1. Introduction
Propolis is a highly valuable biologically active product of animal origin, which is widely used in many preparations for oral and topical application. Its composition is very complex and it is influenced by geographical factors and botanical origin. Continental or temperate climate propolis, also called poplar type, is the largest source of propolis in Europe [
1]. The most significant biologically active components of this type of propolis are polyphenols, including flavonoid aglycones (flavones, flavonols and flavanones), substituted cinnamic acids and their esters [
2,
3]. There is no uniform type of propolis in Croatia. As Croatia is situated in the south-east of Europe and has a coastal part with the influence of the Mediterranean, the composition of propolis gradually changes from continental to mountainous and finally Mediterranean type [
4]. It is very difficult to compare previously reported papers on the chemical composition of Croatian propolis due to the different origin of the samples, extraction methods and solvents used, but also the way of expressing the content of polyphenolic compounds [
5,
6].
Due to dominant resinous and waxy composition, propolis is hardly consumed in its native form and it is necessary to purify it by extraction. Polyphenols are partially polar compounds, and as a part of resin, are poorly soluble in water and have low bioaccessibility after oral administration. These are the reasons why propolis has limited use for therapeutic purposes. Commonly used procedures for the extraction of propolis samples are maceration (MCR) and Soxhlet extraction. However, newer extraction techniques such as microwave-assisted extraction (MAE) or ultrasound-assisted extraction (UAE) are increasingly being used due to lower consumption of organic solvents, reduced time of extraction and higher yields [
7,
8,
9]. Ethanolic extracts of propolis (EEP) are widely used in various studies of the biological activity of propolis since most propolis compounds are soluble in ethanol (EtOH). On the other hand, these extracts are often not appropriate because EtOH can cause allergies, and is generally undesirable in preparations intended for children and in veterinary medicine [
10].
Numerous methods, such as particle size reduction, nanosuspension, use of surfactants, salt formation or solid dispersion, are used to improve pharmacokinetic properties and maintain high concentration ranges of polyphenols [
11]. During the last few years encapsulation techniques have become a promising and very effective tool for increasing the solubility of polyphenols with the ability to form inclusion complexes thus allowing them to be used as active ingredients in functional foods or medicines.
Cyclodextrins are biocompatible and non-toxic cyclic oligosaccharides that form inclusion complexes by entrapping the organic compound in their hydrophobic cavity. Since the outer surface of cyclodextrins is hydrophilic, solubility in water for some types of cyclodextrins is very high [
12]. Until now, several studies have been conducted related to the biological activity of the inclusion complex of propolis and cyclodextrin, as well as individual polyphenolic compounds and different types of cyclodextrin, like α-, β-, γ-cyclodextrin and their derivatives [
13,
14].
The potential biological activity of propolis extracts depends on the synergy of the polyphenolic fraction but also on its bioaccessibility profile, which can be low and divers among polyphenols [
15,
16]. Simple
in vitro models have become an important tool in gastrointestinal (GI) stability research and the prediction of bioaccessibility of certain food components or food supplements [
17,
18].
In numerous scientific papers, the bioaccessibility of polyphenols was determined during
in vitro simulated gastrointestinal digestion (GID) in food of plant origin and medicinal herbs, but very few papers determined the bioaccessibility of polyphenols from propolis [
19,
20,
21,
22]. For these reasons, our research was directed towards the preparation of a water-soluble propolis complex with the carrier 2-hydroxypropyl-β-cyclodextrin (HP-β-CD), by obtaining stable lyophilisate which will have a high concentration of polyphenolic compounds and have a pleasant taste. Furthermore, we have also investigated gastrointestinal stability (bioaccessibility) and dialysability of polyphenolic groups from two samples of Croatian continental propolis during
in vitro simulated GID after oral administration of the inclusion complex of propolis extract and HP-β-CD by determining total phenolic-flavonoid content.
2. Materials and Methods
2.1. Reagents and Chemicals
The reagents and standards used in this study were obtained as follows: galangin from Sigma Aldrich, China, pinocembrin from Sigma Aldrich, Uzbekistan, Folin-Ciocalteu reagent Sigma-Aldrich, Buchs, Switzerland, sodium carbonate, aluminium chloride (AlCl3) and sulfuric acid from Sigma-Aldrich, Steinheim, Germany, 2,4-dinitrophenylhydrazine (2,4-DNPH) and quercetin from Sigma-Aldrich, India, potassium hydroxide from Kemika, Zagreb, Croatia and HP-β-CD was purchased from Wacker Chemie AG, Burghausen, Germany. Ethanol, absolute, for analysis was purchased from Supelco, Darmstadt, Germany and water (HPLC grade) was obtained from Honeywell, Seelze, Germany. α-amylase from human saliva, pepsin from porcine mucosa, pancreatin, porcine (8x United States Pharmacopoeia) and beef bile, dry, unfractionated were purchased from Sigma-Aldrich, Saint Louis, Missouri, SAD and cellulose dialysis membrane (molecular weight cutoff of 3.5 kDa) was obtained from Spectrum Labs, New Brunswick, NJ, SAD. All other reagents and solvents used were of analytical grade.
2.2. Propolis Samples
Samples of raw propolis were obtained from local beekeepers during 2019 and 2020 from two regions of Croatia: Valpovo (Osijek-Baranja County, 45°40′N 18°25′E) and near Zagreb (the City of Zagreb, 45°49′0″N 15°59′0″E). The samples were placed in sealed plastic bags and stored at -10 to -20 °C until analysis.
2.3. Preparation of Inclusion Complex with HP-β-CD
Frozen propolis was homogenized into powder and 0.5 g was extracted in 5 mL of 70% EtOH by UAE for one hour at room temperature. The extract was centrifuged at 4472 x g for 20 minutes at 4 °C and stored over night at -10 to -20 °C. The next day, the extract was centrifuged under the same conditions. The supernatant was quantitatively separated from the residue and waxes by filtration through filter paper (Whatman No.4) in a volumetric flask and the volume was made up to 10 mL obtaining EEP. The extract was subsequently evaporated to dryness in a rotary evaporator under reduced pressure at 50 °C. Propolis dry extract was suspended in 50 mL of 2.0% m/V HP-β-CD aqueous solution and the suspension was sonicated for 2 hours. The obtained suspension was frozen at -80 °C and subsequently lyophilized using a freeze dryer at -50 °C and 0.1-0.3 mbar. Water extract was prepared in the same manner as the complex but without cyclodextrin. The powder, referred to as Valpovo (Vlp) or Zagreb (Zg) complex, was kept at -20 °C until further use.
2.4. In Vitro Digestion Protocol
Propolis complexes (Valpovo and Zagreb) were analyzed according to the method developed by the COST INFOGEST international network [
23]. This static
in vitro procedure included three sequential phases of digestion: oral, gastric, and intestinal. Before performing the procedure, simulated saliva fluid (SSF), gastric fluid (SGF) and intestinal fluid (SIF) were prepared for each phase at a defined temperature and adjusted pH,
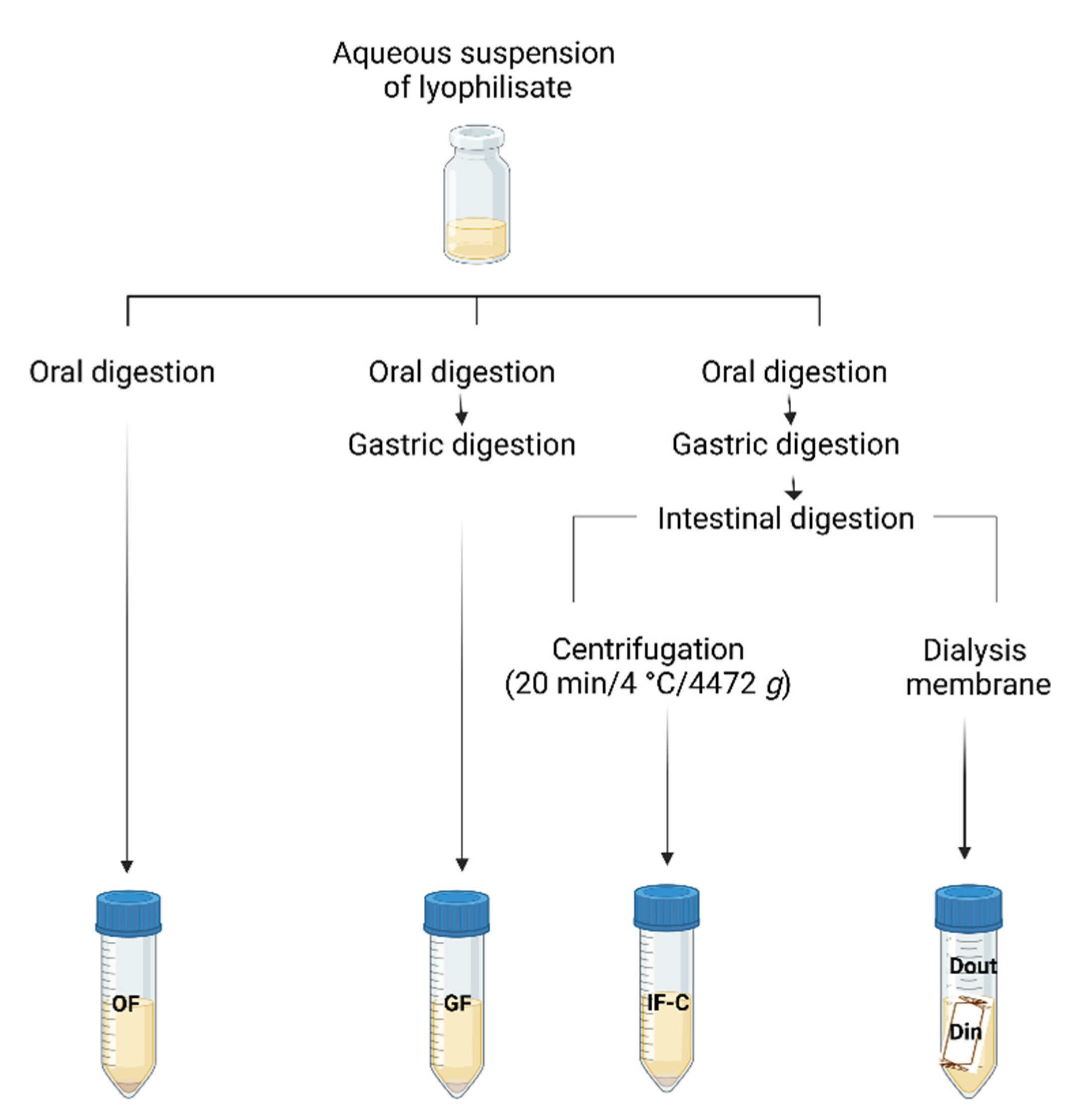
Table S1 (Supplementary Material). The enzyme solutions were freshly prepared and kept in an ice bath until use. During the experiment, separate test tubes were used for each digestion phase to ensure the homogeneity of the sample during each phase.
The procedure was initiated by adding a 5 mL sample (aqueous suspension of the complex EEP with HP-β-CD) to a 50 mL amber centrifuge tube and was mixed with 4.0 mL of SSF. After that, 0.5 mL of α-amylase aqueous solution (1500 U/mL in SSF), 25 μL of 0.3 mol/L CaCl2 and 225 μL of distilled water were added to the mixture to make the ratio of sample to SSF 1:1 (v/v). The mixture was incubated in a water bath at 37 °C for 5 minutes with a uniform shake. To simulate the gastric phase (GF), 8.0 mL of SGF, 0.667 mL of pepsin solution (2000 U/mL in SGF), 5 μL of 0.3 mol/L CaCl2 and 0.448 mL of distilled water were added to the mixture from OF. The mixture was incubated in a water bath at 37 °C for 2 hours with stirring.
Two separate protocols were performed to simulate the intestinal phase (IF):
The volume of 8.0 mL SIF was added to GF, followed by adding 5.0 mL of pancreatin solution (800 U/mL in SIF), 3.0 mL of 160 mmol/L bile salts, 40 μL of 0.3 mol/L CaCl2, 0.15 mL of 1 mol/L NaOH, and 3.16 mL of distilled water. A sodium hydroxide solution was added to the mixture to neutralize the mixture to pH 7.0. The reaction mixture was again incubated in a water bath at 37 °C for 2 hours.
Enzymes and reagents were added to the GF in the same concentrations as in the 1st model. However, a semi-permeable membrane for dialysis, which simulated the wall of the small intestine, was filled with NaHCO3 and NaCl solution and immersed in the test tube with the GF. Simulated intestinal fluid, pancreatin and bile salts were then added in concentrations as already described in the IF-C model. The reaction tube with membrane was placed in a water bath at 37 °C for 2 hours with agitation. The solution outside and inside the dialysis tubing (referred to as “Dout samples” and “Din samples”, respectively) were taken to represent the material that remained within the gastrointestinal tract and the fraction that was accessible for absorption, respectively.
At the end of each phase the fractions OF, GF, IF-C, Din and Dout were placed in an ice bath for 10 minutes and centrifuged at 4472 x g for 20 minutes at 4 °C to separate soluble (bioaccessible) from insoluble compounds, then aliquots were stored at -80 °C until analysis. The concentration of individual standards in the EESS and HP-β-CD complex during the in vitro digestion simulation was determined taking into account the obtained average concentrations of polyphenolic compounds in the EEP complex with HP-β-CD (described in section Results 3.1.,
Table 1.). The blank sample contained water, enzymes and digestive fluids. Before spectrophotometric analysis, aliquots of the samples were diluted or directly analyzed without dilution, depending on the analyte concentration in the individual phase.
Figure 1.
Schematic representation of the in vitro gastrointestinal digestion. Created with BioRender.com.
Figure 1.
Schematic representation of the in vitro gastrointestinal digestion. Created with BioRender.com.
2.4.1. Bioaccessibility Index
Bioaccessibility (%) was described as the content of phenolic compounds released in the
in vitro digestion process compared to the content of phenolic compounds in the tested samples, and the percentage of bioaccessibility was calculated according to the following equation:
2.5. Determination of Total Phenolic Content
Determination of the total phenolic content (TP) was carried out using Folin-Ciocalteu's reagent according to the modified method described by Popova et al. [
24]. For sample analysis, a volume of 0.1 mL was diluted with 80% ethanol in a 10 mL volumetric flask so that the concentration corresponded to the working range of the method. An aliquot of 0.5 mL of the resulting solution was added in a 25 mL volumetric flask to which 7.5 mL of distilled water was previously added, after which 2 mL of Folin-Ciocalteu reagent and 3 mL of 20% aqueous solution of sodium carbonate were added. The volume was made up with distilled water and stored in the dark at room temperature for 2 hours (± 3 min). The absorbance of the resulting solution was measured at 760 nm. The results for the TP content are expressed in μg PC:GN/mL of the inclusion complex aqueous solution.
2.6. Determination of Total Flavonols and Flavones
The content of total flavones and flavonols (TFF) was estimated based on the procedure used by Popova et al. [
24] with some modifications. The calibration curve for TFFs was prepared with QC as a reference. For TFF analysis, a volume of 0.1 mL was diluted with 80% ethanol in a 10 mL volumetric flask and the volume was made up with the same solvent. An aliquot of 1.0 mL of the resulting solution was diluted with 10 mL of methanol, 0.5 mL of 5% methanolic solution of aluminium chloride was added and the volume was made up to 25 mL with methanol. The reaction mixture was stored for 30 minutes in the dark at room temperature. The absorbance of the resulting solution was measured at 415 nm. The results for the TFF content are expressed in μg QC/mL of the complex aqueous solution.
2.7. Determination of Total Flavanones and Dihydroflavonols
The quantitative analysis for the determination of flavanones/dihydroflavonols content (TFD) was carried out according to the method reported by Bankova et al. [
25], with some modifications. Methanolic solution of PC was used as a reference standard for the preparation of the calibration curve. The sample for analysis of a volume of 1.0 mL was diluted with 80% ethanol in a 10 mL volumetric flask. The 2,4-DNPH reagent was prepared by dissolving 1 g of 2,4-DNPH in 2 mL of 96% sulfuric acid in a volumetric flask with a volume of 100 mL, and the resulting mixture was diluted with methanol. The reaction mixture consisting of 1 mL of diluted sample solution and 2 mL of 2,4-DNPH solution heated in a water bath at 50 °C / 50 min. After cooling to room temperature, the mixture was diluted with a 10% ethanolic solution of potassium hydroxide (80% ethanol). The volume of 1.0 mL of the resulting solution was then diluted with methanol in a 25 mL volumetric flask, and the resulting solution was centrifuged (4472 x
g, 10 minutes). The absorbance of the supernatant was measured at 495 nm. The results for the TFD content are expressed in μg PC/mL of the complex aqueous solution.
2.8. Statistical Analysis
Samples were analyzed in triplicate and data were expressed as mean ± standard deviation (SD). The results were analyzed using the statistical program Stata 13.1 (Stata Corp. USA). Polyphenolic content in individual phases were compared using the Kruskall Wallis (KW) non-parametric test and Dunn's post hoc test of mutual phases. Probability values less than 0.05 (p < 0.05) were considered statistically significant.
3. Results
3.1. Influence of HP-β-CD on Polyphenolic Contents
The effect of HP-β-CD, water and 70% EtOH on extraction of polyphenols was shown in
Table 1 by estimation of TPs, TFFs and TFDs contents. As the aim of this study was to prepare propolis dry extract with minimal solvent consumption and the use of non-toxic chemicals, the analysis of the EEP complex showed that HP-β-CD effectively encapsulated polyphenols from propolis extract (
Table 1) obtaining high contents of TP and TFF. The TP content in the analyzed undigested Vlp complex was 15477.22 ± 10.50 μg PC:GN/mL and 20104.91 ± 13.75 μg PC:GN/mL for sample Zg complex (
Table 1;
Table 2). The contents of TFDs and TFFs in water extract were significantly lower (p < 0.05), unlike to TPs where no significant difference was observed compared to EtOH extract and HP-β-CD complex. The content of TFF, calculated using QC as a reference, was 4626.10 ± 12.12 μg QC/mL in the Zg complex and 3795.19 ± 20 μg QC/mL in the sample Vlp (
Table 3). The results for 70%EtOH extract were obtained in study reported by Perak et.al. [
26]. The content of TFD in 70% EtOH extract was significantly higher than TFDs in complex or water extract. However, the contents of TFDs in HP-β-CD complex were significantly lower compared to 70% EtOH.
3.2. Effect of In Vitro GID on Total Phenolic Content
The content of TPs in complexes of the undigested sample, US, and fractions from OF, GF, IF-C, Din and Dout phases of digestion were determined by the Folin-Ciocalteu method as presented in
Table 2. During the
in vitro digestion, all analyzed samples showed a slight increase in TP content compared to the US for both models of the intestinal phase, centrifugation and dialysis.
3.3. Effect of In Vitro GID on Total Flavones and Flavonols Content
During the GID, TFF content slightly decreased in Zg and Vlp complexes, and stagnated for the standard mixture. In
Table 3, a significant increase of the bioaccessible TFFs was observed in the IF-C fraction compared to Din in Zg sample (
p < 0.05).
A greater instability was observed during GF of GID, TFFs were significantly decreased in the sample Zg and increased in std mix (p < 0.05), however, compared to the US there were no statistically significant differences (p > 0.05), which proved the stability of TFFs during stages of GID.
3.4. Effect of In Vitro GID on Total Flavanones and Dihydroflavonols Content
During
in vitro GID, there was a significant increase in TFD content in both propolis complexes and the standard mixture. The exception in the obtained results is the content of TFD in Din fraction of the standard mixture, TFDs were extremely low, which was confirmed by statistical analysis (
Table 4). The content of TFDs in US was very similar for all three samples and there was no significant difference (
p > 0.05) between them. After OF, the content of TFD only slightly increased in the complexes Zg and Vlp, while in the std mix the content significantly increased from 426.96 μg PC/mL to 1060.17 μg PC/mL. In acidic gastric conditions, the content of TFD compared to the initial US increased significantly, there was an enhanced release of flavanones and dihydroflavonols from the propolis matrix. The intestinal phase, IF-C showed further increase in the content of TFD for the complexes, however, in the std mix sample, TFDs have maintained similar trend compared to GF (a very slight increase compared to GF).
3.5. Bioaccessibility of Polyphenols
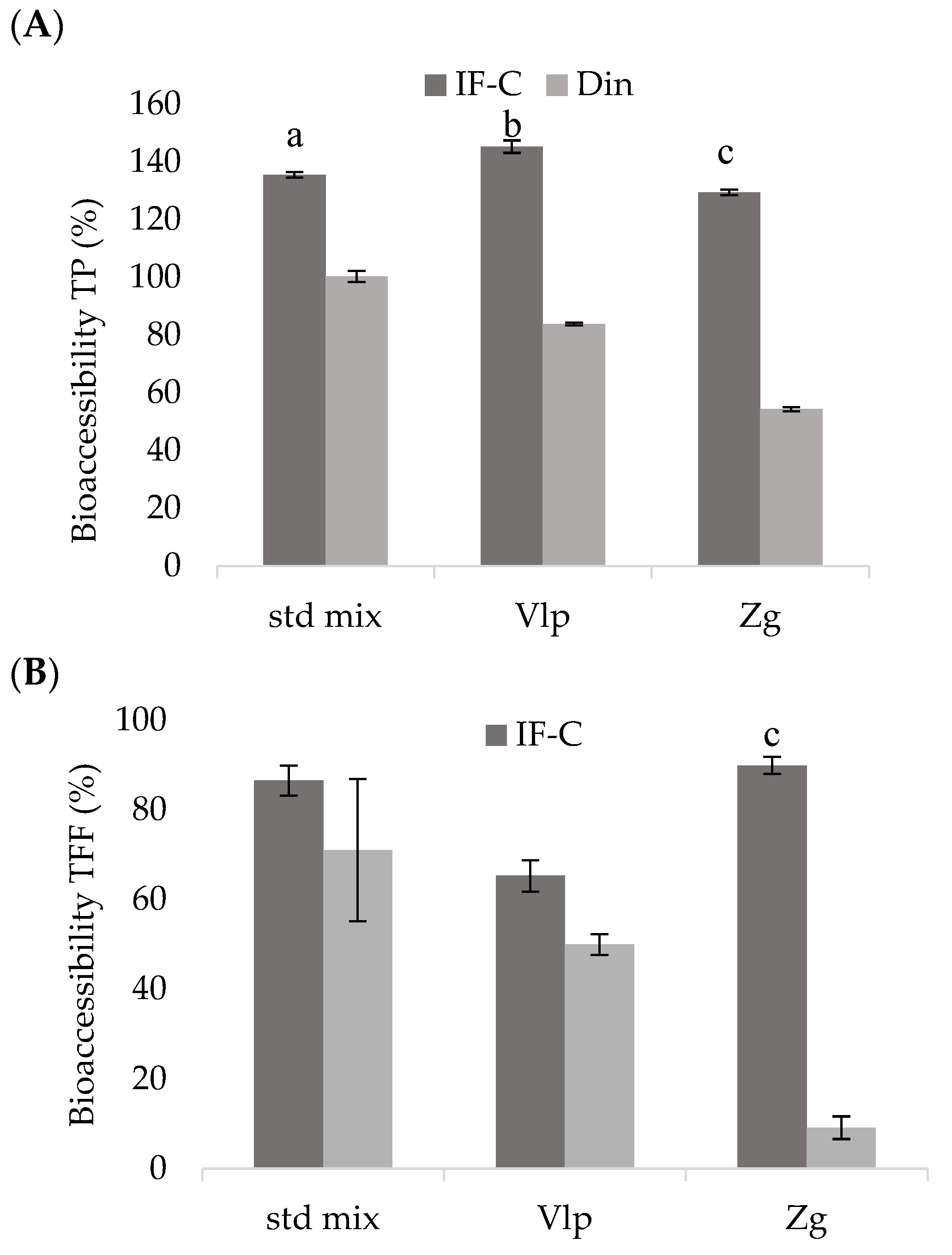
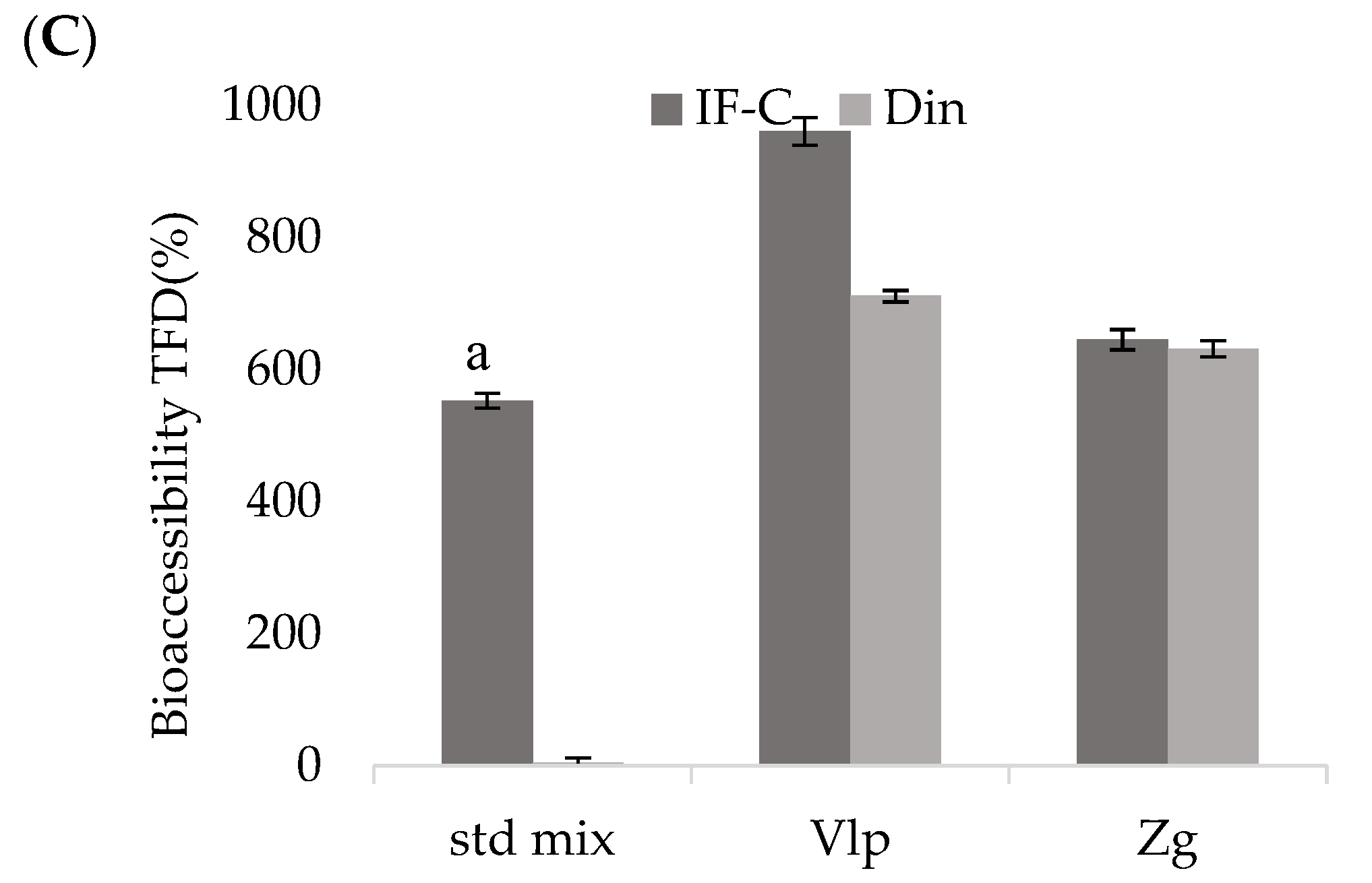
In vitro bioaccessibility and potential absorption of polyphenols after simulated
in vitro GID of the following complexes: standard mixture of selected polyphenols, ethanolic extracts of propolis Valpovo and Zagreb are shown in
Figure 2. Total phenolics, calculated according to Equation 1, were above 100% in IF-C fraction for all three complexes (
Figure 2A). In the Din fraction, the highest values of bioaccessible polyphenols were in the std mix, and the differences between bioaccessible contents in IF-C and Din were statistically significant (
p < 0.05) for all analyzed complexes. Total phenolics in GID fractions presented in
Table 2 showed that TPs are stable during GID, and the increase in content after IF-C compared to US indicated high bioaccessibility, i.e., polyphenols were effectively released from the matrix. In Din fraction, the content of dialyzable TPs was found from 54% in the Zg complex to 100% in the std mix.
The content of bioaccessible TFFs in all three complexes was high, ranging from 65% to 90% when IF-C model was performed. In the dialysis model, significantly lower value of bioaccessible TFFs were obtained in the Zg complex (p < 0.05) when compared to IF-C, while the TFF in Vlp and std mix samples have shown better recoveries.
Dialyzable fraction Din in the std mix showed very low TFDs, but in the complexes the situation was different, TFDs in IF-C and Din were very similar, without significant differences (p > 0.05) between them for both Vlp and Zg complexes. Although TFDs in Din fraction were high, the Dout fraction also contained a large content of bioaccessible compounds indicating that most of TFDs in this study remained outside the dialysis membrane, did not pass through the membrane. The increase in bioaccessible TPs, TFFs and TFDs during GF and IF-C, compared to US, indicated successful release of polyphenols passing through the gastrointestinal tract.
4. Discussion
Bioaccessibility research during simulated
in vitro digestion included two continental samples of propolis, near Valpovo (Vlp) and near Zagreb (Zg). The samples of raw propolis investigated in this study were prepared in the form of an oral preparation, an aqueous suspension of a lyophilized complex of a EEP and an aqueous solution of HP-β-CD (20 mg/mL). The advantage of the preparation of the complex is that HP-β-CD helped to improve the solubility of the lipophilic polyphenolic compounds and masked the bitter taste and intense smell of propolis, making the described processing of propolis more acceptable for oral use than the alcoholic solution (
Table 1). Experiments were also carried out on an aqueous suspension of a lyophilized complex of a 70% ethanolic extract of a mixture of selected polyphenol standards and an aqueous solution of HP-β-CD (20 mg/mL), to examine the influence of the synergy of all analyzed polyphenols without the propolis matrix. Spectrophotometric methods estimate all compounds of the appropriate phenolic structure present in the propolis sample and given that std mix complex was prepared as a mixture of only selected standards PC, KR, GN, CA and CAPE, the initial US sample and the digestion fractions had significantly lower TPs and TFFs compared to Vlp and Zg propolis complexes (
Table 2,
Table 3 and
Table 4).
Considering that the previous reports on assessing the bioaccessibility and bioavailability of polyphenols in various food and plant materials confirmed very variable but also low values (from 2 to 20%) [
27], it was very challenging to perform this research. To the best of our knowledge, this is the first research that describes the contents of bioaccessible and dialyzable polyphenols in the complex of EEP and HP-β-CD. Therefore, our results were compared with similar studies which investigated polyphenols in different extracts of propolis, honey, pollen, medicinal plants or food but without cyclodextrin carrier. In the study by Ozdal et al. [
21], that investigated the bioaccessibility of TP and total flavonoids (TF) of raw propolis, an increase in TPs and TFs was confirmed during GF and IF, however, compared to the results of the undigested sample, the bioaccessibility was low. The research concluded that it would be more efficient to prepare the propolis sample in the form of extracts to increase the bioaccessibility of polyphenols.
The conditions and protocol of the
in vitro method described in this study were chosen based on the standardized COST INFOGEST method to be widely applicable to both human and animal organisms [
23]. By simulating
in vitro digestion in different foods or plant extracts, several simulation models for the evaluation of the bioaccessible content of polyphenols in IF were established: centrifugation or dialysis membrane. In IF-C, the sample from the intestinal phase is centrifuged and the supernatant representing soluble compounds, which can potentially be absorbed, is separated from the sediment with insoluble, i.e., non-absorbable compounds. In the dialysis model, the dialyzable fraction (Din) represents the amount of the sample that has passed through the semipermeable membrane and is available for absorption from the small intestine, i.e., it is bioavailable. On the other hand, the fraction outside the membrane (Dout) represents the amount of the sample that is further digested in the large intestine. Cyclodextrins (CD) create inclusion complexes with lipophilic molecules without the formation of covalent bonds, and the molecules inside the cavity are in dynamic equilibrium with free molecules in the solution. The EEP-HP-β-CD complex showed stability under OF conditions thus hydrolysis by α-amylase did not occur. As reported earlier by Shahidi and Peng [
28], molecules of polyphenols are stable in acidic conditions and in this study the complex showed stability in GF compared to OF. In the IF, the polyphenols remained stable and no degradation occurred due to the influence of slightly alkaline pH (around 7.5-8.0), showing that the polyphenol molecules in the complex have continuous stability during all three phases of GID (
Table 2,
Table 3 and
Table 4).
Despite the relatively high values of bioaccessible polyphenols after IF-C, their dialyzability through the semipermeable membrane (Din fraction) turned out to be lower, and a considerable content of bioaccessible polyphenols was obtained in the Dout fraction (
Table 2,
Table 3 and
Table 4).
By this study centrifugation model showed that the two propolis complexes had similar bioaccessibility profiles without statistical significance. However, the impact on cyclodextrin ability to pass through biological membranes can be very variable [
29,
30]. In general, cyclodextrins increase the solubility of poorly soluble compounds in water and improve the oral bioaccessibility in some cases, but limit it in others. However, since similar research has not been conducted, it is not possible to compare this study with the reported ones, but all the results are credible since they were obtained by reliable, validated procedures and analyzed by acceptable statistical methods.
5. Conclusions
Propolis samples used in this investigation were of continental origin, a poplar type of propolis. Using the HP-β-CD as a carrier and following the sample preparation method that included lyophilization, a stable propolis-cyclodextrin complex was prepared, resistant to acid-base conditions of GID, interaction with enzymes and other ingredients of digestive fluids. Spectrophotometric methods were used to determine major polyphenolic groups. High contents of TPs, TFFs and TFDs were estimated during all phases of the implemented in vitro GID method with two different models: centrifugation and dialysis tubing in obtained complexes.
Bioaccessibility of TP, TFF and TFD showed higher values for the centrifugation model compared to dialysis. Since similar research has not been conducted, our research provides new insights for improving the solubility of polyphenols from propolis samples which leads to increasing their bioavailability and bioactivity. The investigation will be continued on determining individual polyphenols of the inclusion complex using liquid chromatography, optimizing the amount of cyclodextrin and ratio of propolis/cyclodextrin, but also analyzing the potential biological activity of the complex.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org. Table S1: Preparation of simulated solutions: simulated saliva fluid (SSF), simulated gastric fluid (SGF) and simulated intestinal fluid (SIF).
Author Contributions
Conceptualization, E.P.J., K.Š., S.T., I.Ž. and A.V.; methodology, E.P.J. and K.Š.; validation, E.P.J. and K.Š.; formal analysis, E.P.J. and K.Š.; investigation, E.P.J., K.Š., S.S., D.F., M.P., A.V.; data curation, E.P.J.; writing—original draft preparation, E.P.J., K.Š., I.Ž. and M.A.; writing—review and editing, K.Š., M.A., I.Ž., A.V. and M.B.; visualization, E.P.J., K.Š., S.S., D.F. and M.P.; supervision, S.T. and M.B.; statistical analysis, M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was summarized under a PhD thesis supported by the Croatian Veterinary Institute, Zagreb, Croatia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors extend their appreciation to the Croatian Veterinary Institute, Zagreb, Croatia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Salatino, A.; Fernandes-Silva, C.C.; Righi, A.A.; Salatino, M.L.F. Propolis research and the chemistry of plant products. Nat. Prod. Rep. 2011, 28, 925. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.; Popova, M.; Bogdanov, S.; Sabatini, A.G. Chemical composition of European propolis: expected and unexpected results. Z. Naturforsch. C J. Biosci. 2002, 57, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Trusheva, B.; Bankova, V. Content of biologically active compounds in Bulgarian propolis: a basis for its standardization. Bulg. Chem. Commun. 2017, 49, 115–120. [Google Scholar]
- Saftić, L.; Peršurić, Ž.; Fornal, E.; Pavlešić, T.; Kraljević Pavelić, S. Targeted and untargeted LC-MS polyphenolic profiling and chemometric analysis of propolis from different regions of Croatia. J. Pharm. Biomed. Anal. 2019, 165, 162–172. [Google Scholar] [CrossRef]
- Pavlović, R.; Borgonovo, G.; Leoni, V.; Giupponi, L.; Ceciliani, G.; Sala, S.; Bassoli, A.; Giorgi, A. Effectiveness of Different Analytical Methods for the Characterization of Propolis: A Case of Study in Northern Italy. Molecules 2020, 25, 504. [Google Scholar] [CrossRef]
- Jug, M.; Zovko Končić, M.; Kosalec, I. Modulation of antioxidant, chelating and antimicrobial activity of poplar chemo-type propolis by extraction procures. LWT - Food Sci. Technol. 2014, 57, 530–537. [Google Scholar] [CrossRef]
- Escriche, I.; Juan-Borras, M. Standardizing the analysis of phenolic profile in propolis. Int. Food Res. J. 2018, 106, 834–841. [Google Scholar] [CrossRef]
- Blekić, M.; Režek Jambrak, A.; Chemat, F. Mikrovalna ekstrakcija bioaktivnih spojeva. Croat. J. Food Sci. Technol. 2011, 3, 32–47. [Google Scholar]
- Bankova, V.; Trusheva, B.; Popova, M. Propolis extraction methods: a review. J. Apicult. Res. 2021, 60, 734–743. [Google Scholar] [CrossRef]
- Šuran, J.; Cepanec, I.; Mašek, T.; Starčević, K.; Tlak Gajger, I.; Vranješ, M.; Radić, B.; Radić, S.; Kosalec, I.; Vlainić, J. Nonaqueous polyethylene glycol as a safer alternative to ethanolic propolis extracts with comparable antioxidant and antimicrobial activity. Antioxidants 2021, 10, 978. [Google Scholar] [CrossRef]
- Sareen, S.; Mathew, G.; Joseph, L. Improvement in solubility of poor water-soluble drugs by solid dispersion. Int. J. Pharm. Investig. 2012, 2, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Gao, R.; Shah, N.; et al. Anticancer activity in honeybee propolis: functional insights to the role of Caffeic Acid Phenethyl Ester and its complex with γ-cyclodextrin. Integr. Cancer Ther 2018, 17, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.-Y.; Luo, Y.; Liu, Y.; Wang, X.-T.; Liu, F.; Guo, M.-Z.; Wang, Z.; Liu, A.-J.; Zhang, Y.-M. Inclusion of chrysin in β-cyclodextrin and its biological activities. J. Drug Deliv. Sci. Technol. 2016, 31, 176–186. [Google Scholar] [CrossRef]
- Abbas, Z.S.; Sulaiman, G.M.; Jabir, M.S.; Mohammed, S.A.A.; Khan, R.A.; Mohammed, H.A.; Al-Subaiyel, A. Galangin/β-cyclodextrin inclusion complex as a drug-delivery system for improved solubility and biocompatibility in breast cancer treatment. Molecules 2022, 27, 4521. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, K.; Sagcan, N.; Ozulku, G.; Sagdic, O.; Toker, O.; Muz, M. Bioactive and bioaccessibility characteristics of honeybee pollens collected from different regions of Turkey. J. Food Meas. 2018, 12, 581–587. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Giampieri, F.; Zhang, J.; Ansary, J.; Pacetti, M.; Quiles, J.L.; Simal-Gandara, J.; Battino, M. Effect of in vitro gastrointestinal digestion on the bioaccessibility of phenolic compounds and antioxidant activity of Manuka honey. eFood 2020, 1, 85–93. [Google Scholar] [CrossRef]
- Bouayed, J.; Deußer, H.; Hoffmann, L.; Bohn, T. Bioaccessible and dialysable polyphenols in selected apple varieties following in vitro digestion vs. their native patterns. Food Chem. 2012, 131, 1466–1472. [Google Scholar] [CrossRef]
- Gayoso, S.; Claerbout, A.M.; Calvo, M.I.; Cavero, R.Y.; Astiasarán, I.; Ansorena, D. Bioaccessibility of rutin, caffeic acid and rosmarinic acid: Influence of the in vitro gastrointestinal digestion models. J. Funct. Foods. 2016, 26, 428–438. [Google Scholar] [CrossRef]
- Zhang, Q.; Xing, B.; Sun, M.; Zhou, B.; Ren, G.; Qin, P. Changes in bio-accessibility, polyphenol profile and antioxidants of quinoa and djulis sprouts during in vitro simulated gastrointestinal digestion. Food Sci. UStr. 2020, 8, 4232–4241. [Google Scholar] [CrossRef] [PubMed]
- Quatrin, A.; Rampelotto, C.; Pauletto, R.; Maurer, L.; Nichelle, S.; Klein, B.; Rodrigues, R.; Maróstica Jr, M.; Fonseca, B.; Menezes, C.; Mello, R.; Rodrigues, E.; Bochi, V.; EmaUSelli, T. Bioaccessibility and catabolism of phenolic compounds from jaboticaba (Myrciaria trunciflora) fruit peel during in vitro gastrointestinal digestion and colonic fermentation. J. Funct. Foods 2019, 65, 103714. [Google Scholar] [CrossRef]
- Ozdal, T.; Duygu Ceylan, F.; Eroglu, N.; Kaplan, M.; Olgun, E.O.; Capanoglu, E. Investigation of antioxidant capacity, bioaccessibility and LC-MS/MS phenolic profile of Turkish propolis. Int. Food Res. J. 2019, 122, 528–536. [Google Scholar] [CrossRef]
- Boyraci, G.M.; Er Kemal, M.; Degirmenci, A. Evaluating bioactivity and bioaccessibility properties of Turkish propolis extracts prepared with various solvents. JAN 2019, 2, 7–11. [Google Scholar]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; Clemente, A.; Corredig, M.; Dupont, D.; Dufour, C.; Edwards, C.; Golding, M.; Karakaya, S.; Kirkhus, B.; Le Feunteun, S.; Lesmes, U.; Macierzanka, A.; Mackie, A.R.; Martins, C.; Marze, S.; McClements, D.J.; Ménard, O.; Minekus, M.; Portmann, R.; Santos, C.N.; Souchon, I.; Singh, R.P.; Vegarud, G.E.; Wickham, M.S.J.; Weitschies, W.; Recio, I. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Bankova, V.; Butovska, D.; Petkov, V.; Nikolova-Damyanova, B.; Sabatini, A.G.; Marcazzan, G.L.; Bogdanov, S. Validated methods for the quantification of biologically active constituents of poplar-type propolis. Phytochem. Anal. 2004, 15, 235–40. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.; Bertelli, D.; Borba, R.; Conti, B.J.; da Silva Cunha, I.B.; Danert, C.; Nogueira Eberlin, M.; Falcão, S.I.; Isla, M.I.; Nieva Moreno, M.I.; et al. Standard methods for Apis mellifera propolis research. In The COLOSS BEEBOOK, Volume III, Dietemann, V.; Ellis, J.D.; Neumann, P. Eds.; Standard methods for Apis mellifera hive product research. J. Apic. Res. 2016, 56, 3. [Google Scholar]
- Perak Junaković, E.; Šandor, K.; Vujnović, A.; Oršolić, N.; Andrišić, M.; Žarković, I.; Vretenar Špigelski, K.; Fajdić, D.; Sinković, S.; Terzić, S. Spectrophotometric determination of main polyphenol groups in propolis samples from different regions of Croatia. Vet. Arhiv 2023, 93, 257–270. [Google Scholar] [CrossRef]
- Chen, X.; Xiong, J.; He, L.; Zhang, Y.; Li, X.; Zhang, L.; Wang, F. Effects of In Vitro Digestion on the Content and Biological Activity of Polyphenols from Acacia mearnsii Bark. Molecules 2018, 23, 1804. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Peng, H. Bioaccessibility and bioavailability of phenolic compounds. Journal of Food Bioactives 2018, 4, 11–68. [Google Scholar] [CrossRef]
- Loftsson, T.; Moya-Ortega, M.D.; Alvarez-Lorenzo, C.; Concheiro, A. Pharmacokinetics of cyclodextrins and drugs after oral and parenteral administration of drug/cyclodextrin complexes. J. Pharm. Pharmacol. 2016, 68, 544–555. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).