1. Introduction
In the pathogenesis of neurodegenerative disorders including Alzheimer's disease (AD), an age-related disorder characterized by memory deficits, exposure to neurotoxicants of natural or industrial origin or those produced by human activities holds a key role (1–6). The neurotoxic substances including reactive oxygen species (ROS) act either directly on neural cells or produce oxidative stress and interfere with various metabolic processes of fundamental significance for the functional integrity of the nervous system (2–6). They also disrupt various brain functions usually related to abnormalities of ion channel function, neurotransmitter release and receptor activation (1).
The brain is particularly susceptible to oxidative stress due to its high energy demands and the presence of lipids that are susceptible to oxidation. It is known that lipid peroxidation can produce reactive compounds that can modify the activity of ion channels and membrane fluidity, among others. Oxidative stress can also affect the organization and distribution of membrane-associated proteins, such as receptors and transporters, thus further exacerbating neuronal function (7). In particular, in the case of AD, the accumulation of β-amyloid proteins in the brain, a hallmark of this disease, is thought to contribute to oxidative stress and neurotoxicity, because these proteins can stimulate the production of reactive oxygen species (ROS). It is considered that these mechanisms play significant roles in the progressive neurodegeneration observed in AD. Therefore, pharmacological interventions targeting oxidative stress may have therapeutic potential in the treatment of AD (8). To date, the use of antioxidants in the treatment regimes to retard the progression of AD remains a complicated goal. It is of note that some antioxidants have also metal-chelating capacity, which may be beneficial in certain therapeutic settings (9,10). There is still though no effective cure for AD and the available options focus on alleviating and retarding the symptoms.
Since ancient times, traditional medicine aims worldwide at strengthening memory. Natural therapy, employing in most cases herbal preparations, has been extensively used in the treatment of memory deficits that are observed in dementia, amnesia and AD (11,12). They are utilized in these disorders mainly because of their antioxidant properties (3–6). Previous studies employing in vitro and in vivo models of AD reported that several Crocus, Olea europea and Salvia plant species exhibit beneficial effects that were primarily attributed to the neuroprotective, antioxidant and anti-inflammatory properties of crude extracts or isolated and characterized active compounds (3,6,11,12). In particular, in the case of Salvia, also known as Sage, these effects have been attributed to compounds known as salvianolic acids that have antioxidant properties and can protect the brain from oxidative stress and improve memory and cognitive functions in patients with mild to moderate AD (13). It is conceivable that more research is needed to fully understand the mechanisms underlying the potential benefits of these plant extracts, because they appear to be promising as natural alternatives or complementary to the traditional treatment of AD.
In this study the potential antioxidant and neuroprotective properties of several medicinal plants, widely used in folk medicine, were investigated employing an in vitro model of AD. We have assessed the effects of Salvia, Crocus sativus and Olea europea leaf extracts and several major reactive compounds against superoxide (H2O2)- and β-amyloid-induced toxicity on differentiated human SH-SY5Y neuroblastoma cells . Our results suggest that Salvia fruticosa, Salvia officinalis and Salvia argentea leaf extracts, along with the reactive compounds trans-crocin-3, trans-crocin-4 and oleuropein display significant neuroprotective and antioxidant properties.
2. Materials and Methods
2.1. Reagents and Materials
All solvents were purchased from Fisher Scientific. For extraction and isolation procedures, Dichloromethane (DCM), Diethylether, Ethanol (EtOH), Ethyl Acetate (EtOAc), Acetone, Methanol (MeOH), and n-Heptane (n-Hept) were of analytical grade, while deionized water was used to prepare all aqueous solutions. For preparative HPLC analyses, Acetonitrile (AcN), Methanol (MeOH), and Water were of appropriate grade. Vanillin standard and Sulfuric acid (H2SO4) were obtained from Sigma-Aldrich. Analytical Thin Layer chromatography (TLC) was performed on Merck 60 F254 pre-coated silica gel plates (Merck Millipore, Billerica, MA, USA). TLC spots were observed at 254 nm and 366 nm and were visualized by heating silica gel plates sprayed with vanillin 10% H2SO4 in EtOH. 1D (1H, 13C) and 2D (COSY, HSQC-DEPT135, HMBC) NMR spectra were carried out on a Bruker Avance III-600 spectrometer (Karlsruhe, Germany) in 600 μL of deuterated chloroform. 1H and 13C NMR spectra were acquired at 600.15 MHz and 150.91 MHz, respectively. Chemical shifts (δ) are expressed in ppm while coupling constants (J) in Hz.
The human neuroblastoma SH-SY5Y cell line was provided by Dr. Th. Michailidis (University of Ioannina, Ioannina, Greece). 7PA2-CHO cells, which were isolated in the laboratory of Dr. D. Selkoe (Harvard University, Boston, USA), were kindly provided by Dr. K. Vekrellis (BRFAA, Athens, Greece). Dulbecco's Modified Eagle's Medium (DMEM), Ham's F-12 Nutrient Mixture, Fetal Bovine Serum (FBS) and Phosphate Buffered Saline (PBS) were purchased from Gibco (Grand Island, NY, USA). For cell viability assays, CellTiter 96® AQueous One Solution Reagent (G3581) containing a novel tetrazolium compound, [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS)], and an electron coupling reagent, phenazine ethosulfate (PES), was purchased from Promega (Madison, WI, USA). The tested compounds were dissolved in DMSO in 0.25–50 mM stock solutions. Stock solutions were stored at −20°C. The final concentration of DMSO (vehicle) that was used in the present biological studies was 0.2% v/v.
2.2. Plant Material
Crocus sativus L. (Iridaceae) stigmas (saffron) were provided from the Cooperative De Safran (Krokos Kozanis, West Macedonia, Greece). The final product obtained by the dehydration of the harvested saffron stigmas (first at 20°C and later at 35°C) until the moisture reaches the level of about 10%. Olea europaea var. Koroneiki L. (Oleaceae) leaves were collected in 2018 at the region of Attica and left to dry in a well-ventilated shady place. Subsequently, the dried leaves were ground using an AllenWest type SCIS grinder with a sieve of 3 mm. Olive oil used for the extraction of TPF (Total Phenolic Fraction) was freshly produced from olive fruits of Olea europaea var. Koroneiki and was kindly provided by Pharmagnose S.A. (Inofyta, Greece). Seven species of genus Salvia (Labiatae) were studied in the present study. The aerial parts of each species were collected from their natural environment and were authenticated by the botanist Dr. Eleftherios Kalpoutzakis. In more details, Salvia officinalis was collected at altitude 800-900 m from Mt. Tzoumerka, Central Greece, in June 2018 (Voucher specimen: ΤΖ 019), Salvia rigens and Salvia verbenaca were collected in April 2018 at a region near to Varkiza (Attica, Greece) (Voucher specimens: KL 208 and KL 207 respectivelly), Salvia fruticosa was collected in July 2018 from Mt. Psiloritis (Crete, Greece) near to village Zaros (Voucher specimens: KL 053), Salvia argentea was collected at altitude 1400 m from Mt. Parnonas (south Peloponese, Greece) in June 2018 (Voucher specimens: KLPY 289), Salvia amplexicaulis was collected in June 2018 from Mt. Vurnos (North Greece) at an altitude of 1100 m (Voucher specimens:SM 001), and Salvia pomifera was collected in August 2018 at a region near to the village of Spili in central Crete (Voucher specimens: F 016). Specimens of the collected plants were stored in the herbarium of the Division of Pharmacognosy and Natural Products Chemistry, Department of Pharmacy, National Kapodistrian University Athens, Greece. The plant material was dried under shadow and pulverized by a ground mill.
2.3. Extraction of Plant Material
The extraction of C. sativus stigmas was carried out in two steps following a well-established process described previously by Karkoula et.al.(14). In details, 20 g of dried stigmas were initially defatted with 1L of Diethylether for 2 h at 40oC (Diethilether extract). Subsequently the defatted stigmas were extracted with 2.5 L of Methanol/Water in ratio 1/1 for 24 h at 25oC in the dark place. The extraction eluent was then filtered, evaporated under vacuum until the complete removing of methanol and lyophilized yielding totally 11.7 g of the C. sativus hydrophilic extract.
The Oleuropein enriched extract was obtained from olive leaves following a previously described extraction process (15)with slight modifications. In details, 200 gr of grounded leaves were subjected to ultrasound-assisted extraction (UAE). The extraction was carried out with 1.5 L of acetone at room temperature and lasted 1 h. Then, the extraction eluent was filtered, evaporated to dryness and weighted yielding 18.1 g of Acetone extract. In order to increase the oleuropein content, the obtained Acetone extract was further defatted with a solution of dichloromethane/methanol 98/2 resulting in the recovery of 10.6 g of defatted olive leaves extract.
A liquid-liquid extraction process developed by Angelis et. al. (16,17) was applied for the recovery of TPF from Extra Virgin Olive oil. The extraction was carried out in a pilot-scale extractor ACE-BXP190 (Rousselet-Robatel Kromaton, Annonay, France) with parallel pumping of the feed oil phase (n-Hept/EVOO in ratio 3:2 v/v) and aqueous extraction phase (EtOH/H2O in ratio 3:2 v/v) into the extractor bowl. By setting the flow rate of both phases at 5 L/min and the rotation speed at 1050 rpm, 80 L of EVOO (diluted in 120 L of n-Hept) were extracted rapidly (40 min) resulting in the recovery of 200 L of enriched in polyphenols aqueous extraction phase. Then, the enriched aqueous phase was evaporated under vacuum leading in the recovery of 72 g of Total Polyphenolic Fraction (TPF).
The plant material of Salvia species were subjected to ultrasound-assisted extraction (UAE) using Ethanol as extraction solvent. For each species 20 g of the pulverized aerial parts were extracted with 200 mL of Ethanol for 30 min at room temperature using a laboratory ultrasonic bath. The supernatant was filtrated, and the solvent was evaporated under vacuum (at 40oC) to dryness. The procedure was repeated three times yielding 2.24 g of ethanolic extract (11.2 % w/w) for S. officinalis, 2.02 g of extract (10.1 % w/w) for S. fruticosa, 1.96 g of extract (9.8 % w/w) for S. argentea, 1.82 g of extract (9.1 % w/w) for S. rigens, 2.06 g of extract (10.3 % w/w) for S. verbenaca, 1.9 g of extract (9.5 % w/w) for S. amplexicaulis, and 1.92 g of ethanolic extract (9.6 % w/w) for S. pomifera. Details for chromatographic isolation of secondary metabolites are given as Supplementary material.
2.4. Differentiation of human SH-SY5Y neuroblastoma cells
Human SH-SY5Y neuroblastoma cells maintained in a growth medium consisting of DMEM:F12 (1:1), supplemented with Fetal Bovine Serum (FBS, 10%), penicillin/streptomycin (1%) and L-glutamine (at a final concentration of 2 mM), were incubated at 37oC and 5% CO2. Differentiation of SH-SY5Y cells to cholinergic neurons was induced by retinoic acid (5 μM). The undifferentiated SH-SY5Y cells were cultured (seeding density of 7.5 × 104 cells/well) in DMEM:F12 (1:1) without FBS, supplemented with retinoic acid, penicillin/streptomycin (1%) and L-glutamine (at a final concentration of 2 mM) for 4-5 days. Differentiation medium was changed every 48 h.
Isolation of the Aβ peptides containing supernatant of 7PA2-CHO cell cultures
7PA2-CHO, mutant APP-expressing cells, were cultured in 10 cm plates containing DMEM growth medium supplemented with FBS (10%), penicillin/streptomycin (1%), L-glutamine (at a final concentration of 2 mM) and G418 (100 μg/ml). After two passages, they were shifted to medium without G418 for at least 24 h and after extensive washing, they were incubated overnight in 10 ml of DMEM:F12 (1:1) medium supplemented with 1% penicillin/streptomycin and L-glutamine (at a final concentration of 2 mM) . The supernatant of the cells, containing APP and a mixture of Aβ peptides in oligomeric forms, was collected and centrifuged in order to remove cells and cell debri (18). The final cleared supernatant was aliquoted and stored at
-20oC. The same protocol was used for the isolation of the supernatant of naive CHO cell cultures (CHO-K1), which was used as control.
2.5. Compound toxicity
The potential toxicity of the compounds and plant extracts tested in this study was assessed using differentiated human SH-SY5Y neuroblastoma cells (7.5 × 104 cells/well), which were cultured in 96-well plates. Five days following the differentiation of SH-SY5Y cells, the incubation medium was changed to DMEM:F12 (1:1) without FBS, which was supplemented with penicillin/streptomycin (1%) and L-glutamine (final concentration 2 mM). The compounds and plant extracts were added at various concentrations. As a vehicle, either DMSO or DMSO: ethanol (3:1), at a final concentration of 0.2% v/v was used. After 48 h, cell viability was assessed at 492 nm using the MTS assay.
2.6. Aβ peptide- and H2O2-induced toxicity
Human SH-SY5Y neuroblastoma cells (7.5 × 104 cells/well of a 96-well plate) were differentiated for 5 days. The medium was changed to DMEM:F12 (1:1) without FBS, supplemented only with penicillin/streptomycin (1%) and L-glutamine (at a final concentration of 2 mM) and after 24hr, the medium was aspirated, the cells were washed with Phosphate Buffered Saline (PBS) and were pre-incubated for 4h in DMEM:F12 (1:1) medium containing the medicinal plant extracts and compounds tested at various concentrations or the vehicles used. Subsequently, either H2O2 (final concentration 750 μМ), the 7PA2-CHO supernatant (final concentration 75% v/v) or the control CHO-K1 supernatant (final concentration 75% v/v) was added. Following incubation for 48hr, cell viability was assessed using the MTS assay at 492 nm.
2.7. Cell viability
MTS reagent (20 μl of a solution 1.90 mg/ml MTS) and 300 μM PES in Dulbecco’s PBS (pH 6.0) was added into control and differentiated SH-SY5Y treated cells (total volume 120 μl) seeded in 96-well plates and the plates were incubated at 37 oC and 5% CO2 for 90 min. Absorbance was measured in an ELISA spectrophotometer at 492 nm. All MTS assays were performed using triplicate samples and the Data were expressed as % cell viability. DMSO-ethanol (3:1) or DMSO medium-treated cells (controls) represented 100% cell viability.
2.8. Statistical analysis
All experiments were repeated at least twice using different cell batches and samples in triplicates. The present data are presented as the mean ± SEM and their statistical analysis was performed using the one-way analysis of variance (ANOVA) followed by the Bonferroni’s and Tuckey’s post-hoc tests. The significance level for all analyses was set at probability of less than 0.05 (* P < 0.05, ** P < 0.01, *** P < 0.001).
3. Results
3.1. Assessment of the effects of extracts and compounds on H2O2-induced cell toxicity
H2O2 is a widely used oxidative stress inducer, which can generate exogenous free radicals that cause cytotoxicity. It constitutes a popular model to study the oxidative stress -induced neuronal cell death (10,19).
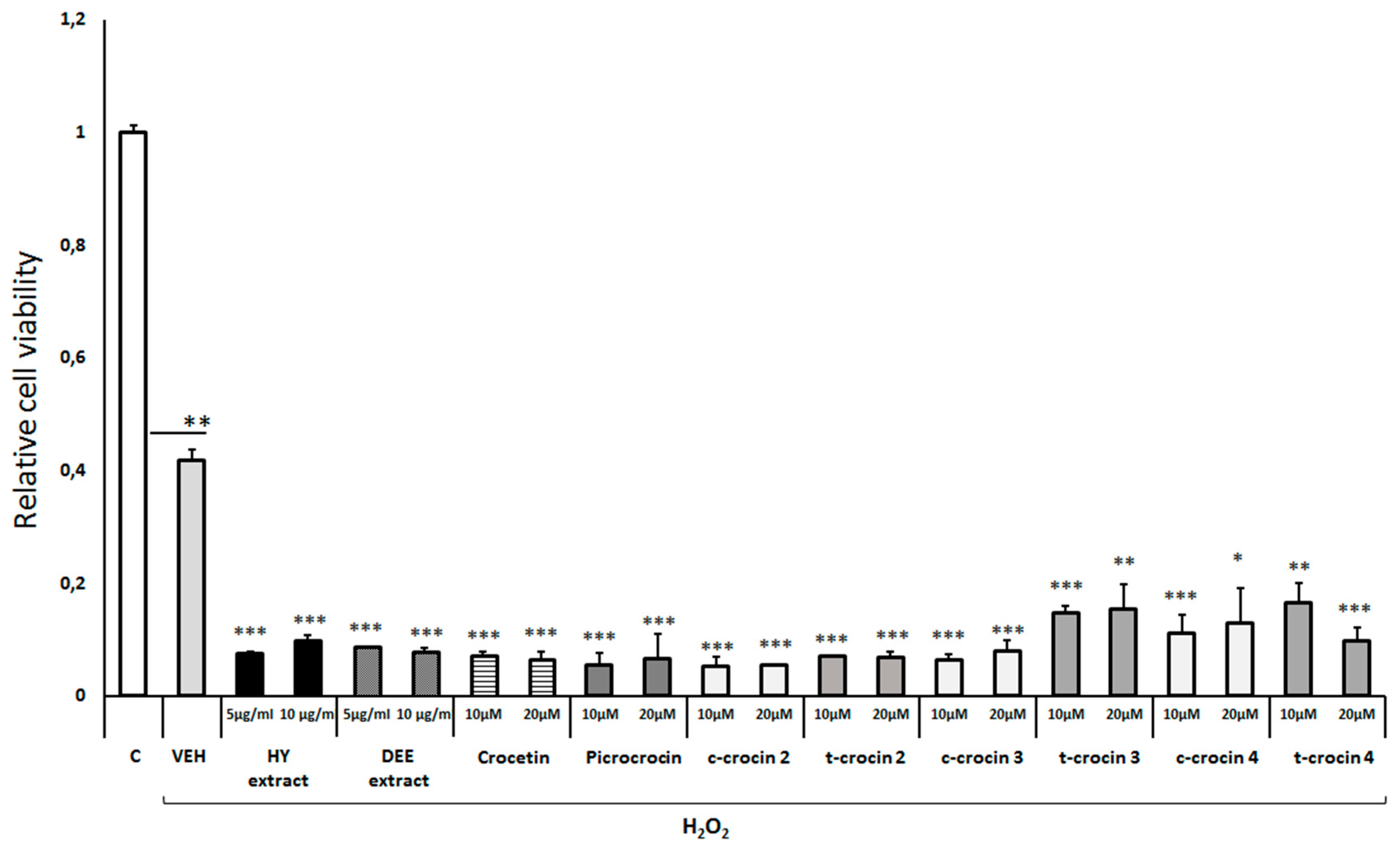
Multiple dose-response experiments indicated that treatment of differentiated human SH-SY5Y neuroblastoma cells with 750 μМ H
2O
2 reduced cell viability by 40-60%, as expected (
Figure 1)(10). Notably, pretreatment of the cells with either the hydrophilic or diethyl ester extract of
Crocus sativus augmented the H
2O
2-induced cell toxicity (
Figure 1). Furthermore, treatment of differentiated cells with either crocetin, picrocrocin, cis-crocin 2, trans-crocin 2, cis-crocin 3, trans-crocin 3, cis-crocin 4 or trans-crocin 4 did not provide any protection against the H
2O
2-induced reduction of cell viability, but exacerbated the H
2O
2-induced toxicity (
Figure 1).
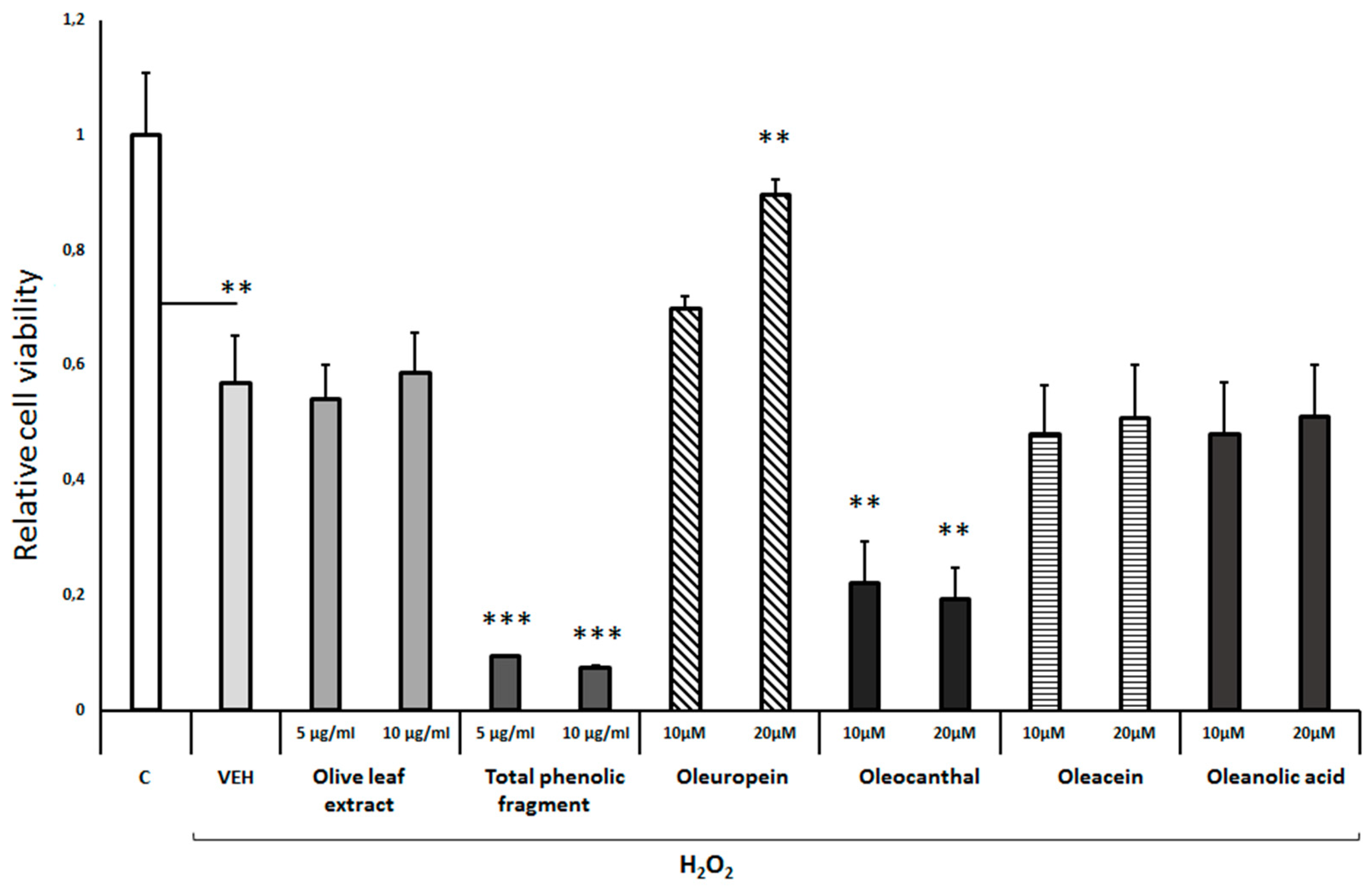
On the other hand, pretreatment of differentiated cells with either the
Olea europea leaf extract, oleacein or oleanolic acid did not affect the H
2O
2-induced cell toxicity (
Figure 2). It should be noted though, that exposure of differentiated cells to either the total phenolic fragment of the olive leaf extract or oleocanthal exacerbated the H
2O
2-induced toxicity (
Figure 2). Of all the compounds tested from
Olea europea extracts, only oleuropein at a concentration of 20μM efficiently protected the differentiated cells against the H
2O
2-induced toxicity (
Figure 2).
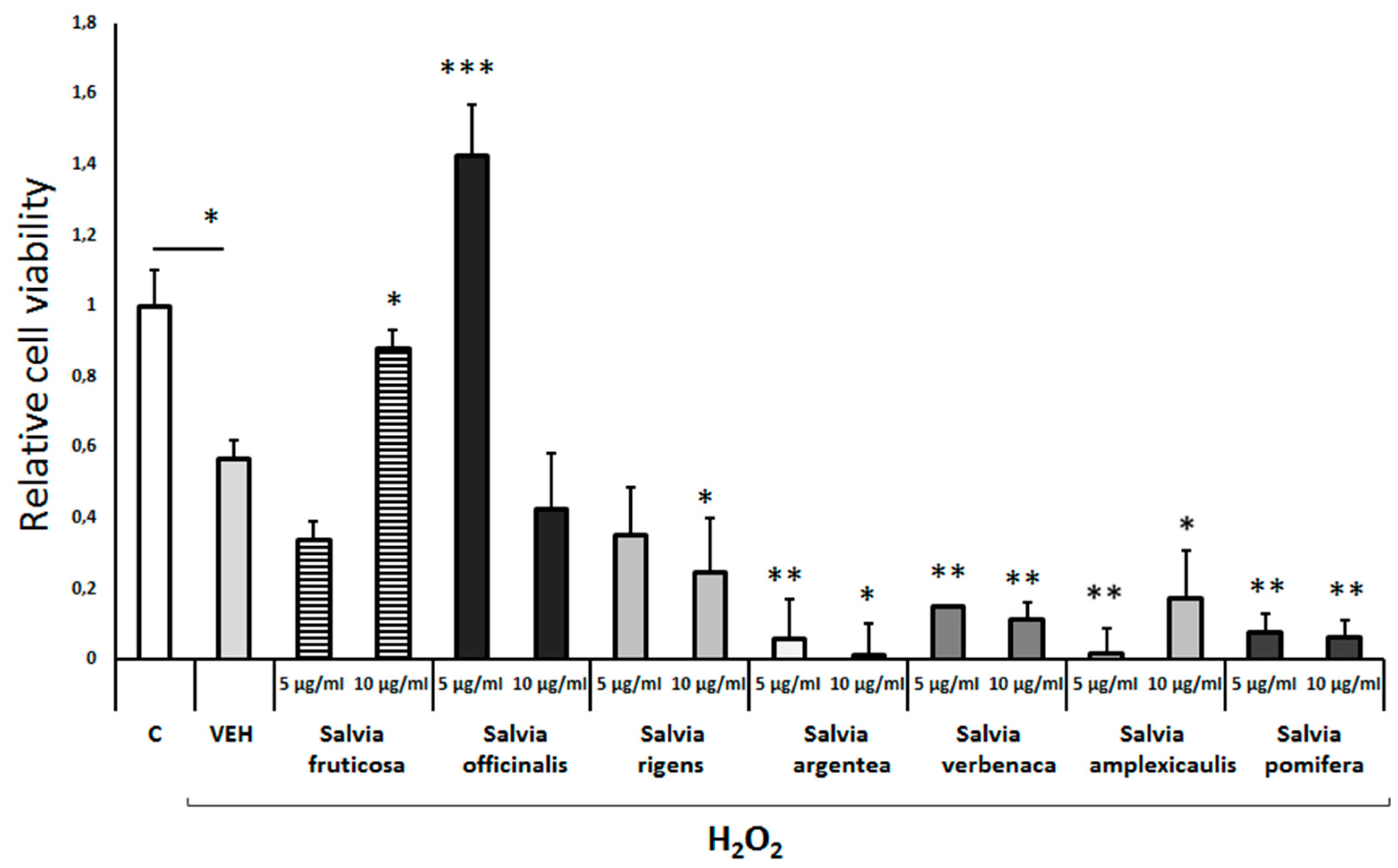
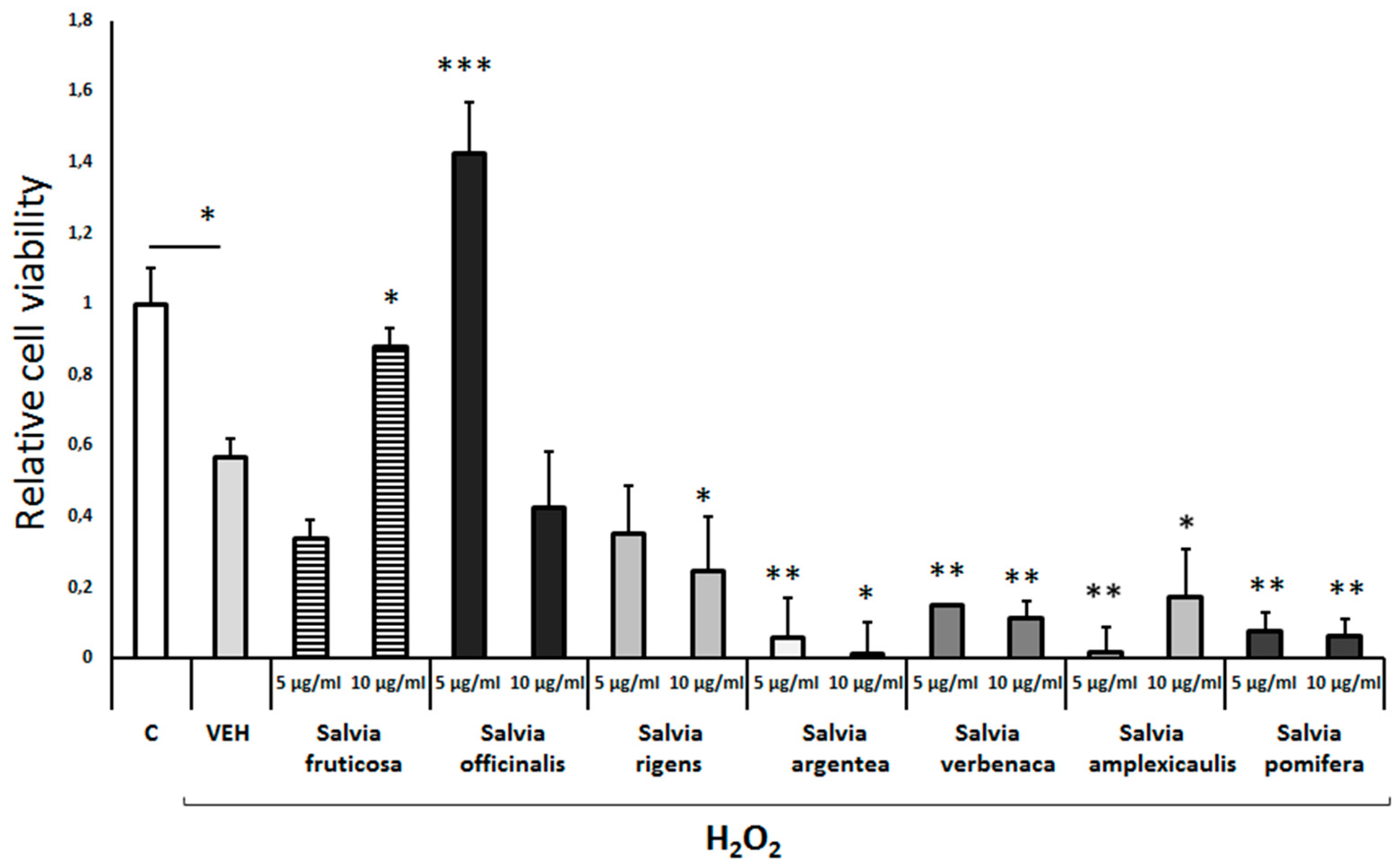
Exposure of differentiated cells to either
Salvia fruticosa or
Salvia officinalis leaf extract (10μg/ml and 5μg/ml, respectively) completely prevented the H
2O
2-induced cell toxicity (
Figure 3). Of note, the
Salvia officinalis extract induced a significant trophic effect on these cells in the presence of H
2O
2. All the other Salvia leaf extracts tested (
S. rigens, S. argentea, S. verbenaca, S. amplexicaulis and
S. pomifera) augmented the H
2O
2-induced cell toxicity (
Figure 3).
3.2. Assessment of the effects of extracts and compounds on Aβ-amyloid-induced cell toxicity
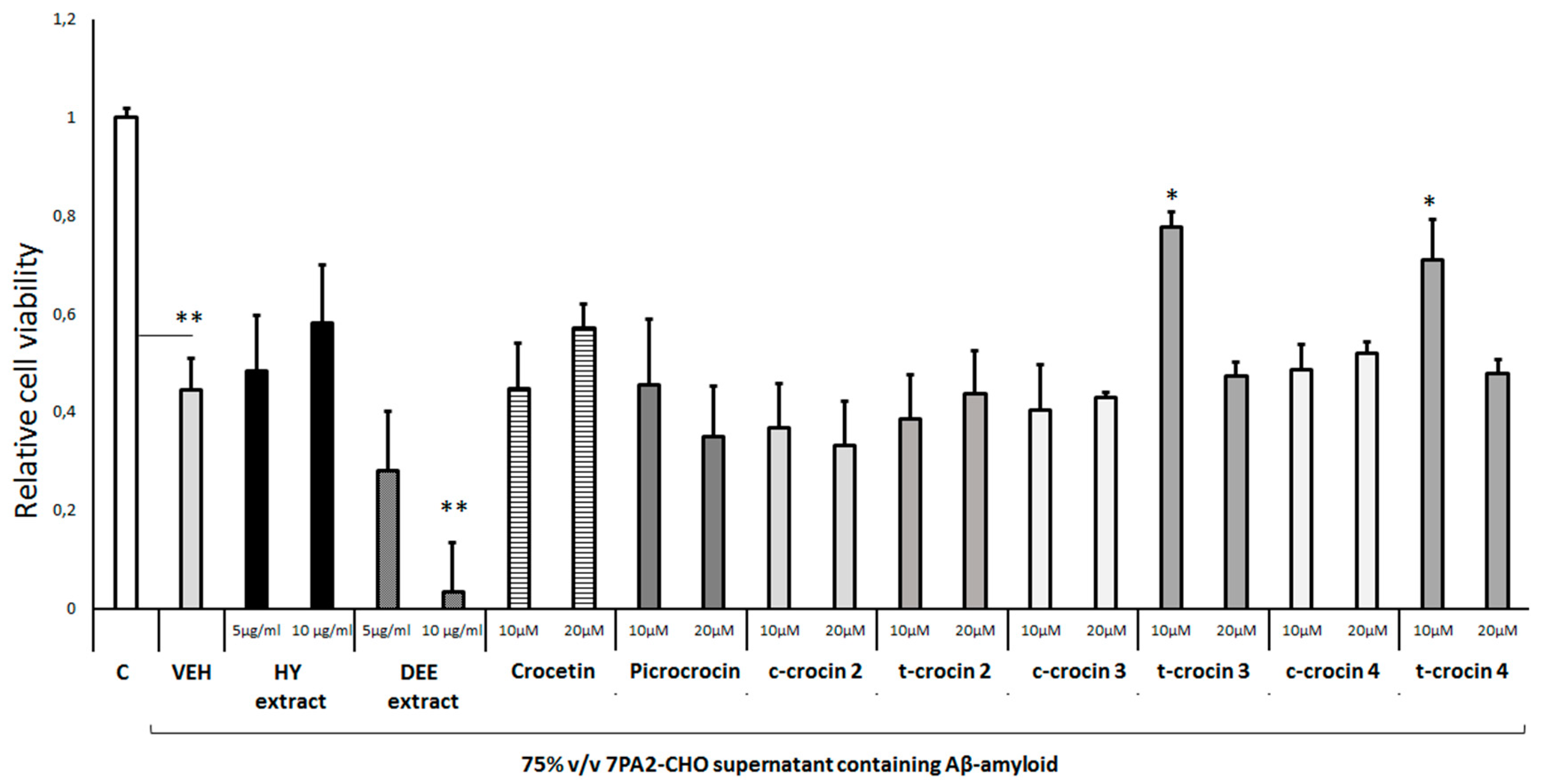
Treatment of differentiated cells with a natural mix of Aβ-amyloid peptides present in the supernatant of the stable cell line CHO 7PA2 (18) markedly reduced cell viability to approximately 45% of control (
Figure 4) as reported previously (10). Of all the reactive compounds of
Crocus sativus tested, only trans-crocin 3 and trans-crocin 4 at 10μM protected the cells from the Aβ-induced cell toxicity by increasing cell viability to 70-80% of control level (
Figure 4). All other compounds had no effect, while interestingly, exposure of the cells to the diethyl ether extract (DEE) of
Crocus sativus leaves exacerbated the Aβ-induced cell toxicity (
Figure 4).
Treatment of the differentiated cells with either the total olive leaf extract, oleacein or oleanolic acid had no effect on the Aβ-induced cell toxicity (
Figure 5). Notably, exposure of the cells to the total phenolic fragment of the olive leaf extract or oleocanthal markedly augmented the Aβ-induced reduction of cell viability (
Figure 5). It is of interest though to note that oleuropein was able to protect to some extent the cells from the Aβ-induced toxicity (
Figure 5).
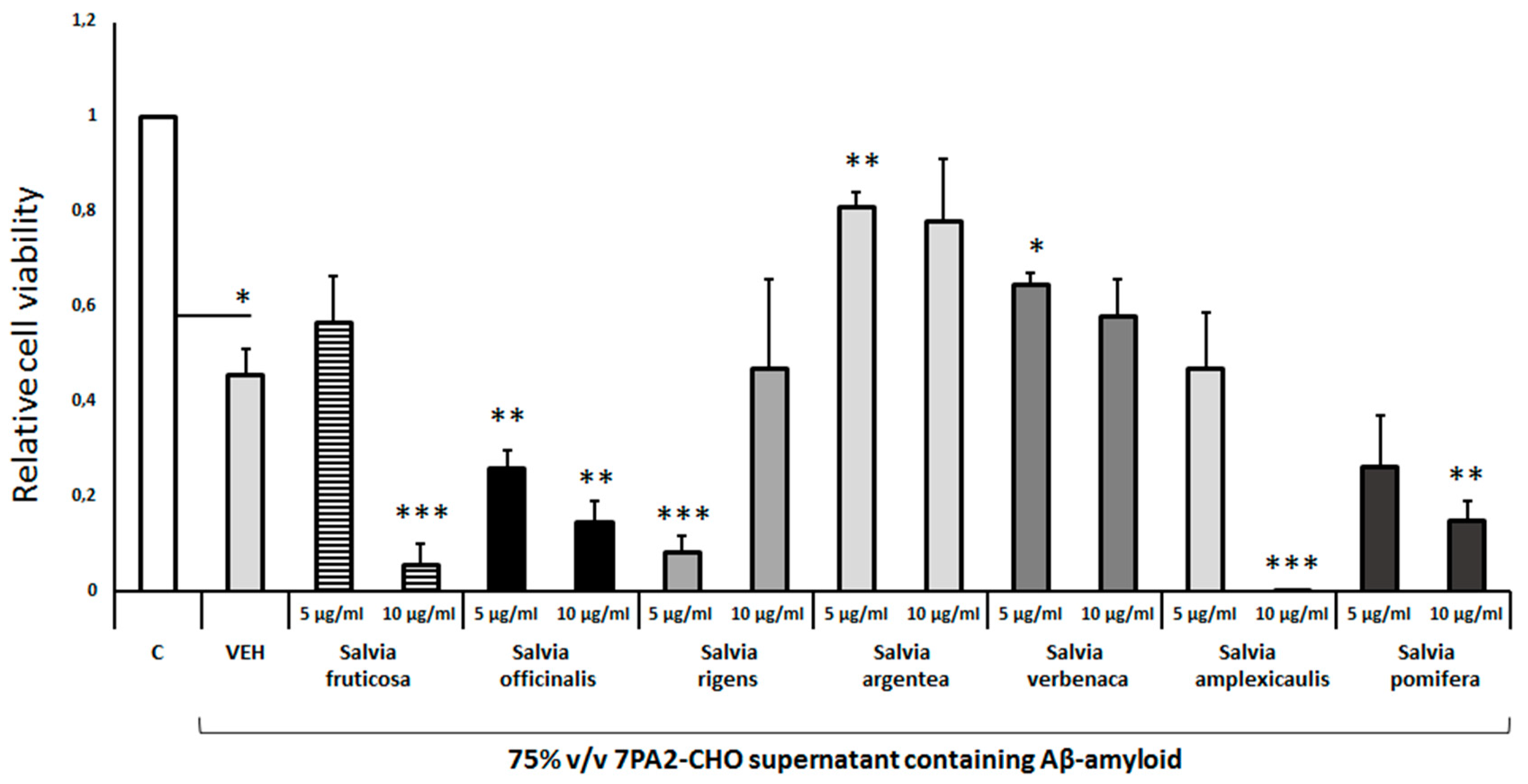
Pretreatment of differentiated cells with
Salvia argentea and
Salvia verbenaca leaf extracts protected to some extent from the Aβ-induced toxicity (
Figure 6). But, the extracts from other Salvia species, such as
Salvia fruticosa,
Salvia rigens,
Salvia amplexicaulis did not provide any protection (
Figure 6). Interestingly,
Salvia officinalis and
Salvia pomifera enhanced the Aβ-induced cell toxicity in the concentrations tested (
Figure 6). It appears that the different Salvia spp leaf extracts differ significantly in their effects on the Aβ-induced cell toxicity in a concentration-dependent manner.
3.3. Assessment of extract- and compound-induced cell toxicity
To assess the potential cellular toxicity of crude extracts and isolated fractions or individual compounds, differentiated human SH-SY5Y neuroblastoma cells were incubated with different concentrations of them for 48 h. Exposure of the cells to the hydrophilic extract of
Crocus sativus (10μg/ml) markedly reduced cell viability about 70%, whereas this extract at a concentration of 5μg/ml had no effect (Suppl.
Figure S1A). The diethyl ester extract of
Crocus sativus and all the active compounds of this medicinal plant tested, including crocetin, picrocrocin, cis-crocin 2/3/4 and trans-crocin 3/4 did not display any toxic effect on the differentiated cells, except for trans-crocin 2, which at a concentration of 20μM slightly reduced cell viability by 15% (Suppl.
Figure S1A).
Treatment of the cells with the extract of olive leaves, oleuropein, oleacein or oleanolic acid did not reduce cell viability. On the contrary, cell viability was increased by 20-40%, suggesting a significant trophic effect of olive leaf extract and oleuropein (Suppl.
Figure S1B). Interestingly, the total phenolic fraction of the olive leaf extract and oleocanthal appear to display a strong toxic effect on the differentiated cells as they reduced cell viability by about 80-90% (Suppl.
Figure S1B).
Salvia species leaf extracts were tested at a concentration of 5μg/ml and 10μg/ml. Although these extracts at the low concentration did not affect cell viability (Suppl.
Figure S1C), they markedly reduced it by 30-65% at the high concentration (Suppl.
Figure S1C). Notably, the
Salvia fruticosa leaf extract at the high concentration displays the strongest toxic effect (approximately 80% reduction of cell viability; Suppl.
Figure S1C).
4. Discussion
Previous preclinical and clinical studies indicated the beneficial impact of several plant species including Crocus, Olea and Salvia in AD that has been primarily attributed to neuroprotective, antioxidant and anti-inflammatory properties of crude extracts or isolated and characterized active compounds (3,6,11,12). However, only a few comparative studies have been performed to assess these properties of medicinal plant extracts and compounds, as well as their toxicity in differentiated human neuronal cells. Such comparative studies using relevant cellular models may well reveal additional compounds or extracts that warrant further development and also highlight specific aspects of neuroprotective action of medicinal plant extracts. In this study, we embarked on a comprehensive investigation of the antioxidant and neuroprotective properties of several medicinal plants, widely used in folk medicine, using a well-established in vitro model of AD, the differentiated human SH-SY5Y neuroblastoma cells. We have focused on the effect of Olea europea, Crocus sativus and seven Salvia species leaf extracts and several of their bioactive constituents on the H2O2- and Aβ-amyloid-induced neurotoxicity. These two neuroxicity assays are well established and highly relevant to AD.
4.1. Toxicity of the extracts and individual compounds in differentiated human SH-SY5Y neuroblastoma cells
Cellular toxicity of extracts and individual compounds is often studied using proliferating human or rodent cell lines, as their ability to inhibit cellular growth can serve as an indication of their toxicity. Critical factors, such as the specific cell type, the duration of treatment and the composition of culture medium (i.e., the concentration of serum) may significantly affect the toxicity outcomes (20). In the present study human SH-SY5Y neuroblastoma cells were employed, which were differentiated to a neuron-like cell population by adding retinoic acid in the cell culture in the absence of serum (10,21,22). Under these conditions, toxicity (i.e., decrease of cell viability) likely corresponds to deregulation of neuronal survival pathways and it is irrelevant to antiproliferative effects observed in proliferating human or murine cell lines used in other studies, including non-differentiated, proliferating SH-SY5Y neuroblastoma cells (23–25).
Current study indicated that extracts and individual compounds from
Crocus sativus exhibited no significant toxicity at the low concentrations tested, while all Salvia extracts exhibited a moderate dose-dependent toxicity (Suppl.
Figure S1). Previous studies, using proliferating cell lines, such as human melanoma cell lines and non-cancerous human fibroblasts (26), indicated that some Salvia species, (e.g
Salvia pomifera and
Salvia fruticosa) may exhibit a dose- and time-dependent toxicity with IC
50 values in the range of 7-20 μg/ml. Our results suggest lower levels of cytotoxicity of Salvia extracts in differentiated human SH-SY5Y cells compared to other human cell lines. Interestingly, extracts and individual compounds from
Olea europea leaves, namely the total phenolic fraction and oleocanthal, exhibited high toxicity even at low concentrations (Suppl.
Figure S1). It should be noted that oleocanthal represents a constituent of the total phenolic fraction of Olea leaves (27). Previous studies using SH-SY5Y cells reported no effects on cell viability by submicromolar or micromolar (1-10 μΜ) concentrations of oleocanthal (28,29). The apparent discrepancy between our results and those of previous studies (29) could be attributed to the different time of incubation and the composition of the differentiating medium, which in the latter case contained 1% fetal bovine serum. It has been postulated that the effects of olive oil phenols, including oleocanthal, can be neutralized by binding to proteins (30). Thus, even small amounts of serum may sequester oleocanthal and other phenolic compounds and hence, blunt their cytotoxicity
in vitro. In sharp contrast to oleocanthal, oleuropein, oleacein and oleanolic acid were virtually devoid of any toxicity at the tested concentrations, as shown previously in neuronal cell lines (31–33).
4.2. Antioxidant and neuroprotective properties of Crocus sativus extracts and individual compounds
We assessed the possible effects of
Crocus sativus extracts and several individual compounds against the H
2O
2-induced oxidative stress and toxicity using differentiated human SH-SY5Y cells. Surprisingly, under these conditions, we found that all
Crocus extracts and individual compounds augmented the H
2O
2-induced cell toxicity (
Figure 1). It is suggested that this synergistic effect is not associated with a direct reaction of H
2O
2 with the compounds, since in this experimental protocol the cells were preincubated with the extracts or compounds for 4h and then, they were challenged with H
2O
2. To our knowledge, the synergistic toxicity of crocins at low micromolar concentrations with that of H
2O
2 has not been previously reported. Notably, only few studies have assessed the potential protective effect of
Crocus sativus extracts and individual compounds against oxidative stress using neuronal cells, despite the significant antioxidant properties of crocins that are observed in animal and cellular models of neurodegenerative disorders (34,35). Previous studies employing neuronal retinal ganglion cells or proliferating SH-SY5Y cells reported a protective effect of low crocin concentrations (0.1-1 μM) against H
2O
2-induced toxicity (36), or other types of oxidative stress (36,37).
Despite the apparent synergistic toxicity of
Crocus sativus extracts and individual compounds with H
2O
2, specific crocins appeared to exhibit significant neuroprotective activity against Aβ-induced toxicity. Trans-crocin-3 and -4 at 10μM protected cells from the Aβ-induced toxicity by increasing cell viability to 70-80% (
Figure 4). It has been reported that crocins and specifically, trans-crocin-4 display the capacity to interfere with amyloid-forming peptides either by binding to hydrophobic regions of Aβ (the hydrophobic carotene backbone) thus inhibiting the formation of fibrils or by interacting with water molecules in the immediate environment of Aβ molecules via the sugar units (38,39). Furthermore, the trans-crocin-4-mediated inhibition of GSK3β, an important kinase for the aberrant phosphorylation of Tau, may explain the protective effect of this compound (40,41). To our knowledge, this is the first report that trans-crocin-3 exhibits significant neuroprotection in
in vitro cellular AD models. Trans-crocin-3 may utilize similar mechanisms with trans-crocin-4, but this hypothesis requires further investigation in the framework of a new study (38,39,41).
4.3. Antioxidant and neuroprotective properties of Olea europea leaf extracts and individual compounds
Oleacein and Oleanolic acid did not exhibit any effect on H
2O
2-induced cell toxicity, whereas oleuropein appears to display a significant antioxidant effect (
Figure 2). Interestingly, oleuropein exhibited also significant neuroprotection against the Aβ-induced toxicity (
Figure 5). Of note, these results have been replicated in an parallel independent study by our group (42). These findings indicate that neuroprotection against oxidative stress and Aβ peptides, along with the induction of neural plasticity indices in several brain areas belong to the broad spectrum of the beneficial effects of oleuropein. Some, but not all, of these functions appear to depend on PPARα activation, since oleuropein can act as a PPARα agonist, thus modulating a variety of functions in the central and peripheral nervous system (43,44). These results are consistent with similar cellular studies and suggest that oleuropein has anti-amyloidogenic and antioxidant properties (45,46).
4.4. Antioxidant and neuroprotective properties of Salvia species extracts
As far as the possible antioxidant and neuroprotective properties of
Salvia spp leaf extracts is concerned, we found a significant diversification between the different Salvia species. In particular,
Salvia fruticosa (10μg/ml) and
Salvia officinalis (5μg/ml) extracts exhibited a mild antioxidant effect as they efficiently protected the differentiated SH-SY5Y cells from the H
2O
2-mediated cell toxicity (
Figure 3) (47). These results are consistent with those from similar studies reporting
Salvia officinalis antioxidant effects on a broad variety of different cell types (48). In contrast though, other
Salvia species exhibited negligible protective actions or even exacerbated oxidative toxicity (
Figure 3). It is of note that the antioxidant capacity of the
Salvia species polar extracts has been investigated mainly using
in vitro chemical and enzymatic assays and less using cellular
in vitro models (48). Interestingly, the antioxidant effects of
Salvia fruticosa and
Salvia officinalis extracts may be attributed to their flavonoid and phenolic compounds. These compounds bear Zn, Cu and Mg, which are essential in the antioxidant mechanisms (49–51). It is likely that differences in the abundance of specific flavonoid and phenolic compounds in the distinct
Salvia species could explain the diversity in the antioxidant properties within different
Salvia species (52). Along with their antioxidant properties, flavonoids, could also act as acetylcholinesterase inhibitors. These actions combined could make flavonoids promising multipotent drugs for the prevention and symptomatic treatment of AD (50). Indeed, we found that
Salvia argentea and
Salvia verbenaca leaf extracts exhibited significant neuroprotection against Aβ-induced toxicity, whereas other
Salvia species had no similar effects even at high concentrations (
Figure 6). Salvianolic acid B is a major constituent of the
Salvia extracts that dissociates amyloid fibrils of the full-length Aβ42 peptide at the low micromolar range (49,50). The above results suggest a significant diversification of antioxidant and neuroprotective effects of the distinct
Salvia species at least, when evaluated in differentiated human SH-SY5Y neuroblastoma cells.
5. Conclusions
In conclusion, several medicinal plant extracts and some of their main constituents appear to display protective effects against oxidative stress and the Aβ-induced toxicity in human differentiated SH-SY5Y cells. Oleuropein exhibited a consistent protective effect against both toxicities, while trans-crocin 3 and trans-crocin 4 significantly reduced the Aβ-induced neuronal cell toxicity. A significant diversification of the distinct Salvia spp extracts regarding their protective properties against cell toxicity was also observed. While Salvia fruticosa and Salvia officinalis exhibit a mild antioxidant effect, Salvia argentea and Salvia verbenaca exhibit significant neuroprotection against Aβ-induced toxicity. Surprisingly, and unlike most reports in the literature, we found that some extracts or individual compounds exhibited significant levels of toxicity under certain experimental conditions. For example, oleocanthal, isolated from the total phenolic fraction of Olea europea leaf extract, was highly toxic at low micromolar concentrations in all assays tested, while the Crocus sativus extract and its compounds potentiated significantly the H2O2-induced toxicity on differentiated SH-SY5Y cells. Apparently, taken together these results cannot be directly extrapolated to the in vivo animal and human condition. They should be though considered in depth and be the basis of further investigation in the framework of in vitro and in vivo studies. It is possible that under certain limiting or pathological conditions in vivo, some of these extracts and compounds are toxic. On the other hand, the significant neuroprotective properties of the extracts and compounds detected using the in vitro AD model of this study should be further evaluated in clinical studies.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
References
- Spencer PS, Lein P. Neurotoxicity. Encyclopedia of Toxicology. 2014 Dec 31.
- Jellinger, K. Basic mechanisms of neurodegeneration: A critical update. J Cell Mol Med. 2010 Mar 1;14:457–87.
- Obulesu M o, Rao D. Effect of plant extracts on Alzheimer′s disease: An insight into therapeutic avenues. J Neurosci Rural Pract. 2011 Mar 4;2:56–61.
- Kolaç U, Ustuner M, Tekin N, Ustuner D, Çolak E, Entok E. The Anti-Inflammatory and Antioxidant Effects of Salvia officinalis on Lipopolysaccharide-Induced Inflammation in Rats. J Med Food. 2017 Nov 13;20.
- Sarbishegi M, Mehraein F, Soleimani M. Antioxidant Role of Oleuropein on Midbrain and Dopaminergic Neurons of Substantia Nigra in Aged Rats. Iran Biomed J. 2014 Jan 1;18:16–22.
- Akram M, Nawaz A. Effects of medicinal plants on Alzheimer′s disease and memory deficits. Neural Regen Res. 2017 Apr 1;12.
- Chen X, Guo C, Kong J. Oxidative stress in neurodegenerative diseases. Neural Regen Res. 2012 Feb 15;7:376–85.
- Tönnies E, Trushina E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J Alzheimers Dis. 2017 Dec 3;Preprint:1–17.
- Behl, C. Oxidative stress in Alzheimer’s disease: implications for prevention and therapy. Subcell Biochem. 2005 Feb 1;38:65–78.
- Pavlidis N, Kofinas A, Papanikolaou M, Miras H, Drouza C, Kalampounias A, et al. Synthesis, characterization and pharmacological evaluation of quinoline derivatives and their complexes with copper(ΙΙ) in in vitro cell models of Alzheimer’s disease. J Inorg Biochem. 2021 Feb 1;217:111393.
- Gregory J, Vengalasetti Y, Bredesen D, Rao R. Neuroprotective Herbs for the Management of Alzheimer’s Disease. Biomolecules. 2021 Apr 8;11:543.
- Pandey SN, Rangra NK, Singh S, Arora S, Gupta V. Evolving Role of Natural Products from Traditional Medicinal Herbs in the Treatment of Alzheimer’s Disease. ACS Chem Neurosci [Internet]. 2021 Aug 4;12(15):2718–28. Available from. [CrossRef]
- Lopresti, A. Salvia (Sage): A Review of its Potential Cognitive-Enhancing and Protective Effects. Drugs in R & D. 2016 Nov 26;17.
- Karkoula E, Angelis A, Koulakiotis N, Gikas E, Halabalaki M, Tsarbopoulos A, et al. Rapid isolation and characterization of crocins, picrocrocin and crocetin from saffron using centrifugal partition chromatography and LC-MS. J Sep Sci. 2018 Sep 19;41.
- Boka VI, Argyropoulou A, Gikas E, Angelis A, Aligiannis N, Skaltsounis AL. Employment of High-Performance Thin-Layer Chromatography for the Quantification of Oleuropein in Olive Leaves and the Selection of a Suitable Solvent System for Its Isolation with Centrifugal Partition Chromatography. Planta Med. 2015 Nov 18;81.
- Angelis A, Michailidis D, Antoniadi L, Stathopoulos P, Tsantila V, Nuzillard JM, et al. Pilot continuous
centrifugal liquid-liquid extraction of extra virgin olive oil biophenols and gram-scale recovery of pure
oleocanthal, oleacein, MFOA, MFLA and hydroxytyrosol. Sep Purif Technol. 2020 Sep 1.
- Angelis A, Hamzaoui M, Aligiannis N, Nikou T, Michailidis D, Gerolimatos P, et al. An integrated process for the recovery of high added-value compounds from olive oil using solid support free liquid-liquid extraction and chromatography techniques. J Chromatogr A. 2017 Feb 22;1491.
- Welzel A, Maggio J, Ganesh M S, Walker D, Ostaszewski B, Li S, et al. Secreted Amyloid β-Proteins in a
Cell Culture Model Include N-Terminally Extended Peptides That Impair Synaptic Plasticity. Biochemistry.
2014 May 19;53.
- Li H ning, Liu Y, Zhang Z peng, Wang Z peng, Hao J zheng, Li F ran, et al. Synthesis, biological evaluation
and structure-activity relationship studies of hederacolchiside E and its derivatives as potential anti-
Alzheimer agents. Eur J Med Chem [Internet]. 2018;143:376–89. Available from:
https://www.sciencedirect.com/science/article/pii/S0223523417309431.
- Obidike Ezenyi I, Oluwakanyinsola S. Screening of Herbal Medicines for Potential Toxicities. In 2013.
- Teppola H, Sarkanen JR, Jalonen T, Linne ML. Morphological Differentiation Towards Neuronal Phenotype of SH-SY5Y Neuroblastoma Cells by Estradiol, Retinoic Acid and Cholesterol. Neurochem Res. 2016 Apr 1;41.
- Kalinovskii A, Osmakov D, Koshelev S, Lubova K, Korolkova Y, Kozlov S, et al. Retinoic Acid-Differentiated Neuroblastoma SH-SY5Y Is an Accessible In Vitro Model to Study Native Human Acid-Sensing Ion Channels 1a (ASIC1a). Biology (Basel). 2022 Jan 20;11.
- Kovalevich J, Langford D. Considerations for the Use of SH-SY5Y Neuroblastoma Cells in Neurobiology. Methods Mol Biol. 2013 Aug 26;1078:9–21.
- Pieńkowska N, Fahnestock M, Mahadeo C, Zaborniak I, Chmielarz P, Bartosz G, et al. Induction of Oxidative Stress in SH-SY5Y Cells by Overexpression of hTau40 and Its Mitigation by Redox-Active Nanoparticles. Int J Mol Sci. 2022 Dec 26;24:359.
- Wang H, Shao B, Yu H, Xu F, Wang P, Yu K, et al. Neuroprotective role of hyperforin on aluminum maltolate-induced oxidative damage and apoptosis in PC12 cells and SH-SY5Y cells. Chem Biol Interact. 2018 Nov 1;299.
- Koutsoulas A, Carnecka M, Slanina J, Tóth J, Slaninová I. Characterization of Phenolic Compounds and Antiproliferative Effects of Salvia pomifera and Salvia fruticosa Extracts. Molecules. 2019 Aug 12;24:2921.
- Koutsoni O, Karampetsou K, Kyriazis I, Stathopoulos P, Aligiannis N, Halabalaki M, et al. Evaluation of
total phenolic fraction derived from extra virgin olive oil for its antileishmanial activity. Phytomedicine.
2018 May 1;47.
- Grewal R, Reutzel M, Dilberger B, Hein H, Zotzel J, Marx S, et al. Purified oleocanthal and ligstroside protect against mitochondrial dysfunction in models of early Alzheimer’s disease and brain ageing. Exp Neurol. 2020 Feb 1;328:113248.
- Giusti L, Angeloni C, Barbalace M, Lacerenza S, Ciregia F, Ronci M, et al. A Proteomic Approach to Uncover Neuroprotective Mechanisms of Oleocanthal against Oxidative Stress. Int J Mol Sci. 2018 Aug 8;19.
- Peyrot des Gachons C, O’Keefe A, Slade L, Beauchamp G. Protein suppresses both bitterness and oleocanthal-elicited pungency of extra virgin olive oil. Sci Rep. 2021 Jun 4;11.
- Cirmi S, Celano M, Lombardo G, Maggisano V, Procopio A, Russo D, et al. Oleacein inhibits STAT3, activates the apoptotic machinery and exerts anti-metastatic effects in the SH-SY5Y human neuroblastoma cells. Food Funct. 2020 Mar 13;11.
- Muñoz-Garcia R, Sánchez-Hidalgo M, Montoya T, Alcarranza M, Ortega-Vidal J, Altarejos J, et al. Effects of Oleacein, a New Epinutraceutical Bioproduct from Extra Virgin Olive Oil, in LPS-Activated Murine Immune Cells. Pharmaceuticals. 2022 Oct 28;15:1338.
- Gu S. Oleanolic Acid Improved Inflammatory Response and Apoptosis of PC12 Cells Induced by OGD/R
Through Downregulating miR-142-5P. Nat Prod Commun [Internet]. 2021 May 1;16(5):1934578X211018019. [CrossRef]
- Hashemzaei M, Mamoulakis C, Tsarouhas K, Georgiadis G, Lazopoulos G, Tsatsakis A, et al. Crocin: A
fighter against inflammation and pain. Food and Chemical Toxicology [Internet]. 2020;143:111521.
Available from: https://www.sciencedirect.com/science/article/pii/S0278691520304117.
- Hatziagapiou K, Kakouri E, Lambrou G, Bethanis K, Tarantilis P. Antioxidant Properties of Crocus Sativus L. and Its Constituents and Relevance to Neurodegenerative Diseases; Focus on Alzheimer’s and Parkinson’s Disease. Curr Neuropharmacol. 2018 Mar 20;16.
- Lv B, Chen T, Xu Z, Huo F, Wei Y, Yang X. Crocin protects retinal ganglion cells against H2O2-induced damage through the mitochondrial pathway and activation of NF-κB. Int J Mol Med. 2015 Nov 23;37.
- Heidari S, Mehri S, Shariaty V, Hosseinzadeh H. Preventive effects of crocin on neuronal damages induced by D-galactose through AGEs and oxidative stress in human neuroblastoma cells (SH-SY5Y). J Pharmacopuncture. 2018 Mar 1;21:18–25.
- Papandreou M, Kanakis C, Polissiou M, Efthimiopoulos S, Cordopatis P, Margarity M, et al. Inhibitory Activity on Amyloid-?? Aggregation and Antioxidant Properties of Crocus sativus Stigmas Extract and Its Crocin Constituents. J Agric Food Chem. 2006 Dec 1;54:8762–8.
- Koulakiotis N, Purhonen P, Gikas E, Hebert H, Tsarbopoulos A. Crocus-derived compounds alter the aggregation pathway of Alzheimer’s Disease: associated beta amyloid protein. Sci Rep. 2020 Oct 23;10.
- Chalatsa I, Arvanitis D, Koulakiotis N, Giagini A, Skaltsounis A, Papadopoulou-Daifoti Z, et al. The Crocus sativus Compounds trans-Crocin 4 and trans-Crocetin Modulate the Amyloidogenic Pathway and Tau Misprocessing in Alzheimer Disease Neuronal Cell Culture Models. Front Neurosci. 2019 Mar 1;13:249.
- Liu R, Barkhordarian H, Emadi S, Park CB, Sierks MR. Trehalose differentially inhibits aggregation and
neurotoxicity of beta-amyloid 40 and 42. Neurobiol Dis [Internet]. 2005;20(1):74–81. Available from:
https://www.sciencedirect.com/science/article/pii/S0969996105000628.
- Malliou F; ACE; KA; KA; LG; GFJ; MTM; DM; SLA; KM. Oleuropein Promotes Neural Plasticity and Neuroprotection via PPARα-Dependent and Independent Pathways. Preprints.org [Internet]. 2023 [cited 2023 Jul 26]; Available from, 2023. [CrossRef]
- Malliou F, Andreadou I, Gonzalez FJ, Lazou A, Xepapadaki E, Vallianou I, et al. The olive constituent
oleuropein, as a PPARα agonist, markedly reduces serum triglycerides. J Nutr Biochem [Internet].
2018;59:17–28. Available from: https://www.sciencedirect.com/science/article/pii/S0955286317306484.
- Zolezzi J, Silva C, Ordenes D, Godoy J, Carvajal F, Santos M, et al. Peroxisome Proliferator-Activated
Receptor (PPAR) γ and PPARα Agonists Modulate Mitochondrial Fusion-Fission Dynamics: Relevance to
Reactive Oxygen Species (ROS)-Related Neurodegenerative Disorders? PLoS One. 2013 May 13;8.
- Omar SH, Scott C, Hamlin A, Obied H. Olive biophenols reduces alzheimer’s pathology in SH-SY5Y cells and APPswe mice. Int J Mol Sci. 2018 Dec 30;20:125.
- Omar SH, Kerr P, Scott C, Hamlin A, Obied H. Olive (Olea europaea L.) Biophenols: A Nutriceutical against Oxidative Stress in SH-SY5Y Cells. Molecules. 2017 Oct 29;22:1858.
- Lopresti, A. Salvia (Sage): A Review of its Potential Cognitive-Enhancing and Protective Effects. Drugs in R & D. 2016 Nov 26;17.
- Afonso A, Pereira O, Cardoso S. Salvia Species as Nutraceuticals: Focus on Antioxidant, Antidiabetic and Anti-Obesity Properties. Applied Sciences. 2021 Oct 9;11:9365.
- Bednarikova Z, Gancar M, Wang R, Zheng L, Tang Y, Luo Y, et al. Extracts from Chinese herbs with anti-amyloid and neuroprotective activities. Int J Biol Macromol. 2021 Mar 1;179.
- Uriarte-Pueyo I, Calvo M. Flavonoids as Acetylcholinesterase Inhibitors. Curr Med Chem. 2011 Nov 16;18:5289–302.
- Ravipati AS, Zhang L, Koyyalamudi SR, Jeong SC, Reddy N, Bartlett J, et al. Antioxidant and anti-inflammatory activities of selected Chinese medicinal plants and their relation with antioxidant content. BMC Complement Altern Med [Internet]. 2012;12(1):173. Available from. [CrossRef]
- Mervić M, Bival Štefan M, Kindl M, Blažeković B, Marijan M, Vladimir-Knežević S. Comparative Antioxidant, Anti-Acetylcholinesterase and Anti-α-Glucosidase Activities of Mediterranean Salvia Species. Plants. 2022 Feb 25;11:625.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).