Submitted:
27 July 2023
Posted:
28 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
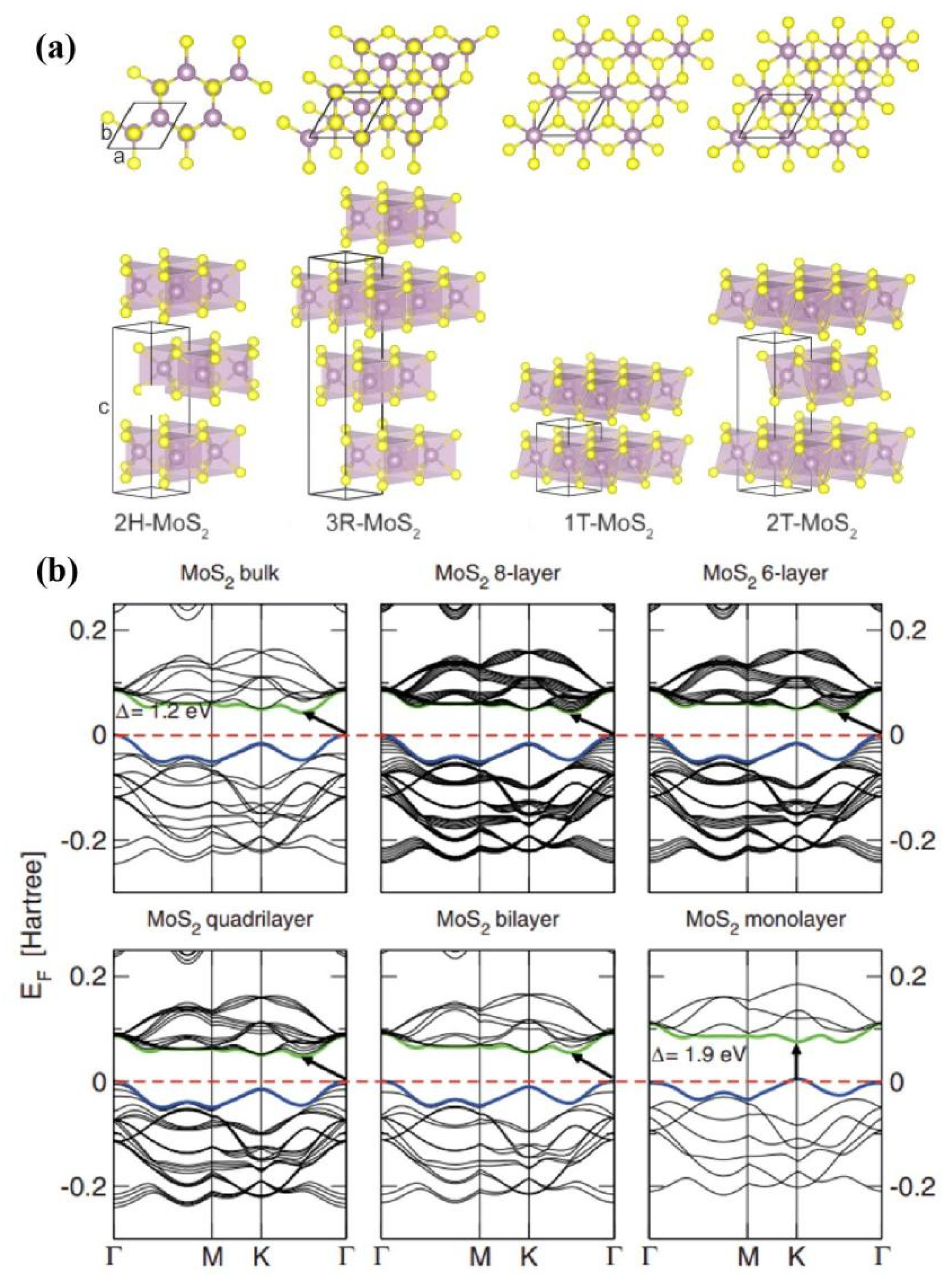
2. Synthesis and characteristics of molybdenum disulfide
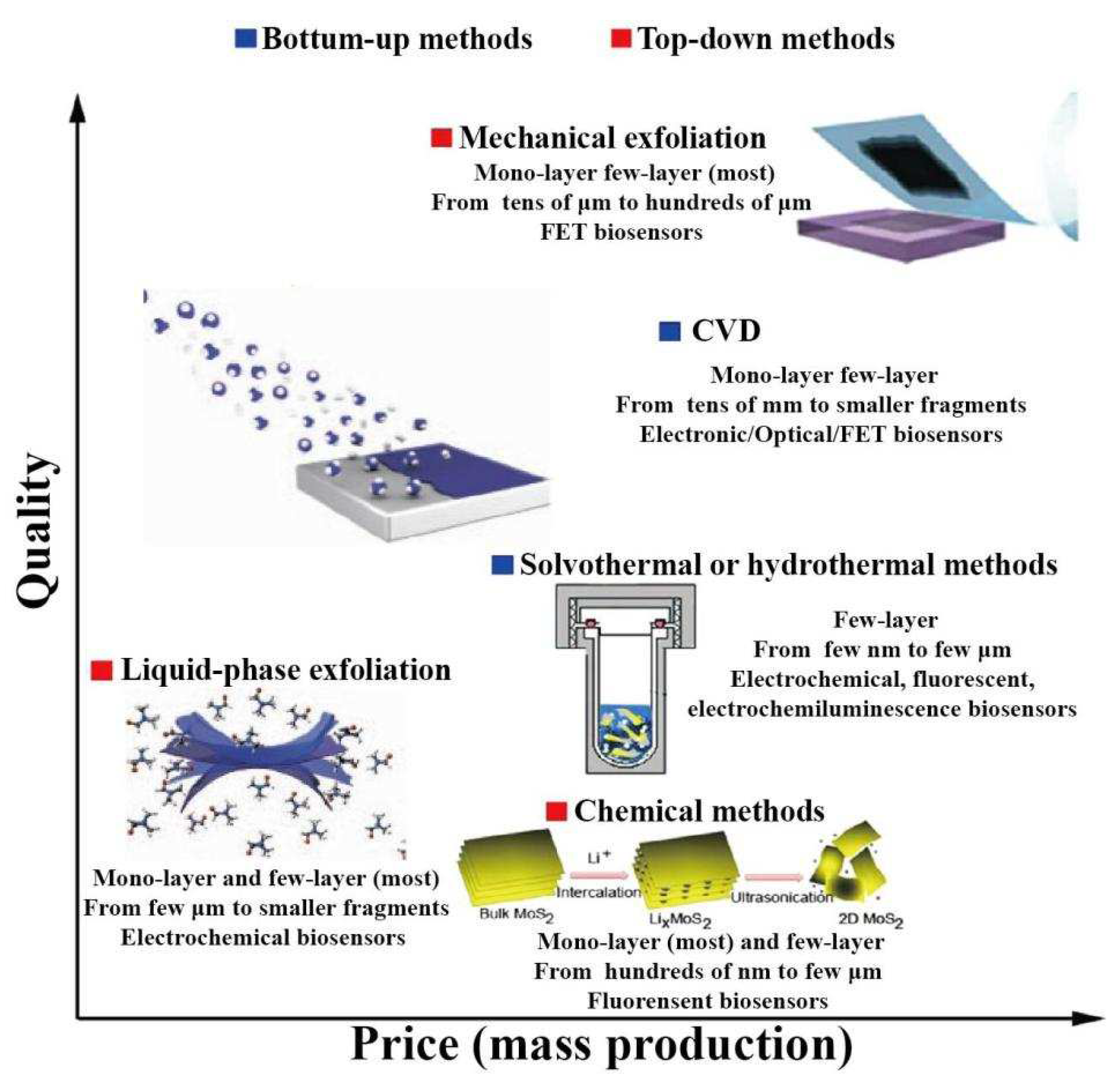
2.1. Top-down methods
2.2. Bottom-up methods
3. Electrochemical biosensors for cancer biomarkers detection based on MoS2
3.1. Potentiometry
3.2. Amperometry
3.3. Impedimetry
3.4. Photoelectrochemistry (PEC)
4. Optical biosensors for cancer biomarkers detection based on MoS2
4.1. Colorimetry
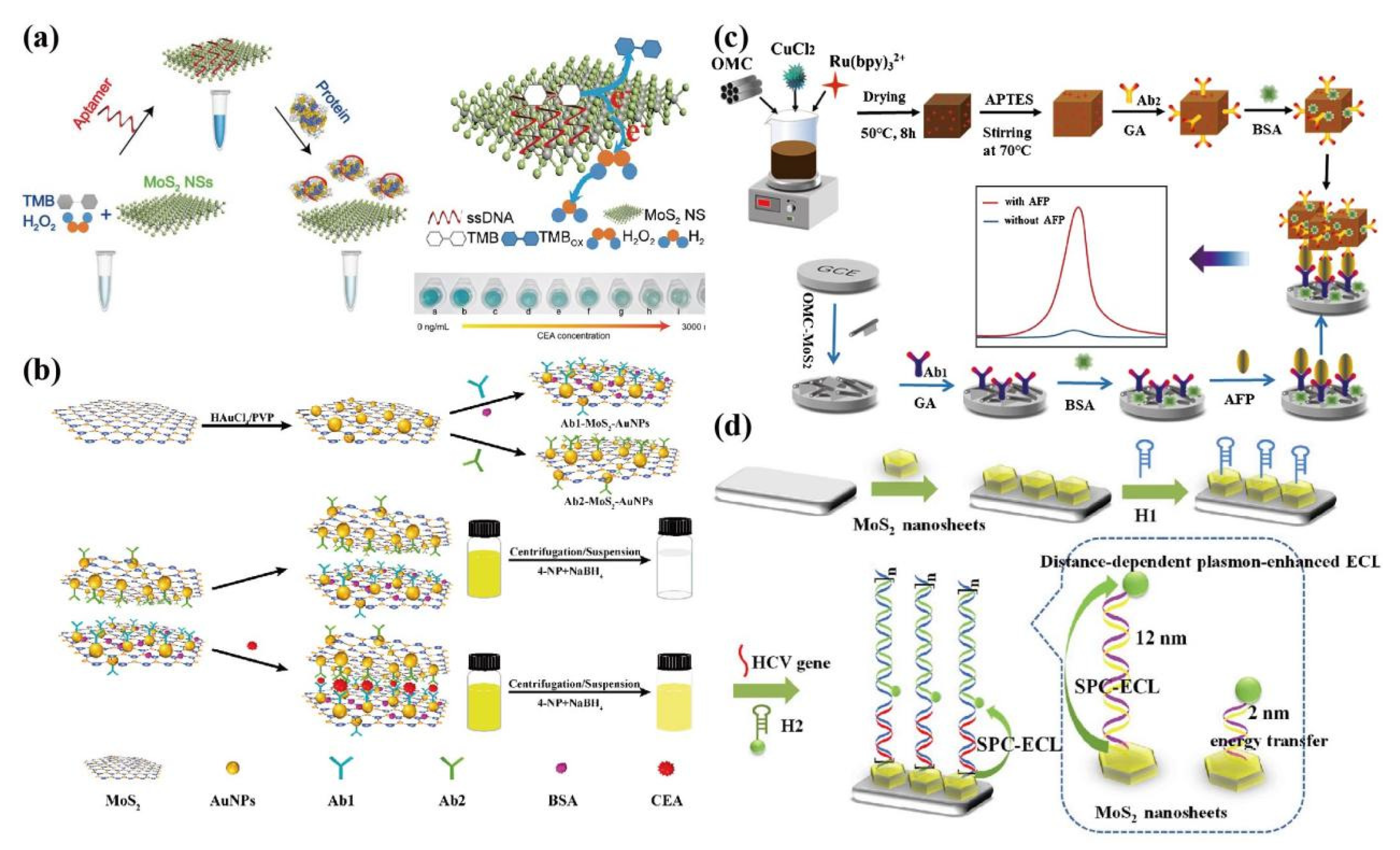
4.2. ECL
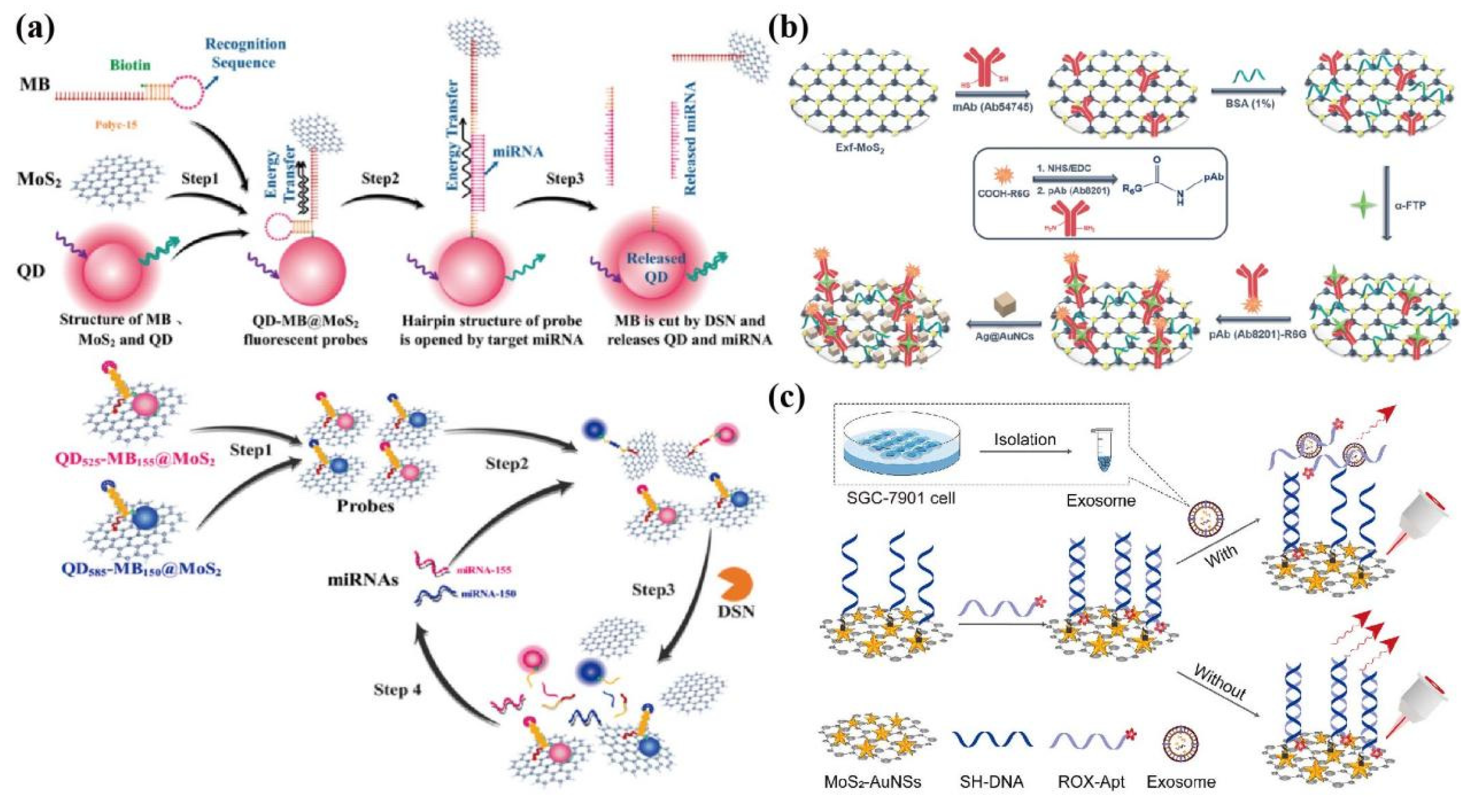
4.3. Fluorescence
4.4. SERS
4.5. SPR
5. Miscellaneous biosensors for cancer biomarkers detection based on MoS2
6. Discussion and Outlook
7. Conclusion
Author Contributions
Funding
Abbreviations
References
- Jayanthi, V.S.; Das, A.B.; Saxena, U. Recent advances in biosensor development for the detection of cancer biomarkers. Biosensors and Bioelectronics 2017, 91, 15–23. [Google Scholar] [CrossRef]
- Hasan, M.R.; Ahommed, M.S.; Daizy, M.; Bacchu, M.S.; Ali, M.R.; Al-Mamun, M.R.; Aly Saad Aly, M.; Khan, M.Z.H.; Hossain, S.I. Recent development in electrochemical biosensors for cancer biomarkers detection. Biosensors and Bioelectronics: X 2021, 8, 100075. [Google Scholar] [CrossRef]
- Rabie, A.M.I.; Ali, A.S.M.; Al-Zeer, M.A.; Barhoum, A.; EL-Hallouty, S.; Shousha, W.G.; Berg, J.; Kurreck, J.; Khalil, A.S.G. Spontaneous Formation of 3D Breast Cancer Tissues on Electrospun Chitosan/Poly(ethylene oxide) Nanofibrous Scaffolds. ACS Omega 2022, 7(2), 2114–2126. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, R.; Esimbekova, E.N.; Kratasyuk, V.A. Rapid biosensing tools for cancer biomarkers. Biosensors and Bioelectronics 2017, 87, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Henry, N.L.; Hayes, D.F. Cancer biomarkers. Mol Oncol 2012, 6(2), 140–146. [Google Scholar] [CrossRef]
- Sharifi, M.; Avadi, M.R.; Attar, F.; Dashtestani, F.; Ghorchian, H.; Rezayat, S.M.; Saboury, A.A; Falahati, M. Cancer diagnosis using nanomaterials based electrochemical nanobiosensors. Biosensors and Bioelectronics 2019, 126, 773–784. [Google Scholar] [CrossRef]
- Agnolon, V.; Contato, A.; Meneghello, A.; Tagliabue, E.; Toffoli, G.; Gion, M.; Polo, F.; Fabricio, A.S.C. ELISA assay employing epitope-specific monoclonal antibodies to quantify circulating HER2 with potential application in monitoring cancer patients undergoing therapy with trastuzumab. Sci Rep 2020, 10(1), 3016. [Google Scholar] [CrossRef]
- Dueck, M.E.; Lin, R.; Zayac, A.; Gallagher, S.; Chao, A.K.; Jiang, L.X.; Datwani, S.S.; Hung, P.; Stieglitz, E. Precision cancer monitoring using a novel, fully integrated, microfluidic array partitioning digital PCR platform. Sci Rep 2019, 9, 19606. [Google Scholar] [CrossRef]
- Selvakumar, S.C.; Preethi, K.A.; Ross, K.; Tusubira, D.; Khan, M.W.A.; Mani, P.; Rao, T.N.; Sekar, D. CRISPR/Cas9 and next generation sequencing in the personalized treatment of Cancer. Mol Cancer 2022, 21(1), 83. [Google Scholar] [CrossRef]
- Udo, R.; Katsumata, K.; Kuwabara, H.; Enomoto, M.; Ishizaki, T.; Sunamura, M.; Nagakawa, Y.; Soya, R.; Sugimoto, M.; Tsuchida, A. Urinary charged metabolite profiling of colorectal cancer using capillary electrophoresis-mass spectrometry. Sci Rep 2020, 10(1), 21057. [Google Scholar] [CrossRef]
- Barhoum, A.; Altintas, Z.; Shalini Devi, K.S.; Forster, R.J. Electrochemiluminescence biosensors for detection of cancer biomarkers in biofluids: Principles, opportunities, and challenges. Nano Today 2023, 50, 101874. [Google Scholar] [CrossRef]
- Yang, G.J.; Xiao, Z.Q.; Tang, C.L.; Deng, Y.; Huang, H.; He, Z.Y. Recent advances in biosensor for detection of lung cancer biomarkers. Biosensors and Bioelectronics 2019, 141, 111416. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: recommended definitions and classification. Biosens Bioelectron 2001, 16(1-2), 121–131. [Google Scholar] [CrossRef]
- Choi, S.; Goryll, M.; Sin, L.Y.M.; Wong, P.K.; Chae, J. Microfluidic-based biosensors toward point-of-care detection of nucleic acids and proteins. Microfluid Nanofluidics 2011, 10(2), 231–247. [Google Scholar] [CrossRef] [PubMed]
- Kukkar, M.; Tuteja, S.K.; Sharma, A.L.; Kumar, V.; Paul, A.K.; Kim, K.-H.; Sabherwal, P.; Deep, A. A New Electrolytic Synthesis Method for Few-Layered MoS2 Nanosheets and Their Robust Biointerfacing with Reduced Antibodies. ACS Applied Materials & Interfaces 2016, 8(26), 16555–16563. [Google Scholar] [CrossRef]
- Turner, A.P. Biosensors: sense and sensibility. Chem Soc Rev. 2013, 42(8), 3184–3196. [Google Scholar] [CrossRef] [PubMed]
- Vaddiraju, S.; Legassey, A.; Wang, Y.; Qiang, L.; Burgess, D.J.; Jain, F. Papadimitrakopoulos F. Design and fabrication of a high-performance electrochemical glucose sensor. J Diabetes Sci Technol 2011, 5(5), 1044–1051. [Google Scholar] [CrossRef]
- Bozal-Palabiyik, B.; Uslu, B.; Marrazza, G. Chapter 11 - Nanosensors in Biomarker Detection. In New Developments in Nanosensors for Pharmaceutical Analysis; Academic Press, 2019; pp. 327–380. [Google Scholar] [CrossRef]
- Kausar, M. Pesticidal activity of Pakistani Bacillus thuringiensis isolates against Helicoverpa armigera (Hubner) and Earias vittella (Lepidoptera: Noctuidae). IOSR Journal of Pharmacy and Biological Sciences 2013, 4, 9–12. [Google Scholar] [CrossRef]
- Kaya, S.I.; Ozcelikay, G.; Mollarasouli, F.; Bakirhan, N.K.; Ozkan, S.A. Recent achievements and challenges on nanomaterial based electrochemical biosensors for the detection of colon and lung cancer biomarkers. Sensors and Actuators B: Chemical 2022, 351, 130856. [Google Scholar] [CrossRef]
- Nawz, T.; Safdar, A.; Hussain, M.; Sung Lee, D.; Siyar, M. Graphene to Advanced MoS2: A Review of Structure, Synthesis, and Optoelectronic Device Application. Crystals 2020, 10, 902. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: synthesis, properties, and applications. Adv Mater 2010, 22(35), 3906–3924. [Google Scholar] [CrossRef]
- Gan, X.R.; Zhao, H.M.; Quan, X. Two-dimensional MoS2: A promising building block for biosensors. Biosensors and Bioelectronics 2017, 89, 56–71. [Google Scholar] [CrossRef]
- Samadi, M.; Sarikhani, N.; Zirak, M.; Zhang, H.; Zhang, H.L.; Moshfegh, A.Z. Group 6 transition metal dichalcogenide nanomaterials: synthesis, applications and future perspectives. Nanoscale Horiz 2018, 3(2), 90–204. [Google Scholar] [CrossRef] [PubMed]
- Joswig, J.O.; Lorenz, T.; Wendumu, T.B.; Gemming, S.; Seifert, G. Optics, mechanics, and energetics of two-dimensional MoS2 nanostructures from a theoretical perspective. Acc Chem Res 2015, 48(1), 48–55. [Google Scholar] [CrossRef] [PubMed]
- Ataca, C.; Şahin, H.; Ciraci, S. Stable, Single-Layer MX2 Transition-Metal Oxides and Dichalcogenides in a Honeycomb-Like Structure. The Journal of Physical Chemistry C 2012, 116(16), 8983–8999. [Google Scholar] [CrossRef]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous lattice vibrations of single- and few-layer MoS2. ACS Nano 2010, 4(5), 2695–2700. [Google Scholar] [CrossRef]
- Huang, Y.H.; Peng, C.C.; Chen, R.S.; Huang, Y.S.; Ho, C.H. Transport properties in semiconducting NbS2 nanoflakes. Appl. Phys. Lett. 2014, 105(9), 093106. [Google Scholar] [CrossRef]
- Chen, F.; Xia, J.; Ferry, D.K.; Tao, N. Dielectric screening enhanced performance in graphene FET. Nano Lett. 2009, 9(7), 2571–2574. [Google Scholar] [CrossRef]
- Kalantar-zadeh, K.; Ou, J.Z. Biosensors Based on Two-Dimensional MoS2. ACS Sensors 2016, 1(1), 5–16. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, P.; Su, Z.; Wei, G. Synthesis and sensor applications of MoS2-based nanocomposites. Nanoscale 2015, 7(44), 18364–18378. [Google Scholar] [CrossRef]
- Krishnan, U.; Kaur, M.; Singh, K.; Kumar, M.; Kumar, A. A synoptic review of MoS2: Synthesis to applications. Superlattices and Microstructures 2019, 128, 274–297. [Google Scholar] [CrossRef]
- Andrey, N.; Gotthard Seifert, E. Density-functional study of LixMoS2 intercalates (0⩽x⩽1). Computational and Theoretical Chemistry 2012, 999, 13–20. [Google Scholar] [CrossRef]
- Mortazavi, M.; Wang, C.; Deng, J.K.; Shenoy, V.B.; Medhekar, N.V. Ab initio characterization of layered MoS2 as anode for sodium-ion batteries. Journal of Power Sources 2014, 268, 279–286. [Google Scholar] [CrossRef]
- Zhao, W.; Pan, J.; Fang, Y.; Che, X.; Wang, D.; Bu, K.; Huang, F. Metastable MoS2 : Crystal Structure, Electronic Band Structure, Synthetic Approach and Intriguing Physical Properties. Chemistry 2018, 24(60), 15942–15954. [Google Scholar] [CrossRef]
- Shi, S.L.; Sun, Z.X.; Hu, Y.H. Synthesis, stabilization and applications of 2-dimensional 1T metallic MoS2. Journal of Materials Chemistry A 2018, 6, 47. [Google Scholar] [CrossRef]
- Sha, R.; Bhattacharyya, T.K. MoS2-based nanosensors in biomedical and environmental monitoring applications. Electrochimica Acta 2020, 349, 136370. [Google Scholar] [CrossRef]
- Tang, H.; Morrison, S.R. Optimization of the anisotropy of composite MoS2 films. Thin Solid Films 1993, 227(1), 90–94. [Google Scholar] [CrossRef]
- Sundaram, R.S.; Engel, M.; Lombardo, A.; Krupke, R.; Ferrari, A.C.; Avouris, P.; Steiner, M. Electroluminescence in single layer MoS2. Nano Lett. 2013, 13(4), 1416–1421. [Google Scholar] [CrossRef]
- Kuc, A.; Zibouche, N.; Heine, T. Influence of quantum confinement on the electronic structure of the transition metal sulfide. Phys. Rev. B 2011, 83, 245213. [Google Scholar] [CrossRef]
- Zhang, X.; Biekert, N.; Choi, S.; Naylor, C.H.; De-Eknamkul, C.; Huang, W.; Zhang, X.; Zheng, X.; Wang, D.; Johnson, A.T.C.; et al. Dynamic Photochemical and Optoelectronic Control of Photonic Fano Resonances via Monolayer MoS2 Trions. Nano Lett 2018, 18(2), 957–963. [Google Scholar] [CrossRef]
- Nalwa, H.S. A review of molybdenum disulfide (MoS2) based photodetectors: from ultra-broadband, self-powered to flexible devices. RSC Adv. 2020, 10(51), 30529–30602. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.P.; Gaharwar, A.K. Light-Responsive Inorganic Biomaterials for Biomedical Applications. Adv Sci (Weinh). 2020, 7(17), 2000863. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, D.; Damien, D.; Shaijumon, M.M. MoS2 quantum dot-interspersed exfoliated MoS2 nanosheets. ACS Nano. 2014, 8(5), 5297–303. [Google Scholar] [CrossRef] [PubMed]
- Nath, M.; Govindaraj, A.; Rao, C. Simple Synthesis of MoS2 and WS2 Nanotubes. Advanced Materials. 2001, 13, 283–286. [Google Scholar] [CrossRef]
- Lee, H.P.; Lokhande, G.; Singh, K.A.; Jaiswal, M.K.; Rajput, S.; Gaharwar, A.K. Light-Triggered In Situ Gelation of Hydrogels using 2D Molybdenum Disulfide (MoS2) Nanoassemblies as Crosslink Epicenter. Adv Mater. 2021, 33(23), e2101238. [Google Scholar] [CrossRef]
- Shounak, R.; Kaivalya, A.D.; Singh, K.A.; Lee, H.P.; Jaiswal, A.; Gaharwar, A.K. Nano-bio interactions of 2D molybdenum disulfide. Advanced Drug Delivery Reviews 2022, 187, 114361. [Google Scholar] [CrossRef]
- Najmaei, S.; Yuan, J.; Zhang, J.; Ajayan, P.; Lou, J. Synthesis and defect investigation of two-dimensional molybdenum disulfide atomic layers. Acc Chem Res. 2015, 48(1), 31–40. [Google Scholar] [CrossRef]
- Novoselov, V, K.S.; Fal’ko, L.; Colombo, I.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [CrossRef]
- Magda, G.Z.; Pető, J.; Dobrik, G.; Hwang, C.; Biró, L.P.; Tapasztó, L. Exfoliation of large-area transition metal chalcogenide single layers. Sci Rep. 2015, 5, 14714. [Google Scholar] [CrossRef]
- Matte, H.R.; Gomathi, A.; Manna, A.K.; Late, D.J.; Datta, R.; Pati, S.K.; Rao, C.N. MoS2 and WS2 analogues of graphene. Angewandte Chemie 2010, 49, 4059–4062. [Google Scholar] [CrossRef]
- Varrla, E.; Backes, C.; Paton, K.R.; Harvey, A.; Gholamvand, Z.; McCauley, J.; Coleman, J.N. Chemistry of Materials 2015, 27(3), 1129–1139. [CrossRef]
- Forsberg, V.; Zhang, R.; Bäckström, J.; Dahlström, C.; Andres, B.; Norgren, M.; Andersson, M.; Hummelgård, M.; Olin, H. Exfoliated MoS2 in Water without Additives. PLoS One. 2016, 11(4), e0154522. [Google Scholar] [CrossRef] [PubMed]
- Varrla, E.; Backes, C.; Paton, K.R.; Harvey, A.; Gholamvand, Z.; McCauley, J.; Coleman, J.N. Large-Scale Production of Size-Controlled MoS2 Nanosheets by Shear Exfoliation. Chemistry of Materials 2015, 27(3), 1129–1139. [Google Scholar] [CrossRef]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nature Mater 2014, 13, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Howe, R.C.T.; Woodward, R.I.; Kelleher, E.J.R.; Torrisi, F.; Hu, G.; Popov, S.V.; Taylor, J.R.; Hasan, T. Solution processed MoS2-PVA composite for sub-bandgap mode-locking of a wideband tunable ultrafast Er:fiber laser. Nano Res. 2015, 8, 1522–1534. [Google Scholar] [CrossRef]
- Liu, N.; Kim, P.; Kim, J.H.; Ye, J.H.; Kim, S.; Lee, C.J. Large-area atomically thin MoS2 nanosheets prepared using electrochemical exfoliation. ACS Nano. 2014, 8(7), 6902–6910. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.N.; Lotya, M.; O'Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science. 2011, 331(6017), 568–571. [Google Scholar] [CrossRef]
- Eda, G.; Yamaguchi, H.; Voiry, D.; Fujita, T.; Chen, M.; Chhowalla, M. Photoluminescence from chemically exfoliated MoS2. Nano Lett. 2011, 11(12), 5111–5116. [Google Scholar] [CrossRef]
- Fan, X.B.; Xu, P.T.; Zhou, D.; Sun, Y.F.; Li, Y.G.C.; Nguyen, M.A.T.; Terrones, M.; Mallouk, T.E. Fast and Efficient Preparation of Exfoliated 2H MoS2 Nanosheets by Sonication-Assisted Lithium Intercalation and Infrared Laser-Induced 1T to 2H Phase Reversion. Nano Letters 2015, 15(9), 5956–5960. [Google Scholar] [CrossRef]
- Zeng, Z.; Yin, Z.; Huang, X.; Li, H.; He, Q.; Lu, G.; Boey, F.; Zhang, H. Single-Layer Semiconducting Nanosheets: High-Yield Preparation and Device Fabrication. Angew. Chem. Int. Ed. 2011, 50, 11093–11097. [Google Scholar] [CrossRef]
- Shi, Y.M.; Li, H.N.; Li, L.J. Recent advances in controlled synthesis of two-dimensional transition metal dichalcogenides via vapour deposition techniques. Chemical Society Reviews 2015, 44, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Liu, Z.; Najmaei, S.; Ajayan, P.M.; Lou, J. Large-area vapor-phase growth and characterization of MoS(2) atomic layers on a SiO(2) substrate. Small. 2012, 8(7), 966–971. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Zhang, W.J.; Huang, J.K.; Liu, K.K.; Lee, Y.H.; Liang, C.T.; Chu, C.W.; Li, L.J. Wafer-scale MoS2 thin layers prepared by MoO3 sulfurization. Nanoscale 2012, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Endler, I.; Leonhardt, A.; König, U.; van den Berg, H.; Pitschke, W.; Sottke, V. Chemical vapour deposition of MoS2 coatings using the precursors MoCl5 and H2S. Surface and Coatings Technology 1999, 120-121, 482–488. [Google Scholar] [CrossRef]
- Liu, H.F.; Wong, S.L.; Chi, D.Z. CVD Growth of MoS2-based Two-dimensional Materials. Chem. Vap. Deposition 2015, 21, 241–259. [Google Scholar] [CrossRef]
- Vattikuti, S.V.P.; Byon, C.; Reddy, C.V.; Venkatesh, B.; Shim, J. Synthesis and structural characterization of MoS2 nanospheres and nanosheets using solvothermal method. J Mater Sci 2015, 50, 5024–5038. [Google Scholar] [CrossRef]
- Liao, H.W.; Wang, Y.F.; Zhang, S.Y.; Qian, Y.T. A Solution Low-Temperature Route to MoS2 Fiber. Chemistry of Materials 2001, 13, 6–8. [Google Scholar] [CrossRef]
- Jeong, S.; Yoo, D.; Jang, J.; Kim, M.Y.; Cheon, J. Well-Defined Colloidal 2-D Layered Transition-Metal Chalcogenide Nanocrystals via Generalized Synthetic Protocols. Journal of the American Chemical Society 2012, 134(44), 18233–18236. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Q.; Du, X.; Liu, M. Application of electrochemical biosensors in tumor cell detection. Thorac Cancer. 2020, 11(4), 840–850. [Google Scholar] [CrossRef]
- Chai, H.; Tang, Y.G.; Guo, Z.Z.; Miao, P. Ratiometric Electrochemical Switch for Circulating Tumor DNA through Recycling Activation of Blocked DNAzymes. Analytical Chemistry 2022, 94(6), 2779–2784. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Torad, N.L.; Xia, W.; Aslam, M.A.; Kaneti, Y.V.; Hou, Z.; Ding, Z.; Da, B.; Fatehmulla, A.; et al. Rational Design of Nanoporous MoS2/VS2 Heteroarchitecture for Ultrahigh Performance Ammonia Sensors. Small. 2020, 16(12), e1901718. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Feng, L.; Ji, D.72; Zhang, Y.; Chen, W.; Dai, Y.; Janyasupab, M.; Li, X.; Wen, W.; Liu, C.C. Phase-Regulated Sensing Mechanism of MoS2 Based Nanohybrids toward Point-of-Care Prostate Cancer Diagnosis. Small. 2020, 16(18), e2000307. [Google Scholar] [CrossRef]
- Li, F.; Zhang, L.; Li, J.; Lin, X.Q.; Li, X.Z.; Fang, Y.Y.; Huang, J.W.; Li, W.Z.; Tian, M.; Jin, J.; Li, R. Synthesis of Cu–MoS2/rGO hybrid as non-noble metal electrocatalysts for the hydrogen evolution reaction. Journal of Power Sources 2015, 292, 15–22. [Google Scholar] [CrossRef]
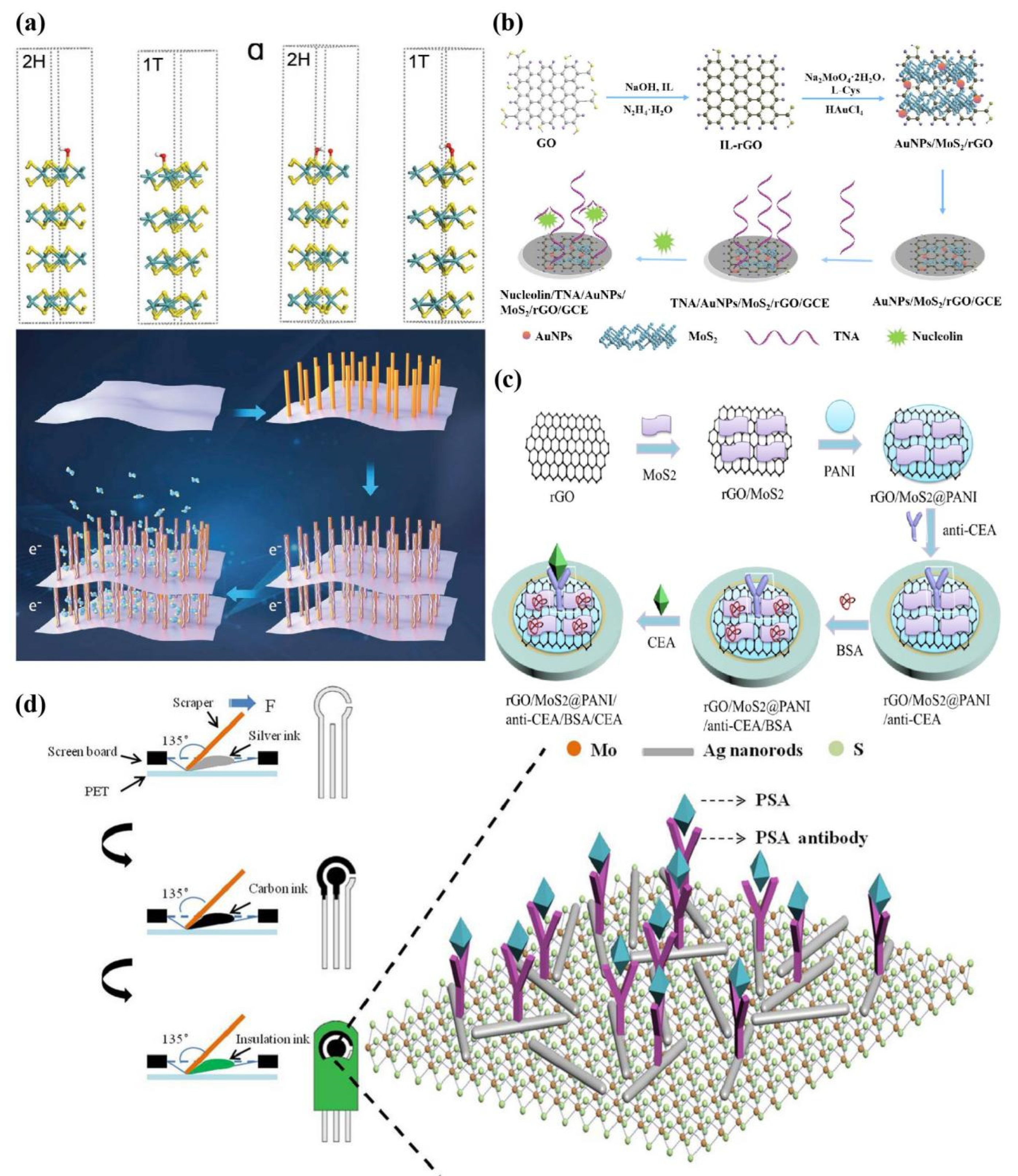
- Su, X.J.; Han, Y.J.; Liu, Z.G.; Fan, L.F.; Guo, Y.J. One-pot synthesized AuNPs/MoS2/rGO nanocomposite as sensitive electrochemical aptasensing platform for nucleolin detection. Journal of Electroanalytical Chemistry 2020, 859, 113868. [Google Scholar] [CrossRef]
- Jing, P.; Yi, H.; Xue, S.; Chai, Y.; Yuan, R.; Xu, W. A sensitive electrochemical aptasensor based on palladium nanoparticles decorated graphene-molybdenum disulfide flower-like nanocomposites and enzymatic signal amplification. Anal Chim Acta. 2015, 853, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.J.; Cao, K.H.; Li, W.J.; Ma, C.Y.; Qiao, X.W.; Li, H.L.; Hong, C.G. Optimal film thickness of rGO/MoS2 @ polyaniline nanosheets of 3D arrays for carcinoembryonic antigen high sensitivity detection. Microchemical Journal 2020, 155, 104694. [Google Scholar] [CrossRef]
- Gui, J.C.; Han, L.; Du, C.X.; Yu, X.N.; Hu, K.; Li, L.H. An efficient label-free immunosensor based on ce-MoS2/AgNR composites and screen-printed electrodes for PSA detection. J Solid State Electrochem 2021, 25, 973–982. [Google Scholar] [CrossRef]
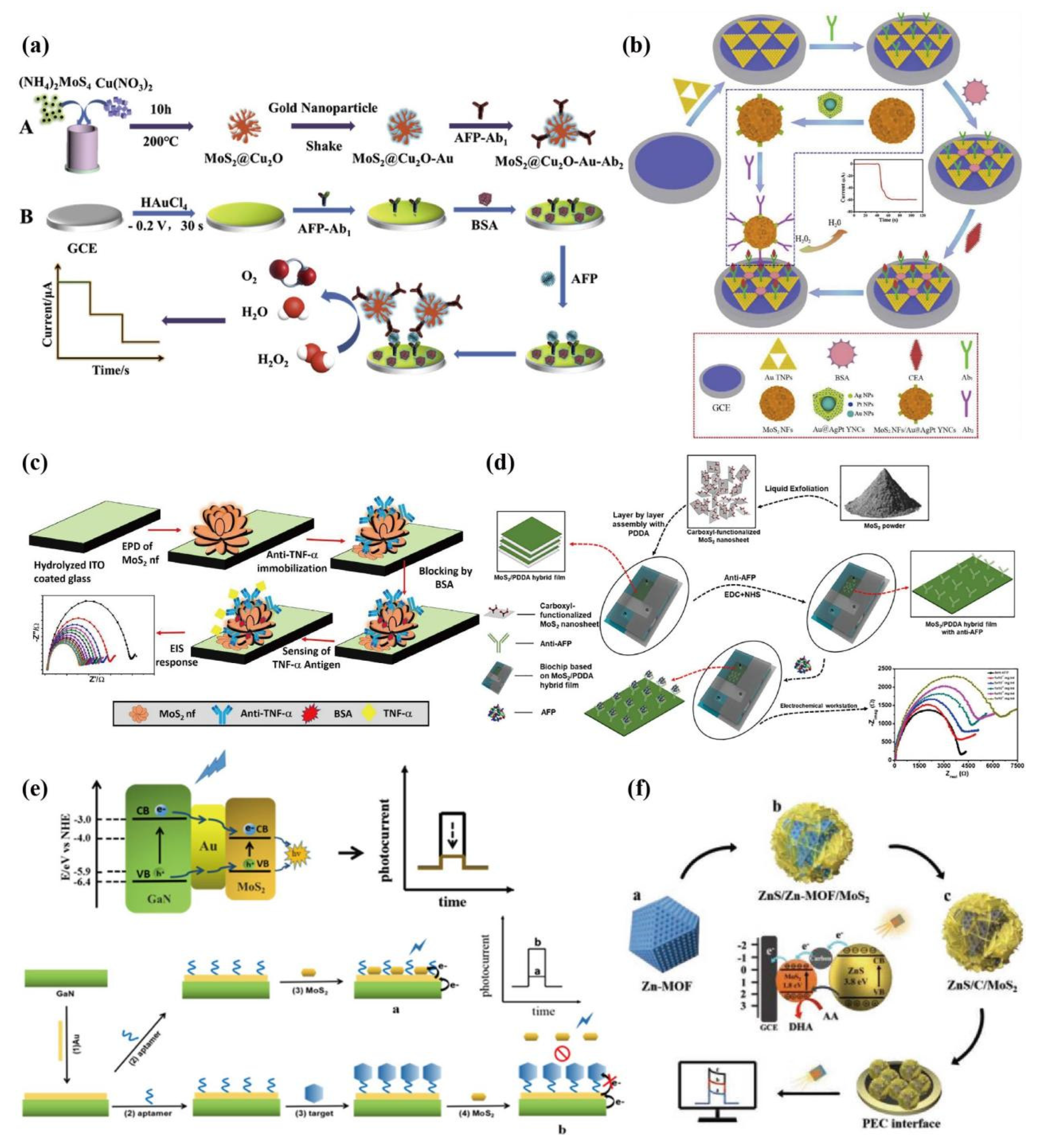
- Ma, N.; Zhang, T.; Fan, D.W.; Kuang, X.; Ali, A.; Wu, D.; Wei, Q. Triple amplified ultrasensitive electrochemical immunosensor for alpha fetoprotein detection based on MoS2@Cu2O-Au nanoparticles. Sensors and Actuators B: Chemical 2019, 297, 126821. [Google Scholar] [CrossRef]
- Ma, E.; Wang, P.; Yang, Q.S.; Yu, H.X.; Pei, F.B.; Li, Y.Y.; Liu, Q.; Dong, Y.H. Electrochemical immunosensor based on MoS2 NFs/Au@AgPt YNCs as signal amplification label for sensitive detection of CEA. Biosensors and Bioelectronics 2019, 142, 111580. [Google Scholar] [CrossRef]
- Jia, Q.J.; Huang, S.J.; Hu, M.Y.; Song, Y.P.; Wang, M.H.; Zhang, Z.H.; He, L.H. Polyoxometalate-derived MoS2 nanosheets embedded around iron-hydroxide nanorods as the platform for sensitively determining miRNA-21. Sensors and Actuators B: Chemical 2020, 323, 128647. [Google Scholar] [CrossRef]
- Sri, S.; Chauhan, D.; Lakshmi, G.B.V.S.; Thakar, A.; Solanki, P.R. MoS2 nanoflower based electrochemical biosensor for TNF alpha detection in cancer patients. Electrochimica Acta 2022, 405, 139736. [Google Scholar] [CrossRef]
- Hu, T.; Zhang, M.; Wang, Z.; Chen, K.; Li, X.; Ni, Z.H. Layer-by-layer self-assembly of MoS2/PDDA hybrid film in microfluidic chips for ultrasensitive electrochemical immunosensing of alpha-fetoprotein. Microchemical Journal 2020, 158, 105209. [Google Scholar] [CrossRef]
- Hu, D.; Cui, H.; Wang, X.; Luo, F.; Qiu, B.; Cai, W.; Huang, H.; Wang, J.; Lin, Z. Highly Sensitive and Selective Photoelectrochemical Aptasensors for Cancer Biomarkers Based on MoS2/Au/GaN Photoelectrodes. Anal Chem. 2021, 93(19), 7341–7347. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, C.; Li, P.; Wu, T.; Yang, N.; Wang, X.; Wang, Y.; Li, C. MOF Photochemistry: ZnS/C/MoS2 Nanocomposite Derived from Metal–Organic Framework for High-Performance Photo-Electrochemical Immunosensing of Carcinoembryonic Antigen. Small 2019, 15, 1970257. [Google Scholar] [CrossRef]
- Su, S.; Han, X.Y.; Lu, Z.W.; Liu, W.; Zhu, D.; Chao, J.; Fan, C.; Wang, L.H.; Song, S.P.; Weng, L.; et al. Facile Synthesis of a MoS2-Prussian Blue Nanocube Nanohybrid-Based Electrochemical Sensing Platform for Hydrogen Peroxide and Carcinoembryonic Antigen Detection. ACS Applied Materials & Interfaces 2017, 9(14), 12773–12781. [Google Scholar] [CrossRef]
- Wang, Y.g.; Zhao, G.h.; Zhang, Y.; Pang, X.h.; Cao, W.; Du, B.; Wei, Q. Sandwich-type electrochemical immunosensor for CEA detection based on Ag/MoS2@Fe3O4 and an analogous ELISA method with total internal reflection microscopy. Sensors and Actuators B: Chemical 2018, 266, 561–569. [Google Scholar] [CrossRef]
- Liu, L.; Wei, Y; Jiao, S.; Zhu, S.Y.; Liu, X.L. A novel label-free strategy for the ultrasensitive miRNA-182 detection based on MoS2/Ti3C2 nanohybrids. Biosensors and Bioelectronics 2019, 137, 45–51. [Google Scholar] [CrossRef]
- Su, S.; Sun, Q.; Wan, L.; Gu, X.d.; Zhu, D.; Zhou, Y.; Chao, J.; Wang, L.H. Ultrasensitive analysis of carcinoembryonic antigen based on MoS2-based electrochemical immunosensor with triple signal amplification. Biosensors and Bioelectronics 2019, 140, 111353. [Google Scholar] [CrossRef]
- Lin, Y.; Xiong, C.; Shi, J.; Zhang, J.J.; Wang, X.H. Electrochemical immunosensor based on Pd@Pt/MoS2-Gr for the sensitive detection of CEA. J Solid State Electrochem 2021, 25, 2075–2085. [Google Scholar] [CrossRef]
- Li, S.; Hu, C.; Chen, C.; Zhang, J.w.; Bai, Y.C.; Tan, C.S.; Ni, G.J.; He, F.; Li, W.F.; Ming, D. Molybdenum Disulfide Supported on Metal-Organic Frameworks as an Ultrasensitive Layer for the Electrochemical Detection of the Ovarian Cancer Biomarker CA125. ACS Applied Bio Materials 2021, 4(7), 5494–5502. [Google Scholar] [CrossRef]
- Mehmandoust, M.; Karimi, F.; Erk, N. A zinc oxide nanorods/molybdenum disulfide nanosheets hybrid as a sensitive and reusable electrochemical sensor for determination of anti-retroviral agent indinavir. Chemosphere 2022, 300, 134430. [Google Scholar] [CrossRef]
- Li, W.J.; Qiao, X.W.; Hong, C.L.; Ma, C.Y.; Song, Y.J. A sandwich-type electrochemical immunosensor for detecting CEA based on CeO2-MoS2 absorbed Pb2+. Analytical Biochemistry 2020, 592, 113566. [Google Scholar] [CrossRef]
- Wang, Y.G.; Wang, Y.L.; Wu, D.; Ma, H.; Zhang, Y.; Fan, D.W.; Pang, X.H; Du, B.; Wei, Q. Label-free electrochemical immunosensor based on flower-like Ag/MoS2/rGO nanocomposites for ultrasensitive detection of carcinoembryonic antigen. Sensors and Actuators B: Chemical 2018, 255(1), 125–132. [Google Scholar] [CrossRef]
- Gao, Z.Q.; Li, Y.Y.; Zhang, X.B.; Feng, J.H.; Kong, L.; Wang, P.; Chen, Z.W.; Dong, Y.H.; Wei, Q. Ultrasensitive electrochemical immunosensor for quantitative detection of HBeAg using Au@Pd/MoS2@MWCNTs nanocomposite as enzyme-mimetic labels. Biosensors and Bioelectronics 2018, 102, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Pei, F.B.; Wang, P.; Ma, E.H.; Yang, Q.S.; Yu, H.X.; Liu, J.; Yin, H.H.; Li, Y.Y.; Liu, Q.; Dong, Y.H. A sensitive label-free immunosensor for alpha fetoprotein detection using platinum nanodendrites loaded on functional MoS2 hybridized polypyrrole nanotubes as signal amplifier. Journal of Electroanalytical Chemistry 2019, 835, 197–204. [Google Scholar] [CrossRef]
- Soni, A.; Pandey, C.M.; Pandey, M.K.; Sumana, G. Highly efficient Polyaniline-MoS2 hybrid nanostructures based biosensor for cancer biomarker detection. Analytica Chimica Acta 2019, 1055, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, T.; Wang, X.; Wei, Q.; Wang, Y.Y.; Li, C.Y.; Sun, D. Ultrathin-layered carbon intercalated MoS2 hollow nanospheres integrated with gold nanoparticles for photoelectrochemical immunosensing of squamous cell carcinoma antigen. Sensors and Actuators B: Chemical 2019, 297, 126716. [Google Scholar] [CrossRef]
- Hou, S.N.; Wang, P.L.; Nie, Y.X.; Guo, Y.P.; Ma, Q. A novel work function tuning strategy-based ECL sensor with sulfur dots and Au NP@MoS2 nanosheet heterostructure for triple-negative breast cancer diagnosis. Chemical Engineering Journal 2022, 446(1), 136906. [Google Scholar] [CrossRef]
- Singh, A.K.; Mittal, S.; Das, M.; Saharia, A.; Tiwari, M. Optical biosensors: a decade in review. Alexandria Engineering Journal 2023, 67, 673–691. [Google Scholar] [CrossRef]
- Lv, Q.; Chen, L.S.; Liu, H.X.; Zou, L.L. Peony-like 3D-MoS2/graphene nanostructures with enhanced mimic peroxidase performance for colorimetric determination of dopamine. Talanta 2022, 247, 123553. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Y.; Wu, F.; Ni, Y.; Kokot, S. A colorimetric method of analysis for trace amounts of hydrogen peroxide with the use of the nano-properties of molybdenum disulfide. Analyst. 2015, 140(4), 1119–26. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.; Su, D.; Zhang, Y.; Lu, H.; Yan, X.; Bai, J.; Gao, Y.; Lu, G. The DNA controllable peroxidase mimetic activity of MoS2 nanosheets for constructing a robust colorimetric biosensor. Nanoscale 2020, 12(37), 19420–19428. [Google Scholar] [CrossRef]
- Su, S.; Li, J.; Yao, Y.; Sun, Q.; Zhao, Q.; Wang, F.; Li, Q.; Liu, X.; Wang, L. Colorimetric Analysis of Carcinoembryonic Antigen Using Highly Catalytic Gold Nanoparticles. ACS Applied bio Materials 2019, 2(1), 292–298. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, S.; Wang, X.; Wei, L.; Kong, Q.; Ye, M.; Luo, X.; Xu, J.; Zhang, C.; Xian, Y. pH-Sensitive Dye-Based Nanobioplatform for Colorimetric Detection of Heterogeneous Circulating Tumor Cells. ACS Sens 2021, 6(5), 1925–1932. [Google Scholar] [CrossRef]
- Ma, C.; Cao, Y.; Gou, X.; Zhu, J.J. Recent Progress in Electrochemiluminescence Sensing and Imaging. Anal Chem. 2020, 92(1), 431–454. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y. Recent Progress of Novel Electrochemiluminescence Nanoprobes and Their Analytical Applications. Front Chem. 2021, 8, 626243. [Google Scholar] [CrossRef]
- Lv, X.; Li, Y.; Cui, B.; Fang, Y.; Wang, L. Electrochemiluminescent sensor based on an aggregation-induced emission probe for bioanalytical detection. Analyst. 2022, 147(11), 2338–2354. [Google Scholar] [CrossRef]
- Du, L.; Zhang, H.; Wang, Z.; Zhuang, T.; Wang, Z. Boosting the electrochemiluminescence of luminol by high-intensity focused ultrasound pretreatment combined with 1T/2H MoS2 catalysis to construct a sensitive sensing platform. Ultrason Sonochem. 2023, 92, 106264. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, Z.; Tan, T.; Zhu, J.J. Recent Progress in Plasmonic based Electrochemiluminescence Biosensors: A Review. Biosensors (Basel) 2023, 13(2), 200. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, H.; Zhang, Y.; Wang, Z.; Gao, H.; Rong, S.; Meng, L.; Dai, J.; Pan, H.; Chang, D. A sandwich-configuration electrochemiluminescence immunoassay based on Cu2O@OMC-Ru nanocrystals and OMC-MoS2 nanocomposites for determination of alpha-fetoprotein. Mikrochim Acta. 2021, 188(6), 213. [Google Scholar] [CrossRef] [PubMed]
- Srinidhi, G.; Sudalaimani, S.; Giribabu, K.; Basha, S.J.S.; Suresh, C. Amperometric determination of hydrazine using a CuS-ordered mesoporous carbon electrode. Mikrochim Acta. 2020, 187(6), 359. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, Y.; Wang, M.; Zhang, Q.; Ma, Q. Distance-dependent plasmon-enhanced electrochemiluminescence biosensor based on MoS2 nanosheets. Biosens Bioelectron. 2020, 148, 111823. [Google Scholar] [CrossRef]
- Muhammad, M.; Khan, S.; Shehzadi, S.A.; Gul, Z.; Al-Saidi, H.M.; Kamran, A.W.; Alhumaydhi, F.A. Recent advances in colorimetric and fluorescent chemosensors based on thiourea derivatives for metallic cations: A review. Dyes and Pigments 2022, 205, 110477. [Google Scholar] [CrossRef]
- Yao, W.; Ling, J.; Zhang, W.; Ding, Y. Highly sensitive fluorescent aptasensor based on MoS2 nanosheets for one-step determination of methamphetamine. Anal Sci. 2022, 38(1), 99–104. [Google Scholar] [CrossRef]
- Liang, J.; Yan, R.; Chen, C.; Yao, X.; Guo, F.; Wu, R.; Zhou, Z.; Chen, J.; Li, G. A novel fluorescent strategy for Golgi protein 73 determination based on aptamer/nitrogen-doped graphene quantum dots/molybdenum disulfide @ reduced graphene oxide nanosheets. Spectrochim Acta A Mol Biomol Spectrosc 2023, 294, 122538. [Google Scholar] [CrossRef]
- Wang, J.J.; Liu, Y.; Ding, Z.; Zhang, L.; Han, C.; Yan, C.; Amador, E.; Yuan, L.; Wu, Y.; Song, C.; et al. The exploration of quantum dot-molecular beacon based MoS2 fluorescence probing for myeloma-related Mirnas detection. Bioact Mater. 2022, 17, 360–368. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat Chem. 2013, 5(4), 263–75. [Google Scholar] [CrossRef]
- Ge, J.; Hu, Y.; Deng, R.; Li, Z.; Zhang, K.; Shi, M.; Yang, D.; Cai, R.; Tan, W. Highly Sensitive MicroRNA Detection by Coupling Nicking-Enhanced Rolling Circle Amplification with MoS2 Quantum Dots. Anal Chem. 2020, 92(19), 13588–13594. [Google Scholar] [CrossRef]
- Strobbia, P.; Cupil-Garcia, V.; Crawford, B.M.; Fales, A.M.; Pfefer, T.J.; Liu, Y.; Maiwald, M.; Sumpf, B.; Vo-Dinh, T. Accurate in vivo tumor detection using plasmonic-enhanced shifted-excitation Raman difference spectroscopy (SERDS). Theranostics 2021, 11(9), 4090–4102. [Google Scholar] [CrossRef]
- Er, E.; Sánchez-Iglesias, A.; Silvestri, A.; Arnaiz, B.; Liz-Marzán, L.M.; Prato, M.; Criado, A. Metal Nanoparticles/MoS2 Surface-Enhanced Raman Scattering-Based Sandwich Immunoassay for α-Fetoprotein Detection. ACS Appl Mater Interfaces 2021, 13(7), 8823–8831. [Google Scholar] [CrossRef]
- Pan, H.; Dong, Y.; Gong, L.; Zhai, J.; Song, C.; Ge, Z.; Su, Y.; Zhu, D.; Chao, J.; Su, S.; et al. Sensing gastric cancer exosomes with MoS2-based SERS aptasensor. Biosens Bioelectron 2022, 215, 114553. [Google Scholar] [CrossRef]
- Medetalibeyoglu, H.; Kotan, G.; Atar, N.; Yola, M.L. A novel sandwich-type SERS immunosensor for selective and sensitive carcinoembryonic antigen (CEA) detection. Anal Chim Acta. 2020, 1139, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Samy, O.; Zeng, S.; Birowosuto, M.D.; El Moutaouakil, A. A Review on MoS2 Properties, Synthesis, Sensing Applications and Challenges. Crystals 2021, 11, 355. [Google Scholar] [CrossRef]
- Chiu, N.F.; Yang, H.T. High-Sensitivity Detection of the Lung Cancer Biomarker CYFRA21-1 in Serum Samples Using a Carboxyl-MoS2 Functional Film for SPR-Based Immunosensors. Front Bioeng Biotechnol 2020, 8, 234. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.L.; Jiang, L.F.; Zong, L.P.; Zeng, H.B.; Shu, G.F.; Marks, R.; Zhang, X.J.; Shan, D. MoS2 quantum dots-combined zirconium-metalloporphyrin frameworks: Synergistic effect on electron transfer and application for bioassay. Sensors and Actuators B: Chemical 2018, 273, 566–573. [Google Scholar] [CrossRef]
- Hou, F.; Hu, X.B.; Ma, S.H.; Cao, J.T.; Liu, Y.M. Construction of electrochemiluminescence sensing platform with in situ generated coreactant strategy for sensitive detection of prostate specific antigen. Journal of Electroanalytical Chemistry 2020, 858, 113817. [Google Scholar] [CrossRef]
- Nie, Y.X.; Zhang, X.; Zhang, Q.; Liang, Z.H.; Ma, Q.; Su, X.G. A novel high efficient electrochemiluminescence sensor based on reductive Cu(I) particles catalyzed Zn-doped MoS2 QDs for HPV 16 DNA determination. Biosensors and Bioelectronics 2020, 160, 112217. [Google Scholar] [CrossRef]
- Hou, S.N.; Wang, P.L.; Nie, Y.X.; Guo, Y.P.; Ma, Q. A novel work function tuning strategy-based ECL sensor with sulfur dots and Au NP@MoS2 nanosheet heterostructure for triple-negative breast cancer diagnosis. Chemical Engineering Journal 2022, 446(1), 136906. [Google Scholar] [CrossRef]
- Liu, W.; Su, M.L.; Chen, A.; Peng, K.F.; Chai, Y.Q.; Yuan, R. Highly Efficient Electrochemiluminescence Based on Luminol/MoS2 Quantum Dots@Zeolitic Imidazolate Framework-8 as an Emitter for Ultrasensitive Detection of MicroRNA. Analytical Chemistry 2022, 94, 9106–9113. [Google Scholar] [CrossRef]
- Lianjing Zhao, Dehao Kong, Zepei Wu, Guannan Liu, Yuan Gao, Xu Yan, Fangmeng Liu, Xiaomin Liu, Chenguang Wang, Jiuwei Cui, et al. Interface interaction of MoS2 nanosheets with DNA based aptameric biosensor for carbohydrate antigen 15–3 detection. Microchemical Journal 2020, 155, 104675. [CrossRef]
- Peng, X.; Wang, Y.; Wen, W.; Chen, M.M.; Zhang, X.; Wang, S. Simple MoS2-Nanofiber Paper-Based Fluorescence Immunosensor for Point-of-Care Detection of Programmed Cell Death Protein 1. Anal Chem. 2021, 93(25), 8791–8798. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, H.; Li, X.; Chen, Y.; Gu, C.; Wei, G.; Zhou, J.; Jiang, T. Nonmetallic SERS-based immunosensor byintegrating MoS2 nanoflower and nanosheet towards the direct serum detection of carbohydrate antigen 19-9. Biosens Bioelectron. 2021, 193, 113481. [Google Scholar] [CrossRef]
- van der Zande, A.M.; Huang, P.s.Y.; Chenet, D.A.; Berkelbach, T.C.; You, Y.M.; Lee, G.H.; Heinz, T.F.; Reichman, D.R.; Muller, D.A.; Hone, J.C. Grains and grain boundaries in highly crystalline monolayer molybdenum disulphide. Nature Mater 2013, 12, 554–561. [Google Scholar] [CrossRef] [PubMed]
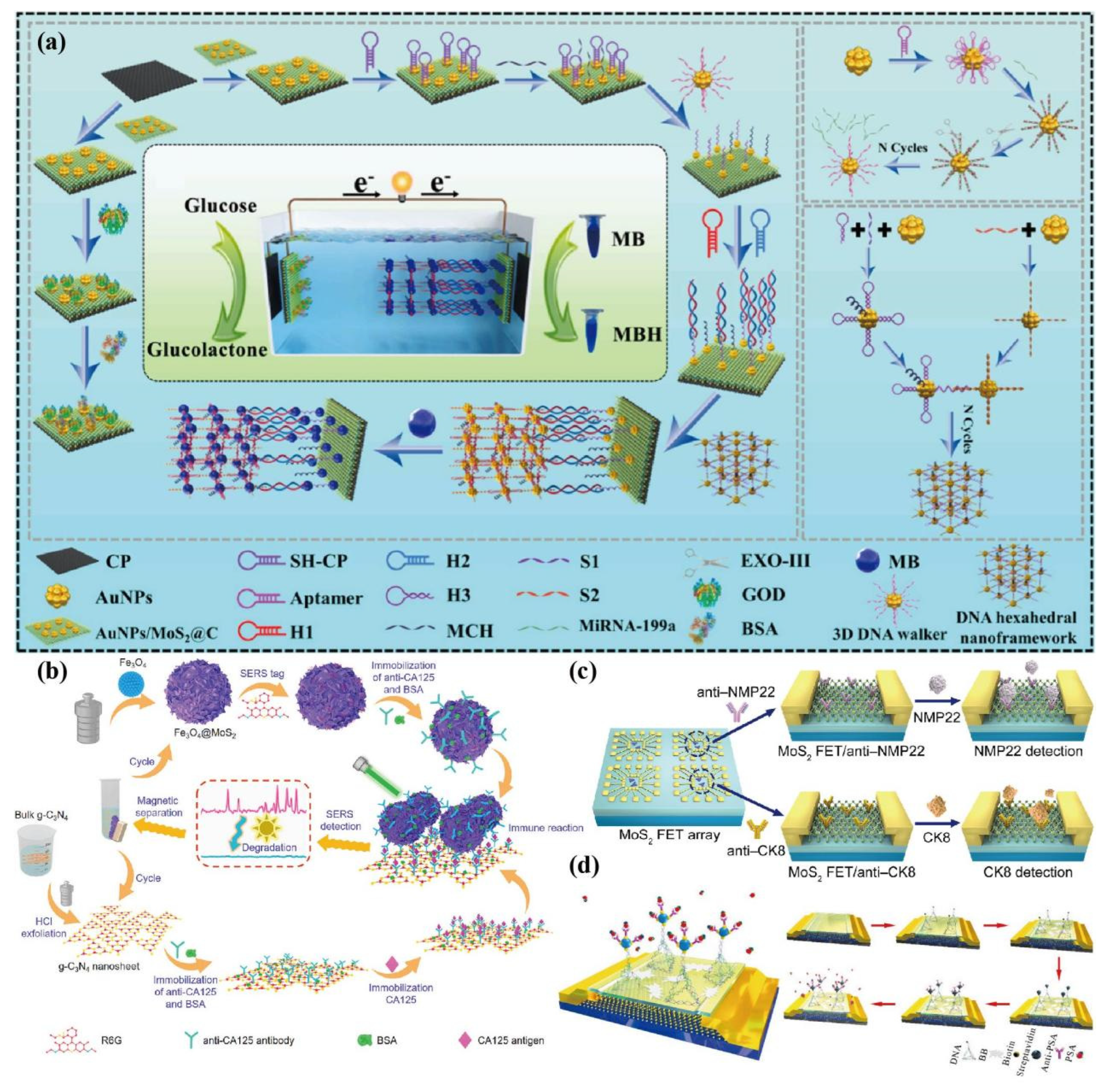
- Shi, J.Y; Qin, W.L.; Lin, Y.; Li, M.X.; Wu, Y.Y.; Luo, H.; Yan, J.; Huang, K.J.; Tan, X.X. Enhancing biosensing with fourfold amplification and self-powering capabilities: MoS2@C hollow nanorods-mediated DNA hexahedral framework architecture for amol-level liver cancer tumor marker detection. Analytica Chimica Acta 2023, 1271, 341413. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, H.; Xu, T.; Zhang, Y.; Gu, C.; Jiang, T. SERS-based recyclable immunoassay mediated by 1T-2H mixed-phase magnetic molybdenum disulfide probe and 2D graphitic carbon nitride substrate. Biosens Bioelectron. 2023, 227, 115160. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, B.; Li, Y.; Liang, H.G.; Yang, Y.B.; Yuan, Q. Construction of MoS2 field effect transistor sensor array for the detection of bladder cancer biomarkers. Sci. China Chem. 2020, 63, 997–1003. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, D.Z.; Xu, Y.; Yin, Z.W.; Dou, W.; Habiba, U.M.E.; Pan, C.Y.; Zhang, Z.k.; Mou, H.; Deng, H.y.; et al. DNA-based functionalization of two-dimensional MoS2 FET biosensor for ultrasensitive detection of PSA. Applied Surface Science 2021, 548, 149169. [Google Scholar] [CrossRef]
- Kashefi-Kheyrabadi, L.; Kim, J.; Chakravarty, S.; Park, S.y.; Gwak, H.; Kim, S.; Mohammadniaei, M. Lee, M.H.; Hyun, K.A.; Jung, H. Detachable microfluidic device implemented with electrochemical aptasensor (DeMEA) for sequential analysis of cancerous exosomes. Biosensors and Bioelectronics 2020, 169, 112622. [CrossRef]
- Yagati, A.K.; Go, A.; Vu, N.H.; Lee, M.H. A MoS2–Au nanoparticle-modified immunosensor for T3 biomarker detection in clinical serum samples. Electrochimica Acta 2020, 342, 136065. [Google Scholar] [CrossRef]
- Jandas, P.J.; Luo, J.T.; Prabakaran, K.; Chen, F.; Fu, Y.Q. Highly stable, love-mode surface acoustic wave biosensor using Au nanoparticle-MoS2-rGO nano-cluster doped polyimide nanocomposite for the selective detection of carcinoembryonic antigen. Materials Chemistry and Physics 2020, 246, 122800. [Google Scholar] [CrossRef]
- Torul, H.; Yarali, E.; Eksin, E.; Ganguly, A.; Benson, J.; Tamer, U.; Papakonstantinou, P.; Erdem, A. Paper-Based Electrochemical Biosensors for Voltammetric Detection of miRNA Biomarkers Using Reduced Graphene Oxide or MoS2 Nanosheets Decorated with Gold Nanoparticle Electrodes. Biosensors 2021, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.X.; Cui, K.; Liu, Y.; Li, L.; Zhang, L.; Hao, S.J.; Ge, S.G.; Yu, J.H. Bi2S3@MoS2 Nanoflowers on Cellulose Fibers Combined with Octahedral CeO2 for Dual-Mode Microfluidic Paper-Based MiRNA-141 Sensors. ACS Applied Materials & Interfaces 2021, 13(28), 32780–32789. [Google Scholar] [CrossRef]
- Ma, J.L.; Xue, D.; Xu, T.; Wei, G.D.; Gu, C.J.; Zhang, Y.L.; Jiang, T. Nonmetallic SERS-based biosensor for ultrasensitive and reproducible immunoassay of ferritin mediated by magnetic molybdenum disulfide nanoflowers and black phosphorus nanosheets. Colloids and Surfaces B: Biointerfaces 2023, 227, 113338. [Google Scholar] [CrossRef]
- Rahman, M.T.; Kumar, R.; Kumar, M.; Qiao, Q.Q. Two-dimensional transition metal dichalcogenides and their composites for lab-based sensing applications: Recent progress and future outlook. Sensors and Actuators A: Physical 2021, 318, 112517. [Google Scholar] [CrossRef]
- Nakada, G.; Igarashi, Y.; Imai, H.; Oaki, Y. Materials-Informatics-Assisted High-Yield Synthesis of 2D Nanomaterials through Exfoliation. Adv. Theory Simul. 2019, 2, 1800180. [Google Scholar] [CrossRef]
- Sethulekshmi, A.S.; Saritha, A.; Joseph, K.; Aprem, A.S.; Sisupal, S.B. MoS2 based nanomaterials: Advanced antibacterial agents for future. Journal of Controlled Release 2022, 348, 158–185. [Google Scholar] [CrossRef] [PubMed]
- Manzeli, S.; Dumcenco, D.; Migliato Marega, G.; Kis, A. Self-sensing, tunable monolayer MoS2 nanoelectromechanical resonators. Nat Commun. 2019, 10(1), 4831. [Google Scholar] [CrossRef] [PubMed]
- Siao, M.D.; Shen, W.C.; Chen, R.S.; Chang, Z.W.; Shih, M.C.; Chiu, Y.P.; Cheng, C.M. Two-dimensional electronic transport and surface electron accumulation in MoS2. Nat Commun 2018, 9, 1442. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Li, Y. Impact of Doping Concentration on Electronic Properties of Transition Metal-Doped Monolayer Molybdenum Disulfide. IEEE Transactions on Electron Devices 2018, 65, 733–738. [Google Scholar] [CrossRef]
- Hu, T.; Zhang, R.; Li, J.P.; Cao, J.Y.; Qiu, F. Photodetectors based on two-dimensional MoS2 and its assembled heterostructures. Chip 2022, 1(3), 100017. [Google Scholar] [CrossRef]
- Kaur, J.; Singh, M.; Dell'Aversana, C.; Benedetti, R.; Giardina, P.; Rossi, M.; Valadan, M.; Vergara, A.; Cutarelli, A.; Montone, A.M.I.; et al. Biological interactions of biocompatible and water-dispersed MoS2 nanosheets with bacteria and human cells. Sci Rep. 2018, 68(1), 16386. [Google Scholar] [CrossRef] [PubMed]
- Mohan, M.; Shetti, N.P.; Aminabhavi, T.M. Recent developments in MoS2-based flexible supercapacitors. Materials Today Chemistry 2023, 27, 101333. [Google Scholar] [CrossRef]
| Method | Analytes | Electrode/Label | Linear range | LOD | Ref. |
|---|---|---|---|---|---|
| Chronoamperometry | AMACR | Pt NWs array@2H-MoS2/SPE | 0.70 ng/µL-12.50 ng/µL | 0.5 pg/µL | [73] |
| CV | CEA | Ab/rGO/MoS2@PANI/GCE | 0.001 ng/mL-80 ng/mL | 0.3 pg/mL | [77] |
| CV | PSA | Ab/ce-MoS2/AgNR/SPE | 0.1 ng/mL-1000 ng/mL | 0.051 ng/mL | [78] |
| DPV | CEA | Ab/MoS2-PBNCs/GCE | 0.005 ng/mL-10 ng/mL | 0.54 pg/mL | [86] |
| DPV | CEA | Ag/MoS2@Fe3O4-Ab2/CEA/Ab1/Ag/MGCE | 0.0001 ng/mL-20 ng/mL | 0.03 pg/mL | [87] |
| DPV | miRNA-182 | ssRNA/MoS2/Ti3C2/GCE | 1 fM-0.1 nM | 0.43 fM | [88] |
| DPV | CEA | MoS2-AuNPs/HRP-Ab2/CEA/Ab1/MoS2-AuNPs/GCE | 10 fg/mL-1 ng/mL | 1.2 fg/mL | [89] |
| DPV | Nucleolin | TNA/AuNPs/MoS2/rGO/GCE | 0.5 nM-1.0 μM | 0.16 nM | [75] |
| DPV | CEA | Ab/Pd@Pt/MoS2-Gr/GCE | 0.00001 ng/mL-100 ng/mL | 0.005 pg/mL | [90] |
| DPV | CA125 | Ab/CuBTC@MoS2-AuNPs/SPE | 0.5 mU/mL-500 U/mL | 0.5 mU/mL | [91] |
| DPV | anti-retroviral agent indinavir | ZnO NRs/MoS2 NSs/SPE | 0.01-0.66 μM & 0.66-7.88 μM |
0.007 μM | [92] |
| SWV | CEA | CeO2-MoS2-Pb2+-Ab2/CEA/Ab1/AuNPs/GCE | 0.001 ng/mL-80 ng/mL | 0.3 pg/mL | [93] |
| Amperometry | CEA | Ab/Ag/MoS2/rGO/GCE | 0.01 pg/mL-100 ng/mL | 1.6 fg/mL | [94] |
| Amperometry | HBeAg | Au@Pd/MoS2@MWCNTs-Ab2/HBeAg/Ab1/p-GO@Au/GCE | 0.1 pg/mL-500 pg/mL | 26 fg/mL | [95] |
| Amperometry | AFP | MoS2@Cu2O-Au-Ab2/AFP/Ab1/AuNPs/GCE | 0.1 pg/mL-50 ng/mL | 0.037 pg/mL | [79] |
| Amperometry | AFP | Ab/Pt NDs/PDDA/MoS2@PPy NTs/GCE | 50 fg/mL-50 ng/mL | 17 fg/mL | [96] |
| Amperometry | CEA | MoS2 NFs/Au@AgPt YNCs -Ab2/CEA/Ab1/AuTNPs/GCE |
10 fg/mL-100 ng/mL | 3.09 fg/mL | [80] |
| EIS | CML | pDNA/PANI-MoS2/ITO | 10−17 M-10−6 M | 3×10−18 M | [97] |
| EIS | AFP | Ab/MoS2/PDDA/Ag/AgCl wire | 0.1 ng/mL-10 ng/mL | 0.033 ng/mL | [83] |
| EIS | miRNA-21 | cDNA/pd-MoS2@β-FeOOH/Au | 1 fM-5 nM | 0.11 fM | [81] |
| EIS | TNF-ɑ | MoS2 NFs | 0.01-200 pg/ml | 0.202 pg/ml | [82] |
| PEC | CEA | ALP-Au-Ab2/CEA /Ab1/ZnS/C/MoS2/GCE |
2.0 pg/mL-10.0 ng/mL | 1.30 pg/mL | [85] |
| PEC | SCCA | Ab/AuNPs/C/MoS2/GCE | 0.005 ng/mL-8 ng/mL | 1.8 pg/mL | [98] |
| PEC | AFP | DNA/Au/GaN | 1.0 ng/mL-150 ng/mL | 0.3 ng/mL | [84] |
| PEC | MCF-7 cells | PM6:Y6/anti-EpCAM-MNs /Au NPs/Au-aptamer |
10-10000 cell/mL | 9 cell/mL | [99] |
| Method | Analytes | Electrode/Label | Linear range | LOD | Ref. |
|---|---|---|---|---|---|
| Colorimetry | CEA | Au NPs-MoS2-Ab2/ CEA/Ab1/MoS2-Au NPs |
5 pg/mL-10 ng/mL | 0.5 pg/mL | [104] |
| Colorimetry | CEA | DNA/MoS2 NSs | 50 ng/mL-1000 ng/mL | 50 ng/mL | [103] |
| Colorimetry | CTC | TP/SYL3C-MoS2 | 5-104cells/mL | 2 cells/mL | [105] |
| ECL | CEA | Ab/MOF-545-Zn@MQDs/GCE | 0.18 ng/mL-1000 ng/mL | 0.45 pg/mL | [126] |
| ECL | PSA | GOD-SiO2-Ab2/PSA /Ab1/MoS2-AuNPs/GCE |
0.5 pg/mL-10.0 ng/mL | 0.20 pg/mL | [127] |
| ECL | HCV gene | S-BN QDs-hairpin DNA2 (H2)/MoS2 Ns | 0.5 pmoL/L-1 nmoL/L | 0.17 pmoL/L. | [114] |
| ECL | HPV 16 DNA | Zn-doped MoS2 QDs & QD-DNA/reductive Cu(I) particles | 0.1 nmolL-200 nmolL | 0.03 nmol/L | [128] |
| ECL | miRNA-210 | S dots/Au NP@MoS2 NSs | 0.1 pM-10 nM | 0.03 pM | [129] |
| ECL | miRNA-21 | luminophore/MoS2 QDs@Zeolitic Imidazolate 2 Framework-8 | Buffer (0.1 mM PBS), co-reactant (2 mM H2O ) | 14.6 aM | [130] |
| Fluorescence | CA15-3 | DNA/MoS2 NSs | 0.01 U/mL-0.1 U/mL | 0.0039 U/mL | [131] |
| Fluorescence | PD-1 | MoS2-NFP | 125-8000 pg/mL | 85.5 pg/mL | [132] |
| Fluorescence | miRNA-155 & miRNA-150 | QD-MB @MoS2 | 10 fM-1 nM | 7.19 fM & 5.84 fM |
[118] |
| SERS | CEA | MoS2 NFs@Au NPs/MBA-Ab2/CEA /Ab1/Fe3O4 NPs@Au NPs/ d-Ti3C2TX Mxene |
0.0001 ng/mL-100.0 ng/mL | 0.033 pg/mL | [125] |
| SERS | CA19-9 | R6G-tagged MoS2 NF | 5×10−3-100 IU/mL | 3.43×10−4 IU/mL | [133] |
| SERS | exosomes | MoS2-AuNSs/ROX-Apt | 55-5.5×105 particles/μL | 17 particles/μL | [124] |
| SPR | CYFRA21-1 | Ab/COOH-MoS2/Au/Cr/BK7 | 0.05 pg/mL-100 ng/mL | 0.05 pg/mL | [125] |
| Method | Analytes | Electrode/Label | Linear range | LOD | Ref. |
|---|---|---|---|---|---|
| FET array | NMP22 & CK8 | MoS2 NSs-FET | 10−6-10−1 pg/mL | 0.027-0.019 aM | [137] |
| DeMEA/ microfluidic |
exosome | GASI microfluidic channel/anti-EpCAM | 1×102-1×109 exosomes/μL | 17 exosomes/μL | [139] |
| EIS/CV | T3 | Au-MoS2/anti-T3 electrodes | 0.01-100 ng/mL | 2.5 pg/mL | [140] |
| SAW | CEA | AuNP–MoS2-rGO/PI | 0.1 ng/ml-80 ng/ml. | 0.084 ng/ml | [141] |
| bio-FET | PSA | MoS2/B-SA system with DNA tetrahedron | 1 fg/mL-100 ng/mL (PBS) 1 fg/mL-100 ng/mL (serum) |
1 fg/mL | [138] |
| Paper-Based/DPV | miRNA-155 miRNA-21 |
AuNPs/RGO/PE AuNPs/MoS2/PE |
33.8 nM-135.3 nM 135.6 nM-406.8 nM |
12.0 nM 25.7 nM |
[142] |
| Microfluidic/ electrochemical/visual |
miRNA-141 | Bi2S3@MoS2 NFs/CeO2 | 10 fM-1 nM 0.5 fM-1 nM |
0.12 fM 2.65 fM |
[143] |
| signal amplification/ 3D DNA walker |
microRNA-199a | AuNPs/MoS2@C | 0.0001-100 pM | 4.94 amol/L | [135] |
| SERS/ELISA | ferritin | MoS2 @Fe3O4/BP | 10-10−4 μg/mL | 7.3×10−5 μg/mL | [144] |
| SERS/ELISA | CA125 | Fe3O4@MoS2/g-C3N4 NSs | 10−3-102 IU/mL | 4.96×10−4 IU/mL | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





