Submitted:
27 July 2023
Posted:
31 July 2023
You are already at the latest version
Abstract
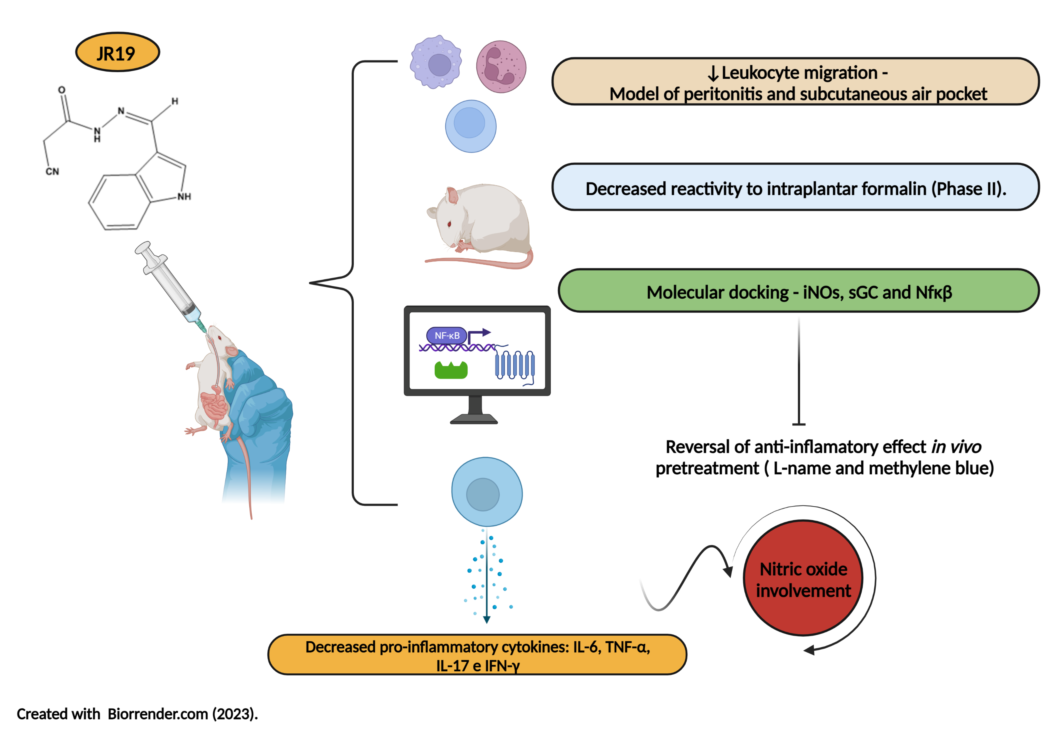
Keywords:
1. Introduction
2. Materials and Methods
2.1. Substances
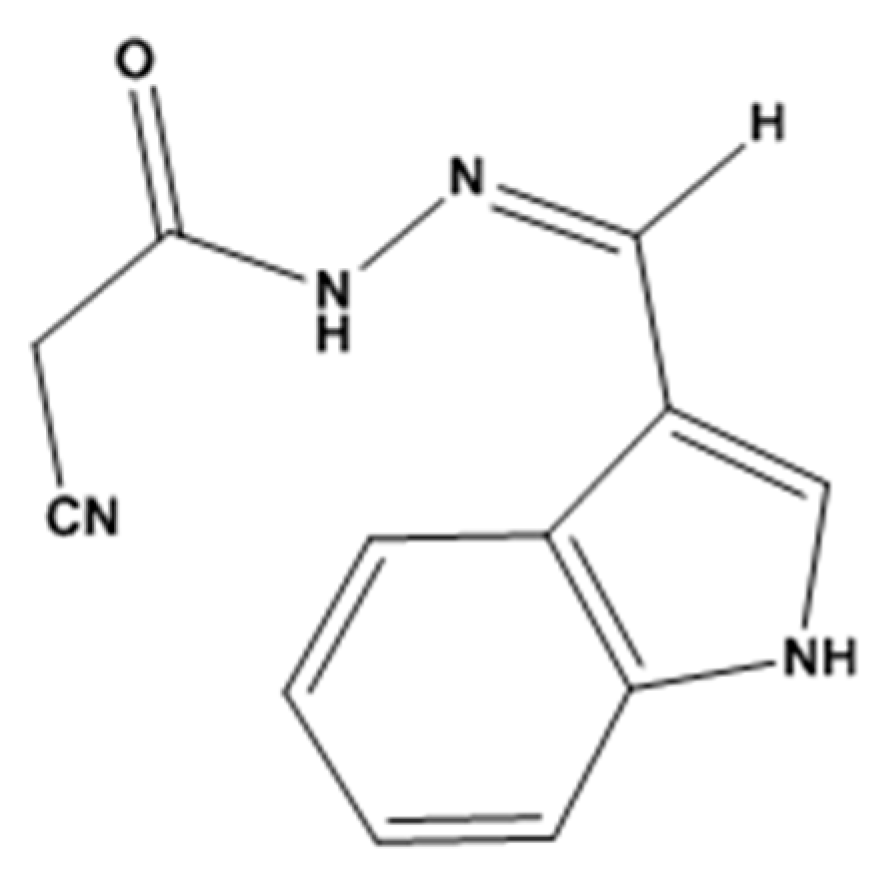
2.2. Biological activity
2.2.1. Animals
2.2.2. Inflammatory models
Carrageenan-induced peritonitis in mice
Subcutaneous air pocket
Formalin-induced nociception
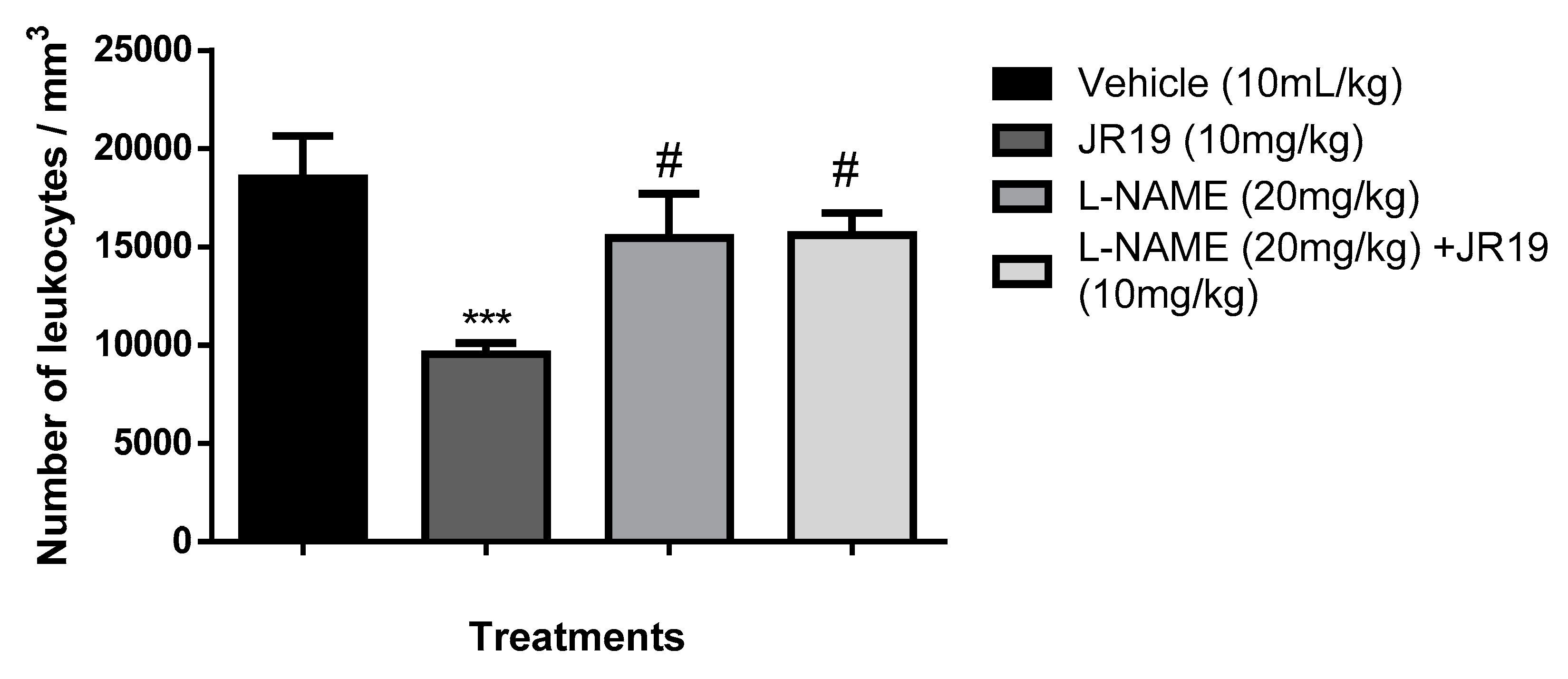
Investigation of the sGC/NOs pathway in the anti-inflammatory effect
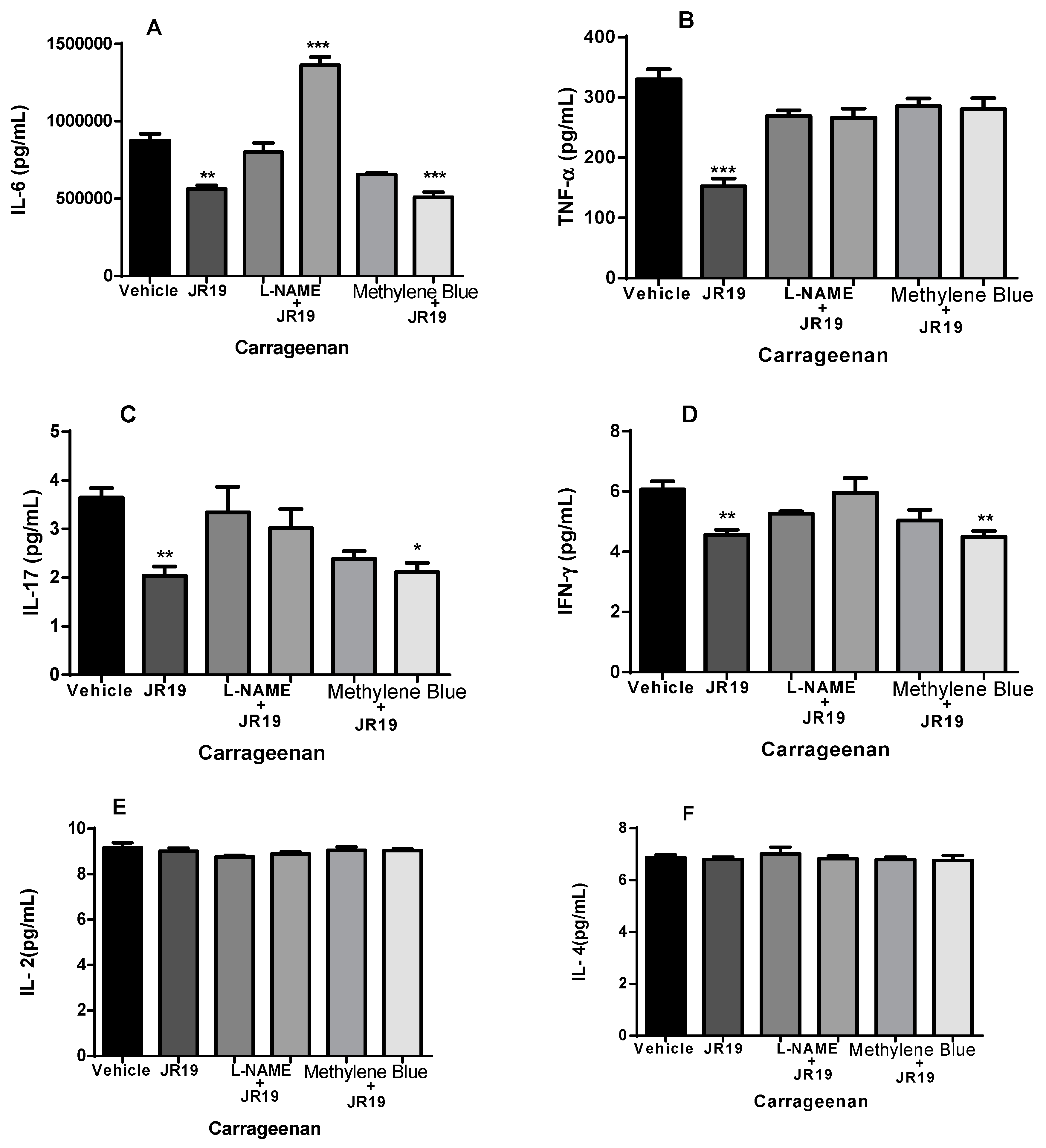
Dosage of cytokines in peritoneal exudate
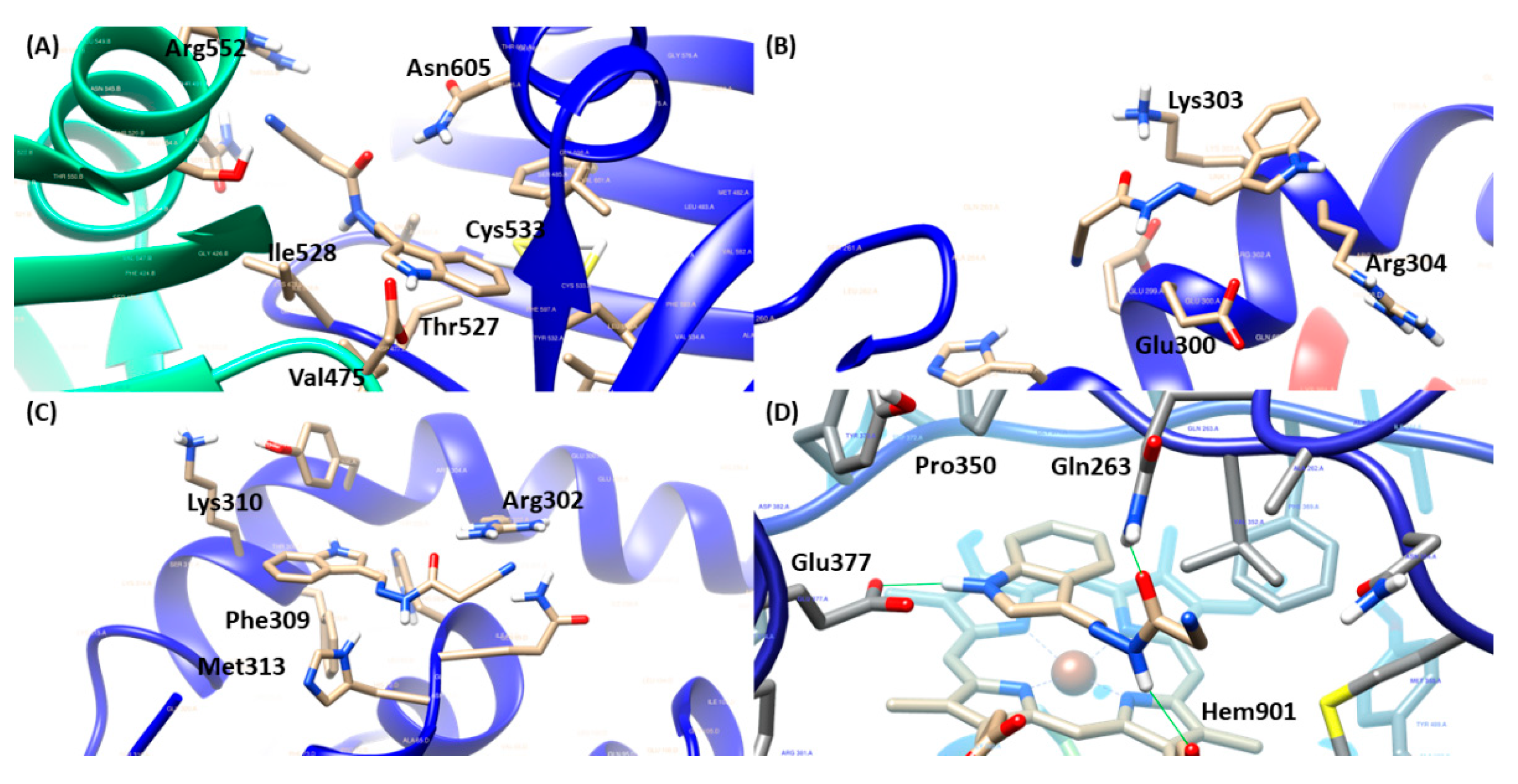
2.3. Docking studies
2.4. Statistical Analysis
3. Results
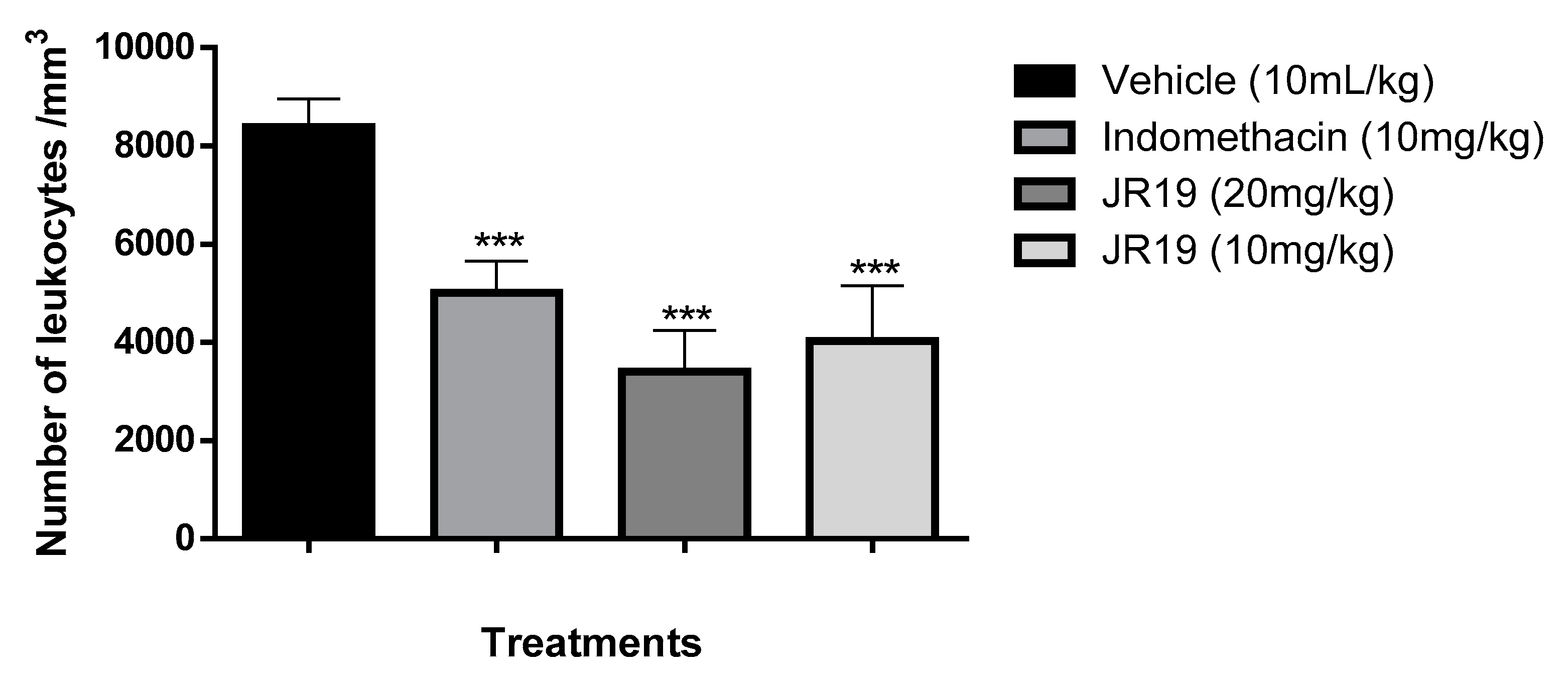
3.1. Carrageenan-induced peritonitis
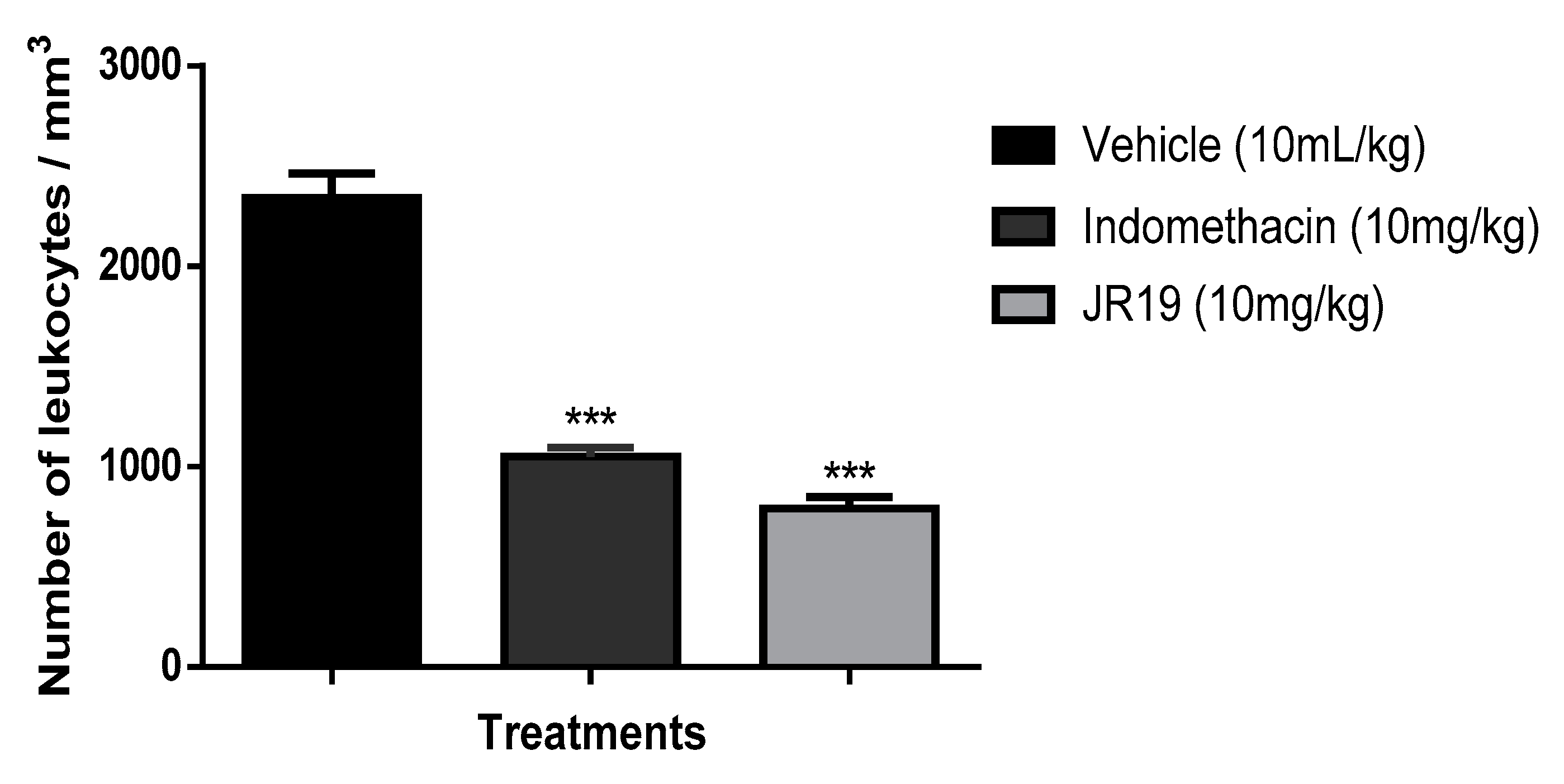
3.2. Subcutaneous air pouch
3.3. Formalin-induced nociception
3.4. Docking molecular
3.5. Investigation of the participation of the oxidonitrergic pathway in the anti-inflammatory effect of JR19
3.6. Cytokine dosage
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fikry, E.M.; Hasan, W.A.; Mohamed, E.G. Rutin and meloxicam attenuate paw inflammation in mice: Affecting sorbitol dehydrogenase activity. J. Biochem. Mol. Toxicol. 2018, 32, e22029. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J. 2017, 31, 1273–1288. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Song, L.; Wang, X.; Xu, Z.; Wang, S.; Wang, J.; Xu, H.; Zheng, Y.; Wang, Y. Cardiac injury prediction and lymphocyte immunity and inflammation analysis in hospitalized patients with coronavirus disease 2019 (COVID-19). Int. J. Cardiol. 2021, 326, 237–242. [Google Scholar] [CrossRef] [PubMed]
- El Menyiy, N.; El Allam, A.; Aboulaghras, S.; Jaouadi, I.; Bakrim, S.; El Omari, N.; Shariati, M.A.; Miftakhutdinov, A.; Wilairatana, P.; Mubarak, M.S.; et al. Inflammatory auto-immune diseases of the intestine and their management by natural bioactive compounds. BioMedicine 2022, 151, 113158. [Google Scholar] [CrossRef]
- da Silva, P.R.; NunesPazos, N.D.; Gonzaga, T.K.S.D.N.; de Andrade, J.C. ; Monteiro,.B.; Portela, A.C.R.; Pires, H.F.O.; Maia, M.d.S.; da Fonsêca, D.V.; Scotti, M.T.; et al. Anxiolytic and antidepressant-like effects of monoterpene tetrahydrolinalool and in silico approach of new potential targets. Curr. Top. Med. Chem. 1530. [Google Scholar] [CrossRef]
- Alessandri, A.L.; Sousa, L.P.; Lucas, C.D.; Rossi, A.G.; Pinho, V.; Teixeira, M.M. Resolution of inflammation: Mechanisms and opportunity for drug development. Pharmacol. Ther. 2013, 139, 189–212. [Google Scholar] [CrossRef]
- Gomes, F.I.F.; Cunha, F.Q.; Cunha, T.M. Peripheral nitric oxide signaling directly blocks inflammatory pain. Biochem. Pharmacol. 2020, 176, 113862. [Google Scholar] [CrossRef]
- Sansbury, B.E.; Cummins, T.D.; Tang, Y.; Hellmann, J.; Holden, C.R.; Harbeson, M.A.; Chen, Y.; Patel, R.P.; Spite, M.; Bhatnagar, A.; et al. Overexpression of Endothelial Nitric Oxide Synthase Prevents Diet-Induced Obesity and Regulates Adipocyte Phenotype. Circ. Res. 2012, 111, 1176–1189. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N. Proresolving Lipid Mediators and Mechanisms in the Resolution of Acute Inflammation. Immunity 2014, 40, 315–327. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147–114147. [Google Scholar] [CrossRef]
- Patrignani, P.; Patrono, C. Cyclooxygenase inhibitors: From pharmacology to clinical read-outs. Biochim. et Biophys. Acta (BBA) - Mol. Cell Biol. Lipids 2015, 1851, 422–432. [Google Scholar] [CrossRef]
- Kowalski, M.L.; Makowska, J.S. Seven Steps to the Diagnosis of NSAIDs Hypersensitivity: How to Apply a New Classification in Real Practice? Allergy, Asthma Immunol. Res. 2015, 7, 312–320. [Google Scholar] [CrossRef]
- Nakhostin, A.; Mirseyyedhosein, S.; Toolabi, M.; Khodabakhsh, P.; Aghamiri, H.; Ghaffari, S.; Shafaroodi, H.; Shayesteh, A.; Amini, M.; Shafiee, A.; et al. Synthesis, conformational assignment, and anti-inflammatory activities of N-arylidene-2-(4-chloro-2-(2-substituted phenoxy)phenyl)acetic acid hydrazides. Med. Chem. Res. 2016, 25, 2220–2236. [Google Scholar] [CrossRef]
- Kumar, A.; Alam, M.S.; Hamid, H.; Chugh, V.; Tikla, T.; Kaul, R.; Dhulap, A.; Sharma, S.K. Design and synthesis of anti–inflammatory 1,2,3–triazolylpyrrolobenzodiazepinone derivatives and impact of molecular structure on COX–2 selective targeting. J. Mol. Struct. 2023, 1272. [Google Scholar] [CrossRef]
- Moraes, A.D.T.d.O.; de Miranda, M.D.S.; Jacob. T.T.; Amorim, C.A.d.C.; de Moura, R.O.; da Silva, S..S.; Soares, M.B.P.; de Almeida, S.M.V.; Souza, T.R.C.d.L.; de Oliveira, J.F.; et al. Synthesis, in vitro and in vivo biological evaluation, COX-1/2 inhibition and molecular docking study of indole-N-acylhydrazone derivatives. Bioorganic Med. Chem. 2018, 26, 5388–5396. [Google Scholar] [CrossRef]
- Oliveira-Tintino, C.D.d.M.; Pessoa, R.T.; Fernandes, M.N.M.; Alcântara, I.S.; da Silva, B.A.F.; de Oliveira, M.R.C.; Martins, A.O.B.P.B.; da Silva, M.D.S.; Tintino, S.R.; Rodrigues, F.F.G.; et al. Anti-inflammatory and anti-edematogenic action of the Croton campestris A. St.-Hil (Euphorbiaceae) essential oil and the compound β-caryophyllene in in vivo models. Phytomedicine 2018, 41, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Sarmento-Filha, M.J.; Torres-Rêgo, M.; Daniele-Silva, A.; de Queiroz-Neto, M.F.; Rocha, H.A.O.; Camara, C.A.; Araújo, R.M.; da Silva-Júnior, A.A.; Silva, T.M.S.; Fernandes-Pedrosa, M.d.F. Phytochemical analysis by UPLC-QTOF-MS/MS and evaluation of antioxidant and anti-inflammatory activities of the extract and fractions from flowers of Cochlospermum vitifolium. South Afr. J. Bot. 2022, 148, 293–306. [Google Scholar] [CrossRef]
- Torres-Rêgo, M.; Furtado, A.A.; Bitencourt, M.A.O.; Lima, M.C.J.d.S.; de Andrade, R.C.L.C.; de Azevedo, E.P.; Soares, T.d.C.; Tomaz, J.C.; Lopes, N.P.; da Silva-Júnior, A.A.; et al. Anti-inflammatory activity of aqueous extract and bioactive compounds identified from the fruits of Hancornia speciosa Gomes (Apocynaceae). BMC Complement. Altern. Med. 2016, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Raymundo, L.J.; Guilhon, C.C.; Alviano, D.S.; Matheus, M.E.; Antoniolli, A.R.; Cavalcanti, S.C.; Alves, P.B.; Alviano, C.S.; Fernandes, P.D. Characterisation of the anti-inflammatory and antinociceptive activities of the Hyptis pectinata (L.) Poit essential oil. J. Ethnopharmacol. 2011, 134, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.S.H.d.S.; Malta, D.J.D.N.; Laranjeira, L.P.M.; Maia, M.B.S.; Colaço, N.C.; de Lima, M.D.C.A.; Galdino, S.L.; Pitta, I.d.R.; Gonçalves-Silva, T. Anti-inflammatory and antinociceptive activities of indole–imidazolidine derivatives. Int. Immunopharmacol. 2011, 11, 1816–1822. [Google Scholar] [CrossRef]
- The Formalin Test in Mice: Dissociation Between;
- Guerra, A.S.H.d.S.; Malta, D.J.D.N.; Laranjeira, L.P.M.; Maia, M.B.S.; Colaço, N.C.; de Lima, M.D.C.A.; Galdino, S.L.; Pitta, I.d.R.; Gonçalves-Silva, T. Anti-inflammatory and antinociceptive activities of indole–imidazolidine derivatives. Int. Immunopharmacol. 2011, 11, 1816–1822. [Google Scholar] [CrossRef] [PubMed]
- Mendes, S.; Bomfim, R.; Jesus, H.; Alves, P.; Blank, A.; Estevam, C.; Antoniolli, A.; Thomazzi, S. Evaluation of the analgesic and anti-inflammatory effects of the essential oil of Lippia gracilis leaves. J. Ethnopharmacol. 2010, 129, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Dissertação Fernanda Virginia Barreto Mota.
- Jain, M.; Parmar, H.S. Evaluation of antioxidative and anti-inflammatory potential of hesperidin and naringin on the rat air pouch model of inflammation. Inflamm. Res. 2011, 60, 483–491. [Google Scholar] [CrossRef]
- Vandooren, J.; Berghmans, N.; Dillen, C.; Van Aelst, I.; Ronsse, I.; Israel, L.L.; Rosenberger, I.; Kreuter, J.; Lellouche, J.-P.; Michaeli, S.; et al. Intradermal air pouch leukocytosis as an in vivo test for nanoparticles. Int. J. Nanomed. 2013, 8, 4745–4756. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and Molecular Mechanisms of Pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Ronchetti, S.; Migliorati, G.; Delfino, D. Association of inflammatory mediators with pain perception. BioMedicine 2017, 96, 1445–1452. [Google Scholar] [CrossRef]
- Allerston, C.K.; von Delft, F.; Gileadi, O. Crystal Structures of the Catalytic Domain of Human Soluble Guanylate Cyclase. PLOS ONE 2013, 8, e57644. [Google Scholar] [CrossRef]
- Malek, S.; Huang, D.-B.; Huxford, T.; Ghosh, S.; Ghosh, G. X-ray Crystal Structure of an IκBβ·NF-κB p65 Homodimer Complex. J. Biol. Chem. 2003, 278, 23094–23100. [Google Scholar] [CrossRef]
- Qu, B.; Wang, S.; Zhu, H.; Yin, T.; Zhou, R.; Hu, W.; Lu, C. Core Constituents of Caragana sinica Root for Rheumatoid Arthritis Treatment and the Potential Mechanism. ACS Omega 2022, 8, 2586–2595. [Google Scholar] [CrossRef]
- Singh, M.; Padhy, G.; Vats, P.; Bhargava, K.; Sethy, N.K. Hypobaric hypoxia induced arginase expression limits nitric oxide availability and signaling in rodent heart. Biochim. et Biophys. Acta (BBA) - Gen. Subj. 2014, 1840, 1817–1824. [Google Scholar] [CrossRef]
- Garcin, E.D.; Arvai, A.S.; Rosenfeld, R.J.; Kroeger, M.D.; Crane, B.R.; Andersson, G.; Andrews, G.; Hamley, P.J.; Mallinder, P.R.; Nicholls, D.J.; et al. Anchored plasticity opens doors for selective inhibitor design in nitric oxide synthase. Nat. Chem. Biol. 2008, 4, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F. Targeting the interleukin pathway in the treatment of asthma. Lancet 2015, 386, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Paulino, N.; Abreu, S.R.L.; Uto, Y.; Koyama, D.; Nagasawa, H.; Hori, H.; Dirsch, V.M.; Vollmar, A.M.; Scremin, A.; Bretz, W.A. Anti-inflammatory effects of a bioavailable compound, Artepillin C, in Brazilian propolis. Eur. J. Pharmacol. 2008, 587, 296–301. [Google Scholar] [CrossRef]
- Francischetti, I.; Bitu MORENO, J.; Scholz, M.; Bonetti YOSHIDA, W.; Paulo, S.; Francischetti Rua Doutor Augusto Barreto, I. Os Leucócitos e a Resposta Inflamatória Na Lesão de Isquemia-Reperfusão Leukocytes and the Inflammatory Response in Ischemia-Reperfusion Injury; 2010; Vol. 25;
- A Muller, W. Leukocyte-Endothelial Cell Interactions in the Inflammatory Response. Lab. Investig. 2002, 82, 521–533. [Google Scholar] [CrossRef]
- Sherwood, E.R.; Toliver-Kinsky, T. Mechanisms of the inflammatory response. Best Pr. Res. Clin. Anaesthesiol. 2004, 18, 385–405. [Google Scholar] [CrossRef] [PubMed]
- Zindel, J.; Kubes, P. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annu. Rev. Pathol. Mech. Dis. 2019, 15, 493–518. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Oncotarget 7204 Www.Impactjournals.Com/Oncotarget Inflammatory Responses and Inflammation-Associated Diseases in Organs; 2018; Vol. 9;
- Sedgwick, A.D.; Lees, P. A Comparison of Air Pouch, Sponge and Pleurisy Models of Acute Carrageenan Inflammation in the Rat; 1986; Vol. 18;
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: a critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- da Silva, P.R.; Santo, R.F.D.E.; Melo, C.d.O.; Cavalcante, F.E.P.; Costa, T.B.; Barbosa, Y.V.; Silva, Y.M.S.d.M.e.; de Sousa, N.F.; Villarreal, C.F.; de Moura, R.O.; et al. The Compound (E)-2-Cyano-N,3-diphenylacrylamide (JMPR-01): A Potential Drug for Treatment of Inflammatory Diseases. Pharmaceutics 2022, 14, 188. [Google Scholar] [CrossRef]
- Da, E.H.; Ramos, S. Avaliação Das Atividades Biológicas Do Óleo Essencial Do Látex de Mangifera Indica L. (Var. 2014. [Google Scholar]
- Baral, P.; Udit, S.; Chiu, I.M. Pain and immunity: implications for host defence. Nat. Rev. Immunol. 2019, 19, 433–447. [Google Scholar] [CrossRef]
- Salomé, D.d.C.; de Freitas, R.H.C.N.; Fraga, C.A.M.; Fernandes, P.D. Novel Regioisomeric Analogues of Naphthyl-N-Acylhydrazone Derivatives and Their Anti-Inflammatory Effects. Int. J. Mol. Sci. 2022, 23, 13562. [Google Scholar] [CrossRef]
- Cordeiro, N.d.M.; Freitas, R.H.; Fraga, C.A.M.; Fernandes, P.D. New 2-amino-pyridinyl-N-acylhydrazones: Synthesis and identification of their mechanism of anti-inflammatory action. BioMedicine 2020, 123, 109739. [Google Scholar] [CrossRef] [PubMed]
- Ha Yoon, M.; il Choi, J. Pharmacologic Interaction between Cannabinoid and Either Clonidine or Neostigmine in the Rat Formalin Test; 2003; Vol. 99;
- Kang, S.; Kim, C.H.; Lee, H.; Kim, D.Y.; Han, J.I.; Chung, R.K.; Lee, G.Y. Antinociceptive Synergy Between the Cannabinoid Receptor Agonist WIN 55,212-2 and Bupivacaine in the Rat Formalin Test. Obstet. Anesthesia Dig. 2007, 104, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, H.A.; Nascimento, M.V.M.; Tavares, A.; Galdino, P.M.; de Paula, J.R.; Costa, E.A. Effects of ethanolic extract of leaves of Lafoensia pacari A. St.-Hil., Lythraceae (pacari), in pain and inflammation models. Rev. Bras. de Farm. 2010, 20, 328–333. [Google Scholar] [CrossRef]
- Da Silva, T.F.; Júnior, W.B.; Alexandre-Moreira, M.S.; Costa, F.N.; Monteiro, C.E.d.S.; Ferreira, F.F.; Barroso, R.C.R.; Noël, F.; Sudo, R.T.; Zapata-Sudo, G.; et al. Novel Orally Active Analgesic and Anti-Inflammatory Cyclohexyl-N-Acylhydrazone Derivatives. Molecules 2015, 20, 3067–3088. [Google Scholar] [CrossRef]
- Domínguez-Luis, M.; Herrera-García, A.; Arce-Franco, M.; Armas-González, E.; Rodríguez-Pardo, M.; Lorenzo-Díaz, F.; Feria, M.; Cadenas, S.; Sánchez-Madrid, F.; Díaz-González, F. Superoxide anion mediates the L-selectin down-regulation induced by non-steroidal anti-inflammatory drugs in human neutrophils. Biochem. Pharmacol. 2013, 85, 245–256. [Google Scholar] [CrossRef]
- Ghasemi, M. Ghasemi, M. Nitric Oxide: Antidepressant Mechanisms and Inflammation. In Advances in Pharmacology; Academic Press Inc., 2019; Vol. 86, pp. 121–152 ISBN 9780128166680.
- Biava, M.; Porretta, G.C.; Poce, G.; Battilocchio, C.; Alfonso, S.; Rovini, M.; Valenti, S.; Giorgi, G.; Calderone, V.; Martelli, A.; et al. Novel Analgesic/Anti-Inflammatory Agents: Diarylpyrrole Acetic Esters Endowed with Nitric Oxide Releasing Properties. J. Med. Chem. 2011, 54, 7759–7771. [Google Scholar] [CrossRef]
- Janakiram, N.B.; Rao, C. v. INOS-Selective Inhibitors for Cancer Prevention: Promise and Progress. Future Med Chem 2012, 4, 2193–2204. [Google Scholar] [CrossRef]
- Król, M.; Kepinska, M. Human Nitric Oxide Synthase—Its Functions, Polymorphisms, and Inhibitors in the Context of Inflammation, Diabetes and Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 56. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.; de Almeida, M.; Silva, J.; Albino, S.; Espírito-Santo, R.; Lima, M.; Villarreal, C.; Moura, R.; Santos, V. (E)-2-Cyano-3-(1H-Indol-3-yl)-N-Phenylacrylamide, a Hybrid Compound Derived from Indomethacin and Paracetamol: Design, Synthesis and Evaluation of the Anti-Inflammatory Potential. Int. J. Mol. Sci. 2020, 21, 2591. [Google Scholar] [CrossRef]
- Ahluwalia, A.; Foster, P.; Scotland, R.S.; Mclean, P.G.; Mathur, A.; Perretti, M.; Moncada, S.; Hobbs, A.J. Antiinflammatory Activity of Soluble Guanylate Cyclase: CGMP-Dependent down-Regulation of P-Selectin Expression and Leukocyte Recruitment; 2004; Vol. 101;
- Florentino, I.F.; Galdino, P.M.; De Oliveira, L.P.; Silva, D.P.; Pazini, F.; Vanderlinde, F.A.; Lião, L.M.; Menegatti, R.; Costa, E.A. Involvement of the NO/cGMP/KATP pathway in the antinociceptive effect of the new pyrazole 5-(1-(3-fluorophenyl)-1H-pyrazol-4-yl)-2H-tetrazole (LQFM-021). Nitric Oxide 2015, 47, 17–24. [Google Scholar] [CrossRef]
- Iwata, M.; Suzuki, S.; Asai, Y.; Inoue, T.; Takagi, K. Involvement of Nitric Oxide in a Rat Model of Carrageenin-Induced Pleurisy. Mediat. Inflamm. 2010, 2010, 1–11. [Google Scholar] [CrossRef]
- Florentino, I.F.; Silva, D.P.; Silva, D.M.; Cardoso, C.S.; Moreira, A.L.; Borges, C.L.; Soares, C.M.d.A.; Galdino, P.M.; Lião, L.M.; Ghedini, P.C.; et al. Potential anti-inflammatory effect of LQFM-021 in carrageenan-induced inflammation: The role of nitric oxide. Nitric Oxide 2017, 69, 35–44. [Google Scholar] [CrossRef]
- Carreau, A.; Kieda, C.; Grillon, C. Nitric oxide modulates the expression of endothelial cell adhesion molecules involved in angiogenesis and leukocyte recruitment. Exp. Cell Res. 2011, 317, 29–41. [Google Scholar] [CrossRef]
- Schioppa, T.; Sozio, F.; Barbazza, I.; Scutera, S.; Bosisio, D.; Sozzani, S.; Del Prete, A. Molecular Basis for CCRL2 Regulation of Leukocyte Migration. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Secco, D.D.; Paron, J.A.; de Oliveira, S.H.; Ferreira, S.H.; Silva, J.S.; Cunha, F.d.Q. Neutrophil migration in inflammation: nitric oxide inhibits rolling, adhesion and induces apoptosis. Nitric Oxide 2003, 9, 153–164. [Google Scholar] [CrossRef]
- Matsui, T.C.; Coura, G.M.; Melo, I.S.; Batista, C.R.; Augusto, P.S.A.; Godin, A.M.; Araújo, D.P.; César, I.C.; Ribeiro, L.S.; Souza, D.G.; et al. Nicorandil inhibits neutrophil recruitment in carrageenan-induced experimental pleurisy in mice. Eur. J. Pharmacol. 2015, 769, 306–312. [Google Scholar] [CrossRef]
- Suk, K.; Kim, S.Y.; Kim, H. Regulation of IL-18 Production by IFNg and PGE 2 in Mouse Microglial Cells: Involvement of NF-KB Pathway in the Regulatory Processes; 2001; Vol. 77;
- Santos, L.A.M.; Ribeiro, E.L.; Barbosa, K.P.S.; Fragoso, I.T.; Gomes, F.O.d.S.; Donato, M.A.M.; Silva, B.S.; Silva, A.K.S.; Rocha, S.W.S.; França, M.E.R.; et al. Diethylcarbamazine inhibits NF-κB activation in acute lung injury induced by carrageenan in mice. Int. Immunopharmacol. 2014, 23, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Albensi, B.C. What Is Nuclear Factor Kappa B (NF-ΚB) Doing in and to the Mitochondrion? Front Cell Dev Biol 2019, 7. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in Inflammation, Autoimmunity and Cancer. Int Immunol 2021, 33, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, B.C.; Lopes, A.L.; Macedo, R.C.O.; Correa, C.S.; Ramis, T.R.; Ribeiro, J.L.; Reischak-Oliveira, A. Marcadores Inflamatórios, Função Endotelial e Riscos Cardiovasculares. J Vasc Bras 2014, 13, 108–115. [Google Scholar] [CrossRef]
- Le, K.T.T.; Chu, X.; Jaeger, M.; Plantinga, J.A.; Matzaraki, V.; Withoff, S.; Joosten, L.A.B.; Netea, M.G.; Wijmenga, C.; Li, Y.; et al. Leukocyte-Released Mediators in Response to Both Bacterial and Fungal Infections Trigger IFN Pathways, Independent of IL-1 and TNF-α, in Endothelial Cells. Front. Immunol. 2019, 10, 2508. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.M.; Campos Pellanda, L.; Gus, I.; Portal, V.L. Inflammatory Markers of Cardiovascular Disease in the Elderly;
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and Atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Wöhrl, S.; Bielory, L. Mast Cell Biology at Molecular Level: A Comprehensive Review. Clin Rev Allergy Immunol 2020, 58, 342–365. [Google Scholar]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS Crosstalk in Inflammation. Trends Cell Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef]
- Zelová, H.; Hošek, J. TNF-α signalling and inflammation: Interactions between old acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef]
- van Loo, G.; Bertrand, M.J.M. Death by TNF: A Road to Inflammation. Nat Rev Immunol 2023, 23, 289–303. [Google Scholar] [PubMed]
- Schurgers, E.; Billiau, A.; Matthys, P.; Dolati, S.; Sadreddini, S.; Rostamzadeh, D.; Ahmadi, M.; Jadidi-Niaragh, F.; Yousefi, M.; Rosillo, M.A.; et al. Collagen-Induced Arthritis as an Animal Model for Rheumatoid Arthritis: Focus on Interferon-γ. J. Interf. Cytokine Res. 2011, 31, 917–926. [Google Scholar] [CrossRef]
- Larkin, J.; Ahmed, C.M.; Wilson, T.D.; Johnson, H.M. Regulation of Interferon Gamma Signaling by Suppressors of Cytokine Signaling and Regulatory T Cells. Front. Immunol. 2013, 4. [Google Scholar] [CrossRef]
- Ohms, M.; Möller, S.; Laskay, T. An Attempt to Polarize Human Neutrophils Toward N1 and N2 Phenotypes in vitro. Front. Immunol. 2020, 11, 532. [Google Scholar] [CrossRef]
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2023, 23, 38–54. [Google Scholar] [CrossRef]
- Schaper, F.; Rose-John, S. Interleukin-6: Biology, signaling and strategies of blockade. Cytokine Growth Factor Rev. 2015, 26, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Stenken, J.A.; Poschenrieder, A.J. Bioanalytical chemistry of cytokines – A review. Anal. Chim. Acta 2015, 853, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.H.C.N.; Cordeiro, N.M.; Carvalho, P.R.; Alves, M.A.; Guedes, I.A.; Valerio, T.S.; Dardenne, L.E.; Lima, L.M.; Barreiro, E.J.; Fernandes, P.D.; et al. Discovery of naphthyl-N -acylhydrazone p38α MAPK inhibitors with in vivo anti-inflammatory and anti-TNF-α activity. Chem. Biol. Drug Des. 2018, 91, 391–397. [Google Scholar] [CrossRef] [PubMed]

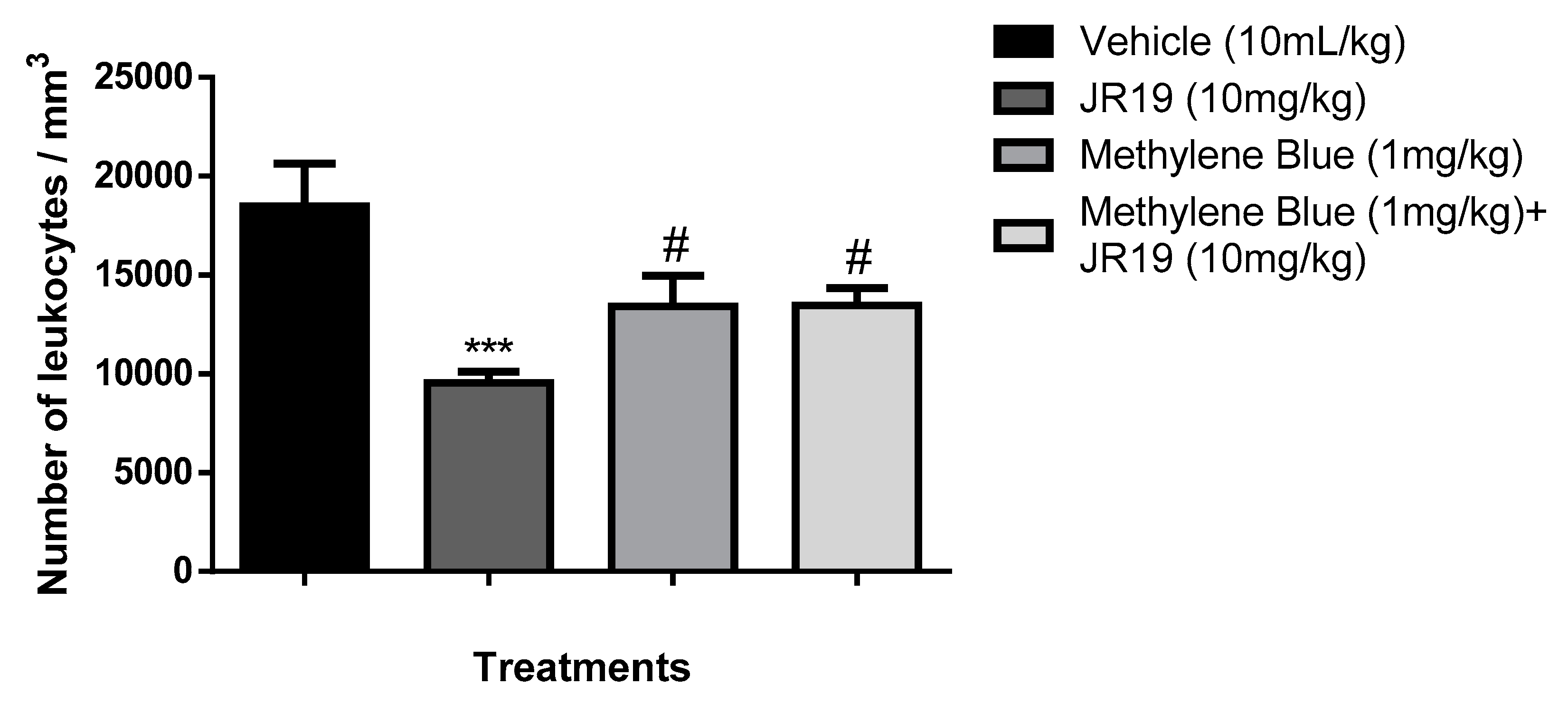
| Molecular Targets | ||||
|---|---|---|---|---|
| Compounds | sGC | NFκB/p65 | iNOS | RMSD (Å ) |
| JR19 | 55.2887 | 32.0025 (pose 1) 42.3261 (pose 2) |
74.6536 | - |
| AR-C95791 | 82.3597 | 0.3297 | ||
| L-NAME | 66.1212 | - | ||
| Nigakinone | 32.5710 (pose 1) 43.4235 (pose 2) |
- | ||
| Methylene blue | 46.9947 | - | ||
| Molecular Targets | ||||
|---|---|---|---|---|
| Compounds | sGC | NFκB/p65 (pose 1) | NF-κB/p65 (pose 2) | iNOS |
| JR-19 | Arg5521, Ile5281,3, Thr5271, Asn6051, Val4752,3 and Cys5332 | Glu3001, Lys3031,2 and Arg3043 | Arg3021, Phe3092, Met3132 and Lys3103 | Gln2631, Hem9011,2, Glu3771 and Pro3503 |
| AR-C95791 | Glu3771, Tyr3471, Asp3821, Trp3721, Hem9012,3, Phe3693, Val3523 and Pro3503 | |||
| L-NAME | Glu3771,2, Gly3711, Tyr3731, Tyr3471, Trp3721, Phe3691,3, Pro3501,3, Hem9011,2, Val3523, Asp3823 | |||
| Nigakinone | Glu3002, Lys3031,2 and Arg3043 | Arg3021, Tyr3062 and His681 | ||
| Methylene blue | Asp5301, Asp4861, Asn6051, Ile5281, Glu5261, Leu5961, Ala5313, Cys5332, Phe4842 and Val4753 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





