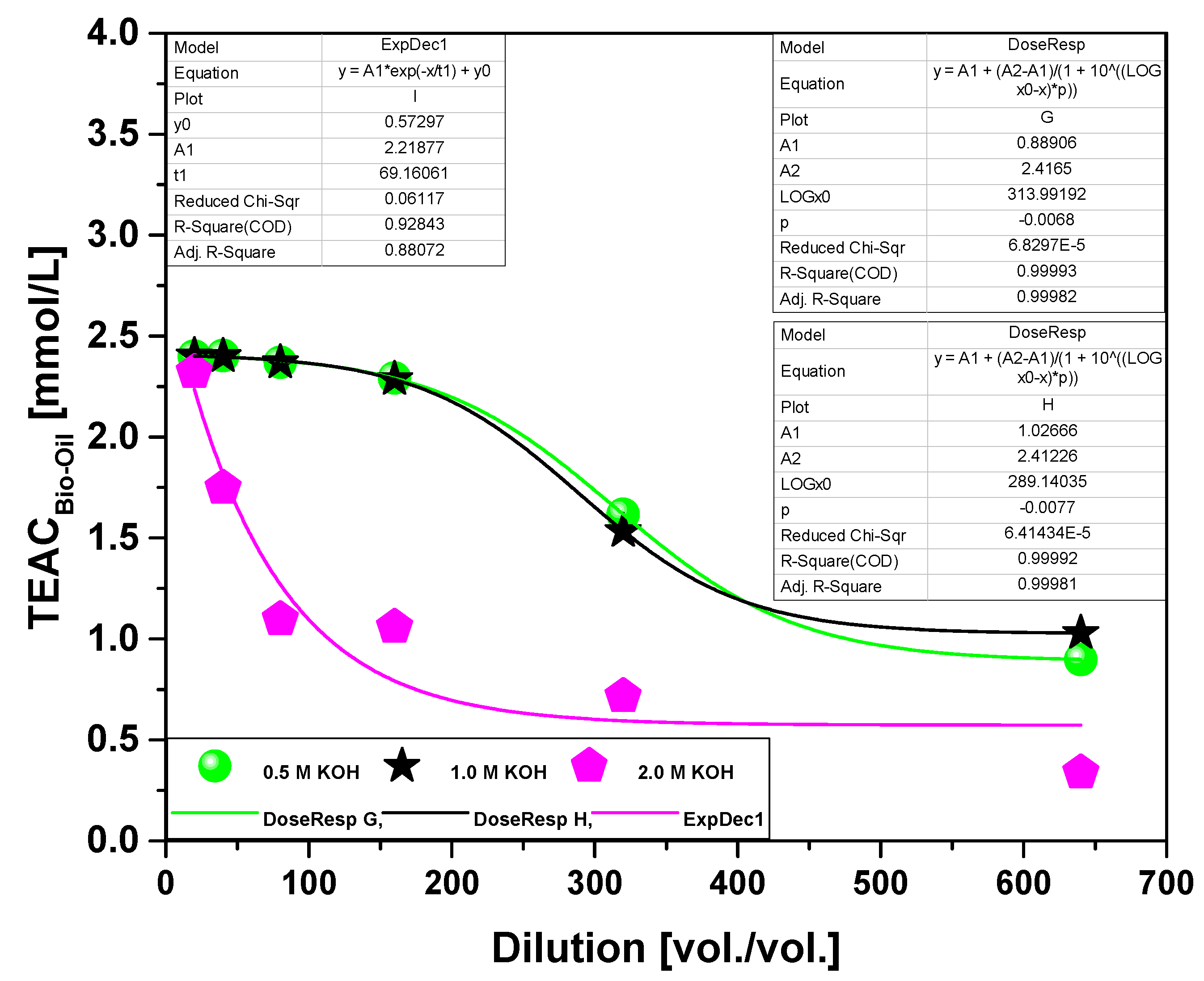
3.2.2.2. Effect of molarity on antioxidant activity of the bio-oil
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Andrezza de Melo Barbosa, Viviane Siqueira Magalhães Rebelo, Lucieta Guerreiro Martorano,Virginia Mansanares Giacon. Caracterização de partículas de açaí visando seu potencial uso na construção civil. revista Matéria, v.24, n.3, 2019, ISSN 1517-7076 artigo e-12435.
- Tavares, F.F.d.C.; de Almeida, M.D.C.; da Silva, J.A.P.; Araújo, L.L.; Cardozo, N.S.M.; Santana, R.M.C. Thermal treatment of açaí (Euterpe oleracea) fiber for composite reinforcement. Polímeros 2020, 30, e2020003. [Google Scholar] [CrossRef]
- Pompeu, D.; Silva, E.; Rogez, H. Optimisation of the solvent extraction of phenolic antioxidants from fruits of Euterpe oleracea using Response Surface Methodology. Bioresour. Technol. 2009, 100, 6076–6082. [Google Scholar] [CrossRef]
- Marcelo Morita Lindolfo, Gilson Sérgio Bastos de Matos, Wendel Valter da Silveira Pereira, Antonio Rodrigues Fernandes. Productivity and nutrition of fertigated açaí palms according to boron fertilization. Rev. Bras. Frutic., Jaboticabal, 2020, v. 42, n. 2: (e-601).
- Michael Heinrich, Tasleem Dhanji, Ivan Casselman. Açai (Euterpe oleracea Mart.)—A phytochemical and phar-macological assessment of the species’ health claims. Phytochemistry Letters 2011, 4, 10–21. [CrossRef]
- Sabbe, S.; Verbeke, W.; Deliza, R.; Matta, V.; Van Damme, P. Effect of a health claim and personal characteristics on consumer acceptance of fruit juices with different concentrations of açaí (Euterpe oleracea Mart.). Appetite 2009, 53, 84–92. [Google Scholar] [CrossRef]
- Del Pozo-Insfran, D.; Percival, S.S.; Talcott, S.T. Açai (Euterpe oleracea Mart.) Polyphenolics in Their Glycoside and Aglycone Forms Induce Apoptosis of HL-60 Leukemia Cells. J. Agric. Food Chem. 2006, 54, 1222–1229. [Google Scholar] [CrossRef]
- de Castro, D.A.R.; Ribeiro, H.J.d.S.; Ferreira, C.C.; Cordeiro, M.d.A.; Guerreiro, L.H.H.; Pereira, A.M.; dos Santos, W.G.; Santos, M.C.; de Carvalho, F.B.; Junior, J.O.C.S.; et al. Machado. Fractional Distillation of Bio-Oil Produced by Pyrolysis of Açaí (Euterpe oleracea) Seeds. Fractionation, Editor Hassan Al-Haj Ibrahim: Fractionation, Intechopen ISBN: 978-1-78984-965-3. [CrossRef]
- Guerreiro, L.H.H.; Baia, A.C.F.; Assunção, F.P.d.C.; Rodrigues, G.d.O.; e Oliveira, R.L.; Junior, S.D.; Pereira, A.M.; de Sousa, E.M.P.; Machado, N.T.; de Castro, D.A.R.; et al. Investigation of the Adsorption Process of Biochar Açaí (Euterpea olerácea Mart.) Seeds Produced by Pyrolysis. Energies 2022, 15, 6234. [Google Scholar] [CrossRef]
- Bufalino, L.; Guimaraes, A.A.; de Silva, B.M.; de Souza, R.L.F.; de Melo, I.C.N.A.; de Oliveira, D.N.P.S.; Trugilho, P.F. Local variability of yield and physical properties of açaí waste and improvement of its energetic attributes by separation of lignocellulosic fibers and seeds. J. Renew. Sustain. Energy 2018, 10, 053102. [Google Scholar] [CrossRef]
- de Castro, D.A.R.; Ribeiro, H.J.d.S.; Guerreiro, L.H.H.; Bernar, L.P.; Bremer, S.J.; Santo, M.C.; Almeida, H.d.S.; Duvoisin, S.; Borges, L.E.P.; Machado, N.T. Production of Fuel-Like Fractions by Fractional Distillation of Bio-Oil from Açaí (Euterpe oleracea Mart.) Seeds Pyrolysis. Energies 2021, 14, 3713. [Google Scholar] [CrossRef]
- Silva, C.d.M.S.d.; de Castro, D.A.R.; Santos, M.C.; Almeida, H.d.S.; Schultze, M.; Lüder, U.; Hoffmann, T.; Machado, N.T. Process Analysis of Main Organic Compounds Dissolved in Aqueous Phase by Hydrothermal Processing of Açaí (Euterpe oleraceae, Mart.) Seeds: Influence of Process Temperature, Biomass-to-Water Ratio, and Production Scales. Energies 2021, 14, 5608. [Google Scholar] [CrossRef]
- de Lima, A.C.P.; Bastos, D.L.R.; Camarena, M.A.; Bon, E.P.S.; Cammarota, M.C.; Teixeira, R.S.S.; Gutarra, M.L.E. Physicochemical characterization of residual biomass (seed and fiber) from açaí (Euterpe oleracea) processing and assessment of the potential for energy production and bioproducts. Biomass- Convers. Biorefinery 2021, 11, 925–935. [Google Scholar] [CrossRef]
- Ferdinand, F.W.; Van de Steene, L.; Blaise, K.K.; Siaka, T. Prediction of pyrolysis oils higher heating value with gas chromatography–mass spectrometry. Fuel 2012, 96, 141–145. [Google Scholar] [CrossRef]
- Bernar, L.P.; Ferreira, C.C.; Costa, A.F.d.F.; Ribeiro, H.J.d.S.; dos Santos, W.G.; Pereira, L.M.; Pereira, A.M.; Moraes, N.L.; Assunção, F.P.d.C.; da Mota, S.A.P.; et al. Catalytic Upgrading of Residual Fat Pyrolysis Vapors over Activated Carbon Pellets into Hydrocarbons-like Fuels in a Two-Stage Reactor: Analysis of Hydrocarbons Composition and Physical-Chemistry Properties. Energies 2022, 15, 4587. [Google Scholar] [CrossRef]
- Ferreira, C.C.; Bernar, L.P.; Costa, A.F.d.F.; Ribeiro, H.J.d.S.; Santos, M.C.; Moraes, N.L.; Costa, Y.S.; Baia, A.C.F.; Mendonça, N.M.; da Mota, S.A.P.; et al. Improving Fuel Properties and Hydrocarbon Content from Residual Fat Pyrolysis Vapors over Activated Red Mud Pellets in Two-Stage Reactor: Optimization of Reaction Time and Catalyst Content. Energies 2022, 15, 5595. [Google Scholar] [CrossRef]
- Sato, M.K.; de Lima, H.V.; Costa, A.N.; Rodrigues, S.; Pedroso, A.J.S.; Maia, C.M.B.d.F. Biochar from Acai agroindustry waste: Study of pyrolysis conditions. Waste Manag. 2019, 96, 158–167. [Google Scholar] [CrossRef]
- Sato, M.K.; de Lima, H.V.; Costa, A.N.; Rodrigues, S.; Mooney, S.J.; Clarke, M.; Pedroso, A.J.S.; de Freitas Maia, C.M.B. Biochar as a sustainable alternative to açaí waste disposal in Amazon, Brazil. Process Safety and Environmental Protection 2020, 139, 36–46. [Google Scholar] [CrossRef]
- Ortiz, L.R.; Torres, E.; Zalazar, D.; Zhang, H.; Rodriguez, R.; Mazza, G. Influence of pyrolysis temperature and bio-waste composition on biochar characteristics. Renew. Energy 2020, 155, 837–847. [Google Scholar] [CrossRef]
- Queiroz, L.S.; de Souza, L.K.; Thomaz, K.T.C.; Lima, E.T.L.; Filho, G.N.d.R.; Nascimento, L.A.S.D.; Pires, L.H.d.O.; Faial, K.D.C.F.; da Costa, C.E. Activated carbon obtained from amazonian biomass tailings (acai seed): Modification, characterization, and use for removal of metal ions from water. J. Environ. Manag. 2020, 270, 110868. [Google Scholar] [CrossRef]
- Pessôa, T.S.; Ferreira, L.E.d.L.; da Silva, M.P.; Neto, L.M.P.; Nascimento, B.F.D.; Fraga, T.J.M.; Jaguaribe, E.F.; Cavalcanti, J.V.; Sobrinho, M.A.d.M. Açaí waste beneficing by gasification process and its employment in the treatment of synthetic and raw textile wastewater. J. Clean. Prod. 2019, 240, 118047. [Google Scholar] [CrossRef]
- Zavarize, D.G. Insights on preparation and characteristics of KOH-doped carbons derived from an abundant agroindustrial waste in Brazil: Amazon açaí berry seeds. Bioresour. Technol. Rep. 2021, 13, 100611. [Google Scholar] [CrossRef]
- de Souza, L.K.; Gonçalves, A.A.; Queiroz, L.S.; Chaar, J.S.; Filho, G.N.d.R.; da Costa, C.E. Utilization of acai stone biomass for the sustainable production of nanoporous carbon for CO2 capture. Sustain. Mater. Technol. 2020, 25, e00168. [Google Scholar] [CrossRef]
- de Souza, T.N.V.; Vieira, M.G.A.; da Silva, M.G.C.; Brasil, D.D.S.B.; de Carvalho, S.M.L. H3PO4-activated carbons produced from açai stones and Brazil nut shells: Removal of basic blue 26 dye from aqueous solutions by adsorption. Environ. Sci. Pollut. Res. 2019, 26, 28533–28547. [Google Scholar] [CrossRef]
- Araujo, R.O.; Chaar, J.d.S.; Queiroz, L.S.; Filho, G.N.d.R.; da Costa, C.E.F.; da Silva, G.C.; Landers, R.; Costa, M.J.; Gonçalves, A.A.; de Souza, L.K. Low temperature sulfonation of acai stone biomass derived carbons as acid catalysts for esterification reactions. Energy Convers. Manag. 2019, 196, 821–830. [Google Scholar] [CrossRef]
- de Souza, T.N.V.; de Carvalho, S.M.L.; Vieira, M.G.A.; da Silva, M.G.C.; do Brasil, D.S.B. Adsorption of basic dyes onto activated carbon: Experimental and theoretical investigation of chemical reactivity of basic dyes using DFT-based descriptors. Appl. Surf. Sci. 2018, 448, 662–670. [Google Scholar] [CrossRef]
- Nascimento, B.F.D.; de Araujo, C.M.B.; Nascimento, A.C.D.; da Silva, F.L.H.; de Melo, D.J.N.; Jaguaribe, E.F.; Cavalcanti, J.V.F.L.; Sobrinho, M.A.d.M. Detoxification of sisal bagasse hydrolysate using activated carbon produced from the gasification of açaí waste. J. Hazard. Mater. 2020, 409, 124494. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.A.d.S.; Thim, G.P.; Alvarez-Mendez, M.O.; Coutinho, A.d.R.; de Moraes, N.P.; Rodrigues, L.A. Preparation, characterization, and application of low-cost açaí seed-based activated carbon for phenol adsorption. Int. J. Environ. Res. 2018, 12, 755–764. [Google Scholar] [CrossRef]
- de Souza, L.K.C.; Martins, J.C.; Oliveira, D.P.; Ferreira, C.S.; Gonçalves, A.A.S.; Araujo, R.O.; Chaar, J.d.S.; Costa, M.J.F.; Sampaio, D.V.; Passos, R.R.; et al. Hierarchical porous carbon derived from acai seed biowaste for supercapacitor electrode materials. J. Mater. Sci. Mater. Electron. 2020, 31, 12148–12157. [Google Scholar] [CrossRef]
- Serrão, A.C.M.; Silva, C.M.S.; Assunção, F.P.d.C.; Ribeiro, H.J.d.S.; Santos, M.C.; Almeida, H.d.S.; Junior, S.D.; Borges, L.E.P.; de Castro, D.A.R.; Machado, N.T. Process analysis of pyrolysis of Açaí (Euterpe Oleracea, Mart) seeds: Influence of temperature on the yield of reaction products and physico-chemical properties of Bio-Oil. Brazilian Journal of Development, Curitiba. Braz. J. Dev. 2021, 7, 18200–18220. [Google Scholar] [CrossRef]
- Valdez, G.D.; Valois, F.P.; Bremer, S.J.; Bezerra, K.C.A.; Guerreiro, L.H.H.; Santos, M.C.; Bernar, L.P.; Feio, W.P.; Moreira, L.G.S.; Mendonça, N.M.; et al. Improving the Bio-Oil Quality of Residual Biomass Pyrolysis by Chemical Activation: Effect of Alkalis and Acid Pre-Treatment. Energies 2023, 16, 3162. [Google Scholar] [CrossRef]
- de Sousa, J.L.; Guerreiro, L.H.H.; Bernar, L.P.; Ribeiro, H.J.d.S.; e Oliveira, R.L.; Santos, M.C.; Almeida, H.d.S.; Junior, S.D.; Borges, L.E.P.; Castro, D.A.R.; et al. Chemical Analysis of Bio-Oil Produced by Pyrolysis of Açaí (Euterpe Oleracea, Mart) Seeds. Braz. J. Dev. 2021, 7, 15549–15565. [Google Scholar] [CrossRef]
- Chandrasekaran, S.R.; Murali, D.; Marley, K.A.; Larson, R.A.; Doll, K.M.; Moser, B.R.; Scott, J.; Sharma, B.K. Antioxidants from Slow Pyrolysis Bio-Oil of Birch Wood: Application for Biodiesel and Biobased Lubricants. ACS Sustain. Chem. Eng. 2016, 4, 1414–1421. [Google Scholar] [CrossRef]
- del Pozo, C.; Bartrolí, J.; Alier, S.; Puy, N.; Fàbregas, E. Production of antioxidants and other value-added compounds from coffee silverskin via pyrolysis under a biorefinery approach. Waste Manag. 2020, 109, 19–27. [Google Scholar] [CrossRef]
- Patra, J.K.; Kim, S.H.; Hwang, H.; Choi, J.W.; Baek, K.-H. Volatile Compounds and Antioxidant Capacity of the Bio-Oil Obtained by Pyrolysis of Japanese Red Pine (Pinus Densiflora Siebold and Zucc.). Molecules 2015, 20, 3986–4006. [Google Scholar] [CrossRef] [PubMed]
- Malagón-Romero, D.; Torres-Velasquez, A.C.; Tinoco-Navarro, L.K.; Arrubla-Vélez, J.P. Pyrolysis of Colombian spent coffee grounds (SCGs), characterization of bio-oil, and study of its antioxidant properties. Int. J. Sustain. Energy 2023, 42, 811–829. [Google Scholar] [CrossRef]
- Hossain, M.M.; Scott, I.M.; Berruti, F.; Briens, C. Application of Novel Pyrolysis Reactor Technology to Concentrate Bio-oil Components with Antioxidant Activity from Tobacco, Tomato and Coffee Ground Biomass. Waste Biomass- Valorization 2018, 9, 1607–1617. [Google Scholar] [CrossRef]
- Patra, J.K.; Kim, S.H.; Hwang, H.; Choi, J.W.; Baek, K.-H. Volatile Compounds and Antioxidant Capacity of the Bio-Oil Obtained by Pyrolysis of Japanese Red Pine (Pinus Densiflora Siebold and Zucc.). Molecules 2015, 20, 3986–4006. [Google Scholar] [CrossRef]
- del Pozo, C.; Bartrolí, J.; Alier, S.; Puy, N.; Fàbregas, E. Production, identification, and quantification of antioxidants from torrefaction and pyrolysis of grape pomace. Fuel Process. Technol. 2021, 211, 106602. [Google Scholar] [CrossRef]
- Chen, A.; Liu, X.; Zhang, H.; Wu, H.; Xu, D.; Li, B.; Zhao, C. Influence of pyrolysis temperature on bio-oil produced from hazelnut shells: Physico-chemical properties and antioxidant activity of wood vinegar and tar fraction. J. Renew. Sustain. Energy 2021, 13, 043102. [Google Scholar] [CrossRef]
- de Andrade Cordeiro, M.; de Almeida, O.; de Castro, D.A.R.; da Silva Ribeiro, H.J.; Machado, N.T. Produção de Etanol através da Hidrólise Enzimática do Caroço de Açaí (Euterpe oleracea, Mart. ). Rev. Bras. Energ. Renov. 2019, 8, 122–152. [Google Scholar]
- Almeida, H.d.S.; Corrêa, O.; Eid, J.; Ribeiro, H.; de Castro, D.; Pereira, M.; Pereira, L.; Mâncio, A.d.A.; Santos, M.; Souza, J.d.S.; et al. Production of biofuels by thermal catalytic cracking of scum from grease traps in pilot scale. J. Anal. Appl. Pyrolysis 2016, 118, 20–33. [Google Scholar] [CrossRef]
- Almeida, H.d.S.; Corrêa, O.; Eid, J.; Ribeiro, H.; de Castro, D.; Pereira, M.; Pereira, L.; Aâncio, A.d.A.; Santos, M.; da Mota, S.; et al. Performance of thermochemical conversion of fat, oils, and grease into kerosene-like hydrocarbons in different production scales. J. Anal. Appl. Pyrolysis 2016, 120, 126–143. [Google Scholar] [CrossRef]
- Almeida, H.d.S.; Corrêa, O.; Ferreira, C.; Ribeiro, H.; de Castro, D.; Pereira, M.; Mâncio, A.d.A.; Santos, M.; da Mota, S.; Souza, J.d.S.; et al. Diesel-like hydrocarbon fuels by catalytic cracking of fat, oils, and grease (FOG) from grease traps. J. Energy Inst. 2017, 90, 337–354. [Google Scholar] [CrossRef]
- da Mota, S.; Mancio, A.; Lhamas, D.; de Abreu, D.; da Silva, M.; dos Santos, W.; de Castro, D.; de Oliveira, R.; Araújo, M.; Borges, L.E.; et al. Production of green diesel by thermal catalytic cracking of crude palm oil (Elaeis guineensis Jacq) in a pilot plant. J. Anal. Appl. Pyrolysis 2014, 110, 1–11. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A Novel Method for Measuring Antioxidant Capacity and its Application to Monitoring the Antioxidant Status in Premature Neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Mancio, A.; da Mota, S.; Ferreira, C.; Carvalho, T.; Neto, O.; Zamian, J.; Araújo, M.; Borges, L.; Machado, N. Separation and characterization of biofuels in the jet fuel and diesel fuel ranges by fractional distillation of organic liquid products. Fuel 2018, 215, 212–225. [Google Scholar] [CrossRef]
- Mâncio, A.; da Costa, K.; Ferreira, C.; Santos, M.; Lhamas, D.; da Mota, S.; Leão, R.; de Souza, R.; Araújo, M.; Borges, L.; et al. Process analysis of physicochemical properties and chemical composition of organic liquid products obtained by thermochemical conversion of palm oil. J. Anal. Appl. Pyrolysis 2017, 123, 284–295. [Google Scholar] [CrossRef]
- Santos, M.; Lourenço, R.; de Abreu, D.; Pereira, A.; de Castro, D.; Pereira, M.; Almeida, H.; Mâncio, A.; Lhamas, D.; da Mota, S.; et al. Gasoline-like hydrocarbons by catalytic cracking of soap phase residue of neutralization process of palm oil (Elaeis guineensis Jacq). J. Taiwan Inst. Chem. Eng. 2017, 71, 106–119. [Google Scholar] [CrossRef]
- Ferreira, C.; Costa, E.; de Castro, D.; Pereira, M.; Mâncio, A.; Santos, M.; Lhamas, D.; da Mota, S.; Leão, A.; Duvoisin, S.; et al. Deacidification of organic liquid products by fractional distillation in laboratory and pilot scales. J. Anal. Appl. Pyrolysis 2017, 127, 468–489. [Google Scholar] [CrossRef]
- Costa, M.E.G.; Assunção, F.P.d.C.; Teribele, T.; Pereira, L.M.; de Castro, D.A.R.; Santo, M.C.; da Costa, C.E.F.; Shultze, M.; Hofmann, T.; Machado, N.T. Characterization of Bio-Adsorbents Produced by Hydrothermal Carbonization of Corn Stover: Application on the Adsorption of Acetic Acid from Aqueous Solutions. Energies 2021, 14, 8154. [Google Scholar] [CrossRef]
- Teribele, T.; Costa, M.E.G.; Silva, C.d.M.S.d.; Pereira, L.M.; Bernar, L.P.; de Castro, D.A.R.; Assunção, F.P.d.C.; Santos, M.C.; Brandão, I.W.d.S.; Fonseca, C.J.N.; et al. Hydrothermal Carbonization of Corn Stover: Structural Evolution of Hydro-Char and Degradation Kinetics. Energies 2023, 16, 3217. [Google Scholar] [CrossRef]
- Costa, A.F.d.F.; Ferreira, C.C.; da Paz, S.P.A.; Santos, M.C.; Moreira, L.G.S.; Mendonça, N.M.; Assunção, F.P.d.C.; Freitas, A.C.G.d.A.d.; Costa, R.M.R.; Brandão, I.W.d.S.; et al. Catalytic Upgrading of Plastic Waste of Electric and Electronic Equipment (WEEE) Pyrolysis Vapors over Si–Al Ash Pellets in a Two-Stage Reactor. Energies 2023, 16, 541. [Google Scholar] [CrossRef]
- Assunção, F.P.d.C.; Pereira, D.O.; da Silva, J.C.C.; Ferreira, J.F.H.; Bezerra, K.C.A.; Bernar, L.P.; Ferreira, C.C.; Costa, A.F.d.F.; Pereira, L.M.; da Paz, S.P.A.; et al. A Systematic Approach to Thermochemical Treatment of Municipal Household Solid Waste into Valuable Products: Analysis of Routes, Gravimetric Analysis, Pre-Treatment of Solid Mixtures, Thermochemical Processes, and Characterization of Bio-Oils and Bio-Adsorbents. Energies 2022, 15, 7971. [Google Scholar] [CrossRef]
- Prakongkep, N.; Gilkes, R.J.; Wiriyakitnateekul, W. Agronomic benefits of durian shell biochar. J. Met. Mater. Miner. 2014, 24. [Google Scholar] [CrossRef]
- Lee, J.H.; Cha, Y.L.; Kang, Y.-M.; Roh, K.C. Study on the reaction mechanism of the potassium bicarbonate alkali activation process in black liquor. APL Mater. 2022, 10, 101105. [Google Scholar] [CrossRef]
- Dı́az-Terán, J.; Nevskaia, D.; Fierro, J.; López-Peinado, A.; Jerez, A. Study of chemical activation process of a lignocellulosic material with KOH by XPS and XRD. Microporous Mesoporous Mater. 2003, 60, 173–181. [Google Scholar] [CrossRef]
- Piyali Das, Anuradda Ganesh. Bio-oil from pyrolysis of cashew nutshell—A near fuel. Biomass and Bioenergy 2003, 25, 113–117. [CrossRef]
- Asadullah, M.; Rahman, M.; Ali, M.; Motin, M.; Sultan, M.; Alam, M. Production of bio-oil from fixed bed pyrolysis of bagasse. Fuel 2007, 86, 2514–2520. [Google Scholar] [CrossRef]
- Ji-Lu, Z. Bio-oil from fast pyrolysis of rice husk: Yields and related properties and improvement of the pyrolysis system. J. Anal. Appl. Pyrolysis 2007, 80, 30–35. [Google Scholar] [CrossRef]
- Mohamad Azri Sukiran, Chow Mee Chin, Nor Kartini Abu Bakar. Bio-oils from Pyrolysis of Oil Palm Empty Fruit Bunches. American Journal of Applied Sciences 2009, 6, 869–875. [CrossRef]
- Kim, S.-J.; Jung, S.-H.; Kim, J.-S. Fast pyrolysis of palm kernel shells: Influence of operation parameters on the bio-oil yield and the yield of phenol and phenolic compounds. Bioresour. Technol. 2010, 101, 9294–9300. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Panda, A.K.; Singh, R. Optimization of process for the production of bio-oil from eucalyptus wood. J. Fuel Chem. Technol. 2010, 38, 162–167. [Google Scholar] [CrossRef]
- Heo, H.S.; Park, H.J.; Dong, J.-I.; Park, S.H.; Kim, S.; Suh, D.J.; Suh, Y.-W.; Kim, S.-S.; Park, Y.-K. Fast pyrolysis of rice husk under different reaction conditions. J. Ind. Eng. Chem. 2010, 16, 27–31. [Google Scholar] [CrossRef]
- Ortega, J.V.; Renehan, A.M.; Liberatore, M.W.; Herring, A.M. Physical and chemical characteristics of aging pyrolysis oils produced from hardwood and softwood feedstocks. J. Anal. Appl. Pyrolysis 2011, 91, 190–198. [Google Scholar] [CrossRef]
- Rajeev Sharma, Pratik N. Sheth. Thermo-Chemical Conversion of Jatropha Deoiled Cake: Pyrolysis vs. Gasification. International Journal of Chemical Engineering and Applications 2015, 6.
- Paenpong, C.; Pattiya, A. Effect of pyrolysis and moving-bed granular filter temperatures on the yield and properties of bio-oil from fast pyrolysis of biomass. J. Anal. Appl. Pyrolysis 2016, 119, 40–51. [Google Scholar] [CrossRef]
- Garg, R.; Anand, N.; Kumar, D. Pyrolysis of babool seeds (Acacia nilotica) in a fixed bed reactor and bio-oil characterization. Renew. Energy 2016, 96, 167–171. [Google Scholar] [CrossRef]
- Varma, A.K.; Mondal, P. Pyrolysis of sugarcane bagasse in semi batch reactor: Effects of process parameters on product yields and characterization of products. Ind. Crop. Prod. 2017, 95, 704–717. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, Y.; Li, T.; Ren, Z. Upgrading of liquid fuel from the pyrolysis of biomass. Bioresour. Technol. 2005, 96, 545–550. [Google Scholar] [CrossRef]
- Torri, C.; Fabbri, D. Biochar enables anaerobic digestion of aqueous phase from intermediate pyrolysis of biomass. Bioresour. Technol. 2014, 172, 335–341. [Google Scholar] [CrossRef]
- Zhou, H.; Brown, R.C.; Wen, Z. Anaerobic digestion of aqueous phase from pyrolysis of biomass: Reducing toxicity and improving microbial tolerance. Bioresour. Technol. 2019, 292, 121976. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2020, 12, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Rosario, A.C.R.S.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef]
- Caleja, C.; Ribeiro, A.; Barreiro, M.F.; Ferreira, I.C. Phenolic Compounds as Nutraceuticals or Functional Food Ingredients. Curr. Pharm. Des. 2017, 23, 2787–2806. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Leng, S.; Bai, J.; Zhou, H.; Zhong, X.; Zhuang, G.; Wang, J. Role of pretreatment with acid and base on the distribution of the products obtained via lignocellulosic biomass pyrolysis. RSC Adv. 2015, 5, 24984–24989. [Google Scholar] [CrossRef]
- Douglas Alberto Rocha de Castro. Tese de Doutorado: ESTUDO DO PROCESSO DE PIRÓLISE DE SEMENTES DE AÇAÍ (Eutero olerace Mart.) PARA PRODUÇÃO DE BIOCOMBUSTÍVEIS. PRODERNA/ITEC, UFPA, 2019. Available online: https://proderna.propesp.ufpa.br/index.php/br/teses-e-dissertacoes/teses/273-2019.



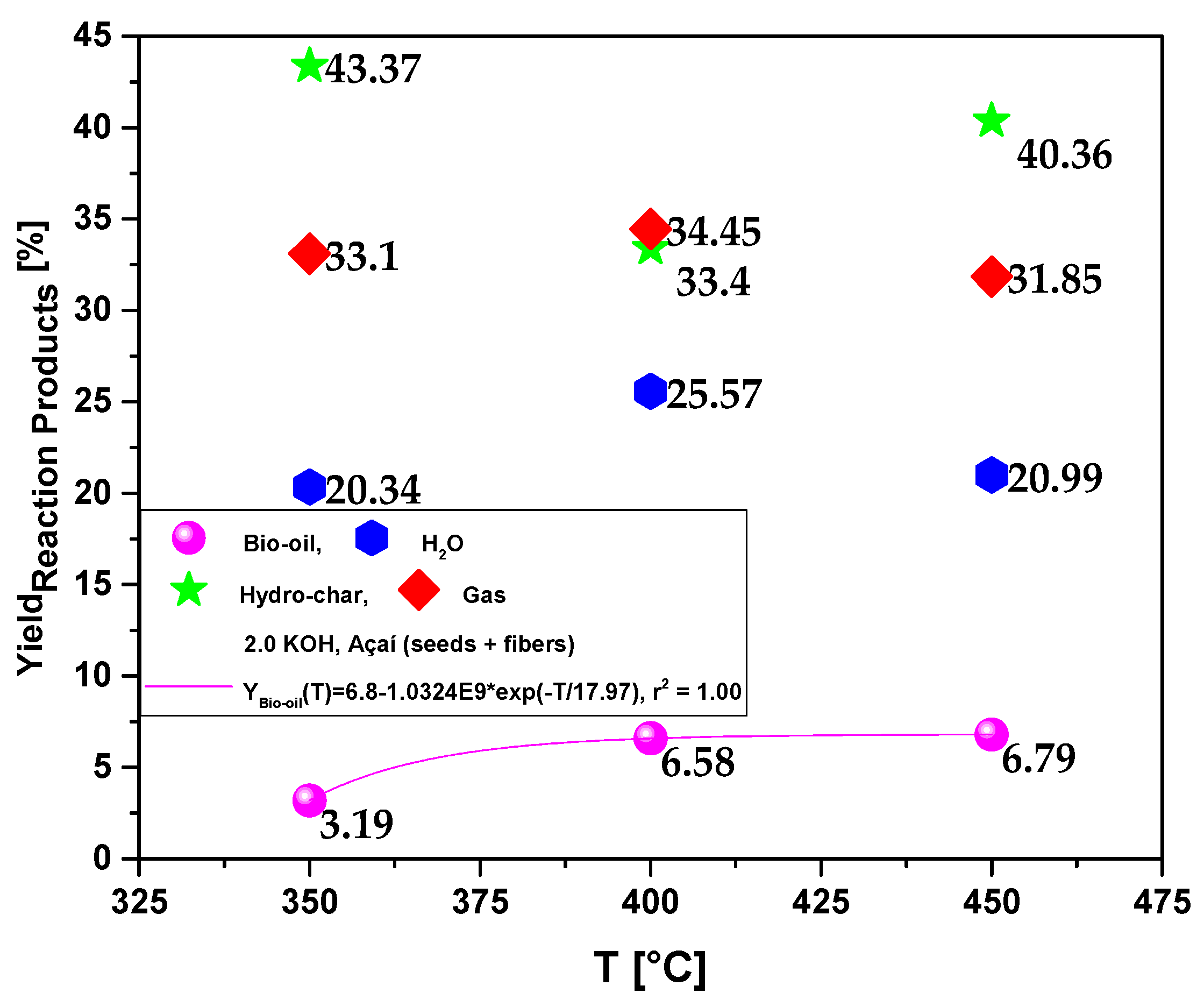
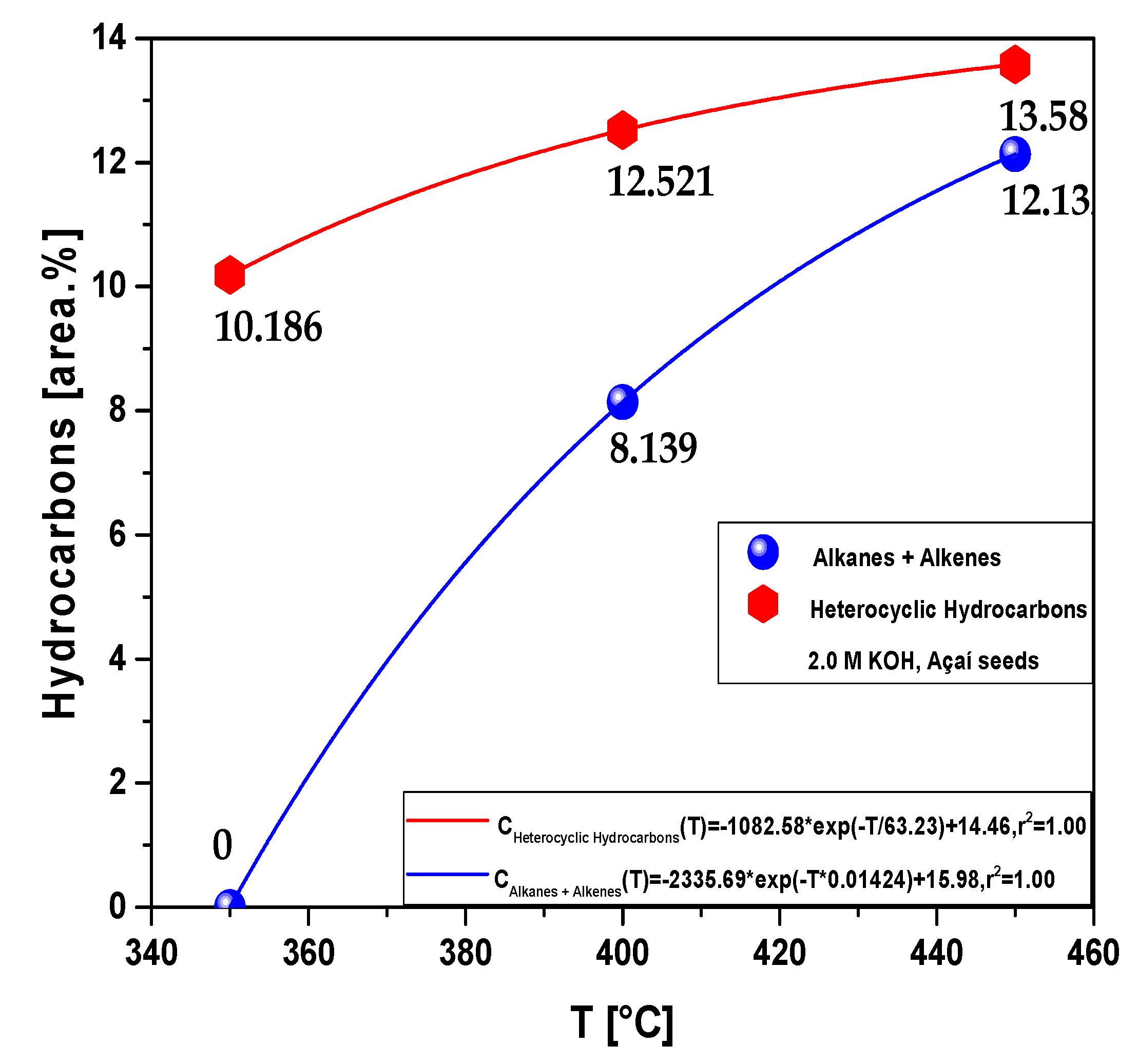
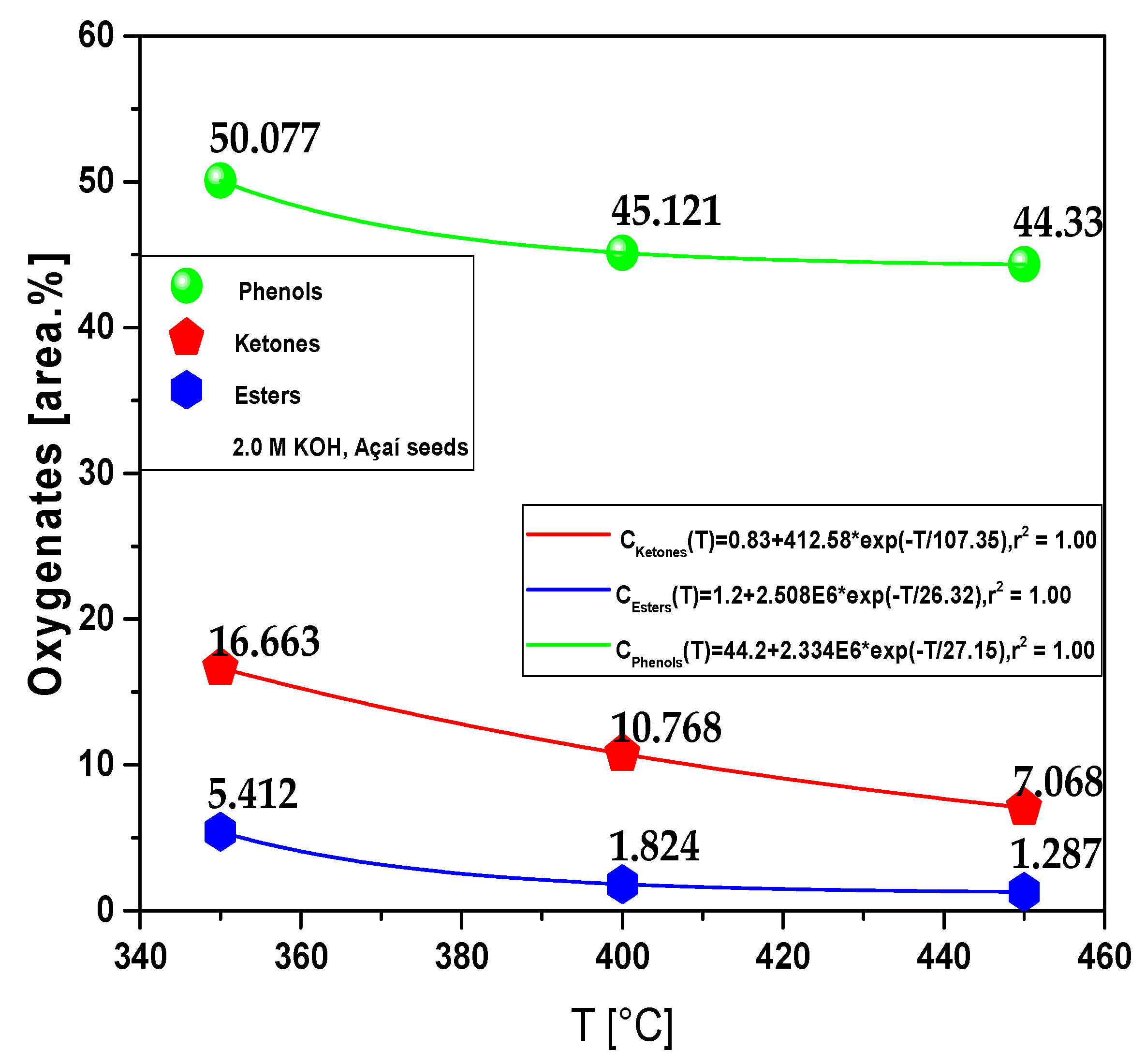
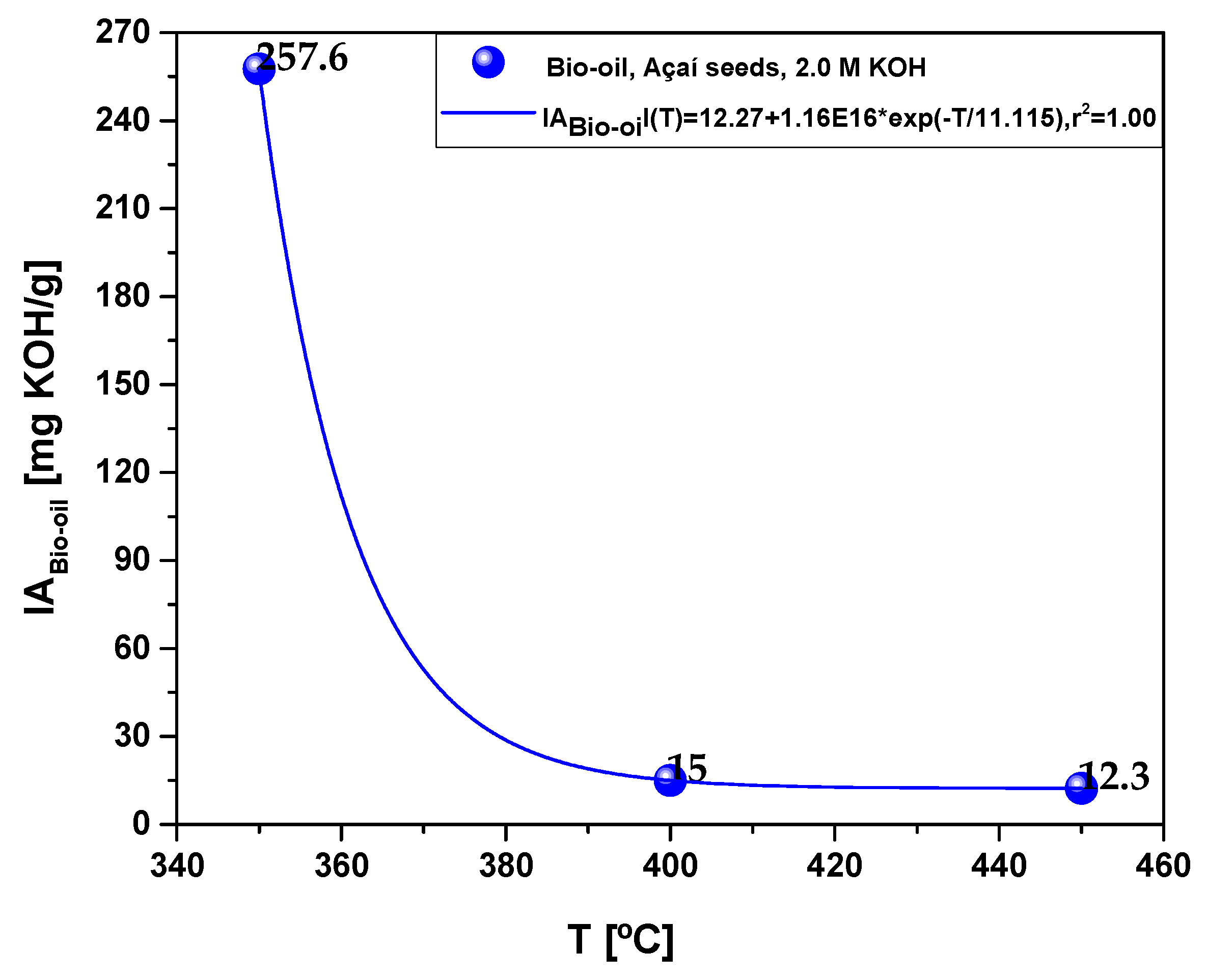
| Process Parameters | 2.0 M KOH | ||
|---|---|---|---|
| 350 °C | 400 °C | 450 °C | |
| Mass of Açaí seeds (g) | 40.12 | 40.12 | 40.06 |
| Cracking time (min) | 62 | 67 | 72 |
| Solid weight (Coke) (g) | 17.40 | 13.40 | 16.17 |
| Liquid weight (Bio-oil) (g) | 1.28 | 2.64 | 2.72 |
| Weight of H2O (g) | 8.16 | 10.26 | 8.41 |
| Weight of gas (g) | 13.28 | 13.82 | 12.76 |
| Bio-oil Yield (wt.%) | 3.19 | 6.58 | 6.79 |
| H2O Yield (wt.%) | 20.34 | 25.57 | 20.99 |
| Bio-char Yield(wt.%) | 43.37 | 33.40 | 40.36 |
| Gas Yield(wt.%) | 33.10 | 34.45 | 31.85 |
| Acidity (mg KOH/g) | 257.6 | 15.0 | 12.3 |
| Chemical Composition Ci (area.%) | 2.0 M KOH | ||
|---|---|---|---|
| 350 °C | 400 °C | 450 °C | |
| Alcohols | 2.34 | 20.74 | 26.62 |
| Carboxylic Acids | 4.05 | 15.02 | 9.23 |
| Ketones | 52.81 | 44.38 | 19.69 |
| Oxygenates | 40.80 | 19.86 | 44.46 |
| 100.00 | 100.00 | 100.00 | |
| Acidity (mg KOH/g) | 118.9 | 26.8 | 17.9 |
| Dilution | TEAC (µM/L) 2.0 M KOH | ||
|---|---|---|---|
| 350 °C | 400 °C | 450 °C | |
| 1:20 | 2.35 | 2.33 | 2.32 |
| 1:40 | 2.31 | 2.29 | 1.74 |
| 1:80 | 2.30 | 2.24 | 1.33 |
| 1:160 | 2.33 | 1.36 | 1.05 |
| 1:320 | 2.06 | 1.01 | 0.71 |
| 1:640 | 1.25 | 0.36 | 0.33 |
| Process Parameters | 450 °C | ||
|---|---|---|---|
| 0.5 M | 1.0 M | 2.0 M | |
| Mass of Açaí seeds (g) | 33.285 | 40.040 | 40.06 |
| Cracking time (min) | 72 | 72 | 72 |
| Solid weight (Coke) (g) | 9.650 | 13.080 | 16.17 |
| Bio-oil weight (g) | 3.431 | 2.720 | 2.72 |
| H2O weight (g) | 12.290 | 11.99 | 8.41 |
| Gas weight (g) | 7.914 | 12.25 | 12.76 |
| Bio-oil Yield(wt.%) | 10.31 | 6.79 | 6.79 |
| H2O Yield(wt.%) | 36.92 | 29.94 | 20.99 |
| Bio-char Yield(wt.%) | 29.99 | 32.67 | 40.36 |
| Gas Yield(wt.%) | 23.78 | 30.59 | 31.85 |
| Acidity (mg KOH/g) | 112.7 | 103.7 | 12.3 |
| Dilution | TEAC (mmol/L) 450 °C | ||
|---|---|---|---|
| 0.5 M KOH | 1.0 M KOH | 2.0 M KOH | |
| 1:20 | 2.40 | 2.40 | 2.32 |
| 1:40 | 2.40 | 2.40 | 1.74 |
| 1:80 | 2.37 | 2.37 | 1.33 |
| 1:160 | 2.29 | 2.29 | 1.05 |
| 1:320 | 1.62 | 1.53 | 0.71 |
| 1:640 | 0.90 | 1.03 | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





