1. Introduction
An inspiration for undertaking the current study were the latest scientific reports clearly indicating that the soil ecosystem is under threat due to over-fertilization [
1,
2,
3,
4]. It should be mentioned that the increase in the use of fertilizers on farm fields over the past 50 years has led not only to increased yields but also to serious environmental problems. Farmers are the primary decision-makers in chemical fertilizer application [
5]. However, many of them still mistakenly believe that a higher dose of a fertilizer will guarantee a higher yield. Meanwhile, the main goal of the European Commission's "Field to Table" strategy adopted recently is to reduce nutrient losses by at least 50%, while ensuring that soil fertility has not deteriorated. This is intended to reduce fertilizer use by at least 20% by 2030. The technological progress and changing climatic conditions also cause farmers to constantly seek new (optimal) agrotechnical solutions [
5]. One of them is the no-till (NT) system, which combines elements of environmental protection with the improvement of economic and organizational factors on the farm [
6,
7,
8,
9] and mitigates negative climate change (less CO
2 and fumes are released into the atmosphere). The use of NT has increased in agricultural systems over the last 30 years [
10]. In contrast to NT, many researchers have proved that conventional intensive agricultural practices (primarily excessive use of fertilizers and pesticides as well as plowing - P system) in agricultural areas have already led to a significant decline in the quality and biodiversity of these soils [
9,
11,
12,
13,
14].
Indeed, an excess of nitrogen (N) fertilizers in particular causes a reduction in the amount of plant metabolites and a decrease in the biological activity of soils, all of which contribute to maintaining the stability of soil structure and guaranteeing its proper functioning [
9,
14,
15]. Therefore, some studies have recently been focused on the reduction of chemical fertilization through the replacement thereof by bio-organic fertilizers as a solution that can limit the use of chemical fertilizers while maintaining soil fertility [
16]. Fachini et al. [
17] have evidenced that biochar fertilizers are an excellent alternative to traditional chemical fertilizers due to their poor solubility and minimal risk of contaminating groundwater through leaching.
The biological activity of soils, which is directly related to fertility, is largely influenced by anthropogenic activities, primarily unskillful and irrational agricultural cultivation. Thus, to obtain information about the current biological state of soils and their potential fertility, it is recommended that e.g. respiration activity (RA) [
18,
19] and the concentration of humic acids (HA-like) [
20,
21] should be measured. Respiration is a universal process carried out by all soil-dwelling heterotrophic organisms using available carbon derived from organic matter [
18]. Simply, the higher the RA, the higher the abundance of living organisms in the soil environment, which is linked to the occurrence of so-called hot spots in soils [
22] characterized by the greatest abundance of microorganisms and the fastest rate of metabolic processes (the most fertile areas). HA-like are organic molecules playing an essential role in improving both soil properties and plant growth [
21]. The sources of HA-like include coal, lignite, soils, and organic materials [
21]. It has been evidenced that HA-like can have a positive effect on soil physical, chemical, and biological characteristics, including texture, structure, water holding capacity, cation exchange capacity, pH, carbon, enzymes, nitrogen cycling, and nutrient availability [
20,
21]. Consequently, the knowledge of HA in agricultural soils is extremely important but there are currently no studies investigating the relationship of HA-like content with reduced nitrogen fertilization, time in the vegetation season (beginning and end), and their correlation with yields, which is one of the aspects of the present paper.
Moreover, understanding and assessing the impact of reduced fertilizer application rates on soil fertility is not possible without recognizing the most important chemical characteristics of soils (e.g., pH, carbon and nutrient element content). The reaction (pH) of agriculturally exploited soils is generally acidic, limiting the uptake of nutrients, which are converted into a form inaccessible to plant roots and microorganisms [
3,
6,
12,
22,
23]. This usually results in a decrease in yields [
9,
16]. Importantly, nitrogen fertilization is well known for its impact on soil acidity in the arable layer (0-15 cm topsoil) [
10,
23]. Additionally, agroecosystems intensively fertilized with N may act as a source of acidifying gaseous pollutants [
23]. However, it has been revealed that the reduction of chemical fertilizers combined with the use of bio-organic fertilizers prevents soil acidification and effectively improves soil chemical properties [
16]. Concurrently, the carbon and nutrient contents in arable (mineral) soils are not high, which is also reflected in the quality of the soil and its crop yields [
13,
16,
23]. Therefore, the knowledge of individual environmental parameters allows specific activities to be undertaken to reduce the adverse effects of individual factors and mitigate the worsening environmental problem (e.g. soil acidification, nutrient depletion).
In the present study, the most important chemical and biological parameters were monitored before maize sowing and after harvesting. The statistical analysis allowed the determination of correlations between these parameters and the maize farming system (NT, P). This approach facilitated the verification of the hypothesis that, in addition to the economic aspect, reduced fertilization rates may be sufficient to maintain fertility and good quality of agriculturally exploited soils and guarantee high yields of maize.
Consequently, the main objective of the study was to check whether the 20% lower nitrogen fertilization (in agreement with the EU “From field to table” directive) may ensure good yields on the one hand and, on the other hand, may contribute to maintenance of soil fertility in monoculture maize cultivation in the NT system versus the traditional P system.
2. Materials and Methods
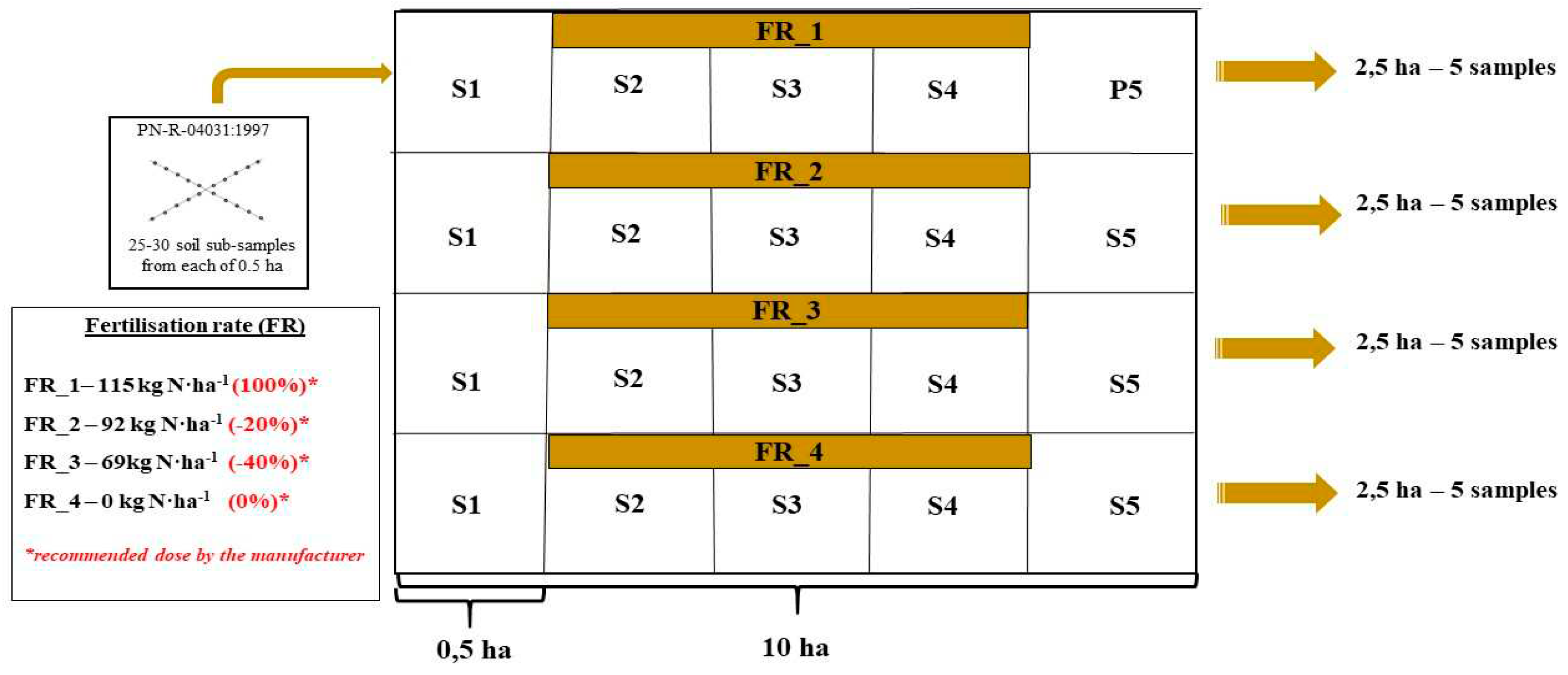
The field experiment was carried out in the arable fields of the Potulicka Foundation Group located in Janin (53°17′02″N 17°43′36″E) in NW Poland (kujawsko-pomorskie voivodeship). Two neighboring fields (10 ha each) were established for plowing (P) and no-tillage (NT) maize cultivation. The scheme of a single field with a description of the reduced fertilization pattern and rules of soil sampling is presented in
Figure 1. The large-scale area of agricultural soils (20 ha) managed by the Potulicka Foundation and dedicated to the current experiment is a guarantee of the representativity of the obtained results (taking into account the heterogeneity of the soil environment). Moreover, the Potulicka Foundation agricultural acreage is predominantly (>95%) mapped using GPS, and the precision farming system is successfully applied in the whole area [
24]; hence, precise doses of fertilizers were applied and samples for analysis were collected from the same places.
Within each of the 10 ha fields (
Figure 1), 2.5-ha "micro-plots" were exposed to the following N treatments: (1) control sites with no fertilization (0.0 kg ha
-1) from which 5 composite samples were taken and variable N rates: (2) amount suggested by the fertilizer manufacturer (115.0 kg ha
-1) represented by 5 composite samples, (3) standard fertilizer rate reduced by 20% (in agreement with the EC directive) represented by 5 composite samples, and (4) standard fertilizer rate reduced by 40% (also 5 composite samples). The greatest possible representativeness of soil material was guaranteed by the single soil sub-sampling from 25-30 randomly selected sites from each experimental plot (0.5 ha area) separated from the 2.5 ha micro-plot (
Figure 1) at a surface soil depth of 0-20 cm. The soil was collected according to PN-R-04031:1997, using an automatic sampler (Wintex 100, AgroTechnology, Poland) with an Egner stick [
25]. Soil samples were taken in two periods: (1) before maize sowing (April 2022) and (2) after harvesting the crop (November 2022). Finally, twenty soil samples represented the P system, whereas another twenty samples pertained to the NT maize cultivation system.
Since 2015, the fields have remained in a monoculture of maize planted for grain (except in 2019, when maize was harvested for silage). The maize variety grown in the experimental fields is Dekalb DKC 3730 from Bayer (Monsanto). In the studied fields, basal fertilization with urea 46% N was applied at NT sowing and dosed directly by a cultivator aggregated with a seeder to a depth of about 20 cm. In the P sowing variant, a traditional fertilizer spreader was used before tillage and the fertilizer was mixed with an approximately 5–10 cm soil layer with a cultivator. In both technologies, the doses of 0.0, 150.0, 200.0, and 250.0 kg ha-1 of urea corresponded to the N content of 0.0, 69.0, 92.0, and 115.0 kg N ha-1.
Soil acidity (pH) was determined from a 1:2.5 soil suspension (10 g of soil, 25 ml of water) prepared in distilled water. An automatic multifunctional potential meter (Hach, Lange) equipped with a glass measuring electrode was applied [
12].
Water Holding Capacity (WHC) was determined using the under-bed (suction) method. Approximately 10-cm high plastic cylinders, onto which permeable nylon fabric membranes were placed and attached with a rubber band, were used for the measurements. The cylinders were weighed, filled with soil, and then weighed again. The determination of WHC was carried out after preparation of a cuvette. Its bottom was covered with sand at such a height that the sand covered the filler of the bottle turned upside down, thus regulating a constant water level. The cylinders of soil were placed in the cuvette prepared in this way and incubated for at least 10 days until a constant mass was obtained. After completion of the incubation, WHC was calculated using the following formulas: Mw = Mt – Ms, where, Mw is the mass of water in grams, Mt is the total mass of the container and wet soil in grams, Ms is the total mass of the container and dry soil in grams (1 gram of water is equal to 1 milliliter of water, therefore, Vw = Mw). Consequently, the percentage of holding capacity (WHC%) = (Vw/Vt) * 100, where Vw is the volume of water and Vt is the total volume of saturated soil.
The total carbon (TC) content was measured using an automatic carbon analyzer TOC-VCSH SSM 5000 A (Shimadzu, Japan). 150 mg of soil was pulverized, dried, and combusted at 900◦C in a column containing a platinum and cobalt oxide catalyst. All carbon compounds were converted into carbon dioxide and detected by an infrared detector [
24].
Easily Degradable Carbon (EDC) was measured spectrophotometrically (λ = 550 nm, UV-1800, Shimadzu, Japan) in extracts prepared (2.5 g of air-dry soil sample (dw) mixed with 2 ml of 0.2 KMnO
4 in 1 M CaCl
2 (pH 7.2) and diluted to 20 ml using distilled water) according to the Weil et al. [
26] method. Details are described in Wolińska et al. [
22].
The content of N forms (NH
4-N and NO
3-N) and phosphorus (P-Olsen) were determined colorimetrically using an AutoAnalyser 3 System (Bran+Luebbe, Germany) in the prepared soil extracts (35 g of fresh soil (fw) and 100 ml of water). More methodological details are presented in our previous work [
27].
The total concentrations of potassium (K), magnesium (Mg), and calcium (Ca) were established using the flame atomic absorption spectrometry (FAAS) technique (ZA-3300 Hitachi, Japan) after microwave mineralization of the soil material (Ethos One, Milestone, Italy) in a mixture of HNO3:HCl:HF (2:1:5 mL).
Total Sulfur (TS) content was determined at the District Chemical and Agricultural Station in Lublin. The determination was carried out using the nephelometric technique. The principle of the method is to oxidize organic sulfur to SO4 and determine its content nephelometrically as BaSO4. The oxidation process while maintaining sulfur as SO4 was carried out in an electric muffle furnace (Nabertherm) at 500ºC in the presence of sodium bicarbonate and oxygen from the air. Readings were taken on a spectrophotometer capable of measuring absorbance at 490 nm (Specol 11).
Respiration Activity (AR) in the studied soils was determined with the gas chromatography (GC) technique. Briefly, 5 g of each soil sample was placed in 60-ml dark sterile bottles, tightly closed, and incubated at 20°C for one week [
27]. Both at the beginning of the experiment and after 7 days, the level of accumulated CO
2 was monitored in the headspace of the soil samples (GC, Varian CP-3800, USA). Based on the differences between the concentration of CO
2 at the start and end of the experiment, AR was calculated and expressed as the mass of produced CO
2 per the mass of fresh soil used in the experiment and per unit of time (mg CO
2 per kg of fresh soil per day).
The chemical preparation of HA-like materials was based on the Kononova and Belchikova [
28] method with modifications. Briefly, the soil samples were incubated with 0.1 M NaOH in a ratio of 10 mL of liquid per g soil for 4 h with shaking at 180 rpm·min
-1 (25°C). The supernatant was separated by centrifugation (10 min, 4000 rpm), acidified with 1 M HCl to set pH < 2, and allowed to stand overnight (HA-like precipitate). The HA-like precipitate was obtained by centrifugation at 12,000 rpm (10 min), after which it was washed with water at pH 1. The decantation process was then performed. The HA-like precipitate was stored (4°C, dark) until use. The susceptibility of the HA-like solution (0.02% HA-like, in 0.5M NaHCO
3 with 5% H
2O
2) precipitates to 5% H
2O
2 oxidation was determined by measuring the absorbance (at λ=465 and λ=665 nm, BioSpectrometers, Eppendorf) of the HA-like solutions [
28]. The absorbance was measured after 1, 6, and 24 h of oxidant treatment with simultaneous measurement of HA-like material absorbance without oxidant treatment (0.02% HA-like, in 0.5M NaHCO
3). The measured absorbance values and indices were used as proxies for estimating the degree of humification (E4/E6). All the above-mentioned measurements were taken in triplicate.
The data were statistically processed by means of SPSS 27 PL (IBM). The requirements of parametric tests were checked using Shapiro-Wilk and Levene’s statistics. In order to assess the effects of cultivation and the level of fertilization and to compare changes over time, a MANOVA with Tukey’s post hoc test was used. Then correlation analysis was conducted by calculating either Person’s r or Spearman’s rho coefficient, depending on data normality. A correlation matrix was prepared using R software [
30]. Significance was accepted at p < 0.05, and all figures present average values of the given parameter ± standard deviation (SD). For the correlation analysis, the significance of the r-values was indicated as follows: * - p < 0.05, ** - p < 0.01.
3. Results
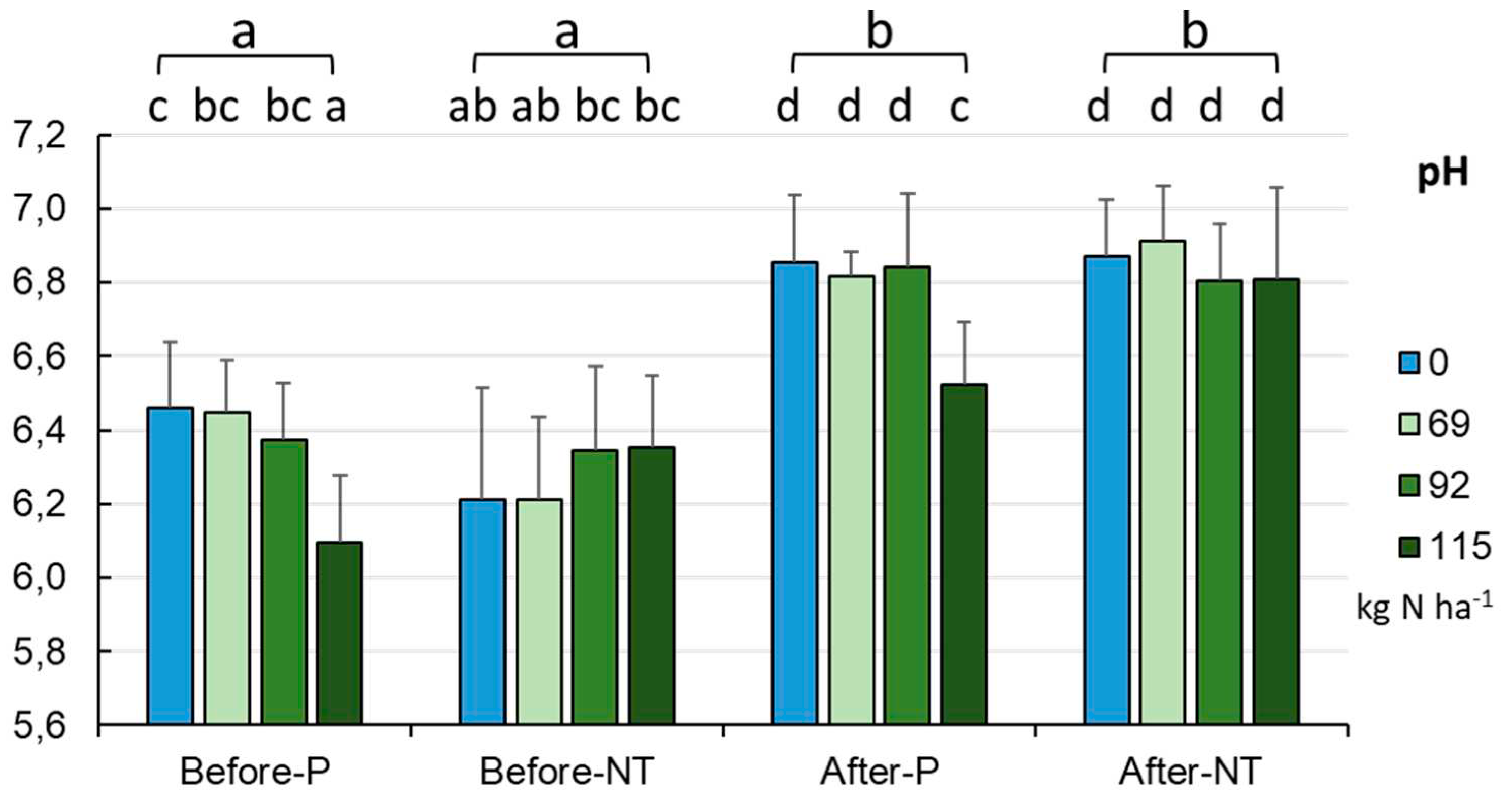
The soil pH value in the studied fields ranged from 5.69 to 6.77 (mean 6.31±0.23), as illustrated in
Figure 2. There were no statistically significant differences in the pH values between the fields depending on the farming method (pH - approx. 6.35±0.22 in the P variant and approx. 6.28±0.24, p = 0.131 in NT). However, the analysis of the effect of reduced fertilization revealed a significant effect of the fertilizer dose on the pH in the P field (p < 0.001). It was noted that the pH decreased in a statistically significant manner to 6.09 only at the highest fertilizer dose, compared to the value of 6.37-6.46 in the NT variant. Taking into account three factors (cultivation method, fertilizer application rate, and sampling dates), homogeneity in the pH of soils sampled after maize harvesting was revealed (exception: P system with 115.0 kg of N ha
-1). In turn, significant variation was observed in the soils sampled before maize sowing in spring, as their acidity ranged from the lowest pH (6.09) in the P system with the application of 115.0 kg N ha
-1 to the highest acidity (6.46) in the control non-fertilized soils (p < 0.001,
Figure 2). In general, irrespective of the N dose, the acidity was by approx. 0.4 pH unit higher (close to neutral conditions) at the end of the maize vegetation season than at the start of vegetation (
Figure 2).
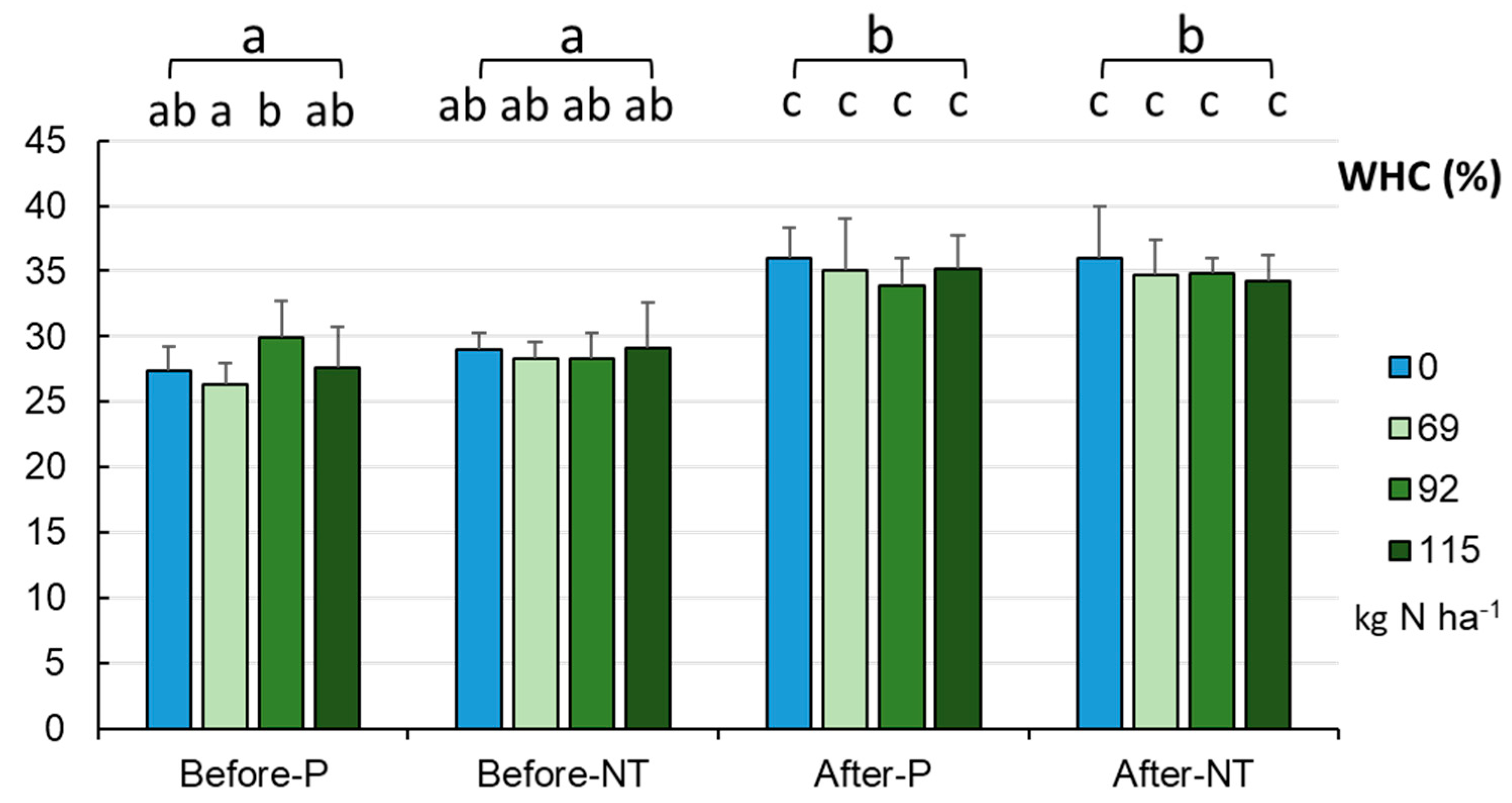
A similar trend was confirmed in the WHC level (
Figure 3). A significant increase in WHC from 28.21±2.51% in spring (before maize sowing) to 34.97±2.79% in autumn was recorded after maize harvesting (p < 0.001). In fact, this was the only factor differentiating the soils without differences within the groups (fertilizer dose) or between the cultivation methods (P, NT) in a given season (before sowing, after harvesting). The lowest WHC values were recorded in spring in the NT variant (26-27%) and the highest values of the parameter were noted in autumn without the addition of fertilizer in both systems of maize cultivation (36%).
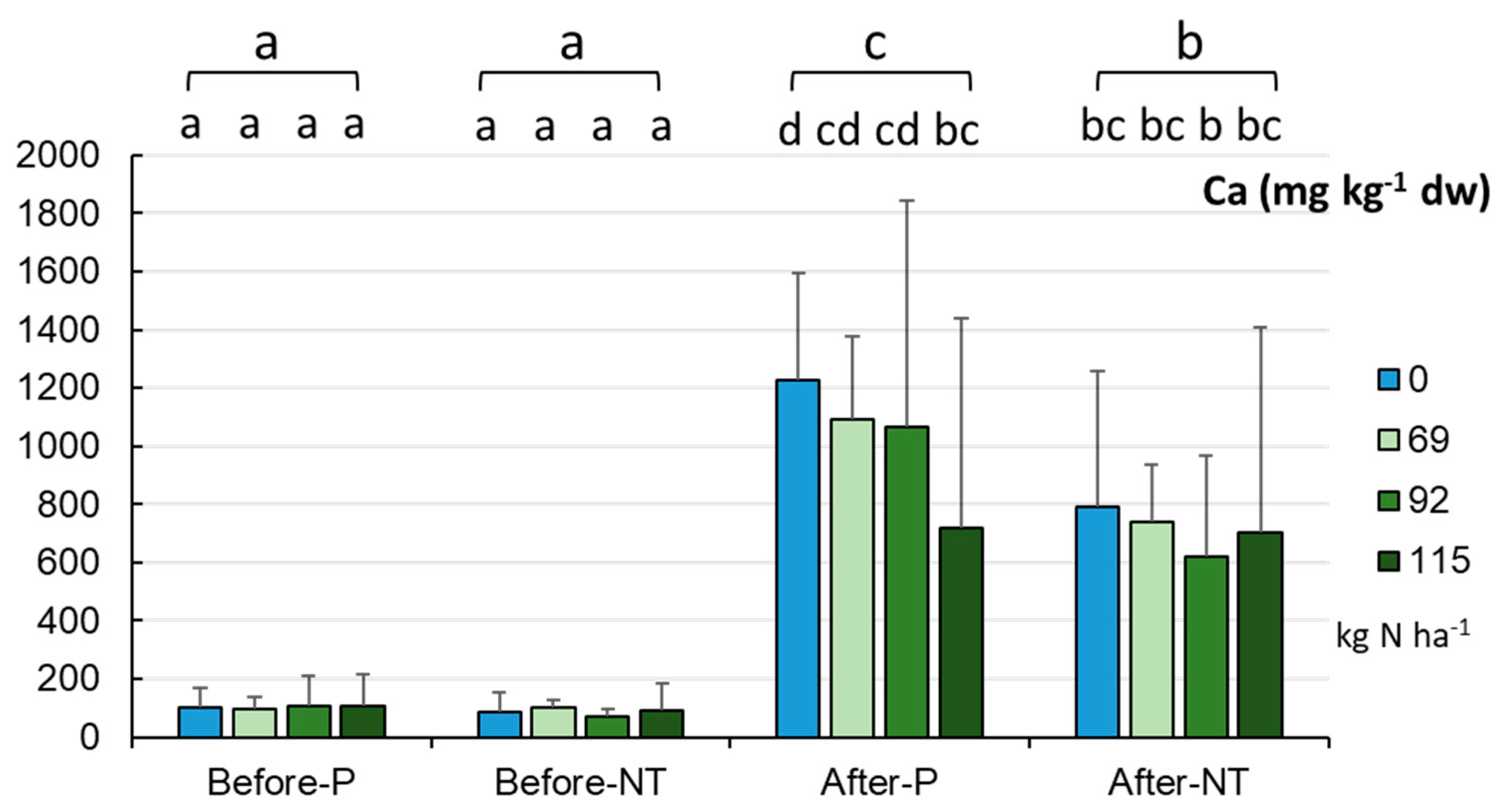
The analysis of the macronutrient abundance in the P and NT fields resulted in the determination of Ca, Mg, and K levels at the beginning and end of the maize vegetation season and with reference to the fertilizer dose. The Ca content in the studied field conditions presented in
Figure 4. In spring, the soils contained an average of 95.89±57.62 mg Ca kg
-1 dw irrespective of the cultivation system and the fertilizer dose. After the maize harvest, significantly higher Ca levels were recorded, i.e. on average 869.8±477.3 mg kg
-1 dw (p < 0.001). Soil samples taken from the two different tillage systems in autumn exhibited variation, with higher levels in the P variant (c.a. 1025 mg kg
-1 dw) than in the NT system (c.a. 713 mg kg
-1 dw, p < 0.001). The analysis of the complete set-up indicated lower Ca content in the NT and 92 kg N ha-1 variant (at both the beginning and end of the maize vegetation season), and the highest content in autumn in the unfertilized part of the P field (1224 mg kg
-1 dw). In general, there was a trend towards the lowest Ca values in spring and the highest concentration in autumn in both the P and NT systems (p < 0.001,
Figure 4).
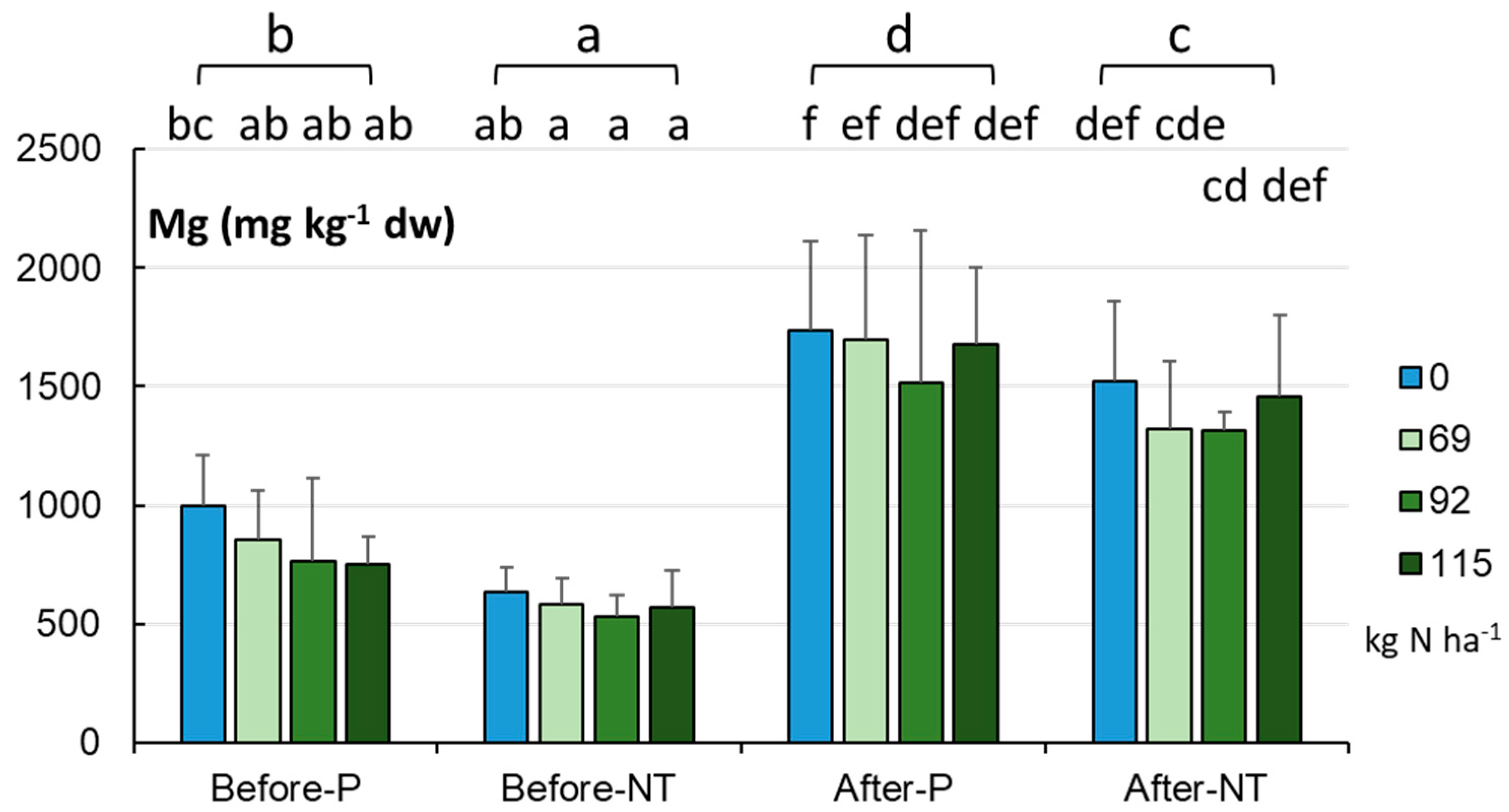
The soil Mg level at both the beginning and end of the maize vegetation season and at the various N rates is presented in
Figure 5. Similar to the content of Ca, the Mg concentration in the arable fields was significantly higher in autumn (1529.2±402.2 mg kg
-1 dw) than in spring (711.3±235.2 mg kg
-1 dw, p < 0.001). The analysis of the effect of reduced N fertilization on Mg levels both before maize sowing and after harvesting showed that the 20 and 40% fertilization reduction resulted in a slight (statistically insignificant) decrease in the content of this element, which was particularly evident in autumn (
Figure 5).
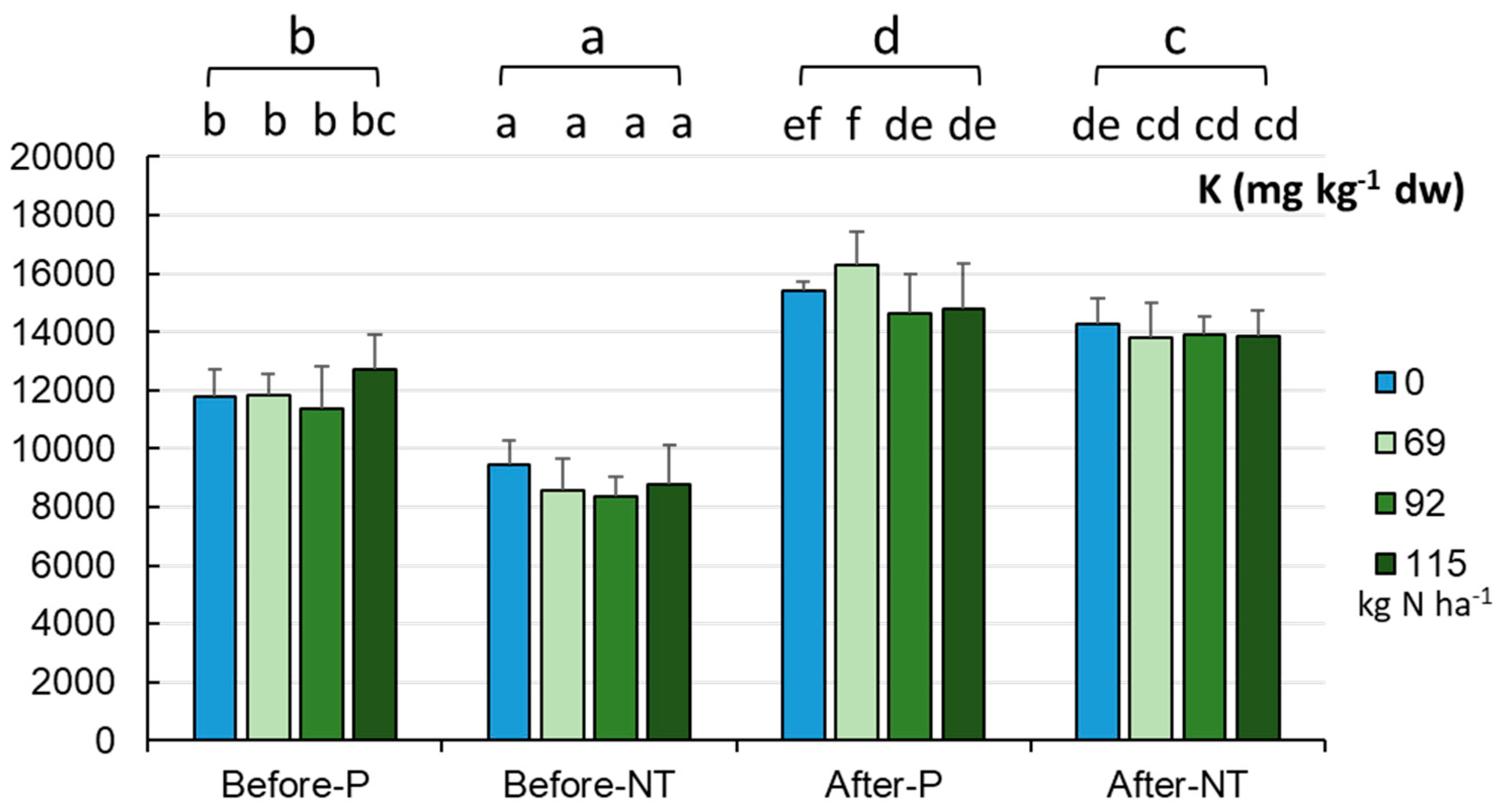
The K content in the maize monoculture soils exhibited a similar trend of changes to that of the elements reported above (
Figure 6). From spring to autumn, the K levels increased from 10343.1±1936.9 mg kg
-1 dw to 14623.36±1315.96 mg kg
-1 dw (p < 0.001). The K content displayed variation between the seasons and cultivation methods (two-factor system). The lowest values were recorded before maize sowing in NT, followed by P, and higher values were noted after harvesting in NT and P (p < 0.001). The detailed analysis revealed the lowest K content (after harvest) in the range of 8386-9438 mg kg
-1 dw, and the highest level in the P system and the 69.0 kg ha
-1 N dose variant (16310 mg kg
-1 dw). Summarizing, the reduction of the N fertilization dose undoubtedly does not reduce the potassium levels in soils (
Figure 6).
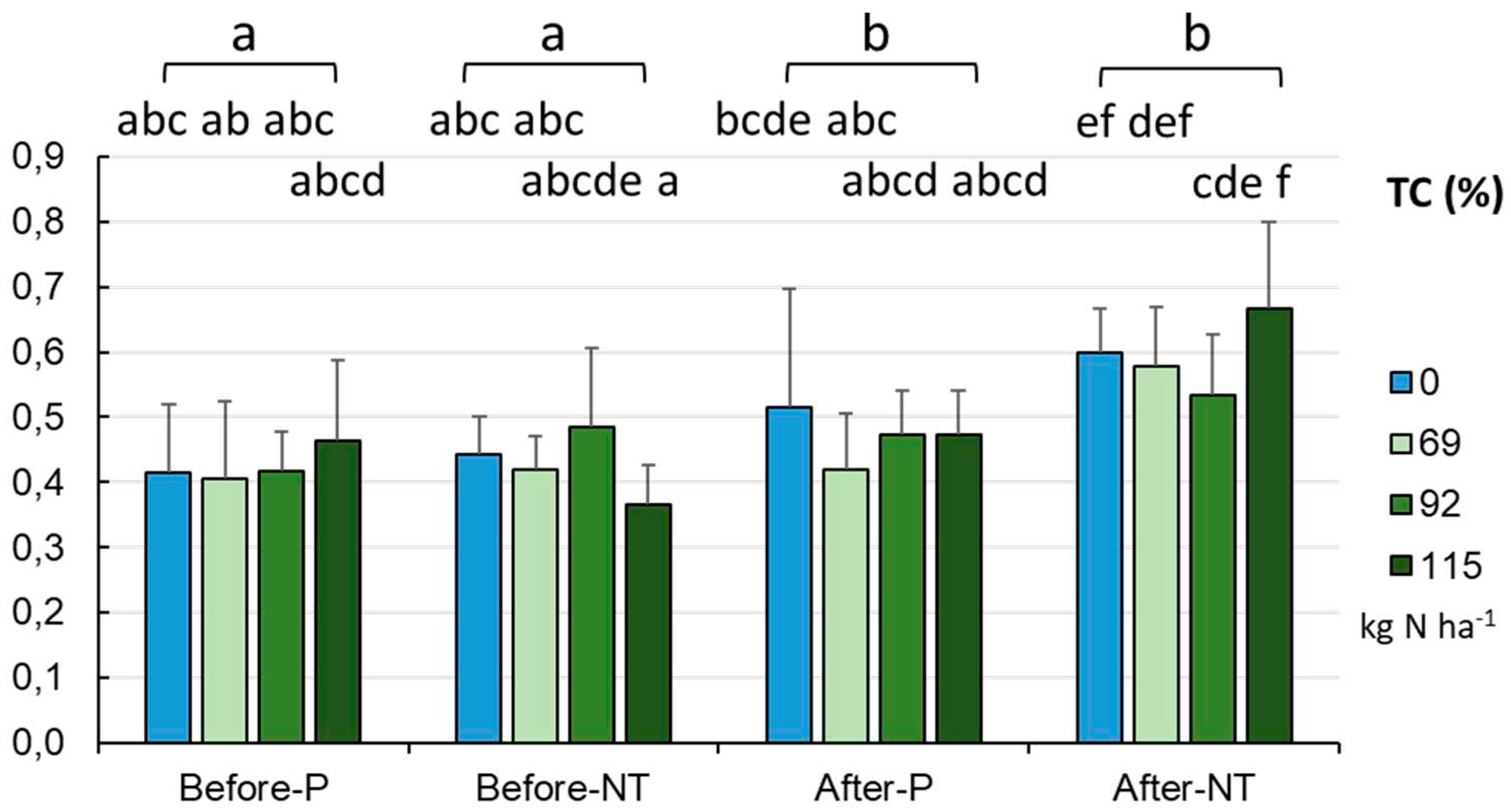
The entire TC pool was constituted by total organic carbon (TOC), whose concentration did not exceed the level of 1% (due to the mineral character of the soils), with an average content of about 0.532±0.127% (
Figure 7). The soils in the NT system showed statistically significantly higher TC content (mean 0.594±0.109%) than in the P system (mean 0.471±0.113%, p < 0.001). A statistically significant effect of the fertilization level was only observed in the NT field (after harvesting), with the lowest TC content of 0.534% at 92.0 kg N ha
-1 and the highest level (0.666%) at 115.0 kg N ha
-1 (p < 0.01).
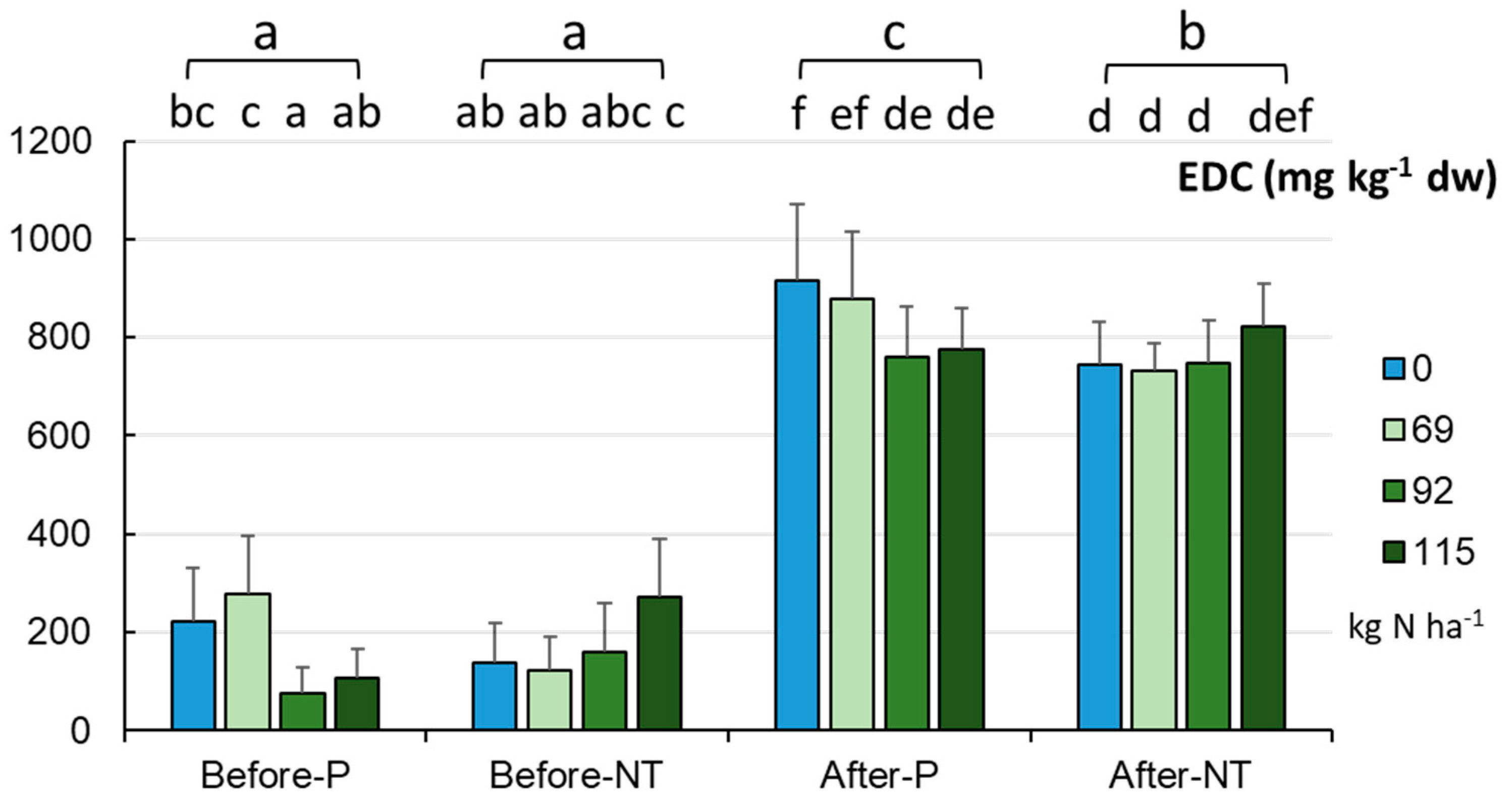
To complement the data on the content of carbon in the tested soils, its bioavailable (useful) fraction for microorganisms and plants was determined (
Figure 8). It was found that the EDC concentrations varied mainly between the sampling time points (before sowing and after harvesting). The pool of bioavailable carbon was significantly higher in autumn (mean 796.996±114.102 mg kg
-1 dw) than in spring (mean 172.012±114.10 mg kg
-1 dw, p < 0.001). No differences were noted between the cultivation systems in spring (170.5 and 173.5 mg kg
-1 dw), while in autumn EDC reached maximum values in the P system (average 832 mg kg
-1 dw) and in NT (761.8 mg kg
-1 dw, p < 0.001). It was revealed that, at the end of the growing season, the reduced fertilizer application rates did not significantly affect the level of the bioavailable form of carbon (
Figure 8).
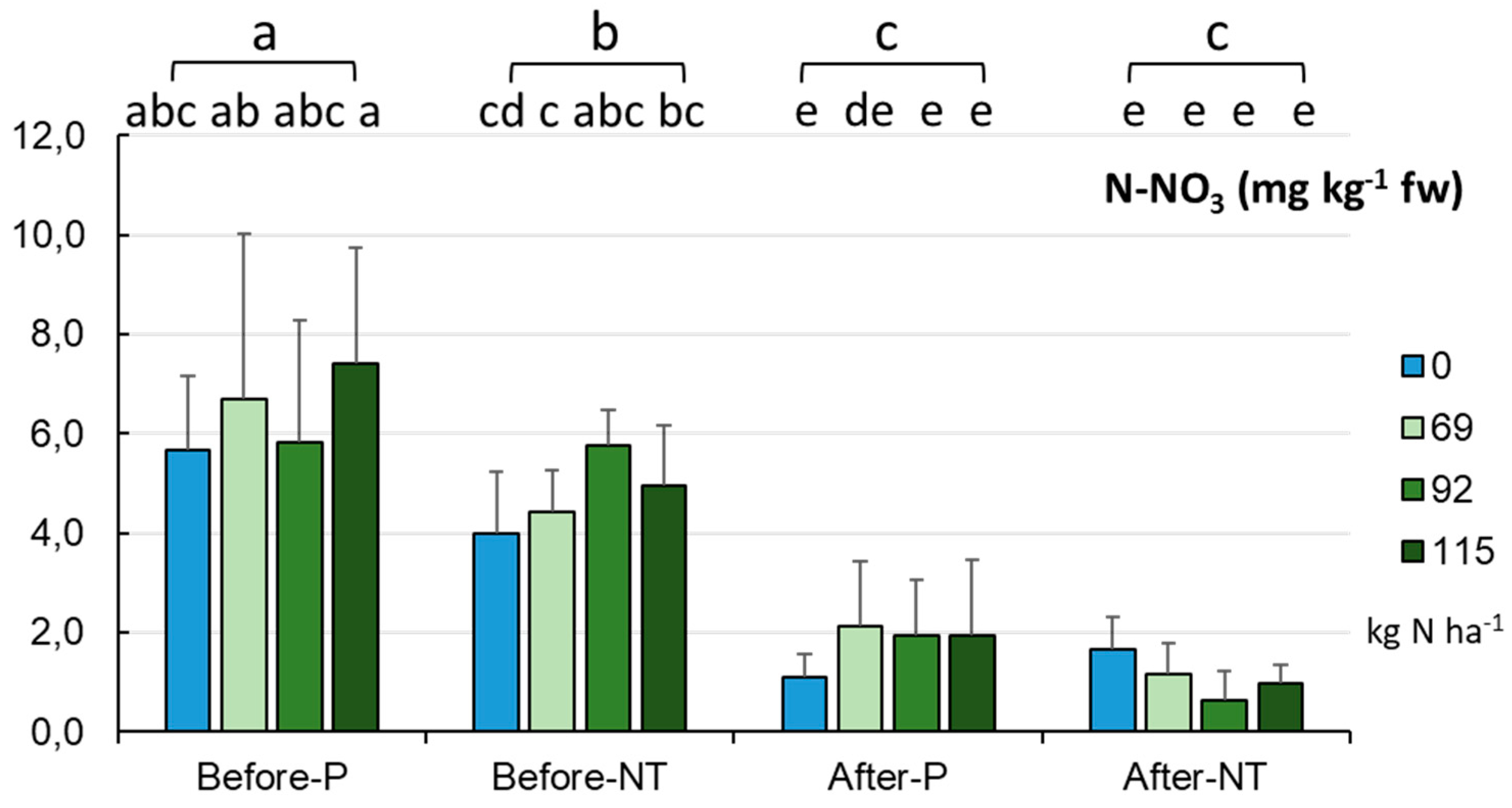
As part of the comprehensive characterization of the chemical traits of the studied soils, their content of N (nitrate, ammonium) and P forms was also determined, focusing on their bioavailable (and therefore most useful) form for plants (called Olsen P). The average nitrate (V) content (
Figure 9) decreased from 5.59±2.13 mg kg
-1 fw in spring to 1.44±1.03 mg kg
-1 fw in autumn (p < 0.001). This trend was maintained at every N fertilization rate tested. The highest values of N-NO
3 were recorded before maize sowing in the P system (6.39 mg kg
-1 fw), followed by significantly lower values in NT (4.78 mg kg
-1 fw), whilst the lowest values were noted after harvesting (p < 0.001) with no significant variation between the cultivation modes during this period (1.1-1.78 mg kg
-1 fw). This trend was also confirmed by the full factorial analysis indicating the lowest nitrate values at the end of the growing season in NT with the N dose addition of 92.0 kg ha
-1 (0.637 mg kg
-1 fw), and the highest levels were recorded in P at the beginning of vegetation with the application of 115.0 kg N ha
-1 (7.42 mg kg
-1 fw, p < 0.001,
Figure 9).
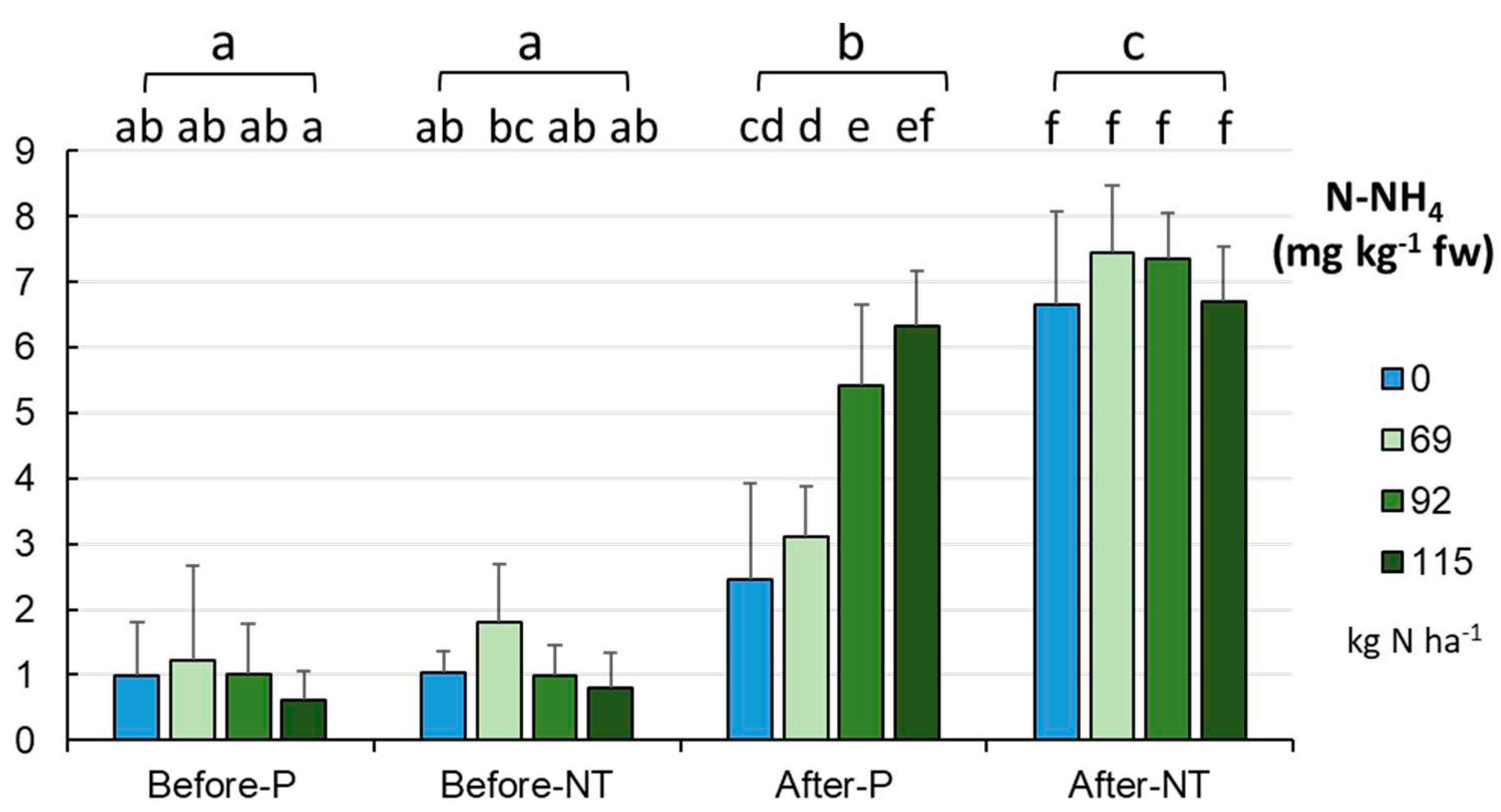
The ammonium nitrogen content increased from spring to autumn in a statistically significant manner (
Figure 10) from 1.054±0.837 to 5.685±2.067 mg kg
-1 fw (p < 0.001). After harvesting the maize (in autumn), significant differences were found between the cultivation methods (p < 0.001), i.e. there were higher N-NH
4 levels (7.038 mg kg
-1 fw) in NT than in P (4.33 mg kg
-1 fw).
This trend was maintained regardless of the N fertilization rate (
Figure 10). However, it should be noted that the 40% reduction of fertilization in the P system (in autumn) resulted in a significant decrease in the soil ammonium nitrogen content. In contrast, this relationship was not confirmed in the NT system.
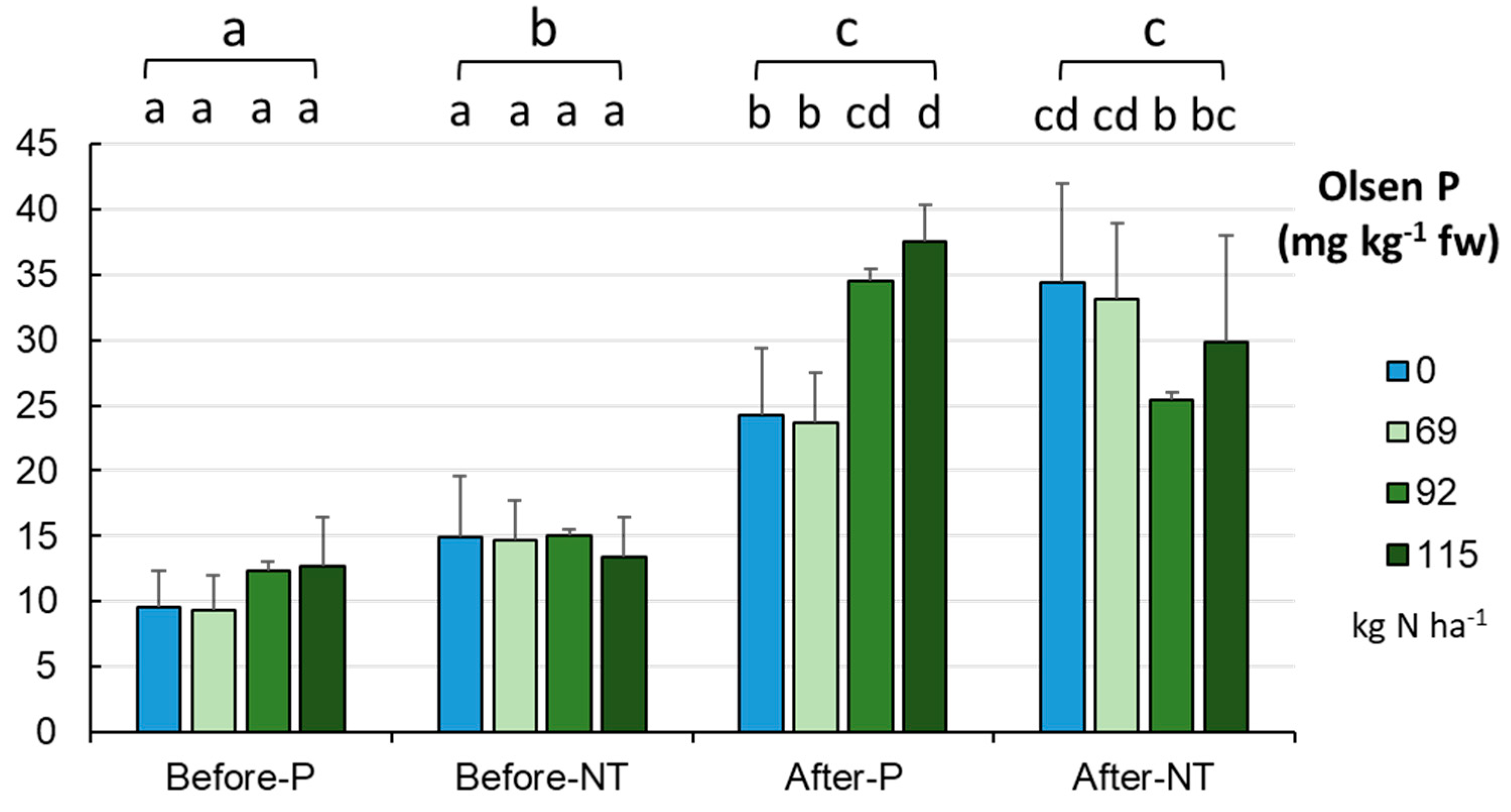
After harvesting the maize crop in autumn, the pool of bioavailable Olsen phosphorus (
Figure 11) increased from the initial (spring) values from 12.764±4.155 to 30.331±7.857 mg kg
-1 fw (p < 0.001). Soils collected in spring differed significantly (p < 0.001) in the Olsen P content between the fields studied (P, NT); however, no such differences were found between the samples collected in autumn. The analysis of all the variables revealed that there were no significant differences in soil samples exposed to the different levels of fertilization and collected before maize sowing, while samples taken after maize harvesting seemed to be more diverse in this respect (p < 0.001). In the P system, the Olsen P level was significantly lower in the control soils (without fertilization) and in the low fertilization variant, while a significant reduction in the bioavailable phosphorus content was recorded at 92.0 kg N ha
-1 in NT. At the same time, the Olsen P level was significantly higher in samples taken in autumn than in spring (
Figure 11).
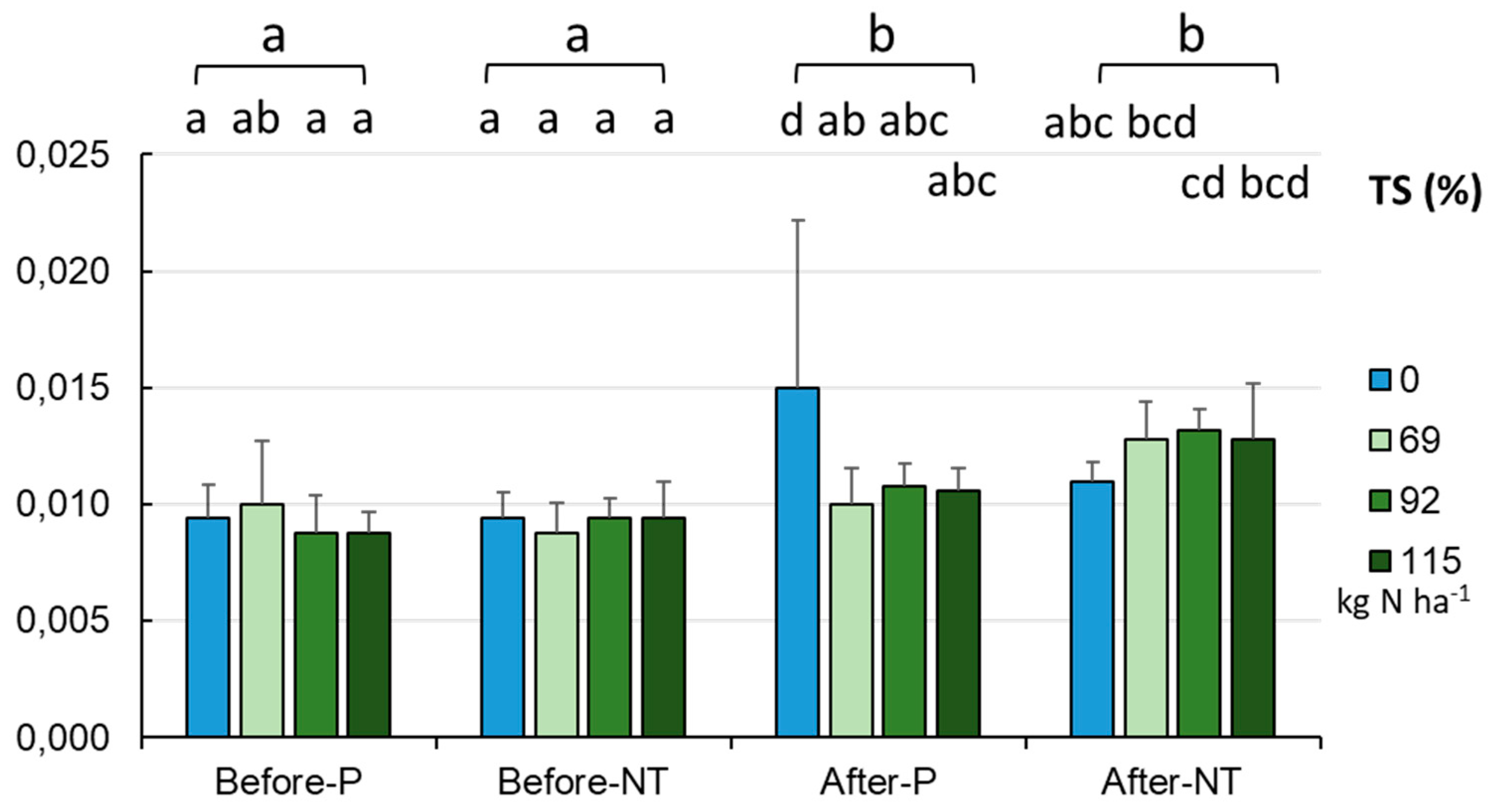
There was also a significant increase in the TS content (
Figure 12) from spring to autumn (0.009±0.001% in spring and 0.012±0.003% in autumn, p < 0.001) with no significant differences between the cultivation methods at the different sampling dates. The sulfur levels were similar in spring (0.0088-0.009%), slightly higher in autumn in the P system (0.01-0.011%), and the highest in the NT system (0.012-0.015%, p < 0.001). Taking into account the N fertilization rate, it was found that the reduced N doses did not affect the TS level (
Figure 12).
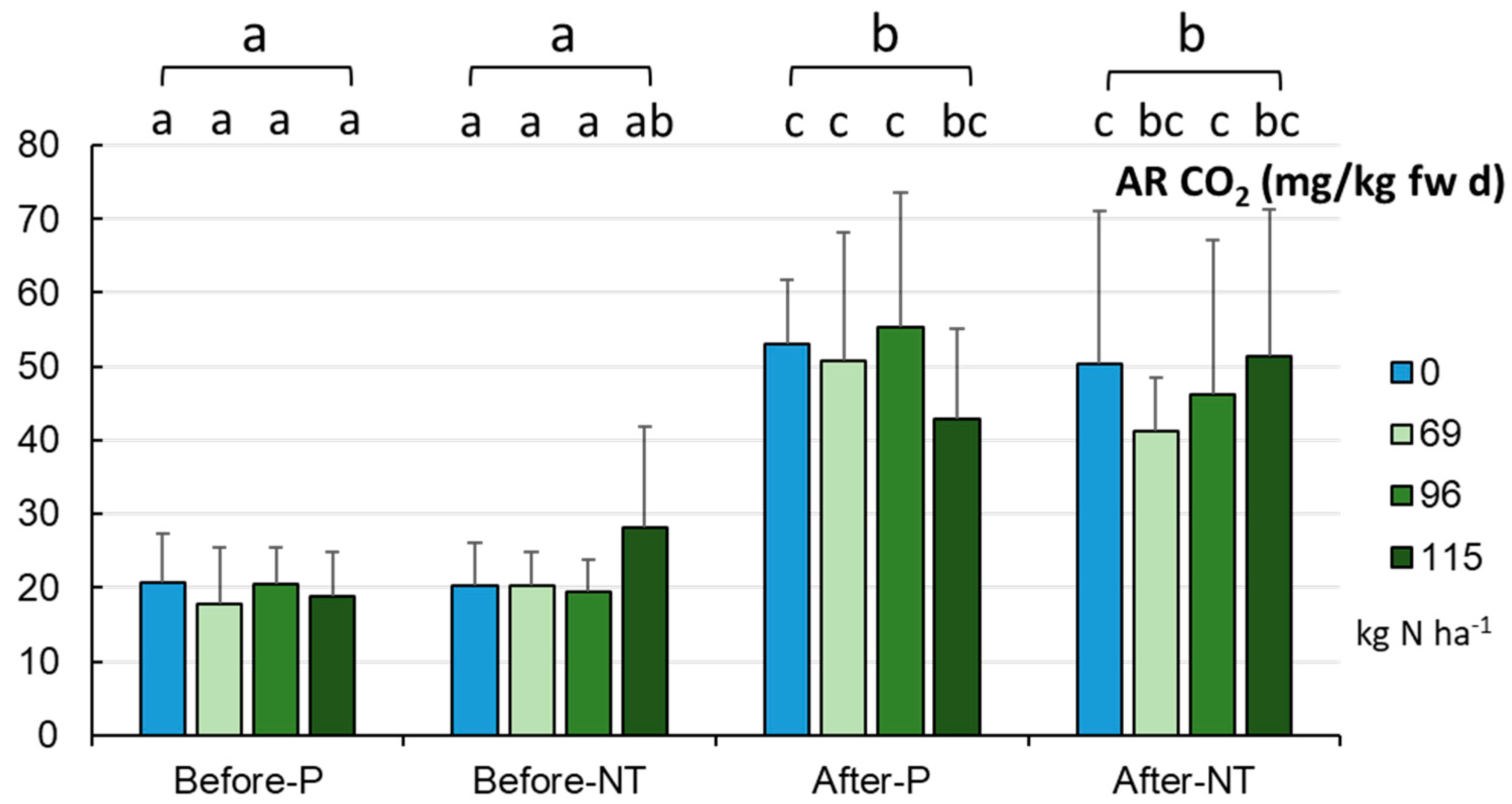
The respiratory activity (AR) of the tested soils (
Figure 13) was significantly higher after maize harvesting in autumn (48.9±16.7 mg CO
2 kg
-1 fw d
-1) than before maize sowing in spring (20.7±7.7 mg CO
2 kg
-1 fw d
-1, p < 0.001), showing no variation between the cropping systems in each term of the vegetation season. It was shown that the highest N dose recommended by the manufacturer (115.0 kg ha
-1) in the P system resulted in a decrease in AR, whereas the reduced doses (in accordance with the UE suggestion: by 20% and even by 40%) had a positive impact on the level of AR (
Figure 13). In the NT system, the 20% reduction in N fertilization was shown to have no statistically significant effect on AR.
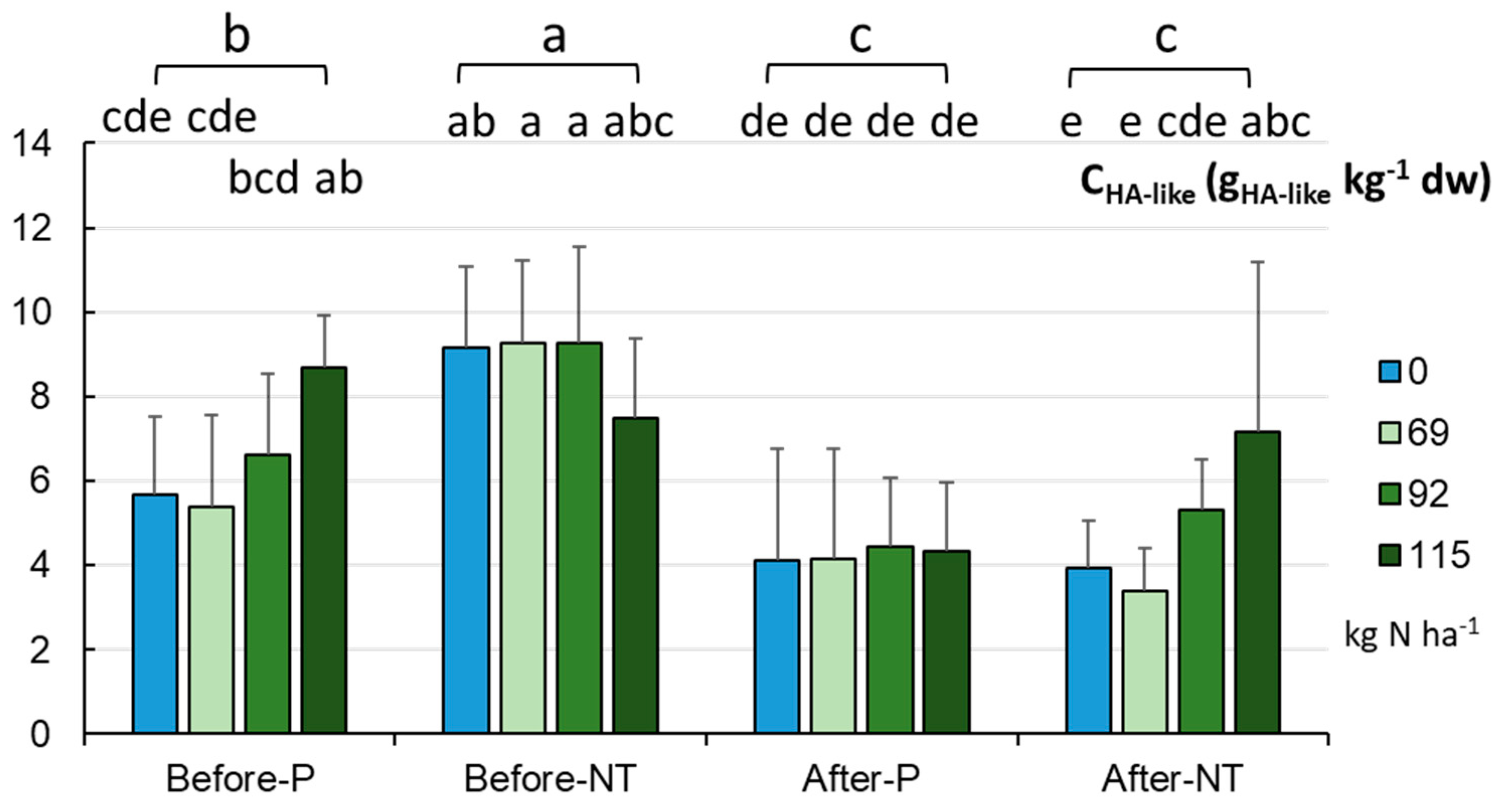
The level of HA-like substances obtained in the experiment is presented in
Figure 14. The content of HA-like substances was in the range of 2.37-13.66 gHA-like kg
-1 (mean 7.69±2.42 g gHA-like kg
-1) and was significantly higher (p< 0.001) in the NT than P system (8.79±2.11 and 6.58±.21 gHA-like kg
-1, respectively). The HA-like values ranged between 5.39 and 13.66 gHA-like·kg
-1 in the NT field and between 2.37 and 11.68 gHA-like kg
-1 in the P variants. Our results suggest that, before maize sowing in the NT variant, it is not necessary to apply the dose of N suggested by the manufacturer, as it resulted in a lower level of HA-like substances, whilst it is more reasonable to reduce N fertilization by 20 or even 40% at the beginning of the vegetation season, in contrast to the end of vegetation when the maximal N dose resulted in the highest content of HA-like substances in the soil.
The P experimental field showed different HA-like values depending on the amount of fertilization (p < 0.05), i.e. the highest level of humic substances was recorded at the dose of 115.0 kg N ha
-1 (8.67 gHA-like kg
-1) compared to the other doses (5.37-6.60 g gHA-like kg
-1). The analysis of the full model showed significantly lower HA-like values in the P field at 69 kg N ha
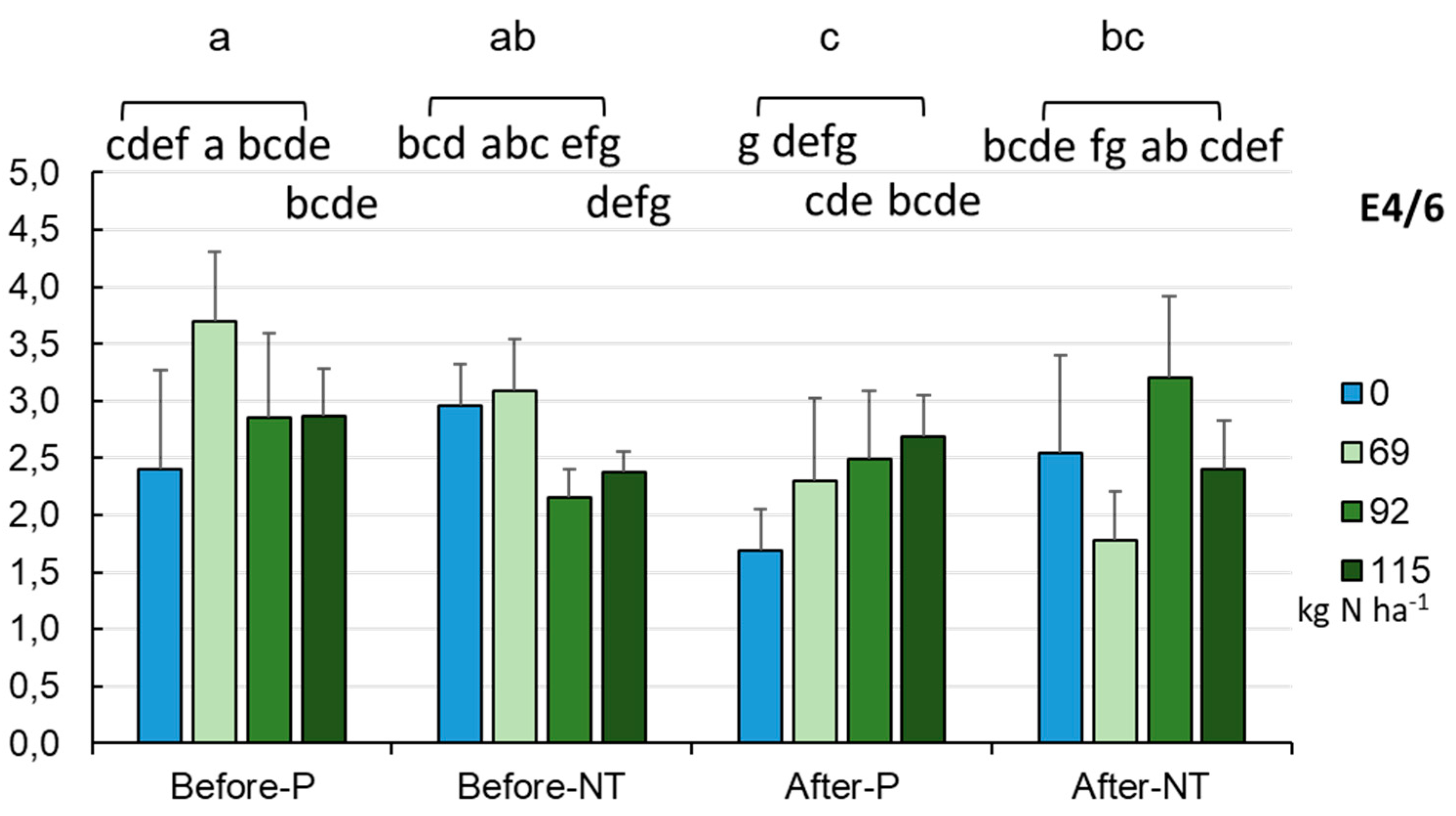
-1, while significantly higher values were recorded in the NT system with fertilization between 0.0 and 115.0 kg·ha-1 (p < 0.001). The E4/6 ratios of HA-like substances were between 0.396 and 4.880 (2.801±0.692). Significantly higher values (p <0.05) were recorded for HA-like materials isolated from the P soils (2.957±0.813, range: 0.396-4.880), while lower values were recorded for HA-like substances obtained from the NT soils (2.646±0.506, range: 1.727-4.000). Furthermore, significantly different E4/6 ratios of HA-like substances were obtained in the soils treated with the different amounts of fertilization (p < 0.001). In the case of the HA-like substances isolated from the P system, a significantly higher E4/6 ratio was recorded when 69.0 kg N ha
-1 was applied (3.699), while the E4/6 ratio for the HA-like materials obtained from the NT field variant was significantly higher in the control combination (2.961) and at 69.0 kg N ha
-1 (3.091). The analysis of the full model data evidenced statistically significant differences between the E4/6 ratios obtained from the NT at 92.0 and 69.0 kg N ha
-1 (3.699, p < 0.001) (
Figure 15).
In order to examine the relationship between the studied soil properties and the yield obtained (after maize harvesting,
Table S1), a correlation analysis was performed for the seasons separately (Supplementary material,
Figures S1 and S2) and for the entire data set (
Figure 16). In the description of the data, the measure of the correlation and its power and direction are given, and statistical significance is denoted as follows: * p < 0.05, ** p < 0.01. To avoid duplication of the correlations, they are presented based on half of the matrix. Before maize sowing (spring,
Figure S1), positive correlations were found among pH and the level of Ca (r = 0.232*), Mg (r = 0.209*), EDC (r = 0.279**), and absorbance coefficient E4/6 related to the level of humic substances (r = 0.186*). In turn, negative relationships were shown between soil acidity and Olsen P (r = -0,436**) as well as the HA-like level (r = -0.323**). At the beginning of the vegetation season, WHC correlated directly proportionally with HA-like levels (r = 0.353**), AR (r = 0.231*), and TC (r= 0.191*). The strongest positive correlations in the spring season were determined between the abundance of Mg and Ca (r = 0.589**) and Mg and K (r= 0.626**), whereas the strongest negative correlations were found between Olsen P and Mg (r = -0.599**), K (r = -0.532**), and Ca (r = -0.471**). Equally noteworthy is the correlation between EDC and TS (r = 0.485**). After maize harvesting (autumn,
Figure S2), it was also possible to determine correlations with the maize crop obtained in 2022 (
Table S1). The knowledge of the yields was available owing to the principles of precision agriculture applied on the farm of CGFP Ltd, which made it possible to estimate that the average maize yields amounted to 4095.75 kg ha
-1 in the P system and 2162.05 kg ha
-1 in the NT system (
Table S1), suggesting that the P system is better for monoculture maize cultivation. The yield was affected by the drought phenomenon that occurred in the kujawsko-pomorskie voivodeship in 2022. Interestingly, according to the data, both fields (P, NT) were characterized by excellent yields without additional fertilization (
Table S1). However, when the maximum fertilization rate suggested by the fertilizer supplier was assumed as 100%, it was shown that the 20% reduction in N fertilization in the P system resulted in an approximately 18% decrease in the yield, while the 40% reduction rate decreased the yield by approximately 6.7%. The situation was different in the case of the NT system, where the reduction of fertilization by 20% contributed to an approx. 20.4% increase in the maize yield versus the manufacturer's recommended rate, while the 40% reduction of the fertilization dose resulted in a 21.3% increase in the yield (
Table S1).
The correlation analysis evidenced that the maize crop was mainly affected by the content of Mg (r = 0.740**) and Ca (r = 0.494**), followed by Olsen P (r = -0.353**) and EDC (r = 0.363**). Other directly proportional statistically significant relationships were found for the effects of K (r = 0.296*), TS (r = 0.258**), WHC (r = 0.235**), and TC (r = 0.219*) on the maize yield (
Figure S2). In addition to the correlations related to the yield, after the maize harvest, the most significant relationships were noted between pH and Ca (r = 0.534**) and Olsen P content (r = -0.527**). Furthermore, a significant correlation was determined between Ca and EDC and Olsen P levels. Mg was found to be significantly correlated with EDC, while K was significantly correlated with the ammonium nitrogen content (
Figure S2).
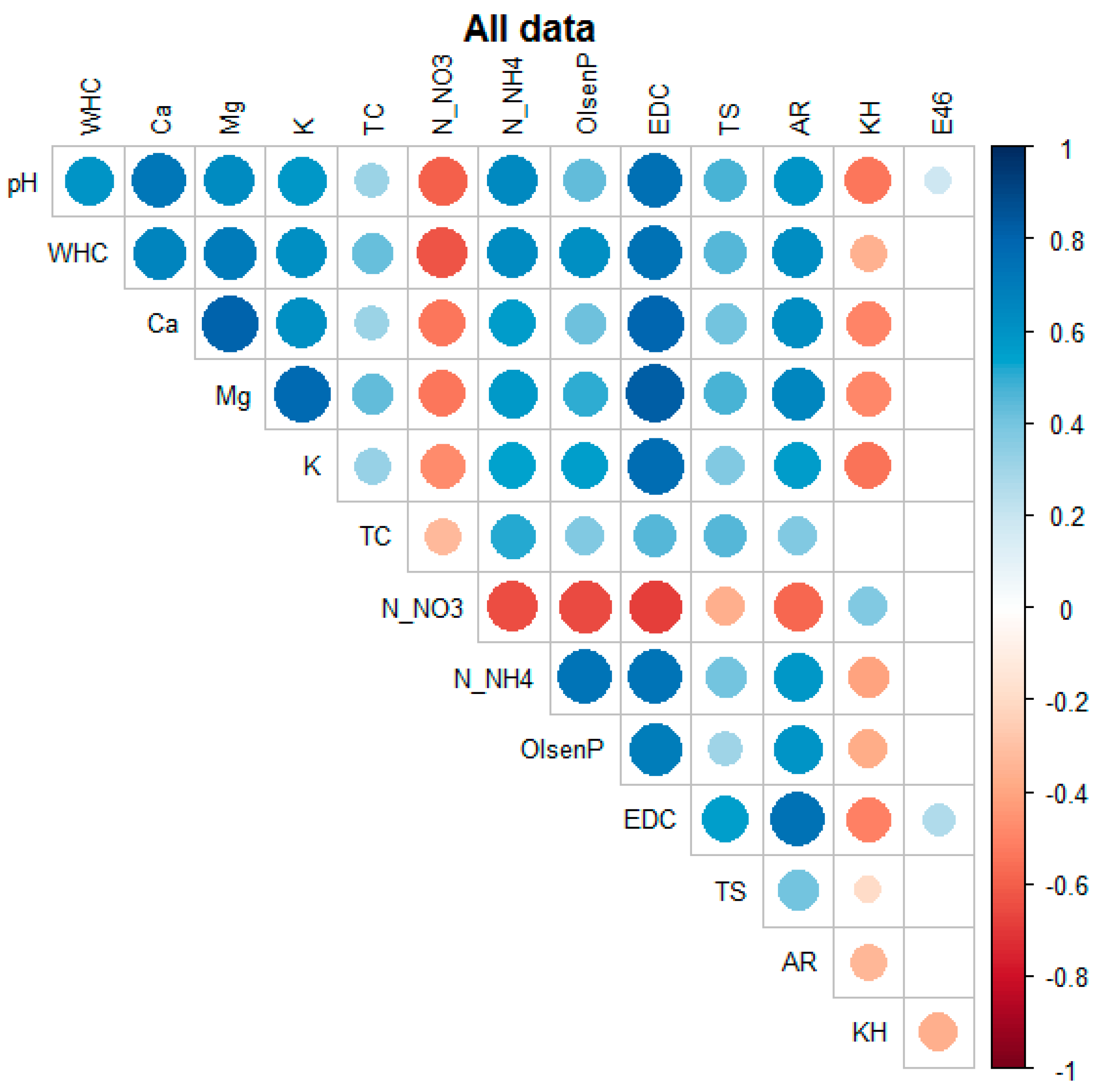
The compilation of the entire dataset showed numerous statistically significant correlations between the variables under study, as presented in
Figure 16. It was evidenced that all the studied factors were important for maintaining good soil quality and interdependent, as confirmed by these correlations. Therefore, the factors should be constantly monitored. In general, soil pH was highly correlated with EDC (r = 0.760**), Ca (r = 0.725**), N-NH
4 (r = 0.644**), Mg (r = 0.636**), N-NO
3 (r = -0.596**), AR (r = 0.598**), WHC (r = 0.591**), and K (r = 0.583**) and negatively correlated with the content of HA-like substances (r = -0.530**). The WHC of the studied soils was strongly positively associated with EDC (r = 0.749**), Mg (r = 0.703**), Ca (r = 0.662**), N-NH
4 (r = 0.639**), AR (r = 0.624**), K (r = 0.616**), Olsen P (r = 0.615**), TS (r = 0.453**), and TC (r = 0.430**) and negatively correlated with N-NO
3 (r = -0.621**). The N-NO
3 levels seemed to be significantly negatively correlated with EDC (r = -0.686**), Olsen P (r = -0.659**), N-NH
4 (r = -0.644**), and AR (r = -0.574**). The N-NH
4 content was most strongly associated with EDC (r = 0.735**), Olsen P (r = 0.734**), and AR (r = 0.583**), followed by TS (r = 0.406**), HA-like substances (r = -0.405**), and E4/6 (r = 0.199**). Olsen phosphorus was additionally correlated with EDC (r = 0.694**), AR (r = 0.593**), TS (r = 0.300**), HA-like content (r = -0.374**), and E4/6 (r = 0.143*). The EDC form of carbon was strongly dependent on AR (r = 0.744**), TS (r = 0.556**), and HA-like materials (r = -0.502**) and weakly correlated with E4/6 (r = 0.135*). The total sulfur content was positively related to AR (r = 0.407**) and negatively correlated with the content of humic substances (r = -0.191**). Finally, AR was negatively correlated with HA-like materials (r = -0.334**).
4. Discussion
In this study, we intended to verify whether a reduction in N fertilization by at least 20%, as recommended by the EC under the "From Field to Table" strategy, affects the yield of maize and the chemical and biological parameters of soils that guarantee their fertility. Moreover, in planning the field experiment, we went a step further from the EC recommendation and simultaneously tested a 40% reduction in N fertilization. The undoubted advantage of this study are the results achieved in a multi-area field experiment conducted by CGFP Ltd (each field is an area of 10 hectares) rather than from small experimental plots, which incomparably improves the quality and precision of conclusions, since we worked on representative soil material, taking into account many environmental variables.
There is no doubt that fertilization is an important agricultural aspect that cannot be completely disregarded if farmers want to achieve satisfactory harvests. Optimal N management is critical for efficient crop production and agricultural pollution control [
31]. However, fertilizers are often overused on smallholder farms, which not only results in lower crop yields but also causes damage to the environment and human health [
31,
32]. Literature data emphasize that tillage is an important management practice, with conventional tillage (CT) helping to manage weeds, prepare the soil for sowing, and mix soil nutrients and crop residues [
9,
33]. However, CT can also decrease soil water retention, accelerate soil erosion (given that there is minimal crop residue retained on the soil surface), increase oxidation of organic matter, and deplete N and other nutrients in soils [
9]. In contrast, no-tillage (NT) management can create a cooler microclimate in the topsoil with higher water content, i.e. more favorable for microbial growth compared to CT [
9,
33]. These authors studied a 48-y wheat cropping trial in semi-arid subtropical Australia with application of different tillage practices (NT and CT) and N fertilization rates (0 and 90 kg N ha
-1) in a Vertisol at a soil depth from 0 to 10 cm. They found that TOC significantly increased only when the N fertilizer was applied under NT. Similarly, we also noted that, in the case of maize monoculture, NT favors the presence of a higher TC pool. Finally, the authors concluded that NT and N fertilization exerted an important impact on soil properties and functioning over decades in Vertisol in the semi-arid subtropical region [
9]. Su et al. [
33], who compared crop yield data from the NT and CT systems, noted that the adoption of NT practice overall led to a yield decrease. This is consistent with findings reported by other researchers [
34,
35,
36], and the same trend was evidenced in our study in the maize monoculture after one year of observation.
Our experiment evidenced that the combination of the three factors (time of soil sampling, cultivation system, and N dose) had an impact on soil chemical and biological features. Indeed, the majority of the studied parameters (WHC, Ca, Mg, K, EDC, N-NH
4, Olsen P, TS, AR) reached higher levels after maize harvesting (autumn) than at the beginning of the vegetation season (spring) when an increase in the content of N-NO
3 and HA-like substances was found. Our results may be supported by the findings reported by [
37,
38], who noted analogical differences in pH, TOC, N forms, P, K, Mg, Ca, and AR when comparing spring and autumn soil sampling. We also emphasized that, in the case of the P cultivation system, the highest dose of N fertilization (115.0 kg ha
-1) resulted in a lower pH value; therefore, when farming maize with plowing, it is worth reducing the fertilization rate to prevent soil acidification. Furthermore, it has been shown that soil acidity drastically reduces maize crop yields and that minimum tillage has a positive effect on soil acidity [
9,
39]. Interestingly, in the NT system, the pH value at the highest fertilization rate was similar to that in the 20% fertilization reduction variant, which suggests that the cultivation system has a crucial role in soil acidity. It is worth mentioning here that soil acidity is accelerated by crop production practices, mainly by application of nitrogenous fertilizers to enhance crop productivity [
39]. Qiao et al. [
40] reported that N additions in tea plantations acidified soils (a significant decrease by 0.41 pH unit on average) and produced soil nutrient imbalance [
40]. Kou et al. [
41] concluded that N fertilizer reduction directly decreased the amount of NO
3-N in the soil and then alleviated the soil acidification caused by excessive use of N fertilizers. In contrast, no significant difference in TN was observed upon 25% reduction of chemical nitrogen fertilizers in the study conducted by Liu et al. [
42]. It was also assumed that soil pH declined significantly when the fertilizer level exceeded 200 kg N ha
-1 [
23].
The analysis of the effect of 20% reduction of N fertilization (in accordance with the EC recommendation) showed that this amount is better (than the dose suggested by the fertilizer manufacturer) for maintaining Ca levels (likewise in the case of the 40% reduction) as well as the Olsen P pool and AR levels. In recent years, studies have been conducted to determine the effects of N enrichment on AR in soil ecosystems [
43,
44]. However, the effect of N addition on AR remains still controversial and unclear. AR was sometimes found to increase during the first year of N fertilization but decrease in subsequent years [
43,
45]; hence, we decided to continue our experiment for the next vegetation season. Moreover, with respect to the TC content in the field under the NT system, it was observed that the 20% N fertilization reduction resulted in a lower carbon pool after the maize harvest. Due to the lack of similar studies (large-scale experiment with fertilizer reduction recommended by the EC), it is currently difficult to compare our observations with other data. However, our field experiment is being continued in the next maize vegetation season (in the same fields), which will allow verification of the present results and observed trends.
Nevertheless, it was found that the reduction in the N fertilization did not affect the level of most of the monitored parameters, e.g. WHC (no threat to the water-holding properties of soils), did not deteriorate the quality of soils in terms of Mg abundance, and did not deplete the K pool. Our study also showed that the fertilizer reduction did not have a statistically significant effect on the carbon fraction of EDCs or the nitrate abundance in the soils. Our data are compatible with the study conducted by Zhang et al. [
9], who indicated that tillage did not have a significant effect on carbon pooling in the soil environment. The variable rates of reduced fertilization were not significantly related to the TS levels. There was also no effect of the N dose on the humic substance levels in the P field, although a decrease in the HA-like substances was observed in the NT system after the 20 and 40% fertilization reductions, while the highest humic substance levels were recorded at the standard fertilization rate.
While analyzing the values of the E4/6 coefficient in the two tillage systems: P and NT and at the two sampling dates: before sowing and after harvesting the crop, it can be concluded that the HA-like substances in the soils cultivated in the NT system were characterized by a higher E4/6 coefficient after harvesting the maize. It should be added that this correlation is not homogeneous in this cropping system, as the relationship between the land use and the degree of humic polymerization varied depending on the location of soil sampling in specific rasters. As demonstrated by Watanabe et al. [
46], an increase in the degree of humification with a decreasing amount of HA-like substances due to changes in land use suggests selective decomposition of humic acid molecules or parts with a low degree of humification. The higher values of the E4/6 ratio of the HA-like substances analyzed in the soils after the maize harvest may indicate the presence of non-humidified material due to the presence of proteins and carbohydrates. Similar conclusions were made by Morán-Vieyra et al. [
47], who indicated that a higher value of the E4/6 ratio in soil material from agricultural soils. Humic acids extracted from different soils are characterized by different E4/6 ratios. The cause of this phenomenon is the different structure of HA-like molecules and their sizes. It has been indicated that components susceptible to oxidation significantly affect the activity of soil microorganisms and thus soil fertility [
29]. Moreover, E4/6 values greater than 6 suggest the predominance of fulvic acid-type compounds, while values less than 6 point to the predominance of humic acids in the humic substance solution studied. The data in
Figure 1 show that the E4/6 parameter in all the studied soils had values below 6, indicating an advanced stage of humification of organic matter, with a predominance of highly polymerized humic acid-like compounds. As suggested by Licznar et al. [
48], the low values of this parameter indicate the occurrence of degradation processes that are not conducive to the humification of organic matter and its transformation into high-molecular HA-like substances. HA-like substances with higher values of this ratio characterize fertile and biologically active soils. Many authors have suggested that the value of the E4/6 ratio, calculated for HA-like solutions, is a criterion that characterizes soil type and humus quality. It is generally accepted that the value of this quotient is inversely proportional to the molecular weight of these compounds. Given the assumption made by Licznar et al. [
48] and the values of the E4/6 coefficient below 3 determined in the studied soils with regard to HA-like substances, it can be assumed that there are degradation processes in both fields (P, NT), which do not support the humification of organic matter and its transformation into high molecular humus compounds. Hence, samples with a reduced E4/6 ratio may require supplementation with humus-enhancing preparations. Importantly, an increase in the E4/6 ratio was observed in the NT system, which may indicate the first signs of humus level restoration.
The above observations may be linked to the fact that CGFP Ltd continuously monitors fields (precision farming) and responds to decreases or increases in individual soil parameters. The effectiveness of precision farming techniques used on the farm is also reflected in the condition of soils treated as controls (without the fertilizer), which are rich in nutrient elements. Consequently, as shown by our results, it seems reasonable to reduce N fertilization according to the EC “From field to table” strategy, especially since literature data proved that higher rates of N fertilization cause soil secondary salinization and acidification, and thus inhibit soil enzyme activities, functional diversity of microbial communities, and nitrification capacity[
49].