Introduction (472 words)
Atherosclerosis is a systemic disorder characterized by the accumulation of lipid-rich plaques within the arterial walls. This pathological process occurs throughout the body, causing the progressive narrowing and stiffening of arteries. One specific manifestation of atherosclerosis is peripheral artery disease (PAD). It primarily affects the arteries outside the heart and brain, most commonly in the lower extremities [
1]. PAD is a chronic and progressive disease, it leads to reduced blood flow to the legs and feet, resulting in claudication, ischemic ulcers, tissue necrosis, and, if left untreated, it significantly contributes to morbidity and mortality, leading to considerable rates of disability and death attributed to stroke and myocardial infarction [
2]. Compared to coronary artery disease (CAD), patients with PAD are at a significantly higher risk of cardiovascular events [
1,
3,
4,
5], and their long-term prognosis is even less favorable [
6].
Recent studies have shown that lipid-lowering therapy by statins significantly increased overall survival of PAD patients [
7] but many patients with PAD don’t experience any symptoms at an early disease stage [
8]. Indeed, PAD tends to be frequently underdiagnosed and undertreated, despite its severity as a systemic condition. Consequently, there is a pressing need for improved recognition and therapy of PAD to mitigate its detrimental impact on public health.
A detailed comparison between PAD and CAD has recently demonstrated that ceramide-based lipid profiles are able to discriminate between PAD and CAD [
9]. Ceramides are sphingolipids and have recently been described as novel lipid biomarkers involved in several signaling processes, exerting a wide range of biological functions [
10]. They modulate cellular and whole-body metabolism and are associated with a number of diseases [
11]. They are also found in coronary plaques and are involved in their formation [
12], but the family of ceramides is large and its members are distributed differently among lipoprotein particles [
13]. From a biological and clinical point of view, they can be divided into harmful species indicating an increased cardiovascular risk and more neutral or benign forms [
14,
15,
16]. Previous studies have shown that the prognostic test score named Coronary Event Risk Test (CERT), which combines risk-associated and benign ceramides, is superior to traditional cardiovascular risk markers such as LDL-cholesterol (LDL-C) in predicting cardiovascular risk [
17,
18].
However, in PAD patients, the prognosis of clinical outcomes for individual patients remains challenging and for successful treatment knowledge about the overall risk of PAD patients is important. As of yet, there is no single biomarker that is considered ideal for the diagnosis and management of PAD, and the commonly used ankle-brachial index (ABI) has its own set of limitations [
19].
In the present observational study comprising a 10-year follow up we, therefore, aimed at investigating the use of CERT in a well-characterized cohort of patients with sonographically verified PAD for the prediction of all-cause mortality risk, also taking into account patients’ statin treatment status before enrollment.
Methods (966 words)
Study subjects
Between October 2006 and December 2010, we enrolled 379 Caucasian patients who underwent routine duplex sonography for the evaluation of suspected or established PAD at the Academic Teaching Hospital Feldkirch and in whom PAD was sonographically verified, as described previously [
20]. Patients with acute coronary syndrome or with secondary inflammation or wounds were excluded. The follow-up time was limited to 10 years and ended in November 2020. During the follow-up period, we recorded all-cause death (primary endpoint) as well as major cardiovascular events (MACE) and cardiovascular death (secondary endpoints). MACE were defined as a composite of three factors: cardiovascular mortality, nonfatal myocardial infarction, and nonfatal stroke. Cardiovascular mortality was specifically defined as death resulting from myocardial infarction, sudden cardiac death, congestive heart failure due to CAD, or fatal ischemic stroke. Myocardial infarction was diagnosed in the presence of at least two of three criteria: (i) standard electrocardiographic criteria, (ii) ischaemic cardiac pain and (iii) creatinine kinase isoenzyme MB activity of at least twice the upper limit of normal. Stroke was defined as a neurological deficit lasting longer than 48 hours with a confirmative computer tomography or magnetic resonance image. The collection of data on both the date and cause of death was done annually through the utilization of a national registry (Statistik Austria, Vienna, Austria), hospital registries, and telephone contacts. Additionally, standardized interviews were conducted every two years to obtain information on non-fatal events.
Clinical and laboratory analyses
Venous blood samples were collected after an overnight fast of 12 hours before sonography was performed, and laboratory measurements were performed using fresh serum samples. Low-density lipoprotein cholesterol (LDL-C) was measured using enzymatic hydrolysis and precipitation techniques on a Hitachi-Analyzer 717 or 911 (QuantolipLDL, QuantolipHDL; Roche, Basel, Switzerland). Ceramides (Cer(d18:1/16:0), Cer(d18:1/18:0), Cer(d18:1/24:1), and Cer(d18:1/24:0)) were determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS). For LC-MS/MS analysis, lipids were extracted from serum or plasma samples as described previously [
18]. In short, 10 µl of sample was combined with 590 µl isopropanol:ethylacetate (8:2), containing the corresponding isotopically labeled standard for each ceramide. Samples were then mixed by aspirating them three times, followed by 10 min centrifugation at 3000g. Supernatants were transferred to Eppendorf Twintec PCR plate and sealed with a heat sealing foil (Hamburg, Germany), prior to analysis with LC-MS/MS. Ceramide concentrations were calculated using standard straight calibration based on internal standard concentration. The analyzed lipids and ions used in this study are presented in
supplementary Table 1. LC-MS/MS analysis was conducted on a Sciex MSTrap 5500 mass spectrometer coupled to Shimadzu nexera 2 UHPLC system. Electrospray ionization in positive ion mode was used with multiple reaction monitoring. Instrument and data acquisition were controlled using Analyst® (version 1.6). The following settings were applied to all compounds in the analysis: Curtain gas, 35; ion spray voltage, 5000V; temperature, 300°C; gas 1 and gas 2, 50; declustering potential, 30; entrance potential, 10; collision exit potential, 20. Collision energy was set separately to each lipid (
supplementary Table 1). Chromatographic separation was performed on an Acquity BEH C18 2.1x50 mm id.1.7 µm column. Temperature was set to 60°C. Mobile phases consisted of (A) 10 mM Ammonium acetate with 0.1% formic acid and (B) 10 mM Ammonium acetate in acetonitrile:2-propanol (4:3, v/v) with 0.1% formic acid. Injection volume was 3 µl and flow rate was 500 µl/min. The following gradient was applied: A/B (22/78%) from 0 to 1.5 min, then B to 85% at 2 min and to 100% at 2.5 min. B was held at 100% from 2.5 min to 4.0 min, and then dropped to 78% at 4.1 min and held until 4.6 min. The selection of ceramides and their combined use in scores (CERT) has been described previously by others [
21]. In short, the CERT score consists of three single ceramides and three ceramide/ceramide ratios. It assigns patients to a 13-step score ranging from 0-12 and, more generally, to one out of four risk categories: Category 1 = low risk (score 0-2), category 2 = medium risk (score 3-6), category 3 = high risk (score 7-9), and category 4 = very high risk (score 10-12)). A more detailed description is provided elsewhere [
22].
Type 2 diabetes mellitus (T2DM) was diagnosed according to the WHO definition [
23]. Hypertension was diagnosed by adhering to the NCEP-ATPIII criteria for high blood pressure, or by identifying the use of antihypertensive treatment. Renal function was assessed based on estimated glomerular filtration rate (eGFR) and classified as chronic kidney disease (CKD) in patients with eGFR less than 60 ml/min/1.73 m
2, according to the guidelines of the Kidney Disease Outcomes Quality Initiative (KDOQI) [
24]. Body mass index (BMI) was calculated as body weight (kg)/height
2 (m).
Statistical analyses
Normal distribution was checked using the Kolmogorov–Smirnov test. Non-normally distributed variables were described using median and interquartile range (IQR). Differences were tested for statistical significance with Chi-squared tests for categorical, with the Welch test for two continuous variables. In case of more than two continuous variables, we used the non-parametric Jonckheere-Terpstra test. Correlation was tested using the Pearson test. Survival curves were generated using the Kaplan-Meier method and compared by log-rank-Mantel-Cox tests. Hazard ratios (HRs) and 95% confidence intervals (CIs) of the HRs were derived from univariable and multivariable Cox proportional hazards models. Significance was defined as a two-tailed P value < 0.05. No imputation was applied and all data were analyzed by complete-case analysis. All statistical analyses were performed with SPSS 28.0 for Windows (IBM Corp., USA), and R statistical software v. 3.5.1 (
http://www.r-project.org).
Ethics approval and consent statements
The present study conforms to the ethical guidelines of the 1975 Declaration of Helsinki and has been approved by the Ethics Committee of Vorarlberg, Austria (EK-2-22013/0008). Written informed consent was given by all participants. The authors affirm that all participants provided informed consent for publication of anonymized data.
Results (752 words)
Follow-up
In total, we have enrolled 379 PAD patients. The median age of our patients at baseline was 67 years. They were followed for a maximum of ten years resulting in 2825 patient-years. The median follow-up time of our patients was 9.0 years with an interquartile range of 5.3 to 10.0 years. Follow-up data were available for 373 patients and only 6 were lost during follow-up (follow-up rate 98%). Out of these 373 patients, 159 (42%) died from any cause during follow-up, 138 (36%) suffered a MACE and 66 (17%) succumbed to a cardiovascular death.
CERT categories predict overall mortality
To evaluate the power of the CERT score in PAD patients we stratified all patients according to the 4 risk categories of the CERT score (low risk, moderate risk, high risk, and very high risk), which is only based on ceramide measures and does not use any demographic or anthropometric data. Baseline characteristics of all patients and of the 4 subgroups are shown in
Table 1. There was no significant difference between the groups regarding patients’ sex, BMI, hypertension prevalence, LDL-C, statin use, or smoking status. In contrast age and the prevalence of T2DM and CKD increased with increasing CERT categories ranging from 1 (low risk) to 4 (very high risk).
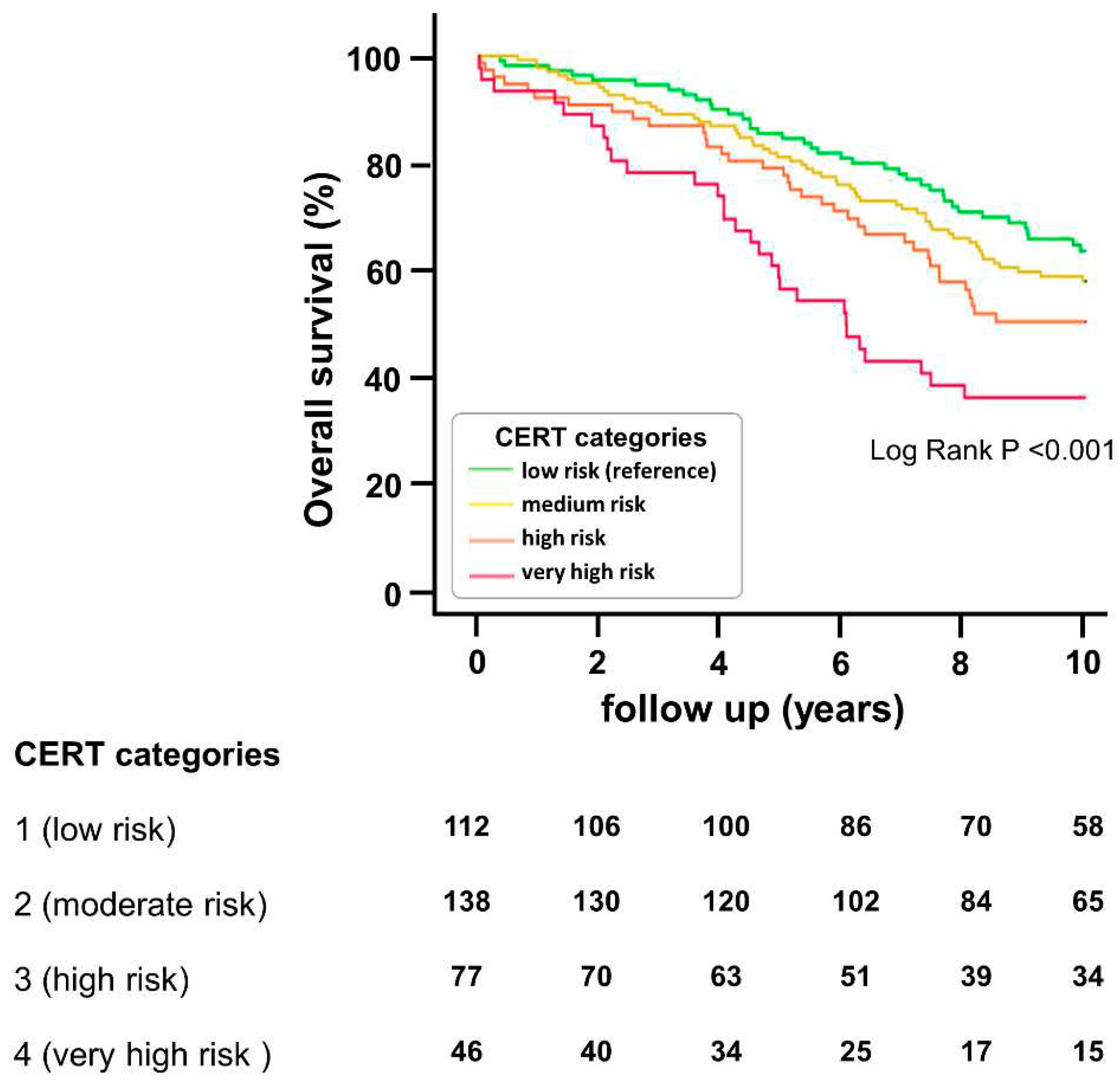
We then analyzed patients’ survival during the 10 years of follow-up. Kaplan Meier survival curves are depicted in
Figure 1. They demonstrate that overall survival significantly decreases with increasing CERT categories from low risk to very high risk. Comparable results are seen in the case of the secondary endpoints cardiovascular mortality and MACE. Respective survival curves are given in the
supplementary Figure 1. Applying a Cox regression, we found that each one-category increase in CERT (ranging from 1 to 4) resulted in a 35% rise in overall mortality risk (HR=1.351 [1.157-1.578], p>0.001). Alternatively, applying the more detailed 13-step scoring scale of CERT (ranging from 0 to 12) every 1-step increase in the risk scale was associated with a 9% rise in overall mortality risk (HR=1.089 [1.040-1.114], p>0.001).
To account for possible confounding effects, we have built several adjustment models (
Table 2). We found that in a multivariate model including age, sex, BMI, LDL-C, status of hypertension, CKD, T2DM, smoking, and statin treatment, CERT was still a significant predictor of overall mortality applying the 4-category scale (HR=1.215 [1.035-1.427], p=0.017) and the 13-step scale (HR=1.055 [1.007-1.106], p=0.024), respectively. Apart from CERT, only age (HR=1.070 [1.047-1.093], p<0.001) and sex (HR=2.343 [1.501-3.659], p<0.001) were significant risk predictors in this model whereas all other variables including LDL-C failed. Further models describing the association between CERT and the primary endpoint overall mortality as well as between CERT and the secondary endpoints cardiovascular mortality and MACE demonstrate comparable results (
Table 2).
Statin effect is stronger in higher-risk groups
Furthermore, we analyzed the effect of statin on the outcome of patients. Seventy percent of our PAD patients were already under statin therapy before enrollment. When comparing the pre-baseline-treated to the untreated patient subgroup, we found that mean LDL-C was significantly lower in the treated group than in the untreated one (99±34 mg/dL vs. 123±43 mg/dL, p<0.001) applying a one-sided Welch test. Regarding the 4-category scale CERT in treated vs. untreated patients, there was no significant difference (2.1±1.0 vs. 2.2±0.9, p=0.113). Similarly, regarding the distribution of the four CERT categories in our patients, the frequencies appeared to be roughly comparable between treated patients and untreated patients and we did not find a statistically significant difference (supplementary Figure 2).
Concerning the outcome, we found that statin treatment before enrollment was associated with a 26% lower mortality risk during follow-up, though this just failed significance in the total study population (HR=0.742 [0.533-1.032], p>0.076). We then separated patients according to the four CERT categories. Taking into account this stratification, we found that the impact of statin treatment increased with increasing CERT categories (
Figure 2) and was highest in category 4 (HR=0.442 [0.200-0.987], p=0.046). Moreover, the interaction between statin treatment and the CERT categories was significant (p=0.040) meaning that the CERT categories had a significant impact on the association between statin treatment status and patients’ outcome. In contrast, the LDL-C concentration at baseline was not correlated with CERT (r=0.035, p=0.495) and not associated with patients’ outcomes, neither in the total cohort (HR per 10mg/dL increase = 0.976 [0.934-1.019], p=0.270) nor in any of the subgroups (
Figure 2) and there was also no significant interaction between the CERT categories and LDL-C (p=0.310).
Discussion (1650 words)
Main findings
From our data, we conclude that in PAD patients the ceramide-based risk score CERT is a significant and powerful predictor for the 10-year overall survival and also for cardiovascular survival and MACE. This predictive power was not observed with LDL-C. Regarding the effect of statin on patients’ outcomes, we also found that the categorization of the PAD patients according to CERT had a significant impact: The protective effect of statin treatment was more pronounced with increasing CERT risk categories.
Ceramides as risk predictors
In this study, we have used the CERT score for assigning 379 PAD patients to one of four risk categories. This categorization according to CERT has significantly predicted overall mortality as well as cardiovascular mortality and MACE, which is in line with previous data reporting the usage of CERT in patients with CAD [
18] and ACS [
25]. Considering the clinical significance of ceramides in predicting cardiovascular risk, there is a pertinent inquiry regarding their molecular characteristics. Ceramides are bioactive lipids [
10] and mediate the building of signal platforms based on lipid rafts [
26]. They also regulate cellular functions via the activation of protein phosphatase of the PP1 and PP2A family [
27] and downstream targets including the serine/threonine protein kinase Akt [
11]. This impact on signaling pathways may explain in part their described role in metabolism and their predictive power regarding the development or prevention of diseases [
11]. Given their role (i) as a kind of a hub in lipid metabolism [
10], (ii) in the uptake of lipids into the endothelial cell, (iii) in mediating lipotoxic events [
11,
28], and (iv) in promoting arterial dysfunction [
29], they have recently been described as important and valuable predictors for increased cardiovascular event risk [
12,
29,
30]. This study adds further color to the picture demonstrating the value of ceramides, in particular the ceramide-based risk score CERT, as predictors of cardiovascular and overall risk in PAD patients.
For making clinical decisions, stratification of the baseline risk is suggested to be most applicable [
31]. In our study, we describe that the power of statin treatment was stronger with higher CERT categories reflecting a higher cardiovascular risk. This is in line with the well-known principle that the statin-mediated relative risk reduction for fatal endpoints is greater in people with a higher risk. The absolute benefits of statins are different concerning the cardiovascular risk. This is true in primary prevention and also accounts for secondary prevention populations with previous cardiovascular disease events in which the benefit-to-harm balance of treatment is more favorable than in individuals, who have a lower average risk of cardiovascular disease [
31,
32]. Furthermore, in terms of cost-effectiveness, a previous study evaluated the health-economic justification of statin treatment in patients with borderline cardiovascular risk and found that statin therapy was more cost-effective in individuals with a higher risk of cardiovascular disease [
33].
That said, a high cardiovascular risk may be not necessarily linked with a high LDL-C concentration and thus an existing high cardiovascular risk may be masked and treatment may be done less consequent or even not initiated [
34,
35]. In contrast to ceramides, LDL-C was no significant predictor in our study and there was no correlation between LDL-C and the CERT risk score. It has become more and more clear now that in populations of cardiovascular disease patients, age, comorbidities, and lipid-lowering treatment impair LDL-C-based risk prediction and other risk predictors may be more valuable [
35,
36,
37]. Ceramides and ceramide-based risk scores including CERT are novel kinds of lipid biomarkers that have already been supposed to outperform risk prediction by LDL-C [
38] which are even particularly effective in discriminating PAD from CAD [
9].
As the cardiovascular risk of PAD is higher than that of CAD we suggest for PAD patients characterized to be in a high CERT category that statin treatment should be mandatory.
However, our study shows a statistical interaction between statins and ceramides but cannot necessarily link the biological statin action to ceramide function. In contrast to LDL-C, it is known that statins do not directly block ceramide synthesis, though there is a certain indirect statin effect on ceramide levels [
39]. In our study, we did not see a clear and significant difference between statin-naïve patients and those under therapy concerning ceramide levels represented by CERT categories.
Ceramides as treatment targets
The levels of ceramides in the bloodstream can be influenced by various factors, including lifestyle changes like dietary modifications and regular exercise as well as certain medications.
In the context of the impact of diet, it is estimated that Western diets contain 200 to 400mg sphingolipids per day, with the majority being sphingomyelin which is further broken down to ceramide [
40]. It is, however, not yet clear how much dietary ceramides per se contribute to the body's overall ceramide levels or how effectively they are absorbed or secreted after digestion. Nevertheless, it has been demonstrated that increased saturated fat intake in overweight subjects is able to elevate the level of circulating ceramides in plasma [
41]. Conversely, a Mediterranean diet may mitigate the potential deleterious effects of elevated plasma ceramide concentrations on CVD [
42]. For instance, an 8-week fruit and vegetable intervention in young adults, caused a decline in ceramide concentrations, along with improvements in overall metabolic health and inflammatory status [
43]. Though certain dietary interventions have already shown their general impact on circulating ceramide levels, it has been mentioned that further research with larger sample sizes is required to gain a more comprehensive understanding of the effects of specific nutrients on the concentrations of specific ceramides [
44].
Apart from intake by diet, ceramides are also synthesized by de novo or salvage pathways specifically at the endoplasmic reticulum's surface. This process involves the combination of serine and palmitoyl-CoA, resulting in the formation of a ceramide precursor that possesses an additional fatty acyl group [
45,
46]. Once generated, ceramides are part of the eucaryotic cell membrane but they are also present in circulating lipoprotein particles such as LDL-C. Hence drugs like statins, ezetimibe, and PCSK9 inhibitors aimed at reducing cholesterol levels have the potential to lower serum ceramides as well [
13,
14,
47]. As mentioned above, statins do not directly inhibit ceramide synthesis, but at least have a limited indirect effect [
39]. In our study, we just found a slight but not significant lower CERT levels in treated than in untreated patients. On the other hand, it has already been shown that inhibiting PCSK-9, which interferes with LDL-receptor degradation, reduces not only LDL-C but also, to an even larger extent, ceramide concentrations [
14]. This implies that there might be a connection between the action of PCSK-9, the up-regulation of LDL receptors, and the levels of some circulating ceramides. Such a relationship could potentially contribute to the cardiovascular risk reduction observed with the use of PCSK-9 inhibitors. Moreover, an indirect reduction of ceramide concentration may also be achieved by ezetimibe [
48,
49]. However, both these drug classes had no impact on our study results since recruitment of our patients took place between 2006 and 2010 when ezetimibe was not broadly prescribed in Austria and PCSK-9 inhibitors were not even approved for prescription. Apart from that, fibrates, which are used to manage lipid levels, are an additional drug class reported to be associated with significant reductions in ceramide concentrations [
50]. However, given the poor role fibrates have demonstrated in reducing the cardiovascular risk [
51] it seems to be questionable whether fibrates may become useful as a ceramide-modulating drug class for lowering the CVD risk.
On the other hand, drugs that directly target ceramide levels are currently being developed and this is based on the primary objective that atherosclerosis development can indeed be reduced by inhibiting de novo ceramide biosynthesis [
52]. Thus we strongly believe that the clinical significance of ceramides may gain further attention, though the role of ceramide as a target for reducing the risk of atherosclerotic diseases and in particular treating PAD patients remains to be investigated in more detail.
Strengths and limitations
The present study has strengths and limitations. A particular strength of this study is the 98% follow-up rate. Additional strengths are that the study comprises a very well-characterized patient cohort, that all patients were in a stable stage, and that PAD was sonographically proven. One limitation is the fact that we selected European patients with PAD, which consequently does not reflect the general population, of course. The mean annual death rate among our study patients was 4.7%, which is about four times higher than in the general population of the EU (1.2%) [
53]. Despite these limitations, it is important to emphasize that PAD patients are a high-risk population and require special clinical attention. Another limitation of our study is its observational design, which precludes any conclusions about the causal relationships between ceramides and the observed outcomes. Additionally, our analyses were based on single measurements and did not consider changes in parameters over time, which may have impacted the observed outcomes. We also did not have access to data on patient adherence to medical treatment, which could have influenced the outcomes. We also had no data about the genetic background of our patients in particular concerning ceramides. We also want to mention that we have only analyzed a limited set of ceramides, previously described in the literature to be associated with cardiovascular risk. There are many more species whose roles remain to be analyzed. Finally, the underlying pathophysiological mechanisms linking ceramides and outcome as well as the different environmental factors on the circulating ceramide concentrations require further investigation.
Conclusion
In summary, our findings demonstrate that CERT serves as a robust predictor of the 10-year prognosis of patients with PAD beyond conventional clinical risk factors. These results support the established link between ceramides and adverse outcomes in high-risk patients. The use of ceramides, specifically CERT, as a tool for risk assessment and decision-making represents a promising avenue for future research.
Author Contributions
A.L. researched data and wrote the manuscript. A.M. and C.H.S. designed and organized the project and reviewed/edited the manuscript. K.G., EM.B., C.H., and S.G. performed analyses and reviewed/edited the manuscript. R.J. organized and supervised ceramide analysis and reviewed/edited the manuscript. P.F. organized the project, contributed to discussion, and reviewed/edited the manuscript. H.D. supervised the project, contributed to discussion, and is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
The present study did not receive any particular funding. The VIVIT research institute is financially supported by the State Government of Vorarlberg (Bregenz, Austria), which, however, exerted no influence on the present work in any way.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We are grateful to the State Government of Vorarlberg (Bregenz, Austria) for continuously supporting our research.
Conflicts of Interest
A.L. has nothing to disclose. A.M. has nothing to disclose. C.H.S. has nothing to disclose. K.G. has nothing to disclose. EM. B. has nothing to disclose. C.H. has nothing to disclose. S.G. has nothing to disclose. P.F. has nothing to disclose. H.D. has nothing to disclose. R.L. is employed by Zora Biosciences.
Abbreviations list
CAD: Coronary artery disease
CERT: Coronary event risk test
CKD: Chronic kidney disease
CVD: Cardiovascular disease
HR: Hazard ratio
LC-MS/MS: liquid chromatography-tandem mass spectrometry
LDL: Low-density lipoprotein
MACE: Major adverse cardiovascular events
T2DM: Type 2 diabetes mellitus
PAD: Peripheral artery disease
References
- Weitz, J.I.; Byrne, J.; Clagett, G.P.; Farkouh, M.E.; Porter, J.M.; Sackett, D.L.; Strandness, D.E.; Taylor, L.M. Diagnosis and Treatment of Chronic Arterial Insufficiency of the Lower Extremities: A Critical Review. Circulation 1996, 94, 3026–3049. [Google Scholar] [CrossRef] [PubMed]
- Criqui, M.H. Peripheral Arterial Disease--Epidemiological Aspects. Vasc Med 2001, 6, 3–7. [Google Scholar] [CrossRef] [PubMed]
- CAPRIE Steering Committee A Randomised, Blinded, Trial of Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events (CAPRIE). Lancet 1996, 348, 1329–1339. [CrossRef] [PubMed]
- Steg, Ph.G.; Bhatt, D.L.; Wilson, P.W.F.; D’Agostino, R.; Ohman, E.M.; Röther, J.; Liau, C.-S.; Hirsch, A.T.; Mas, J.-L.; Ikeda, Y.; et al. One-Year Cardiovascular Event Rates in Outpatients with Atherothrombosis. JAMA 2007, 297, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, L.; Mader, A.; Larcher, B.; Mächler, M.; Vonbank, A.; Zanolin-Purin, D.; Leiherer, A.; Muendlein, A.; Drexel, H.; Saely, C.H. Type 2 Diabetes and the Risk of Cardiovascular Events in Peripheral Artery Disease versus Coronary Artery Disease. BMJ Open Diabetes Res Care 2021, 9. [Google Scholar] [CrossRef]
- Welten, G.M.; Schouten, O.; Hoeks, S.E.; Chonchol, M.; Vidakovic, R.; van Domburg, R.T.; Bax, J.J.; van Sambeek, M.R.H.M.; Poldermans, D. Long-Term Prognosis of Patients with Peripheral Arterial Disease: A Comparison in Patients with Coronary Artery Disease. J Am Coll Cardiol 2008, 51, 1588–1596. [Google Scholar] [CrossRef]
- Isma, N.; Barani, J.; Mattiasson, I.; Lindblad, B.; Gottsäter, A. Lipid-Lowering Therapy Is Related to Inflammatory Markers and 3-Year Mortality in Patients With Critical Limb Ischemia. https://doi.org/10.1177/0003319707306144 2008, 59, 542–548. [CrossRef]
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.C.; Olin, J.W.; Puschett, J.B.; et al. ACC/AHA 2005 Practice Guidelines for the Management of Patients With Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic). Circulation 2006, 113, e463–654. [Google Scholar] [CrossRef]
- Leiherer, A.; Muendlein, A.; Brandtner, E.M.; Saely, C.H.; Ramadani, H.; Vonbank, A.; Mader, A.; Dopheide, J.F.; Jylhä, A.; Lääperi, M.; et al. Lipid Profiles of Patients with Manifest Coronary versus Peripheral Atherosclerosis – Is There a Difference? J Intern Med 2021, 290, 1249–1263. [Google Scholar] [CrossRef]
- Canals, D.; Salamone, S.; Hannun, Y.A. Visualizing Bioactive Ceramides. Chem Phys Lipids 2018, 216, 142–151. [Google Scholar] [CrossRef]
- Bikman, B.T.; Summers, S.A. Ceramides as Modulators of Cellular and Whole-Body Metabolism. Journal of Clinical Investigation 2011, 121, 4222–4230. [Google Scholar] [CrossRef]
- Schissel, S.L.; Tweedie-Hardman, J.; Rapp, J.H.; Graham, G.; Williams, K.J.; Tabas, I. Rabbit Aorta and Human Atherosclerotic Lesions Hydrolyze the Sphingomyelin of Retained Low-Density Lipoprotein: Proposed Role for Arterial-Wall Sphingomyelinase in Subendothelial Retention and Aggregation of Atherogenic Lipoproteins. Journal of Clinical Investigation 1996, 98, 1455–1464. [Google Scholar] [CrossRef]
- Hilvo, M.; Simolin, H.; Metso, J.; Ruuth, M.; Öörni, K.; Jauhiainen, M.; Laaksonen, R.; Baruch, A. PCSK9 Inhibition Alters the Lipidome of Plasma and Lipoprotein Fractions. Atherosclerosis 2018, 269, 159–165. [Google Scholar] [CrossRef]
- Tarasov, K.; Ekroos, K.; Suoniemi, M.; Kauhanen, D.; Sylvänne, T.; Hurme, R.; Gouni-Berthold, I.; Berthold, H.K.; Kleber, M.E.; Laaksonen, R.; et al. Molecular Lipids Identify Cardiovascular Risk and Are Efficiently Lowered by Simvastatin and PCSK9 Deficiency. J Clin Endocrinol Metab 2014, 99, e45–52. [Google Scholar] [CrossRef]
- Mundra, P.A.; Barlow, C.K.; Nestel, P.J.; Barnes, E.H.; Kirby, A.; Thompson, P.; Sullivan, D.R.; Alshehry, Z.H.; Mellett, N.A.; Huynh, K.; et al. Large-Scale Plasma Lipidomic Profiling Identifies Lipids That Predict Cardiovascular Events in Secondary Prevention. JCI Insight 2018, 3, e121326. [Google Scholar] [CrossRef]
- Tippetts, T.S.; Holland, W.L.; Summers, S.A. The Ceramide Ratio: A Predictor of Cardiometabolic Risk. J Lipid Res 2018, 59, 1549–1550. [Google Scholar] [CrossRef]
- Cheng, J.M.; Suoniemi, M.; Kardys, I.; Vihervaara, T.; de Boer, S.P.M.; Akkerhuis, K.M.; Sysi-Aho, M.; Ekroos, K.; Garcia-Garcia, H.M.; Oemrawsingh, R.M.; et al. Plasma Concentrations of Molecular Lipid Species in Relation to Coronary Plaque Characteristics and Cardiovascular Outcome: Results of the ATHEROREMO-IVUS Study. Atherosclerosis 2015, 243, 560–566. [Google Scholar] [CrossRef]
- Hilvo, M.; Meikle, P.J.; Pedersen, E.R.; Tell, G.S.; Dhar, I.; Brenner, H.; Schöttker, B.; Lääperi, M.; Kauhanen, D.; Koistinen, K.M.; et al. Development and Validation of a Ceramide- and Phospholipid-Based Cardiovascular Risk Estimation Score for Coronary Artery Disease Patients. Eur Heart J 2019, 41, 371–380. [Google Scholar] [CrossRef]
- Hazarika, S.; Annex, B.H. Biomarkers and Genetics in Peripheral Artery Disease. Clin Chem 2017, 63, 236–244. [Google Scholar] [CrossRef]
- Saely, C.H.; Sternbauer, S.; Vonbank, A.; Heinzle, C.; Zanolin-Purin, D.; Larcher, B.; Mader, A.; Leiherer, A.; Muendlein, A.; Drexel, H. Type 2 Diabetes Mellitus Is a Strong Predictor of LDL Cholesterol Target Achievement in Patients with Peripheral Artery Disease. J Diabetes Complications 2020, 34. [Google Scholar] [CrossRef]
- Hilvo, M.; Vasile, V.C.; Donato, L.J.; Hurme, R.; Laaksonen, R. Ceramides and Ceramide Scores: Clinical Applications for Cardiometabolic Risk Stratification. Front Endocrinol (Lausanne) 2020, 11. [Google Scholar] [CrossRef]
- Laaksonen, R.; Ekroos, K.; Sysi-Aho, M.; Hilvo, M.; Vihervaara, T.; Kauhanen, D.; Suoniemi, M.; Hurme, R.; März, W.; Scharnagl, H.; et al. Plasma Ceramides Predict Cardiovascular Death in Patients with Stable Coronary Artery Disease and Acute Coronary Syndromes beyond LDL-Cholesterol. Eur Heart J 2016, 37, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Zimmet, P.Z. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus. Provisional Report of a WHO Consultation. Diabetic Medicine 1998, 15, 539–553. [Google Scholar] [CrossRef]
- KDOQI KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. American Journal of Kidney Diseases 2007, 49, S12–S154. [CrossRef] [PubMed]
- Gencer, B.; Morrow, D.A.; Braunwald, E.; Goodrich, E.L.; Hilvo, M.; Kauhanen, D.; Sabatine, M.S.; Laaksonen, R.; O’Donoghue, M.L. Plasma Ceramide and Phospholipid-Based Risk Score and the Risk of Cardiovascular Death in Patients after Acute Coronary Syndrome. Eur J Prev Cardiol 2022, 29, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Matsumori, N. Inimitable Impacts of Ceramides on Lipid Rafts Formed in Artificial and Natural Cell Membranes. Membranes (Basel) 2022, 12. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and Their Metabolism in Physiology and Disease. Nat Rev Mol Cell Biol 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Park, T.S.; Hu, Y.; Noh, H.L.; Drosatos, K.; Okajima, K.; Buchanan, J.; Tuinei, J.; Homma, S.; Jiang, X.C.; Abel, E.D.; et al. Ceramide Is a Cardiotoxin in Lipotoxic Cardiomyopathy. J Lipid Res 2008, 49, 2101–2112. [Google Scholar] [CrossRef]
- Bharath, L.P.; Ruan, T.; Li, Y.; Ravindran, A.; Wan, X.; Nhan, J.K.; Walker, M.L.; Deeter, L.; Goodrich, R.; Johnson, E.; et al. Ceramide-Initiated Protein Phosphatase 2A Activation Contributes to Arterial Dysfunction in Vivo. Diabetes 2015, 64, 3914–3926. [Google Scholar] [CrossRef]
- Mehra, V.C.; Jackson, E.; Zhang, X.M.; Jiang, X.C.; Dobrucki, L.W.; Yu, J.; Bernatchez, P.; Sinusas, A.J.; Shulman, G.I.; Sessa, W.C.; et al. Ceramide-Activated Phosphatase Mediates Fatty Acid-Induced Endothelial VEGF Resistance and Impaired Angiogenesis. American Journal of Pathology 2014, 184, 1562–1576. [Google Scholar] [CrossRef]
- Byrne, P.; Cullinan, J.; Smith, S.M. Statins for Primary Prevention of Cardiovascular Disease. BMJ 2019, 367. [Google Scholar] [CrossRef]
- Navarese, E.P.; Robinson, J.G.; Kowalewski, M.; Kołodziejczak, M.; Andreotti, F.; Bliden, K.; Tantry, U.; Kubica, J.; Raggi, P.; Gurbel, P.A. Association between Baseline LDL-C Level and Total and Cardiovascular Mortality after LDL-C Lowering a Systematic Review and Meta-Analysis. JAMA - Journal of the American Medical Association 2018, 319, 1566–1579. [Google Scholar] [CrossRef] [PubMed]
- Kohli-Lynch, C.N.; Bellows, B.K.; Thanassoulis, G.; Zhang, Y.; Pletcher, M.J.; Vittinghoff, E.; Pencina, M.J.; Kazi, D.; Sniderman, A.D.; Moran, A.E. Cost-Effectiveness of Low-Density Lipoprotein Cholesterol Level-Guided Statin Treatment in Patients With Borderline Cardiovascular Risk. JAMA Cardiol 2019, 4, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Bhatt, D.L.; Pradhan, A.D.; Glynn, R.J.; MacFadyen, J.G.; Nissen, S.E. Inflammation and Cholesterol as Predictors of Cardiovascular Events among Patients Receiving Statin Therapy: A Collaborative Analysis of Three Randomised Trials. The Lancet 2023, 401, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Johannesen, C.Di.L.; Langsted, A.; Mortensen, M.B.; Nordestgaard, B.G. Association between Low Density Lipoprotein and All Cause and Cause Specific Mortality in Denmark: Prospective Cohort Study. BMJ 2020, 371, m4266. [Google Scholar] [CrossRef]
- Abdelhafiz, A.H.; Loo, B.E.; Hensey, N.; Bailey, C.; Sinclair, A. The U-Shaped Relationship of Traditional Cardiovascular Risk Factors and Adverse Outcomes in Later Life. Aging Dis 2012, 3, 454–464. [Google Scholar]
- Leiherer, A.; Ulmer, H.; Muendlein, A.; Saely, C.H.; Vonbank, A.; Fraunberger, P.; Foeger, B.; Brandtner, E.M.; Brozek, W.; Nagel, G.; et al. Value of Total Cholesterol Readings Earlier versus Later in Life to Predict Cardiovascular Risk. EBioMedicine 2021, 67, 103371. [Google Scholar] [CrossRef]
- Summers, S.A. Could Ceramides Become the New Cholesterol? Cell Metab 2018, 27, 276–280. [Google Scholar] [CrossRef]
- Tippetts, T.S.; Holland, W.L.; Summers, S.A. Cholesterol – the Devil You Know; Ceramide – the Devil You Don’t. Trends Pharmacol Sci 2021, 42, 1082–1095. [Google Scholar] [CrossRef]
- Norris, G.H.; Blesso, C.N. Dietary and Endogenous Sphingolipid Metabolism in Chronic Inflammation. Nutrients 2017, Vol. 9, Page 1180 2017, 9, 1180. [Google Scholar] [CrossRef]
- Luukkonen, P.K.; Sädevirta, S.; Zhou, Y.; Kayser, B.; Ali, A.; Ahonen, L.; Lallukka, S.; Pelloux, V.; Gaggini, M.; Jian, C.; et al. Saturated Fat Is More Metabolically Harmful for the Human Liver Than Unsaturated Fat or Simple Sugars. Diabetes Care 2018, 41, 1732–1739. [Google Scholar] [CrossRef]
- Wang, D.D.; Toledo, E.; Hruby, A.; Rosner, B.A.; Willett, W.C.; Sun, Q.; Razquin, C.; Zheng, Y.; Ruiz-Canela, M.; Guasch-Ferré, M.; et al. Plasma Ceramides, Mediterranean Diet, and Incident Cardiovascular Disease in the PREDIMED Trial. Circulation 2017, 135, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- Mathews, A.T.; Famodu, O.A.; Olfert, M.D.; Murray, P.J.; Cuff, C.F.; Downes, M.T.; Haughey, N.J.; Colby, S.E.; Chantler, P.D.; Olfert, I.M.; et al. Efficacy of Nutritional Interventions to Lower Circulating Ceramides in Young Adults: FRUVEDomic Pilot Study. Physiol Rep 2017, 5. [Google Scholar] [CrossRef]
- Hilvo, M.; Vasile, V.C.; Donato, L.J.; Hurme, R.; Laaksonen, R. Ceramides and Ceramide Scores: Clinical Applications for Cardiometabolic Risk Stratification. Front Endocrinol (Lausanne) 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Kitatani, K.; Idkowiak-Baldys, J.; Hannun, Y.A. The Sphingolipid Salvage Pathway in Ceramide Metabolism and Signaling. Cell Signal 2008, 20, 1010–1018. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Principles of Bioactive Lipid Signalling: Lessons from Sphingolipids. Nature Reviews Molecular Cell Biology 2008 9:2 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Ng, T.W.K.; Ooi, E.M.M.; Watts, G.F.; Chan, D.C.; Weir, J.M.; Meikle, P.J.; Barrett, P.H.R. Dose-Dependent Effects of Rosuvastatin on the Plasma Sphingolipidome and Phospholipidome in the Metabolic Syndrome. J Clin Endocrinol Metab 2014, 99, E2335–E2340. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Niccoli, G. Ezetimibe and Plaque Regression: Cholesterol Lowering or Pleiotropic Effects? J Am Coll Cardiol 2015, 66, 508–510. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.B.; Fink, C.S.; Trautwein, E.A.; Ntanios, F.Y. β-Sitosterol Stimulates Ceramide Metabolism in Differentiated Caco2 Cells. Journal of Nutritional Biochemistry 2005, 16, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Croyal, M.; Kaabia, Z.; León, L.; Ramin-Mangata, S.; Baty, T.; Fall, F.; Billon-Crossouard, S.; Aguesse, A.; Hollstein, T.; Sullivan, D.R.; et al. Fenofibrate Decreases Plasma Ceramide in Type 2 Diabetes Patients: A Novel Marker of CVD? Diabetes Metab 2018, 44, 143–149. [Google Scholar] [CrossRef]
- Drexel, H.; Saely, C.H.; Agewall, S. Fibrates: One More Lost Paradise in Lipid Treatment. Eur Heart J Cardiovasc Pharmacother 2023, 9, 121. [Google Scholar] [CrossRef]
- Song, J.-H.; Kim, G.-T.; Park, K.-H.; Park, W.-J.; Park, T.-S. Bioactive Sphingolipids as Major Regulators of Coronary Artery Disease. Biomol Ther (Seoul) 2021. [Google Scholar] [CrossRef]
- Eurostat Mortality and Life Expectancy Statistics - Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Mortality_and_life_expectancy_statistics (accessed on 30 March 2023).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).