1. Introduction
Alpha-1 antitrypsin (AAT) deficiency (MIM 613490) is an autosomal co-dominant disorder classified as a rare disease. AAT is an inhibitor of serine proteases, especially neutrophilic elastase. When an imbalance occurs between protease and antiprotease levels, pulmonary pathology can develop early1,2. The most common manifestation is emphysema, which becomes evident by the third or fourth decade. A less common manifestation of this entity is cirrhosis or liver failure, which occurs in children and adults. The liver pathology is due to toxic gain of function related to intrahepatic polymerization of some of the variant proteins3.
AAT is the most abundant serum antiproteinase4. It is an acute phase glycoprotein formed by 394 amino acids, arranged in 3 beta sheets and a mobile reactive zone. AAT is produced in hepatocytes and is encoded by the SERPINA1 (Pi) gene (OMIM 107400) that is located on chromosome 14q32.1. Its plasma concentration should range between 100 to 200 mg per deciliter and the liver produces approximately 34mg of AAT per kilogram of body weight per day, although this amount may be increased in response to inflammatory or tumor type processes. The AAT presented in serum is 40% of the total, with the remaining 60% remaining in the extracellular space. The AAT deficiency is due to mutations in the gene that encodes it. To date more than 70 variants have been described in the SERPINA 1 gene, of which, at least 30 have pathological consequences5–7. The most common genetic mutations causing decreased release of AAT to serum are called Pi*Z (c.1096G>A;p.Glu366Lys) and Pi*S (c.863A>T;p.Glu288Val). In addition, Pi*ZZ and Pi*SZ are the most frequent severe deficient phenotypes.
Common methods for diagnosing AAT deficiency include quantification of serum, phenotyping, and genotyping of AAT8. In the case of the diagnosis of a congenital deficiency, it is assumed that values below 35% of normality indicate the possibility of Pi*ZZ. In this determination it is necessary to consider that the AAT is an acute phase reactant and therefore it can be elevated with non-specific inflammatory or infectious processes. High artifacts values have also been described in pregnancy and the use of oral contraceptives6. In addition, only with quantification, although the measurement is performed very accurately, patients with heterozygous mutations that have AAT levels at the low limit of normality can be overlooked9. In general, homozygous people have low serum AAT levels, and after that discovery, the AAT phenotype for confirming AAT deficiency is studied10. The molecular analysis of the AAT gene is the reference method to identify the less frequent allelic variants related to the deficiency, the null variants, or for the characterization of new variants11–13.
It is clear the importance of diagnosing patients with AAT deficiency, to start treatment if indicated, to be able to study their first-degree relatives and offer them genetic counseling which implies complete care focused on their diagnosis and establishing preventive measures. It is estimated that in Spain the AAT deficiency affects 1 in 2500 people, but according to the Spanish Registry of Patients with Alpha-1 Antitrypsin Deficiency only 500 people have been diagnosed with the Pi*ZZ mutation14.
The serum protein electrophoresis (SPE) is a laboratory technique that allows proteins to be separated based on their displacement when they are subjected to an electric field. Classify circulating proteins as albumin and globulins. A normal SPE can be considered with the following values: Albumin 53-69%; 35-46 g/L, Alpha-1 globulin: 3-4%; 1-3 g/L, Alpha-2 globulin: 6-11%; 4-7.5 g/L, Beta globulin: 8-13%; 5-9 g/L, and Gamma globulin: 12-19%; 8-12 g/L. Since alpha-1 antitrypsin is the most abundant globulin in the Alpha-1 group, if a patient has a lower than normal AAT concentration, it can be represented by a band in the diminished Alpha-1. This determination is therefore qualitative and may indicate normal, intermediate or low enzyme values. The SPE is a test that is requested by various medical specialties, mainly in the search for monoclonal bands, and occasionally low values of the alpha region are detected incidentally. The objective of this study is to assess a detection strategy for patients with mutations in SERPINA1 gene based on the incidental finding of low values of the Alpha-1 region of the SPE because it is an underdiagnosed and potentially treatable disease.
2. Material and Methods
This study was performed on all the SPE (capillary serum electrophoresis in free solution) carried out for any reason from September 2019 to February 2020 in the Laboratory of Biochemistry of the “Lozano Blesa” University Hospital of Zaragoza, Spain. We selected the group of subjects older than 18 years in which the quantification of the Alpha-1 globulin band was <3%. Subsequently, the concentration of AAT was determined in them, and those in which a concentration lower than 100 mg/dL were detected as subjects under study.
After the identification of the possible cases, they were contacted by telephone and those who agreed to participate in the study had a blood sample taken, in addition to conducting a clinical interview recording the family history. The blood collection tubes were labelled, registering the day and time of extraction, and being processed and stored in the appropriate conditions. This study was carried out following the principles of the Helsinki Declaration, being approved by the Ethics and Research Committee of Aragón (CEICA)(Approval Code: CI PI20/219; Approval Date: v3.14/05/2020).
To determine the serum AAT concentration, an immunonephelometry method was used in BN* II and BN ProSpec® systems. The genetic study of the coding exons and adjacent intronic regions of the
SERPINA1 gene (ENST00000448921.5) was carried out with the following primers (
Table 1).
The amplification conditions have been as follows: 5 minutes of preamplification at 95 °C, 35 cycles of amplification (30 seconds at 95 °C, 30 seconds at 60 °C and 30 seconds at 72 °C) and finally a post-amplification cycle (10 minutes at 72 °C). After subsequent verification of the correct amplification, sequencing was performed by capillary electrophoresis (ABI 3500 XL from Applied Biosystems).
Given the characteristics of the study, a descriptive statistical analysis with the SPSS 22.0 program was carried out, being that for the quantitative variables the mean, standard deviation, minimum and maximum were calculated. In the case of qualitative variables, the percentages were calculated.
3. Results
A total of 12800 SPE were studied, of which a percentage of Alpha-1 globulins less than 3% was detected in 40 of them (0,31%). After determining the concentration of AAT, 14 subjects (0,11%) showed levels below 100 mg/dL and only 11 of them participated in the study. This was since one of the patients had died (due to “idiopathic” liver cirrhosis associating pulmonary emphysema) and the other two refused consent.
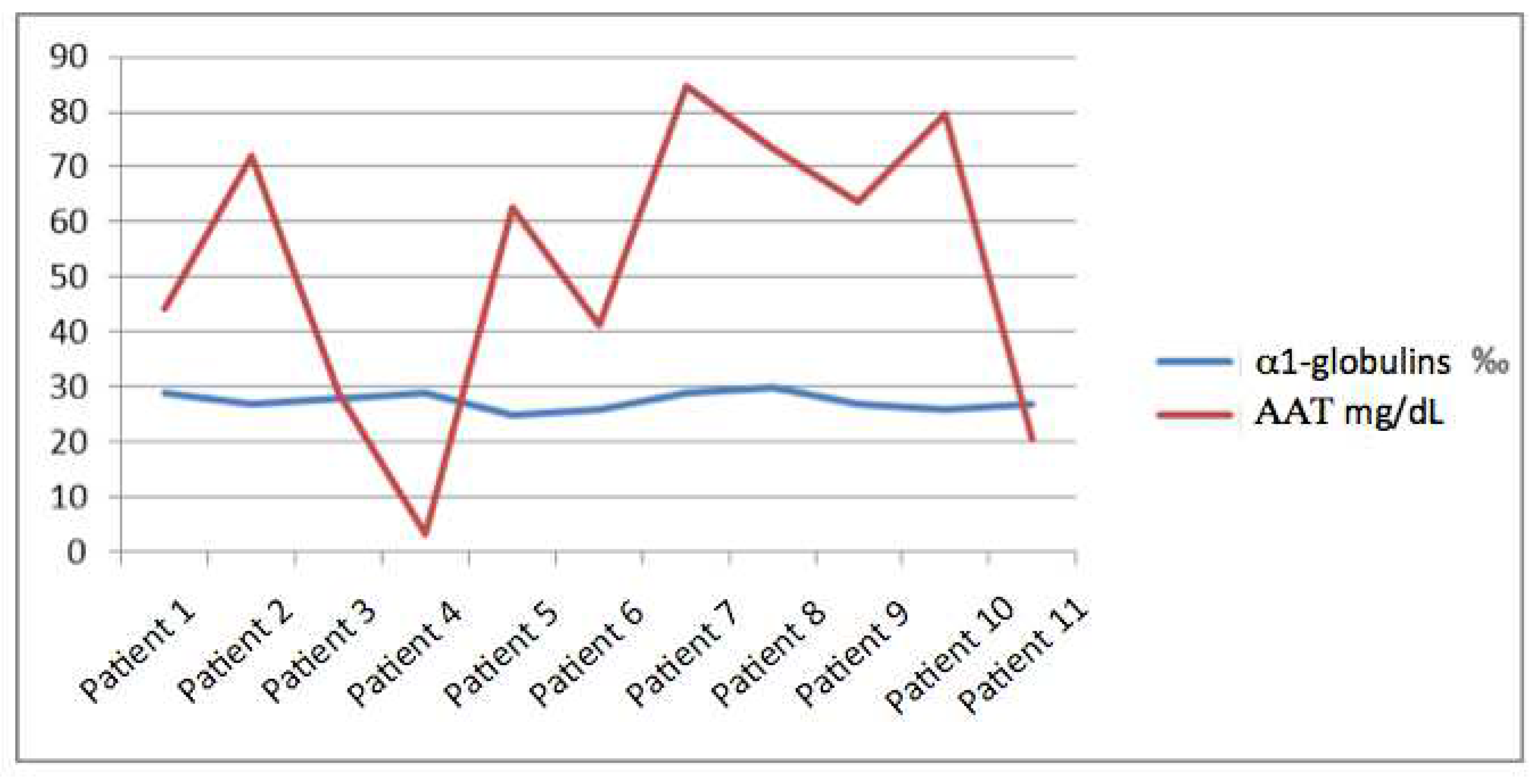
Regarding the concentration of AAT, its mean was 53.7 mg/dL with a maximum of 84.6 mg/dL and a minimum of < 10mg/dL, with a standard deviation of 26.18. We did not find a relationship between it and the percentage of Alpha-1 globulins and the range in which this percentage moves is narrow (since it does not vary by more than 0.5%) and instead the margin of AAT is much wider with a variation between the maximum and minimum value of more than 70 points (
Figure 1).
Of the 11 patients detected, 4 were women (36%) and 7 men (64%). Regarding age, they did not present a distribution with a specific pattern, although it is true that most patients were in middle ages of life, with an average of 54 years and a maximum of 90 years and a minimum of 19 respectively (standard deviation of 22.13). The clinical characteristics of the patients and relatives were studied, where it is remarkable the fact that two patients suffered infertility problems, two had liver fibrosis, one was affected by psoriasis, and other with celiac sprue. After a familial medical record, no lung or liver disease was discovered in five first-degree relatives but some entities were discovered in the family members of the other six (
Table 2).
After the demonstration of the enzyme deficiency, the genetic study was carried out, resulting in the finding of mutations in 10 of the 11 patients. With these data, it can be affirmed that in this series, 91% of the altered AAT concentrations anticipated the presence of a mutation in any of the exons of the
SERPINA1 gene (
Table 3).
Heterozygous mutations were detected in 7 patients (NM_001002235.2); in 3 the c.1096G>A mutation (p.Glu366Lys; Pi*Z), in 2 the c.863A>T mutation (p.Glu288Val), in one the c.221_223delTCT (p.Phe76del) mutation and in another the c.1066G>A mutation not currently described. In one patient the c.863 A>T mutation was detected in homozygosis and two double heterozygous patients were detected c.863A>T/c.1096G>A. The variant c.1066G>A (p.Ala356Thr) has been detected in patient number 3. This variant has not been reported so far in patients with suspected deficiency of AAT and is not present in the population databases. The aforementioned variant affects the first nucleotide of exon 7 and alters the consensus sequence of recognition of the splicing acceptor of said exon. Bioinformatic splicing site calculation programs predict an alteration of the splicing site. Following the recommendations of the American College of Medical Genetics and Genomics15 this variant must be classified as probably pathogenic. Due to the generation of a truncated protein, the most probable phenotype would be the "Null".
After the great proportionality between the band of Alpha-1 globulins and the presence of a mutation in the
SERPINA1 gene, we predicted the phenotype of each patient according to the AAT concentrations and the available literature
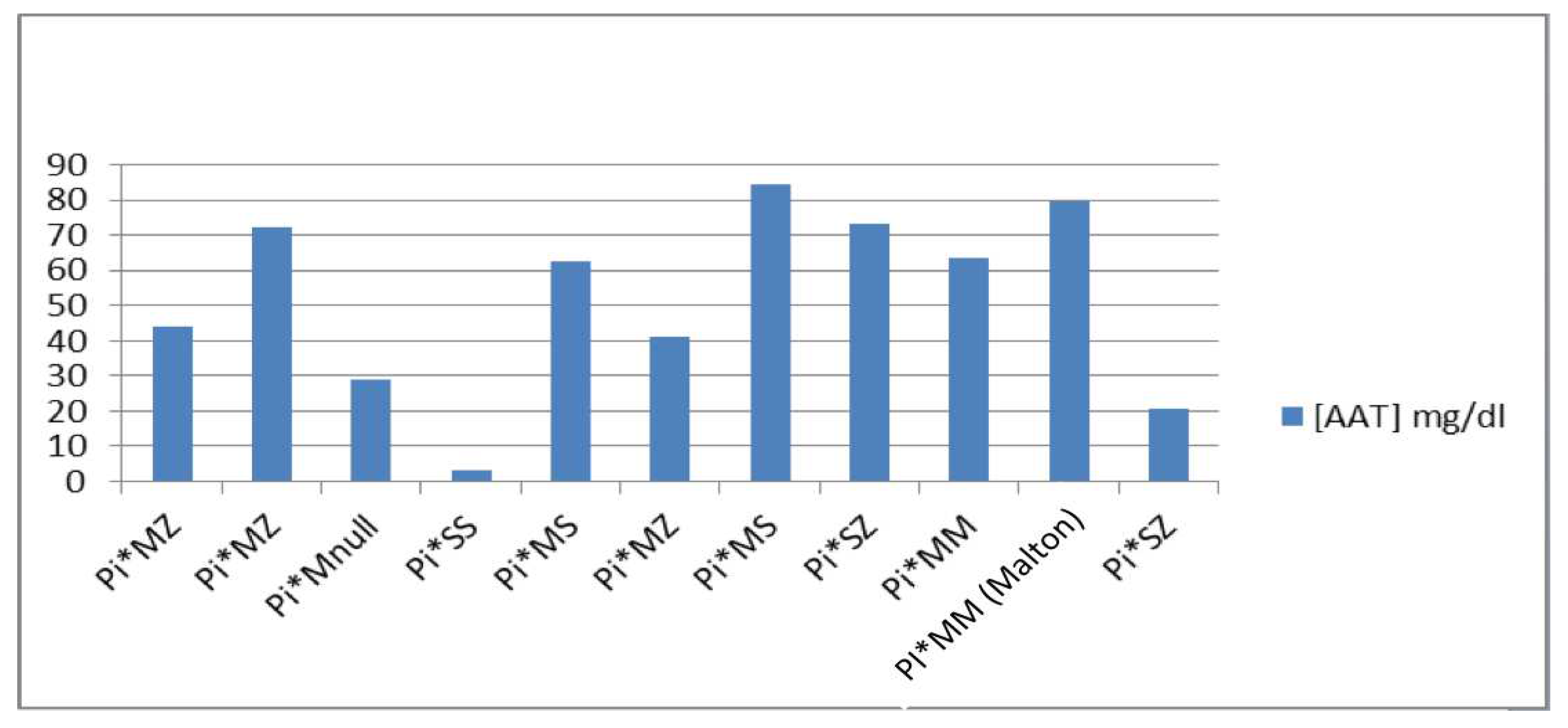
6. The phenotypes we found in the study were: 1) Pi*MZ: it has an average value of [AAT] of 52.53 mg/dL, the maximum value being 72.1 and the minimum being 41.3; 2) Pi*MS: it has an average value of [AAT] of 73.65 mg/dL, the maximum value being 84.6 and the minimum 62.7; 3) Pi*MM (Malton): only one case with an AAT concentration of 79.8 mg/dL is presented; 4) Pi*SS: only one case with a concentration of AAT <10 mg/dL is presented; 5) Pi*SZ: it has an average value of [AAT] of 46.95 mg/dL, the maximum value being 73.4 and the minimum 20.5; and 6) Pi*MNull: only one case with an AAT concentration of 28.9 mg/dL is presented (
Figure 2).
4. Discussion
In this study we try to verify if the SPE would be a useful tool as an opportunistic screening method for the deficiency of AAT, and we have found validity. Anyway we want to make clear that it only represents an opportunity and not an alternative to diagnostic methods. From a public health point of view, the set of severe consequences that lack of diagnosis entails represents an incentive to standardize the screening of this disease. AAT deficiency is currently an entity that is diagnosed in the final stages of an evolved lung disease16,17, with a treatment that significantly decreases mortality in treated patients18 and slows the evolution of pulmonary emphysema19. Besides, there is no data on how the disease would evolve by administering replacement therapy in patients who have not yet presented symptoms. With an early diagnosis, patients would benefit from the implementation of preventive measures and a targeted and specific action from an early time.
In any case, seeing our results, what is evident is that in every patient who undergoes a SPE the band of Alpha-1 globulins should be carefully evaluated. In addition, if an alteration is discovered, it will be necessary to determine AAT. On the other hand, it should be imperative to take this entity into account in any patient with lung and / or liver problems. There are few bibliographic citations involving the usefulness of the SPE in the screening of AAT deficiency and they consider this method as not very sensitive and specific20–23. In Italy population, prevalence studies have been carried out using different methods and creating controversy. In this way Coude24 et al suggest that in high-risk areas adult population screening program employing up-to-date genetic methods may be useful and on the other hand, Ferrarotti25 illustrates that routine SPE is a suitable method for quantification of the Alpha-1 globulin band and the detection of AAT deficiency.
In our study we have shown that it is effective due to its high specificity (91%) and allows us to guide the rest of the tests - such as genetic ones - that should be limited due to their high cost. In other similar studies22 where similar values of AAT are taken as the cut-off point, the percentage of patients who subsequently presented mutations in the SERPINA1 gene was very significant.
Regarding the impact of our study, it is important to consider that a decrease in the Alpha-1 globulins of the SPE, confirmed with a lower than normal AAT concentration, is related to any type of mutation in the SERPINA1 gene. If confirmed in larger population studies, there is possibility to elaborate a new diagnostic algorithm that can have a better and earlier approach in these patients. Taking into account the estimated figures of population with AAT deficiency, it could be deduced the great impact that this disease has not only at the level of health but also at the level of economic resources since the current diagnosis based on the phenotyping of patients is three times more expensive than the method proposed in this work. According to the bibliography6, the Null variants have an unknown relevance and the one detected by us has not been described in the literature or in the population databases. In this case, it is a variant in a heterozygous state and the concentration for which it codes is significantly low (28.9 mg/dL). In this regard, we can affirm that certain variants are well studied while others are very far from their estimate. For all these reasons, one of the most relevant aspects of our study is the fact that when heterozygous variants appear, the estimation of mutations from the concentration of AAT is very complicated without establishing ranges and without being able to ensure which is the probable Phenotype of the patients.
Concerning the limitations, we must mention the following: first, it is a cross-sectional study in which we perform an approach of the current population with a small sample size, although we must bear in mind that the AAT deficiency is included in the called “rare disorders”. In this sense, we aspire to carry out a future study with a greater representation of the Aragon´s population (Spain). Secondly, we must remark that SPE will be useful in screening for AAT deficiency only if it´s carried out in a basal state of health, since AAT is an acute phase reactant protein.
As final considerations, the AAT deficiency is a little known entity that provides a useful model for the protein linkages, the effect of mutations, and therapeutic strategies for other conformational diseases. The number of patients who are diagnosed is minimal compared to official estimates and the fact that the substitute treatment gives good results marks the need to identify the patients who can benefit from it. Identifying patients, investigating the process, and offering a specific and targeted treatment and approach are the keys to the future of this disease. In this study, a solution to the first of the three points has been proposed and it is foreseeable that the other two factors will progress successively to this. Considering the AAT deficiency as one of the most frequent life-threatening congenital diseases in adulthood and taking into account that there are only a few patients diagnosed in Spain, it is demonstrated that our screening protocol has been highly effective.
5. Conclusions
The serum protein electrophoresis is a useful tool for the opportunistic diagnosis of AAT deficiency. In all patients with Alpha-1 globulins <3%, the concentration of AAT should be quantified, since this cut-off point tends to anticipate a lower than normal concentration of AAT in baseline health conditions.
The relationship between a decrease in the concentration of AAT and the presence of mutations in the SERPINA1 gene is close to 91%, but estimating the phenotype using the concentration of AAT is difficult, especially in heterozygous mutations.
References
- Torres-Duran M, López-Campos JL, Barrecheguren M, Miravitlles M, Martínez-Delgado B, Castillo S, et al. Alpha-1 antitrypsin deficiency: outstanding questions and future directions. Orphanet J Rare Dis 2018; 13: 114. [CrossRef]
- Figueira Gonçalves JM, Martínez Bugallo F, García-Talavera I, and Rodríguez González J. Alpha1-Antitrypsin deficiency associated with null alleles. Arch Bronconeumol 2017; 53: 700-2.
- Irving JA, Haq I, Dickens JA, Faull SV, and Lomas DA. Altered native stability is the dominant basis for susceptibility of α1-antitrypsin mutants to polymerization. Biochem J 2014; 460: 103-15. [CrossRef]
- Lomas D, and Parfrey H. Alpha 1-antitrypsin deficiency, Molecular Pathophysiology. Thorax 2004; 59: 529-35.
- Beatriz de Rienzo Modelo, Alberto M. González Chávez, Juan I. Monjarás Guerra, Nicolás González Jáuregui López, Adrián Méndez Cedillo, Ramón I. Lemus Ramírez, et al. La importancia de la deficiencia de Alfa-1 antitripsina en el desarrollo de la enfermedad pulmonar obstructiva crónica y otras patologías pulmonares. Medigraphic Neumología y Cirugía de Tórax 2008; 67: 16-23.
- Vidal R, Blanco I, Casas F, Jardí R, and Miravitlles M; Committee on the National Registry of Individuals with Alpha-1 Antitrypsin Deficiency. Guidelines for the diagnosis and management of alpha-1 antitrypsin deficiency. Arch Bronconeumol 2006; 42: 645-59.
- American Thoracic Society/European respiratory Society Statement. Standards for the diagnosis an management of individuals with alpha 1- antitrypsin deficiency. Am J Resp Clin Care Med. 2003; 168: 818-900.
- Snyder MR, Katzmann JA, Butz ML, Wiley C, Yang P, Dawson DB, et al. Diagnosis of alpha-1-antitrypsin deficiency: an algorithm of quantification, genotyping, and phenotyping. Clin Chem 2006; 52: 2236–42.
- Silverman EK, and Sandhaus RA. Alpha1-Antitrypsin Deficiency. N Engl J Med 2009; 360: 2749-57.
- Reilly JJ Silverman EK, and Shapiro SD. Chronic Obstructive Pulmonary Disease. In: Harrison´s Principles of Internal Medicine 2015 (19th Edition). McGraw-Hill Editors. ISBN: 978-0-07-1 8021 6- 1.
- Jardi R, Rodríguez-Frias F, Casas F, Cotrina M, Vidal R, Miravitlles M, et al. Molecular characterization of two variants of alpha-1-antitrypsin deficiency: Pi Mpalermo and Pi Plovel. Med Clin (Barc) 1997; 109: 463-6.
- Jardi R, Rodríguez-Frias F, López-Talavera JC, Miravitlles M, Cortina M, Costa X, et al. Characterization of the new alpha-1-antitrypsin-deficient PI M-type allele, PI M (vall d’hebron) (Pro(369)-->Ser). Hum Hered 2000; 50: 320-1. [CrossRef]
- Jardi R, Rodríguez F, Miravitlles M, Vidal R, Cotrina M, Quer J, et al. Identification and molecular characterization of the new alpha-1-antitrypsin deficient allele PI Y barcelona (Asp256-->Val and Pro391-->His). Mutations in brief no. 174. Online. Hum Mutat 1998; 12: 213.
- Lara B, Blanco I, Martínez MT, Rodríguez E, Bustamante A, Casas F, et al. Spanish Registry of Patients With Alpha-1 Antitrypsin Deficiency: Database Evaluation and Population Analysis. Arch Bronconeumol. 2017; 53: 13-8. [CrossRef]
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17: 405-24. [CrossRef]
- The Alpha-1 antitrypsin deficiency Registry study group. Survival and FEV1 decline in individuals with severe deficiency of alpha-1 antitrypsin. Am J Respir Crit Care Med 1998; 158: 49-59.
- Wencker M, Furhmann B, and Banik N. For the Wissenschafliche Arbeitsgemeinschaft zur Therapie von Lungenerkrankunger. Longitudinal follow-up of patients with alpha-1-protease inhibitor deficiency before and during therapy with alpha-1-protease inhibitor. Chest 2001; 119: 373-44. [CrossRef]
- Stoller JK, Fallat R, Schluchter MD, O’Brien RG, Connor JT, Gross N, et al. Augmentation therapy with alpha-1 antitrypsin: patterns of use and adverse events. Chest. 2003; 123: 1425-34.
- Gotzsche PC, and Johansen HK. Intravenous alpha-1 antitrypsin augmentation therapy for treating patients with alpha-1 antitrypsine deficiency and lung disease. Cochrane Database Syst Rev 2016; 9: CD007851.
- Miravitlles M, Jardi R, Rodríguez-Frías F, Torrella M, Pelegri D, and Vidal R. Usefulness of the quantification of the alpha-1 serous protein band in the screening of alpha-1-antitrypsin deficiency. Arch Bronconeumol 1998; 34: 536-40.
- Jenkins MA. Clinical applications of capillary electrophoresis. Status at the new millennium. Mol Biotechnol 2000; 15: 201–209. [CrossRef]
- González-Sagrado M, López-Hernández S, Martín-Gil FJ, Tasende J, Bañuelos MC, Fernández-García N, et al. Alpha1-antitrypsin deficiencies masked by a clinical capillary electrophoresis system (CZE 2000). Clin Biochem 2000; 33: 79–80. [CrossRef]
- Slev PR, Williams BG, Harville TO, Ashwood ER, Bornhorst JA. Efficacy of the detection of the alpha1-antitrypsin "Z" deficiency variant by routine serum protein electrophoresis. Am J Clin Pathol 2008; 130: 568–572.
- Corda L, Medicina D, La Piana GE, Bertella E, Moretti G, Bianchi L, et al. Population Genetic Screening for alpha1-antitrypsin Deficiency in a High-Prevalence Area. Respiration 2011; 82: 418-25. [CrossRef]
- Ferrarotti I, Poplawska-Wisniewska B, Trevisan MT, Koepke J, Dresel M, Koczulla R, et al. How Can We Improve the Detection of Alpha1-Antitrypsin Deficiency? PLoS One 2015; 10: e0135316.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).