Submitted:
26 July 2023
Posted:
27 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animals and Epidemiological Data
2.2. Sample Collection and DNA Extraction
2.3. Real-Time PCR Analysis
2.4. Statistical Analysis
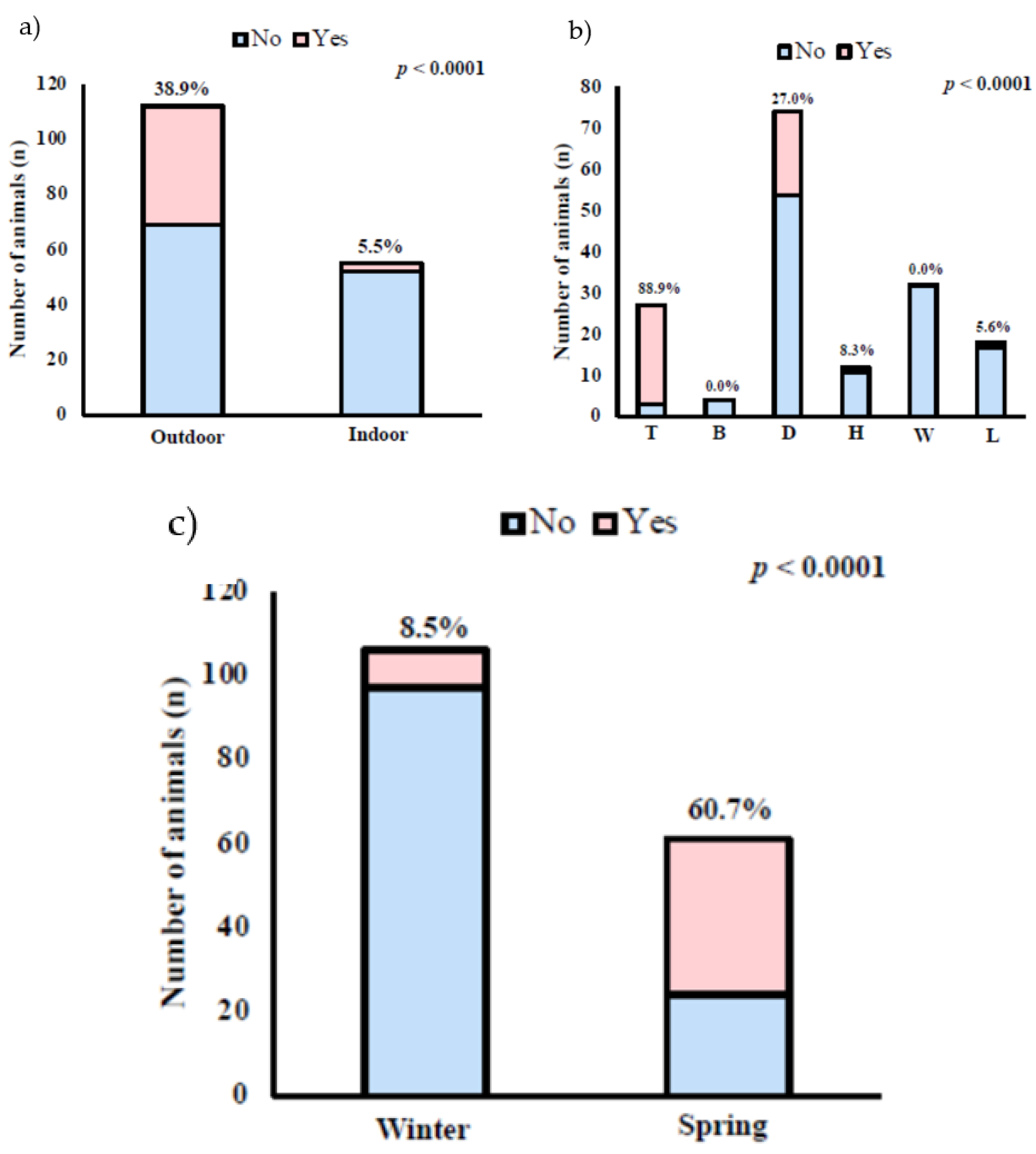
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akhoundi, M.; Kuhls, K.; Cannet, A.; Votýpka, J.; Marty, P.; Delaunay, P.; Sereno, D. A Historical Overview of the Classification, Evolution, and Dispersion of Leishmania Parasites and Sandflies. PLOS Neglected Tropical Diseases 2016, 10, e0004349. https://doi.org/10.1371/journal.pntd.0004349. [CrossRef]
- Baneth, G.; Nachum-Biala, Y.; Shabat Simon, M.; Brenner, O.; Gaier, S.; Rojas, A.; Yasur-Landau, D. Leishmania Major Infection in a Dog with Cutaneous Manifestations. Parasit Vectors 2016, 9, 246. https://doi.org/10.1186/s13071-016-1541-2. [CrossRef]
- Gramiccia, M. Recent Advances in Leishmaniosis in Pet Animals: Epidemiology, Diagnostics and Anti-Vectorial Prophylaxis. Vet Parasitol 2011, 181, 23–30. https://doi.org/10.1016/j.vetpar.2011.04.019. [CrossRef]
- Mancianti, F.; Gramiccia, M.; Gradoni, L.; Pieri, S. Studies on Canine Leishmaniasis Control. 1. Evolution of Infection of Different Clinical Forms of Canine Leishmaniasis Following Antimonial Treatment. Trans. R. Soc. Trop. Med. Hyg. 1988, 82, 566–567. https://doi.org/10.1016/0035-9203(88)90510-x. [CrossRef]
- Ready, P.D. Leishmaniasis Emergence in Europe. Euro Surveill 2010, 15, 19505.
- Ahuir-Baraja, A.E.; Ruiz, M.P.; Garijo, M.M.; Llobat, L. Feline Leishmaniosis: An Emerging Public Health Problem. Vet Sci 2021, 8, 173. https://doi.org/10.3390/vetsci8090173. [CrossRef]
- Giner, J.; Villanueva-Saz, S.; Fernández, A.; Gómez, M.A.; Podra, M.; Lizarraga, P.; Lacasta, D.; Ruiz, H.; Del Carmen Aranda, M.; de Los Ángeles Jimenez, M.; et al. Detection of Anti-Leishmania Infantum Antibodies in Wild European and American Mink (Mustela Lutreola and Neovison Vison) from Northern Spain, 2014-20. J Wildl Dis 2022, 58, 198–204. https://doi.org/10.7589/JWD-D-21-00027. [CrossRef]
- Ortuño, M.; Nachum-Biala, Y.; García-Bocanegra, I.; Resa, M.; Berriatua, E.; Baneth, G. An Epidemiological Study in Wild Carnivores from Spanish Mediterranean Ecosystems Reveals Association between Leishmania Infantum, Babesia Spp. and Hepatozoon Spp. Infection and New Hosts for Hepatozoon Martis, Hepatozoon Canis and Sarcocystis Spp. Transbound Emerg Dis 2022, 69, 2110–2125. https://doi.org/10.1111/tbed.14199. [CrossRef]
- Athanasiou, L.V.; Katsogiannou, E.G.; Tsokana, C.N.; Boutsini, S.G.; Bisia, M.G.; Papatsiros, V.G. Wild Rabbit Exposure to Leishmania Infantum, Toxoplasma Gondii, Anaplasma Phagocytophilum and Babesia Caballi Evidenced by Serum and Aqueous Humor Antibody Detection. Microorganisms 2021, 9, 2616. https://doi.org/10.3390/microorganisms9122616. [CrossRef]
- Martín-Sánchez, J.; Torres-Medina, N.; Morillas-Márquez, F.; Corpas-López, V.; Díaz-Sáez, V. Role of Wild Rabbits as Reservoirs of Leishmaniasis in a Non-Epidemic Mediterranean Hot Spot in Spain. Acta Trop 2021, 222, 106036. https://doi.org/10.1016/j.actatropica.2021.106036. [CrossRef]
- Otranto, D.; Testini, G.; Buonavoglia, C.; Parisi, A.; Brandonisio, O.; Circella, E.; Dantas-Torres, F.; Camarda, A. Experimental and Field Investigations on the Role of Birds as Hosts of Leishmania Infantum, with Emphasis on the Domestic Chicken. Acta Trop 2010, 113, 80–83. https://doi.org/10.1016/j.actatropica.2009.09.014. [CrossRef]
- Mendoza-Roldan, J.A.; Zatelli, A.; Latrofa, M.S.; Iatta, R.; Bezerra-Santos, M.A.; Annoscia, G.; Gernone, F.; Votýpka, J.; Modrý, D.; Tichá, L.; et al. Leishmania (Sauroleishmania) Tarentolae Isolation and Sympatric Occurrence with Leishmania (Leishmania) Infantum in Geckoes, Dogs and Sand Flies. PLoS Negl Trop Dis 2022, 16, e0010650. https://doi.org/10.1371/journal.pntd.0010650. [CrossRef]
- Gazzonis, A.L.; Bertero, F.; Moretta, I.; Morganti, G.; Mortarino, M.; Villa, L.; Zanzani, S.A.; Morandi, B.; Rinnovati, R.; Vitale, F.; et al. Detecting Antibodies to Leishmania Infantum in Horses from Areas with Different Epizooticity Levels of Canine Leishmaniosis and a Retrospective Revision of Italian Data. Parasit Vectors 2020, 13, 530. https://doi.org/10.1186/s13071-020-04385-8. [CrossRef]
- Koehler, K.; Stechele, M.; Hetzel, U.; Domingo, M.; Schönian, G.; Zahner, H.; Burkhardt, E. Cutaneous Leishmaniosis in a Horse in Southern Germany Caused by Leishmania Infantum. Vet Parasitol 2002, 109, 9–17. https://doi.org/10.1016/s0304-4017(02)00246-7. [CrossRef]
- Rolão, N.; Martins, M.J.; João, A.; Campino, L. Equine Infection with Leishmania in Portugal. Parasite 2005, 12, 183–186. https://doi.org/10.1051/parasite/2005122183. [CrossRef]
- Solano-Gallego, L.; Fernández-Bellon, H.; Serra, R.; Gállego, M.; Ramis, A.; Fondevila, D.; Ferrer, L. Cutaneous Leishmaniosis in Three Horses in Spain. Equine Vet J 2003, 35, 320–323. https://doi.org/10.2746/042516403776148336. [CrossRef]
- Müller, N.; Welle, M.; Lobsiger, L.; Stoffel, M.H.; Boghenbor, K.K.; Hilbe, M.; Gottstein, B.; Frey, C.F.; Geyer, C.; von Bomhard, W. Occurrence of Leishmania Sp. in Cutaneous Lesions of Horses in Central Europe. Vet Parasitol 2009, 166, 346–351. https://doi.org/10.1016/j.vetpar.2009.09.001. [CrossRef]
- Gazzonis, A.L.; Morganti, G.; Porcellato, I.; Roccabianca, P.; Avallone, G.; Gavaudan, S.; Canonico, C.; Rigamonti, G.; Brachelente, C.; Veronesi, F. Detection of Leishmania Spp. in Chronic Dermatitis: Retrospective Study in Exposed Horse Populations. Pathogens 2022, 11, 634. https://doi.org/10.3390/pathogens11060634. [CrossRef]
- Aguilar, C.M.; Rangel, E.F.; Deane, L.M. Cutaneous Leishmaniasis Is Frequent in Equines from an Endemic Area in Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz 1986, 81, 471–472. https://doi.org/10.1590/s0074-02761986000400015. [CrossRef]
- Yoshida, E.L.; Correa, F.M.; Marques, S.A.; Stolf, H.O.; Dillon, N.L.; Momen, H.; Grimaldi, G. Human, Canine and Equine (Equus Caballus) Leishmaniasis Due to Leishmania Braziliensis (= L. Braziliensis Braziliensis) in the South-West Region of São Paulo State, Brazil. Mem Inst Oswaldo Cruz 1990, 85, 133–134. https://doi.org/10.1590/s0074-02761990000100026. [CrossRef]
- Falqueto, A.; Varejão, J.B.; Sessa, P.A. Cutaneous Leishmaniasis in a Horse (Equus Caballus) from Endemic Area in the State of Espirito Santo, Brazil. Mem Inst Oswaldo Cruz 1987, 82, 443. https://doi.org/10.1590/s0074-02761987000300020. [CrossRef]
- Soares, I.R.; Silva, S.O.; Moreira, F.M.; Prado, L.G.; Fantini, P.; Maranhão, R. de P.A.; da Silva Filho, J.M.; Melo, M.N.; Palhares, M.S. First Evidence of Autochthonous Cases of Leishmania (Leishmania) Infantum in Horse (Equus Caballus) in the Americas and Mixed Infection of Leishmania Infantum and Leishmania (Viannia) Braziliensis. Vet Parasitol 2013, 197, 665–669. https://doi.org/10.1016/j.vetpar.2013.06.014. [CrossRef]
- de Pinho, F.A.; Mendes, M.O.; de Magalhães, V.L.P.; Tinôco, A.A.C.; Seoane, J.H.L.; Rêgo, F.D.; Soares, R.P.; Barrouin-Melo, S.M. Clinical Evolution of Equine Leishmaniasis with Self-Limiting Cutaneous Disease Caused by Leishmania Infantum in Northeastern Brazil: A Case Report. Vet Parasitol Reg Stud Reports 2023, 41, 100881. https://doi.org/10.1016/j.vprsr.2023.100881. [CrossRef]
- Escobar, T.A.; Dowich, G.; Dos Santos, T.P.; Zuravski, L.; Duarte, C.A.; Lübeck, I.; Manfredini, V. Assessment of Leishmania Infantum Infection in Equine Populations in a Canine Visceral Leishmaniosis Transmission Area. BMC Vet Res 2019, 15, 381. https://doi.org/10.1186/s12917-019-2108-1. [CrossRef]
- Vedovello Filho, D.; Jorge, F.A.; Lonardoni, M.V.C.; Teodoro, U.; Silveira, T.G.V. American Cutaneous Leishmaniasis in Horses from Endemic Areas in the North-Central Mesoregion of Paraná State, Brazil. Zoonoses Public Health 2008, 55, 149–155. https://doi.org/10.1111/j.1863-2378.2008.01106.x. [CrossRef]
- Aguilar, C.M.; Rangel, E.F.; Garcia, L.; Fernandez, E.; Momen, H.; Grimaldi Filho, G.; De Vargas, Z. Zoonotic Cutaneous Leishmaniasis Due to Leishmania (Viannia) Braziliensis Associated with Domestic Animals in Venezuela and Brazil. Mem Inst Oswaldo Cruz 1989, 84, 19–28. https://doi.org/10.1590/s0074-02761989000100005. [CrossRef]
- Schulz, A.; Mellenthin, K.; Schönian, G.; Fleischer, B.; Drosten, C. Detection, Differentiation, and Quantitation of Pathogenic Leishmania Organisms by a Fluorescence Resonance Energy Transfer-Based Real-Time PCR Assay. J Clin Microbiol 2003, 41, 1529–1535. https://doi.org/10.1128/JCM.41.4.1529-1535.2003. [CrossRef]
- Lopes, A.P.; Sousa, S.; Dubey, J.P.; Ribeiro, A.J.; Silvestre, R.; Cotovio, M.; Schallig, H.D.F.H.; Cardoso, L.; Cordeiro-da-Silva, A. Prevalence of Antibodies to Leishmania Infantum and Toxoplasma Gondii in Horses from the North of Portugal. Parasit Vectors 2013, 6, 178. https://doi.org/10.1186/1756-3305-6-178. [CrossRef]
- Kouam, M.K.; Diakou, A.; Kanzoura, V.; Papadopoulos, E.; Gajadhar, A.A.; Theodoropoulos, G. A Seroepidemiological Study of Exposure to Toxoplasma, Leishmania, Echinococcus and Trichinella in Equids in Greece and Analysis of Risk Factors. Vet Parasitol 2010, 170, 170–175. https://doi.org/10.1016/j.vetpar.2010.02.004. [CrossRef]
- Aharonson-Raz, K.; Baneth, G.; Lopes, A.P.; Brancal, H.; Schallig, H.; Cardoso, L.; Steinman, A. Low Seroprevalence of Leishmania Infantum and Toxoplasma Gondii in the Horse Population in Israel. Vector Borne Zoonotic Dis 2015, 15, 726–731. https://doi.org/10.1089/vbz.2015.1826. [CrossRef]
- Miranda, D.E. de O.; Sales, K.G. da S.; Faustino, M.A. da G.; Alves, L.C.; Brandão-Filho, S.P.; Dantas-Torres, F.; de Carvalho, G.A. Ecology of Sand Flies in a Low-Density Residential Rural Area, with Mixed Forest/Agricultural Exploitation, in North-Eastern Brazil. Acta Trop 2015, 146, 89–94. https://doi.org/10.1016/j.actatropica.2015.03.011. [CrossRef]
- Ximenes, M.F.; Souza, M.F.; Castellón, E.G. Density of Sand Flies (Diptera: Psychodidae) in Domestic and Wild Animal Shelters in an Area of Visceral Leishmaniasis in the State of Rio Grande Do Norte, Brazil. Mem Inst Oswaldo Cruz 1999, 94, 427–432. https://doi.org/10.1590/s0074-02761999000400001. [CrossRef]
- Manna, L.; Reale, S.; Viola, E.; Vitale, F.; Foglia Manzillo, V.; Pavone, L.M.; Michele, P.L.; Caracappa, S.; Gravino, A.E. Leishmania DNA Load and Cytokine Expression Levels in Asymptomatic Naturally Infected Dogs. Vet Parasitol 2006, 142, 271–280. https://doi.org/10.1016/j.vetpar.2006.06.028. [CrossRef]
- Meteorología, A.E. de Agencia Estatal de Meteorología - AEMET. Gobierno de España Available online: https://www.aemet.es/es/portada (accessed on 23 June 2023).
- Humanes-Navarro, A.M.; Herrador, Z.; Redondo, L.; Cruz, I.; Fernández-Martínez, B. Estimating Human Leishmaniasis Burden in Spain Using the Capture-Recapture Method, 2016-2017. PLoS One 2021, 16, e0259225. https://doi.org/10.1371/journal.pone.0259225. [CrossRef]
- Gálvez, R.; Montoya, A.; Cruz, I.; Fernández, C.; Martín, O.; Checa, R.; Chicharro, C.; Migueláñez, S.; Marino, V.; Miró, G. Latest Trends in Leishmania Infantum Infection in Dogs in Spain, Part I: Mapped Seroprevalence and Sand Fly Distributions. Parasit Vectors 2020, 13, 204. https://doi.org/10.1186/s13071-020-04081-7. [CrossRef]
- Edo, M.; Marín-García, P.J.; Llobat, L. Is the Prevalence of Leishmania Infantum Linked to Breeds in Dogs? Characterization of Seropositive Dogs in Ibiza. Animals (Basel) 2021, 11, 2579. https://doi.org/10.3390/ani11092579. [CrossRef]
- Fernández-Bellon, H.; Solano-Gallego, L.; Bardagí, M.; Alberola, J.; Ramis, A.; Ferrer, L. Immune Response to Leishmania Infantum in Healthy Horses in Spain. Vet Parasitol 2006, 135, 181–185. https://doi.org/10.1016/j.vetpar.2005.09.007. [CrossRef]
- Nardoni, S.; Altomonte, I.; Salari, F.; Martini, M.; Mancianti, F. Serological and Molecular Findings of Leishmania Infection in Healthy Donkeys (Equus Asinus) from a Canine Leishmaniosis Endemic Focus in Tuscany, Italy: A Preliminary Report. Pathogens 2019, 8, 99. https://doi.org/10.3390/pathogens8030099. [CrossRef]
- Biral, N.V.; Azevedo Santos, H.; Senne, N.A.; Paulino, P.G.; Camilo, T.A.; Tassinari, W. de S.; Silva, V.L.; Santos, F.N.; Angelo, I. da C. A Cross-Sectional Study of Leishmania Spp. in Draft Horses from the Distrito Federal, Brazil: Seroprevalence, Spatial Distribution, and Associated Factors. Prev Vet Med 2021, 195, 105467. https://doi.org/10.1016/j.prevetmed.2021.105467. [CrossRef]
- Lopes, K.F.C.; Delai, R.M.; Fazoli, K.G.Z.; Rey, L.M.R.; Lopes-Mori, F.M.R.; Benitez, A. do N.; Borges Neto, A.; Bernardes, J.C.; Caldart, E.T.; Mitsuka-Breganó, R.; et al. Urban Horses As Environmental Bioindicators for Leishmaniasis. Vector Borne Zoonotic Dis 2021, 21, 534–538. https://doi.org/10.1089/vbz.2020.2760. [CrossRef]
- de Assis, J.; de Queiroz, N.M.G.P.; da Silveira, R. de C.V.; Nunes, C.M.; Oliveira, T.M.F. de S.; Junior, A.C.F. de N.; Neves, M.F.; Machado, R.Z.; Buzetti, W.A.S. [Comparative study of diagnostic methods for visceral leishmaniasis in dogs from Ilha Solteira, SP]. Rev Bras Parasitol Vet 2010, 19, 17–25. https://doi.org/10.4322/rbpv.01901004. [CrossRef]
- Srivastava, P.; Dayama, A.; Mehrotra, S.; Sundar, S. Diagnosis of Visceral Leishmaniasis. Trans R Soc Trop Med Hyg 2011, 105, 1–6. https://doi.org/10.1016/j.trstmh.2010.09.006. [CrossRef]
- Symeonidou, I.; Angelou, A.; Theodoridis, A.; Sioutas, G.; Papadopoulos, E. Canine Leishmaniosis in Greece: An Updated Countrywide Serological Study and Associated Risk Factors. Pathogens 2021, 10, 1129. https://doi.org/10.3390/pathogens10091129. [CrossRef]
- Piscopo, T.V.; Mallia Azzopardi, C. Leishmaniasis. Postgrad Med J 2007, 83, 649–657. https://doi.org/10.1136/pgmj.2006.047340corr1. [CrossRef]
- Trájer, A.J. The Alteration of the Suitability Patterns of Leishmania Infantum Due to Climate Change in Iran. Int J Environ Health Res 2022, 32, 1567–1580. https://doi.org/10.1080/09603123.2021.1897535. [CrossRef]
- WHO Leishmaniasis Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 11 May 2023).
- Daoudi, M.; Outammassine, A.; Amane, M.; Hafidi, M.; Boussaa, S.; Boumezzough, A. Climate Change Influences on the Potential Distribution of the Sand Fly Phlebotomus Sergenti, Vector of Leishmania Tropica in Morocco. Acta Parasitol 2022, 67, 858–866. https://doi.org/10.1007/s11686-022-00533-5. [CrossRef]
- Apostolopoulos, N.; Mitropoulou, A.; Thom, N.; Moritz, A. Update on Therapy and Prevention of Canine Leishmaniasis. Tierarztl Prax Ausg K Kleintiere Heimtiere 2018, 46, 315–322. https://doi.org/10.15654/TPK-180089. [CrossRef]
- Oerther, S.; Jöst, H.; Heitmann, A.; Lühken, R.; Krüger, A.; Steinhausen, I.; Brinker, C.; Lorentz, S.; Marx, M.; Schmidt-Chanasit, J.; et al. Phlebotomine Sand Flies in Southwest Germany: An Update with Records in New Locations. Parasit Vectors 2020, 13, 173. https://doi.org/10.1186/s13071-020-04058-6. [CrossRef]
- Talas, J.; Mielcarek, K.; Wu, J.; Brunner, M.; Steinhoff, M.; Zouboulis, C.C. [Cutaneous leishmaniasis in Germany-still a travel-related disease]. Hautarzt 2022, 73, 146–151. https://doi.org/10.1007/s00105-021-04890-6. [CrossRef]
| Variable | Categories | Number of Horses (%) | |
|---|---|---|---|
| Gender | Male | Castrated | 72 (66.1%) |
| No castrated | 37 (33.9%) | ||
| Female | 58 (34.7%) | ||
| Age | Foal (<5 years) | 21 (12.6%) | |
| Young (5-12 years) | 40 (24.0%) | ||
| Adult (13-21) | 80 (47.9%) | ||
| Elder (>21 years) | 26 (9.6%) | ||
| Breed | Purebreed (125) | Anglo-Arabian | 1 (0.6%) |
| Belgian Warmblood | 2 (1.2%) | ||
| Spanish Sport Horse | 7 (4.2%) | ||
| Connemara | 1 (0.6%) | ||
| Haflinger | 1 (0.6%) | ||
| Hannoverian | 4 (2.4%) | ||
| Hispano-Breton | 8 (4.8%) | ||
| Hispanic-Arabic | 6 (3.6%) | ||
| Holsteiner | 1 (0.6%) | ||
| Gypsy Cob | 2 (1.2%) | ||
| Jaca navarra | 2 (1.2%) | ||
| KWPN | 5 (3.0%) | ||
| PRE | 74 (44.3%) | ||
| Percheron | 1 (0.6%) | ||
| Pony | 4 (2.4%) | ||
| Frech Saddle Horse | 5 (3.0%) | ||
| Arabian | 1 (0.6%) | ||
| Crossbred | 42 (25.1%) | ||
| Use | Teaching | 27 (16.2%) | |
| Breeding | 4 (2.4%) | ||
| Dressage | 74 (44.3%) | ||
| Hitch | 12 (7.2%) | ||
| Walking | 32 (19.2%) | ||
| Leap | 18 (10.8%) | ||
| Type of housing | Outdoor | 112 (67.1%) | |
| Indoor | 55 (32.9%) | ||
| Living with dogs | Yes | 159 (95.2%) | |
| No | 8 (4.8%) | ||
| Periodo of the year | Winter | 106 (63.5%) | |
| Spring | 61 (36.5%) | ||
| Number of Positive Animals (%) | ||
|---|---|---|
| Purebred (125) | Anglo-Arabian | 0 |
| Belgian Warmblood | 0 | |
| Spanish Sport Horse | 4 (57.1%) | |
| Connemara | 0 | |
| Haflinger | 1 (100%) | |
| Hannoverian | 0 | |
| Hispano-Breton | 0 | |
| Hispanic-Arabic | 0 | |
| Holsteiner | 0 | |
| Gypsy Cob | 1 (50%) | |
| Jaca navarra | 0 | |
| KWPN | 0 | |
| PRE | 25 (32.9%) | |
| Percheron | 1 (100%) | |
| Pony | 3 (75%) | |
| Frech Saddle Horse | 0 | |
| Arabian | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





