Submitted:
29 July 2023
Posted:
31 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Methods
2.2.1. Fourier-transform infrared (FTIR) spectroscopy
2.2.2. Ellipsometric Porosimetry (EP)
2.2.3. X-ray Photoelectron Spectroscopy (XPS)
2.2.4. Photoluminescence Spectroscopy (PL)
3. Results
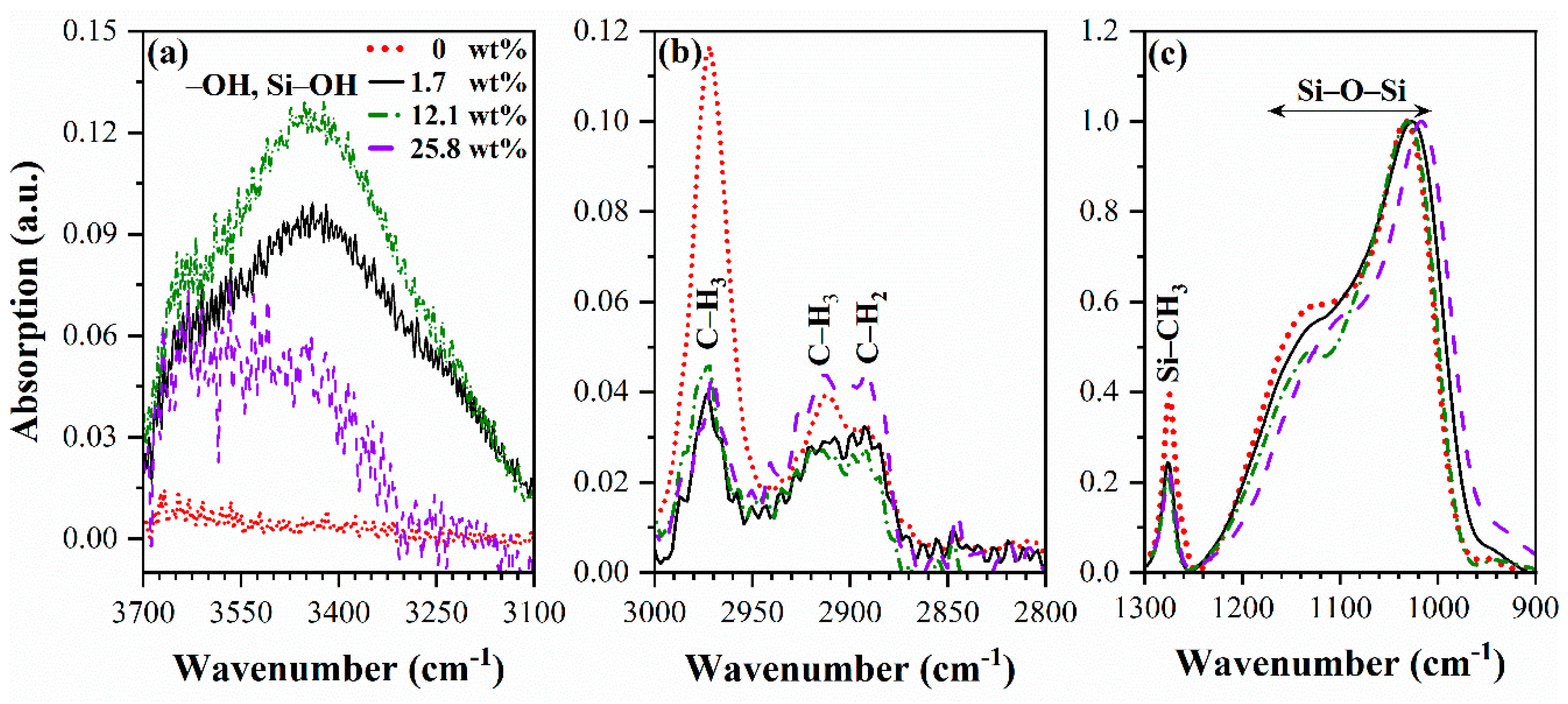
3.1. Chemical composition (FTIR data)
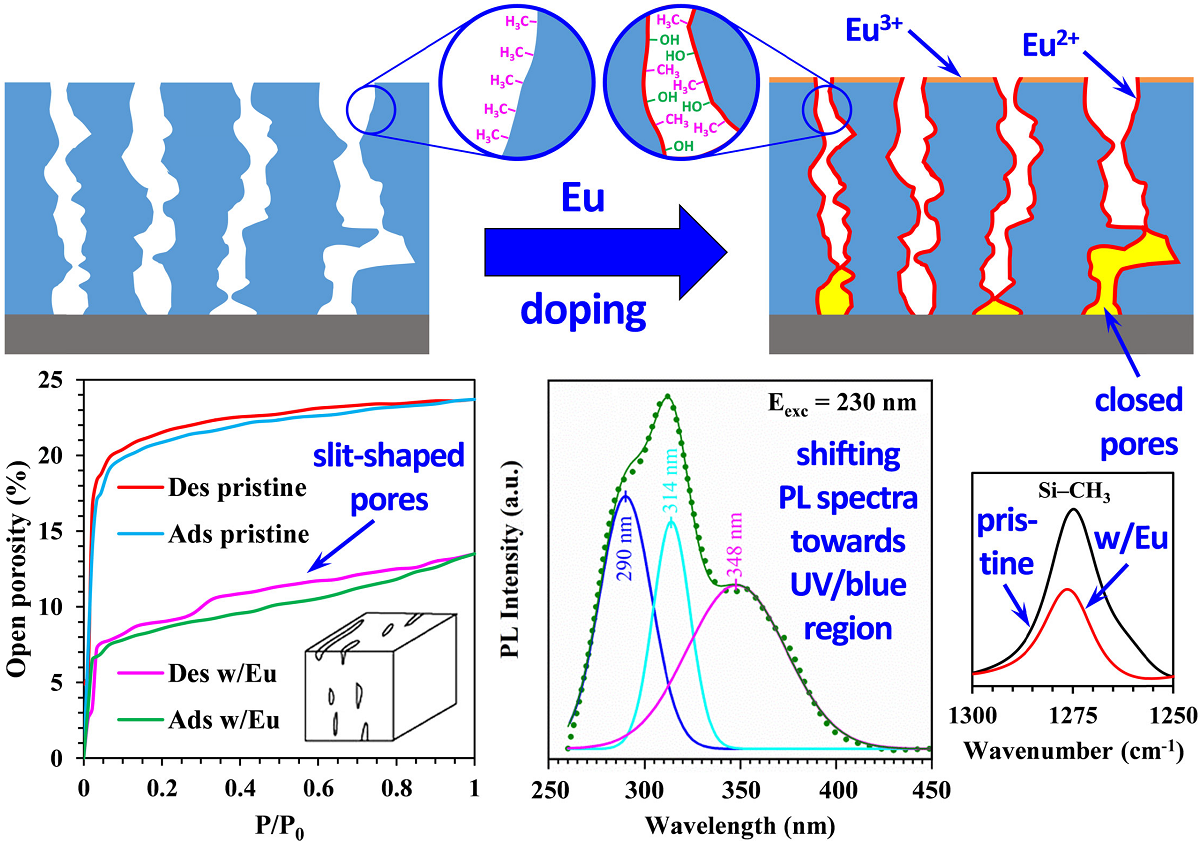
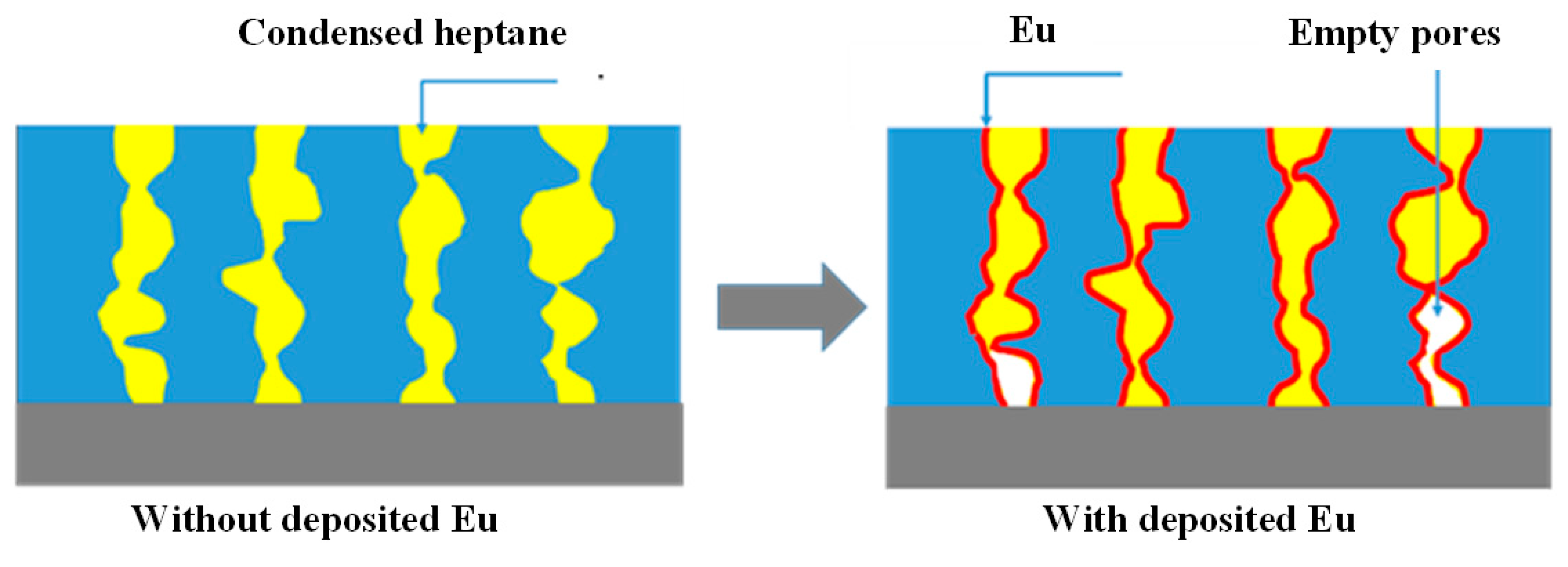
3.2. Porosity and pore size distribution
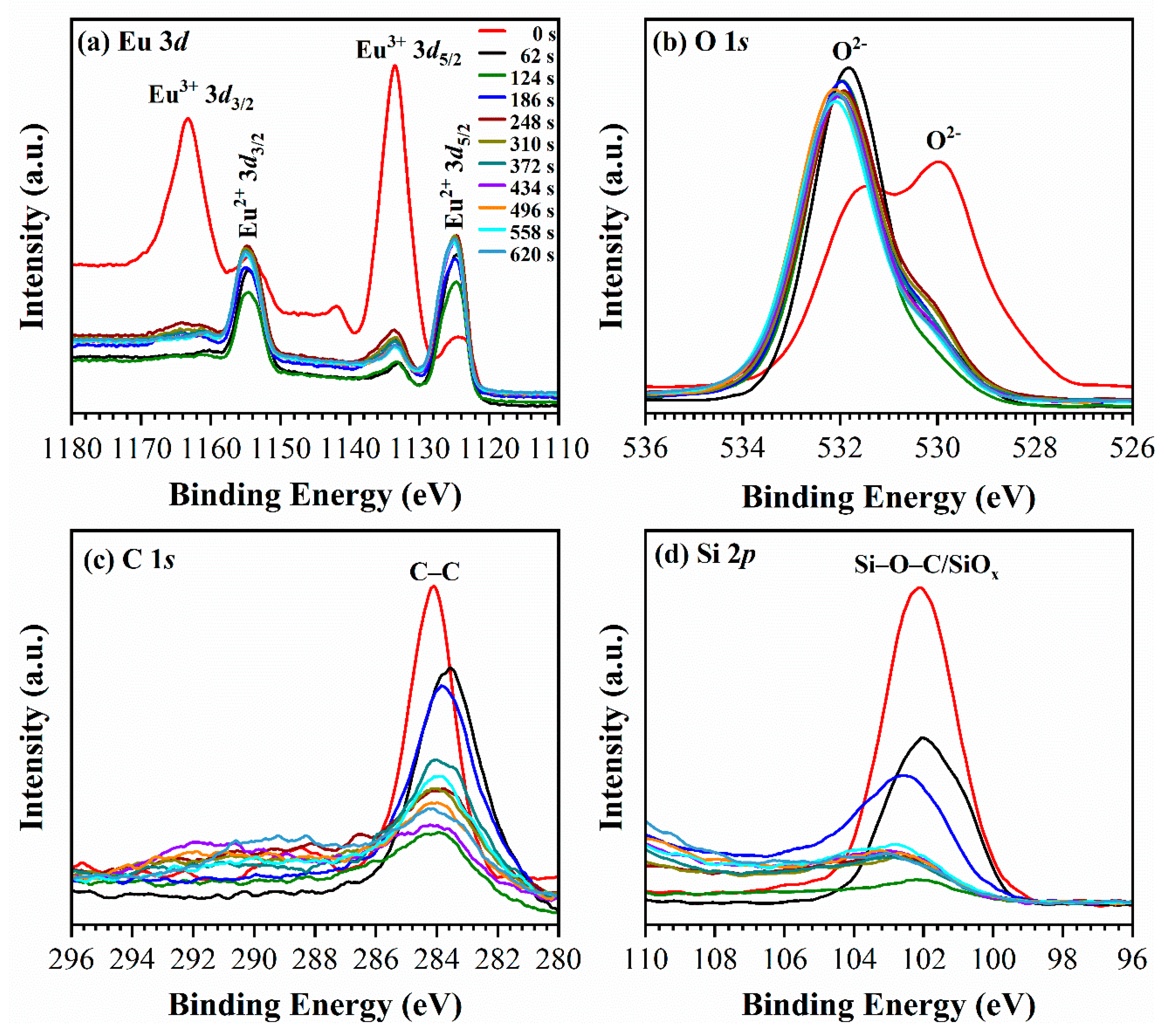
3.3. X-ray Photoelectron Spectroscopy Analysis (XPS)
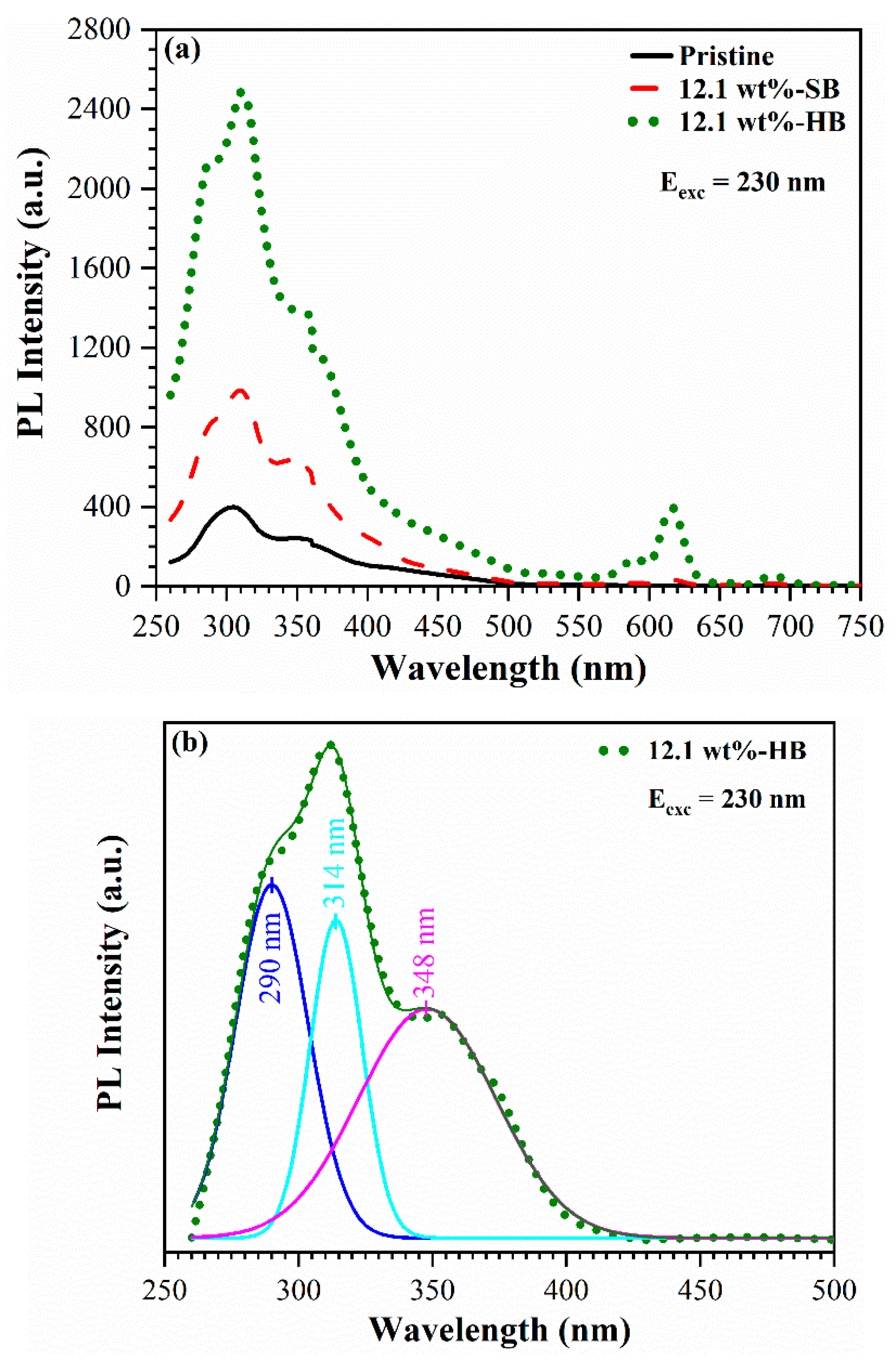
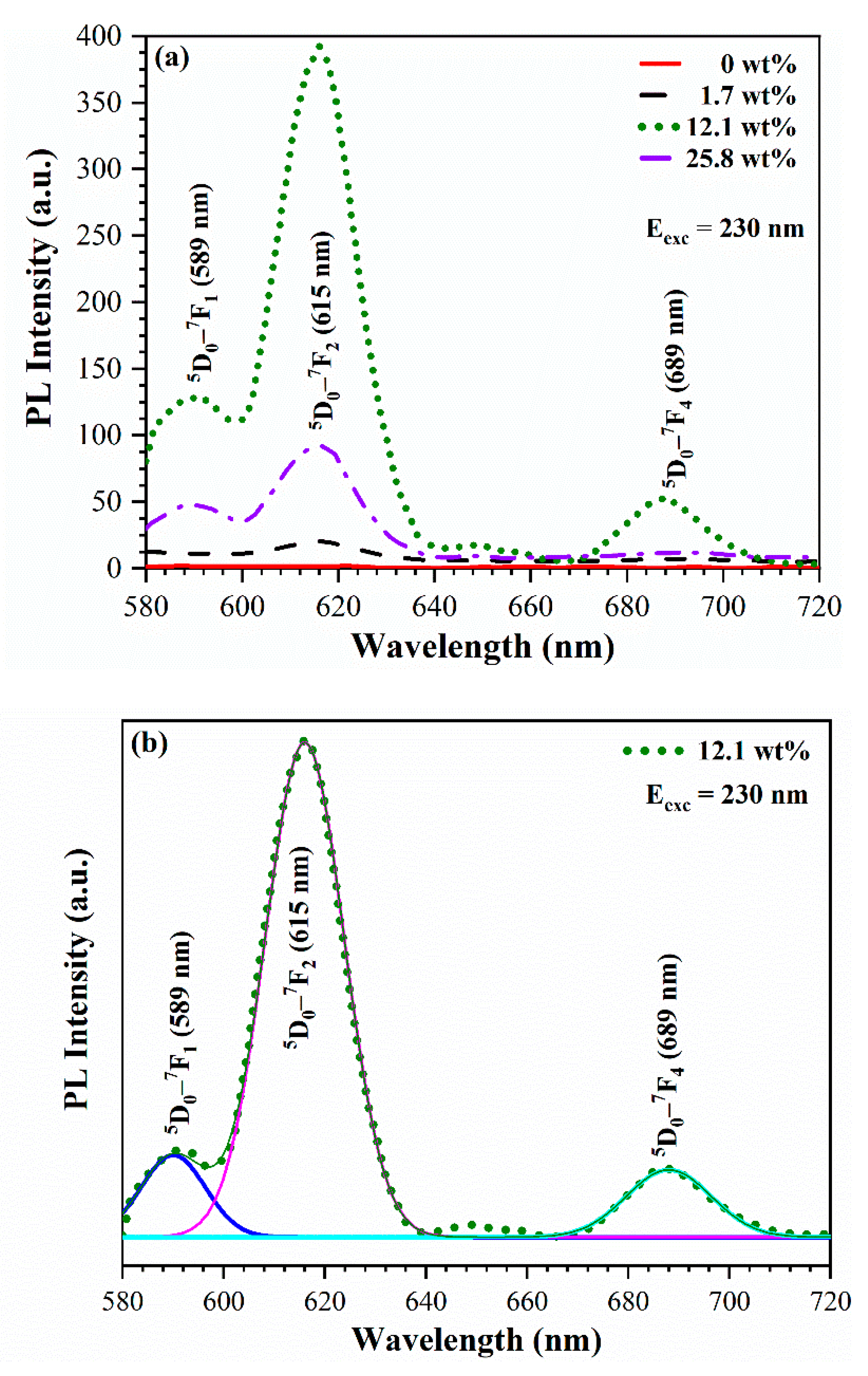
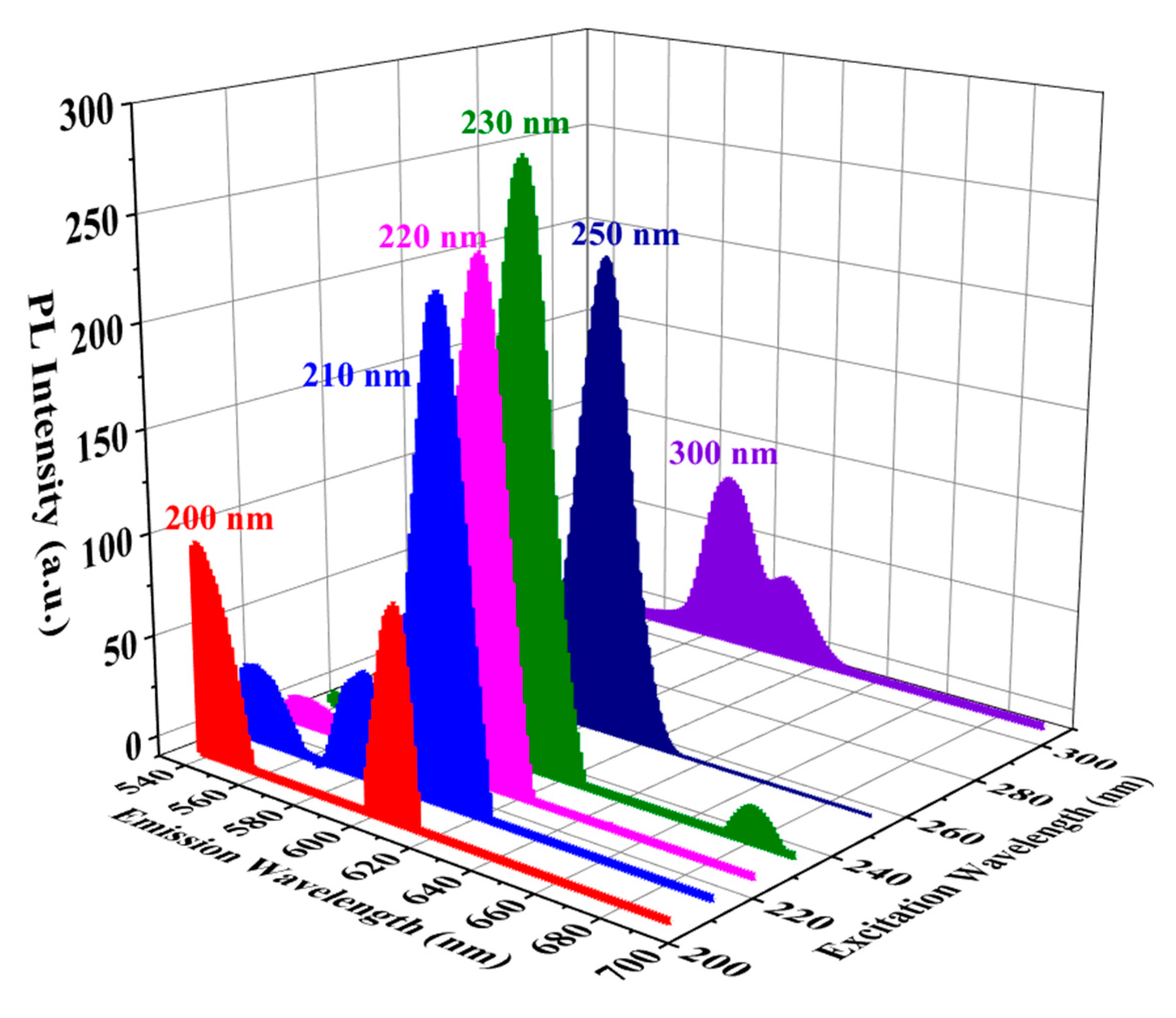
3.4. UV-induced luminescence
3.4.1. Effect of oxygen plasma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Z.; Yin, B.; Michel, J. On-chip light sources for silicon photonics. Light Sci. Appl. 2015, 4, e358–e358. [Google Scholar] [CrossRef]
- Boninelli, S.; Bellocchi, G.; Franzò, G.; Miritello, M.; Iacona, F. New strategies to improve the luminescence efficiency of Eu ions embedded in Si-based matrices. J. Appl. Phys. 2013, 113, 143503. [Google Scholar] [CrossRef]
- Bellocchi, G.; Fabbri, F.; Miritello, M.; Iacona, F.; Franzò, G. Multicolor Depth-Resolved Cathodoluminescence from Eu-Doped SiOC Thin Films. ACS Appl. Mater. Interfaces 2015, 7, 18201–18205. [Google Scholar] [CrossRef]
- Gallis, S.; Nikas, V.; Kaloyeros, A.E. Silicon Oxycarbide Thin films and Nanostructures: Synthesis, Properties and Applications. In Modern Technologies for Creating the Thin-film Systems and Coatings; InTech, 2017.
- Prucnal, S.; Sun, J.M.; Skorupa, W.; Helm, M. Switchable two-color electroluminescence based on a Si metal-oxide-semiconductor structure doped with Eu. Appl. Phys. Lett. 2007, 90. [Google Scholar] [CrossRef]
- Nazarov, A.N.; Tiagulskyi, S.I.; Tyagulskyy, I.P.; Lysenko, V.S.; Rebohle, L.; Lehmann, J.; Prucnal, S.; Voelskow, M.; Skorupa, W. The effect of rare-earth clustering on charge trapping and electroluminescence in rare-earth implanted metal-oxide-semiconductor light-emitting devices. J. Appl. Phys. 2010, 107. [Google Scholar] [CrossRef]
- Rebohle, L.; Lehmann, J.; Prucnal, S.; Kanjilal, A.; Nazarov, A.; Tyagulskii, I.; Skorupa, W.; Helm, M. Blue and red electroluminescence of Europium-implanted metal-oxide-semiconductor structures as a probe for the dynamics of microstructure. Appl. Phys. Lett. 2008, 93. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.; Jin, L.; Yang, D. Structure and luminescence evolution of annealed Europium-doped silicon oxides films. Opt. Express 2010, 18, 27191. [Google Scholar] [CrossRef]
- Bellocchi, G.; Franzò, G.; Boninelli, S.; Miritello, M.; Cesca, T.; Iacona, F.; Priolo, F. Structural and luminescence properties of undoped and Eu-doped SiOC thin films. IOP Conf. Ser. Mater. Sci. Eng. 2014, 56, 012009. [Google Scholar] [CrossRef]
- Blasse, G.; Grabmaier, B.C. Luminescent Materials; Springer Berlin Heidelberg: Berlin, Heidelberg, 1994; ISBN 978-3-540-58019-5. [Google Scholar]
- Gaft, M.; Reisfeld, R.; Panczer, G. Interpretation of Luminescence Centers. In; 2015; pp. 221–420.
- Kumar, G. B., Raoa, B. V., Babub, B. C., Hungerford, G., Nandyala, S. H. and Santos, J.D. Luminescence and energy transfer phenomena in lanthanide ions doped phosphor and glassy materials. In; Nandyala, S.H., Ed.; Materials Research Forum LLC., 2017; pp. 159–189.
- Stojadinović, S.; Vasilić, R. Eu2+ photoluminescence in Al2O3 coatings obtained by plasma electrolytic oxidation. J. Lumin. 2018, 199, 240–244. [Google Scholar] [CrossRef]
- Baklanov, M.R.; Ho, P.S.; Zschech, E. Advanced Interconnects for ULSI Technology; Baklanov, M.R., Ho, P.S., Zschech, E., Eds.; John Wiley & Sons, Ltd: Chichester, UK, 2012; ISBN 9781119963677. [Google Scholar]
- Grill, A.; Gates, S.M.; Ryan, T.E.; Nguyen, S. V.; Priyadarshini, D. Progress in the development and understanding of advanced low k and ultralow k dielectrics for very large-scale integrated interconnects - State of the art. Appl. Phys. Rev. 2014, 1, 011306. [Google Scholar] [CrossRef]
- Volksen, W.; Miller, R.D.; Dubois, G. Low Dielectric Constant Materials. Chem. Rev. 2010, 110, 56–110. [Google Scholar] [CrossRef]
- Goethals, F.; Ciofi, I.; Madia, O.; Vanstreels, K.; Baklanov, M.R.; Detavernier, C.; Van Der Voort, P.; Van Driessche, I. Ultra-low-k cyclic carbon-bridged PMO films with a high chemical resistance. J. Mater. Chem. 2012, 22, 8281. [Google Scholar] [CrossRef]
- Vishnevskiy, A.S.; Naumov, S.; Seregin, D.S.; Wu, Y.H.; Chuang, W.T.; Rasadujjaman, M.; Zhang, J.; Leu, J.; Vorotilov, K.A.; Baklanov, M.R. Effects of methyl terminal and carbon bridging groups ratio on critical properties of porous organosilicate-glass films. Materials (Basel). 2020, 13, 1–21. [Google Scholar] [CrossRef]
- Li, H.; Knaup, J.M.; Kaxiras, E.; Vlassak, J.J. Stiffening of organosilicate glasses by organic cross-linking. Acta Mater. 2011, 59, 44–52. [Google Scholar] [CrossRef]
- Vanstreels, K.; Wu, C.; Gonzalez, M.; Schneider, D.; Gidley, D.; Verdonck, P.; Baklanov, M.R. Effect of Pore Structure of Nanometer Scale Porous Films on the Measured Elastic Modulus. Langmuir 2013, 29, 12025–12035. [Google Scholar] [CrossRef]
- Kaczmarek, A.M.; Van Der Voort, P. Light-emitting lanthanide periodic mesoporous organosilica (PMO) hybrid materials. Materials (Basel). 2020, 13. [Google Scholar] [CrossRef]
- Lu, Y.; Fan, H.; Doke, N.; Loy, D.A.; Assink, R.A.; LaVan, D.A.; Brinker, C.J. Evaporation-Induced Self-Assembly of Hybrid Bridged Silsesquioxane Film and Particulate Mesophases with Integral Organic Functionality. J. Am. Chem. Soc. 2000, 122, 5258–5261. [Google Scholar] [CrossRef]
- Baklanov, M.R.; Mogilnikov, K.P.; Polovinkin, V.G.; Dultsev, F.N. Determination of pore size distribution in thin films by ellipsometric porosimetry. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. 2000, 18, 1385–1391. [Google Scholar] [CrossRef]
- Baklanov, M. R., Mogilnikov, K. P. Mogilnikov, Vishnevskiy, A.S. Challenges in porosity characterization of thin films: cross-evaluation of different techniques. J. Vac. Sci. Technol. A 2023.
- Grill, A.; Neumayer, D.A. Structure of low dielectric constant to extreme low dielectric constant SiCOH films: Fourier transform infrared spectroscopy characterization. J. Appl. Phys. 2003, 94, 6697–6707. [Google Scholar] [CrossRef]
- Marsik, P.; Verdonck, P.; De Roest, D.; Baklanov, M.R. Porogen residues detection in optical properties of low-k dielectrics cured by ultraviolet radiation. Thin Solid Films 2010, 518, 4266–4272. [Google Scholar] [CrossRef]
- ALOthman, Z. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials (Basel). 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Particle Technology Series; Springer Netherlands: Dordrecht, 2004; Volume 16, ISBN 978-90-481-6633-6. [Google Scholar]
- Esquivel, D.; Kaczmarek, A.M.; Jiménez-Sanchidrián, C.; Van Deun, R.; Romero-Salguero, F.J.; Van Der Voort, P. Eu3+ @PMO: synthesis, characterization and luminescence properties. J. Mater. Chem. C 2015, 3, 2909–2917. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Hu, H.; Wang, J.; Wang, X. Novel photoactive lanthanide hybrids covalently grafted on functionalized periodic mesoporous organosilicons (PMOs) by Schiff-base derivative. J. Porous Mater. 2017, 24, 487–496. [Google Scholar] [CrossRef]
- Sheng, G.; Dong, H.; Shen, R.; Li, Y. Microscopic insights into the temperature-dependent adsorption of Eu(III) onto titanate nanotubes studied by FTIR, XPS, XAFS and batch technique. Chem. Eng. J. 2013, 217, 486–494. [Google Scholar] [CrossRef]
- Bilewska, K.; Wolna, E.; Edely, M.; Ruello, P.; Szade, J. EuNiO3 thin films- growth and characterization. J. Phys. Conf. Ser. 2011, 289, 012014. [Google Scholar] [CrossRef]
- Guo, X.; Jakes, J.E.; Banna, S.; Nishi, Y.; Shohet, J.L. Effects of plasma and vacuum-ultraviolet exposure on the mechanical properties of low-k porous organosilicate glass. J. Appl. Phys. 2014, 116. [Google Scholar] [CrossRef]
- Rasadujjaman, M.; Zhang, J.; Spassky, D.A.; Naumov, S.; Vishnevskiy, A.S.; Vorotilov, K.A.; Yan, J.; Zhang, J.; Baklanov, M.R. UV-Excited Luminescence in Porous Organosilica Films with Various Organic Components. Nanomaterials 2023, 13, 1419. [Google Scholar] [CrossRef]
- Wan, N.; Xu, J.; Lin, T.; Zhang, X.; Xu, L. Energy transfer and enhanced luminescence in metal oxide nanoparticle and rare earth codoped silica. Appl. Phys. Lett. 2008, 92. [Google Scholar] [CrossRef]
- Blasse, G. , Dirksen, G. J., Van Vliet, J.P.M. The luminescence of Europium nitrate Hexahydrate, Eu(NO3)3ˑ6H2O. Inorganica Chim. Acta 1988, 142, 165–168. [Google Scholar] [CrossRef]
- Lu, Q.; Wang, Z.; Wang, P.; Li, J. Structure and Luminescence Properties of Eu3+-Doped Cubic Mesoporous Silica Thin Films. Nanoscale Res. Lett. 2010, 5, 761–768. [Google Scholar] [CrossRef]
- Liu, W.; Kaczmarek, A.M.; Van Der Voort, P.; Van Deun, R. Chemical sensors based on periodic mesoporous organosilica @NaYF 4 :Ln3+ nanocomposites. Dalt. Trans. 2022, 51, 11467–11475. [Google Scholar] [CrossRef] [PubMed]
- Mulder, J.T.; Meijer, M.S.; van Blaaderen, J.J.; du Fossé, I.; Jenkinson, K.; Bals, S.; Manna, L.; Houtepen, A.J. Understanding and Preventing Photoluminescence Quenching to Achieve Unity Photoluminescence Quantum Yield in Yb:YLF Nanocrystals. ACS Appl. Mater. Interfaces 2023, 15, 3274–3286. [Google Scholar] [CrossRef] [PubMed]
- Fneich, H.; Gaumer, N.; Chaussedent, S.; Blanc, W.; Mehdi, A. Europium-Doped Sol-Gel SiO2-Based Glasses: Effect of the Europium Source and Content, Magnesium Addition and Thermal Treatment on Their Photoluminescence Properties. Molecules 2018, 23, 1768. [Google Scholar] [CrossRef] [PubMed]
- Sipina, E. V.; Spassky, D.A.; Krutyak, N.R.; Morozov, V.A.; Zhukovskaya, E.S.; Belik, A.A.; Manylov, M.S.; Lazoryak, B.I.; Deyneko, D. V. Abnormal Eu3+ → Eu2+ Reduction in Ca9−xMnxEu(PO4)7 Phosphors: Structure and Luminescent Properties. Materials (Basel). 2023, 16, 1383. [Google Scholar] [CrossRef]
- Maex, K.; Baklanov, M.R.; Shamiryan, D.; Lacopi, F.; Brongersma, S.H.; Yanovitskaya, Z.S. Low dielectric constant materials for microelectronics. J. Appl. Phys. 2003, 93, 8793–8841. [Google Scholar] [CrossRef]
- Ishizaka, T.; Nozaki, R.; Kurokawa, Y. Luminescence properties of Tb3+ and Eu3+-doped alumina films prepared by sol-gel method under various conditions and sensitized luminescence. J. Phys. Chem. Solids 2002, 63, 613–617. [Google Scholar] [CrossRef]
- Yu, M.; Lin, J.; Fu, J.; Zhang, H.J.; Han, Y.C. Sol–gel synthesis and photoluminescent properties of LaPO 4 :A (A = Eu3+, Ce3+, Tb3+ ) nanocrystalline thin films. J. Mater. Chem. 2003, 13, 1413–1419. [Google Scholar] [CrossRef]
- Jia, W.; Liu, H.; Felofilov, S.P.; Meltzer, R.; Jiao, J. Spectroscopic study of Eu3+-doped and Eu3+,Y3+-codoped SiO2 sol–gel glasses. J. Alloys Compd. 2000, 311, 11–15. [Google Scholar] [CrossRef]
- Baklanov, M.R.; De Marneffe, J.F.; Shamiryan, D.; Urbanowicz, A.M.; Shi, H.; Rakhimova, T. V.; Huang, H.; Ho, P.S. Plasma processing of low-k dielectrics. J. Appl. Phys. 2013, 113. [Google Scholar] [CrossRef]
- Braginsky, O. V.; Kovalev, A.S.; Lopaev, D. V.; Malykhin, E.M.; Mankelevich, Y.A.; Rakhimova, T. V.; Rakhimov, A.T.; Vasilieva, A.N.; Zyryanov, S.M.; Baklanov, M.R. The mechanism of low-k SiOCH film modification by oxygen atoms. J. Appl. Phys. 2010, 108. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Li, K.; Lian, H.; Shang, M.; Lin, J. Crystal-Site Engineering Control for the Reduction of Eu 3+ to Eu2+ in CaYAlO4 : Structure Refinement and Tunable Emission Properties. ACS Appl. Mater. Interfaces 2015, 7, 2715–2725. [Google Scholar] [CrossRef] [PubMed]
- Dereń, P.J.; Stefańska, D.; Ptak, M.; Wiśniewski, P. Method to Measure the Degree of Reduction of Eu3+ to Eu2+ : How Anion and Cation Vacancies Influence the Degree of Reduction. J. Phys. Chem. C 2021, 125, 24505–24514. [Google Scholar] [CrossRef]
- Zaitoun, M.A.; Kim, T.; Lin, C.T. Observation of Electron−Hole Carrier Emission in the Eu3+-Doped Silica Xerogel. J. Phys. Chem. B 1998, 102, 1122–1125. [Google Scholar] [CrossRef]
- Zaitoun, M.A.; Goken, D.M.; Bailey, L.S.; Kim, T.; Lin, C.T. Thermoanalysis and Emission Properties of Eu3+ /Eu2+ in Eu3+ -Doped Xerogels. J. Phys. Chem. B 2000, 104, 189–196. [Google Scholar] [CrossRef]
- He, J.; Wang, Y.; Liu, Y.; Wang, K.; Li, R.; Fan, J.; Xu, S.; Zhang, L. Tailoring the Luminescence of Europium Ions in Mesoporous AlPO4 Monolithic Glass. J. Phys. Chem. C 2013, 117, 21916–21922. [Google Scholar] [CrossRef]
- Zhang, Q.; Qiao, Y.; Qian, B.; Dong, G.; Ruan, J.; Liu, X.; Zhou, Q.; Chen, Q.; Qiu, J.; Chen, D. Luminescence properties of the Eu-doped porous glass and spontaneous reduction of Eu3+ to Eu2+. J. Lumin. 2009, 129, 1393–1397. [Google Scholar] [CrossRef]
- Biswas, A.; Friend, C.S.; Prasad, P.N. Spontaneous reduction of Eu3+ ion in Al co-doped sol–gel silica matrix during densification. Mater. Lett. 1999, 39, 227–231. [Google Scholar] [CrossRef]
- Wang, C.; Peng, M.; Jiang, N.; Jiang, X.; Zhao, C.; Qiu, J. Tuning the Eu luminescence in glass materials synthesized in air by adjusting glass compositions. Mater. Lett. 2007, 61, 3608–3611. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, X.; Jing, G.; Liu, E.; Fan, J.; Zhang, G.; Hou, X. Transition from Eu3+ to Eu2+ in SiO2 Matrix Prepared by Sol–Gel. J. Nanosci. Nanotechnol. 2014, 14, 3642–3647. [Google Scholar] [CrossRef]
- Jousseaume, V.; Zenasni, A.; Gourhant, O.; Favennec, L.; R., B.M. Ultra-Low-k by CVD: Deposition and Curing. In Advanced Interconnects for ULSI Technology; Baklanov, M.R., Ho, P.S., Zschech, E., Eds.; John Wiley & Sons., Ltd.: UK, 2012 ISBN 9781119963677.
- Palov, A.; Rakhimova, T. V.; Krishtab, M.B.; Baklanov, M.R. Dependence of dielectric constant of SiOCH low-k films on porosity and pore size. J. Vac. Sci. Technol. B, Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2015, 33, 020603. [Google Scholar] [CrossRef]
- Kumar, B.P.; Kumar, A.P.; Bindu, P.H.; Mukherjee, A.K.; Patra, A.S. Red Light Emission of POSS Triol Chelated with Europium. Asian J. Nanosci. Mater. 2019, 2, 244–256. [Google Scholar] [CrossRef]
- Horrocks, W.D.; Schmidt, G.F.; Sudnick, D.R.; Kittrell, C.; Bernheim, R.A. Laser-induced lanthanide ion luminescence lifetime measurements by direct excitation of metal ion levels. A new class of structural probe for calcium-binding proteins and nucleic acids. J. Am. Chem. Soc. 1977, 99, 2378–2380. [Google Scholar] [CrossRef] [PubMed]
- Dorenbos, P. Energy of the first 4f7→4f65d transition of Eu2+ in inorganic compounds. J. Lumin. 2003, 104, 239–260. [Google Scholar] [CrossRef]







| Sample Number | Eu(NO3)3·6H2O Content, wt% | Thickness, nm | RI | Full Porosity, % |
Open Porosity, % |
Pore Diameter, nm |
|---|---|---|---|---|---|---|
| 1 | 0 | 478.0 | 1.325 | 26.5±1 | 23.7±1 | 1.8±0.1 |
| 2 | 1.7 | 493.6 | 1.329 | 25.7±1 | 24.3±1 | 1.9±0.1 |
| 3 | 12.1 | 485.2 | 1.333 | 25.9±1 | 20.7±2 | 1.8±0.1 |
| 4 | 25.8 | 465.7 | 1.320 | 27.6±1 | 13.5±2 | 1.8±0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





