Submitted:
27 July 2023
Posted:
31 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
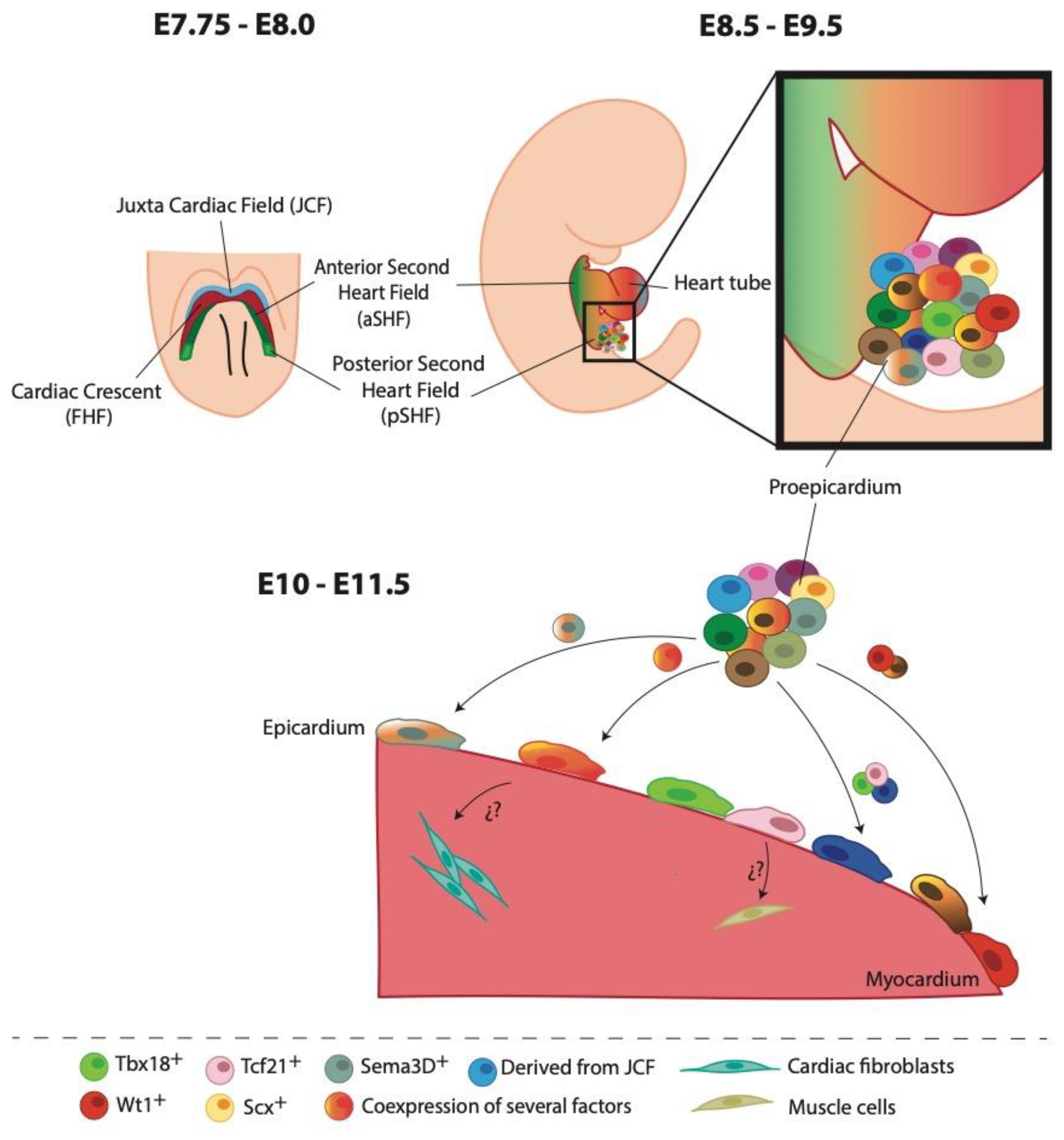
2. Epicardium during cardiac development and regeneration
3. Determining epicardial heterogeneity: do different cell types constitute the epicardial layer?
4. Does the embryonic origin of the epicardium underlie its cell heterogeneity?
4. Reflections and future perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Trembley, M.A.; Velasquez, L.S.; Bentley, K.L.d.M.; Small, E.M. Myocardin-related transcription factors control the motility of epicardium-derived cells and the maturation of coronary vessels. Development 2015, 142, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Grieskamp, T.; Rudat, C.; Lüdtke, T.H.-W.; Norden, J.; Kispert, A.; P, Q.; M, T.; E, S.; G, L.; G, D.; et al. Notch Signaling Regulates Smooth Muscle Differentiation of Epicardium-Derived Cells. Circ. Res. 2011, 108, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Moore-Morris, T.; Cattaneo, P.; Puceat, M.; Evans, S.M. Origins of cardiac fibroblasts. J. Mol. Cell. Cardiol. 2015, 91, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Carmona, R.; Barrena, S.; Gambero, A.J.L.; Rojas, A.; Muñoz-Chápuli, R. Epicardial cell lineages and the origin of the coronary endothelium. FASEB J. 2020, 34, 5223–5239. [Google Scholar] [CrossRef] [PubMed]
- Streef, T.J.; Smits, A.M. Epicardial Contribution to the Developing and Injured Heart: Exploring the Cellular Composition of the Epicardium. Front. Cardiovasc. Med. 2021, 8. [Google Scholar] [CrossRef]
- Redpath, A.N.; Smart, N. Recapturing embryonic potential in the adult epicardium: Prospects for cardiac repair. STEM CELLS Transl. Med. 2020, 10, 511–521. [Google Scholar] [CrossRef]
- Zhou, B.; Ma, Q.; Rajagopal, S.; Wu, S.M.; Domian, I.; Rivera-Feliciano, J.; Jiang, D.; von Gise, A.; Ikeda, S.; Chien, K.R.; et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 2008, 454, 109–113. [Google Scholar] [CrossRef]
- Carmona, R.; Guadix, J.A.; Cano, E.; Ruiz-Villalba, A.; Portillo-Sánchez, V.; Pérez-Pomares, J.M.; Muñoz-Chápuli, R. The embryonic epicardium: an essential element of cardiac development. J. Cell. Mol. Med. 2010, 14, 2066–2072. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- von Gise, A.; Pu, W.T.; H, Z.; K, L.; B, Z.; L, Z.; M, O.; L, Y.; Q, M.; N, S.; et al. Endocardial and Epicardial Epithelial to Mesenchymal Transitions in Heart Development and Disease. Circ. Res. 2012, 110, 1628–1645. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.-J.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [PubMed]
- del Moral, S.D.; Barrena, S.; Hernández-Torres, F.; Aránega, A.; Villaescusa, J.M.; Doblas, J.J.G.; Franco, D.; Jiménez-Navarro, M.; Muñoz-Chápuli, R.; Carmona, R. Deletion of the Wilms’ Tumor Suppressor Gene in the Cardiac Troponin-T Lineage Reveals Novel Functions of WT1 in Heart Development. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef]
- Masters, M.; Riley, P.R. The epicardium signals the way towards heart regeneration. Stem Cell Res. 2014, 13, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Poss, K.D. The epicardium as a hub for heart regeneration. Nat. Rev. Cardiol. 2018, 15, 631–647. [Google Scholar] [CrossRef]
- Tricarico, D.; Servidei, S.; Tonali, P.; Jurkat-Rott, K.; Camerino, D.C. Impairment of skeletal muscle adenosine triphosphate–sensitive K+ channels in patients with hypokalemic periodic paralysis. J. Clin. Investig. 1999, 103, 675–682. [Google Scholar] [CrossRef]
- van Wijk, B.; Gunst, Q.D.; Moorman, A.F.M.; Hoff, M.J.B.v.D. Cardiac Regeneration from Activated Epicardium. PLOS ONE 2012, 7, e44692. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S.M.; von Gise, A.; VanDusen, N.; Zhou, B.; Pu, W.T. Epicardium is required for cardiac seeding by yolk sac macrophages, precursors of resident macrophages of the adult heart. Dev. Biol. 2016, 413, 153–159. [Google Scholar] [CrossRef]
- Huang, G.N.; Thatcher, J.E.; McAnally, J.; Kong, Y.; Qi, X.; Tan, W.; DiMaio, J.M.; Amatruda, J.F.; Gerard, R.D.; Hill, J.A.; et al. C/EBP Transcription Factors Mediate Epicardial Activation During Heart Development and Injury. Science 2012, 338, 1599–1603. [Google Scholar] [CrossRef]
- Cai, W.; Tan, J.; Yan, J.; Zhang, L.; Cai, X.; Wang, H.; Liu, F.; Ye, M.; Cai, C.-L. Limited Regeneration Potential with Minimal Epicardial Progenitor Conversions in the Neonatal Mouse Heart after Injury. Cell Rep. 2019, 28, 190–201. [Google Scholar] [CrossRef]
- Sereti, K.-I.; Nguyen, N.B.; Kamran, P.; Zhao, P.; Ranjbarvaziri, S.; Park, S.; Sabri, S.; Engel, J.L.; Sung, K.; Kulkarni, R.P.; et al. Analysis of cardiomyocyte clonal expansion during mouse heart development and injury. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Kanisicak, O.; Khalil, H.; Ivey, M.J.; Karch, J.; Maliken, B.D.; Correll, R.N.; Brody, M.J.; Lin, S.-C.J.; Aronow, B.J.; Tallquist, M.D.; et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat. Commun. 2016, 7, 12260. [Google Scholar] [CrossRef] [PubMed]
- Senyo, S.E.; Steinhauser, M.L.; Pizzimenti, C.L.; Yang, V.K.; Cai, L.; Wang, M.; Wu, T.-D.; Guerquin-Kern, J.-L.; Lechene, C.P.; Lee, R.T. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 2013, 493, 433–436. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Huang, X.; Kanisicak, O.; Li, Y.; Wang, Y.; Li, Y.; Pu, W.; Liu, Q.; Zhang, H.; Tian, X.; et al. Preexisting endothelial cells mediate cardiac neovascularization after injury. J. Clin. Investig. 2017, 127, 2968–2981. [Google Scholar] [CrossRef]
- Moerkamp, A.T.; Lodder, K.; van Herwaarden, T.; Dronkers, E.; Dingenouts, C.K.E.; Tengström, F.C.; van Brakel, T.J.; Goumans, M.-J.; Smits, A.M. Human fetal and adult epicardial-derived cells: a novel model to study their activation. Stem Cell Res. Ther. 2016, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Menasche, B.L.; Crisman, L.; Gulbranson, D.R.; Davis, E.M.; Yu, H.; Shen, J. Fluorescence Activated Cell Sorting (FACS) in Genome-Wide Genetic Screening of Membrane Trafficking. Curr. Protoc. Cell Biol. 2018, 82, e68. [Google Scholar] [CrossRef] [PubMed]
- Packer, J.; Trapnell, C. Single-Cell Multi-omics: An Engine for New Quantitative Models of Gene Regulation. Trends Genet. 2018, 34, 653–665. [Google Scholar] [CrossRef]
- Wen, L.; Tang, F. Single-cell sequencing in stem cell biology. Genome Biol. 2016, 17, 71–71. [Google Scholar] [CrossRef]
- Weinberger, M.; Simões, F.C.; Patient, R.; Sauka-Spengler, T.; Riley, P.R. Functional Heterogeneity within the Developing Zebrafish Epicardium. Dev. Cell 2020, 52, 574–590. [Google Scholar] [CrossRef]
- Gambardella, L.; McManus, S.A.; Moignard, V.; Sebukhan, D.; Delaune, A.; Andrews, S.; Bernard, W.G.; Morrison, M.A.; Riley, P.R.; Göttgens, B.; et al. BNC1 regulates cell heterogeneity in human pluripotent stem cell derived-epicardium. Development 2019, 146. [Google Scholar] [CrossRef]
- Braitsch, C.M.; Combs, M.D.; Quaggin, S.E.; Yutzey, K.E. Pod1/Tcf21 is regulated by retinoic acid signaling and inhibits differentiation of epicardium-derived cells into smooth muscle in the developing heart. Dev. Biol. 2012, 368, 345–357. [Google Scholar] [CrossRef]
- Bochmann, L.; Sarathchandra, P.; Mori, F.; Lara-Pezzi, E.; Lazzaro, D.; Rosenthal, N. Revealing New Mouse Epicardial Cell Markers through Transcriptomics. PLOS ONE 2010, 5, e11429. [Google Scholar] [CrossRef] [PubMed]
- Tyser, R.C.V.; Ibarra-Soria, X.; McDole, K.; Jayaram, S.A.; Godwin, J.; Brand, T.A.H.v.D.; Miranda, A.M.A.; Scialdone, A.; Keller, P.J.; Marioni, J.C.; et al. Characterization of a common progenitor pool of the epicardium and myocardium. Science 2021, 371. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, W.; Li, W.; Li, Y.; Priest, J.R.; Zhou, B.; Wang, J.; Zhou, Z. Single-Cell RNA-Seq of the Developing Cardiac Outflow Tract Reveals Convergent Development of the Vascular Smooth Muscle Cells. Cell Rep. 2019, 28, 1346–1361. [Google Scholar] [CrossRef] [PubMed]
- Rudat, C.; Grieskamp, T.; Röhr, C.; Airik, R.; Wrede, C.; Hegermann, J.; Herrmann, B.G.; Schuster-Gossler, K.; Kispert, A. Upk3b Is Dispensable for Development and Integrity of Urothelium and Mesothelium. PLOS ONE 2014, 9, e112112–e112112. [Google Scholar] [CrossRef]
- Mantri, M.; Scuderi, G.J.; Abedini-Nassab, R.; Wang, M.F.Z.; McKellar, D.; Shi, H.; Grodner, B.; Butcher, J.T.; De Vlaminck, I. Spatiotemporal single-cell RNA sequencing of developing chicken hearts identifies interplay between cellular differentiation and morphogenesis. Nat. Commun. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Sun, X.; Malandraki-Miller, S.; Kennedy, T.; Bassat, E.; Klaourakis, K.; Zhao, J.; Gamen, E.; Vieira, J.M.; Tzahor, E.; Riley, P.R. The extracellular matrix protein agrin is essential for epicardial epithelial-to-mesenchymal transition during heart development. Development 2021, 148. [Google Scholar] [CrossRef]
- Lupu, I.-E.; Redpath, A.N.; Smart, N. Spatiotemporal Analysis Reveals Overlap of Key Proepicardial Markers in the Developing Murine Heart. Stem Cell Rep. 2020, 14, 770–787. [Google Scholar] [CrossRef]
- Asp, M.; Giacomello, S.; Larsson, L.; Wu, C.; Fürth, D.; Qian, X.; Wärdell, E.; Custodio, J.; Reimegård, J.; Salmén, F.; et al. A Spatiotemporal Organ-Wide Gene Expression and Cell Atlas of the Developing Human Heart. Cell 2019, 179, 1647–1660. [Google Scholar] [CrossRef]
- Smart, N.; Bollini, S.; Dubé, K.N.; Vieira, J.M.; Zhou, B.; Davidson, S.; Yellon, D.; Riegler, J.; Price, A.N.; Lythgoe, M.F.; et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature 2011, 474, 640–644. [Google Scholar] [CrossRef]
- Ramjee, V.; Li, D.; Manderfield, L.J.; Liu, F.; Engleka, K.A.; Aghajanian, H.; Rodell, C.B.; Lu, W.; Ho, V.; Wang, T.; et al. Epicardial YAP/TAZ orchestrate an immunosuppressive response following myocardial infarction. J. Clin. Investig. 2017, 127, 899–911. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Suffee, N.; Moore-Morris, T.; Jagla, B.; Mougenot, N.; Dilanian, G.; Berthet, M.; Proukhnitzky, J.; Le Prince, P.; Tregouet, D.A.; Pucéat, M.; et al. Reactivation of the Epicardium at the Origin of Myocardial Fibro-Fatty Infiltration During the Atrial Cardiomyopathy. Circ. Res. 2020, 126, 1330–1342. [Google Scholar] [CrossRef] [PubMed]
- Bollini, S.; Vieira, J.M.N.; Howard, S.; Dubè, K.N.; Balmer, G.M.; Smart, N.; Riley, P.R. Re-Activated Adult Epicardial Progenitor Cells Are a Heterogeneous Population Molecularly Distinct from Their Embryonic Counterparts. Stem Cells Dev. 2014, 23, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- Bertaud, A.; Joshkon, A.; Heim, X.; Bachelier, R.; Bardin, N.; Leroyer, A.S.; Blot-Chabaud, M. Signaling Pathways and Potential Therapeutic Strategies in Cardiac Fibrosis. Int. J. Mol. Sci. 2023, 24, 1756. [Google Scholar] [CrossRef]
- Cao, J.; Navis, A.; Cox, B.D.; Dickson, A.L.; Gemberling, M.; Karra, R.; Bagnat, M.; Poss, K.D. Single epicardial cell transcriptome sequencing identifies Caveolin-1 as an essential factor in zebrafish heart regeneration. Development 2015, 143, 232–243. [Google Scholar] [CrossRef]
- Owenier, C.; Hesse, J.; Alter, C.; Ding, Z.; Marzoq, A.; Petzsch, P.; Köhrer, K.; Schrader, J. Novel technique for the simultaneous isolation of cardiac fibroblasts and epicardial stromal cells from the infarcted murine heart. Cardiovasc. Res. 2019. [Google Scholar] [CrossRef]
- Hesse, J.; Owenier, C.; Lautwein, T.; Zalfen, R.; Weber, J.F.; Ding, Z.; Alter, C.; Lang, A.; Grandoch, M.; Gerdes, N.; et al. Single-cell transcriptomics defines heterogeneity of epicardial cells and fibroblasts within the infarcted murine heart. eLife 2021, 10. [Google Scholar] [CrossRef]
- Simões, F.C.; Riley, P.R. The ontogeny, activation and function of the epicardium during heart development and regeneration. Development 2018, 145, dev155994. [Google Scholar] [CrossRef]
- Smits, A.M.; Dronkers, E.; Goumans, M.-J. The epicardium as a source of multipotent adult cardiac progenitor cells: Their origin, role and fate. Pharmacol. Res. 2018, 127, 129–140. [Google Scholar] [CrossRef]
- Maya-Ramos, L.; Cleland, J.; Bressan, M.; Mikawa, T. Induction of the Proepicardium. J. Dev. Biol. 2013, 1, 82–91. [Google Scholar] [CrossRef]
- Bressan, M.; Liu, G.; Mikawa, T. Early Mesodermal Cues Assign Avian Cardiac Pacemaker Fate Potential in a Tertiary Heart Field. Science 2013, 340, 744–748. [Google Scholar] [CrossRef] [PubMed]
- 52. Wei, K.; Díaz-Trelles, R.; Liu, Q.; Diez-Cuñado, M.; Scimia, M.C.; Cai, W.; Sawada, J.; Komatsu, M.; Boyle, J.J.; Zhou, B.; Ruiz-Lozano, P.; Mercola, M. Developmental origin of age-related coronary artery disease. Cardiovascular Research 2015, 107, 107–294. [Google Scholar]
- Wei, K.; Díaz-Trelles, R.; Liu, Q.; Diez-Cuñado, M.; Scimia, M.C.; Cai, W.; Sawada, J.; Komatsu, M.; Boyle, J.J.; Zhou, B.; Ruiz-Lozano, P.; Mercola, M. Nkx2-5- andIsl1-expressing cardiac progenitors contribute to proepicardium. Biochem.Biophys. Res. Commun. 2008, 375, 450–453. [Google Scholar] [CrossRef]
- Katz, T.C.; Singh, M.K.; Degenhardt, K.; Rivera-Feliciano, J.; Johnson, R.L.; Epstein, J.A.; Tabin, C.J. Distinct Compartments of the Proepicardial Organ Give Rise to Coronary Vascular Endothelial Cells. Dev. Cell 2012, 22, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.-L.; Martin, J.C.; Sun, Y.; Cui, L.; Wang, L.; Ouyang, K.; Yang, L.; Bu, L.; Liang, X.; Zhang, X.; et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature 2008, 454, 104–108. [Google Scholar] [CrossRef]
- Cossette, S.; Misra, R. The identification of different endothelial cell populations within the mouse proepicardium. Dev. Dyn. 2011, 240, 2344–2353. [Google Scholar] [CrossRef]
- Vicente-Steijn, R.; Scherptong, R.W.C.; Kruithof, B.P.T.; Duim, S.N.; Goumans, M.J.T.H.; Wisse, L.J.; Zhou, B.; Pu, W.T.; Poelmann, R.E.; Schalij, M.J.; et al. Regional differences in WT-1 and Tcf21 expression during ventricular development: implications for myocardial compaction. PLOS ONE 2015, 10, e0136025. [Google Scholar] [CrossRef]
- Kikuchi, K.; Gupta, V.; Wang, J.; Holdway, J.E.; Wills, A.A.; Fang, Y.; Poss, K.D. tcf21+ epicardial cells adopt non-myocardial fates during zebrafish heart development and regeneration. Development 2011, 138, 2895–2902. [Google Scholar] [CrossRef]
- MacNeill, C.; French, R.; Evans, T.; Burch, A.W.A.J.B. Modular Regulation of cGATA-5 Gene Expression in the Developing Heart and Gut. Dev. Biol. 2000, 223, 463. [Google Scholar] [CrossRef]
- Peeters, M.-. .-P.F.M.V.; Mentink, M.M.T.; Poelmann, R.E.; Groot, A.C.G.-D. Cytokeratins as a marker for epicardial formation in the quail embryo. Anat. Embryol. 1995, 191, 503–508. [Google Scholar] [CrossRef]
- Balmer, G.M.; Bollini, S.; Dubé, K.N.; Martinez-Barbera, J.P.; Williams, O.; Riley, P.R. Dynamic haematopoietic cell contribution to the developing and adult epicardium. Nat. Commun. 2014, 5, 4054. [Google Scholar] [CrossRef]
- Niderla-BieliŃska, J.; Jankowska-Steifer, E.; Flaht-Zabost, A.; Gula, G.; Czarnowska, E.; Ratajska, A. Proepicardium: Current Understanding of its Structure, Induction, and Fate. Anat. Rec. 2018, 302, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Plavicki, J.S.; Hofsteen, P.; Yue, M.S.; A Lanham, K.; E Peterson, R.; Heideman, W. Multiple modes of proepicardial cell migration require heartbeat. BMC Dev. Biol. 2014, 14, 18–18. [Google Scholar] [CrossRef]
- Pérez-Pomares, J.M.; Phelps, A.; Sedmerova, M.; Wessels, A. Epicardial-like cells on the distal arterial end of the cardiac outflow tract do not derive from the proepicardium but are derivatives of the cephalic pericardium. Dev. Dyn. 2003, 227, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Peralta, M.; González-Rosa, J.M.; Marques, I.J.; Mercader, N. The Epicardium in the Embryonic and Adult Zebrafish. J. Dev. Biol. 2014, 2, 101–116. [Google Scholar] [CrossRef]
- Cui, Y.; Zheng, Y.; Liu, X.; Yan, L.; Fan, X.; Yong, J.; Hu, Y.; Dong, J.; Li, Q.; Wu, X.; et al. Single-Cell Transcriptome Analysis Maps the Developmental Track of the Human Heart. Cell Rep. 2019, 26, 1934–1950. [Google Scholar] [CrossRef] [PubMed]
- Farbehi, N.; Patrick, R.; Dorison, A.; Xaymardan, M.; Janbandhu, V.; Wystub-Lis, K.; Ho, J.W.; E Nordon, R.; Harvey, R.P. Single-cell expression profiling reveals dynamic flux of cardiac stromal, vascular and immune cells in health and injury. eLife 2019, 8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




