Submitted:
28 July 2023
Posted:
31 July 2023
You are already at the latest version
Abstract
Keywords:
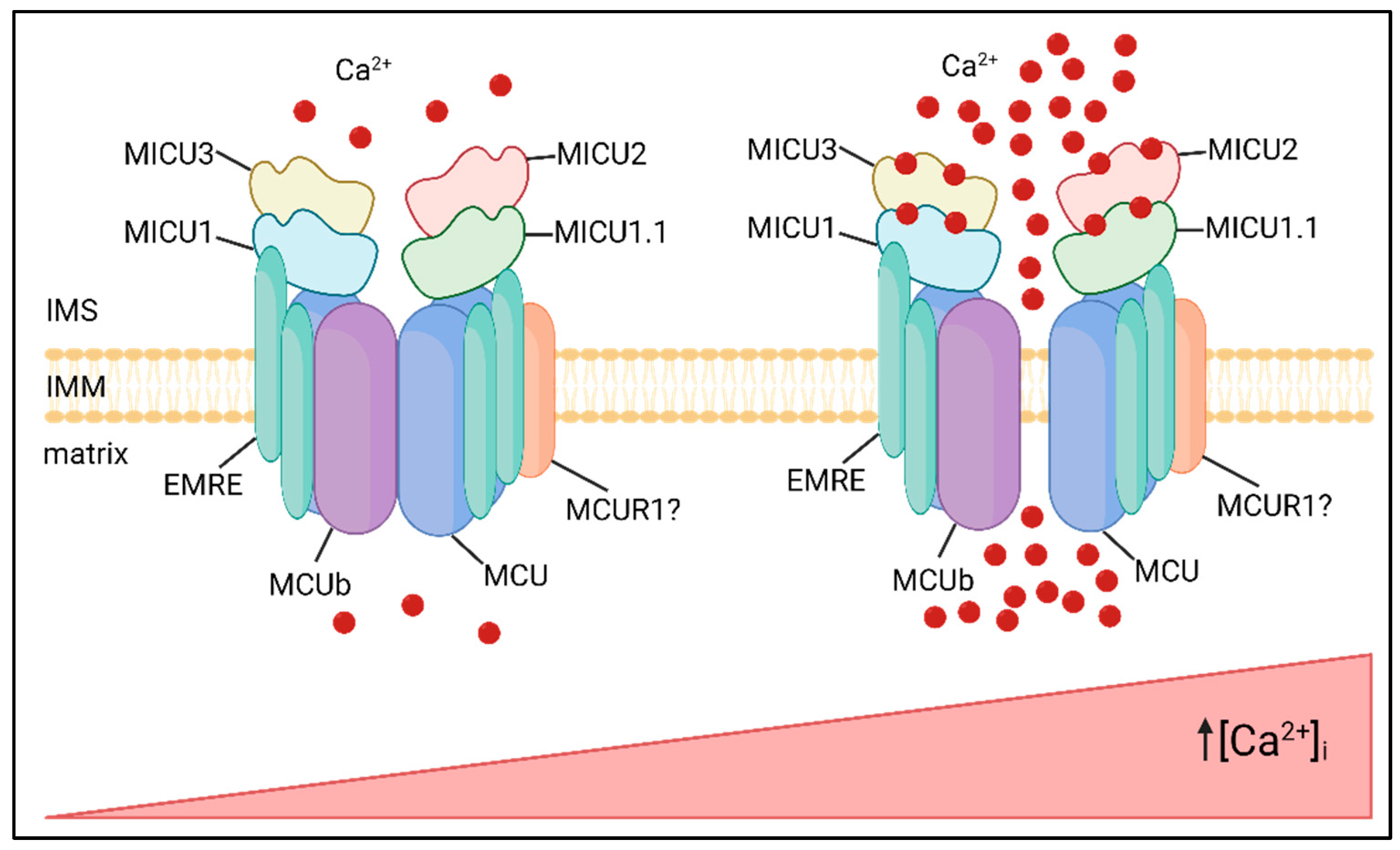
1. MCU Complex Structure and Function
2. Cardiovascular Diseases
3. Metabolic Diseases
4. Cancer
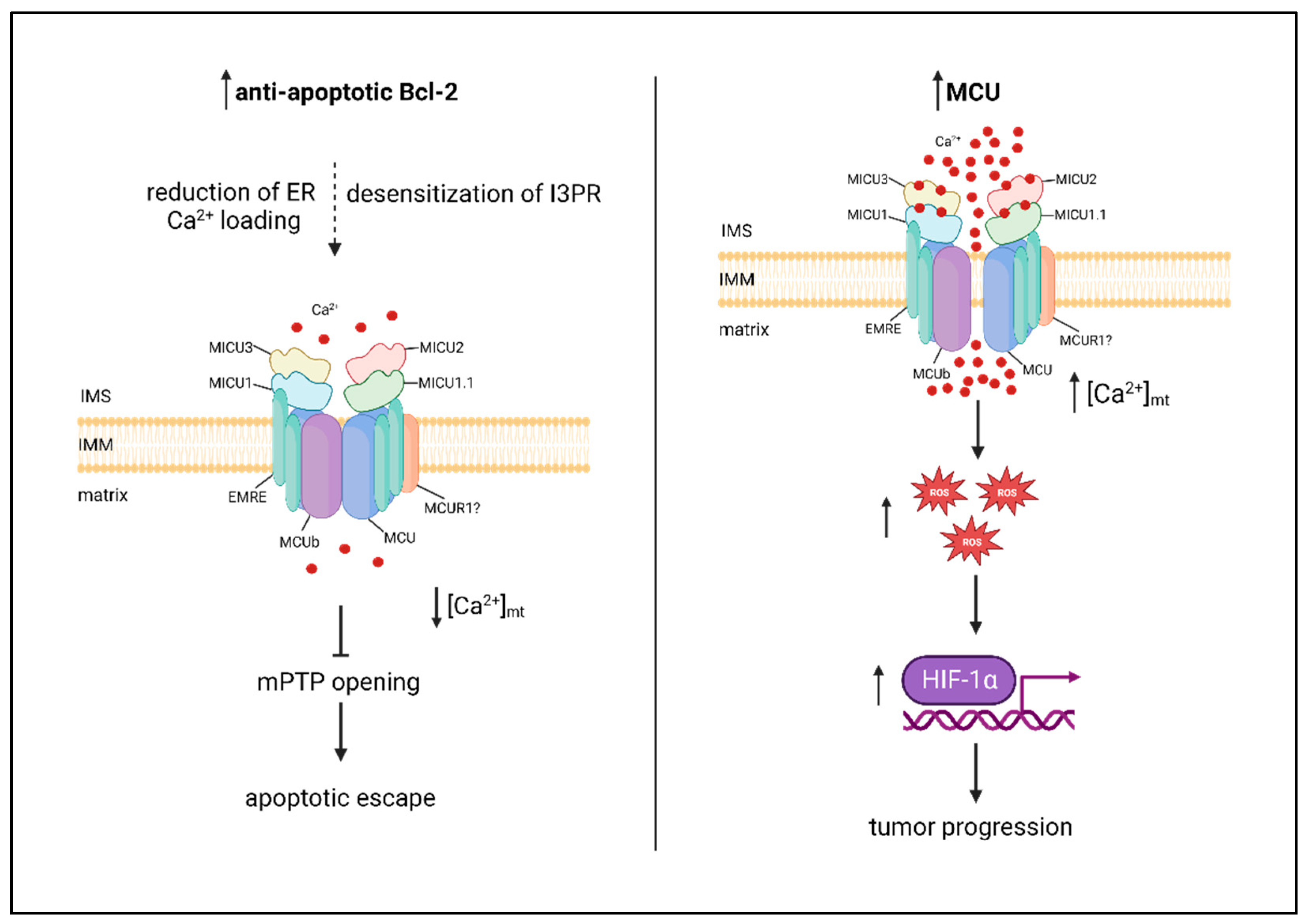
4.1. Breast Cancer
4.2. Pancreatic Cancer
4.3. Colon Cancer
4.4. Hepatocellular Carcinoma
4.5. Other Cancer Types
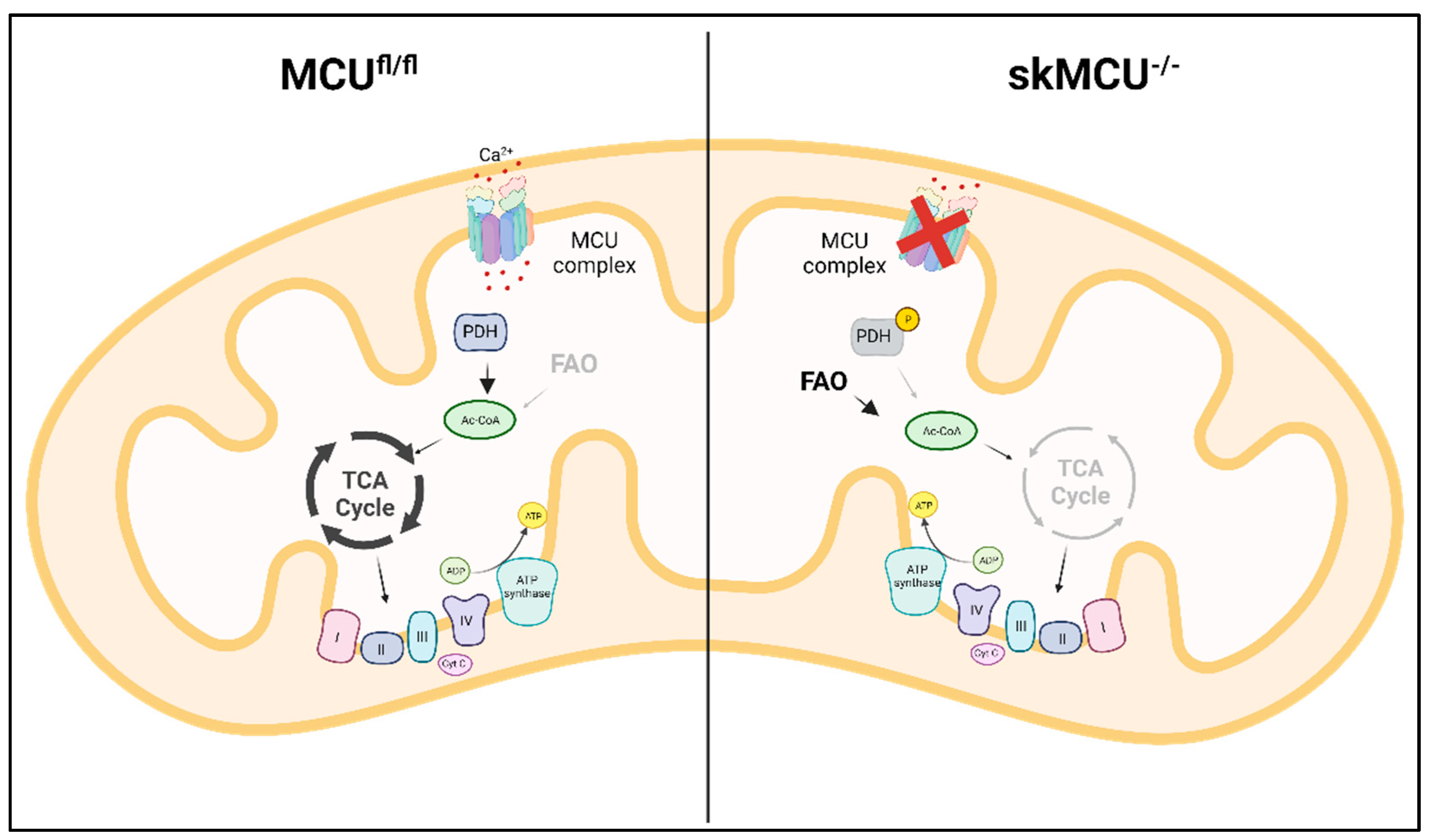
5. Skeletal Muscle Diseases
6. Neurodegenerative Diseases
6.1. Alzheimer’s Disease
6.2. Parkinson’s Disease
6.3. Huntington’s Disease
6.4. Amyotrophic Lateral Sclerosis
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011 Jun 19;476(7360):341-5. PMID: 21685886; PMCID: PMC3486726. [CrossRef]
- De Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011 Jun 19;476(7360):336-40. PMID: 21685888; PMCID: PMC4141877. [CrossRef]
- Baradaran R, Wang C, Siliciano AF, Long SB. Cryo-EM structures of fungal and metazoan mitochondrial calcium uniporters. Nature. 2018 Jul;559(7715):580-584. Epub 2018 Jul 11. PMID: 29995857; PMCID: PMC6336196. [CrossRef]
- Raffaello A, De Stefani D, Sabbadin D, Teardo E, Merli G, Picard A, Checchetto V, Moro S, Szabò I, Rizzuto R. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J. 2013 Aug 28;32(17):2362-76. Epub 2013 Jul 30. PMID: 23900286; PMCID: PMC3771344. [CrossRef]
- Fieni F, Lee SB, Jan YN, Kirichok Y. Activity of the mitochondrial calcium uniporter varies greatly between tissues. Nat Commun. 2012;3:1317. PMID: 23271651; PMCID: PMC3818247. [CrossRef]
- Sancak Y, Markhard AL, Kitami T, Kovács-Bogdán E, Kamer KJ, Udeshi ND, Carr SA, Chaudhuri D, Clapham DE, Li AA, Calvo SE, Goldberger O, Mootha VK. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science. 2013 Dec 13;342(6164):1379-82. Epub 2013 Nov 14. PMID: 24231807; PMCID: PMC4091629. [CrossRef]
- Yamamoto T, Yamagoshi R, Harada K, Kawano M, Minami N, Ido Y, Kuwahara K, Fujita A, Ozono M, Watanabe A, Yamada A, Terada H, Shinohara Y. Analysis of the structure and function of EMRE in a yeast expression system. Biochim Biophys Acta. 2016 Jun;1857(6):831-9. Epub 2016 Mar 18. [CrossRef] [PubMed]
- König T, Tröder SE, Bakka K, Korwitz A, Richter-Dennerlein R, Lampe PA, Patron M, Mühlmeister M, Guerrero-Castillo S, Brandt U, Decker T, Lauria I, Paggio A, Rizzuto R, Rugarli EI, De Stefani D, Langer T. The m-AAA Protease Associated with Neurodegeneration Limits MCU Activity in Mitochondria. Mol Cell. 2016 Oct 6;64(1):148-162. Epub 2016 Sep 15. [CrossRef] [PubMed]
- Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature. 2010 Sep 16;467(7313):291-6. Epub 2010 Aug 8. PMID: 20693986; PMCID: PMC2977980. [CrossRef]
- Csordás G, Golenár T, Seifert EL, Kamer KJ, Sancak Y, Perocchi F, Moffat C, Weaver D, Perez SF, Bogorad R, Koteliansky V, Adijanto J, Mootha VK, Hajnóczky G. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca²⁺ uniporter. Cell Metab. 2013 Jun 4;17(6):976-987. PMID: 23747253; PMCID: PMC3722067. [CrossRef]
- Vecellio Reane D, Vallese F, Checchetto V, Acquasaliente L, Butera G, De Filippis V, Szabò I, Zanotti G, Rizzuto R, Raffaello A. A MICU1 Splice Variant Confers High Sensitivity to the Mitochondrial Ca2+ Uptake Machinery of Skeletal Muscle. Mol Cell. 2016 Nov 17;64(4):760-773. Epub 2016 Nov 3. [CrossRef] [PubMed]
- Plovanich M, Bogorad RL, Sancak Y, Kamer KJ, Strittmatter L, Li AA, Girgis HS, Kuchimanchi S, De Groot J, Speciner L, Taneja N, Oshea J, Koteliansky V, Mootha VK. MICU2, a paralog of MICU1, resides within the mitochondrial uniporter complex to regulate calcium handling. PLoS One. 2013;8(2):e55785. Epub 2013 Feb 7. PMID: 23409044; PMCID: PMC3567112. [CrossRef]
- Patron M, Checchetto V, Raffaello A, Teardo E, Vecellio Reane D, Mantoan M, Granatiero V, Szabò I, De Stefani D, Rizzuto R. MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Mol Cell. 2014 Mar 6;53(5):726-37. Epub 2014 Feb 20. PMID: 24560927; PMCID: PMC3988891. [CrossRef]
- Kamer KJ, Grabarek Z, Mootha VK. High-affinity cooperative Ca2+ binding by MICU1-MICU2 serves as an on-off switch for the uniporter. EMBO Rep. 2017 Aug;18(8):1397-1411. Epub 2017 Jun 14. PMID: 28615291; PMCID: PMC5538426. [CrossRef]
- Payne R, Hoff H, Roskowski A, Foskett JK. MICU2 Restricts Spatial Crosstalk between InsP3R and MCU Channels by Regulating Threshold and Gain of MICU1-Mediated Inhibition and Activation of MCU. Cell Rep. 2017 Dec 12;21(11):3141-3154. PMID: 29241542; PMCID: PMC5734103. [CrossRef]
- Patron M, Granatiero V, Espino J, Rizzuto R, De Stefani D. MICU3 is a tissue-specific enhancer of mitochondrial calcium uptake. Cell Death Differ. 2019 Jan;26(1):179-195. Epub 2018 May 3. PMID: 29725115; PMCID: PMC6124646. [CrossRef]
- Adlakha J, Karamichali I, Sangwallek J, Deiss S, Bär K, Coles M, Hartmann MD, Lupas AN, Hernandez Alvarez B. Characterization of MCU-Binding Proteins MCUR1 and CCDC90B - Representatives of a Protein Family Conserved in Prokaryotes and Eukaryotic Organelles. Structure. 2019 Mar 5;27(3):464-475.e6. Epub 2019 Jan 3. [CrossRef] [PubMed]
- Mallilankaraman K, Cárdenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenár T, Csordás G, Madireddi P, Yang J, Müller M, Miller R, Kolesar JE, Molgó J, Kaufman B, Hajnóczky G, Foskett JK, Madesh M. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat Cell Biol. 2012 Dec;14(12):1336-43. Epub 2012 Nov 25. Erratum in: Nat Cell Biol. 2013 Jan;15(1):123. Erratum in: Nat Cell Biol. 2015 Jul;17(7):953. PMID: 23178883; PMCID: PMC3511605. [CrossRef]
- Paupe V, Prudent J, Dassa EP, Rendon OZ, Shoubridge EA. CCDC90A (MCUR1) is a cytochrome c oxidase assembly factor and not a regulator of the mitochondrial calcium uniporter. Cell Metab. 2015 Jan 6;21(1):109-16. [CrossRef] [PubMed]
- Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta. 2009 Nov;1787(11):1395-401. Epub 2009 Jul 1. PMID: 19576166; PMCID: PMC2730424. [CrossRef]
- Pan X, Liu J, Nguyen T, Liu C, Sun J, Teng Y, Fergusson MM, Rovira II, Allen M, Springer DA, Aponte AM, Gucek M, Balaban RS, Murphy E, Finkel T. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol. 2013 Dec;15(12):1464-72. Epub 2013 Nov 10. PMID: 24212091; PMCID: PMC3852190. [CrossRef]
- Wu Y, Rasmussen TP, Koval OM, Joiner ML, Hall DD, Chen B, Luczak ED, Wang Q, Rokita AG, Wehrens XH, Song LS, Anderson ME. The mitochondrial uniporter controls fight or flight heart rate increases. Nat Commun. 2015 Jan 20;6:6081. Erratum in: Nat Commun. 2015;6:7241. PMID: 25603276; PMCID: PMC4398998. [CrossRef]
- Luongo TS, Lambert JP, Yuan A, Zhang X, Gross P, Song J, Shanmughapriya S, Gao E, Jain M, Houser SR, Koch WJ, Cheung JY, Madesh M, Elrod JW. The Mitochondrial Calcium Uniporter Matches Energetic Supply with Cardiac Workload during Stress and Modulates Permeability Transition. Cell Rep. 2015 Jul 7;12(1):23-34. Epub 2015 Jun 25. PMID: 26119731; PMCID: PMC4517182. [CrossRef]
- Kwong JQ, Lu X, Correll RN, Schwanekamp JA, Vagnozzi RJ, Sargent MA, York AJ, Zhang J, Bers DM, Molkentin JD. The Mitochondrial Calcium Uniporter Selectively Matches Metabolic Output to Acute Contractile Stress in the Heart. Cell Rep. 2015 Jul 7;12(1):15-22. Epub 2015 Jun 25. PMID: 26119742; PMCID: PMC4497842. [CrossRef]
- Luongo TS, Lambert JP, Gross P, Nwokedi M, Lombardi AA, Shanmughapriya S, Carpenter AC, Kolmetzky D, Gao E, van Berlo JH, Tsai EJ, Molkentin JD, Chen X, Madesh M, Houser SR, Elrod JW. The mitochondrial Na+/Ca2+ exchanger is essential for Ca2+ homeostasis and viability. Nature. 2017 May 4;545(7652):93-97. Epub 2017 Apr 26. PMID: 28445457; PMCID: PMC5731245. [CrossRef]
- Lambert JP, Luongo TS, Tomar D, Jadiya P, Gao E, Zhang X, Lucchese AM, Kolmetzky DW, Shah NS, Elrod JW. MCUB Regulates the Molecular Composition of the Mitochondrial Calcium Uniporter Channel to Limit Mitochondrial Calcium Overload During Stress. Circulation. 2019 Nov 19;140(21):1720-1733. Epub 2019 Sep 19. PMID: 31533452; PMCID: PMC6996560. [CrossRef]
- Xue Q, Pei H, Liu Q, Zhao M, Sun J, Gao E, Ma X, Tao L. MICU1 protects against myocardial ischemia/reperfusion injury and its control by the importer receptor Tom70. Cell Death Dis. 2017 Jul 13;8(7):e2923. PMID: 28703803; PMCID: PMC5550843. [CrossRef]
- Rutter, GA. Visualising insulin secretion. The Minkowski Lecture 2004. Diabetologia. 2004 Nov;47(11):1861-72. Epub 2004 Nov 17. [CrossRef] [PubMed]
- Kennedy HJ, Pouli AE, Ainscow EK, Jouaville LS, Rizzuto R, Rutter GA. Glucose generates sub-plasma membrane ATP microdomains in single islet beta-cells. Potential role for strategically located mitochondria. J Biol Chem. 1999 May 7;274(19):13281-91. [CrossRef] [PubMed]
- Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984 Nov 29-Dec 5;312(5993):446-8. [CrossRef] [PubMed]
- Wollheim CB, Sharp GW. Regulation of insulin release by calcium. Physiol Rev. 1981 Oct;61(4):914-73. [CrossRef] [PubMed]
- Georgiadou E, Haythorne E, Dickerson MT, Lopez-Noriega L, Pullen TJ, da Silva Xavier G, Davis SPX, Martinez-Sanchez A, Semplici F, Rizzuto R, McGinty JA, French PM, Cane MC, Jacobson DA, Leclerc I, Rutter GA. The pore-forming subunit MCU of the mitochondrial Ca2+ uniporter is required for normal glucose-stimulated insulin secretion in vitro and in vivo in mice. Diabetologia. 2020 Jul;63(7):1368-1381. Epub 2020 Apr 29. PMID: 32350566; PMCID: PMC7286857. [CrossRef]
- Tarasov AI, Semplici F, Ravier MA, Bellomo EA, Pullen TJ, Gilon P, Sekler I, Rizzuto R, Rutter GA. The mitochondrial Ca2+ uniporter MCU is essential for glucose-induced ATP increases in pancreatic β-cells. PLoS One. 2012;7(7):e39722. Epub 2012 Jul 19. PMID: 22829870; PMCID: PMC3400633. [CrossRef]
- Alam MR, Groschner LN, Parichatikanond W, Kuo L, Bondarenko AI, Rost R, Waldeck-Weiermair M, Malli R, Graier WF. Mitochondrial Ca2+ uptake 1 (MICU1) and mitochondrial ca2+ uniporter (MCU) contribute to metabolism-secretion coupling in clonal pancreatic β-cells. J Biol Chem. 2012 Oct 5;287(41):34445-54. Epub 2012 Aug 17. PMID: 22904319; PMCID: PMC3464549. [CrossRef]
- Vishnu N, Hamilton A, Bagge A, Wernersson A, Cowan E, Barnard H, Sancak Y, Kamer KJ, Spégel P, Fex M, Tengholm A, Mootha VK, Nicholls DG, Mulder H. Mitochondrial clearance of calcium facilitated by MICU2 controls insulin secretion. Mol Metab. 2021 Sep;51:101239. Epub 2021 Apr 28. PMID: 33932586; PMCID: PMC8163986. [CrossRef]
- Wright LE, Vecellio Reane D, Milan G, Terrin A, Di Bello G, Belligoli A, Sanna M, Foletto M, Favaretto F, Raffaello A, Mammucari C, Nitti D, Vettor R, Rizzuto R. Increased mitochondrial calcium uniporter in adipocytes underlies mitochondrial alterations associated with insulin resistance. Am J Physiol Endocrinol Metab. 2017 Dec 1;313(6):E641-E650. Epub 2017 Aug 8. PMID: 28790027; PMCID: PMC6109647. [CrossRef]
- Pinton P, Ferrari D, Magalhães P, Schulze-Osthoff K, Di Virgilio F, Pozzan T, Rizzuto R. Reduced loading of intracellular Ca(2+) stores and downregulation of capacitative Ca(2+) influx in Bcl-2-overexpressing cells. J Cell Biol. 2000 Mar 6;148(5):857-62. PMID: 10704437; PMCID: PMC2174537. [CrossRef]
- Pinton P, Ferrari D, Rapizzi E, Di Virgilio F, Pozzan T, Rizzuto R. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 2001 Jun 1;20(11):2690-701. PMID: 11387204; PMCID: PMC125256. [CrossRef]
- Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003 Apr 4;300(5616):135-9. Epub 2003 Mar 6. [CrossRef] [PubMed]
- Rosa N, Ivanova H, Wagner LE 2nd, Kale J, La Rovere R, Welkenhuyzen K, Louros N, Karamanou S, Shabardina V, Lemmens I, Vandermarliere E, Hamada K, Ando H, Rousseau F, Schymkowitz J, Tavernier J, Mikoshiba K, Economou A, Andrews DW, Parys JB, Yule DI, Bultynck G. Bcl-xL acts as an inhibitor of IP3R channels, thereby antagonizing Ca2+-driven apoptosis. Cell Death Differ. 2022 Apr;29(4):788-805. Epub 2021 Nov 8. PMID: 34750538; PMCID: PMC8990011. [CrossRef]
- Tosatto A, Sommaggio R, Kummerow C, Bentham RB, Blacker TS, Berecz T, Duchen MR, Rosato A, Bogeski I, Szabadkai G, Rizzuto R, Mammucari C. The mitochondrial calcium uniporter regulates breast cancer progression via HIF-1α. EMBO Mol Med. 2016 May 2;8(5):569-85. PMID: 27138568; PMCID: PMC4864890. [CrossRef]
- Stejerean-Todoran I, Zimmermann K, Gibhardt CS, Vultur A, Ickes C, Shannan B, Bonilla Del Rio Z, Wölling A, Cappello S, Sung HM, Shumanska M, Zhang X, Nanadikar M, Latif MU, Wittek A, Lange F, Waters A, Brafford P, Wilting J, Urlaub H, Katschinski DM, Rehling P, Lenz C, Jakobs S, Ellenrieder V, Roesch A, Schön MP, Herlyn M, Stanisz H, Bogeski I. MCU controls melanoma progression through a redox-controlled phenotype switch. EMBO Rep. 2022 Nov 7;23(11):e54746. Epub 2022 Sep 26. PMID: 36156348; PMCID: PMC9638851. [CrossRef]
- Vultur A, Gibhardt CS, Stanisz H, Bogeski I. The role of the mitochondrial calcium uniporter (MCU) complex in cancer. Pflugers Arch. 2018 Aug;470(8):1149-1163. Epub 2018 Jun 21. [CrossRef] [PubMed]
- Filadi R, De Mario A, Audano M, Romani P, Pedretti S, Cardenas C, Dupont S, Mammucari C, Mitro N, Pizzo P. Sustained IP3-linked Ca2+ signaling promotes progression of triple negative breast cancer cells by regulating fatty acid metabolism. Front Cell Dev Biol. 2023 Mar 13;11:1071037. PMID: 36994106; PMCID: PMC10040683. [CrossRef]
- Fernandez Garcia E, Paudel U, Noji MC, Bowman CE, Rustgi AK, Pitarresi JR, Wellen KE, Arany Z, Weissenrieder JS, Foskett JK. The mitochondrial Ca2+ channel MCU is critical for tumor growth by supporting cell cycle progression and proliferation. Front Cell Dev Biol. 2023 Jun 8;11:1082213. PMID: 37363724; PMCID: PMC10285664. [CrossRef]
- Curry MC, Peters AA, Kenny PA, Roberts-Thomson SJ, Monteith GR. Mitochondrial calcium uniporter silencing potentiates caspase-independent cell death in MDA-MB-231 breast cancer cells. Biochem Biophys Res Commun. 2013 May 10;434(3):695-700. Epub 2013 Apr 18. [CrossRef] [PubMed]
- De Mario A, Tosatto A, Hill JM, Kriston-Vizi J, Ketteler R, Vecellio Reane D, Cortopassi G, Szabadkai G, Rizzuto R, Mammucari C. Identification and functional validation of FDA-approved positive and negative modulators of the mitochondrial calcium uniporter. Cell Rep. 2021 Jun 22;35(12):109275. PMID: 34161774; PMCID: PMC8242467. [CrossRef]
- Xue P, Chen Q, Ren X, Liu D, Yang X. A novel protoapigenone analog RY10-4 induces apoptosis of breast cancer cells by exacerbating mitochondrial Ca2+ influx through mitochondrial calcium uniporter. Toxicol Appl Pharmacol. 2021 Dec 15;433:115776. Epub 2021 Oct 28. [CrossRef] [PubMed]
- Tang S, Wang X, Shen Q, Yang X, Yu C, Cai C, Cai G, Meng X, Zou F. Mitochondrial Ca²⁺ uniporter is critical for store-operated Ca²⁺ entry-dependent breast cancer cell migration. Biochem Biophys Res Commun. 2015 Feb 27;458(1):186-93. Epub 2015 Jan 29. [CrossRef] [PubMed]
- Hall DD, Wu Y, Domann FE, Spitz DR, Anderson ME. Mitochondrial calcium uniporter activity is dispensable for MDA-MB-231 breast carcinoma cell survival. PLoS One. 2014 May 6;9(5):e96866. PMID: 24802861; PMCID: PMC4011874. [CrossRef]
- Yu C, Wang Y, Peng J, Shen Q, Chen M, Tang W, Li X, Cai C, Wang B, Cai S, Meng X, Zou F. Mitochondrial calcium uniporter as a target of microRNA-340 and promoter of metastasis via enhancing the Warburg effect. Oncotarget. 2017 Jul 31;8(48):83831-83844. PMID: 29137386; PMCID: PMC5663558. [CrossRef]
- Martin J, Maurhofer O, Bellance N, Benard G, Graber F, Hahn D, Galinier A, Hora C, Gupta A, Ferrand G, Hoppeler H, Rossignol R, Dufour JF, St-Pierre MV. Disruption of the histidine triad nucleotide-binding hint2 gene in mice affects glycemic control and mitochondrial function. Hepatology. 2013 May;57(5):2037-48. [CrossRef] [PubMed]
- Chen L, Sun Q, Zhou D, Song W, Yang Q, Ju B, Zhang L, Xie H, Zhou L, Hu Z, Yao H, Zheng S, Wang W. HINT2 triggers mitochondrial Ca2+ influx by regulating the mitochondrial Ca2+ uniporter (MCU) complex and enhances gemcitabine apoptotic effect in pancreatic cancer. Cancer Lett. 2017 Dec 28;411:106-116. Epub 2017 Sep 23. [CrossRef] [PubMed]
- Xie KF, Guo DD, Luo XJ. SMDT1-driven change in mitochondrial dynamics mediate cell apoptosis in PDAC. Biochem Biophys Res Commun. 2019 Apr 2;511(2):323-329. Epub 2019 Feb 16. [CrossRef] [PubMed]
- Wang X, Li Y, Li Z, Lin S, Wang H, Sun J, Lan C, Wu L, Sun D, Huang C, Singh PK, Hempel N, Trebak M, DeNicola GM, Hao J, Yang S. Mitochondrial Calcium Uniporter Drives Metastasis and Confers a Targetable Cystine Dependency in Pancreatic Cancer. Cancer Res. 2022 Jun 15;82(12):2254-2268. PMID: 35413105; PMCID: PMC9203979. [CrossRef]
- Marchi S, Lupini L, Patergnani S, Rimessi A, Missiroli S, Bonora M, Bononi A, Corrà F, Giorgi C, De Marchi E, Poletti F, Gafà R, Lanza G, Negrini M, Rizzuto R, Pinton P. Downregulation of the mitochondrial calcium uniporter by cancer-related miR-25. Curr Biol. 2013 Jan 7;23(1):58-63. Epub 2012 Dec 13. PMID: 23246404; PMCID: PMC3540261. [CrossRef]
- Yu J, Chen X, Li J, Wang F. CERS6 antisense RNA 1 promotes colon cancer via upregulating mitochondrial calcium uniporter. Eur J Clin Invest. 2023 May;53(5):e13951. Epub 2023 Feb 23. [CrossRef] [PubMed]
- Zhu J, Zhang C, Wang Z, Shi L, Li L, Wu H, Liu M. miR-138-5p targets MCU to inhibit mitochondrial biogenesis and colorectal cancer growth. J Cell Mol Med. 2023 Jun 1. Epub ahead of print. [CrossRef] [PubMed]
- Zeng F, Chen X, Cui W, Wen W, Lu F, Sun X, Ma D, Yuan Y, Li Z, Hou N, Zhao H, Bi X, Zhao J, Zhou J, Zhang Y, Xiao RP, Cai J, Zhang X. RIPK1 Binds MCU to Mediate Induction of Mitochondrial Ca2+ Uptake and Promotes Colorectal Oncogenesis. Cancer Res. 2018 Jun 1;78(11):2876-2885. Epub 2018 Mar 12. [CrossRef] [PubMed]
- Ren T, Zhang H, Wang J, Zhu J, Jin M, Wu Y, Guo X, Ji L, Huang Q, Zhang H, Yang H, Xing J. MCU-dependent mitochondrial Ca2+ inhibits NAD+/SIRT3/SOD2 pathway to promote ROS production and metastasis of HCC cells. Oncogene. 2017 Oct 19;36(42):5897-5909. Epub 2017 Jun 26. [CrossRef] [PubMed]
- Ren T, Wang J, Zhang H, Yuan P, Zhu J, Wu Y, Huang Q, Guo X, Zhang J, Ji L, Li J, Zhang H, Yang H, Xing J. MCUR1-Mediated Mitochondrial Calcium Signaling Facilitates Cell Survival of Hepatocellular Carcinoma via Reactive Oxygen Species-Dependent P53 Degradation. Antioxid Redox Signal. 2018 Apr 20;28(12):1120-1136. Epub 2017 Nov 1. [CrossRef] [PubMed]
- Deng Q, Chen S, Fu C, Jiang J, Zou M, Tan Y, Wang X, Xia F, Feng K, Ma K, Bie P. Long noncoding RNA expression profiles in sub-lethal heat-treated hepatoma carcinoma cells. World J Surg Oncol. 2017 Jul 21;15(1):136. PMID: 28732507; PMCID: PMC5521104. [CrossRef]
- Zhao L, Jiang M, Tian T, Wang G, Mei Y, Fu G, Zhou N. Effects of MCU mediated Ca2+ homeostasis on ovarian cancer cell SKOV3 proliferation, migration and transforming. Curr Mol Med. 2022 Jun 17. Epub ahead of print. [CrossRef] [PubMed]
- Meng K, Hu Y, Wang D, Li Y, Shi F, Lu J, Wang Y, Cao Y, Zhang CZ, He QY. EFHD1, a novel mitochondrial regulator of tumor metastasis in clear cell renal cell carcinoma. Cancer Sci. 2023 May;114(5):2029-2040. Epub 2023 Feb 26. PMID: 36747492; PMCID: PMC10154798. [CrossRef]
- Gherardi G, De Mario A, Mammucari C. The mitochondrial calcium homeostasis orchestra plays its symphony: Skeletal muscle is the guest of honor. Int Rev Cell Mol Biol. 2021;362:209-259. Epub 2021 May 20. [CrossRef] [PubMed]
- Mammucari C, Gherardi G, Zamparo I, Raffaello A, Boncompagni S, Chemello F, Cagnin S, Braga A, Zanin S, Pallafacchina G, Zentilin L, Sandri M, De Stefani D, Protasi F, Lanfranchi G, Rizzuto R. The mitochondrial calcium uniporter controls skeletal muscle trophism in vivo. Cell Rep. 2015 Mar 3;10(8):1269-79. Epub 2015 Feb 26. PMID: 25732818; PMCID: PMC4351162. [CrossRef]
- Gherardi G, Nogara L, Ciciliot S, Fadini GP, Blaauw B, Braghetta P, Bonaldo P, De Stefani D, Rizzuto R, Mammucari C. Loss of mitochondrial calcium uniporter rewires skeletal muscle metabolism and substrate preference. Cell Death Differ. 2019 Jan;26(2):362-381. Epub 2018 Sep 19. PMID: 30232375; PMCID: PMC6329801. [CrossRef]
- Feno S, Munari F, Reane DV, Gissi R, Hoang DH, Castegna A, Chazaud B, Viola A, Rizzuto R, Raffaello A. The dominant-negative mitochondrial calcium uniporter subunit MCUb drives macrophage polarization during skeletal muscle regeneration. Sci Signal. 2021 Nov 2;14(707):eabf3838. Epub 2021 Nov 2. [CrossRef] [PubMed]
- Logan CV, Szabadkai G, Sharpe JA, Parry DA, Torelli S, Childs AM, Kriek M, Phadke R, Johnson CA, Roberts NY, Bonthron DT, Pysden KA, Whyte T, Munteanu I, Foley AR, Wheway G, Szymanska K, Natarajan S, Abdelhamed ZA, Morgan JE, Roper H, Santen GW, Niks EH, van der Pol WL, Lindhout D, Raffaello A, De Stefani D, den Dunnen JT, Sun Y, Ginjaar I, Sewry CA, Hurles M, Rizzuto R; UK10K Consortium; Duchen MR, Muntoni F, Sheridan E. Loss-of-function mutations in MICU1 cause a brain and muscle disorder linked to primary alterations in mitochondrial calcium signaling. Nat Genet. 2014 Feb;46(2):188-93. Epub 2013 Dec 15. [CrossRef] [PubMed]
- Debattisti V, Horn A, Singh R, Seifert EL, Hogarth MW, Mazala DA, Huang KT, Horvath R, Jaiswal JK, Hajnóczky G. Dysregulation of Mitochondrial Ca2+ Uptake and Sarcolemma Repair Underlie Muscle Weakness and Wasting in Patients and Mice Lacking MICU1. Cell Rep. 2019 Oct 29;29(5):1274-1286.e6. PMID: 31665639; PMCID: PMC7007691. [CrossRef]
- Singh R, Bartok A, Paillard M, Tyburski A, Elliott M, Hajnóczky G. Uncontrolled mitochondrial calcium uptake underlies the pathogenesis of neurodegeneration in MICU1-deficient mice and patients. Sci Adv. 2022 Mar 18;8(11):eabj4716. Epub 2022 Mar 18. PMID: 35302860; PMCID: PMC8932652. [CrossRef]
- Ghosh S, Zulkifli M, Joshi A, Venkatesan M, Cristel A, Vishnu N, Madesh M, Gohil VM. MCU-complex-mediated mitochondrial calcium signaling is impaired in Barth syndrome. Hum Mol Genet. 2022 Feb 3;31(3):376-385. PMID: 34494107; PMCID: PMC8825335. [CrossRef]
- Chiu HY, Loh AHP, Taneja R. Mitochondrial calcium uptake regulates tumour progression in embryonal rhabdomyosarcoma. Cell Death Dis. 2022 Apr 30;13(4):419. PMID: 35490194; PMCID: PMC9056521. [CrossRef]
- Britti E, Delaspre F, Tamarit J, Ros J. Mitochondrial calcium signalling and neurodegenerative diseases. Neuronal Signal. 2018 Nov 16;2(4):NS20180061. PMID: 32714593; PMCID: PMC7373239. [CrossRef]
- Area-Gomez E, Del Carmen Lara Castillo M, Tambini MD, Guardia-Laguarta C, de Groof AJ, Madra M, Ikenouchi J, Umeda M, Bird TD, Sturley SL, Schon EA. Upregulated function of mitochondria-associated ER membranes in Alzheimer disease. EMBO J. 2012 Nov 5;31(21):4106-23. Epub 2012 Aug 14. PMID: 22892566; PMCID: PMC3492725. [CrossRef]
- Zampese E, Fasolato C, Kipanyula MJ, Bortolozzi M, Pozzan T, Pizzo P. Presenilin 2 modulates endoplasmic reticulum (ER)-mitochondria interactions and Ca2+ cross-talk. Proc Natl Acad Sci U S A. 2011 Feb 15;108(7):2777-82. Epub 2011 Feb 1. PMID: 21285369; PMCID: PMC3041131. [CrossRef]
- Cheung KH, Shineman D, Müller M, Cárdenas C, Mei L, Yang J, Tomita T, Iwatsubo T, Lee VM, Foskett JK. Mechanism of Ca2+ disruption in Alzheimer's disease by presenilin regulation of InsP3 receptor channel gating. Neuron. 2008 Jun 26;58(6):871-83. PMID: 18579078; PMCID: PMC2495086. [CrossRef]
- Toglia P, Ullah G. The gain-of-function enhancement of IP3-receptor channel gating by familial Alzheimer's disease-linked presenilin mutants increases the open probability of mitochondrial permeability transition pore. Cell Calcium. 2016 Jul;60(1):13-24. Epub 2016 May 7. [CrossRef] [PubMed]
- Shilling D, Müller M, Takano H, Mak DO, Abel T, Coulter DA, Foskett JK. Suppression of InsP3 receptor-mediated Ca2+ signaling alleviates mutant presenilin-linked familial Alzheimer's disease pathogenesis. J Neurosci. 2014 May 14;34(20):6910-23. PMID: 24828645; PMCID: PMC4019804. [CrossRef]
- Ferreiro E, Oliveira CR, Pereira CMF. The release of calcium from the endoplasmic reticulum induced by amyloid-beta and prion peptides activates the mitochondrial apoptotic pathway. Neurobiol Dis. 2008 Jun;30(3):331-342. Epub 2008 Feb 20. [CrossRef] [PubMed]
- Jadiya P, Kolmetzky DW, Tomar D, Di Meco A, Lombardi AA, Lambert JP, Luongo TS, Ludtmann MH, Praticò D, Elrod JW. Impaired mitochondrial calcium efflux contributes to disease progression in models of Alzheimer's disease. Nat Commun. 2019 Aug 29;10(1):3885. PMID: 31467276; PMCID: PMC6715724. [CrossRef]
- Kalia LV, Lang AE. Parkinson's disease. Lancet. 2015 Aug 29;386(9996):896-912. Epub 2015 Apr 19. [CrossRef] [PubMed]
- Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P. Alpha-synuclein overexpression and aggregation exacerbates impairment of mitochondrial functions by augmenting oxidative stress in human neuroblastoma cells. Int J Biochem Cell Biol. 2009 Oct;41(10):2015-24. Epub 2009 May 19. [CrossRef] [PubMed]
- Gandhi S, Wood-Kaczmar A, Yao Z, Plun-Favreau H, Deas E, Klupsch K, Downward J, Latchman DS, Tabrizi SJ, Wood NW, Duchen MR, Abramov AY. PINK1-associated Parkinson's disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell. 2009 Mar 13;33(5):627-38. PMID: 19285945; PMCID: PMC2724101. [CrossRef]
- Soman S, Keatinge M, Moein M, Da Costa M, Mortiboys H, Skupin A, Sugunan S, Bazala M, Kuznicki J, Bandmann O. Inhibition of the mitochondrial calcium uniporter rescues dopaminergic neurons in pink1-/- zebrafish. Eur J Neurosci. 2017 Feb;45(4):528-535. Epub 2016 Dec 28. PMID: 27859782; PMCID: PMC5324670. [CrossRef]
- Matteucci A, Patron M, Vecellio Reane D, Gastaldello S, Amoroso S, Rizzuto R, Brini M, Raffaello A, Calì T. Parkin-dependent regulation of the MCU complex component MICU1. Sci Rep. 2018 Sep 21;8(1):14199. Erratum in: Sci Rep. 2019 Mar 12;9(1):4665. PMID: 30242232; PMCID: PMC6155109. [CrossRef]
- Verma M, Callio J, Otero PA, Sekler I, Wills ZP, Chu CT. Mitochondrial Calcium Dysregulation Contributes to Dendrite Degeneration Mediated by PD/LBD-Associated LRRK2 Mutants. J Neurosci. 2017 Nov 15;37(46):11151-11165. Epub 2017 Oct 16. PMID: 29038245; PMCID: PMC5688524. [CrossRef]
- Walker, FO. Huntington's disease. Lancet. 2007 Jan 20;369(9557):218-28. [CrossRef] [PubMed]
- Panov AV, Gutekunst CA, Leavitt BR, Hayden MR, Burke JR, Strittmatter WJ, Greenamyre JT. Early mitochondrial calcium defects in Huntington's disease are a direct effect of polyglutamines. Nat Neurosci. 2002 Aug;5(8):731-6. [CrossRef] [PubMed]
- Panov AV, Lund S, Greenamyre JT. Ca2+-induced permeability transition in human lymphoblastoid cell mitochondria from normal and Huntington's disease individuals. Mol Cell Biochem. 2005 Jan;269(1-2):143-52. [CrossRef] [PubMed]
- Lim D, Fedrizzi L, Tartari M, Zuccato C, Cattaneo E, Brini M, Carafoli E. Calcium homeostasis and mitochondrial dysfunction in striatal neurons of Huntington disease. J Biol Chem. 2008 Feb 29;283(9):5780-9. Epub 2007 Dec 21. [CrossRef] [PubMed]
- Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001 May 31;344(22):1688-700. [CrossRef] [PubMed]
- Tadić V, Adam A, Goldhammer N, Lautenschlaeger J, Oberstadt M, Malci A, Le TT, Sengupta S, Stubendorff B, Keiner S, Witte OW, Grosskreutz J. Investigation of mitochondrial calcium uniporter role in embryonic and adult motor neurons from G93AhSOD1 mice. Neurobiol Aging. 2019 Mar;75:209-222. Epub 2018 Nov 23. [CrossRef] [PubMed]
- Mühling T, Duda J, Weishaupt JH, Ludolph AC, Liss B. Elevated mRNA-levels of distinct mitochondrial and plasma membrane Ca(2+) transporters in individual hypoglossal motor neurons of endstage SOD1 transgenic mice. Front Cell Neurosci. 2014 Nov 14;8:353. PMID: 25452714; PMCID: PMC4231948. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



