Submitted:
27 July 2023
Posted:
27 July 2023
You are already at the latest version
Abstract
Keywords:
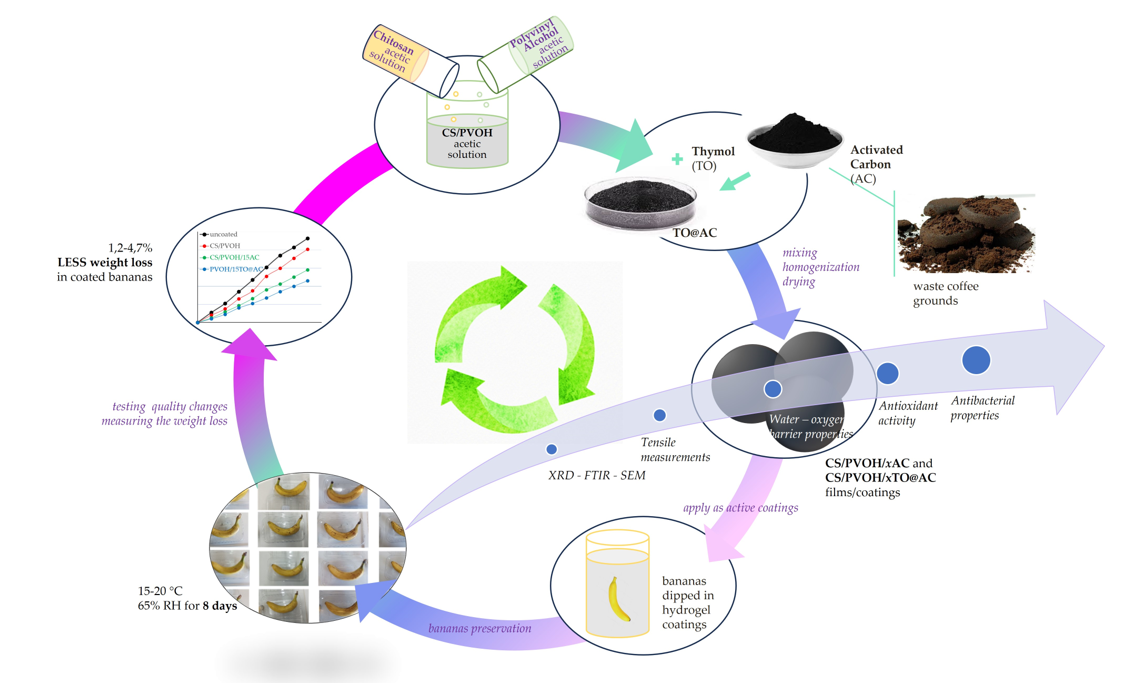
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TO@AC nanohybrid
2.3. Preparation of CS/PVOH/xAC and CS/PVOH/xTO@AC films
2.4. XRD analysis of CS/PVOH/xAC and CS/PVOH/xTO@AC films
2.5. FTIR spectroscopy of CS/PVOH/xAC and CS/PVOH/xTO@AC films
2.6. Tensile measuraments of CS/PVOH/xAC and CS/PVOH/xTO@AC films
2.7. Water Vapor Transmission Rate Measurements and Water Diffusion Coefficient Calculation
2.8. Oxygen Transmission Rate Measurements and Oxygen Permeability Calculation
2.9. Total antioxidant activity of CS/PVOH/xAC and CS/PVOH/xTO@AC films
2.10. Antibacterial Activity Tests of CS/PVOH/xAC and CS/PVOH/xTO@AC films
2.11. Application in fresh bananas preservation
2.12. Statistical analysis
3. Results
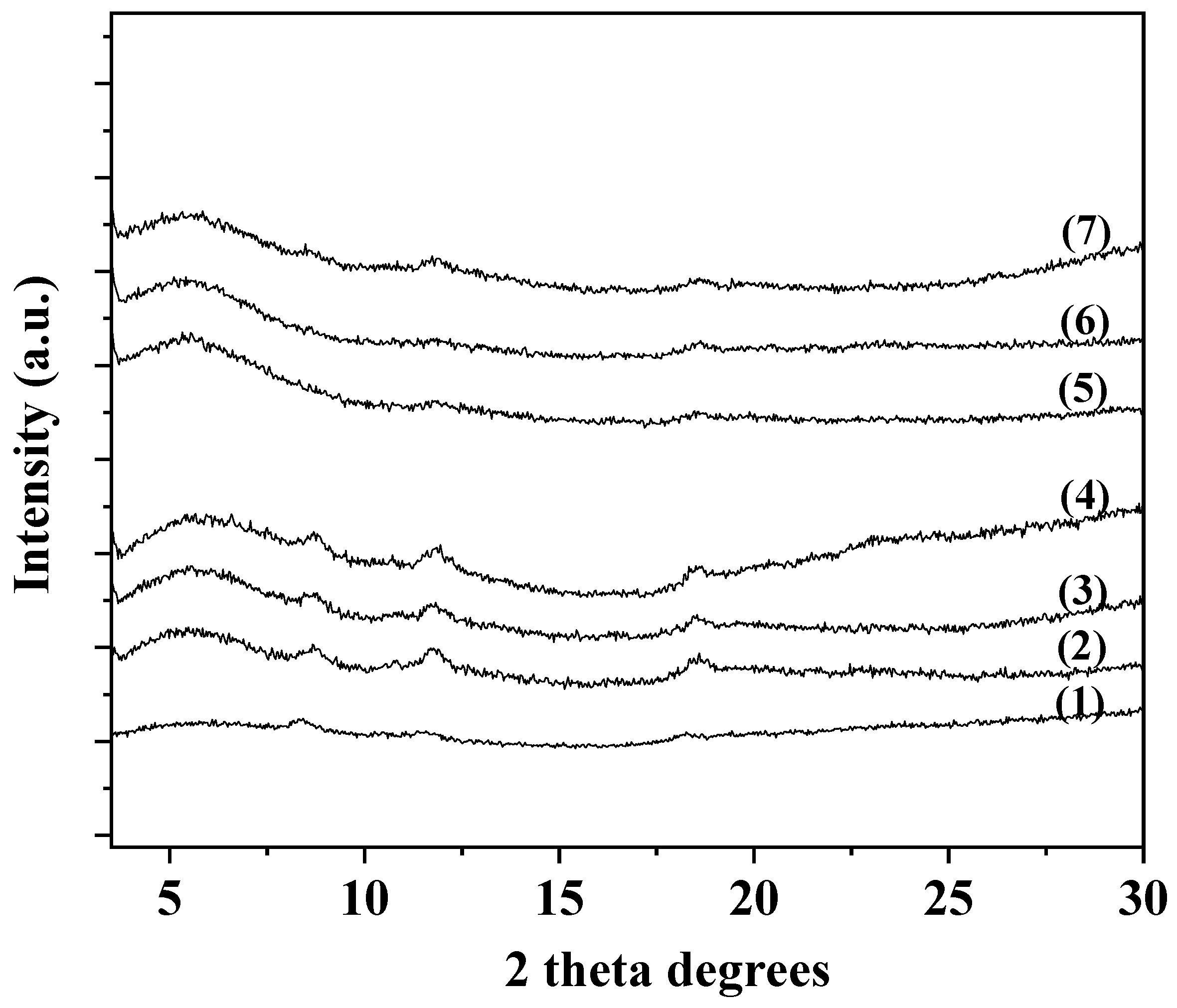
3.1. XRD analysis of CS/PVOH/xAC, CS/PVOH/xTO@AC films
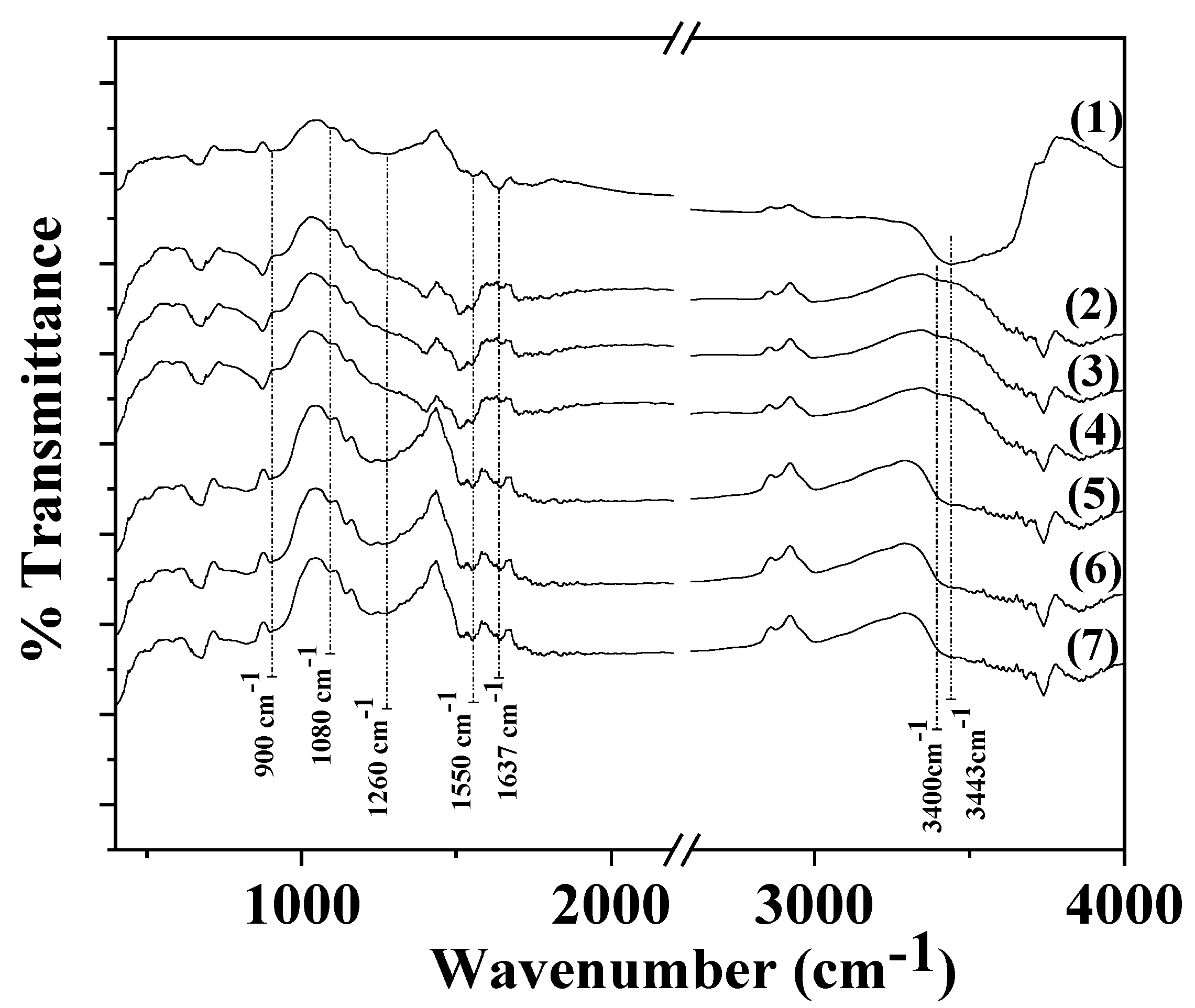
3.2. FTIR spectroscopy of CS/PVOH/xAC, CS/PVOH/xTO@AC films
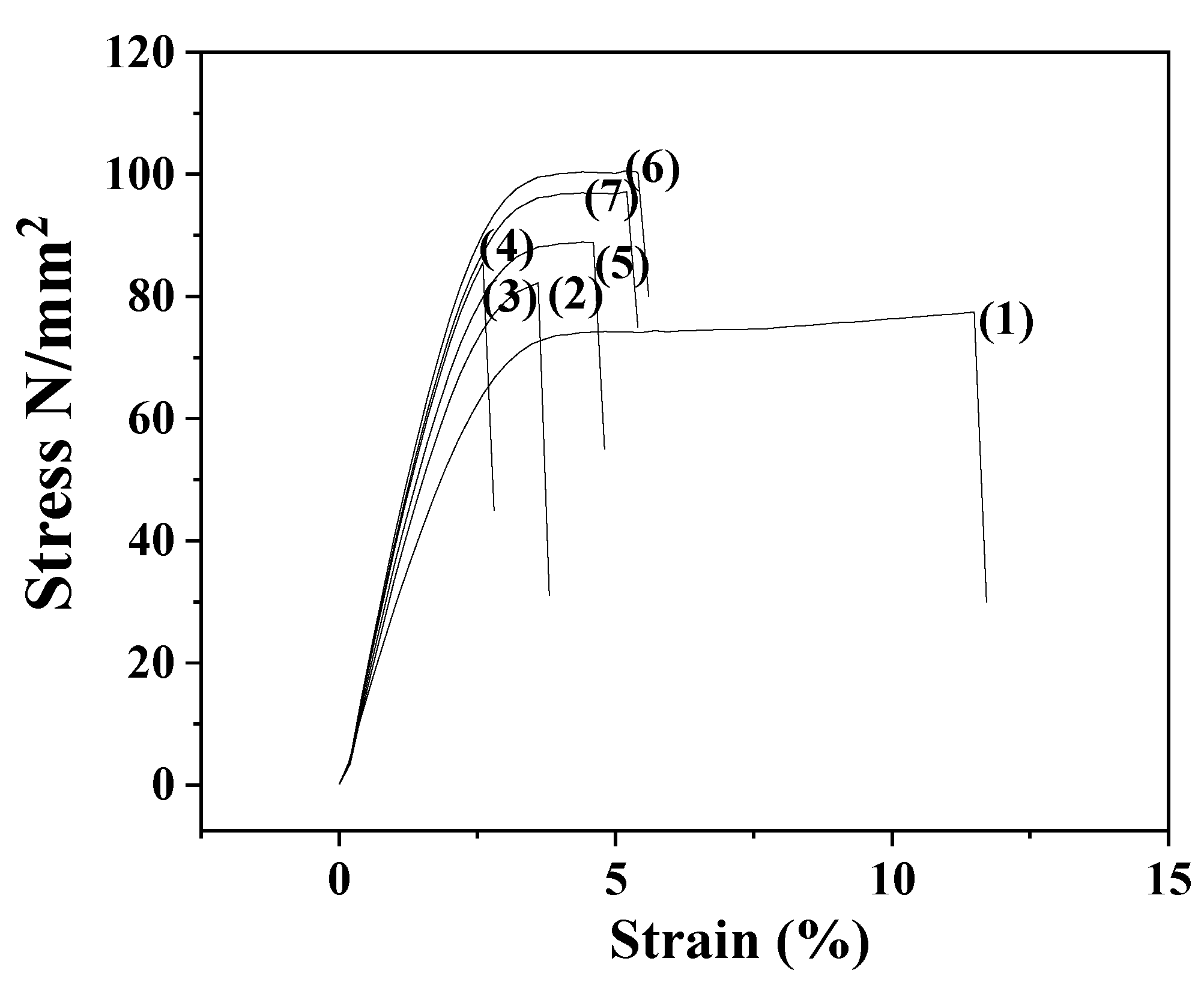
3.3. Tensile properties CS/PVOH/xAC, CS/PVOH/xTO@AC films
| E | σ uts | ε% | |
| CS/PVOH | 2249.3(100.3) | 71.2(1.8) | 11.8(0.9) |
| CS/PVOH/5AC | 2511.0(43.0) | 79.0(5.7) | 4.1(0.7) |
| CS/PVOH/10AC | 2881.3(63.2) | 86.1(6.1) | 3.4(0.1) |
| CS/PVOH/15AC | 2789.0(64.4) | 82.1(4.5) | 5.4(0.9) |
| CS/PVOH/5TO@AC | 2692.0(54.2) | 81.8(7.4) | 5.2(1.5) |
| CS/PVOH10TO@AC | 3041.3(79.2) | 104.3(8.0) | 6.1(0.9) |
| CS/PVOH/15TO@AC | 2938.5(60.5) | 88.0(6.1) | 5.9(0.8) |
3.4. Water – oxygen barrier properties of CS/PVOH/xAC, CS/PVOH/xTO@AC films
| Water Vapor Transmition Rate film thickness (mm) | Water Vapor Transmition Rate (10-6 g/cm2.s) | Diffusion Coefficient (10-4 cm2/s) | Oxygen Transmition Rate -film thickness (mm) | Oxygen Transmition Rate (ml/m2.day) | PeO2 (10-7 cm2/s) | |
|---|---|---|---|---|---|---|
| CS/PVOH | 0.14±0.01 | 1.339±0.024 | 4.36±0.11 | 0.15±0.01 | 38.20±1.91 | 5.73±0.29 |
| CS/PVOH/5AC | 0.15±0.01 | 1.240±0.025 | 4.32±0.11 | 0.15±0.01 | 19.10±0.96 | 2.87±0.14 |
| CS/PVOH/10AC | 0.17±0.01 | 0.896±0.021 | 3.53±0.10 | 0.09±0.01 | 27.50±1.38 | 2.48±0.12 |
| CS/PVOH/15AC | 0.16±0.01 | 0.924±0.014 | 3.43±0.07 | 0.16±0.01 | 10.10±0.51 | 1.62±0.08 |
| CS/PVOH/5TO@AC | 0.12±0.01 | 0.902±0.018 | 3.18±0.07 | 0.14±0.01 | 18.50±0.93 | 2.59±0.13 |
| CS/PVOH/10TO@AC | 0.13±0.01 | 0.735±0.017 | 2.99±0.09 | 0.10±0.01 | 17.50±0.88 | 1.75±0.09 |
| CS/PVOH/15TO@AC | 0.14±0.01 | 0.802±0.012 | 2.17±0.09 | 0.15±0.01 | 10.30±0.50 | 1.49±0.07 |
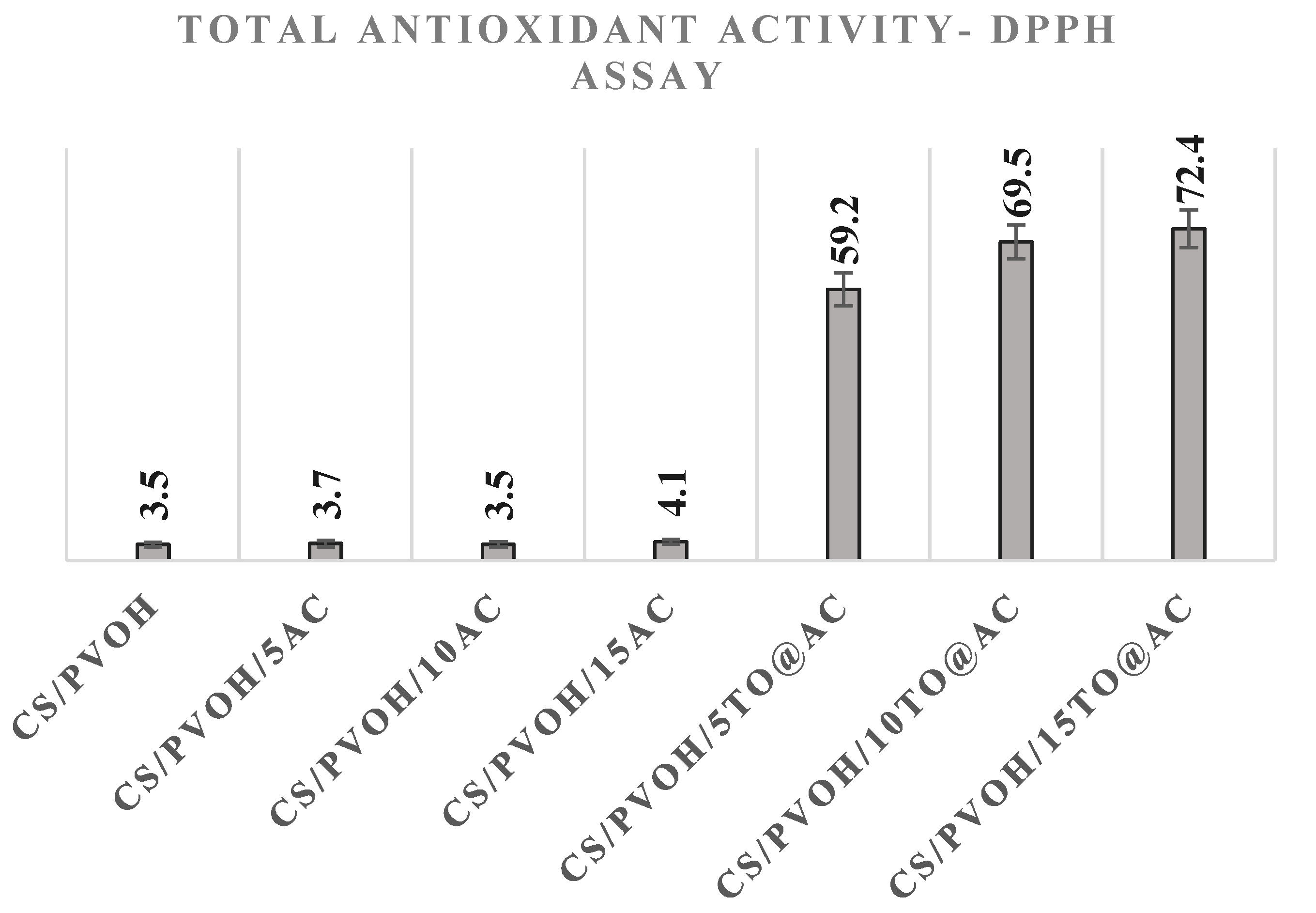
3.5. Total antioxidant activity of CS/PVOH/xAC and CS/PVOH/xTO@AC films
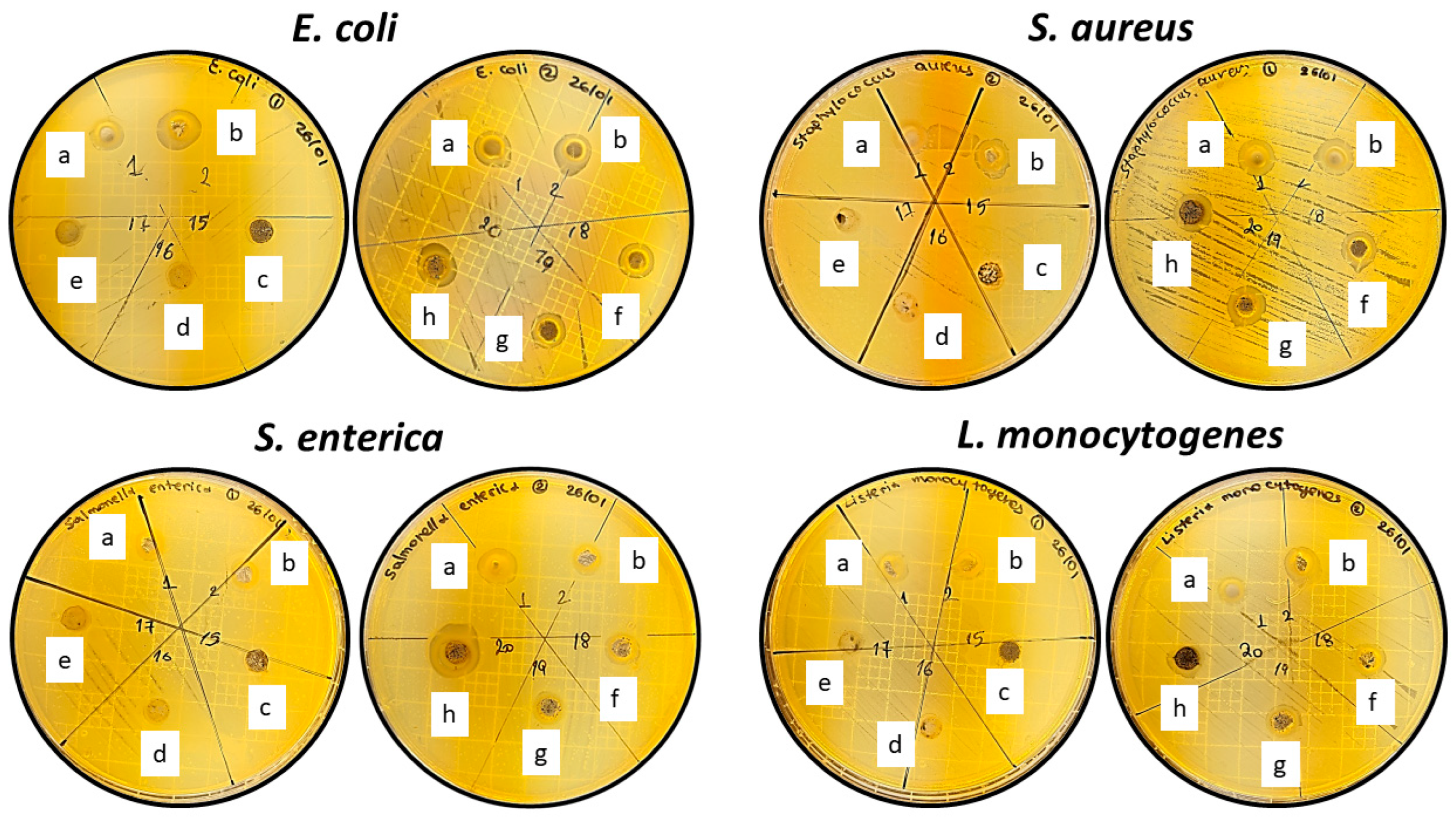
3.6. Antibacterial properties of CS/PVOH/xAC and CS/PVOH/xTO@AC films- Agar diffusion test
| Film material | E. coli | S. aureus | S. enterica | L. monocytogenes |
|---|---|---|---|---|
| Inhibition1 (diameter of clear zone) |
Inhibition1 (diameter of clear zone) |
Inhibition1 (diameter of clear zone) |
Inhibition1 (diameter of clear zone) |
|
| CS | 3.07 ± 0.22a | 3.56 ± 0.43a | 3.40 ± 0.32a | 2.03 ± 0.26a |
| CS/PVOH | 3.63 ± 0.35ab | 3.72 ± 0.25ab | 3.49 ± 0.11ab | 3.78 ± 0.52ab |
| CS/PVOH/5AC | 0 ± 0c | 0 ± 0c | 0 ± 0c | 0 ± 0ac |
| CS/PVOH/10AC | 0 ± 0c | 0 ± 0c | 0 ± 0c | 0 ± 0ac |
| CS/PVOH/15AC | 0 ± 0c | 0 ± 0c | 0 ± 0c | 0 ± 0ac |
| CS/PVOH/5TO@AC | 3.08 ± 0.26abd | 5.43 ± 0.49abd | 4.13 ± 0.68abd | 2.66 ± 0.36a |
| CS/PVOH/10TO@AC | 3.10 ± 0.29abd | 3.63 ± 0.46abd | 3.00 ± 0.16abd | 3.44 ± 0.30ad |
| CS/PVOH/15TO@AC | 3.84 ± 0.60abd | 4.53 ± 0.36abd | 6.07 ± 0.18e | 3.86 ± 0.91ad |
3.7. Visual Evaluation of the obtained Active Coatings against enzymatic browning of fresh bananas
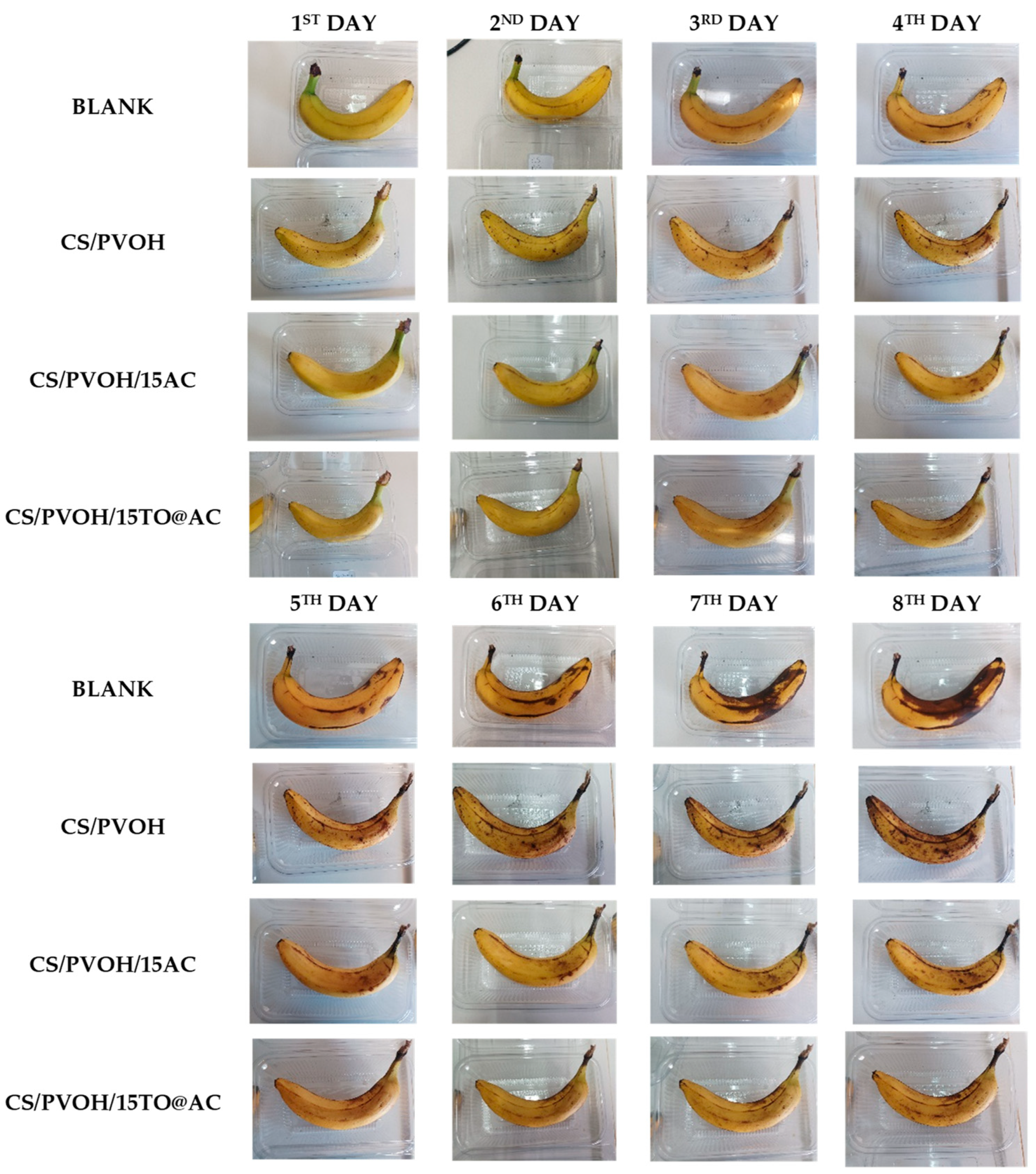
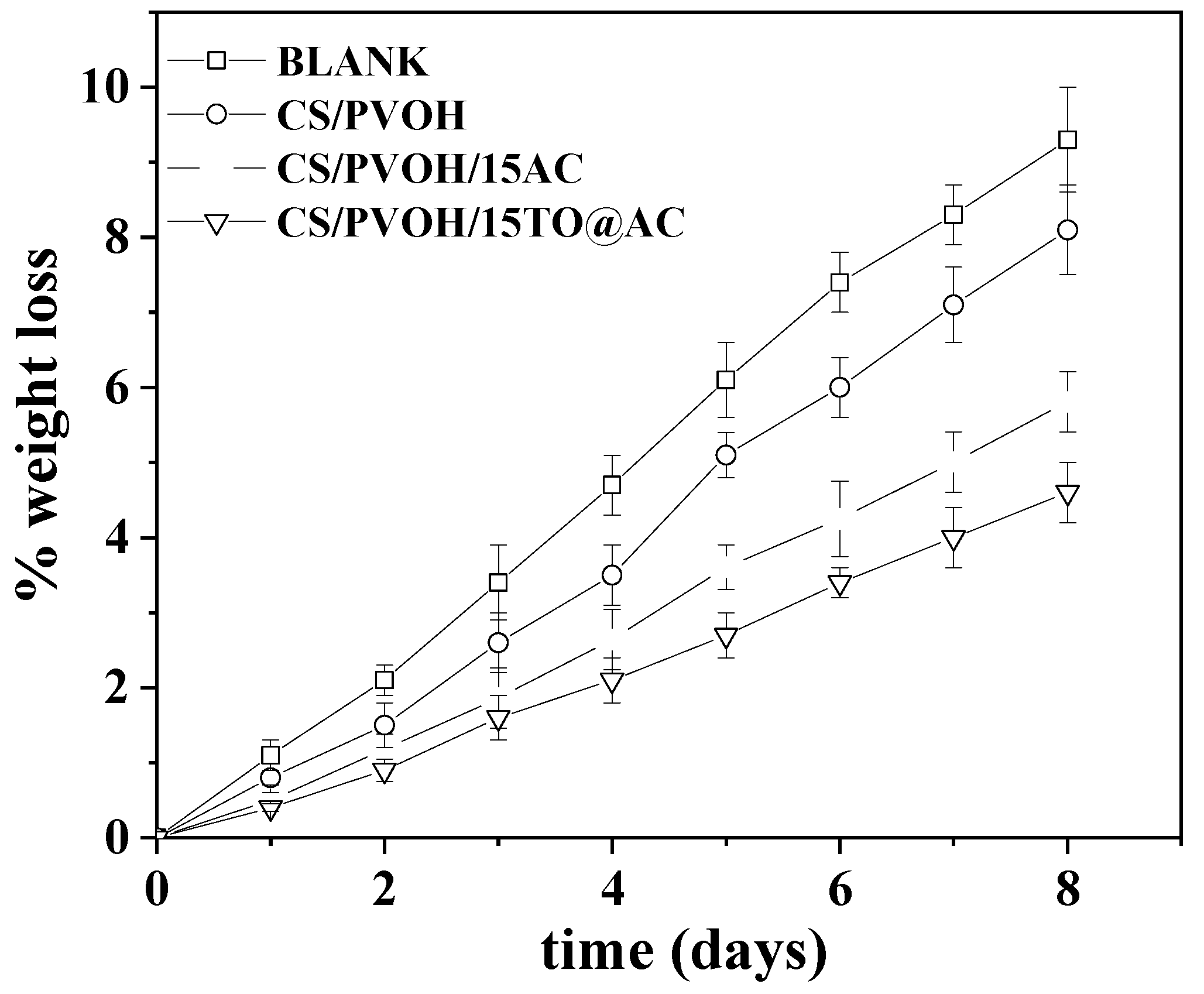
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luckachan, G.E.; Pillai, C.K.S. Biodegradable Polymers- A Review on Recent Trends and Emerging Perspectives. J Polym Environ 2011, 19, 637–676. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent Advances in Biodegradable Polymers for Sustainable Applications. npj Mater Degrad 2022, 6, 1–28. [Google Scholar] [CrossRef]
- Ahmed, Md.W.; Haque, Md.A.; Mohibbullah, Md.; Khan, Md.S.I.; Islam, M.A.; Mondal, Md.H.T.; Ahmmed, R. A Review on Active Packaging for Quality and Safety of Foods: Current Trends, Applications, Prospects and Challenges. Food Packaging and Shelf Life 2022, 33, 100913. [Google Scholar] [CrossRef]
- Ahari, H.; Soufiani, S.P. Smart and Active Food Packaging: Insights in Novel Food Packaging. Frontiers in Microbiology 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Holden, N.M.; White, E.P.; Lange, M.C.; Oldfield, T.L. Review of the Sustainability of Food Systems and Transition Using the Internet of Food. npj Sci Food 2018, 2, 18. [Google Scholar] [CrossRef]
- Nemat, B.; Razzaghi, M.; Bolton, K.; Rousta, K. The Role of Food Packaging Design in Consumer Recycling Behavior—A Literature Review. Sustainability 2019, 11, 4350. [Google Scholar] [CrossRef]
- Gupta, S.; Sharma, S.; Kumar Nadda, A.; Saad Bala Husain, M.; Gupta, A. Biopolymers from Waste Biomass and Its Applications in the Cosmetic Industry: A Review. Materials Today: Proceedings 2022, 68, 873–879. [Google Scholar] [CrossRef]
- Aider, M. Chitosan Application for Active Bio-Based Films Production and Potential in the Food Industry: Review. LWT - Food Science and Technology 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Applications of Chitosan as Food Packaging Materials. In Sustainable Agriculture Reviews 36: Chitin and Chitosan: Applications in Food, Agriculture, Pharmacy, Medicine and Wastewater Treatment; Crini, G., Lichtfouse, E., Eds.; Sustainable Agriculture Reviews; Springer International Publishing: Cham, 2019; pp. 81–123. ISBN 978-3-030-16581-9. [Google Scholar]
- Ananno, A.A.; Masud, M.H.; Chowdhury, S.A.; Dabnichki, P.; Ahmed, N.; Arefin, A.Md.E. Sustainable Food Waste Management Model for Bangladesh. Sustainable Production and Consumption 2021, 27, 35–51. [Google Scholar] [CrossRef]
- Huang, I.Y.; Manning, L.; James, K.L.; Grigoriadis, V.; Millington, A.; Wood, V.; Ward, S. Food Waste Management: A Review of Retailers’ Business Practices and Their Implications for Sustainable Value. Journal of Cleaner Production 2021, 285, 125484. [Google Scholar] [CrossRef]
- Soltani Firouz, M.; Mohi-Alden, K.; Omid, M. A Critical Review on Intelligent and Active Packaging in the Food Industry: Research and Development. Food Research International 2021, 141, 110113. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, L.; Fan, K. Recent Advances in Polysaccharide-Based Edible Coatings for Preservation of Fruits and Vegetables: A Review. Critical Reviews in Food Science and Nutrition 2022, 0, 1–16. [Google Scholar] [CrossRef]
- Giannakas, A.; Vlacha, M.; Salmas, C.; Leontiou, A.; Katapodis, P.; Stamatis, H.; Barkoula, N.-M.; Ladavos, A. Preparation, Characterization, Mechanical, Barrier and Antimicrobial Properties of Chitosan/PVOH/Clay Nanocomposites. Carbohydrate Polymers 2016, 140, 408–415. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Salmas, C.E.; Moschovas, D.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Karakassides, A.; Leontiou, A.; Kehayias, G.; Avgeropoulos, A.; et al. Nanocomposite Film Development Based on Chitosan/Polyvinyl Alcohol Using ZnO@Montmorillonite and ZnO@Halloysite Hybrid Nanostructures for Active Food Packaging Applications. Nanomaterials 2022, 12, 1843. [Google Scholar] [CrossRef]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Garcia-Oliveira, P.; Prieto, M.A. Essential Oils and Their Application on Active Packaging Systems: A Review. Resources 2021, 10, 7. [Google Scholar] [CrossRef]
- de Oliveira, L.H.; Trigueiro, P.; Souza, J.S.N.; de Carvalho, M.S.; Osajima, J.A.; da Silva-Filho, E.C.; Fonseca, M.G. Montmorillonite with Essential Oils as Antimicrobial Agents, Packaging, Repellents, and Insecticides: An Overview. Colloids and Surfaces B: Biointerfaces 2022, 209, 112186. [Google Scholar] [CrossRef]
- Giannakas, A.; Tsagkalias, I.; Achilias, D.S.; Ladavos, A. A Novel Method for the Preparation of Inorganic and Organo-Modified Montmorillonite Essential Oil Hybrids. Applied Clay Science 2017, 146, 362–370. [Google Scholar] [CrossRef]
- Saucedo-Zuñiga, J.N.; Sánchez-Valdes, S.; Ramírez-Vargas, E.; Guillen, L.; Ramos-deValle, L.F.; Graciano-Verdugo, A.; Uribe-Calderón, J.A.; Valera-Zaragoza, M.; Lozano-Ramírez, T.; Rodríguez-González, J.A.; et al. Controlled Release of Essential Oils Using Laminar Nanoclay and Porous Halloysite / Essential Oil Composites in a Multilayer Film Reservoir. Microporous and Mesoporous Materials 2021, 316, 110882. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Salmas, C.E.; Moschovas, D.; Zaharioudakis, K.; Georgopoulos, S.; Asimakopoulos, G.; Aktypis, A.; Proestos, C.; Karakassides, A.; Avgeropoulos, A.; et al. The Increase of Soft Cheese Shelf-Life Packaged with Edible Films Based on Novel Hybrid Nanostructures. Gels 2022, 8, 539. [Google Scholar] [CrossRef]
- Eroglu, N.; Emekci, M.; Athanassiou, C.G. Applications of Natural Zeolites on Agriculture and Food Production. Journal of the Science of Food and Agriculture 2017, 97, 3487–3499. [Google Scholar] [CrossRef]
- Salmas, C.E.; Giannakas, A.E.; Karabagias, V.K.; Moschovas, D.; Karabagias, I.K.; Gioti, C.; Georgopoulos, S.; Leontiou, A.; Kehayias, G.; Avgeropoulos, A.; et al. Development and Evaluation of a Novel-Thymol@Natural-Zeolite/Low-Density-Polyethylene Active Packaging Film: Applications for Pork Fillets Preservation. Antioxidants 2023, 12, 523. [Google Scholar] [CrossRef]
- Chaemsanit, S.; Matan, N.; Matan, N. Activated Carbon for Food Packaging Application: Review. Walailak Journal of Science and Technology (WJST) 2018, 15, 255–271. [Google Scholar] [CrossRef]
- Quevedo, R.; Díaz, O.; Ronceros, B.; Pedreschi, F.; Aguilera, J.M. Description of the Kinetic Enzymatic Browning in Banana (Musa Cavendish) Slices Using Non-Uniform Color Information from Digital Images. Food Research International 2009, 42, 1309–1314. [Google Scholar] [CrossRef]
- Kaewjumpol, G.; Srisamlee, S.; Beckles, D.M.; Luengwilai, K. Enzymatic Browning in Banana Blossoms and Techniques for Its Reduction. Horticulturae 2021, 7, 373. [Google Scholar] [CrossRef]
- Hu, D.; Liu, X.; Qin, Y.; Yan, J.; Li, J.; Yang, Q. A Novel Edible Packaging Film Based on Chitosan Incorporated with Persimmon Peel Extract for the Postharvest Preservation of Banana. Food Quality and Safety 2022, 6, fyac028. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Karabagias, V.K.; Moschovas, D.; Leontiou, A.; Karabagias, I.K.; Georgopoulos, S.; Karydis-Messinis, A.; Zaharioudakis, K.; Andritsos, N.; Kehayias, G.; et al. Thymol@activated Carbon Nanohybrid for Low-Density Polyeth-Ylene Based Active Packaging Films for Pork Fillets Shelf-Life Extension 2023.
- Asimakopoulos, G.; Baikousi, M.; Kostas, V.; Papantoniou, M.; Bourlinos, A.B.; Zbořil, R.; Karakassides, M.A.; Salmas, C.E. Nanoporous Activated Carbon Derived via Pyrolysis Process of Spent Coffee: Structural Characterization. Investigation of Its Use for Hexavalent Chromium Removal. Applied Sciences 2020, 10, 8812. [Google Scholar] [CrossRef]
- Constantinos E., Salmas; Aris, E. Giannakas; Dimitrios Moschovas; Eleni Kollia; Stsvros Georgopoulos; Christina Gioti; Areti Leontiou; Apostolos Avgeropoulos; Anna Kopsacheili; Learda Avdulai; et al. Kiwi Fruits Preservation Using Novel Edible Active Coatings Based on Rich in Thymol Halloysite Nanostructures and Chitosan/Polyvinyl Alcohol Gels. Gels Bioactive Gel Films and Coatings Applied in Active Food Packaging.
- Salmas, C.Ε.; Kollia, E.; Avdylaj, L.; Kopsacheili, A.; Zaharioudakis, K.; Georgopoulos, S.; Leontiou, A.; Katerinopoulou, K.; Kehayias, G.; Karakassides, A.; et al. Thymol@Natural Zeolite Nanohybrids for Chitosan/Poly-Vinyl-Alcohol Based Hydrogels Applied As Active Pads for Strawberries Preservation 2023.
- Salmas, C.E.; Giannakas, A.E.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Proestos, C. Effect of Copper and Titanium-Exchanged Montmorillonite Nanostructures on the Packaging Performance of Chitosan/Poly-Vinyl-Alcohol-Based Active Packaging Nanocomposite Films. Foods 2021, 10, 3038. [Google Scholar] [CrossRef]
- John, J.; Noorjan, N.; Gurumurthy, S.C.; Ramaprasad, A.T. Chitosan-Polyvinyl Alcohol Blend as Beta-Ray Attenuator. Materials Today: Proceedings 2022, 66, 2109–2114. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Kim, S.-K. Chapter Two - Antioxidant Effects of Chitin, Chitosan, and Their Derivatives. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Marine Carbohydrates: Fundamentals and Applications, Part B; Academic Press, 2014; Vol. 73, pp. 15–31. [Google Scholar]
- Sallam, M.F.; Ahmed, H.M.S.; Diab, K.A.; El-Nekeety, A.A.; Abdel-Aziem, S.H.; Sharaf, H.A.; Abdel-Wahhab, M.A. Improvement of the Antioxidant Activity of Thyme Essential Oil against Biosynthesized Titanium Dioxide Nanoparticles-Induced Oxidative Stress, DNA Damage, and Disturbances in Gene Expression in Vivo. Journal of Trace Elements in Medicine and Biology 2022, 73, 127024. [Google Scholar] [CrossRef] [PubMed]
- Unuabonah, E.I.; Ugwuja, C.G.; Omorogie, M.O.; Adewuyi, A.; Oladoja, N.A. Clays for Efficient Disinfection of Bacteria in Water. Applied Clay Science 2018, 151, 211–223. [Google Scholar] [CrossRef]
- Antibacterial and Adsorption Characteristics of Activated Carbon Functionalized with Quaternary Ammonium Moieties | Industrial & Engineering Chemistry Research Available online:. Available online: https://pubs.acs.org/doi/10.1021/ie0608096 (accessed on 17 July 2023).
- Assessment of the Bacteriological Activity Associated with Granular Activated Carbon Treatment of Drinking Water | Applied and Environmental Microbiology Available online:. Available online: https://journals.asm.org/doi/10.1128/aem.56.12.3822-3829.1990 (accessed on 17 July 2023).
- Pathogens | Free Full-Text | Antibacterial Agents Adsorbed on Active Carbon: A New Approach for S. Aureus and E. Coli Pathogen Elimination. Available online: https://www.mdpi.com/2076-0817/10/8/1066 (accessed on 17 July 2023).
- Azmi, N.N.; Mahyudin, N.A.; Wan Omar, W.H.; Mahmud Ab Rashid, N.-K.; Ishak, C.F.; Abdullah, A.H.; Sharples, G.J. Antibacterial Activity of Clay Soils against Food-Borne Salmonella Typhimurium and Staphylococcus Aureus. Molecules 2022, 27, 170. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





