1. Introduction
Muscle damage is one of the most common issues that today’s athletes face, whether they are amateurs or pros [
1]. Indeed, skeletal muscle injuries account for more than 30% of injuries evident in sports medicine consultations, according to Kirkendall DT et al. [
2]. As a result, it is essential to employ the most effective methods of preventing these injuries. Several prior studies have suggested that warming up may play an important role in reducing muscle damage, whereas other investigations have found no positive effects. In fact, the significance of stretching and warming up in reducing musculoskeletal injuries has been debated. Some studies found no reduction in injury risk or the total number of injuries [
3], whereas others found positive effects and reduced neuromuscular injuries after stretching or warming up programs [
4]. Therefore, a number of investigations have found that the justifications supporting the preventive function of warming up and stretching on muscle damage are insufficient [
5,
6]. Several studies have found a link between an increase in free radicals and hence oxidative stress and the development of muscle injury [
7]. Furthermore, studies on the effects of anaerobic exercise on oxidative stress are few in comparison to studies on the effects of aerobic exercise [
8,
9]. These studies, however, reveal an increase in oxidative stress following supramaximal exercise. To defend against the potentially harmful effects of oxidative stress, organisms have anti-free radical defense systems that involve a variety of enzymatic antioxidants (superoxide dismutase (SOD); glutathione peroxidase (GPX); catalase and non-enzymatic antioxidants (Vitamin E and C; Zinc; Selenium; Copper; Uric acid) [
10]. In addition to the role of the body’s anti-free radical system, many strategies for limiting and modalities for preventing the increase in pro-oxidants have been proposed in the sports field; such as variation in duration, intensity, and type of exercise [
11], the type of recovery [
12,
13]. Recent research has focused on proving an increase in total antioxidant status after anaerobic exercise in amateur athletes [
14].
Warming up helps prepare the muscles for the exercise by increasing their contractile capacity [
15] and decreasing muscle and tendon viscoelasticity [
16]. Warming up has been shown to increase the energy supply to the muscles, increase the rate of nerve impulse transmission, reduce internal viscosity, and increase muscle activation, strength, blood supply, and oxygen to the working muscle [
17,
18,
19]. The effects of warming up on cardiorespiratory functions [
20], the psychological state [
21], and the risk of injury [
22] have been well documented. However, its impact on oxidative stress indicators has received little attention. In this regard, we propose to conduct a study to evaluate the potential short-term influence of varied warm-up durations on muscle injury prevention and the evolution of oxidative stress biomarkers after anaerobic exercise in young amateur handball players.
2. Materials and methods:
2.1. Participants:
Fourteen male handball players belonging to the regional selection of Sfax-Tunisia were recruited to participate in this study. The anthropometric characteristics of all participants are shown in
Table 1.
After receiving a verbal description of the protocol, study risks and benefits, all players gave their written consent before participating in our experimental protocol, which was approved by the Research Ethics Committee of the Faculty of Medicine, University of Sfax, Tunisia (protocol number IRB00008931-03). None of the participating players took antioxidant supplements or medications during the study.
2.2. Experimental design:
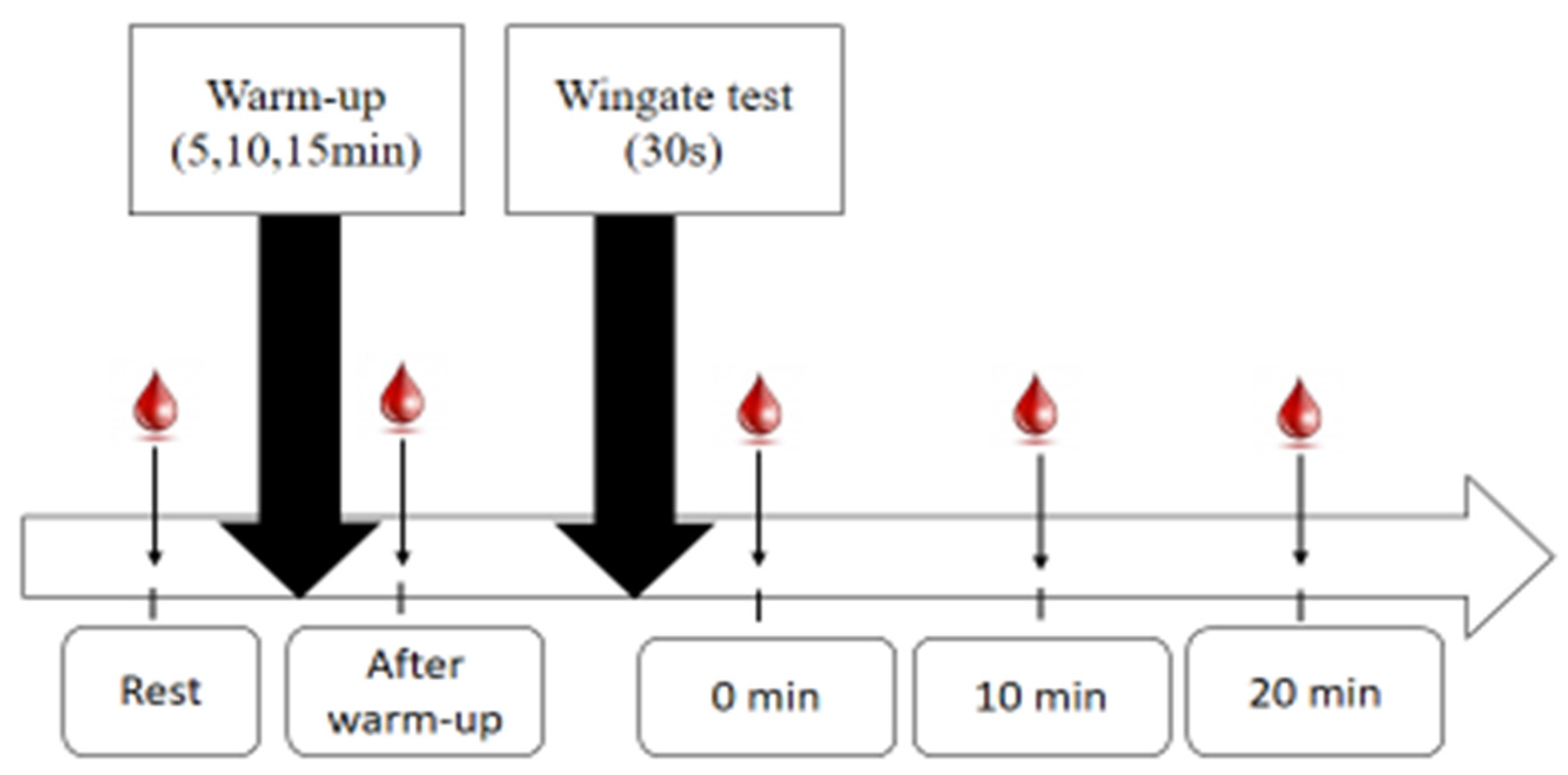
In order to reduce the influence of exogenous factors on biomarkers of oxidative stress, participants were asked to stop eating at least two to three hours before the experiment, not to take alcohol, caffeine, or any other energizing drug, and not to engage in strenuous physical activities during the 24 hours prior to the experiment. Participants performed three sessions, in random order, with a week of rest between each session. Each session consists of a warm-up of 5-min, 10-min or 15-min of pedaling, followed by a Wingate test for 30s on an ergocycle (Monark type 894 E) with an imposed load of 75g/kg of body mass.
Prior to the start of the experiment, the subjects performed an incremental peak test on the ergometer to determine the intensity of the warm-up. To do this, the participant pedaled for 3-min at 50 W, then an increase in the intensity of 25 W every 2-min until exhaustion.
The warm-up intensity was chosen on the basis of the output power recorded in the last step of the incremental test (50% of this power). To neutralize the chrono-biological effects, all sessions were carried out at 9 a.m.
2.3. Anthropometric measurements and body composition:
Body mass was measured with an electronic scale (TANITATBF.350), and standing height was measured with a measuring rod. BMI was calculated using the formula: BMI = Mass (kg) / Height2 (m2).
2.4. Dietary program
The meal should be similar 72 hours before each blood test in order to avoid changes in postprandial oxidative stress. To do this, the participants were present in the dining hall of the Sports Institute of Sfax, -Tunisia, during the three meals. In addition, the participants maintained their usual diet during the training period.
2.5. Blood Sampling and Biochemistry:
The experimental protocol consists of taking several samples: At rest (PR), immediately after the warm-up (PWU), immediately after the Wingate test (P0), and after 10-min and 20-min of stopping the exercise, respectively (P10) and (P20) (
Figure 1). Analyses were performed in the Laboratory of Pharmacology of the Faculty of Medicine of Sfax.
Blood samples were taken under basal conditions (8 a.m.) after 10 hours of fasting and 9 hours of sleep. They were taken from an antecubital vein and immediately centrifuged at 4000 rpm to obtain plasma, then stored in aliquots at -80°C until analysis. Analysis of oxidative stress biomarkers was performed using dosage kits (Randox Laboratories, place-country-region UK). Standard colorimetric dosages were used to measure plasma concentrations of glutathione reductase (GR), glutathione peroxidase (GPx), superoxide dismutase (SOD), and total antioxidant status (TAS). Malondialdehyde (MDA) concentrations were measured by the formula: Sample = (sample peak height x calibrator concentration)/peak height calibrator, and analyzed via HPLC malondialdehyde procedure.
Protein carbonyls after their derivatization with 2,4 dinitrophenylhydrazine (DNPH), were detected by spectrophotometry at 370 nm referring to the basic methods described by Levine RL et al. [
23]. Using tetramethoxypropane as a standard, plasma TBARS were measured using the method of Yagi [
24].
2.6. Statistical analyses:
All statistical tests were processed using STATISTICA Software 20 (Stat-Soft, France). Following normality confirmation was assessed using the Shapiro–Wilk’s W-test, and analysis of the results for each parameter is performed using a two-factor analysis of variance (ANOVA). These ANOVAs took the following form: 3 "warm-up durations" (5-min warm-up; 10-min warm-up and 15-min warm-up) x 5 "blood samples" (PR; PWU; P0; P10; P20) with repeated measurements on both factors. When ANOVA showed significant effects, Bonferroni-type post-hoc tests were applied to make two-by-two comparisons of the experimental data. All the observed differences are considered to be statistically significant for a probability threshold less than 0.05 (p <0.05).
3. Results:
The differences in concentrations of plasma oxidative stress biomarkers for the three warm-up times are summarized in
Table 2.
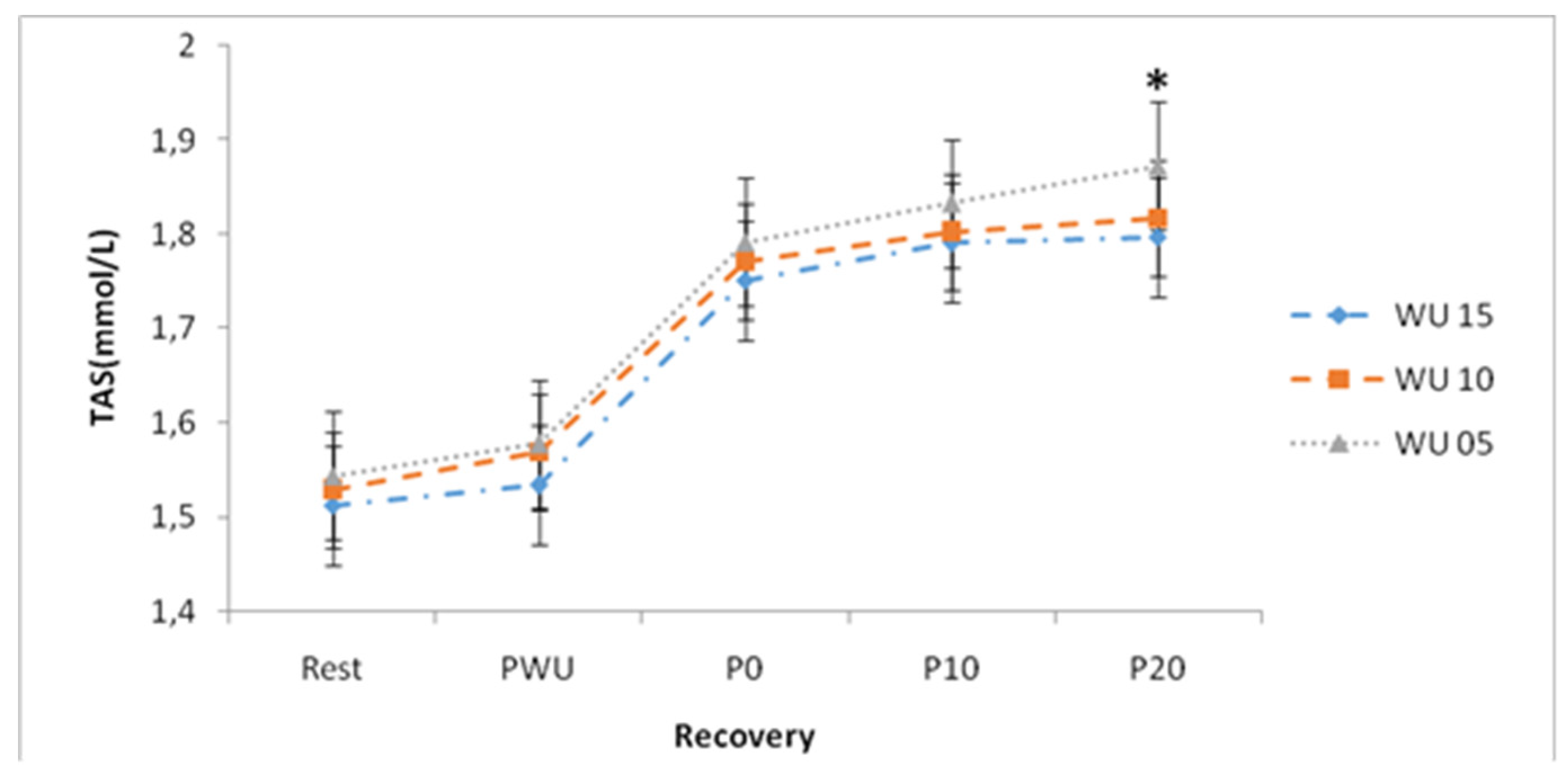
Concerning the TAS concentrations, the ANOVA showed a significant "warm-up" effect (F (2, 26) = 10.17; p <0.01; η2p = 0.44), and a significant "recovery" effect (F (4, 52) = 246.96; p <0.001; η2p = 0.95). Statistical analysis showed a significant difference (p <0.05) between PR and P0 for the three warm-up durations performed and a significant difference between PWU and P0 for the 3 warm-up durations performed.
Following a 15-min warm-up and after 20-min of the effort, the SAT concentrations are significantly different (p <0.05) from those found after 5-min of warm-up. (
Figure 2)
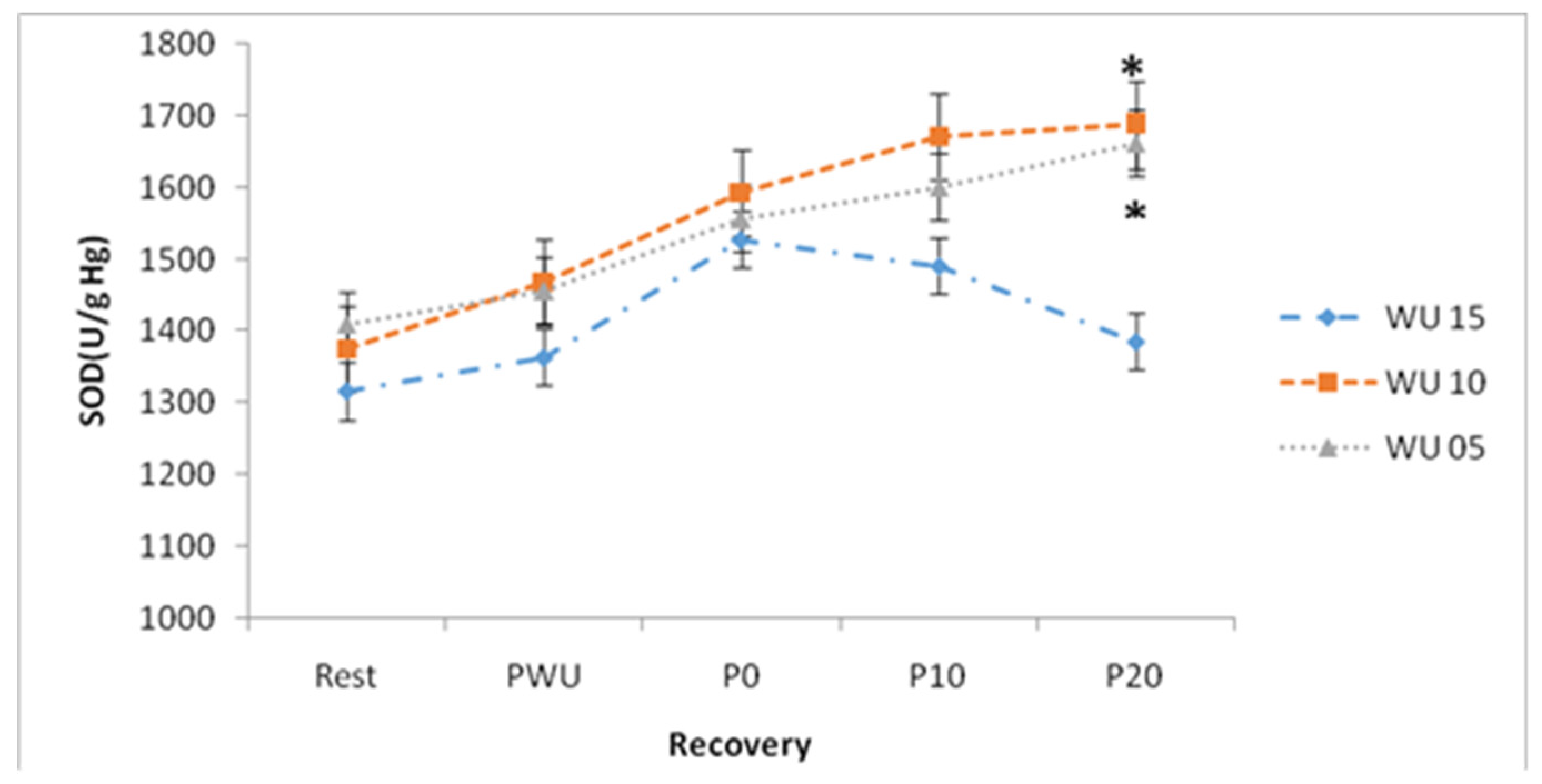
The 5-min, 10-min or 15-min warm-up durations induced variable changes in plasma SOD concentrations during recovery.
Figure 3 shows that 20-min after exercise following a 15-min warm-up, SOD concentrations are lower and significantly different from those found after a 10 or 5-min warm-up (
Figure 3). Statistical analysis revealed that SOD concentrations increased immediately after the Wingate test (p <0.05).
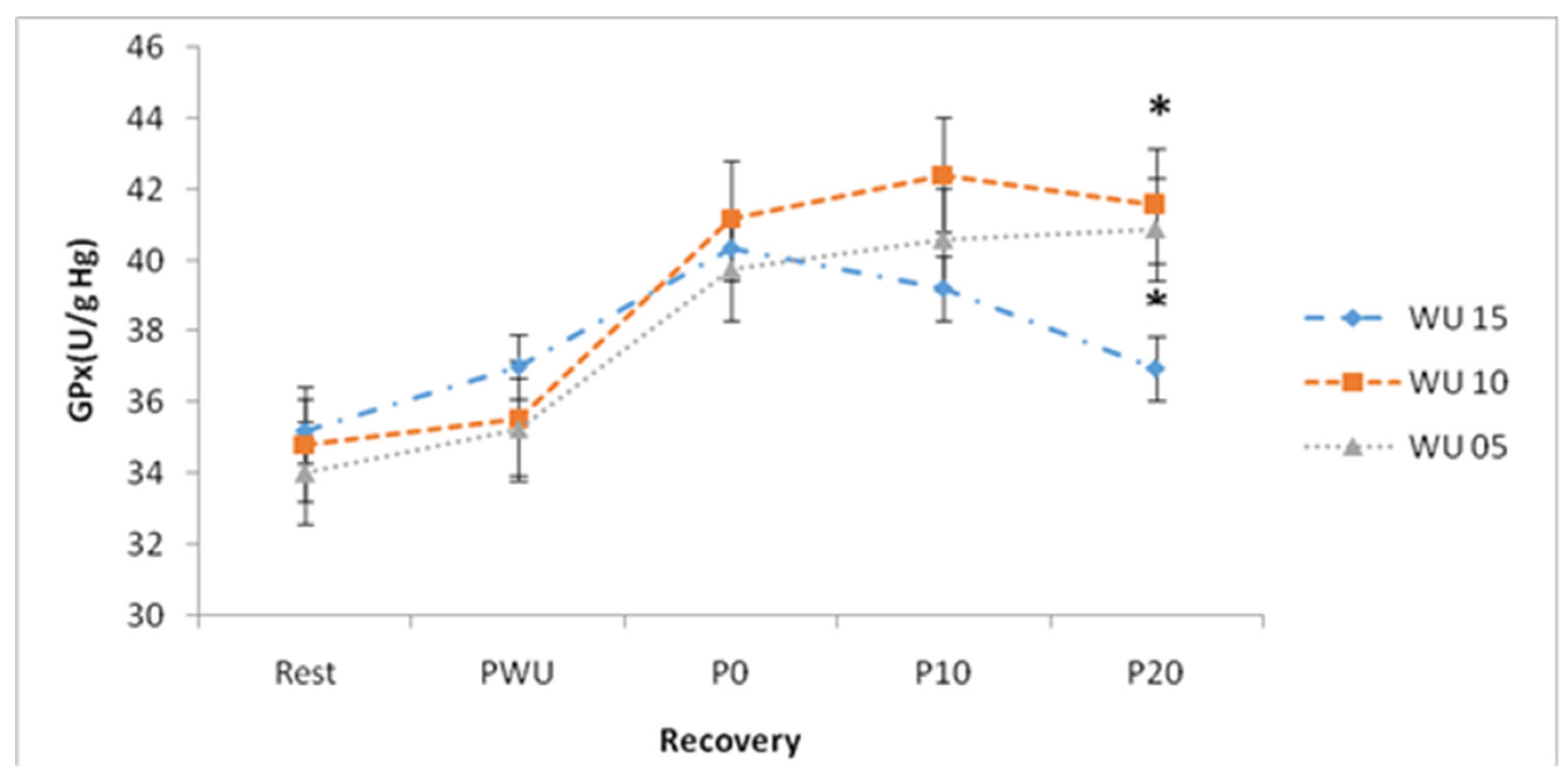
Concerning GPx concentrations, ANOVA showed a significant "Recovery" effect (F (4, 52) = 59.52; p <0.001; η2p = 0.82) and a significant "Warm up × Recovery" interaction (F (8, 104) = 3.61; p <0.001; η2p = 0.22). (
Figure 4)
In addition, a significant difference was recorded between the concentrations of GPx at rest and the concentrations measured after exercise for the 15-min warm-up period.
After 20-min of recovery, the GPx concentrations are higher following a warm-up of 10-min and 5-min compared to those recorded after a 15-min warm-up (p <0.05;
Table 2).
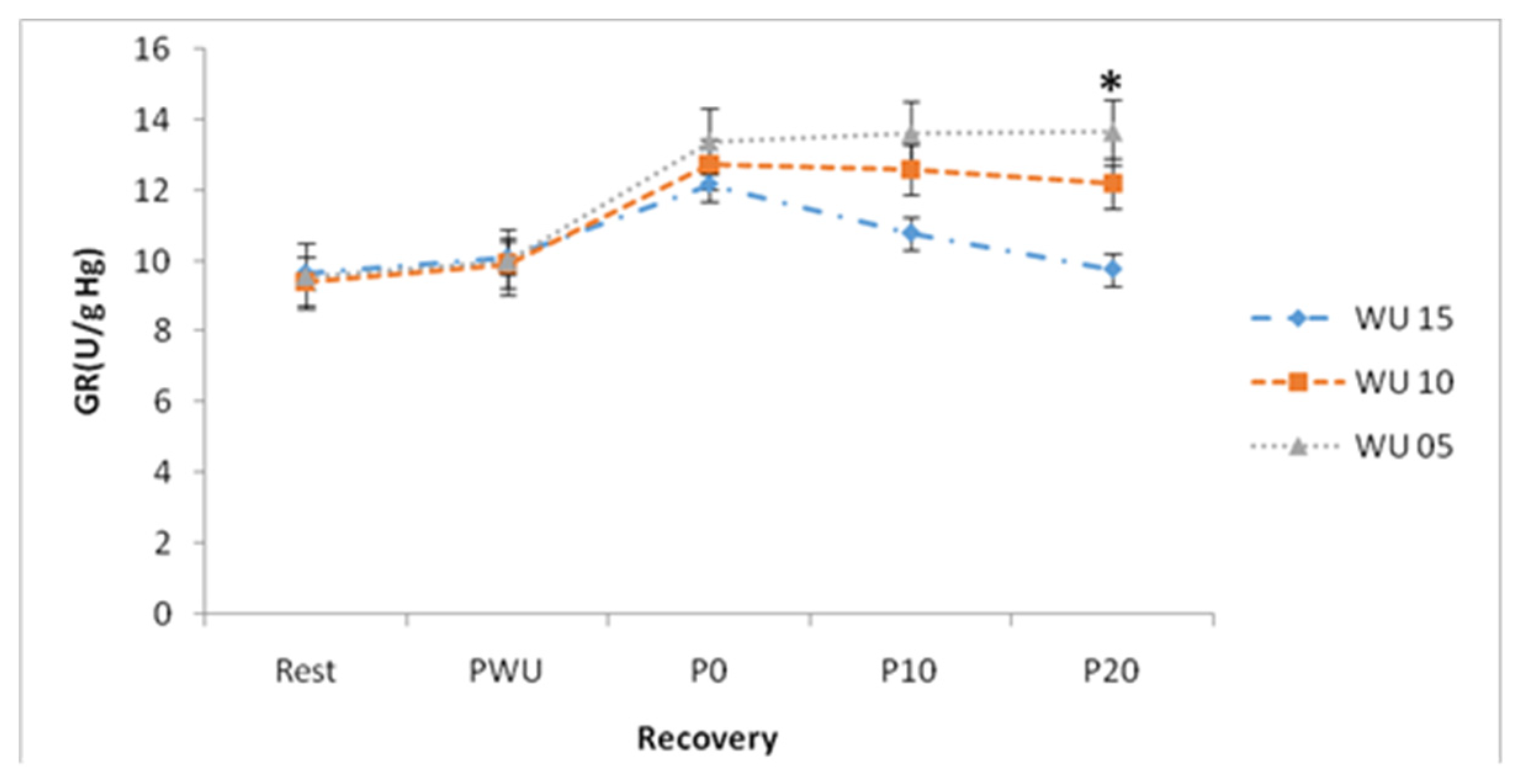
With regard to the concentrations of the GR, the ANOVA showed a significant "Warm up" effect (F (2, 26) = 5.65; p <0.01; η2p = 0.30), a significant "Recovery" effect (F (4, 52) = 13.13; p <0.001; η2p = 0.50) and an insignificant "Warm up × Recovery" interaction (F (8, 104) = 1.88; p >0.05; η2p = 0.13). Statistical analysis showed a significant difference (p <0.05) of GR at P0 compared to PR and PWU for the 5-min warm-up time (
Table 2). Furthermore, a significant difference was recorded in the plasma GR contents after 20-min of stopping the exercise test between the two warm-up times (5-min and 10-min), the values GR of which are respectively 13.67±9.76 (U/g Hg) and 9.76±0.5 (U/g Hg) (
Figure 5).
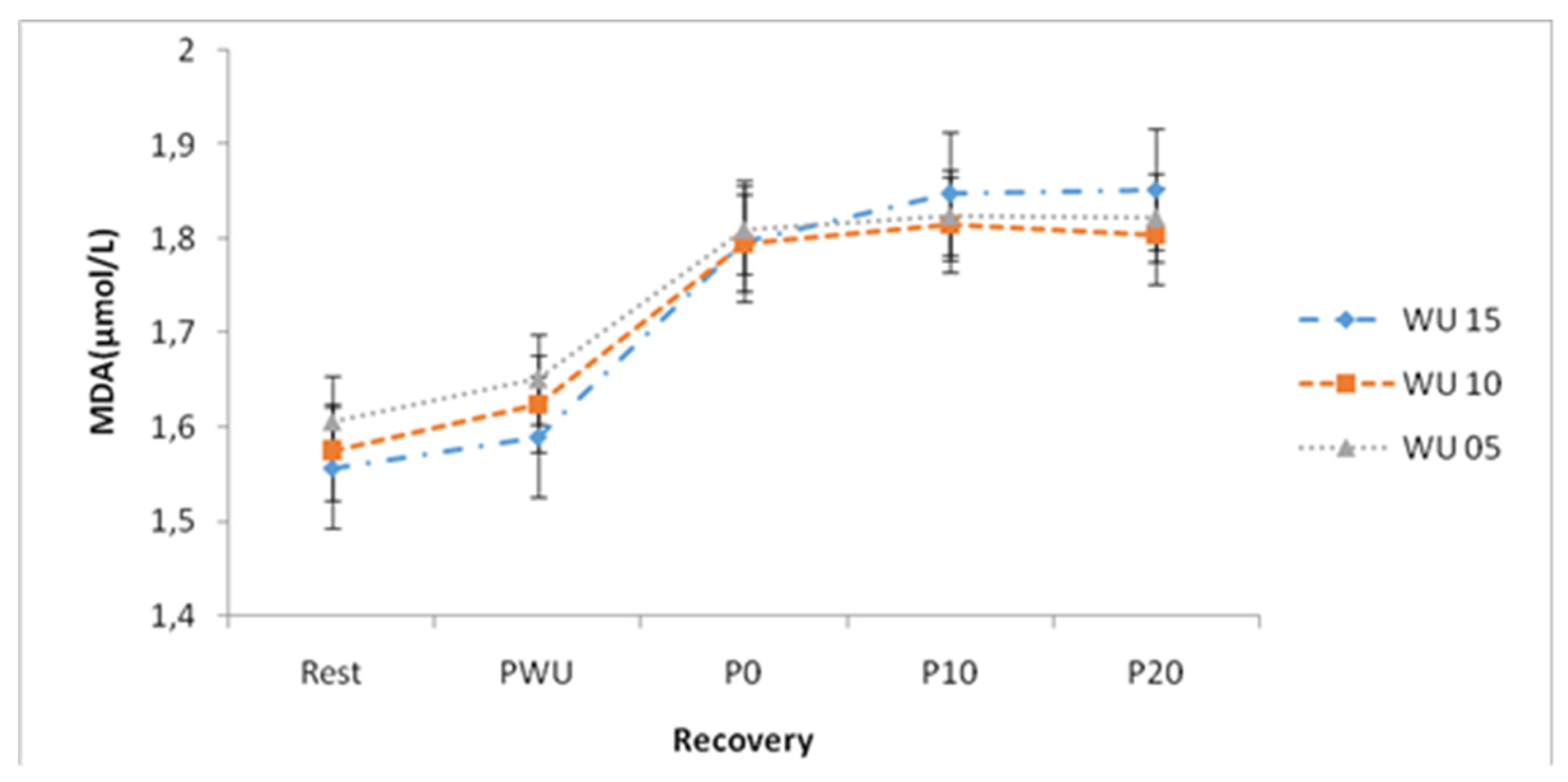
The three warm-up times induced significant increases in MDA concentrations upon stopping exercise (P0) at P10 and P20 up to 20-min of recovery. By comparing MDA concentrations during recovery versus P resting and PWU, ANOVA showed significant differences for the different warm-up times. (
Table 1). The statistical analysis noted that following the three warm-up times, the MDA concentrations increased, from the stop of the Wingate test until 20-min of recovery without significant differences recorded to reach 1.85±0.04 (µmol / L), 1.8±0.05 (µmol / L) and 1.82±0.05 (µmol / L) following 15-min warm-up; 10-min and 5-min respectively. (
Figure 6)
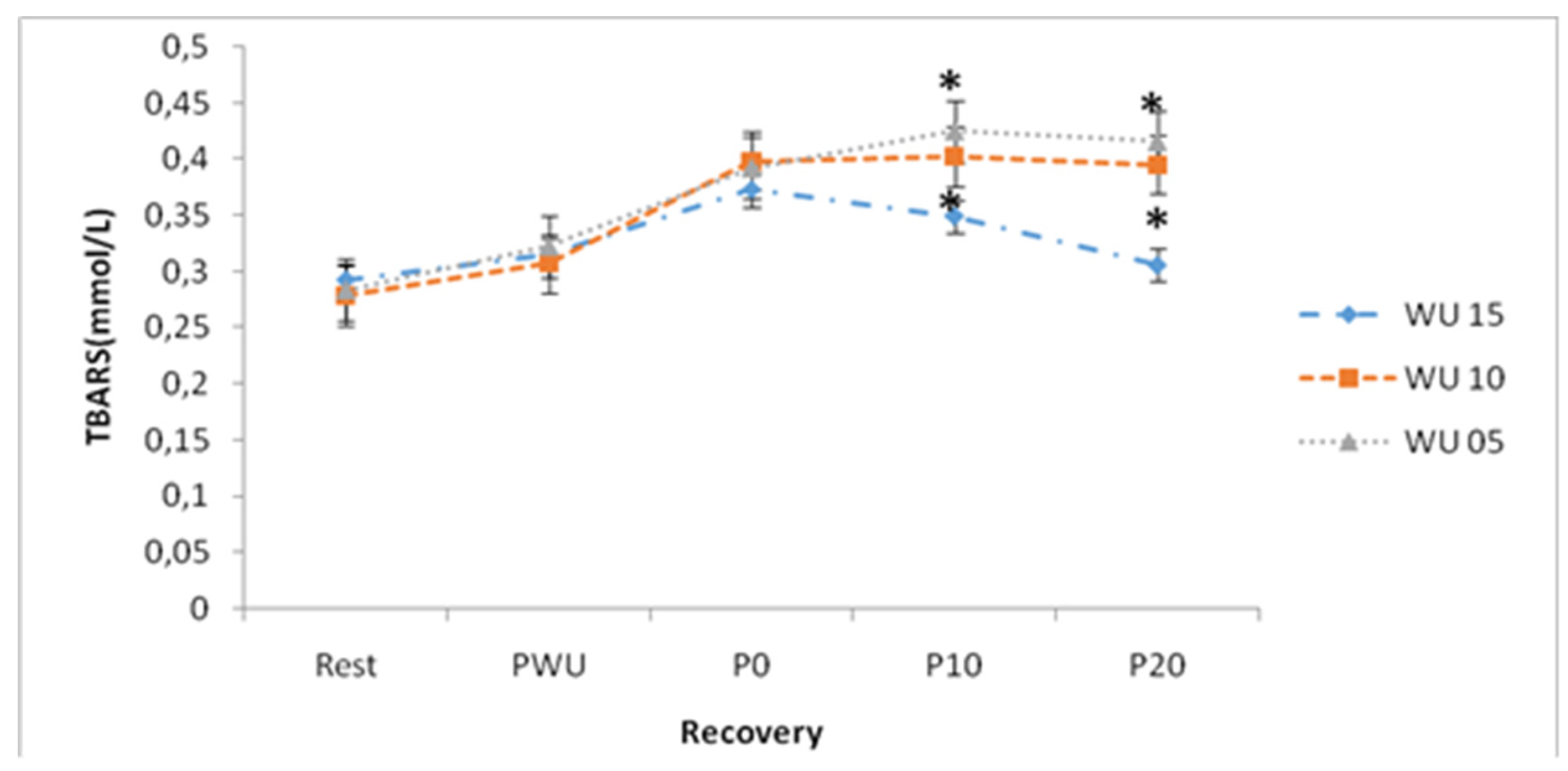
By examining TBARS evolution, ANOVA showed a significant "Warm-up" effect (F (2, 26) = 3.59; p <0.05; η2p = 0.22), a significant "Recovery" effect (F (4, 52) = 161.52; p <0.001; η2p = 0.93) and a significant "Warm-up × Recovery" interaction (F (8, 104) = 12.24; p <0.001; η2p = 0.48) (
Table 2).
Figure 7 shows that after 10-min and 20-min of stopping anaerobic exercise, following a 15-min warm-up, the TBARS concentrations are significantly lower than those recorded after 5 or 10-min warm-ups ( p <0.05).
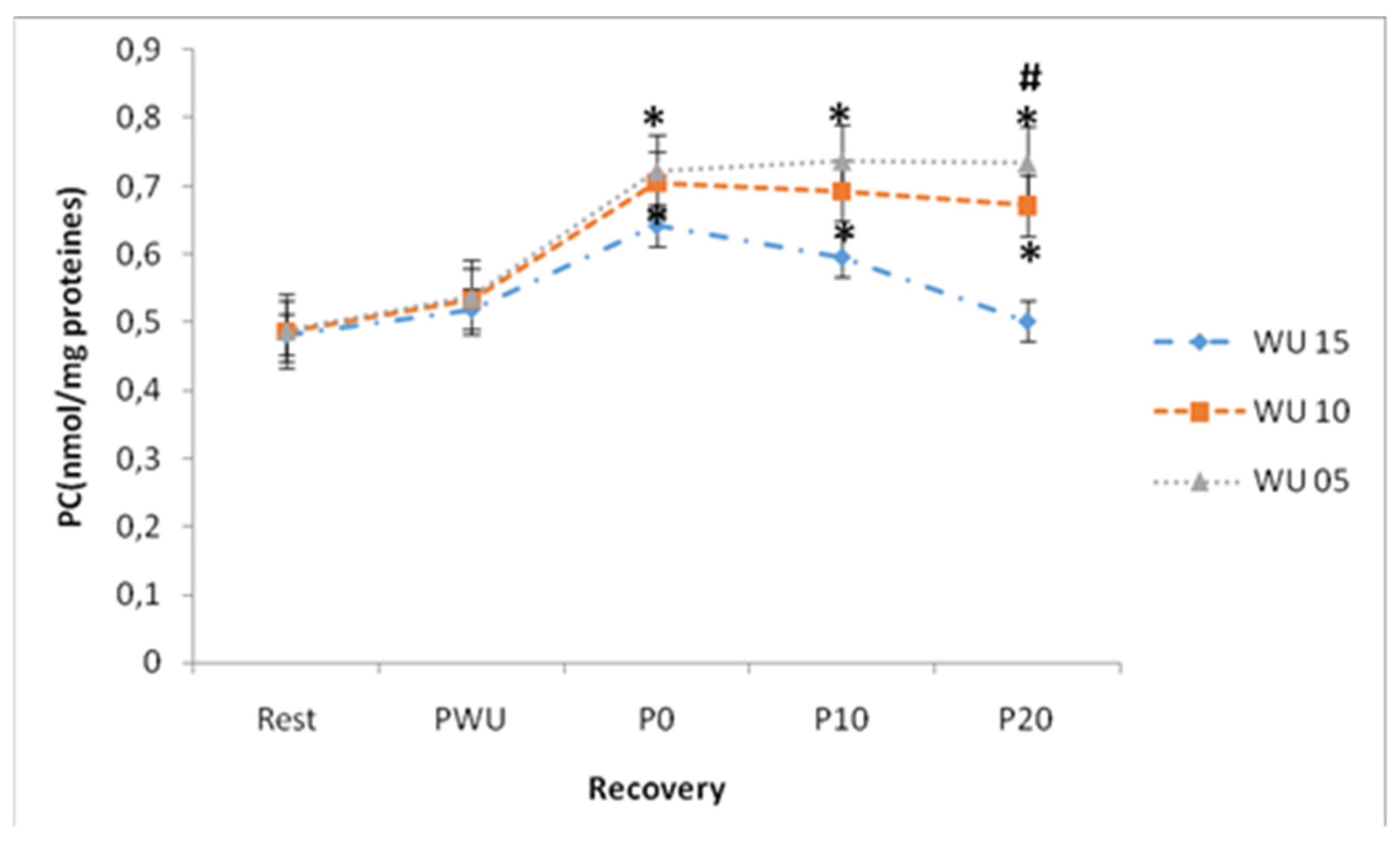
PC concentrations continue to increase until 20-min of recovery, except under the effect of the 15-min warm-up there was a significant drop and a decreasing trend under the effect of the 10-min warm-up, but without statistical significance (Table2). ANOVA showed that the duration of warm-up, recovery, and the "warm-up × recovery" interaction have significant effects on PC concentrations at p <0.001 after the Wingate test. PC concentrations were significantly lower under the effect of the 15-min warm-up. (
Figure 8).
4. Discussion
In addressing this topic, it is evident that there was a lack of studies investigating the effects of warm-up on oxidative stress. besides the role of warming up in minimizing the risk of injury [
22,
25,
26], it also aims to improve physical performance to prepare players for competitions under the best possible conditions [
27]. Muscle damage is one of the main problems met by today's athletes, whether they are amateurs or professionals [
1]. Therefore, it is imperative to use the most effective means to prevent these lesions [
2].
There are a lot of controversies and multiple debates over the effects of warming up. . Therefore, an appropriate warm-up protocol should have an optimal duration without inducing fatigue [
6]. Several studies have highlighted the importance of the duration of the warm-up, [
28,
29]. The duration of the warm-up protocols used for team sports in previous studies varies between 5-min and 40-min [
22,
28,
30,
31,
32,
33]. However, few or no studies have evaluated the effect of warming up on oxidative stress in amateur or professional athletes. In this context, the purpose of this study was to determine the effects of three warm-up durations on the markers of oxidative stress after exercise and on recovery following anaerobic exercise in amateur handball players.
Our results are consistent with the conclusions of several other studies which have demonstrated an increase in the concentration of plasma MDA and SAT and blood concentrations of GPx, SOD and GR immediately following a 30s Wingate test in trained judokas [
34]. An increase in lipid peroxidation (MDA), following sprints, strength exercises, and the Wingate test [
35,
36,
37] and increased plasma concentrations of MDA, SOD, GPX, and GR after the 30s Wingate test in young adults [
38]. The increase in SOD was statistically significant after the two warm-up protocols (5-min and 10 min). On the other hand, a downward trend is observed, during recovery, under the effect of the 15-min warm-up, although the difference is not significant. Nevertheless, our findings are in contrast to those of [
9] in whom a Wingate test causes a decrease in SOD activity without modification of GPX activity. This divergence could be explained, in part, by the diversity of the three protocols carried out, the level of physical aptitude which differs between the subjects as well as the individualized response to exercise which could bring exaggerated reactions of oxidative stress during recovery.
The same finding, concerning blood GR concentrations, was observed experimentally, under the effect of warming up for 10-min and 15-min. However, after 20-min from the end of the exercise, this parameter’s concentrations find their initial values under the effect of the 15-min warm-up with statistically significant differences with those recorded under the effect of 5-min and 10-min warm-up. The present study showed that a Wingate test, preceded by a three-duration warm-up protocol (5-min, 10-min, or 15-min), resulted in an increase in SAT and MDA concentrations up to 20-min recovery. In addition, following the 15-min warm-up protocol and after 20-min of recovery, the decrease in SAT concentrations was statistically significant, although the difference is not significant compared to the other two warm-up times.
The 5-min and 10-min warm-up protocols showed a significant effect on GPx kinetics during recovery. Indeed, its concentrations continue to increase until 20-min of recovery. In contrast, the 15-min warm-up resulted in a partial drop in GPx concentrations from the cessation of anaerobic exercise until 20-min of recovery, although it was not significant relative to P0. The difference in concentrations at P20 after the 15-min warm-up compared to the other two warm-up protocols (5-min and 10-min), was statistically significant (36.97±1.62; 40. 87±3.75 and 41.55±2.55 respectively).
A number of explanations could be presented to explain the wide range of responses. The first is connected to the duration of the warm-up, which varies from protocol to protocol. An inadequate warm-up period appears to impair physical performance and thus have varied impacts on oxidative stress during recovery. The second explanation would be related to the subjects' varying physical abilities. Conditioned athletes will almost certainly require a longer warm-up to attain an appropriate rise in body temperature that results in a significant increase in muscle fiber conduction velocity. Changes in antioxidant concentrations were shown at the end of the three warm-up procedures administered to our individuals. These are characterized by an increase in TBARS and PC from the cessation of anaerobic exercise up to 20-min of recovery, with a substantial difference compared to the 15-min warm-up. The complete drop in concentrations of these two parameters as soon as the activity is finished, to return to their basal value after 20-min of recovery, on the other hand, would be linked to the warm-up duration of 15-min.
It is well accepted that there is a relationship between muscle damage and increased free radicals, which leads to oxidative stress [
39]. The study by Maughan et al. [
40], which discovered a delayed rise in lipid peroxidation produced in plasma during eccentric running exercise, has been proposed as evidence for the involvement of ROS in the muscle injury process. Furthermore, a relationship has been shown between muscle damage and the extent of neutrophil immigration, which can result in the generation of excess ROS during inflammatory processes [
41]. In our study, the results obtained after the 15-min warm-up show the contribution of free radicals, as evidenced by the tendency of antioxidant concentrations to return to their baseline values. This can be explained by the beneficial effects of warm-up, which allows for an increase in temperature, which improves blood flow to active tissues and facilitates oxygen dissociation from hemoglobin [
6], an increase in the rate of nerve transition [
42,
43], and an increase in the speed and strength of muscle contraction. However, the current study’s relatively small sample size may be considered as a limitation. This may have reduced our capacity to detect group differences in our biomarkers of choice. This is a limitation of this investigation that should be evaluated in light of our findings.
5. Conclusion
The two warm-up regimens (5-min and 10-min) were not related to positive modifications in antioxidant capacity during recovery in all of our individuals, according to our findings. A 15-min warm-up regimen, on the other hand, may favorably regulate and lessen the negative effects of oxidative stress, potentially reducing muscle damage after anaerobic activity. This 15-min warm-up program appears to boost antioxidant levels and thus lessen oxidative stress during recovery. The enhancement of a warm-up program (duration and intensity) combined with stretching immediately after the effort is stopped may have greater consequences on the various parameters assessed, in order to remedy the known adverse effects of oxidative stress on muscle damage after anaerobic exercises.
Author Contributions
Conceptualization, AK and ME; methodology, SK; writing-original draft preparation, AK and ME; writing-review and editing, AK, SK, and ME. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The authors wish to express their sincere gratitude to all the participants who took part in this study for their maximal effort and cooperation. The author M. Elloumi would like to thank Prince Sultan University for the support and for paying the APC.
Conflicts of Interest
The authors have no conflicts of interest that are directly relevant to the content of this article.
References
- Garrett, W.E. Muscle strain injuries: Clinical and basics aspects. Med. Sci. Sports. Exerc. 1990, 22, 436–443. [Google Scholar] [CrossRef]
- Kirkendall, D.T.; Garrett, W.E. Clinical perspectives regarding eccentric muscle injury. Clin. Orthop. Relat. Res. 2002, 403, S81–S89. [Google Scholar] [CrossRef]
- Faigenbaum, A.D.; McFarland, J.E.; Kelly, N.A.; Ratamess, N.A.; Kang, J.; Hoffman, J.R. Influence of recovery time on warm-up effects in male adolescent athletes. Pediatr. Exerc. Sci. 2010, 22, 266–277. [Google Scholar] [CrossRef]
- Jones, B.H.; Cowan, D.N.; Tomlinson, J.P.; Robinson, J.R.; Polly, D.W.; Frykman, P.N. Epidemiology of injuries associated with physical training among young men in the army. Med Sci Sports Exerc. 1993, 25, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Herbert, R.D.; Gabriel, M. Effects of stretching before and after exercising on muscle soreness and risk of injury: systematic review. BMJ. 2002, 325, 468. [Google Scholar] [CrossRef]
- Woods, K.; Bishop, P.; Jones, E. Warm Up and stretching in the prevention of muscular injury. Sports Med. 2007, 37, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.J. Free radical mechanisms in exercise related muscle damage in oxidative stress in skeletal muscle; Birkhaeuser Verlag: Basel/Switzerland, 1998; pp. 75–86. [Google Scholar]
- Bloomer, R.J.; Goldfarb, A.H. Anaerobic exercise and oxidative stress: A Review. Can J Appl Physiol. 2004, 29, 245–63. [Google Scholar] [CrossRef] [PubMed]
- Groussard, C.; Rannou-Bekono, F.; Machefer, G.; Chevanne, M.; Vincent, S.; Sergeant, O.; Cillard, J.; Gratas-Delamarche, A. Changes in blood lipid peroxidation markers and antioxidants after a single sprint anaerobic exercise. Eur J Appl Physiol. 2003, 89, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Finaud, J.; Lac, G.; Filaire, G. Oxidative stress. Sports Med. 2006, 36, 327–358. [Google Scholar] [CrossRef]
- El-abed, K.; Trabelsi, K. Kinetics of oxidative stress markers at rest and in response to aerobic and acute exercise in judokas men. Int J of Science and Research. 2014, 3, 756–761. [Google Scholar]
- Bessa, A.L.; Oliveira, V.N.; Agostini, G.G.; Oliveira, R.J.S.; Oliveira, A.C.S.; White, G.E.; Wells, G.D.; Teixeira, D.N.; Espindola, F.S. Exercise intensity and recovery: Biomarkers of injury, inflammation, and oxidative stress. J Strength Cond Res. 2016, 30, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Souissi, W.; Bouzid, M.A.; Farjallah, M.A.; Ben Mahmoud, L.; Boudaya, M.; Engel, F.A.; Sahnoun, Z. Effect of different running exercise modalities on post-exercise oxidative stress markers in trained athletes. Int J Environ Res Public Health. 2020, 17, 3729. [Google Scholar] [CrossRef] [PubMed]
- El-abed, K.; Megdich, M.; Trabelsi, K.; Masmoudi, L.; Hakim, A. Evaluation des effets à court terme de deux techniques d’étirements actifs réalisés lors de l’échauffement, sur Les antioxydants enzymatiques suite à un effort anaérobie: Etude pilote. Science & Sports. 2018, 33, 237–244. [Google Scholar]
- Billat, V. Physiologie et Méthodologie de l’Entraînement: de la Théorie à la Pratique. De Boeck Supérieur.
- Hadala, M.; Barrios, C. Different Strategies for Sports Injury Prevention in an America’s Cup Yachting Crew. Med Sci Sports Exerc. 2009, 41, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Weerapong, P.; Hume, P.A.; Kolt, G.S. Stretching: Mechanisms and benefits for sport performance and injury prevention. Physical Therapy Reviews. 2004, 9, 189–206. [Google Scholar] [CrossRef]
- Fradkin, A.J.; Gabbe, B.J.; Cameron, P.A. Does warming up prevent injury in sport? The evidence from randomised controlled trials. J Sci Med Sport. 2006, 9, 214–220. [Google Scholar] [CrossRef]
- Caliskan, E.; Akkoc, O.; Bayramoglu, Z.; Gozubuyuk, O.B.; Kural, D.; Azamat, S.; Adaletli, I. Effects of static stretching duration on muscle stiffness and blood flow in the rectus femoris in adolescents. Med Ultrason. 2019, 21, 136–143. [Google Scholar] [CrossRef]
- Proske, U.; Morgan, D.L. Do cross-bridges contribute to the tension during stretch of passive muscle? J Muscle Res Cell M. 1999, 20, 433–442. [Google Scholar] [CrossRef]
- Maffiuletti, NA.; Jubeau, M.; Munzinger, U.; Bizzini, M.; Agosti, F.; De Col, A.; Lafortuna, CL.; Sartorio, A. Differences in quadriceps muscle strength and fatigue between lean and obese subjects. Eur J Appl Physiol. 2007, 101, 51–9. [Google Scholar] [CrossRef]
- Steib, S.; Zahn, P.; Zu Eulenburg, C.; Pferfer, K.; Zech, A. Time-dependent postural control adaptations following a neuromuscular warm-up in female handball players: a randomized controlled trial. BMC Sports Sci Med Rehabil. 2016, 8, 33. [Google Scholar] [CrossRef]
- Levine, RL.; Garland, D.; Oliver, CN.; Amici, A.; Climent, I.; Lenz, AG.; et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [PubMed]
- Kunio, Y. Lipid peroxides in Biology and Medicine; United Kingdom Edition. Academic Press INC.: London, UK, 1982. [Google Scholar]
- Soligard, T.; Nilstad, A.; Steffen, K.; Myklebust, G.; Home, I.; Dvorak, J.; Bahr, R. , Andersen, TE. Compliance with a comprehensive warm-up programme to prevent injuries in youth football. Br J Sports Med. 2010, 44, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Thompsen, AG.; Kackley, T.; Palumbo, MA.; Faigenbaum, AD. Acute effects of different warm-up protocols with and without a weighted vest on jumping performance in athletic women. J Strength Cond Res. 2007, 21, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Romaratezabala, E.; Nakamura, FY.; Castillo, D.; Gorostegi-Anduga, I.; Yanci, J. Influence of Warm-Up duration on physical performance and psychological perceptions in handball players. Res Sports Med. 2018, 26, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Pardeiro, M.; Yanci, J. Efectos del calentamiento en el rendimiento físico y en la percepción psicológica en jugadores semi profesionales de fútbol. RICYDE. Revista Internacional De Ciencias Del Deporte. 2017, 48, 104–116. [Google Scholar] [CrossRef]
- Van den Tillaar, R.; Von Heimburg, E. Comparison of two types of warm-up upon repeated-sprint performance in experienced soccer players. J Strength Cond Res. 2016, 30, 2258–2265. [Google Scholar] [CrossRef]
- Dumitru, DC. The importance of a specific warm-up on the performance of the handball goalkeeper. Journal of Physical Education and Sport/Citius Altius Fortius. 2010, 28, 23–31. [Google Scholar]
- Manchado, C.; García-Ruiz, J.; Cortell-Tormo, JM.; Tortosa-Martínez, J. Effect of core training on male handball players’ throwing velocity. J Hum Kinet. 2017, 56, 177–185. [Google Scholar] [CrossRef]
- Zois, J.; Bishop, D.; Aughey, R. High-intensity warm-ups: Effects during subsequent intermittent exercise. Int J Sports Physiol Perform. 2015, 10, 498–503. [Google Scholar] [CrossRef]
- Mascarin, NC.; Vancini, RL.; Lira, CA.; Andrade, MS. Stretch-induced reductions in throwing performance are attenuated by warm-up before exercise. J Strength Cond Res. 2015, 29, 1393–1398. [Google Scholar] [CrossRef]
- El-abed, K.; Ammar, A.; Boukhris, D.; Trabelsi, K.; Masmoudi, L.; Bailey, S.; Hakim, A.; Bragazzi, NL. Independent and combined effects of all-out sprint and low-intensity continuous exercise on plasma oxidative stress biomarkers in trained judokas. Front Physiol. 2019, 10, 842. [Google Scholar] [CrossRef] [PubMed]
- Antoncic-Svetina, M.; Sentija, D.; Cipak, A.; Millicic, D.; Meinitzer, A.; Tatzber, F.; Andrisic, L.; Zelzer, S.; Zarkovic, N. Ergometry induces systemic oxidative stress in healthy human subjects. Tohoku J Exp Med. 2010, 221, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, O.; Chtourou, H.; Chahed, H.; Ferchichi, S.; Chaouachi, A.; Kallel, C.; Miled, A.; Chamari, K.; Souissi, N. High intensity exercise affects diurnal variation of some biological markers in trained subjects. Int J Sports Med. 2012, 33, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, JR.; Im, J.; Kang, J.; Maresh, CM.; Kraemer, WJ.; French, D.; Nioka, S.; Kime, R.; Rundell, KW.; Ratamess, NA.; Faigenbaum, AD.; Chance, B. Comparison of low- and high-intensity resistance exercise on lipid peroxidation: Role of muscle oxygenation. J Strength Cond Res. 2007, 21, 118–122. [Google Scholar] [CrossRef]
- Ammar, A.; Trabelsi, K.; Boukhris, O.; Glen, JM.; Bott, N.; Masmoudi, L.; Hakim, A.; Chtourou, H.; Driss, T.; Hoekelmann, A.; El Abed, K. Effects of aerobic, anaerobic and combined-based exercises on plasma oxidative stress biomarkers in healthy untrained young adults. Int J Environ Res Public Health. 2020, 17, 2601. [Google Scholar] [CrossRef]
- Ammar, A.; Chtourou, H.; Hammouda, O.; Trabelsi, K.; Chiboub, J.; Turki, M.; AbdelKarim, O.; El Abed, K.; Ben Ali, M.; Hoekelmann, A.; Souissi, N. Acute and delayed responses of C-reactive protein, malondialdehyde and antioxidant markers after resistance training session in elite weightlifters: Effect of time of day. Chronobiol Int. 2015, 32, 1–12. [Google Scholar] [CrossRef]
- Maughan, RJ.; Donnelly, AE.; Gleeson, M.; Whiting, PH.; Walker, KA.; Clough, PJ. Delayed-onset muscle damage and lipid peroxidation in man after a downhill run. Muscle Nerve. 2005, 12, 332–336. [Google Scholar] [CrossRef]
- Sakurai, T.; Hollander, J.; Brickson, SL.; Ohno, H.; Ji, L.; Izawa, T.; Best, TM. Best: Changes in nitric oxide and inducible nitric oxide synthase following stretch-induced injury to the tibialis anterior muscle of rabbit. Jpn J Physiol. 2005, 55, 101–107. [Google Scholar] [CrossRef]
- Tillin, M.N.A.; Bishop, D. Factors modulating post-activation potentiation and its effect on performance of subsequent explosive activities. Sports Med. 2009, 39, 147–166. [Google Scholar] [CrossRef]
- Rassier, D.; Macintosh, B. Coexistence of potentiation and fatigue in skeletal muscle. Braz J Med Biol Res. 2000, 33, 499–508. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).