Submitted:
27 July 2023
Posted:
31 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
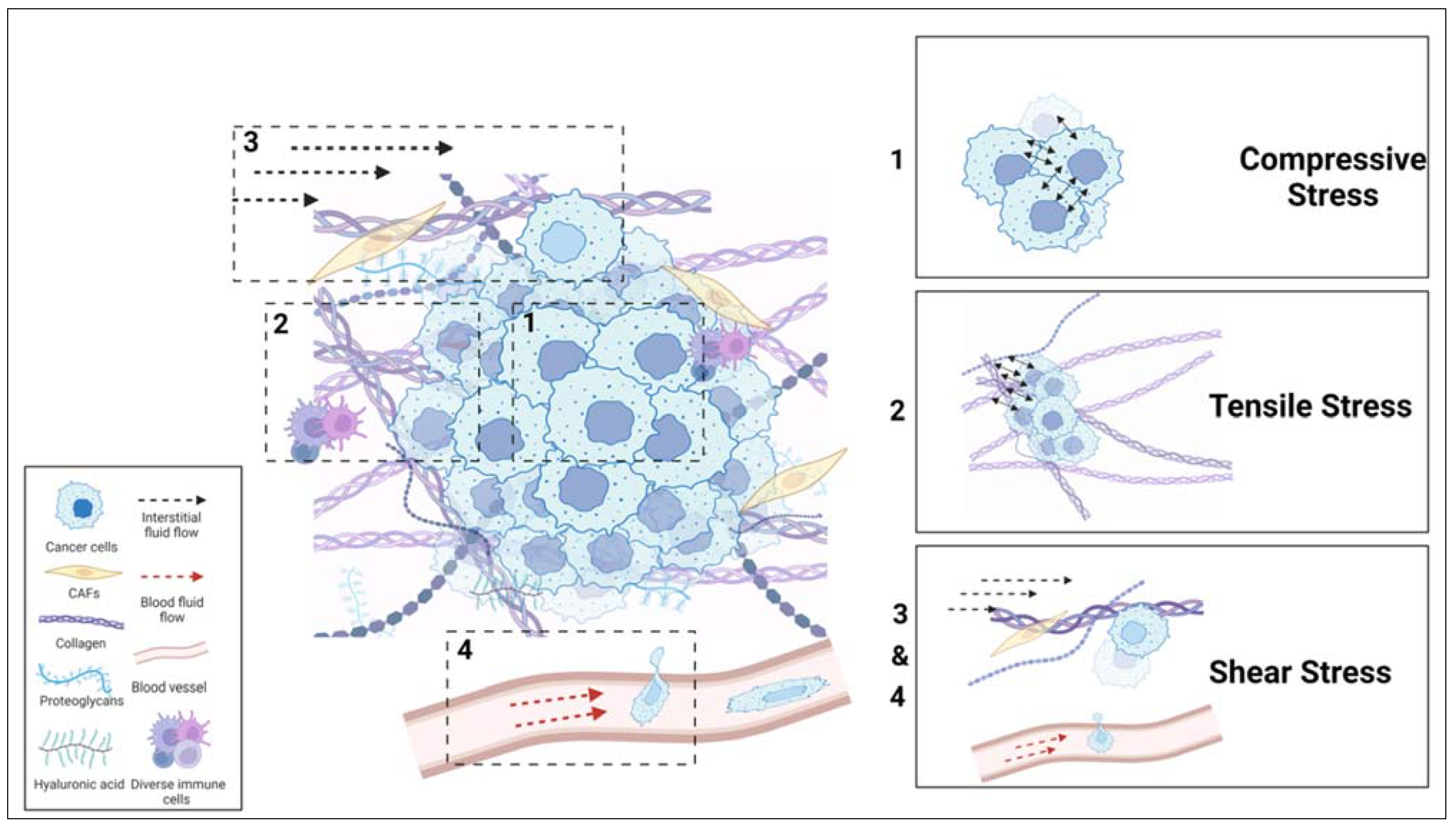
1.1. Mechanobiology of the Tumor Microenvironment
1.2. The Impact of TME on Cancer Cell Behavior
2. Mechanobiological Strategies Targeting Tme
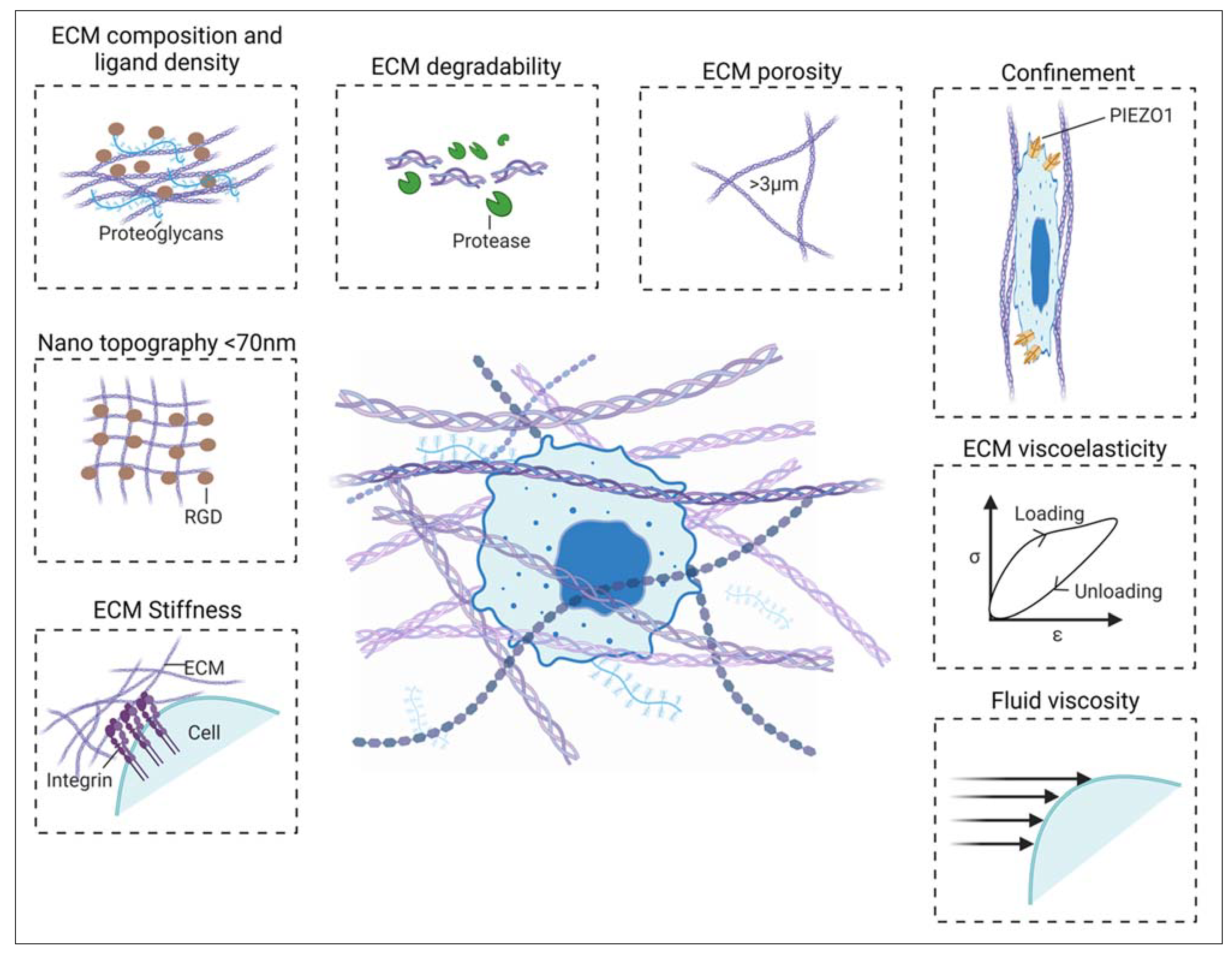
2.1. Extracellular Matrix (ECM)
2.2. Targeting ECM Protein Synthesis and Stiffening
2.3. Physical Disruption of ECM
2.4. Targeting Mechanosensory and Mechanotransducers of ECM Stiffness
3. Cancer Cells
4. Immune Cells
4.1. T Cells
4.2. Dendritic Cells (DCs)
4.3. Tumor-Associated Macrophages (TAMs)
4.4. Cancer-Associated Fibroblasts (CAFs)
CONCLUSION AND OUTLOOK
Author Contributions
Acknowledgment
Conflicts of Interest
Biographies
References
- Shi, X.; Zhang, L.; Li, B.; Feng, X. The Mechanical Problems in Tumor and Tumor Microenvironment. Advances in Mechanics 2018. [CrossRef]
- Liu, Q.; Luo, Q.; Ju, Y.; Song, G. Role of the Mechanical Microenvironment in Cancer Development and Progression. Cancer Biology and Medicine 2020. [CrossRef] [PubMed]
- Stylianopoulos, T.; Martin, J. D.; Chauhan, V. P.; Jain, S. R.; Diop-Frimpong, B.; Bardeesy, N.; Smith, B. L.; Ferrone, C. R.; Hornicek, F. J.; Boucher, Y.; Munn, L. L.; Jain, R. K. Causes, Consequences, and Remedies for Growth-Induced Solid Stress in Murine and Human Tumors. Proc Natl Acad Sci U S A 2012, 109. [Google Scholar] [CrossRef]
- Luo, M.; K. Y. Ho, K.; Tong, Z.; Deng, L.; P. Liu, A. Compressive Stress Enhances Invasive Phenotype of Cancer Cells via Piezo1 Activation. bioRxiv 2019. [Google Scholar] [CrossRef]
- Stylianopoulos, T. The Solid Mechanics of Cancer and Strategies for Improved Therapy. J Biomech Eng 2017, 139. [Google Scholar] [CrossRef]
- Yu, H.; Mouw, J. K.; Weaver, V. M. Forcing Form and Function: Biomechanical Regulation of Tumor Evolution. Trends in Cell Biology 2011. [CrossRef]
- Northcott, J. M.; Dean, I. S.; Mouw, J. K.; Weaver, V. M. Feeling Stress: The Mechanics of Cancer Progression and Aggression. Frontiers in Cell and Developmental Biology 2018. [CrossRef]
- Bera, K.; Kiepas, A.; Godet, I.; Li, Y.; Mehta, P.; Ifemembi, B.; Paul, C. D.; Sen, A.; Serra, S. A.; Stoletov, K.; Tao, J.; Shatkin, G.; Lee, S. J.; Zhang, Y.; Boen, A.; Mistriotis, P.; Gilkes, D. M.; Lewis, J. D.; Fan, C. M.; Feinberg, A. P.; Valverde, M. A.; Sun, S. X.; Konstantopoulos, K. Extracellular Fluid Viscosity Enhances Cell Migration and Cancer Dissemination. Nature 2022, 611. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, Z.; He, Y.; Li, P.; Chen, Y.; Chen, X.; Jiang, Y.; Qin, X.; Li, S.; Li, T.; Wu, C.; Yang, H.; You, F.; Liu, Y. Non-Muscle Myosin II Isoforms Orchestrate Substrate Stiffness Sensing to Promote Cancer Cell Contractility and Migration. Cancer Lett 2022, 524. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, Z.; Chen, Y.; Li, S.; Jiang, Y.; Yang, H.; Wu, C.; You, F.; Zheng, C.; Zhu, J.; Tan, Y.; Qin, X.; Liu, Y. ROCK Isoforms Differentially Modulate Cancer Cell Motility by Mechanosensing the Substrate Stiffness. Acta Biomater 2019, 88. [Google Scholar] [CrossRef]
- Ruoslahti, E. RGD and Other Recognition Sequences for Integrins. Annual Review of Cell and Developmental Biology 1996. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhao, C.; Spatz, J. P.; Wei, Q. Nanopatterned Adhesive, Stretchable Hydrogel to Control Ligand Spacing and Regulate Cell Spreading and Migration. ACS Nano 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Farino Reyes, C. J.; Pradhan, S.; Slater, J. H. The Influence of Ligand Density and Degradability on Hydrogel Induced Breast Cancer Dormancy and Reactivation. Adv Healthc Mater 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Koshy, S. T.; Branco Da Cunha, C.; Shin, J. W.; Verbeke, C. S.; Allison, K. H.; Mooney, D. J. Extracellular Matrix Stiffness and Composition Jointly Regulate the Induction of Malignant Phenotypes in Mammary Epithelium. Nat Mater 2014, 13. [Google Scholar] [CrossRef]
- Koorman, T.; Jansen, K. A.; Khalil, A.; Haughton, P. D.; Visser, D.; Rätze, M. A. K.; Haakma, W. E.; Sakalauskaitè, G.; van Diest, P. J.; de Rooij, J.; Derksen, P. W. B. Spatial Collagen Stiffening Promotes Collective Breast Cancer Cell Invasion by Reinforcing Extracellular Matrix Alignment. Oncogene 2022, 41. [Google Scholar] [CrossRef]
- Wolf, K.; te Lindert, M.; Krause, M.; Alexander, S.; te Riet, J.; Willis, A. L.; Hoffman, R. M.; Figdor, C. G.; Weiss, S. J.; Friedl, P. Physical Limits of Cell Migration: Control by ECM Space and Nuclear Deformation and Tuning by Proteolysis and Traction Force. Journal of Cell Biology 2013, 201. [Google Scholar] [CrossRef]
- Harada, T.; Swift, J.; Irianto, J.; Shin, J. W.; Spinler, K. R.; Athirasala, A.; Diegmiller, R.; Dingal, P. C. D. P.; Ivanovska, I. L.; Discher, D. E. Nuclear Lamin Stiffness Is a Barrier to 3D-Migration, but Softness Can Limit Survival. In Proceedings of the IEEE Annual Northeast Bioengineering Conference, NEBEC; 2014; Vol. 2014-December. [Google Scholar] [CrossRef]
- Weigelin, B.; Bakker, G.-J.; Friedl, P. Intravital Third Harmonic Generation Microscopy of Collective Melanoma Cell Invasion. Intravital 2012, 1. [Google Scholar] [CrossRef]
- Shen, Q.; Hill, T.; Cai, X.; Bui, L.; Barakat, R.; Hills, E.; Almugaiteeb, T.; Babu, A.; Mckernan, P. H.; Zalles, M.; Battiste, J. D.; Kim, Y. T. Physical Confinement during Cancer Cell Migration Triggers Therapeutic Resistance and Cancer Stem Cell-like Behavior. Cancer Lett 2021, 506, 142. [Google Scholar] [CrossRef]
- Balzer, E. M.; Tong, Z.; Paul, C. D.; Hung, W. C.; Stroka, K. M.; Boggs, A. E.; Martin, S. S.; Konstantopoulos, K. Physical Confinement Alters Tumor Cell Adhesion and Migration Phenotypes. FASEB Journal 2012, 26. [Google Scholar] [CrossRef]
- Hung, W. C.; Yang, J. R.; Yankaskas, C. L.; Wong, B. S.; Wu, P. H.; Pardo-Pastor, C.; Serra, S. A.; Chiang, M. J.; Gu, Z.; Wirtz, D.; Valverde, M. A.; Yang, J. T.; Zhang, J.; Konstantopoulos, K. Confinement Sensing and Signal Optimization via Piezo1/PKA and Myosin II Pathways. Cell Rep 2016, 15. [Google Scholar] [CrossRef]
- Elosegui-Artola, A.; Gupta, A.; Najibi, A. J.; Seo, B. R.; Garry, R.; Tringides, C. M.; de Lázaro, I.; Darnell, M.; Gu, W.; Zhou, Q.; Weitz, D. A.; Mahadevan, L.; Mooney, D. J. Matrix Viscoelasticity Controls Spatiotemporal Tissue Organization. Nat Mater 2023, 22. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, M.; Cheng, M.; Gao, Z.; Wang, G. The Viscoelastic Behaviors of Several Kinds of Cancer Cells and Normal Cells. J Mech Behav Biomed Mater 2019, 91. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, B.; Meng, S.; Li, S.; Yang, X. Electrical and Viscoelastic Measurement of Cancer Cells in Epithelial-Mesenchymal Transition Process on a Microfluidic Device. In 2019 8th International Symposium on Next Generation Electronics, ISNE 2019; 2019. [CrossRef]
- Gonzalez-Molina, J.; Zhang, X.; Borghesan, M.; Mendonça da Silva, J.; Awan, M.; Fuller, B.; Gavara, N.; Selden, C. Extracellular Fluid Viscosity Enhances Liver Cancer Cell Mechanosensing and Migration. Biomaterials 2018, 177, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Formenti, S. C.; Hawtin, R. E.; Dixit, N.; Evensen, E.; Lee, P.; Goldberg, J. D.; Li, X.; Vanpouille-Box, C.; Schaue, D.; McBride, W. H.; Demaria, S. Baseline T Cell Dysfunction by Single Cell Network Profiling in Metastatic Breast Cancer Patients. J Immunother Cancer 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Zion, O.; Genin, O.; Kawada, N.; Yoshizato, K.; Roffe, S.; Nagler, A.; Iovanna, J. L.; Halevy, O.; Pines, M. Inhibition of Transforming Growth Factor β Signaling by Halofuginone as a Modality for Pancreas Fibrosis Prevention. Pancreas 2009, 38, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Yi, S.; Gong, L.; Liu, W.; Wang, P.; Liu, N.; Zhao, L.; Wang, P. Inhibition of TGF-β Signaling with Halofuginone Can Enhance the Antitumor Effect of Irradiation in Lewis Lung Cancer. Taylor & Francis 2015, 8, 3549–3559. [Google Scholar] [CrossRef]
- Juárez, P.; Mohammad, K.; Yin, J.; research, P. F.-C. 2012, undefined. Halofuginone Inhibits the Establishment and Progression of Melanoma Bone MetastasesHalofuginone Decreases Melanoma Bone Metastases. AACR.
- Juárez, P.; Fournier, P.; Oncotarget, K. M. 2017, undefined. Halofuginone Inhibits TGF-β/BMP Signaling and in Combination with Zoledronic Acid Enhances Inhibition of Breast Cancer Bone Metastasis. ncbi.nlm.nih.gov.
- Zhang, B.; Halder, S.; Zhang, S.; letters, P. D.-C. 2009, undefined. Targeting Transforming Growth Factor-β Signaling in Liver Metastasis of Colon Cancer. Elsevier.
- Melisi, D.; Ishiyama, S.; Sclabas, G.; … J., F.-M. cancer; 2008, undefined. LY2109761, a Novel Transforming Growth Factor β Receptor Type I and Type II Dual Inhibitor, as a Therapeutic Approach to Suppressing Pancreatic Cancer Metastasis. AACR.
- Korpal, M.; Yan, J.; Lu, X.; Xu, S.; Lerit, D.; medicine, Y. K.-N. 2009, undefined. Imaging Transforming Growth Factor-β Signaling Dynamics and Therapeutic Response in Breast Cancer Bone Metastasis. nature.com.
- Rudman, S. M.; Jameson, M. B.; McKeage, M. J.; Savage, P.; Jodrell, D. I.; Harries, M.; Acton, G.; Erlandsson, F.; Spicer, J. F. A Phase 1 Study of AS1409, a Novel Antibody-Cytokine Fusion Protein, in Patients with Malignant Melanoma or Renal Cell Carcinoma. Clinical Cancer Research 2011, 17. [Google Scholar] [CrossRef]
- Hisatomi, K.; Mukae, H.; Sakamoto, N.; Ishimatsu, Y.; Kakugawa, T.; Hara, S.; Fujita, H.; Nakamichi, S.; Oku, H.; Urata, Y.; Kubota, H.; Nagata, K.; Kohno, S. Pirfenidone Inhibits TGF-Β1-Induced over-Expression of Collagen Type I and Heat Shock Protein 47 in A549 Cells. BMC Pulm Med 2012, 12. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Quimbo, A.; Xia, F.; Yao, J.; Clamme, J.-P.; Zabludoff, S.; Zhang, J.; Ying, W. Anti-HSP47 SiRNA Lipid Nanoparticle ND-L02-S0201 Reverses Interstitial Pulmonary Fibrosis in Preclinical Rat Models. Eur Respiratory Soc. [CrossRef]
- Jiang, Y.; Zhang, H.; Wang, J.; Liu, Y.; Luo, T.; Hua, H. Targeting Extracellular Matrix Stiffness and Mechanotransducers to Improve Cancer Therapy. J Hematol Oncol 2022, 15, 1–15. [Google Scholar] [CrossRef]
- Benson, A. B.; Wainberg, Z. A.; Hecht, J. R.; Vyushkov, D.; Dong, H.; Bendell, J.; Kudrik, F. A Phase II Randomized, Double-Blind, Placebo-Controlled Study of Simtuzumab or Placebo in Combination with Gemcitabine for the First-Line Treatment of Pancreatic Adenocarcinoma. The Oncologist 2017, 241-e15. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J. R.; Benson, A. B.; Vyushkov, D.; Yang, Y.; Bendell, J.; Verma, U. A Phase II, Randomized, Double-Blind, Placebo-Controlled Study of Simtuzumab in Combination with FOLFIRI for the Second-Line Treatment of Metastatic KRAS Mutant Colorectal Adenocarcinoma. The Oncologist 2017, 243-e23. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Han, H.; Koo, H.; Na, J. H.; Yoon, H. Y.; Lee, K. E.; Lee, H.; Kim, H.; Kwon, I. C.; Kim, K. Extracellular Matrix Remodeling in Vivo for Enhancing Tumor-Targeting Efficiency of Nanoparticle Drug Carriers Using the Pulsed High Intensity Focused Ultrasound. Journal of Controlled Release 2017, 263, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Fink, K.; Scott, B.; Health, W.; Ravin, P.; Raizer, J. J. Randomized Phase II Study of Cilengitide, an Integrin-Targeting Arginine-Glycine-Aspartic Acid Peptide, in Recurrent Glioblastoma Multiforme. researchgate.net 2008. [Google Scholar] [CrossRef]
- Cianfrocca, M. E.; Kimmel, K. A.; Gallo, J.; Cardoso, T.; Brown, M. M.; Hudes, G.; Lewis, N.; Weiner, L.; Lam, G. N.; Brown, S. C.; Shaw, D. E.; Mazar, A. P.; Cohen, R. B. Phase 1 Trial of the Antiangiogenic Peptide ATN-161 (Ac-PHSCN-NH2), a Beta Integrin Antagonist, in Patients with Solid Tumours. British Journal of Cancer 2006, 94, 1621–1626. [Google Scholar] [CrossRef]
- Delbaldo, C.; Raymond, E.; Vera, K.; Hammershaimb, L.; Kaucic, K.; Lozahic, S.; Marty, M.; Faivre, S. Phase I and Pharmacokinetic Study of Etaracizumab (AbegrinTM), a Humanized Monoclonal Antibody against Avβ3 Integrin Receptor, in Patients with Advanced Solid Tumors. Invest New Drugs 2008, 26, 35–43. [Google Scholar] [CrossRef]
- Hersey, P.; Sosman, J.; Steven O’day, ; Richards, J.; Bedikian, A.; Gonzalez, R.; Sharfman, W.; Weber, R.; Logan, T.; Buzoianu, M.; Hammershaimb, ; Luz; Kirkwood, J. M. A Randomized Phase 2 Study of Etaracizumab, a Monoclonal Antibody against Integrin Avβ3, ± Dacarbazine in Patients with Stage IV Metastatic Melanoma. Wiley Online Library 2010, 116, 1526–1534. [CrossRef]
- O’day, S.; Pavlick, A.; Loquai, C.; … D., L.-B. journal of; 2011, undefined. A Randomised, Phase II Study of Intetumumab, an Anti-Av-Integrin MAb, Alone and with Dacarbazine in Stage IV Melanoma. nature.com.
- Heidenreich, A.; Rawal, S.; Szkarlat, K.; oncology, N. B.-A. 2013, undefined. A Randomized, Double-Blind, Multicenter, Phase 2 Study of a Human Monoclonal Antibody to Human Aν Integrins (Intetumumab) in Combination with Docetaxel. Elsevier.
- Élez, E.; Kocáková, I.; Höhler, T.; Martens, U. M.; Bokemeyer, C.; Van cutsem, E.; Melichar, B.; Smakal, M.; Csoszi, T.; Topuzov, E.; Orlova, R.; Tjulandin, S.; Rivera, F.; Straub, J.; Bruns, R.; Quaratino, S.; Tabernero, J. Abituzumab Combined with Cetuximab plus Irinotecan versus Cetuximab plus Irinotecan Alone for Patients with KRAS Wild-Type Metastatic Colorectal Cancer: The Randomised Phase I/II POSEIDON Trial. Ann Oncol 2015, 26, 132–140. [Google Scholar] [CrossRef]
- Hussain, M.; Le Moulec, S.; Gimmi, C.; Bruns, R.; Straub, J.; Miller, K. Differential Effect on Bone Lesions of Targeting Integrins: Randomized Phase II Trial of Abituzumab in Patients with Metastatic Castration-Resistant Prostate Cancer. Clinical Cancer Research 2016, 22, 3192–3200. [Google Scholar] [CrossRef]
- Bae, C.; Sachs, F.; Gottlieb, P. A. The Mechanosensitive Ion Channel Piezo1 Is Inhibited by the Peptide GsMTx4. Biochemistry 2011, 50. [Google Scholar] [CrossRef]
- Dayaram, V.; Malloy, C.; Martha, S.; Alvarez, B.; Chukwudolue, I.; Dabbain, N.; Dlovan, D.; Goleva, S.; Hickey, T.; Ho, A.; Kington, P.; Mattingly, M.; Potter, S.; Simpson, L.; Uradu, H.; Doorn, J. Van; Cooper, R. L. Stretch Activated Channels in Proprioceptive Organs of Crab and Crayfish Are Sensitive to Gadolinium but Not Amiloride, Ruthenium Red or Low PH. Impulse (Sydney) 2017, 1–17. [Google Scholar]
- Coste, B.; Xiao, B.; Santos, J. S.; Syeda, R.; Grandl, J.; Spencer, K. S.; Kim, S. E.; Schmidt, M.; Mathur, J.; Dubin, A. E.; Montal, M.; Patapoutian, A. Piezo Proteins Are Pore-Forming Subunits of Mechanically Activated Channels. Nature 2012, 483, 176–181. [Google Scholar] [CrossRef]
- Goyal, N.; Skrdla, P.; Schroyer, R.; Kumar, S.; Fernando, D.; Oughton, A.; Norton, N.; Sprecher, D. L.; Cheriyan, J. Clinical Pharmacokinetics, Safety, and Tolerability of a Novel, First-in-Class TRPV4 Ion Channel Inhibitor, GSK2798745, in Healthy and Heart Failure Subjects. American Journal of Cardiovascular Drugs 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Research, A. M.-C. 2021, undefined. Abstract ND11: The Discovery and Characterization of ION-537: A next Generation Antisense Oligonucleotide Inhibitor of YAP1 in Preclinical Cancer Models. AACR.
- Chapeau, E.; Schmelzle, T. IAG933, an Oral Selective YAP1-TAZ/Pan-TEAD Protein-Protein Interaction Inhibitor (PPIi) with Pre-Clinical Activity in Monotherapy and Combinations with MAPK Inhibitors. 2023. [Google Scholar]
- Vincent, P., Maeder, M.E., Hunt, B., Linn, B., Mangels-Dick, T., Hasan, T., Wang, K.K. and Pogue, B.W. 2021, undefined. CT Radiomic Features of Photodynamic Priming in Clinical Pancreatic Adenocarcinoma Treatment. iopscience.iop.org 2021. [CrossRef]
- Hanada, Y.; Pereira, S.; Pogue, B.; … E., M.-G. 2021, undefined. EUS-Guided Verteporfin Photodynamic Therapy for Pancreatic Cancer. Elsevier.
- Huggett, M.; Jermyn, M.; Gillams, A.; … R., I.-B. journal of; 2014, undefined. Phase I/II Study of Verteporfin Photodynamic Therapy in Locally Advanced Pancreatic Cancer. nature.com.
- Lejbkowicz, F.; Zwiran, M.; biology, S. S.-U. in medicine &; 1993, undefined. The Response of Normal and Malignant Cells to Ultrasound in Vitro. Elsevier.
- Shen, S.; Li, Y.; Xiao, Y.; Zhao, Z.; Zhang, C.; Wang, J.; Biomaterials, H. L.-. 2018, undefined. Folate-Conjugated Nanobubbles Selectively Target and Kill Cancer Cells via Ultrasound-Triggered Intracellular Explosion. Elsevier.
- Nicolai, H.; Steinbach, P.; Knuechel-Clarke, R.; Grimm, D.; Roessler, W.; Wieland, W. F.; Hofstaedter, F. Proliferation of Tumor Spheroids after Shock-Wave Treatment. J Cancer Res Clin Oncol 1994, 120, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Pellegrino, A.; Hallack, A.; Petrinic, N.; Jérusalem, A.; Cleveland, R. O. Response of Single Cells to Shock Waves and Numerically Optimized Waveforms for Cancer Therapy. Biophys J 2018, 114, 1433–1439. [Google Scholar] [CrossRef]
- Gamarra, F.; Spelsberg, F.; Dellian, M.; Goetz, A. E. Complete Local Tumor Remission after Therapy with Extra-corporeally Applied High-energy Shock Waves (HESW). Int J Cancer 1993, 55, 153–156. [Google Scholar] [CrossRef]
- Tijore, A.; Margadant, F.; Yao, M.; Hariharan, A.; Chew, C. A. Z.; Powell, S.; Bonney, G. K.; Sheetz, M. Ultrasound-Mediated Mechanical Forces Selectively Kill Tumor Cells. bioRxiv 2020. [Google Scholar]
- Berrueta, L.; Bergholz, J.; Munoz, D.; Muskaj, I.; Badger, G. J.; Shukla, A.; Kim, H. J.; Zhao, J. J.; Langevin, H. M. Stretching Reduces Tumor Growth in a Mouse Breast Cancer Model. Sci Rep 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Azagury, A.; Amar-Lewis, E.; Yudilevitch, Y.; Isaacson, C.; Laster, B.; Kost, J. Ultrasound Effect on Cancerous versus Non-Cancerous Cells. Ultrasound Med Biol 2016, 42, 1560–1567. [Google Scholar] [CrossRef]
- Lejbkowicz, F. Sensitivity of Normal. 1997, 105 (December), 1575–1578.
- Singh, A.; Tijore, A.; Margadant, F.; Simpson, C.; Chitkara, D.; Low, B. C.; Sheetz, M. Enhanced Tumor Cell Killing by Ultrasound after Microtubule Depolymerization. Bioeng Transl Med 2021, 6, e10233. [Google Scholar] [CrossRef]
- Los, U. M. D. E. C. D. E. No 主観的健康感を中心とした在宅高齢者における 健康関連指標に関する共分散構造分析Title.
- Sultan, L. R.; Karmacharya, M. B.; Hunt, S. J.; Wood, A. K. W.; Sehgal, C. M. Subsequent Ultrasound Vascular Targeting Therapy of Hepatocellular Carcinoma Improves the Treatment Efficacy. mdpi.com 2021. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, Y.; Li, R.; Jia, W.; Zhang, F.; Hu, X.; Reports, L. C.-S. 2022, undefined. High Speed Photo-Mediated Ultrasound Therapy Integrated with OCTA. nature.com.
- Xu, Z. L.; Zhu, X. Q.; Lu, P.; Zhou, Q.; Zhang, J.; Wu, F. Activation of Tumor-Infiltrating Antigen Presenting Cells by High Intensity Focused Ultrasound Ablation of Human Breast Cancer. Ultrasound Med Biol 2009, 35, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhou, L.; Chen, W. R. Host Antitumor Immune Responses to HIFU Ablation. International Journal of Hyperthermia 2007, 23, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhang, Y.; Feng, J.; Wu, F. Dendritic Cells Loaded with Ultrasound-Ablated Tumour Induce in Vivo Specific Antitumour Immune Responses. Ultrasound Med Biol 2010, 36, 441–448. [Google Scholar] [CrossRef]
- Zhang, W.; Shou, W. De; Xu, Y. J.; Bai, W. K.; Hu, B. Low-Frequency Ultrasound-Induced VEGF Suppression and Synergy with Dendritic Cell-Mediated Anti-Tumor Immunity in Murine Prostate Cancer Cells in Vitro. Sci Rep 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Sun, X.; Guo, L.; Shang, M.; Shi, D.; Liang, P.; Jing, X.; Meng, D.; Liu, X.; Zhou, X.; Zhao, Y.; Li, J. Ultrasound Mediated Destruction of Lmw-Ha-Loaded and Folate-Conjugated Nanobubble for Tam Targeting and Reeducation. Int J Nanomedicine 2020, 15, 1967–1981. [Google Scholar] [CrossRef]
- Rinella, L.; Pizzo, B.; Frairia, R.; Delsedime, L.; Calleris, G.; Gontero, P.; Zunino, V.; Fortunati, N.; Arvat, E.; Catalano, M. G. Modulating Tumor Reactive Stroma by Extracorporeal Shock Waves to Control Prostate Cancer Progression. Prostate 2020, 80, 1087–1096. [Google Scholar] [CrossRef]
- Wan, Y. Y.; Flavell, R. A. “Yin-Yang” Functions of Transforming Growth Factor-β and T Regulatory Cells in Immune Regulation. Immunological Reviews 2007. [CrossRef]
- Chen, Y.; Di, C.; Zhang, X.; Wang, J.; Wang, F.; Yan, J. fang; Xu, C.; Zhang, J.; Zhang, Q.; Li, H.; Yang, H.; Zhang, H. Transforming Growth Factor β Signaling Pathway: A Promising Therapeutic Target for Cancer. Journal of Cellular Physiology 2020. [CrossRef]
- Johannsen, M.; Spitaleri, G.; Curigliano, G.; Roigas, J.; Weikert, S.; Kempkensteffen, C.; Roemer, A.; Kloeters, C.; Rogalla, P.; Pecher, G.; Miller, K.; Berndt, A.; Kosmehl, H.; Trachsel, E.; Kaspar, M.; Lovato, V.; González-Iglesias, R.; Giovannoni, L.; Menssen, H. D.; Neri, D.; De Braud, F. The Tumour-Targeting Human L19-IL2 Immunocytokine: Preclinical Safety Studies, Phase i Clinical Trial in Patients with Solid Tumours and Expansion into Patients with Advanced Renal Cell Carcinoma. Eur J Cancer 2010, 46. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular Matrix and Its Therapeutic Potential for Cancer Treatment. Signal Transduct Target Ther 2021, 6. [Google Scholar] [CrossRef]
- Liu, S.; Ren, J.; ten Dijke, P. Targeting TGFβ Signal Transduction for Cancer Therapy. Signal Transduction and Targeted Therapy 2021, 6, 1–20. [Google Scholar] [CrossRef]
- Zion, O.; Genin, O.; Kawada, N.; Yoshizato, K.; Pancreas, S. R.-. 2009, undefined. Inhibition of Transforming Growth Factor β Signaling by Halofuginone as a Modality for Pancreas Fibrosis Prevention. journals.lww.com.
- Castellani, P.; Viale, G.; Dorcaratto, A.; Nicolo, G.; Kaczmarek, J.; Querze, G.; Zardi, L. Erratum: The Fibronectin Isoform Containing the ED-B Oncofetal Domain: A Marker of Angiogenesis (Int. J. Cancer, 59, 612-618 (1994)). International Journal of Cancer. 1995. [CrossRef]
- Glukhova, M. A.; Frid, M. G.; Shekhonin, B. V.; Balabanov, Y. V.; Koteliansky, V. E. Expression of Fibronectin Variants in Vascular and Visceral Smooth Muscle Cells in Development. Dev Biol 1990, 141. [Google Scholar] [CrossRef] [PubMed]
- Rybak, J. N.; Roesli, C.; Kaspar, M.; Villa, A.; Neri, D. The Extra-Domain A of Fibronectin Is a Vascular Marker of Solid Tumors and Metastases. Cancer Res 2007, 67. [Google Scholar] [CrossRef] [PubMed]
- Eigentler, T. K.; Weide, B.; De Braud, F.; Spitaleri, G.; Romanini, A.; Pflugfelder, A.; Gonzaĺez-Iglesias, R.; Tasciotti, A.; Giovannoni, L.; Schwager, K.; Lovato, V.; Kaspar, M.; Trachsel, E.; Menssen, H. D.; Neri, D.; Garbe, C. A Dose-Escalation and Signal-Generating Study of the Immunocytokine L19-IL2 in Combination with Dacarbazine for the Therapy of Patients with Metastatic Melanoma. Clinical Cancer Research 2011, 17. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M. E.; Gorlia, T.; Erridge, S. C.; Perry, J.; Hong, Y. K.; Aldape, K. D.; Lhermitte, B.; Pietsch, T.; Grujicic, D.; Steinbach, J. P. eter; Wick, W.; Tarnawski, R.; Nam, D. H.; Hau, P.; Weyerbrock, A.; Taphoorn, M. J. B.; Shen, C. C.; Rao, N.; Thurzo, L.; Herrlinger, U.; Gupta, T.; Kortmann, R. D.; Adamska, K.; McBain, C.; Brandes, A. A.; Tonn, J. C. hristian; Schnell, O.; Wiegel, T.; Kim, C. Y.; Nabors, L. B. urt; Reardon, D. A.; van den Bent, M. J.; Hicking, C.; Markivskyy, A.; Picard, M.; Weller, M. Cilengitide Combined with Standard Treatment for Patients with Newly Diagnosed Glioblastoma with Methylated MGMT Promoter (CENTRIC EORTC 26071-22072 Study): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol 2014, 15. [Google Scholar] [CrossRef] [PubMed]
- Lawhorn, B. G.; Brnardic, E. J.; Behm, D. J. TRPV4 Antagonists: A Patent Review (2015–2020). Expert Opinion on Therapeutic Patents 2021. [CrossRef] [PubMed]
- Doñate-Macian, P.; Duarte, Y.; Rubio-Moscardo, F.; Pérez-Vilaró, G.; Canan, J.; Díez, J.; González-Nilo, F.; Valverde, M. A. Structural Determinants of TRPV4 Inhibition and Identification of New Antagonists with Antiviral Activity. Br J Pharmacol 2022, 179. [Google Scholar] [CrossRef] [PubMed]
- Article, R. YAP / TAZ as Master Regulators in Cancer : Modulation, Function and Therapeutic Approaches Check for Updates. 2023, 4 (January), 9–26.
- Barry, E. R.; Simov, V.; Valtingojer, I.; Venier, O. Recent Therapeutic Approaches to Modulate the Hippo Pathway in Oncology and Regenerative Medicine. Cells 2021. [Google Scholar] [CrossRef]
- Liu-Chittenden, Y.; Huang, B.; Shim, J. S.; Chen, Q.; Lee, S. J.; Anders, R. A.; Liu, J. O.; Pan, D. Genetic and Pharmacological Disruption of the TEAD-YAP Complex Suppresses the Oncogenic Activity of YAP. Genes Dev 2012, 26. [Google Scholar] [CrossRef]
- Dasari, V. R.; Mazack, V.; Feng, W.; Nash, J.; Carey, D. J.; Gogoi, R. Verteporfin Exhibits YAP-Independent Anti-Proliferative and Cytotoxic Effects in Endometrial Cancer Cells. Oncotarget 2017, 8. [Google Scholar] [CrossRef]
- Zhang, H.; Ramakrishnan, S. K.; Triner, D.; Centofanti, B.; Maitra, D.; Gyorffy, B.; Sebolt-Leopold, J. S.; Dame, M. K.; Varani, J.; Brenner, D. E.; Fearon, E. R.; Omary, M. B.; Shah, Y. M. Tumor-Selective Proteotoxicity of Verteporfin Inhibits Colon Cancer Progression Independently of YAP1. Sci Signal 2015, 8. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Hong, Y.; Wang, M.; Zhang, H.; Ma, J.; Qu, K.; Huang, G.; Lu, T. J. Mechanotherapy in Oncology: Targeting Nuclear Mechanics and Mechanotransduction. Adv Drug Deliv Rev 2023, 194. [Google Scholar] [CrossRef] [PubMed]
- Collis, J.; Manasseh, R.; Liovic, P.; Tho, P.; Ultrasonics, A. O.-. 2010, undefined. Cavitation Microstreaming and Stress Fields Created by Microbubbles. Elsevier.
- Krasovitski, B.; Frenkel, V.; Shoham, S.; Kimmel, E. Intramembrane Cavitation as a Unifying Mechanism for Ultrasound-Induced Bioeffects. Proc Natl Acad Sci U S A 2011, 108, 3258–3263. [Google Scholar] [CrossRef] [PubMed]
- Schneider, F.; Gerriets, T.; Walberer, M.; Mueller, C.; Rolke, R.; Eicke, B. M.; Bohl, J.; Kempski, O.; Kaps, M.; Bachmann, G.; Dieterich, M.; Nedelmann, M. Brain Edema and Intracerebral Necrosis Caused by Transcranial Low-Frequency 20-KHz Ultrasound: A Safety Study in Rats. Stroke 2006, 37, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Burleson, A.; Nusstein, J.; Reader, A.; endodontics, M. B.-J. of; 2007, undefined. The in Vivo Evaluation of Hand/Rotary/Ultrasound Instrumentation in Necrotic, Human Mandibular Molars. Elsevier.
- Li, D.; Pellegrino, A.; Hallack, A.; Petrinic, N.; … A., J.-B. 2018, undefined. Response of Single Cells to Shock Waves and Numerically Optimized Waveforms for Cancer Therapy. Elsevier.
- Hato, T.; Goyal, L.; Greten, T. F.; Duda, D. G.; Zhu, A. X. Immune Checkpoint Blockade in Hepatocellular Carcinoma: Current Progress and Future Directions. Hepatology 2014. [CrossRef]
- Ribas, A.; Wolchok, J. D. Cancer Immunotherapy Using Checkpoint Blockade. Science 2018. [CrossRef]
- Fu, Y.; Peng, Y.; Zhao, S.; Mou, J.; Zeng, L.; Jiang, X.; Yang, C.; Huang, C.; Li, Y.; Lu, Y.; Wu, M.; Yang, Y.; Kong, T.; Lai, Q.; Wu, Y.; Yao, Y.; Wang, Y.; Gou, L.; Yang, J. Combination Foretinib and Anti-PD-1 Antibody Immunotherapy for Colorectal Carcinoma. Front Cell Dev Biol 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Xiong, G.; Cao, Z.; Yang, G.; Zheng, S.; Song, X.; You, L.; Zheng, L.; Zhang, T.; Zhao, Y. PD-1/PD-L1 and Immunotherapy for Pancreatic Cancer. Cancer Letters 2017. [CrossRef]
- Harrison, D. L.; Fang, Y.; Huang, J. T-Cell Mechanobiology: Force Sensation, Potentiation, and Translation. Frontiers in Physics 2019. [CrossRef]
- Hu, K. H.; Butte, M. J. T Cell Activation Requires Force Generation. Journal of Cell Biology 2016, 213. [Google Scholar] [CrossRef]
- Le Floc’H, A.; Huse, M. Molecular Mechanisms and Functional Implications of Polarized Actin Remodeling at the T Cell Immunological Synapse. Cellular and Molecular Life Sciences 2015, 72. [Google Scholar] [CrossRef]
- Tabdanov, E.; Gondarenko, S.; Kumari, S.; Liapis, A.; Dustin, M. L.; Sheetz, M. P.; Kam, L. C.; Iskratsch, T. Micropatterning of TCR and LFA-1 Ligands Reveals Complementary Effects on Cytoskeleton Mechanics in T Cells. Integrative Biology (United Kingdom) 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, C. S. C.; Raychaudhuri, D.; Paul, B.; Chakrabarty, Y.; Ghosh, A. R.; Rahaman, O.; Talukdar, A.; Ganguly, D. Cutting Edge: Piezo1 Mechanosensors Optimize Human T Cell Activation. The Journal of Immunology 2018, 200. [Google Scholar] [CrossRef] [PubMed]
- Tabdanov, E. D.; Rodríguez-Merced, N. J.; Cartagena-Rivera, A. X.; Puram, V. V.; Callaway, M. K.; Ensminger, E. A.; Pomeroy, E. J.; Yamamoto, K.; Lahr, W. S.; Webber, B. R.; Moriarity, B. S.; Zhovmer, A. S.; Provenzano, P. P. Engineering T Cells to Enhance 3D Migration through Structurally and Mechanically Complex Tumor Microenvironments. Nat Commun 2021, 12. [Google Scholar] [CrossRef]
- Hickey, J. W.; Dong, Y.; Chung, J. W.; Salathe, S. F.; Pruitt, H. C.; Li, X.; Chang, C.; Fraser, A. K.; Bessell, C. A.; Ewald, A. J.; Gerecht, S.; Mao, H. Q.; Schneck, J. P. Engineering an Artificial T-Cell Stimulating Matrix for Immunotherapy. Advanced Materials 2019, 31. [Google Scholar] [CrossRef]
- Wang, W.; Wu, F.; Mohammadniaei, M.; Zhang, M.; Li, Y.; Sun, Y.; Tang, B. Z. Genetically Edited T-Cell Membrane Coated AIEgen Nanoparticles Effectively Prevents Glioblastoma Recurrence. Biomaterials 2023, 293. [Google Scholar] [CrossRef]
- Kim, K. Do; Bae, S.; Capece, T.; Nedelkovska, H.; De Rubio, R. G.; Smrcka, A. V.; Jun, C. D.; Jung, W.; Park, B.; Kim, T. Il; Kim, M. Targeted Calcium Influx Boosts Cytotoxic T Lymphocyte Function in the Tumour Microenvironment. Nat Commun 2017, 8. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, Y.; Tan, X.; Zheng, X.; Wang, F.; Ke, K.; Zhang, C.; Liao, N.; Dang, Y.; Shi, Y.; Zheng, Y.; Gao, Y.; Li, Q.; Liu, X.; Liu, J. An Optogenetic Controllable T Cell System for Hepatocellular Carcinoma Immunotherapy. Theranostics 2019, 9. [Google Scholar] [CrossRef]
- Tan, P.; He, L.; Han, G.; Zhou, Y. Optogenetic Immunomodulation: Shedding Light on Antitumor Immunity. Trends in Biotechnology 2017. [CrossRef]
- Wu, Y.; Liu, Y.; Huang, Z.; Wang, X.; Jin, Z.; Li, J.; Limsakul, P.; Zhu, L.; Allen, M.; Pan, Y.; Bussell, R.; Jacobson, A.; Liu, T.; Chien, S.; Wang, Y. Control of the Activity of CAR-T Cells within Tumours via Focused Ultrasound. Nat Biomed Eng 2021, 5. [Google Scholar] [CrossRef]
- Liao, D.; Li, F.; Lu, D.; Zhong, P. Activation of Piezo1 Mechanosensitive Ion Channel in HEK293T Cells by 30 MHz Vertically Deployed Surface Acoustic Waves. Biochem Biophys Res Commun 2019, 518, 541–547. [Google Scholar] [CrossRef]
- Qiu, Z.; Guo, J.; Kala, S.; Zhu, J.; Xian, Q.; Qiu, W.; Li, G.; Zhu, T.; Meng, L.; Zhang, R.; Chan, H. C.; Zheng, H.; Sun, L. The Mechanosensitive Ion Channel Piezo1 Significantly Mediates In Vitro Ultrasonic Stimulation of Neurons. iScience 2019, 21. [Google Scholar] [CrossRef]
- Pan, Y.; Yoon, S.; Sun, J.; Huang, Z.; Lee, C.; Allen, M.; Wu, Y.; Chang, Y. J.; Sadelain, M.; Kirk Shung, K.; Chien, S.; Wang, Y. Mechanogenetics for the Remote and Noninvasive Control of Cancer Immunotherapy. Proc Natl Acad Sci U S A 2018, 115. [Google Scholar] [CrossRef] [PubMed]
- Billadeau, D. D.; Nolz, J. C.; Gomez, T. S. Regulation of T-Cell Activation by the Cytoskeleton. Nature Reviews Immunology 2007. [CrossRef] [PubMed]
- Al-Alwan, M. M.; Liwski, R. S.; Haeryfar, S. M. M.; Baldridge, W. H.; Hoskin, D. W.; Rowden, G.; West, K. A. Cutting Edge: Dendritic Cell Actin Cytoskeletal Polarization during Immunological Synapse Formation Is Highly Antigen-Dependent. The Journal of Immunology 2003, 171. [Google Scholar] [CrossRef] [PubMed]
- Gombos, I.; Detre, C.; Vámosi, G.; Matkó, J. Rafting MHC-II Domains in the APC (Presynaptic) Plasma Membrane and the Thresholds for T-Cell Activation and Immunological Synapse Formation. Immunol Lett 2004, 92, 117–124. [Google Scholar] [CrossRef]
- Jönsson, F.; Gurniak, C. B.; Fleischer, B.; Kirfel, G.; Witke, W. Immunological Responses and Actin Dynamics in Macrophages Are Controlled by N-Cofilin but Are Independent from ADF. PLoS One 2012, 7. [Google Scholar] [CrossRef]
- Baranov, M. V.; Beest, T. M.; Reinieren-Beeren, I.; Cambi, A.; Figdor, C. G.; Den Bogaart, G. Van. Podosomes of Dendritic Cells Facilitate Antigen Sampling. J Cell Sci 2014, 127. [Google Scholar] [CrossRef]
- Xu, Z. L.; Zhu, X. Q.; Lu, P.; Zhou, Q.; Zhang, J.; Wu, F. Activation of Tumor-Infiltrating Antigen Presenting Cells by High Intensity Focused Ultrasound Ablation of Human Breast Cancer. Ultrasound Med Biol 2009, 35. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhou, L.; Chen, W. R. Host Antitumor Immune Responses to HIFU Ablation. International Journal of Hyperthermia 2007, 23. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, Y.; Feng, J.; Wu, F. Dendritic Cells Loaded with Ultrasound-Ablated Tumour Induce in Vivo Specific Antitumour Immune Responses. Ultrasound Med Biol 2010, 36. [Google Scholar] [CrossRef]
- Marinova, M.; Huxold, H. C.; Henseler, J.; Mücke, M.; Conrad, R.; Rolke, R.; Ahmadzadehfar, H.; Rauch, M.; Fimmers, R.; Luechters, G.; Cuhls, H.; Radbruch, L.; Schild, H. H.; Strunk, H. Clinical Effectiveness and Potential Survival Benefit of US-Guided High-Intensity Focused Ultrasound Therapy in Patients with Advanced-Stage Pancreatic Cancer. Ultraschall in der Medizin 2019, 40. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yu, Z.; Liu, N.; Huang, J.; Liang, X.; Liang, X.; Liang, M.; Li, M.; Ni, J. The Efficacy of Low-Frequency Ultrasound as an Added Treatment for Chronic Wounds: A Meta-Analysis. Int Wound J 2023, 20. [Google Scholar] [CrossRef]
- Zhang, W.; Shou, W. De; Xu, Y. J.; Bai, W. K.; Hu, B. Low-Frequency Ultrasound-Induced VEGF Suppression and Synergy with Dendritic Cell-Mediated Anti-Tumor Immunity in Murine Prostate Cancer Cells in Vitro. Sci Rep 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Vogel, V. Spatial Confinement Downsizes the Inflammatory Response of Macrophages. Nat Mater 2018, 17. [Google Scholar] [CrossRef]
- Okamoto, T.; Takagi, Y.; Kawamoto, E.; Park, E. J.; Usuda, H.; Wada, K.; Shimaoka, M. Reduced Substrate Stiffness Promotes M2-like Macrophage Activation and Enhances Peroxisome Proliferator-Activated Receptor γ Expression. Exp Cell Res 2018, 367. [Google Scholar] [CrossRef]
- Sridharan, R.; Cavanagh, B.; Cameron, A. R.; Kelly, D. J.; O’Brien, F. J. Material Stiffness Influences the Polarization State, Function and Migration Mode of Macrophages. Acta Biomater 2019, 89. [Google Scholar] [CrossRef]
- Meli, V. S.; Atcha, H.; Veerasubramanian, P. K.; Nagalla, R. R.; Luu, T. U.; Chen, E. Y.; Guerrero-Juarez, C. F.; Yamaga, K.; Pandori, W.; Hsieh, J. Y.; Downing, T. L.; Fruman, D. A.; Lodoen, M. B.; Plikus, M. V.; Wang, W.; Liu, W. F. YAP-Mediated Mechanotransduction Tunes the Macrophage Inflammatory Response. Sci Adv 2020, 6. [Google Scholar] [CrossRef]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage Polarization: Tumor-Associated Macrophages as a Paradigm for Polarized M2 Mononuclear Phagocytes. Trends in Immunology 2002. [CrossRef]
- Guerriero, J. L.; Sotayo, A.; Ponichtera, H. E.; Castrillon, J. A.; Pourzia, A. L.; Schad, S.; Johnson, S. F.; Carrasco, R. D.; Lazo, S.; Bronson, R. T.; Davis, S. P.; Lobera, M.; Nolan, M. A.; Letai, A. Class IIa HDAC Inhibition Reduces Breast Tumours and Metastases through Anti-Tumour Macrophages. Nature 2017, 543. [Google Scholar] [CrossRef]
- Heusinkveld, M.; van der Burg, S. H. Identification and Manipulation of Tumor Associated Macrophages in Human Cancers. Journal of Translational Medicine 2011. [CrossRef] [PubMed]
- Zhang, Z.; Zhang, J.; He, P.; Han, J.; Sun, C. Interleukin-37 Suppresses Hepatocellular Carcinoma Growth through Inhibiting M2 Polarization of Tumor-Associated Macrophages. Mol Immunol 2020, 122. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Fang, B.; Zhang, Y.; Wang, C.; Zhou, J.; Niu, C.; Gao, Y.; Zhao, D.; He, J.; Wang, J.; Zhang, X.; Li, Q. Mechanical Stretch Promotes Tumoricidal M1 Polarization via the FAK/NF-ΚB Signaling Pathway. FASEB Journal 2019, 33. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Guo, L.; Shang, M.; Shi, D.; Liang, P.; Jing, X.; Meng, D.; Liu, X.; Zhou, X.; Zhao, Y.; Li, J. Ultrasound Mediated Destruction of Lmw-Ha-Loaded and Folate-Conjugated Nanobubble for Tam Targeting and Reeducation. Int J Nanomedicine 2020, 15. [Google Scholar] [CrossRef]
- Labernadie, A.; Kato, T.; Brugués, A.; Serra-Picamal, X.; Derzsi, S.; Arwert, E.; Weston, A.; González-Tarragó, V.; Elosegui-Artola, A.; Albertazzi, L.; Alcaraz, J.; Roca-Cusachs, P.; Sahai, E.; Trepat, X. A Mechanically Active Heterotypic E-Cadherin/N-Cadherin Adhesion Enables Fibroblasts to Drive Cancer Cell Invasion. Nat Cell Biol 2017, 19. [Google Scholar] [CrossRef] [PubMed]
- Barbazan, J.; Pérez-González, C.; Gómez-González, M.; Dedenon, M.; Richon, S.; Latorre, E.; Serra, M.; Mariani, P.; Descroix, S.; Sens, P.; Trepat, X.; Vignjevic, D. M. Cancer-Associated Fibroblasts Actively Compress Cancer Cells and Modulate Mechanotransduction. bioRxiv 2021. [CrossRef]
- Johansson, A. C.; Ansell, A.; Jerhammar, F.; Lindh, M. B.; Grénman, R.; Munck-Wikland, E.; Östman, A.; Roberg, K. Cancer-Associated Fibroblasts Induce Matrix Metalloproteinase-Mediated Cetuximab Resistance in Head and Neck Squamous Cell Carcinoma Cells. Molecular Cancer Research 2012, 10. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Ren, Y.; Geng, H.; Zhang, Q.; Cao, L.; Meng, Z.; Wu, X.; Xu, M.; Xu, K. Cancer-Associated Fibroblasts Contribute to Cisplatin Resistance by Modulating ANXA3 in Lung Cancer Cells. Cancer Sci 2019, 110. [Google Scholar] [CrossRef]
- Long, X.; Xiong, W.; Zeng, X.; Qi, L.; Cai, Y.; Mo, M.; Jiang, H.; Zhu, B.; Chen, Z.; Li, Y. Cancer-Associated Fibroblasts Promote Cisplatin Resistance in Bladder Cancer Cells by Increasing IGF-1/ERβ/Bcl-2 Signalling. Cell Death Dis 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yue, J.; Jiang, Z.; Zhou, R.; Xie, R.; Xu, Y.; Wu, S. CAF-Secreted CXCL1 Conferred Radioresistance by Regulating DNA Damage Response in a ROS-Dependent Manner in Esophageal Squamous Cell Carcinoma. Cell Death Dis 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gan, G.; Wang, B.; Wu, J.; Cao, Y.; Zhu, D.; Xu, Y.; Wang, X.; Han, H.; Li, X.; Ye, M.; Zhao, J.; Mi, J. Cancer-Associated Fibroblasts Promote Irradiated Cancer Cell Recovery Through Autophagy. EBioMedicine 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Boluda, A.; Vaquero, J.; Vimeux, L.; Guilbert, T.; Barrin, S.; Kantari-Mimoun, C.; Ponzo, M.; Renault, G.; Deptula, P.; Pogoda, K.; Bucki, R.; Cascone, I.; Courty, J.; Fouassier, L.; Gazeau, F.; Donnadieu, E. Tumor Stiffening Reversion through Collagen Crosslinking Inhibition Improves t Cell Migration and Anti-Pd-1 Treatment. Elife 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Grither, W. R.; Van Hove, S.; Biswas, H.; Ponik, S. M.; Eliceiri, K. W.; Keely, P. J.; Longmore, G. D. Mechanical Signals Regulate and Activate SNAIL1 Protein to Control the Fibrogenic Response of Cancer-Associated Fibroblasts. J Cell Sci 2016, 129. [Google Scholar] [CrossRef]
- Sewell-Loftin, M. K.; Bayer, S. V. H.; Crist, E.; Hughes, T.; Joison, S. M.; Longmore, G. D.; George, S. C. Cancer-Associated Fibroblasts Support Vascular Growth through Mechanical Force. Sci Rep 2017, 7. [Google Scholar] [CrossRef]
- Rinella, L.; Pizzo, B.; Frairia, R.; Delsedime, L.; Calleris, G.; Gontero, P.; Zunino, V.; Fortunati, N.; Arvat, E.; Catalano, M. G. Modulating Tumor Reactive Stroma by Extracorporeal Shock Waves to Control Prostate Cancer Progression. Prostate 2020, 80. [Google Scholar] [CrossRef]

| Aim | Targeting | Targets | Targeting agents | Stage of clinical trials and cancer type |
|---|---|---|---|---|
| ECM stiffness reduction | Collagen production | TGF-β | Fresolimumab (NCT01401062), (NCT02581787) | I/II, metastatic breast cancer, early-stage non-small cell lung cancer[26] |
| Halofuginone | Animal models of pancreatic, lung, melanoma, and breast cancer[27,28,29,30] | |||
| LY-2109761 | In vitro (liver metastasis of colon cancer, pancreatic cancer metastasis), and animal model (breast cancer bone metastasis)[31,32,33] | |||
| Fibronectin production | Extra domain B (EDB) | AS1409 | I, malignant melanoma or renal cell carcinoma[34] | |
| Collagen chaperons | HSP7 | Pirfenidone, | In vitro, lung fibrosis[35] | |
| ND-L02-s0201 (NCT03241264) | I, fibrosis[36] | |||
| ECM crosslinking | Pan LOX, LOX, LOXL2 | PXS-5505 (NCT04676529) | II, myelofibrosis[37] | |
| PXS-5382A (NCT04183517) | I, healthy individuals | |||
| (NCT02852551) | I, healthy individuals | |||
| Simtuzumab (NCT01472198), (NCT01479465) |
Phase II, pancreatic adenocarcinoma[38], colorectal adenocarcinoma[39] | |||
| ECM structural disruption | Collagen | low power of Pulse-HIFU (20 W/cm2) | Animal model[40] | |
| Mechano-sensors (Stiffness sensors) and Transducers | Integrins | Cilengitide (NCT00093964) | III, glioblastoma[41] | |
| ATN-161 (NCT00352313) | II, malignant glioma[42] | |||
| anti-αVβ3 Ab -Etaracizumab (MEDI-522), | I/II, metastatic melanoma, renal cell, prostate cancer, lymphoma, small intestine cancer, colorectal cancer[43,44] | |||
| anti-α5β1 integrin Ab - Volociximab | II, metastatic pancreatic cancer, ovarian cancer peritoneal neoplasms, melanoma | |||
| anti-αV Ab -Intetumumab (NCT00246012), (NCT00537381) |
II, melanoma[45], prostate cancer[46] | |||
| anti-αV Ab -Abituzumab (NCT01008475), (NCT01360840) |
II, colorectal cancer[47], prostate cancer[48] | |||
| Piezo1 | GsMTx4 | In vitro, transfected HEK293 cells[49] | ||
| Gadolinium and ruthenium red | In vitro on non-cancerous cells, sensory cells[50], Piezo-expressing HE293T cells[51] | |||
| TRPV4 | GSK2798745 (NCT02119260) | II, Healthy subjects and Patients[52] | ||
| YAP/TAZ | ION537 (anti-YAP DNA antisense oligonucleotide) (NCT04659096) |
I, advanced solid tumors[53] | ||
| IAG933(NCT04857372) | I, ongoing, mesothelioma and other solid tumors[54] | |||
| VT398 (NCT04665206) | ||||
| Verteporfin (NCT04590664), (NCT03067051), (NCT03033225) |
I/II, glioblastoma, prostate cancer, pancreatic cancer[55,56,57] | |||
| To utilize mismatching of mechano-phenotype of cancerous and normal cells | Nuclear mechano-transduction | High frequency LIPU |
In vitro, breast carcinoma and a malignant melanoma[58], mice cervical cancer (HeLa cell)[59] |
|
| Shock wave therapy |
In vitro, bladder cancer cell and prostate cancer cell[60], human renal epithelial, cancer cell[61], hamster melanomas[62] |
|||
| Mechanical stretch therapy | In vitro and in vivo, breast cancer cells[63], p53PTEN-/- mice breast cancer model[64] | |||
| Low frequency LIPU |
In vitro and in vivo, breast cancer cell, chick embryo grafted tumors [63], murine mammary sarcoma and murine mammary sarcoma[65], Human foreskin fibroblasts[66], Breast cancer cells, malignant melanoma, breast epithelial cell[67], Hacat and Cal33, In vivo mice injected with Cal33 HNSCC cell line[68] |
|||
| Depletion of blood flow to tumor | Tumor blood vessels | Low intensity ultrasound | Animal models, hepatocellular carcinoma[69], rabbit[70] | |
| Dendritic cells | High intensity-focused ultrasound |
In vitro, primary effector CD4+ T cells obtained from TCR-transgenic OT-II mice[71], primary human CD4+ T cells[72], human breast cancer sample[73] |
||
| Low frequency ultrasound |
In vitro, murine prostate cancer cells (RM-1) and bone marrow derived DCs from BALB/c mice[74] |
|||
| TAM | Ultrasound targeted nanobubble destruction (UTND) | In vitro, mouse macrophage cell line RAW264.7 (M0) and Lewis lung carcinoma (LLC) cell line[75] | ||
| CAFs | Shock wave therapy | In vitro, CAFs extracted from tumors of prostate cancer patients[76] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




