1. Introduction
Respiratory infections are a major reason for infants and children seeking medical advice and visiting health facilities, thus remaining a significant public threat with high morbidity and mortality. Generally, upper respiratory infections are more common than lower respiratory infections. Undoubtedly, there are limited options to reduce a viral outbreak in the absence of massive vaccination strategies. making the early detection of new cases crucial for the implementation of precautions that could halt spread to contacts and allow proper management of -patients. [
1,
2,
3] To achieve this target, in the absence of readily available, new rapid diagnostic tests (RDTs) with sufficiently high specificity and sensitivity for promptly detection of contaminate persons in the community are a huge challenge for public health authorities. [
4]
According to published literature, one of the major causes of childhood acute lower respiratory tract infection and leading cause of hospital admissions among young children globally, is the respiratory syncytial virus (RSV). [
5] – More precisely, three million RSV-associated lower respiratory tract infection hospitalizations and thousands of deaths occur annually in children aged < 5 years around the world. The vast majority of these hospitalizations and deaths occur in low and middle-income countries. [
6]
The peak of adenovirus infections is recorded in winter and spring and contrary to the flu, new cases exist throughout the year. They are responsible for 10% of all childhood fevers, and every child has at least one adenovirus infection by 10 years of age. The typical clinical presentation either resembles of a common cold or include croup, bronchitis, and pneumonia. In children, apart from respiratory infections, adenoviruses cause intestinal infections. [
7] Respiratory, followed by the intestinal infections, are reported as the most common ways of transmission of adenoviruses. Usually, respiratory infections occur by close contact with infectious material from infected person or surface. The secretions from the respiratory tract may contain the virus keeping in mind that the adenoviruses can survive for many hours on objects, such as doorknobs, lab surfaces, and toys. The second way of transmission is the intestinal and usually occurs by fecal-oral contact, associated with poor hygiene practices like washing hands or from ingestion of contaminated food or water. [
8,
9,
10]
Special populations, such as young children are at increased risk of severe illness from influenza or flu as referred to usually. Influenza is a respiratory infection caused by influenza virus A, B, or C. [
11] The viral infections of the lower respiratory system amount to 100,000 cases with 100 associated deaths per million annually for children aged under five years. Influenza and pneumococcal pneumonia are responsible for the majority of deaths. [
12] At the beginning of 20th century, during the influenza pandemic, the highest attack rates of influenza were recorded in school-aged children 5 to 18 years old. [
13] Contrarily, the case series of 2009 pandemic influenza A(H1N1) did not present data on children separately or reported small numbers of children viral infection. [
14] Children with chronic medical conditions or aged <5 years old are at high risk for serious complications and death from influenza. During the pandemic of H1N1, Central Disease Control (CDC) monitors child influenza deaths through its influenza-associated pediatric mortality reporting system. Indicatively, for the period April-August 2009, in the United States, 36 deaths were reported among children aged <18 years, 19% of them were aged <5 years, and 67% had one or more of the high-risk medical condition. [
15] The pandemic H1N1 influenza did not appear to cause more severe disease than seasonal influenza A. Asthma was a significant risk factor for severe disease among children with pandemic H1N1 influenza than among those with seasonal influenza. [
16]
The coronavirus disease 2019 (COVID-19) has impressively increased the burden on healthcare globally. In comparison with the worldwide pandemic, children and adolescents are characterized by mild clinical presentation and more propitious outcomes than adults. [
17] Despite the fact that the vast majority of acute pediatric SARS-CoV-2 infections is generally estimated as mild, the post-infectious associated conditions, including pediatric inflammatory multisystem syndrome and 'long COVID' in children, are more complex and worth paying attention to. [
18] The majority of European countries have stopped providing a surveillance system to monitor the circulation of SARS-CoV-2 and other respiratory viruses and its population impact. Testing and further sequencing are no longer undertaken in community and health care settings across most countries.
The co-infections of respiratory viruses cannot be excluded as a hypothesis in differential diagnosis. Furthermore, - clinical signs and symptoms of viral infections -are similar to those of bacterial infections. It is challenging for the public health authorities to clinically distinguish bacterial from viral infections using a diagnostic method and identify coinfections from multiple viral pathogens. In conclusion, rapid test detection of viral pathogens could offer useful clinical information - and reduce the hospitalization cost. [
19]
The predominant viruses causing acute viral respiratory infections are influenza A and B viruses (Flu-A, Flu-B), Respiratory Syncytial Virus (RSV), and Coronaviruses. Prompt and accurate diagnosis of viral infection can be challenging. Rapid and definite diagnosis of viral infections could improve clinical outcomes. Rapid antigen tests could offer quick and affordable results at the point of care, enabling reliable detection of viral load samples associated with the presence of infectious virus. Therefore, early and proper diagnosis of respiratory infections is expected to reduce the inappropriate use of antibiotics and provide the possibility of using antiviral therapy. [
20]
The CDC following recommendations from the agency’s independent vaccine advisory committee and approvals from the US Food and Drug Administration has approved the use of two new RSV vaccines for older adults and expects them to be available in the next fall. [
21]
In the present study, pending the new guidelines for the RSV vaccine and its probable introduction in the National Immunization program targeting maternal immunization and young children, we aimed to record the incidence of RSV, SARS-CoV-2, Influenza A/B and Adenovirus with Rapid tests and validate the tests with the RT-PCR assay in a representative number of upper respiratory specimens with a wide range of viral loads and identify (co)-infection patterns in young children visiting the private and public pediatrician health sector in central Greece.
2. Materials and Methods
The present study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cross-sectional studies. In this cross-sectional study we used nasal specimen. The present surveillance program was conducted in five private pediatrician dispensaries and one Pediatric care unit department from 10 January to 30 March 2023. The clinical samples were collected from the pediatric department of general Hospital in Larissa Greece and private pediatrician dispensaries from early symptomatic children (presenting with a fever and/or cough and/or headache within 5–7 days). The parents or guardians of the participants were informed about the aims of the study by the physicians. The age and sex of participants were reported as demographic details. The total sample of participants was 784 young children and infants, of which 383 (48.8%) were female and 401 were male (51.2%). The mean age of the -study population was 7.3 + 5.5 years. The participation in the study was optional, and parents signed a written consent after being fully informed about the aim of the study. The statistical analysis was performed using Excel, (Microsoft, Redmond, Washington, DC, USA) and IBM SPSS (version 26) (Microsoft, Redmond, Washington, DC, USA) and sensitivity and specificity with a 95% confidence interval (95% CI) were estimated based on binomial distribution
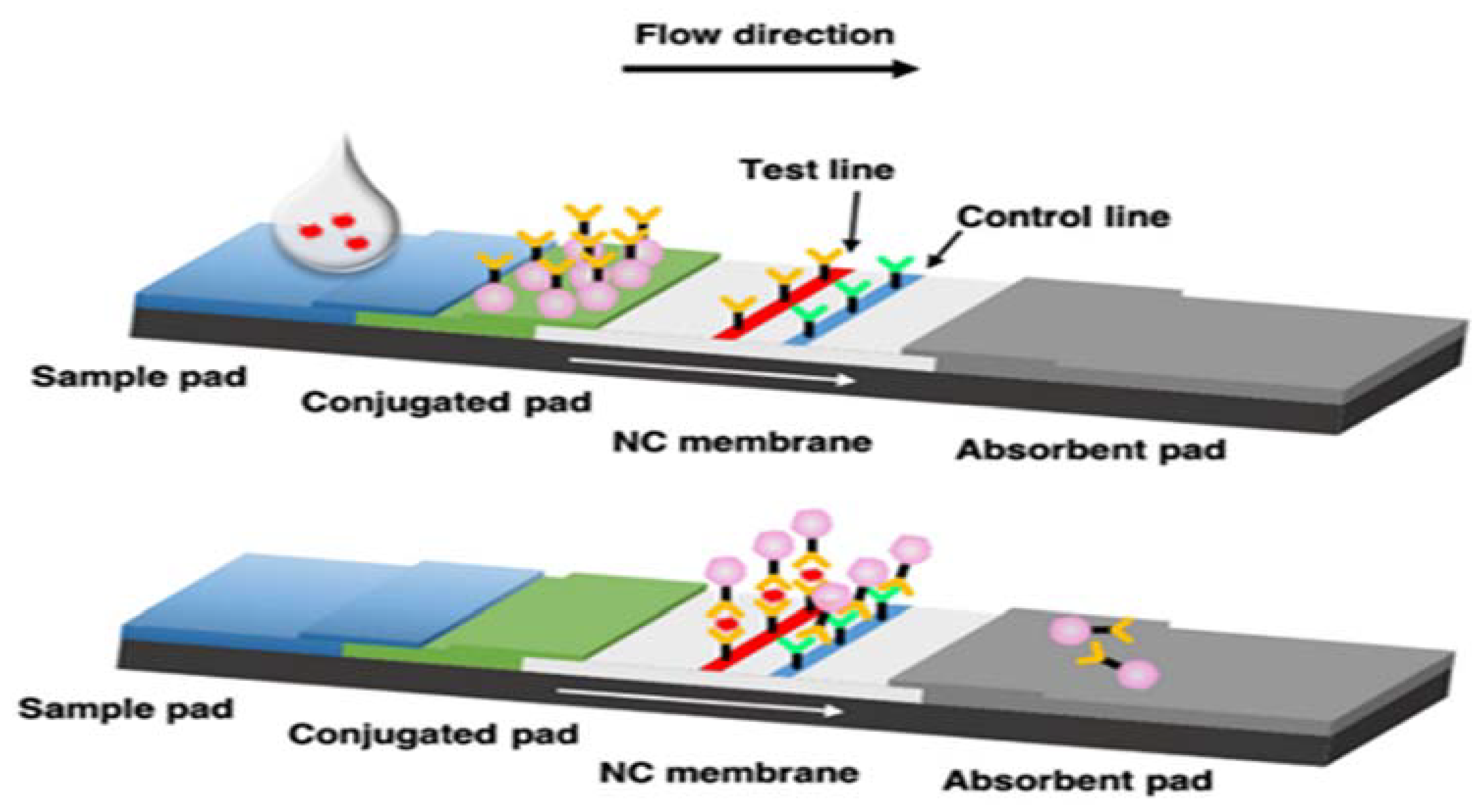
A simplified specimen collection is introduced, since only one swab is required for the detection of four different pathogens. Our methodology, which involves the use of a single swab and consequently one extract during a healthcare visit, as opposed to four distinct tests (samples), is designed to reduce discomfort, introduce a more cost-effective solution, reduce the demand of personnel and eliminate unnecessary stress for all involved parties.
Principal of assay
Antibodies specific to virus proteins are coated on the test line region of the nitrocellulose membrane. During testing, antigens of each virus in the specimen react with the antibodies that are coated onto gold nanoparticles. As the sample flows through the test membrane migrates up to react with the antibodies immobilized on the membrane and generate one colored line in the test region. The presence of this colored line indicates a positive result. To serve as a procedural control, a colored line will always appear in the control region if the test has been performed properly. The test result interpreted after 15 minutes.
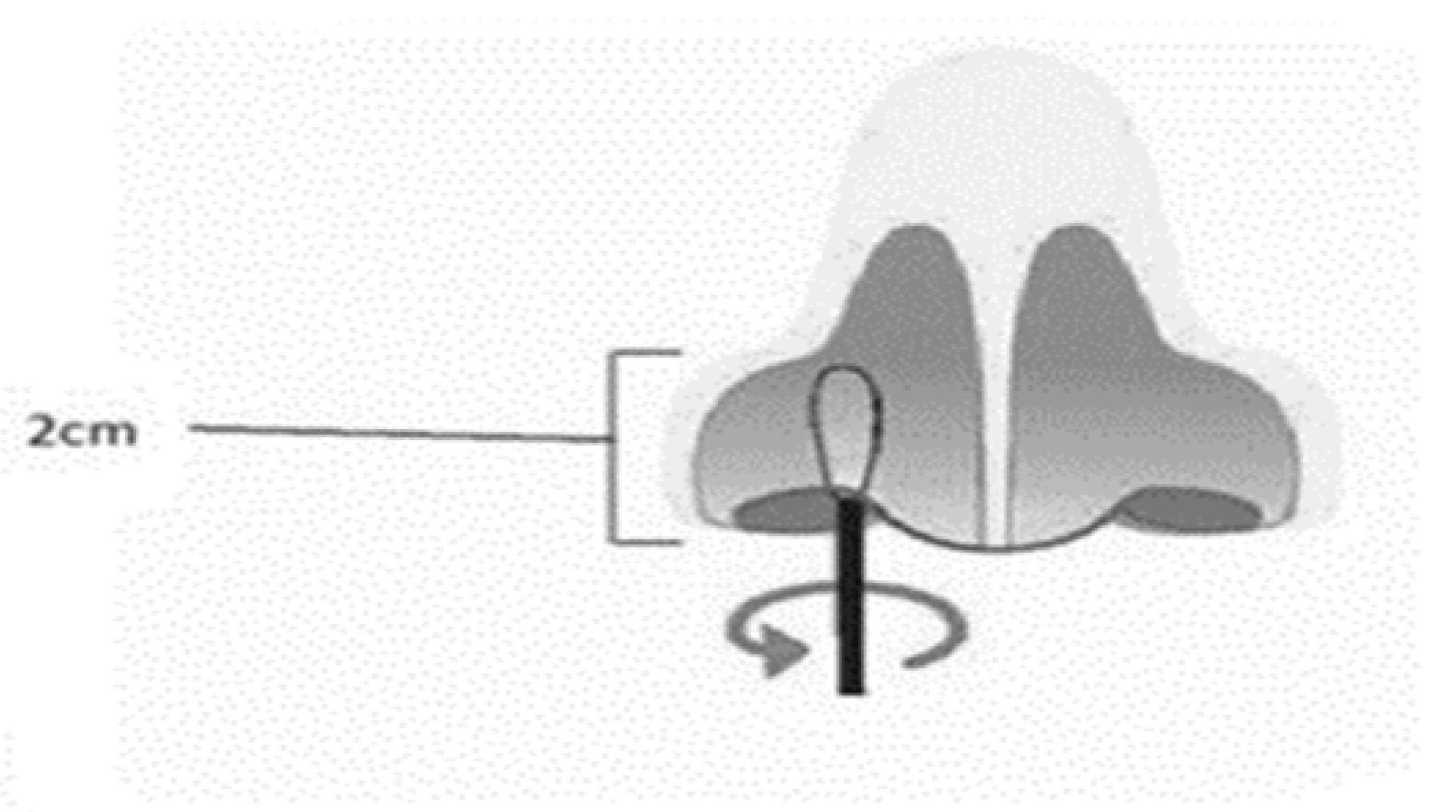
Nasal Mid-Turbinate specimen collection
The patient’s head is tilted back 70 degrees. A sterile swab is removed from the pouch. While gently rotating it, the swab is inserted less than one inch (about 2 cm) into patient’s nostril (until resistance is met at the turbinate’s). The swab is rotated five times against the nasal wall then is slowly removed from the nostril. Using the same swab, the collection procedure is repeated with the second nostril
Figure 2.
Method Procedure
After the specimen collection, the swab is placed in the extraction tube, by rotating the swab forcefully against the side of the tube for 1min. Best results are obtained when the specimen is vigorously extracted in the solution.
The swab is removed, squeezing the sides of the tube to extract as much liquid as possible.
The swab is discarded.
The extraction tube is closed with the dropper cup. Two drops are added in the circular window of the cassette for each test.
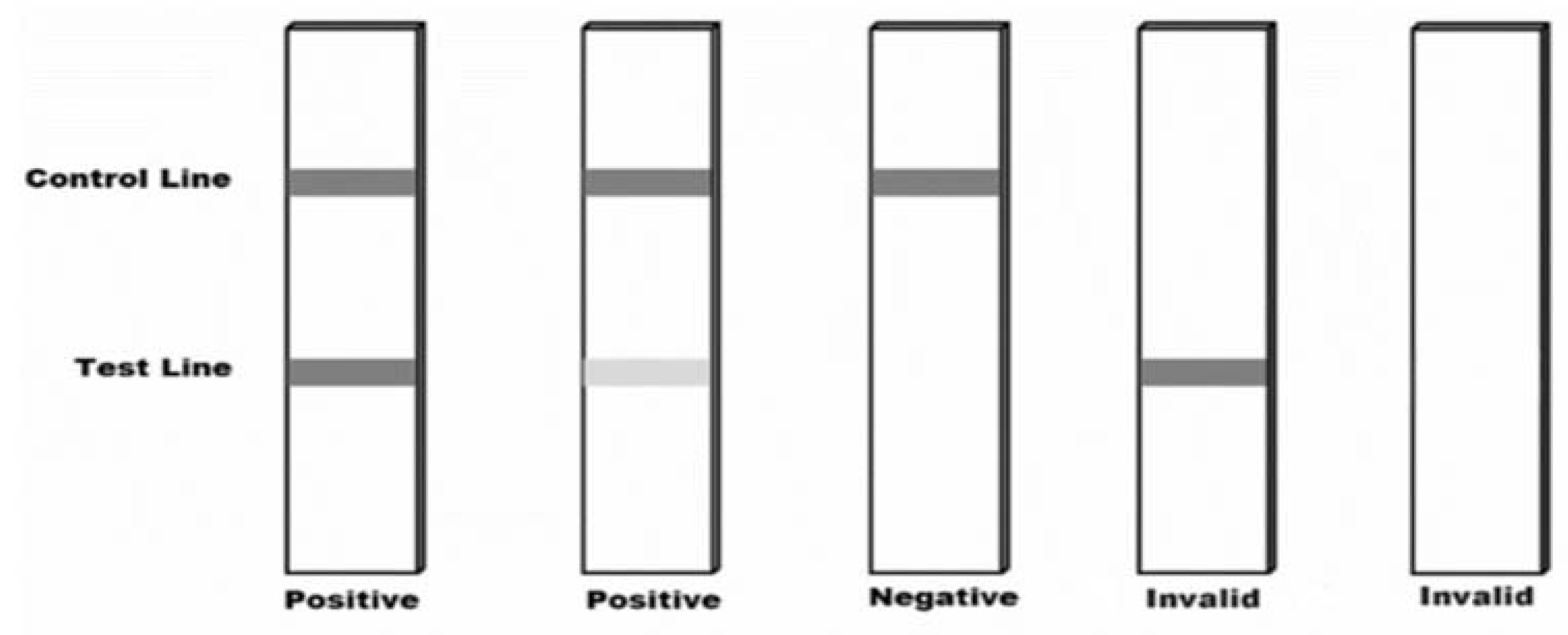
After 15 minutes, the test stick can be visually read and interpreted according to the corresponding
Figure 3.
After the sample selection, stored (at -20 ◦C) and two aliquots were taken. Samples were anonymized and unique code number were given by the physicians and each pair of sample tubes. In this study, clinical performances of four rapid antigen tests were compared to RT-qPCR in upper respiratory specimens from 784 underage individuals from January 2023 until March 2023. The four RDTs (that were used) in this study are manufactured by PROGNOSIS BIOTECH S.A and comply with the requirements in EN ISO 13485:2016. The tests that were used, and their respective functions, are presented below:
Rapid Test FLU_COVID for the detection of Influenza A/B and SARS-CoV-2 antigens in nasal or nasopharyngeal specimen (V16XX).
Rapid Test FLU A_B for the detection of Influenza A/B antigens in nasal or nasopharyngeal specimen (V17XX).
Rapid Test RSV for the detection of Respiratory Syncytial Virus antigen in nasal or nasopharyngeal specimen (V15XX).
Rapid Test ADENOVIRUS for the detection of Adenovirus antigen in nasal or nasopharyngeal specimen (V18XX).
Rapid Test Validation
In detail, two nasal swabs from 784 underage individuals were obtained simultaneously—the first from one nostril according to WHO guidelines for molecular analysis, and the second from the other nostril according to manufacturer’s specifications for antigen testing. [
22] Concerning the molecular analysis, RNA/DNA extraction performed using the NucleoSpin® Virus Isolation Kit (MACHEREY-NAGEL Gmbh & Co). For RT-PCR, the Real SARS CoV-2/Flu/RSV (Operon S.A) and ZENA HAdv qPCR Detection Kit (AMD Advanced Molecular Diagnostics, UK) was used to detect the above viruses respectively, on SaCycler 96 Real Time PCR system from Sacace Biotechnologies Srl.
3. Results
In the present study the rapid test FLU A_B demonstrated high diagnostic accuracy regarding the detection of Influenza A,
Table 1. The sensitivity of the test was 91.15% (95% CI: 84.33% - 95.67%), and the specificity was 98.96% (95% CI: 97.86% - 99.58%). The positive predictive value (PPV) was 93.64% (95% CI: 87.54% - 96.86%), and the negative predictive value (NPV) was 98.52% (95% CI: 97.35%- 99.17%),
Table 2. The rapid test FLU A_B demonstrated high detection rates across most Ct ranges. For samples with Ct<15, the detection rate was 100.00% (95% CI: 15.81% - 100.00%). For samples with 15≤Ct<25, the detection rate was 100.00% (94.64% - 100.00%). For samples with 25≤Ct<30, the detection rate was 100.00% (85.18% - 100.00%). For samples Ct≥30, the detection rate was 52.38% (29.78% - 74.29%). The findings of this study suggest that rapid test FLU A_B has high sensitivity and specificity for the detection of Influenza A. The high Positive Predictive Value (PPV) and Negative Predictive Value (NPV) indicate that the test has a high accuracy in both confirming the disease in patients who test positive and ruling out the disease in patients who test negative. These findings support the use of the Rapid test FLU A_B as a valuable diagnostic tool in clinical settings.
Secondly, we aimed to evaluate the diagnostic performance of a rapid test FLU A_B, for Influenza B (FLUB) in pediatric population. Rapid and accurate diagnosis of Influenza B is crucial for effective patient management and control of disease spread
Table 1. The test demonstrated high sensitivity [91.67%, (95% CI: 73.00% - 98.97%)] and specificity [99.34%, (95% CI: 98.47% - 99.79%)]. The positive predictive value (PPV) was 81.48% (95% CI: 64.56% - 91.40%), and the negative predictive value (NPV) was 99.74% (95% CI: 99.01% - 99.93%)
Table 2. Rapid test FLU A_B, demonstrated high detection rates across most Ct ranges, regarding the detection of Influenza B. For samples with 15≤Ct<25, the detection rate was 100.00% (75.29% - 100.00%). For samples with 25≤Ct<30, the detection rate was 100.00% (29.24% - 100.00%). For samples with Ct≥30, the detection rate was 66.67% (9.43% -98.30%). The findings of this study suggest that rapid test FLU A_B has high detection rates across most Ct ranges, indicating its utility in detecting Influenza B in samples with varying viral loads.
In the present study we demonstrated for the rapid Adenovirus test high sensitivity and specificity in our study population
Table 3. The sensitivity of the test was 92.45% (95% CI: 81.79% to 97.91%), indicating a high ability to correctly identify patients with Adenovirus. The specificity was 99.32% (95% CI: 98.41% - 99.78%), suggesting a high accuracy in correctly identifying patients without the disease. The PPV was 90.74% (95% CI: 80.30% - 95.93%), indicating that among patients who tested positive with the rapid Adenovirus test, a high percentage truly had Adenovirus. The NPV was 99.45% (95% CI: 98.61% - 99.79%), suggesting that among patients who tested negative with the rapid test, a high percentage truly did not have the disease
Table 3.
The rapid Adenovirus test demonstrated high sensitivity and specificity for the detection of Adenovirus in our study population. These findings support its use as a valuable diagnostic tool. The rapid Adenovirus test demonstrated high detection rates across most Ct ranges. For samples with Ct<15, the detection rate was 100.00% (95% CI: 25.00% -100.00%). For samples with 15≤Ct<25, the detection rate was 100.00% (95% CI: 90.97% - 100.00%). For samples with 25≤Ct<30, the detection rate was 88.88% (95% CI: 51.75% to 99.71%). For samples with Ct≥30, the detection rate was 25% (95% CI: 6.31% - 80.58%). The findings of this study suggest that the rapid Adenovirus test has high detection rates across most Ct ranges, indicating its utility in detecting Adenovirus in samples with varying viral loads.
In accordance with the previous results we demonstrated also for the rapid RSV test high sensitivity and specificity in our study population
Table 4. The sensitivity of the test was 92.59% (95% CI: 75.71% - 99.09%), indicating a high ability to correctly identify patients with RSV. The specificity was 99.47% (95% CI: 98.65% to 99.86%), suggesting a high accuracy in correctly identifying patients without the disease. The PPV was 86.21% (95% CI: 70.04% - 94.35%), indicating that among patients who tested positive with the rapid RSV test, a high percentage truly had RSV. The NPV was 99.74% (95% CI: 99.00% to 99.93%), suggesting that among patients who tested negative with the rapid test, a high percentage truly did not have the disease
Table 4. The rapid RSV test demonstrated high detection rates across most Ct ranges. For samples with Ct<15, the detection rate was 100.00% (95% CI: 15.81% - 100.00%). For samples with 15≤Ct<25, the detection rate was 100.00% (95% CI: 80.49% - 100.00%). For samples with 25≤Ct<30, the detection rate was 83.33% (95% CI: 35.87% -99.57%). For samples with Ct≥30, the detection rate was 66.66% (95% CI: 9.43% - 99. 16%). The findings of this study suggest that the rapid RSV test has high detection rates across most Ct ranges, indicating its utility in detecting RSV in samples with varying viral loads. However, there was some variability in detection rates at higher Ct values, suggesting that the test may have reduced sensitivity in samples with lower viral loads
The rapid test for SARS-CoV-2 demonstrated high sensitivity and specificity in our study population, regarding the detection of SARS-CoV-2
Table 5. The sensitivity of the test was 100.00% (95% CI: 79.41% - 100.00%), indicating a high ability to correctly identify patients with SARS-CoV-2. The specificity was 99.74% (95% CI: 99.06% to 99.97%), suggesting a high accuracy in correctly identifying patients without the disease. The PPV was 88.89% (95% CI: 66.71% - 96.96%), indicating that among patients who tested positive with the rapid SARS-CoV-2 test, a high percentage truly had SARS-CoV-2. The NPV was 100.00% (95% CI: 0.00% to 00.00%), suggesting that among patients who tested negative with the rapid test, a high percentage truly did not have the disease
Table 5. The rapid SARS-CoV-2 test demonstrated high detection rates across most Ct ranges. For samples with 15≤Ct<25, the detection rate was 100.00% (95% CI: 54.07% - 100.00%). For samples with 25≤Ct<30, the detection rate was 100.00% (95% CI: 59.04% - 100.00%). For samples with Ct≥30, the detection rate was 100.00% (95% CI: 29.24% - 100.00%). No samples with Ct<15 were included in the study.
For the period of the present study we recorded the highest proportion of incidence for the Influenza-A (110/784 x100=14.3%), followed by the adenovirus with a proportion of (54/784 x100=6.9%) and the Respiratory Syncytial Virus with (29/784 x100= 3.7%) incidence proportion and finally, Influenza- B (27/784x 100=3.44%) and SARS-CoV-2 with incidence proportion (18/784x100=2.3%).We present high Positive Predictive Value (PPV) and Negative Predictive Value (NPV) for the diagnostic tests that indicate that the test has a high accuracy in both confirming the disease in patients who test positive and ruling out the disease in patients who test negative.
4. Discussion
In the present study we have shown promising results in two different directions. On the one hand, we recorded the incidence for Influenza-A and B, adenovirus, RSV and - SARS-CoV-2 in young children for a three months’ period during winter of 2023. On the other hand, we tested the Specificity and Sensitivity of Rapid Tests for these four respiratory diseases. This could be used by Public Health strategies as an implementation tool in order to control the infections in community.
The early diagnosis and isolation of infectious diseases in symptomatic patients to prevent the dissemination of the infection is very important, especially in the emergency department of the hospitals and the private health facilities. [
23] The recent pandemic set a mandate for researchers and the scientific community for rapid, accurate and affordable SARS-CoV-2 diagnostic tools as global priority. Diagnostic tests for infectious diseases, especially for those that active acquired immunity via vaccine does not exist, are essential for widespread testing and contact tracing in public health policies to control the spread of disease. [
24,
25,
26] The gold standard method of real-time reverse transcription polymerase chain reaction (RT-PCR) is a very sensitive method but rapid diagnosis tests, based on antigen detection, are faster, easier to perform and cost effective for the financial capacity of the global economy. [
27]
Furthermore, rapid tests are best performed within the early stages of acute infection, when viral load is at its highest levels (usually the first 5-7 days from symptom onset), after which antigen levels may drop significantly. [
28] In the present study, we demonstrate that rapid tests offer the advantage of early detection of virus infections and help health professionals to start treatment on time as well as to reduce complications.
Given the experience from the recent global health emergency, policies and decisions from public health authorities targeting at keeping the regular viral load activity in semi-closed communities, like workplaces, schools or universities, are based on general measures, such as basic hygiene measures , mask wearing, washing hands and social distancing, [
29,
30,
31] Moreover, proactive broad population surveillance to stop asymptomatic spread and prevent outbreaks is also advisable and has been implemented in many work places that the necessary budget was available. [
32] According to WHO’s chief’s report at the 76th World Health Assembly “The end of COVID-19 as a global health emergency is not the end of COVID-19 as a global health threat, “
The threat of another variant emerging that causes new surges of disease and death remains, and the threat of another pathogen emerging with even deadlier potential remains.” [
33] The Diagnostic tests as developed for SARS-CoV-2 constitute critical component and stable base to the overall prevention and control strategy for new threats and emergencies.
A Plethora of studies published address the financial benefits of the early detection of the infectious diseases. Towards this direction, Paltiel et al with a model study supported that without a testing intervention, the model anticipated 11.6 million infections, 119 000 deaths, and
$10.1 billion in costs (
$6.5 billion in inpatient care and
$3.5 billion in lost productivity) over a 60-day horizon for the SARS-CoV-2 in 2021. [
34] The results of the present study support this opinion and underline the financial benefits of tests and early diagnosis for 4 respiratory viral infections in young children during the winter of 2023.
Another important finding of the present study was the incidence of RSV. There are sparse data for the prevalence and incidence of RSV in community in Greece. We present incidence that accounts for 3.7% of the total sample of the participants. Based on the results of older studies, 61% of infants with bronchiolitis had documented RSV infection with a case fatality rate that accedes up to 0.7% and RSV is the most prevalent virus (56.6%) among children with detected viral infection [
35,
36]. Similar results with the previous study were reported by Tsergouli et al in a hospital in Northern Greece. More precisely, children under the age of 2 years, hospitalized for bronchiolitis, were tested for RSV infection during two RSV seasons (2016-2017 and 2017-2018). RSV was detected in (52.1%) of patients, most of them younger than 6 months. [
37] Statistical data agree with the results of a more recent study for the RSV by Tsagarakis et al in young children. [
38] Contrarily, a study designed for an adult population showed low-level circulation of RSV during the autumn-winter period of 2021. [
39]
Another retrospective study for RSV during a 12-year period (2002-2013) recorded total prevalence of 27% for children tested positive for RSV infection. [
40] In a serum epidemiology study in hospitalized children with atypical community-acquired pneumonia (CAP), IgM antibodies against RSV were detected in 20.7% of the total sample and co-infection was detected in 3 cases, two along with mycoplasma pneumoniae and one with adenovirus. [
41]
The new guidelines dealing with RSV vaccination, after the FDA authorization, constitute priority for age groups the vaccine is addressed to. At the moment, the suggestions refer to the elderly. On the other hand, the epidemiological data emphasize that in children less than one-year RSV is the leading cause of hospitalization and one of the principles causes of clinic appointments in children less than 5 years of age. Furthermore, it is estimated that RSV causes one million lower respiratory tract infections each year resulting in a huge number of hospitalizations as the most common cause of hospitalization in children under 5 years old also. [
42,
43]
WHO published in 2014 a general guidance document that can be used as a reference for making decisions about and planning the introduction of a vaccine into a national immunization programme. [
44] In line with this report, in the present study we present data about the incidence of RSV infections in young children. We also provide data that will be useful in the future to the Public Health authorities in order to consider the introduction of the new RSV vaccine into special population groups expected to profit from its implementation.
Additionally, the incidence of the adenovirus in the present study was 6.9%. An older study aimed to determine the distribution of several respiratory viruses in children diagnosed as having influenza-like illness over the winter period of 2005-2008 recorded similar results and the total proportion of adenovirus was 7.5%. [
45]
Finally, according to WHO, the 2022-2023 influenza season epidemic started prematurely in the European region. At the same time concerns over Respiratory syncytial virus have risen and COVID-19 remained a threat. Along with COVID-19, these viruses are expected to have a high impact on health services and populations. [
46] We recorded low incidence proportion of SARS-CoV 2 in a young child population after three pandemic years despite a modelling estimation about the respiratory infections in community in Wales for the winter 2022-23. [
47] A similar design study with the present by Curatola et al the autumn and winter of 2021–2022 period in pediatric emergency department of a tertiary Italian hospital in children between 0 and 18 years demonstrated 12.0% total prevalence of SARS-CoV 2. [
48]
The present study has several limitations. The main limitation is the study period. Data are lacking as the record system initiated after December 2022, making it impossible to determine the total incidence for the winter period 2022-23. Secondly, the sample of participants related to hospital and dispensaries may not be representive of population, and findings may not apply to other groups. Finally, we cannot exclude the possibility that mistakes using the tests without supervision may have arised and that could have driven to false results.
5. Conclusions
We present incidence of four types of respiratory infectious diseases in child population at the same time of observation for the winter 2022-23 period. The implementation of rapid diagnostic tests (RDTs) may enhance the efficiency and early diagnosis of severe acute respiratory diseases as RDTs are widely accessible and easy to use. There are no clinical signs and symptoms that can distinguish circulating respiratory pathogens and most of the symptoms are common. Accurate laboratory diagnosis from respiratory secretions is thus required and is associated with several potential benefits. Early and confirmed diagnosis may prevent the use of empiric antibiotic treatment or enable its discontinuation if it was already started with a plethora of benefits for the patients and health systems.
Eventually, we tried to evaluate four antigens for respiratory diseases. All the antigens presented high sensitivity and specificity for the four pathogens respectively. The combination of a new multiple rapid test with different antigens, will be probably a useful tool with financial impact for early detection and right treatment in the emergency departments and in primary health care facilities. Finally, we provide data that could be useful for the public health authorities to design against future threats of infectious diseases.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, D.P. and K.I.G; methodology, D.P.; K.I.G software, G.P.; validation, D.P., G.P. and F.M.; formal analysis, D.P.; investigation, D.P.; K.I.G resources, D.P.; G.P; T.T; P.K; G.D; A.K; S.A; F.M; K.I.G; data curation, D.P.; G.P; T.T; P.K;G.D;A.K;S.A;F.M;K.I.G; writing—original draft preparation, D.P.;G.P;F.M;K.I.G writing—review and editing, D.P;G.P.;K.I.G visualization, D.P.;F.M supervision, D.P.;K.I.G project administration, D.P.;K.I.G. All authors have read and agreed to the published version of the manuscript
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the University of Thessaly (No. 2800-01/11/2020; approved on 1 November 2020). The Clinical Evaluation of the samples was performed in collaboration with the research program of Respiratory Medicine Department, Faculty of Medicine, University of Thessaly and the pediatric department of General Hospital of Larissa at the Laboratory of Public Health and adults’ immunization of the University of Thessaly.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank the parents of the children that participated in the study. We would like also to thank health professionals who participated voluntarily and helped with sample collection as follows: Georgios Efstathiou, Anthippi Passa, Zacharoula Bogogiannidou, Christos Karvounis, Maria Tsirevelou, Eleni Raikou, Eleni Chlinou, Anastasia Antoniadou, Styliani Sarri, Loukia Malotziki, Georgia Dellali. The authors would like to thank the PROGNOSIS BIOTECH S.A for the offer of rapid tests.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Peeling, R.W.; Olliaro, P.L.; Boeras, D.I.; Fongwen, N. Scaling up COVID-19 rapid antigen tests: Promises and challenges. Lancet Infect. Dis. 2021, 21, E290–E295. [Google Scholar] [CrossRef]
- Yamayoshi, S.; Sakai-Tagawa, Y.; Koga, M.; Akasaka, O.; Nakachi, I.; Koh, H.; Maeda, K.; Adachi, E.; Saito, M.; Nagai, H.; et al. Comparison of Rapid Antigen Tests for COVID-19. Viruses 2020, 12, 1420. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.E.; Beltran, W.F.G.; Bard, A.Z.; Gogakos, T.; Anahtar, M.N.; Astudillo, M.G.; et al. Clinical sensitivity and interpretation of PCR and serological COVID-19 diagnostics for patients presenting to the hospital. FASEB J. 2020, 34, 13877–13884. [Google Scholar] [CrossRef]
- Scohy A, Anantharajah A, Bodéus M, Kabamba-Mukadi B, Verroken A, Rodriguez-Villalobos H. Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis. J Clin Virol. 2020, 129, 104455. [Google Scholar] [CrossRef]
- Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017, 390, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Li Y, Wang X, Blau DM, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022, 399, 2047–2064. [Google Scholar] [CrossRef] [PubMed]
- Shieh, WJ. Human adenovirus infections in pediatric population - An update on clinico-pathologic correlation. Biomed J. 2022, 45, 38–49. [Google Scholar] [CrossRef]
- Brandt, C.D. , Kim H.W., Vargosko A.J., Jeffries B.C., Arrobio J.O., Rindge B., et al. Infections in 18,000 infants and children in a controlled study of respiratory tract disease. I. Adenovirus pathogenicity in relation to serologic type and illness syndrome. Am J Epidemiol. 1969, 90, 484–500. [Google Scholar] [CrossRef]
- Edwards, K.M. , Thompson J., Paolini J., Wright P.F. Adenovirus infections in young children. Pediatrics. 1985, 76, 420–424. [Google Scholar] [CrossRef]
- Mennechet, F.J.D. , Paris O., Ouoba A.R., Salazar Arenas S., Sirima S.B., Takoudjou Dzomo G.R., et al. A review of 65 years of human adenovirus seroprevalence. Expert Rev Vaccines. 2019, 18, 597–613. [Google Scholar] [CrossRef]
- Influenza in children. Paediatrics & Child Health 2005, 10, 485–487. [CrossRef]
- Troeger, C. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef]
- Dunn FL, Carey DE, Cohen A, et al: Epidemiologic studies of Asian influenza in a Louisiana parish. Am J Hyg. 1959, 70, 351–371.
- Hospitalized patients with novel influenza A (H1N1) virus infection — California, April–May, 2009. MMWR Morb Mortal Wkly Rep. 2009, 58, 536–541.
- Surveillance for pediatric deaths associated with 2009 pandemic influenza A (H1N1) virus infection — United States, April–August 2009. MMWR Morb Mortal Wkly Rep. 2009, 58, 941–947.
- O'Riordan S, Barton M, Yau Y, Read SE, Allen U, Tran D. Risk factors and outcomes among children admitted to hospital with pandemic H1N1 influenza. CMAJ. 2010, 182, 39–44. [Google Scholar] [CrossRef]
- Borrelli M, Corcione A, Castellano F, Fiori Nastro F, Santamaria F. Coronavirus Disease 2019 in Children. front Pediatr. 2021, 9, 668484. [Google Scholar] [CrossRef]
- Howard-Jones AR, Burgner DP, Crawford NW, Goeman E, Gray PE, Hsu P, et al. COVID-19 in children. II: Pathogenesis, disease spectrum and management. J Paediatr Child Health. 2022, 58, 46–53. [Google Scholar] [CrossRef]
- Rebnord IK, Sandvik H, Mjelle AB, Hunskaar S. Out-of-hours antibiotic prescription after screening with C reactive protein: a randomized controlled study. BMJ Open, 2016, 6, e011231. [Google Scholar] [CrossRef]
- Havasi A, Visan S, Cainap C, Cainap SS, Mihaila AA, Pop LA. Influenza A, Influenza B, and SARS-CoV-2 Similarities and Differences - A Focus on Diagnosis. Front Microbiol. 2022, 13, 908525. [Google Scholar] [CrossRef] [PubMed]
- Harris, E. CDC: RSV Vaccine Recommended for Older People. JAMA. 2023. [Google Scholar] [CrossRef]
- World Health Organization in Vitro Diagnostics Detecting Sars-CoV-2 Nucleic Acid and Rapid Diagnostics Tests Detecting Sars-CoV-2 Antigen. Published 9 June 2020. Available at: https://www.who.int/diagnostics_laboratory/EUL/en/ Accessed on July 12, 2023. 9 June.
- Wee, L.E. , Fua T.P., Chua Y.Y., Ho A.F.W., Sim X.Y.J., Conceicao E.P., et al. Containing COVID-19 in the Emergency Department: The Role of Improved Case Detection and Segregation of Suspect Cases. Acad. Emerg. Med. 2020, 27, 379–387. [Google Scholar] [CrossRef]
- Boehme, C. , Hannay E., Sampath R. SARS-CoV-2 testing for public health use: core principles and considerations for defined use settings. Lancet Glob Health. 2021, 9, e247–e249. [Google Scholar] [CrossRef] [PubMed]
- Goldstein N., Burstyn I. On the importance of early testing even when imperfect in a pandemic such as COVID-19. Glob Epidemiol. 2020, 2.
- Adalja, A.A. , Toner E., Inglesby T.V. Priorities for the US health community responding to COVID-19. JAMA. 2020, 323, 1343–1344. [Google Scholar] [CrossRef] [PubMed]
- Artika IM, Dewi YP, Nainggolan IM, Siregar JE, Antonjaya U. Real-Time Polymerase Chain Reaction: Current Techniques, Applications, and Role in COVID-19 Diagnosis. Genes (Basel). 2022, 13, 2387. [Google Scholar] [CrossRef]
- ECDC. Options for the use of rapid antigen detection tests for COVID-19 in the EU/EEA – first update. Available at: https://www.ecdc.europa.eu/en/publications-data/options-use-rapid-antigen-tests-covid-19-eueea-first-update . Accessed on July 14, 2023.
- Papagiannis D, Kotsiou OS, Fradelos EC, Perlepe G, Miziou A, Siachpazidou DS, Gourgoulianis KI. Work place and prevalence of COVID-19 in a rural population in Greece. Rural Remote Health. 2022, 22, 6751. [Google Scholar] [CrossRef]
- Kotsiou OS, Papagiannis D, Fradelos EC, Perlepe G, Miziou A, Siachpazidou DS, Gourgoulianis KI. Understanding COVID-19 Epidemiology and Implications for Control: The Experience from a Greek Semi-Closed Community. J Clin Med. 2021, 10, 2765. [Google Scholar] [CrossRef] [PubMed]
- Zachari S, Papagiannis D, Kotsiou O, Malli F, Fradelos EC, Gourgoulianis KI. Factors of Compliance of Dental Patients in Primary Health Care Services during the Pandemic. Vaccines (Basel). 2023, 11, 844. [Google Scholar] [CrossRef]
- Papagiannis D, Laios T, Tryposkiadis K, Kouriotis K, Roussis X, Basdekis G, et al. COVID-19 Infection among Elite Football Players: A Nationwide Prospective Cohort Study. Vaccines (Basel). 2022, 10, 634. [Google Scholar] [CrossRef]
- WHO. The Seventy-sixth World Health Assembly is being held in Geneva, Switzerland, on . Available at: https://www.who.int/about/governance/world-health-assembly/seventy-sixth-world-health-assembly. Accessed on July 14, 2023. 21–30 May.
- Paltiel AD, Zheng A, Sax PE. Clinical and Economic Effects of Widespread Rapid Testing to Decrease SARS-CoV-2 Transmission. Ann Intern Med. 2021, 174, 803–810. [Google Scholar] [CrossRef]
- Tsolia MN, Kafetzis D, Danelatou K, Astral H, Kallergi K, Spyridis P, Karpathios TE. Epidemiology of respiratory syncytial virus bronchiolitis in hospitalized infants in Greece. Eur J Epidemiol. 2003, 18, 55–61. [Google Scholar] [CrossRef]
- Kouni S, Karakitsos P, Chranioti A, Theodoridou M, Chrousos G, Michos A. Evaluation of viral co-infections in hospitalized and non-hospitalized children with respiratory infections using microarrays. Clin Microbiol Infect. 2013, 19, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Tsergouli K, Pappa S, Haidopoulou K, Gogou M, Giannopoulos A, Papa A. Respiratory Syncytial Virus in Greece, 2016-2018. Intervirology. 2019, 62, 210–215. [Google Scholar] [CrossRef]
- Tsagarakis NJ, Sideri A, Makridis P, Triantafyllou A, Stamoulakatou A, Papadogeorgaki E. Age-related prevalence of common upper respiratory pathogens, based on the application of the Film Array Respiratory panel in a tertiary hospital in Greece. Medicine (Baltimore). 2018, 97, e10903. [Google Scholar] [CrossRef] [PubMed]
- Papachristou E, Rokka C, Sotiriadou T, Maneka L, Vassilakis A, Sapounas S. Low circulation of respiratory syncytial and influenza viruses during autumn-winter 2021 in the industrial workplace and long-term healthcare facilities in Athens, Greece. Front Med (Lausanne). 2023, 9, 1025147. [Google Scholar] [CrossRef]
- Sirimi N, Miligkos M, Koutouzi F, Petridou E, Siahanidou T, Michos A. Respiratory syncytial virus activity and climate parameters during a 12-year period. J Med Virol. 2016, 88, 931–937. [Google Scholar] [CrossRef]
- Almasri M, Papa A, Souliou E, Haidopoulou K, Eboriadou M. Respiratory syncytial virus infection in hospitalized children older than 2 years with community-acquired pneumonia. Hippokratia. 2013, 17, 146–149. [Google Scholar]
- Hall CB, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009, 360, 588–598. [Google Scholar] [CrossRef]
- Nair H, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010, 375, 1545–1555.
- WHO. Principles and considerations for adding a vaccine to a national immunization program: from decision to implementation and monitoring. Available at: https://www.who.int/publications/i/item/9789241506892 . Accessed on July 15 2023.
- Pogka V, Kossivakis A, Kalliaropoulos A, Moutousi A, Sgouras D, Panagiotopoulos T, et al. Respiratory viruses involved in influenza-like illness in a Greek pediatric population during the winter period of the years 2005-2008. J Med Virol. 2011, 83, 1841–1848. [Google Scholar] [CrossRef]
- WHO. Joint statement - Influenza season epidemic kicks off early in Europe as concerns over RSV rise and COVID-19 is still a threat. Available at: https://www.who.int/europe/news/item/01-12-2022-joint-statement---influenza-season-epidemic-kicks-off-early-in-europe-as-concerns-over-rsv-rise-and-covid-19-is-still-a-threat . Accessed on July 16, 2023. 16 July.
- Winter modelling 2022 to 2023 update - gov. wales. Available at: https://www.gov.wales/sites/default/files/publications/2023-01/science-evidence-advice-winter-modelling-update-december-2022.pdf . Accessed on July 16, 2023. 16 July.
- Curatola A, Ferretti S, Graglia B, Capossela L, Menchinelli G, Fiori B, et al. COVID-19 increased in Italian children in the autumn and winter 2021-2022 period when Omicron was the dominant variant. Acta Paediatr. 2023, 112, 290–295. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).