Submitted:
28 July 2023
Posted:
31 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
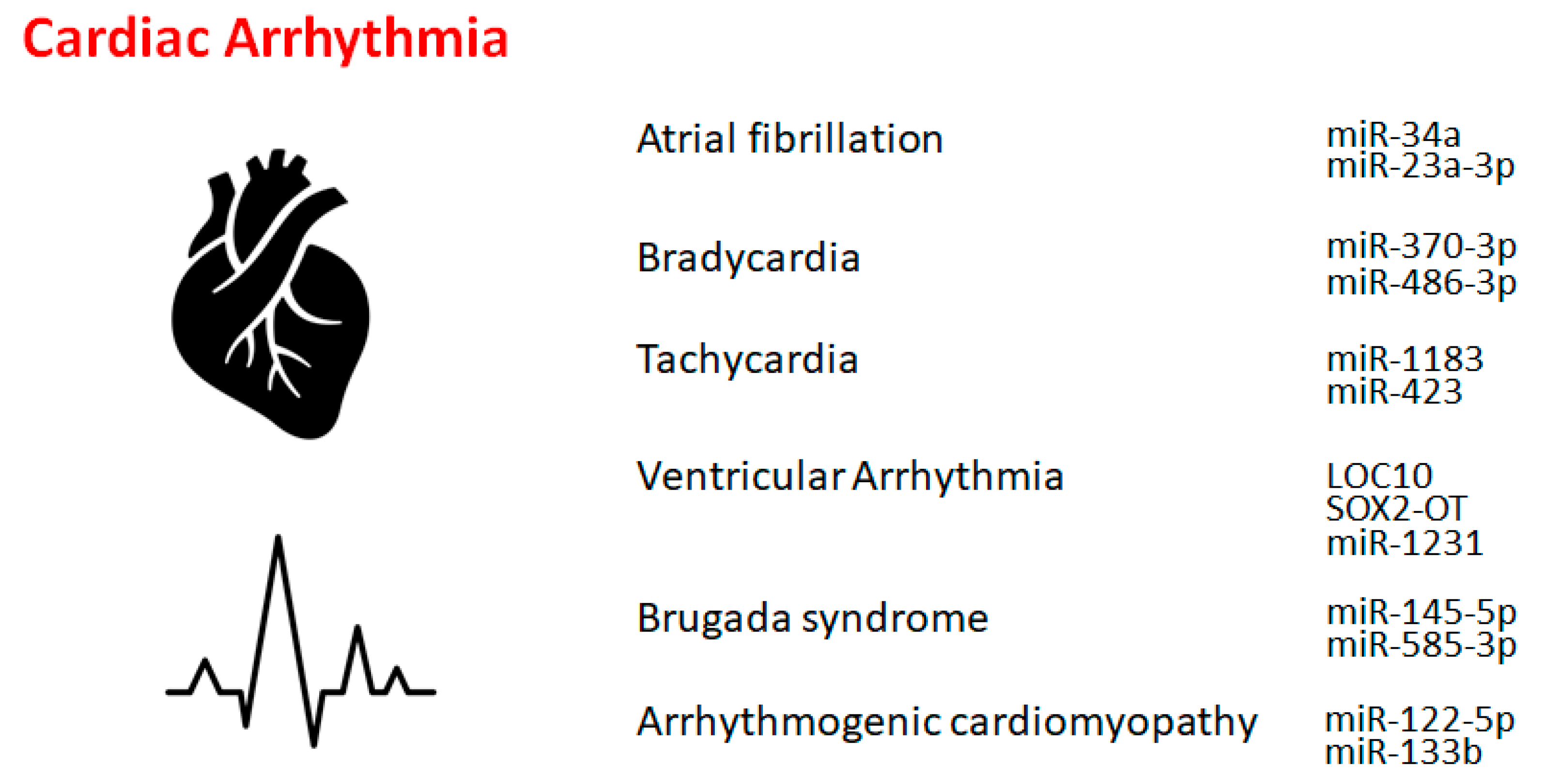
2. Atrial Fibrillation
3. Bradycardia and Tachycardia
4. Other cardiac rhythm disorders
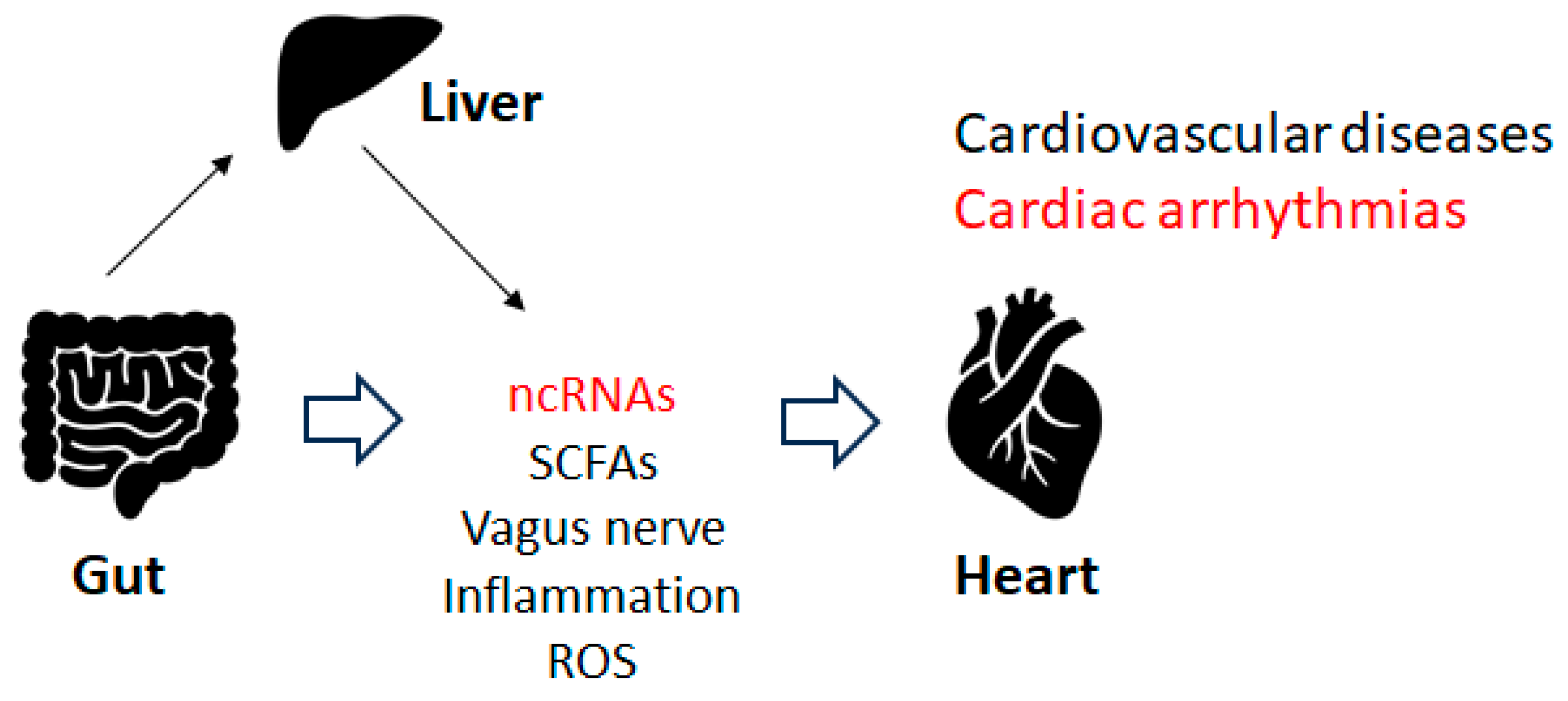
5. Gut microbiota and cardiac arrhythmia
6. Immune pathway and cardiac arrhythmias
7. Future perspectives
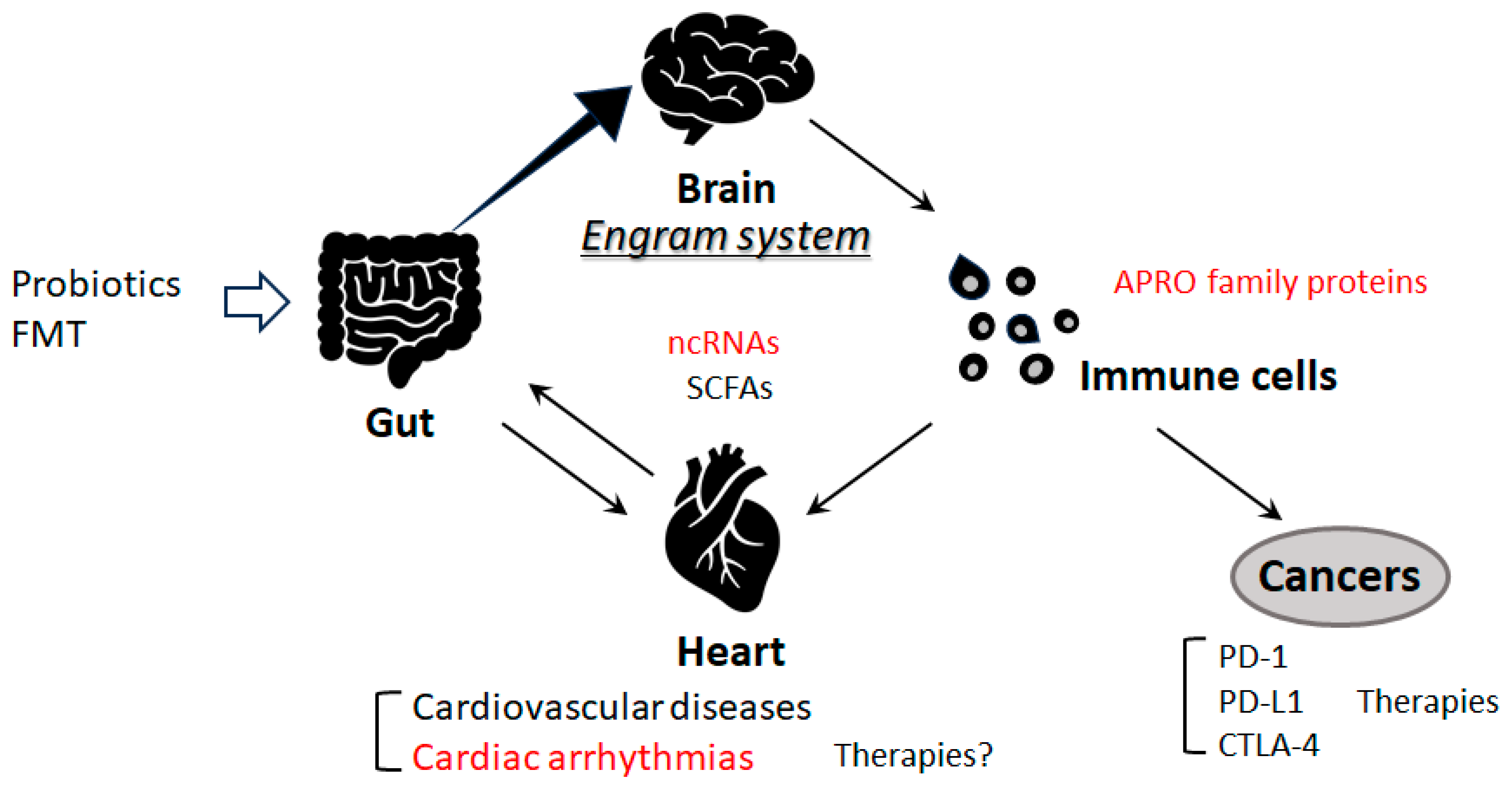
8. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APROs | anti-proliferative proteins |
| BTG1 | B cell translocation gene 1 |
| CNS | central nervous system |
| circRNA | circular RNA |
| CTLA-4 | cytotoxic T lymphocyte-associated protein-4 |
| FMT | fecal microbiota transplantation |
| LOC10 | LOC100911717 |
| lncRNAs | long non-coding RNAs |
| mRNA | messenger RNA |
| ncRNA | non-coding RNA |
| PD-1 | programmed cell death protein 1 |
| PD-L1 | programmed cell death ligand 1 |
| QOL | quality of life |
| ROS | reactive oxygen species |
| SCFAs | short-chain fatty acids |
| siRNA | short interference RNA |
| TASK-1 | tandem of P domains in a fragile inner repairing K+ channel-linked acid-sensitive K+ channel 1 |
| Tob1 | transducer of ErbB2 1 |
| UTR | untranslated region |
References
- Fan, W.; Sun, X.; Yang, C.; Wan, J.; Luo, H.; Liao, B. Pacemaker activity and ion channels in the sinoatrial node cells: MicroRNAs and arrhythmia. Prog. Biophys. Mol. Biol. 2023, 177, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Yaniv, Y.; Lakatta, E.G.; Maltsev, V.A. From two competing oscillators to one coupled-clock pacemaker cell system. Front. Physiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Wilde, A.A.M.; Bezzina, C.R. Genetics of cardiac arrhythmias. Hear. 2005, 91, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, S.; Moukette, B.; Hayasaka, T.; Haskell, A.K.; Mah, J.; Sepúlveda, M.N.; Tang, Y.; Kim, I.-M. Noncoding RNAs as Key Regulators for Cardiac Development and Cardiovascular Diseases. J. Cardiovasc. Dev. Dis. 2023, 10, 166. [Google Scholar] [CrossRef]
- Sallam, T.; Sandhu, J.; Tontonoz, P. Long Noncoding RNA Discovery in Cardiovascular Disease: Decoding Form to Function. Circ. Res. 2018, 122, 155–166. [Google Scholar] [CrossRef]
- Zhang, C.; Han, B.; Xu, T.; Li, D. The biological function and potential mechanism of long non-coding RNAs in cardiovascular disease. J. Cell. Mol. Med. 2020, 24, 12900–12909. [Google Scholar] [CrossRef]
- Correia, C.C.M.; Rodrigues, L.F.; Pelozin, B.R.d.A.; Oliveira, E.M.; Fernandes, T. Long Non-Coding RNAs in Cardiovascular Diseases: Potential Function as Biomarkers and Therapeutic Targets of Exercise Training. Non-Coding RNA 2021, 7, 65. [Google Scholar] [CrossRef]
- Poller, W.; Dimmeler, S.; Heymans, S.; Zeller, T.; Haas, J.; Karakas, M.; Leistner, D.M.; Jakob, P.; Nakagawa, S.; Blankenberg, S.; et al. Non-coding RNAs in cardiovascular diseases: Diagnostic and therapeutic perspectives. Eur. Heart J. 2018, 39, 2704–2716. [Google Scholar] [CrossRef]
- Lu, P.; Ding, F.; Xiang, Y.K.; Hao, L.; Zhao, M. Noncoding RNAs in Cardiac Hypertrophy and Heart Failure. Cells 2022, 11, 777. [Google Scholar] [CrossRef]
- Fan, H.; Liu, X.; Ren, Z.; Fei, X.; Luo, J.; Yang, X.; Xue, Y.; Zhang, F.; Liang, B. Gut microbiota and cardiac arrhythmia. Front. Cell. Infect. Microbiol. 2023, 13. [Google Scholar] [CrossRef]
- Nalliah, C.J.; Sanders, P.; Kalman, J.M. The Impact of Diet and Lifestyle on Atrial Fibrillation. Curr. Cardiol. Rep. 2018, 20, 137. [Google Scholar] [CrossRef] [PubMed]
- Svingen, G.F.; Zuo, H.; Ueland, P.M.; Seifert, R.; Løland, K.H.; Pedersen, E.R.; Schuster, P.M.; Karlsson, T.; Tell, G.S.; Schartum-Hansen, H.; et al. Increased plasma trimethylamine- N -oxide is associated with incident atrial fibrillation. Int. J. Cardiol. 2018, 267, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Gudbjartsson, D.F.; Arnar, D.O.; Helgadottir, A.; Gretarsdottir, S.; Holm, H.; Sigurdsson, A.; Jonasdottir, A.; Baker, A.; Thorleifsson, G.; Kristjansson, K.; et al. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature 2007, 448, 353–357. [Google Scholar] [CrossRef]
- Ruan, Z.-B.; Wang, F.; Gongben, B.-D.; Chen, G.-C.; Zhu, L. Identification of Circulating lncRNA Expression Profiles in Patients with Atrial Fibrillation. Dis. Markers 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Xie, L.; Huang, G.; Gao, M.; Huang, J.; Li, H.; Xia, H.; Xiang, X.; Wu, S.; Ruan, Y. Identification of Atrial Fibrillation-Related lncRNA Based on Bioinformatic Analysis. Dis. Markers 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Chugh, SS.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, EJ.; Gillum, RF.; Kim, YH.; McAnulty, JH Jr.; Zheng, ZJ.; et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014, 129(8), 83. [CrossRef]
- Ye, Q.; Liu, Q.; Ma, X.; Bai, S.; Chen, P.; Zhao, Y.; Bai, C. ; Liu, Y, J Cell Mol Med. 2021, 25(22), 10543. [Google Scholar] [CrossRef]
- Liu, D.; Yang, M.; Yao, Y.; He, S.; Wang, Y.; Cao, Z.; Chen, H.; Fu, Y.; Liu, H.; Zhao, Q. Cardiac Fibroblasts Promote Ferroptosis in Atrial Fibrillation by Secreting Exo-miR-23a-3p Targeting SLC7A11. Oxid Med Cell Longev. 2022, 29; 3961495. [CrossRef]
- Wiedmann, F.; Kraft, M.; Kallenberger, S.; Büscher, A.; Paasche, A.; Blochberger, P.L.; Seeger, T.; Jávorszky, N.; Warnecke, G.; Arif, R.; et al. MicroRNAs Regulate TASK-1 and Are Linked to Myocardial Dilatation in Atrial Fibrillation. J. Am. Hear. Assoc. 2022, 11, e023472. [Google Scholar] [CrossRef] [PubMed]
- Barstow, C.; McDivitt, J.D. Cardiovascular Disease Update: Bradyarrhythmias. FP essentials 2017, 454, 18–23. [Google Scholar]
- Yanni, J.; D’souza, A.; Wang, Y.; Li, N.; Hansen, B.J.; Zakharkin, S.O.; Smith, M.; Hayward, C.; Whitson, B.A.; Mohler, P.J.; et al. Silencing miR-370-3p rescues funny current and sinus node function in heart failure. Sci. Rep. 2020, 10, 1–23. [Google Scholar] [CrossRef]
- Petkova, M.; Atkinson, A.J.; Yanni, J.; Stuart, L.; Aminu, A.J.; Ivanova, A.D.; Pustovit, K.B.; Geragthy, C.; Feather, A.; Li, N.; et al. Identification of Key Small Non-Coding MicroRNAs Controlling Pacemaker Mechanisms in the Human Sinus Node. J. Am. Hear. Assoc. 2020, 9, e016590. [Google Scholar] [CrossRef]
- Aminu, A.J.; Petkova, M.; Atkinson, A.J.; Yanni, J.; Morris, A.D.; Simms, R.T.; Chen, W.; Yin, Z.; Kuniewicz, M.; Holda, M.K.; et al. Further insights into the molecular complexity of the human sinus node – The role of ‘novel’ transcription factors and microRNAs. Prog. Biophys. Mol. Biol. 2021, 166, 86–104. [Google Scholar] [CrossRef]
- Djalinac, N.; Kolesnik, E.; Maechler, H.; Scheruebel-Posch, S.; Pelzmann, B.; Rainer, P.P.; Foessl, I.; Wallner, M.; Scherr, D.; Heinemann, A.; et al. miR-1183 Is a Key Marker of Remodeling upon Stretch and Tachycardia in Human Myocardium. Int. J. Mol. Sci. 2022, 23, 6962. [Google Scholar] [CrossRef]
- Park, H.; Park, H.; Park, J. Circulating microRNA 423 attenuates the phosphorylation of calcium handling proteins in atrial fibrillation. Mol Med Rep. 2022, 25(5), 186. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Tan, Z.; Zhou, J.; Wu, Y.; Hu,Q.; Ling, Q.; Ling, J.; Liu, M.; Ma, J.; Zhang, D.; et al. The regulation of circRNA and lncRNAprotein binding in cardiovascular diseases: Emerging therapeutic targets. Biomed Pharmacother. 2023, 165, 115067.
- Liang, Y.; Wang, B.; Huang, H.; Wang, M.; Wu, Q.; Zhao, Y.; He, Y. Silenced SOX2-OT alleviates ventricular arrhythmia associated with heart failure by inhibiting NLRP3 expression via regulating miR-2355-3p. Immunity, Inflamm. Dis. 2020, 9, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Mao, R.; Zhang, L.; Zhao, R.; Tan, K.; Liu, C.; Xu, J.; Du, G.; Zhang, T. CircNPHP4 in monocyte-derived small extracellular vesicles controls heterogeneous adhesion in coronary heart atherosclerotic disease. Cell Death Dis. 2021, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Qiao, L.; Han, F.; Xie, X.; Wang, W. MiR-1231 regulates L-calcium in ventricular arrhythmia in chronic heart failure. 2021, 112, 305–306. [CrossRef]
- Sarquella-Brugada, G.; Cesar, S.; Zambrano, M.D.; Fernandez-Falgueras, A.; Fiol, V.; Iglesias, A.; Torres, F.; Garcia-Algar, O.; Arbelo, E.; Brugada, J.; et al. Electrocardiographic Assessment and Genetic Analysis in Neonates: a Current Topic of Discussion. Curr. Cardiol. Rev. 2018, 15, 30–37. [Google Scholar] [CrossRef]
- Steinberg, C.; Gaudreault, N.; I Papadakis, A.; Henry, C.; Champagne, J.; Philippon, F.; O’hara, G.; Blier, L.; Plourde, B.; Nault, I.; et al. Leucocyte-derived micro-RNAs as candidate biomarkers in Brugada syndrome. Eur. 2023, 25. [Google Scholar] [CrossRef]
- Scumaci, D.; Oliva, A.; Concolino, A.; Curcio, A.; Fiumara, CV.; ammè, L.; Campuzano, O.; Pascali, VL.; Coll, M.; Iglesias, A.; et al. Integration of "Omics" Strategies for Biomarkers Discovery and for the Elucidation of Molecular Mechanisms Underlying Brugada Syndrome. Proteomics Clin Appl. 2018, 12(6), e1800065. [Google Scholar] [CrossRef] [PubMed]
- Piquer-Gil, M.; Domenech-Dauder, S.; Sepúlveda-Gómez, M.; Machí-Camacho, C.; Braza-Boïls, A.; Zorio, E. Non Coding RNAs as Regulators of Wnt/β-Catenin and Hippo Pathways in Arrhythmogenic Cardiomyopathy. Biomedicines 2022, 10, 2619. [Google Scholar] [CrossRef] [PubMed]
- Asatryan, B.; Asimaki, A.; Landstrom, A.P.; Khanji, M.Y.; Odening, K.E.; Cooper, L.T.; Marchlinski, F.E.; Gelzer, A.R.; Semsarian, C.; Reichlin, T.; et al. Inflammation and Immune Response in Arrhythmogenic Cardiomyopathy: State-of-the-Art Review. Circulation 2021, 144, 1646–1655. [Google Scholar] [CrossRef]
- Broch, K.; Leren, I.S.; Saberniak, J.; Ueland, T.; Edvardsen, T.; Gullestad, L.; Haugaa, K.H. Soluble ST2 is associated with disease severity in arrhythmogenic right ventricular cardiomyopathy. Biomarkers 2017, 22, 367–371. [Google Scholar] [CrossRef]
- Christensen, A.H.; Platonov, P.G.; Jensen, H.K.; Chivulescu, M.; Svensson, A.; Dahlberg, P.; Madsen, T.; Frederiksen, T.C.; Heliö, T.; Lie. H.; et al. Genotype–phenotype correlation in arrhythmogenic right ventricular cardiomyopathy—risk of arrhythmias and heart failure. J. Med Genet. 2021, 59, 858–864. [Google Scholar] [CrossRef]
- Cappelletto, C.; Gregorio, C.; Barbati, G.; Romani, S.; De Luca, A.; Merlo, M.; Mestroni, L.; Stolfo, D.; Sinagra, G. Antiarrhythmic therapy and risk of cumulative ventricular arrhythmias in arrhythmogenic right ventricle cardiomyopathy. Int. J. Cardiol. 2021, 334, 58–64. [Google Scholar] [CrossRef]
- Asimaki, A.; Tandri, H.; Duffy, ER.; Winterfield, JR.; Mackey-Bojack, S.; Picken, MM.; Cooper, LT.; Wilber, DJ.; Marcus, FI.; Basso, C.; et al. Altered desmosomal proteins in granulomatous myocarditis and potential pathogenic links to arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2011, 4(5):, 743. [CrossRef]
- Bueno Marinas, M.; Celeghin, R.; Cason, M.; Thiene, G.; Basso, C.; Pilichou, K. The Role of MicroRNAs in Arrhythmogenic Cardiomyopathy: Biomarkers or Innocent Bystanders of Disease Progression? Int J Mol Sci. 2020, 21(17), 6434. [Google Scholar] [CrossRef]
- Marinas, M.B.; Celeghin, R.; Cason, M.; Bariani, R.; Frigo, A.C.; Jager, J.; Syrris, P.; Elliott, P.M.; Bauce, B.; Thiene, G.; et al. A microRNA Expression Profile as Non-Invasive Biomarker in a Large Arrhythmogenic Cardiomyopathy Cohort. Int. J. Mol. Sci. 2020, 21, 1536. [Google Scholar] [CrossRef]
- Lucking, E.F.; O'Connor, K.M.; Strain, C.R.; Fouhy, F.; Bastiaanssen, T.F.; Burns, D.P.; Golubeva, A.V.; Stanton, C.; Clarke, G.; Cryan, J.F.; et al. Chronic intermittent hypoxia disrupts cardiorespiratory homeostasis and gut microbiota composition in adult male guinea-pigs. EBioMedicine 2018, 38, 191–205. [Google Scholar] [CrossRef]
- Anthony, W.E.; Wang, B.; Sukhum, K.V.; D’souza, A.W.; Hink, T.; Cass, C.; Seiler, S.; Reske, K.A.; Coon, C.; Dubberke, E.R.; et al. Acute and persistent effects of commonly used antibiotics on the gut microbiome and resistome in healthy adults. Cell Rep. 2022, 39, 110649–110649. [Google Scholar] [CrossRef]
- Tierney, BT.; Yang, Z.; Luber, JM.; Beaudin, M.; Wibowo, MC,.; Baek, C.; Mehlenbacher, E.; Patel, CJ.; Kostic, AD. The Landscape of Genetic Content in the Gut and Oral Human Microbiome. Cell Host Microbe. 2019, 26(2), 283. [CrossRef]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef]
- Lal, S.; Kirkup, A.J.; Brunsden, A.M.; Thompson, D.G.; Grundy, D.; Park, S.J.; Yu, Y.; Wagner, B.; Valinsky, W.C.; Lomax, A.E.; et al. Vagal afferent responses to fatty acids of different chain length in the rat. Am. J. Physiol. Liver Physiol. 2001, 281, G907–G915. [Google Scholar] [CrossRef]
- Onyszkiewicz, M.; Gawrys-Kopczynska.; M, Konopelski, P.; Aleksandrowicz, M.; Sawicka, A.; Koźniewska, E.; Samborowska, E.; Ufnal, M. Butyric acid, a gut bacteria metabolite, lowers arterial blood pressure via colon-vagus nerve signaling and GPR41/43 receptors. Pflugers Arch. 2019, 471(11-12), 1441. [CrossRef]
- Tabata, T.; Yamashita, T.; Hosomi, K.; Park, J.; Hayashi, T.; Yoshida, N.; Saito, Y.; Fukuzawa, K.; Konishi, K.; Murakami, H.; et al. Gut microbial composition in patients with atrial fibrillation: effects of diet and drugs. Hear. Vessel. 2020, 36, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Celikyurt, I.; Meier, C.R.; Kühne, M.; Schaer, B. Safety and Interactions of Direct Oral Anticoagulants with Antiarrhythmic Drugs. Drug Saf. 2017, 40, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, T.; Saberi, E.A.; Tabatabaei, A.M.; Tahmasebi, E. Antibiotic use in endodontic treatment during pregnancy: A narrative review. Eur. J. Transl. Myol. 2022, 32. [Google Scholar] [CrossRef]
- Valdivielso, JM.; Balafa, O.; Ekart, R.; Ferro, CJ.; Mallamaci, F.; Mark, PB.; . Rossignol, P.; Sarafidis, P.; Del Vecchio, L. Hyperkalemia in Chronic Kidney Disease in the New Era of Kidney Protection Therapies. Ortiz A.Drugs. 2021, 81(13), 1467. [Google Scholar] [CrossRef] [PubMed]
- Fardi, F.; Khasraghi, L.B.; Shahbakhti, N.; Naseriyan, A.S.; Najafi, S.; Sanaaee, S.; Alipourfard, I.; Zamany, M.; Karamipour, S.; Jahani, M.; et al. An interplay between non-coding RNAs and gut microbiota in human health. Diabetes Res. Clin. Pr. 2023, 201, 110739. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wang, X.; Wang, L.; Sun, X.; Tan, G.; Wei, W.; Zheng, G.; Ma, X.; Tian, D.; Yu, H. Genetics, Epigenetics, Cellular Immunology, and Gut Microbiota: Emerging Links With Graves' Disease. Front Cell Dev Biol. 2022, 9, 794912. [Google Scholar] [CrossRef]
- Wang, X.; Fan, H.; Wang, Y.; Yin, X.; Liu, G.; Gao, C.; Li, X.; Liang, B. Elevated Peripheral T Helper Cells Are Associated With Atrial Fibrillation in Patients With Rheumatoid Arthritis. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- He, Y.; Chen, X.; Guo, X.; Yin, H.; Ma, N.; Tang, M.; Liu, H.; Mei, J. Th17/Treg Ratio in Serum Predicts Onset of Postoperative Atrial Fibrillation After Off-Pump Coronary Artery Bypass Graft Surgery. Heart Lung Circ. 2018, 27(12), 1467. [Google Scholar] [CrossRef]
- Jia, N.; Lin, X.; Ma, S.; Ge, S.; Mu, S.; Yang, C.; Shi, S.; Gao, L.; Xu, J.; Bo, T.; et al. Amelioration of hepatic steatosis is associated with modulation of gut microbiota and suppression of hepatic miR-34a in Gynostemma pentaphylla (Thunb.) Makino treated mice. Nutr. Metab. 2018, 15, 86. [Google Scholar] [CrossRef]
- Li, L.; Li, C.; Lv, M.; Hu, Q.; Guo, L.; Xiong, D. Correlation between alterations of gut microbiota and miR-122-5p expression in patients with type 2 diabetes mellitus. Ann. Transl. Med. 2020, 8, 1481–1481. [Google Scholar] [CrossRef]
- Khudiakov, A.A.; Panshin, D.D.; Fomicheva, Y.V.; Knyazeva, A.A.; Simonova, K.A.; Lebedev, D.S.; Mikhaylov, E.N.; Kostareva, A.A. Different Expressions of Pericardial Fluid MicroRNAs in Patients With Arrhythmogenic Right Ventricular Cardiomyopathy and Ischemic Heart Disease Undergoing Ventricular Tachycardia Ablation. Front. Cardiovasc. Med. 2021, 8. [Google Scholar] [CrossRef]
- Kassan, A.; Ait-Aissa, K.; Kassan, M. Hypothalamic miR-204 Induces Alteration of Heart Electrophysiology and Neurogenic Hypertension by Regulating the Sympathetic Nerve Activity: Potential Role of Microbiota. Cureus 2021, 13. [Google Scholar] [CrossRef]
- Pastori, D.; Carnevale, R.; Nocella, C.; Novo, M.; Santulli, M.; Cammisotto, V.; Menichelli, D.; Pignatelli, P.; Violi, F. Gut-Derived Serum Lipopolysaccharide is Associated With Enhanced Risk of Major Adverse Cardiovascular Events in Atrial Fibrillation: Effect of Adherence to Mediterranean Diet. J. Am. Hear. Assoc. 2017, 6, e005784. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zuo, K.; Zhang, J.; Hu, C.; Wang, P.; Jiao, J.; Liu, Z.; Yin, X.; Liu, X.; Li, K.; et al. Shifts in gut microbiome and metabolome are associated with risk of recurrent atrial fibrillation. J. Cell. Mol. Med. 2020, 24, 13356–13369. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.-X.; Wang, S.; Wei, L.; Cui, Y.-Y.; Chen, Y.-H. Proanthocyanidins: Components, Pharmacokinetics and Biomedical Properties. Am. J. Chin. Med. 2020, 48, 813–869. [Google Scholar] [CrossRef]
- Desantis, V.; Potenza, M.A.; Sgarra, L.; Nacci, C.; Scaringella, A.; Cicco, S.; Solimando, A.G.; Vacca, A.; Montagnani, M. microRNAs as Biomarkers of Endothelial Dysfunction and Therapeutic Target in the Pathogenesis of Atrial Fibrillation. Int. J. Mol. Sci. 2023, 24, 5307. [Google Scholar] [CrossRef]
- Hao, H.; Dai, C.; Han, X.; Li, Y. A novel therapeutic strategy for alleviating atrial remodeling by targeting exosomal miRNAs in atrial fibrillation. Biochim. et Biophys. Acta (BBA) - Mol. Cell Res. 2022, 1869, 119365. [Google Scholar] [CrossRef]
- Tan, A.Y.; Zimetbaum, P. Atrial Fibrillation and Atrial Fibrosis. J. Cardiovasc. Pharmacol. 2011, 57, 625–629. [Google Scholar] [CrossRef]
- Lee, Y.; Choe, J.; Park, O.H.; Kim, Y.K. Molecular Mechanisms Driving mRNA Degradation by m6A Modification. Trends Genet. 2020, 36, 177–188. [Google Scholar] [CrossRef]
- Winkler, G.S. The mammalian anti-proliferative BTG/Tob protein family. J. Cell. Physiol. 2010, 222, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Rouault, J.; Magaud, J.; Berthet, C. In search of a function for the TIS21/PC3/BTG1/TOB family. FEBS Lett. 2001, 497, 67–72. [Google Scholar] [CrossRef]
- Tirone, F. The gene PC3TIS21/BTG2, prototype member of the PC3/BTG/TOB family: Regulator in control of cell growth, differentiation, and DNA repair? J. Cell. Physiol. 2001, 187, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Ezzeddine, N.; Chang, T.-C.; Zhu, W.; Yamashita, A.; Chen, C.-Y.A.; Zhong, Z.; Yamashita, Y.; Zheng, D.; Shyu, A.-B. Human TOB, an Antiproliferative Transcription Factor, Is a Poly(A)-Binding Protein-Dependent Positive Regulator of Cytoplasmic mRNA Deadenylation. Mol. Cell. Biol. 2007, 27, 7791–7801. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Taniguchi, K.; Nagase, N.; Tsuji, A.; Kitagishi, Y.; Matsuda, S. Reactive oxygen species may influence on the crossroads of stemness, senescence, and carcinogenesis in a cell via the roles of APRO family proteins. Explor. Med. 2021, 2, 443–454. [Google Scholar] [CrossRef]
- Ikeda, Y.; Taniguchi, K.; Sawamura, H.; Yoshikawa, S.; Tsuji, A.; Matsuda, S. Presumed Roles of APRO Family Proteins in Cancer Invasiveness. Cancers 2022, 14, 4931. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, W. Expression of B Cell Translocation Gene 1 Protein in Colon Carcinoma and its Clinical Significance. Recent Patents Anti-Cancer Drug Discov. 2020, 15, 78–85. [Google Scholar] [CrossRef]
- Zhao, S.; Xue, H.; Hao, C.-L.; Jiang, H.-M.; Zheng, H.-C. BTG1 Overexpression Might Promote Invasion and Metastasis of Colorectal Cancer via Decreasing Adhesion and Inducing Epithelial–Mesenchymal Transition. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef]
- Jung, Y.Y.; Sung, J.-Y.; Kim, J.-Y.; Kim, H.-S. Down-regulation of B-Cell Translocation Gene 1 by Promoter Methylation in Colorectal Carcinoma. Anticancer. Res. 2018, 38, 691–697. [Google Scholar] [CrossRef]
- Ishida, Y.; Kawakami, H.; Kitajima, H.; Nishiyama, A.; Sasai, Y.; Inoue, H.; Muguruma, K. Vulnerability of Purkinje Cells Generated from Spinocerebellar Ataxia Type 6 Patient-Derived iPSCs. Cell Rep. 2016, 17, 1482–1490. [Google Scholar] [CrossRef]
- Luan, S.-H.; Yang, Y.-Q.; Ye, M.-P.; Liu, H.; Rao, Q.-F.; Kong, J.-L.; Wu, F.-R. ASIC1a promotes hepatic stellate cell activation through the exosomal miR-301a-3p/BTG1 pathway. Int. J. Biol. Macromol. 2022, 211, 128–139. [Google Scholar] [CrossRef]
- Hwang, S.S.,; Lim, J.; Yu, Z.; Kong, P.; Sefik, E.; Xu, H.; Harman, C.C.D.; Kim, .LK.; Lee, G.R.; Li, H.B.; Flavell, R.A. mRNA destabilization by BTG1 and BTG2 maintains T cell quiescence. Science. 2020, 367, 1255-1260. [CrossRef]
- Li, B.-N.; Tang, Q.-D.; Tan, Y.-L.; Yan, L.; Sun, L.; Guo, W.-B.; Qian, M.-Y.; Chen, A.; Luo, Y.-J.; Zheng, Z.-X.; et al. Key Regulatory Differentially Expressed Genes in the Blood of Atrial Septal Defect Children Treated With Occlusion Devices. Front. Genet. 2021, 12, 790426. [Google Scholar] [CrossRef]
- Tzachanis, D.; Boussiotis, V.A. Tob, a member of the APRO family, regulates immunological quiescence and tumor suppression. Cell Cycle 2009, 8, 1019–1025. [Google Scholar] [CrossRef]
- Lee, H.S.; Kundu, J.; Kim, R.N.; Shin, Y.K. Transducer of ERBB2.1 (TOB1) as a Tumor Suppressor: A Mechanistic Perspective. Int. J. Mol. Sci. 2015, 16, 29815–29828. [Google Scholar] [CrossRef]
- Szczyrek, M.; Bitkowska, P.; Chunowski, P.; Czuchryta, P. ; Krawczyk, P, Diet, Microbiome, and Cancer Immunotherapy-A Comprehensive Review. Milanowski J.Nutrients. 2021, 13(7), 2217. [Google Scholar] [CrossRef]
- Grenda, A.; Krawczyk, P. Cancer trigger or remedy: two faces of the human microbiome. Appl. Microbiol. Biotechnol. 2021, 105, 1395–1405. [Google Scholar] [CrossRef]
- Rescigno, M. The microbiota revolution: Excitement and caution. Eur. J. Immunol. 2017, 47, 1406–1413. [Google Scholar] [CrossRef]
- Sawamura, H.; Taniguchi, K.; Ikeda, Y.; Tsuji, A.; Kitagishi, Y.; Matsuda, S. Gut Microbiota Potentiates the Effect of Immune Checkpoint Therapy against Cancers. Recent Prog. Nutr. 2021, 2, 1–1. [Google Scholar] [CrossRef]
- Asai, T.; Yoshikawa, S.; Ikeda, Y.; Taniguchi, K.; Sawamura, H.; Tsuji, A.; Matsuda, S. Encouraging Tactics with Genetically Modified Probiotics to Improve Immunity for the Prevention of Immune-Related Diseases including Cardio-Metabolic Disorders. Biomolecules 2022, 13, 10. [Google Scholar] [CrossRef]
- Wang, X.; Chang, L.; Wan, X.; Tan, Y.; Qu, Y.; Shan, J.; Yang, Y.; Ma, L.; Hashimoto, K. (R)-ketamine ameliorates demyelination and facilitates remyelination in cuprizone-treated mice: A role of gut–microbiota–brain axis. Neurobiol. Dis. 2022, 165, 105635. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Taniguchi, K.; Sawamura, H.; Ikeda, Y.; Tsuji, A.; Matsuda, S. A New Concept of Associations between Gut Microbiota, Immunity and Central Nervous System for the Innovative Treatment of Neurodegenerative Disorders. Metabolites 2022, 12, 1052. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Taniguchi, K.; Sawamura, H.; Ikeda, Y.; Tsuji, A.; Matsuda, S. Encouraging probiotics for the prevention and treatment of immune-related adverse events in novel immunotherapies against malignant glioma. Explor. Target. Anti-tumor Ther. 2022, 3, 817–827. [Google Scholar] [CrossRef]
- Tsuji, A.; Ikeda, Y.; Yoshikawa, S.; Taniguchi, K.; Sawamura, H.; Morikawa, S.; Nakashima, M.; Asai, T.; Matsuda, S. The Tryptophan and Kynurenine Pathway Involved in the Development of Immune-Related Diseases. Int. J. Mol. Sci. 2023, 24, 5742. [Google Scholar] [CrossRef]
- Håvik, B.; Røkke, H.; Dagyte, G.; Stavrum, A.-K.; Bramham, C.; Steen, V. Synaptic activity-induced global gene expression patterns in the dentate gyrus of adult behaving rats: Induction of immunity-linked genes. Neuroscience 2007, 148, 925–936. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




