Submitted:
29 July 2023
Posted:
01 August 2023
You are already at the latest version
Abstract
Keywords:
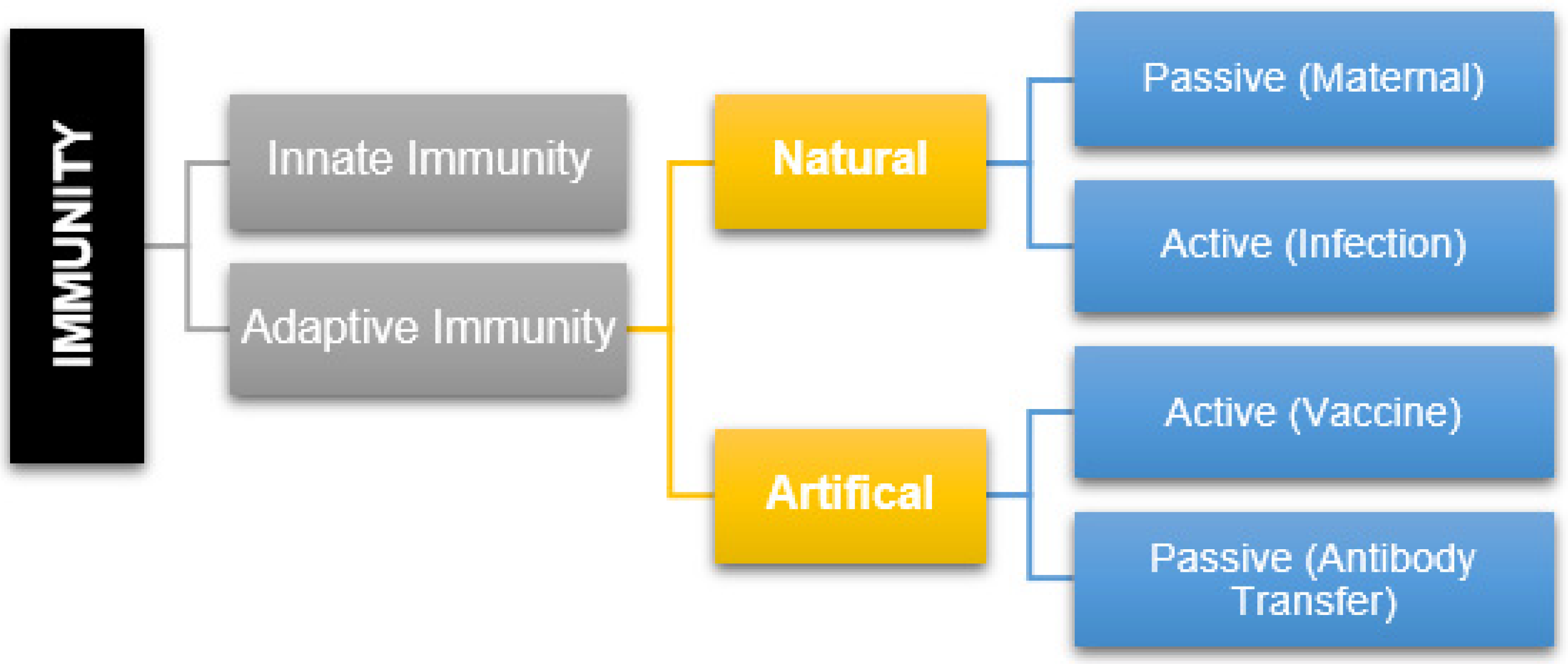
1. Introduction
2. Why PIT Is Crucial?
3. Effecting Factors of the PIT
3.1. Factors up to Dam
3.2. Factors up to litter
4. Evaluation Methods
4.1. Colostral TP and Ig Concentration
4.2. Immunoglobulin Levels in Lamb Blood Serum
5. Discussion and Conclusion
Author Contributions
References
- Kaufmann, P.; Burton, G. Anatomy and genesis of the placenta. ln: E Knobil, JD Neill (eds) The physiology of reproduction. 1994. [CrossRef]
- Leiser, R.; Kaufmann, P. Placental structure: in a comparative aspect. Experimental and Clinical Endocrinology & Diabetes. 1994, 102(03), 122-134. [CrossRef]
- Wooding, F.B.P. The synepitheliochorial placenta of ruminants: binucleate cell fusions and hormone production. Placenta. 1992, 13(2), 101-113. [CrossRef]
- Prado, M.E.; Prado, T.M.; Payton, M.; Confer, A.W. et al. Maternally and naturally acquired antibodies to Mannheimia haemolytica and Pasteurella multocida in beef calves. Veterinary immunology and immunopathology. 2006, 111.3-4, 301-307. [CrossRef]
- Nowak, R.; Poindron. From birth to colostrum: early steps leading to lamb survival. Reproduction Nutrition Development. 2006, 46(4), 431-446. [CrossRef]
- Moreno-Indias, I.; Sánchez-Macías, D.; Castro, N.; Morales-delaNuez, A.; Hernández-Castellano, L.E.; Capote, J.; Argüello, A. Chemical composition and immune status of dairy goat colostrum fractions during the first 10 h after partum. Small Ruminant Research. 2012, 103(2-3), 220-224. [CrossRef]
- Övet, C. Cytokines and growth factors in goat colostrum: a short review. Journal of Bahri Dagdas Animal Research. 2023, 12(1), 89-97.
- García de Jalón, J.A.; De las Heras, M.; Ferrer, L.M.; Sancho, J.F. Síndrome de la boca mojada en corderos. Medicina Veterinaria. 1990, 7.
- Barza, H.; Marinescu, M.; Blaga, L. Disorders of foals 0–10 days of age. Part I. Revista Romana de Medicina Veterinara. 1993, 3, 9-20.
- Zohary, D.; Tchernov, E.; Horwitz, L. K. The role of unconscious selection in the domestication of sheep and goats. Journal of Zoology. 1998, 245(2), 129-135. [CrossRef]
- Haenlein, G.F.W. Past, present, and future perspectives of small ruminant dairy research. Journal of dairy science. 2001, 84(9), 2097-2115. [CrossRef]
- Mellor, D.J. Integration of perinatal events, pathophysiological changes and consequences for the newborn lamb. British Veterinary Journal. 1998, 144(6), 552-569. [CrossRef]
- Dewell, R.D.; Hungerford, L.L.; Keen, J.E.; Laegreid, W.W.; Griffin, D.D.; Rupp, G.P.; Grotelueschen, D.M. Association of neonatal serum immunoglobulin G1 concentration with health and performance in beef calves. Journal of the American Veterinary Medical Association. 2006, 228(6), 914-921. [CrossRef]
- Yalçın, E.; Temizel, E.M. Sütten Kesme Öncesi Dönemde Oğlakların Büyüme Performansına Pasif Transfer Durumunun Etkisi. Uludağ Üniversitesi Veteriner Fakültesi Dergisi. 2010, 29(1), 23-26.
- Gokce, E.; Atakisi, O.; Kirmizigul, A.H.; Erdogan, H.M. Risk Factors Associated with Passive Immunity, Health, Birth Weight and Growth Performance in Lambs: II. Effects of Passive Immunity and Some Risk Factors on Growth Performance During the First 12 Weeks of Life. Kafkas Üniversitesi Veteriner Fakültesi Dergisi. 2013a, 19(4). [CrossRef]
- dos Santos, J.D.C.; Saraiva, E.P.; Pimenta Filho, E.C.; Neta, G.C.X.; Morais, L.K.C.; Teti, H.S.; Fidelis, S.S. Risk factors for neonatal mortality in sheep farming systems in tropical semi-arid regions. The Journal of Agricultural Science. 2023, 1-44. [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nature reviews immunology. 2009, 9(11), 799-809. [CrossRef]
- Nordi, W.M.; Moretti, D.B.; Lima, A.L.; Pauletti, P.; Susin, I.; Machado-Neto, R. Intestinal IgG uptake by small intestine of goat kid fed goat or lyophilized bovine colostrum. Livestock Science. 2012, 144(3), 205-210. [CrossRef]
- Yang, Y.; Zhao, X.; Huang, D.; Wang, J.; Qi, Y.; Jiang, L.; Cheng, G. Changes in intestinal proteins induced by colostrum uptake in neonatal calves: Analysis by two-dimensional gel electrophoresis-based proteomics analysis. Animal Production Science. 2019, 59(8), 1483-1490. [CrossRef]
- Ciuryk, S.; Molik, E.; Kaczor, U.; Bonczar, G. Chemical composition of colostrum and milk of Polish Merino sheep lambing at different times. Archiv fur Tierzucht. 2004, 47(6; SPI), 129-134.
- Hurley, W.L.; Theil, P.K. Perspectives on immunoglobulins in colostrum and milk. Nutrients. 2011, 3(4), 442-474. [CrossRef]
- Gitlin, D.; Kumate, J.; Urrusti, J.; Morales, C. The selectivity of the human placenta in the transfer of plasma proteins from mother to fetus. The Journal of Clinical Investigation. 1964, 43(10), 1938-1951. [CrossRef]
- Silva, S.R.; Sacarrão-Birrento, L.; Almeida, M.; Ribeiro, D.M.; Guedes, C.; Gonzalez Montana, J.R.; de Almeida, A.M. Extensive sheep and goat production: The role of novel technologies towards sustainability and animal welfare. Animals. 2022, 12(7), 885. [CrossRef]
- Pekcan, M.; Fidanci, U. R.; Yuceer, B.; Ozbeyaz, C. Estimation of passive immunity in newborn calves with routine clinical chemistry measurements. Ankara Üniversitesi Veteriner Fakültesi Dergisi. 2013, 60(2), 85-88. [CrossRef]
- Elitok, B. Indicators of passive immunity failure in neonatal calves. Oncol Res Rev. 2018, 1(3), 1-2. [CrossRef]
- Shiels, D.; Loughrey, J.; Dwyer, C. M.; Hanrahan, K.; Mee, J.F.; Keady, T.W. A survey of farm management practices relating to the risk factors, prevalence, and causes of lamb mortality in Ireland. Animals,. 2021, 12(1), 30. [CrossRef]
- Sallam, A.M. Risk factors and genetic analysis of pre-weaning mortality in Barki lambs. Livestock Science. 2019, 230, 103818. [CrossRef]
- Ibrahim, N.H.; Badawy, M.T.; Zakzouk, I.A.; Younis, F.E. Kids’ survivability as affected by their body weight, blood biochemical indices and maternal and kids’ behavior in baladi and shami goats under semi-arid condition. World’s Veterinary Journal. 2020, 10(1), 105-117. [CrossRef]
- Herndon, C.N.; Shanthalingam, S.; Knowles, D.P.; Call, D.R.; Srikumaran, S. Comparison of passively transferred antibodies in bighorn and domestic lambs reveals one factor in differential susceptibility of these species to Mannheimia haemolytica-induced pneumonia. Clinical and Vaccine Immunology. 2011, 18(7), 1133-1138. [CrossRef]
- Demis, C.; Aydefruhim, D.; Wondifra, Y.; Ayele, F.; Alemnew, E.; Asfaw, T. Maternal Immunoglobulin in the Serum of Newborn Lambs and Its Relation With Neonatal Mortality. Online Journal of Animal and Feed Research. 2020, 10(3), 119-124. [CrossRef]
- Agenbag, B.; Swinbourne, A.M.; Petrovski, K.; van Wettere, W.H. Lambs need colostrum: A review. Livestock Science. 2021, 251, 104624. [CrossRef]
- Hernández-Castellano, L.E.; Suárez-Trujillo, A.; Martell-Jaizme, D.; Cugno, G.; Argüello, A.; Castro, N. The effect of colostrum period management on BW and immune system in lambs: from birth to weaning. Animal. 2015, 9(10), 1672-1679. [CrossRef]
- Wesemann, D. R., Portuguese, A. J., Meyers, R. M., Gallagher, M. P., Cluff-Jones, K., Magee, J. M.; Alt, F.W. Microbial colonization influences early B-lineage development in the gut lamina propria. Nature. 2013, 501(7465), 112-115. [CrossRef]
- Castro-Alonso, A.; Castro, N.; Capote, J.; Morales-delaNuez, A.; Moreno-Indias, I.; Sánchez-Macias, D.; Argüello, A. Apoptosis regulates passive immune transfer in newborn kids. Journal of dairy science. 2008, 91(5), 2086-2088. [CrossRef]
- Hernandez-Castellano, L.; M Almeida, A.; Castro, N.; Arguello, A. The colostrum proteome, ruminant nutrition and immunity: a review. Current Protein and Peptide Science. 2014a, 15(1), 64-74. [CrossRef]
- Hernández-Castellano, L.E.; Almeida, A.M.; Ventosa, M.; Coelho, A.V.; Castro, N.; Argüello, A. The effect of colostrum intake on blood plasma proteome profile in newborn lambs: low abundance proteins. BMC Veterinary Research, 2014b, 10, 1-9. [CrossRef]
- Brujeni, G.N.; Jani, S.S.; Alidadi, N.; Tabatabaei, S.; Sharifi, H.; Mohri, M. Passive immune transfer in fat-tailed sheep: Evaluation with different methods. Small Ruminant Research. 2010, 90(1-3), 146-149. [CrossRef]
- Zhu, H.L.; Zhao, X.W.; Han, R.W.; Du, Q.J.; Qi, Y.X.; Jiang, H.N.; Yang, Y.X. Changes in bacterial community and expression of genes involved in intestinal innate immunity in the jejunum of newborn lambs during the first 24 hours of life. Journal of Dairy Science. 2021, 104(8), 9263-9275. [CrossRef]
- Tizard, I.R. Veterinary Immunology-E-Book. 2017, Elsevier Health Sciences.
- Fischer, A.J.; Villot, C.; van Niekerk, J.K.; Yohe, T.T.; Renaud, D.L.; Steele, M.A. Invited Review: Nutritional regulation of gut function in dairy calves: From colostrum to weaning. Applied Animal Science,. 2019, 35(5), 498-510. [CrossRef]
- Castro, I.; Alba, C.; Aparicio, M.; Arroyo, R.; Jiménez, L.; Fernández, L.; Rodríguez, J.M. Metataxonomic and immunological analysis of milk from ewes with or without a history of mastitis. Journal of dairy science. 2019, 102(10), 9298-9311. [CrossRef]
- Banchero, G.E.; Milton, J.T.B.; Lindsay, D.R.; Martin, G.B.; Quintans, G. Colostrum production in ewes: a review of regulation mechanisms and of energy supply. Animal. 2015, 9(5), 831-837. [CrossRef]
- McGovern, F.M.; Campion, F.P.; Lott, S.; Boland, T.M. Altering ewe nutrition in late gestation: I. The impact on pre-and postpartum ewe performance. Journal of animal science. 2015, 93(10), 4860-4872. [CrossRef]
- Banchero, G.E.; Clariget, R.P.; Bencini, R.; Lindsay, D.R.; Milton, J.T.; Martin, G.B. Endocrine and metabolic factors involved in the effect of nutrition on the production of colostrum in female sheep. Reproduction nutrition development. 2006, 46(4), 447-460. [CrossRef]
- Muñoz, C.; Carson, A.F.; McCoy, M.A.; Dawson, L.E.R.; O’Connell, N.E.; Gordon, A.W. Nutritional status of adult ewes during early and mid-pregnancy. 1. Effects of plane of nutrition on ewe reproduction and offspring performance to weaning. Animal. 2008, 2(1), 52-63. [CrossRef]
- Viola, I.; Tizzani, P.; Perona, G.; Lussiana, C.; Mimosi, A.; Ponzio, P.; Cornale, P. Hazelnut skin in ewes’ diet: Effects on colostrum immunoglobulin g and passive transfer of immunity to the lambs. Animals,. 2022, 12(22), 3220. [CrossRef]
- Castellaro, G.; Ochoa, I.; Borie, C.; Parraguez, V.H. Effects of Strategic Supplementation with Lupinus angustifolius and Avena sativa Grains on Colostrum Quality and Passive Immunological Transfer to Newborn Lambs. Animals. 2022, 12(22), 3159. [CrossRef]
- Talore, G.D. On-farm performance evaluation of indigenous sheep and goats in Alaba, Southern Ethiopia (PHD dissertation, Hawassa University). 2009.
- Taye, M.; Abebe, G.; Gizaw, S.; Lemma, S.; Mekoya, A.; Tibbo, M. Growth performances of Washera sheep under smallholder management systems in Yilmanadensa and Quarit districts, Ethiopia. Tropical animal health and production. 2010, 42, 659-667. [CrossRef]
- Abegaz, S.; Hegde, B.P.; Taye, M. Growth and physical body characteristics of Gumuz sheep under traditional management systems in Amhara Regional State, Ethiopia. Livestock Research for Rural Development,. 2011, 23(5), 10.
- Gokce, E.; Atakisi, O.; Kirmizigul, A.H; Erdoğan, H.M. Risk Factors Associated with Passive Immunity, Health, Birth Weight And Growth Performance in Lambs: III. The Relationship among Passive Immunity, Birth Weight Gender, Birth Type, Parity, Dam. Kafkas Üniversitesi Veteriner Fakültesi Dergisi,. 2013b, 19(5). [CrossRef]
- Higaki, S.; Nagano, M.; Katagiri, S.; Takahashi, Y. Effects of parity and litter size on the energy contents and immunoglobulin G concentrations of Awassi ewe colostrum. Turkish Journal of Veterinary & Animal Sciences,. 2013, 37(1), 109-112. [CrossRef]
- Tabatabaei, S.; Nikbakht, G.; Vatankhah, M.; Sharifi, H.; Alidadi, N. Variation in colostral immunoglobulin G concentration in fat tailed sheep and evaluation of methods for estimation of colostral immunoglobulin content. Acta Veterinaria Brno. 2013, 82(3), 271-275. [CrossRef]
- Sjoberg, A.; Van Saun, R.J. Use of brix refractometer in assessing sheep colostrum. In American Association of Bovine Practitioners Conference Proceedings. 2021, (pp. 273-273).
- Chniter, M.; Hammadi, M.; Khorchani, T.; Sassi, M.B.; Hamouda, M.B.; Nowak, R. Aspects of neonatal physiology have an influence on lambs’ early growth and survival in prolific D’man sheep. Small Ruminant Research. 2013, 111(1-3), 162-170. [CrossRef]
- Chniter, M.; Salhi, I.; Harrabi, H.; Khorchani, T.; Lainé, A.L.; Nowak, R.; Hammadi, M. Physiological changes in the peri-partum period and colostral IgG transfer in prolific D’man sheep: effects of parity and litter size. Tropical animal health and production. 2016, 48, 387-394. [CrossRef]
- Sol Morales, M.; Palmquist, D.L.; Weiss, W.P. Milk fat composition on Holstein and Jersey cows with control or depleted copper status and fed whole soybeans or tallow. Journal of Dairy Science. 2000, 83 (9), 2112-2119. [CrossRef]
- Strusińska, D.; Mierzejewska, J.; Skok, A. Concentration of mineral components β-carotene, vitamins A and E in cow colostrum and milk when using mineralvitamin supplements. Medycyna Weterynaryjna, 2004, 60 (2), 202-206.
- Wu, H.; Xu, C.; Wang, J.; Hu, C.; Ji, F.; Xie, J.; Lv, R. Effects of Dietary Probiotics and Acidifiers on the Production Performance, Colostrum Components, Serum Antioxidant Activity and Hormone Levels, and Gene Expression in Mammary Tissue of Lactating Sows. Animals. 2023, 13(9), 1536. [CrossRef]
- Burezq, H.A.; Khalil, F. Improved vaccination protocol to enhance immunity in lambs of Kuwait farms. Iraqi Journal of Veterinary Sciences. 2022, 36(2), 539-548. [CrossRef]
- Yapi, C.V.; Boylan, W.J.; Robinson, R.A. Factors associated with causes of preweaning lamb mortality. Preventive Veterinary Medicine. 1990, 10(1-2), 145-152. [CrossRef]
- Turkson, P.K. Lamb and kid mortality in village flocks in the coastal savanna zone of Ghana. Tropical Animal Health and Production. 2003, 35, 477-490. [CrossRef]
- Turkson, P.K.; Sualisu, M. Risk factors for lamb mortality in Sahelian sheep on a breeding station in Ghana. Tropical Animal Health and Production. 2005, 37, 49-64. [CrossRef]
- Mandal, A.; Prasad, H.; Kumar, A.; Roy, R.; Sharma, N. Factors associated with lamb mortalities in Muzaffarnagari sheep. Small Ruminant Research,. 2007, 71(1-3), 273-279. [CrossRef]
- Nash, M.L.; Hungerford, L.L.; Nash, T.G.; Zinn, G.M. Risk factors for perinatal and postnatal mortality in lambs. Veterinary Record. 1996, 139(3), 64-67. [CrossRef]
- Holmøy, I.H.; Kielland, C.; Stubsjøen, S.M.; Hektoen, L.; Waage, S. Housing conditions and management practices associated with neonatal lamb mortality in sheep flocks in Norway. Preventive Veterinary Medicine,. 2012, 107(3-4), 231-241. [CrossRef]
- Alves, A.C.; Alves, N.G.; Ascari, I.J.; Junqueira, F.B.; Coutinho, A.S.; Lima, R.R.; Abreu, L.R. Colostrum composition of Santa Inês sheep and passive transfer of immunity to lambs. Journal of Dairy science. 2015, 98(6), 3706-3716. [CrossRef]
- Gokce, E.; Atakisi, O. Interrelationships of serum and colostral IgG (passive immunity) with total protein concentrations and health status in lambs. Kafkas Universitesi Veteriner Fakultesi Dergisi. 2019, 25(4), 387-396.
- Mulvaney, F.J.; Morris, S.T.; Kenyon, P.R.; Morel, P.C.H.; West, D.M.; Vinoles, C.; Glover, K.M.M. Comparison between the reproductive performance of ewe hoggets and mature ewes following a progesterone-based oestrus synchronization protocol. New Zealand Journal of Agricultural Research. 2013, 56(4), 288-296. [CrossRef]
- Pain, S.J.; Loureiro, M.F.P.; Kenyon, P.R.; Blair, H.T. The effect of dam age on ewe offspring productive performance and efficiency. In Proceedings of the New Zealand Society of Animal Production. 2015, (Vol. 75, pp. 239-242). New Zealand Society of Animal Production.
- Pettigrew, E.J.; Hickson, R.E.; Blair, H.T.; Griffiths, K.J.; Ridler, A.L.; Morris, S.T.; Kenyon, P.R. Differences in lamb production between ewe lambs and mature ewes. New Zealand Journal of Agricultural Research,. 2021, 64(4), 508-521. [CrossRef]
- Lee, S.H.; Jaekal, J.; Bae, C.S.; Chung, B.H.; Yun, S.C.; Gwak, M.J.; Lee, D.H. Enzyme-linked immunosorbent assay, single radial immunodiffusion, and indirect methods for the detection of failure of transfer of passive immunity in dairy calves. Journal of veterinary internal medicine. 2008, 22(1), 212-218. [CrossRef]
- Cuttance, E.L.; Regnerus, C.; Laven, R.A. A review of diagnostic tests for diagnosing failure of transfer of passive immunity in dairy calves in New Zealand. New Zealand veterinary journal,. 2019, 67(6), 277-286. [CrossRef]
- Agenbag, B.; Swinbourne, A.M.; Petrovski, K.; van Wettere, W.H. Validation of a handheld refractometer to assess Merino ewe colostrum and transition milk quality. Journal of Dairy Science,. 2023, 106(2), 1394-1402. [CrossRef]
- Drikic, M.; Windeyer, C.; Olsen, S.; Fu, Y.; Doepel, L.; De Buck, J. Determining the IgG concentrations in bovine colostrum and calf sera with a novel enzymatic assay. Journal of animal science and biotechnology. 2018, 9, 1-9. [CrossRef]
- Swarnkar, C.P.; Gowane, G.R.; Prince, L.L.L.; Sonawane, G.G. Risk factor analysis for neonatal lamb mortality in Malpura sheep. Indian Journal of Animal Sciences. 2019, 89(6): 640–644. [CrossRef]
- Constantin, N.T.; Sipos, A. Passive Transfer of Immunoglobulıns from Ewe to Lamb. Scientific Works. Series C, Veterinary Medicine. 2021, 67(1).
- Yenilmez, K.; Arslan, S.; Kılıç, S.; Atalay, H. The effect of twinship on mineral matter, immunoglobulin G and lamb birth weight in late pregnant ewes and their newborn lambs. Van Veterinary Journal,. 2021, 32(2), 62-68.
- Abdel-Salam, Z.A.; Abdel-Salam, S.A.M.; Abdel-Mageed, I.I.; Harith, M.A. Evaluation of proteins in sheep colostrum via laser-induced breakdown spectroscopy and multivariate analysis. Journal of advanced research,. 2019, 15, 19-25. [CrossRef]
- Berge, A.C.; Hassid, G.; Leibovich, H.; Solomon, D.; Haines, D.M. A field trial evaluating the health and performance of lambs fed a bovine colostrum replacement. J Anim Res Nutr. 2018, Vol, (3). [CrossRef]
- de Sousa, I.V.P.; Silva, C.B.; Ribeiro, C.V. Influence of the birth order on the total solids concentration of frozen colostrum from Santa Inês ewes. In 55a Reunião Anual da Sociedade Brasileira de Zootecnia, 28° Congresso Brasileiro de Zootecnia, Goiânia, Brasil, Sociedade Brasileira de Zootecnia-SBZ, Associação Brasileira dos Zootecnistas. 2018.
- Torres-Rovira, L.; Pesantez-Pacheco, J.L.; Hernandez, F.; Elvira-Partida, L.; Perez-Solana, M.L.; Gonzalez-Martin, J.V.; Astiz, S. Identification of factors affecting colostrum quality of dairy Lacaune ewes assessed with the Brix refractometer. Journal of Dairy Research,. 2017, 84(4), 440-443. [CrossRef]
- Kessler, E.C.; Bruckmaier, R.M.; Gross, J.J. Comparative estimation of colostrum quality by Brix refractometry in bovine, caprine, and ovine colostrum. Journal of dairy science. 2021, 104(2), 2438-2444. [CrossRef]
- Todaro, M.; Maniaci, G.; Gannuscio, R.; Pampinella, D.; Scatassa, M.L. Chemometric Approaches to Analyse the Composition of a Ewe’s Colostrum. Animals. 2023, 13(6), 983. [CrossRef]
- Harmon, R.S.; Senesi, G.S. Laser-induced breakdown spectroscopy–a geochemical tool for the 21st century. Applied Geochemistry. 2021, 128, 104929. [CrossRef]
- https://www.fao.org/publications/home/fao-flagship-publications/the-state-of-food-security-and-nutrition-in-the-world/2022/en (Accessed on 07 of June 2023).
- Morris, S.T. Overview of sheep production systems. In Advances in sheep welfare. 2017, (pp. 19-35). Woodhead Publishing. [CrossRef]
- Massimini, G.; Britti, D.; Peli, A.; Cinotti, S. Effect of Passive Transfer Status on Preweaning Growth Performance in Dairy Lambs. Journal of the American Veterinary Medical Association,. 2006, 229, 111-115. [CrossRef]
- Castro, N.; Capote, J.; Alvarez, S.; Argüello, A. Effects of lyophilized colostrum and different colostrum feeding regimens on passive transfer of immunoglobulin G in Majorera goat kids. Journal of Dairy Science. 2005, 88(10), 3650-3654. [CrossRef]
- Rodríguez, C.; Castro, N.; Capote, J.; Morales-delaNuez, A.; Moreno-Indias, I.; Sánchez-Macías, D.; Argüello, A. Effect of colostrum immunoglobulin concentration on immunity in Majorera goat kids. Journal of Dairy Science,. 2009, 92(4), 1696-1701. [CrossRef]
- Mukasa-Mugerwa, E.; Lahlou-Kassi, A.; Anindo, D.; Rege, J.E.O.; Tembely, S.; Tibbo, M.; Baker, R.L. Between and within breed variation in lamb survival and the risk factors associated with major causes of mortality in indigenous Horro and Menz sheep in Ethiopia. Small Ruminant Research. 2000, 37(1-2), 1-12. [CrossRef]
- Tibbo, M.; Mukasa-Mugerwa, E.; Woldemeskel, M.; Rege, J.E.O. Risk factors for mortality associated with respiratory disease among Menz and Horro sheep in Ethiopia. The veterinary journal. 2003, 165(3), 276-287. [CrossRef]
- Berhan, A.; Van Arendonk, J. Reproductive performance and mortality rate in Menz and Horro sheep following controlled breeding in Ethiopia. Small Ruminant Research,. 2006, 63(3), 297-303. [CrossRef]
- Piwczyński, D.; Sitkowska, B.; Wiśniewska, E. Application of classification trees and logistic regression to determine factors responsible for lamb mortality. Small ruminant research,. 2012, 103(2-3), 225-231. [CrossRef]
- Vatankhah, M.; Talebi, M.A. Genetic and non-genetic factors affecting mortality in Lori-Bakhtiari lambs. Asian-Australasian Journal of Animal Sciences. 2009, 22(4), 459-464. [CrossRef]
- Ahmed, A.; Egwu, G.O.; Garba, H.S.; Magaji, A.A. Studies on risk factors of mortality in lambs in Sokoto, Nigeria. Nigerian Veterinary Journal,. 2010, 31(1). [CrossRef]
- Abdelqader, A.; Irshaid, R.; Tabbaa, M.J.; Abuajamieh, M.; Titi, H.; Al-Fataftah, A.R. Factors influencing Awassi lambs survivorship under fields conditions. Livestock Science,. 2017, 199, 1-6. [CrossRef]
- Hunter, A.G.; Reneau, J.K.; Williams, J.B. Factors affecting IgG concentration in day-old lambs. Journal of Animal Science. 1977, 45(5), 1146-1151. [CrossRef]
- Nouri, M.; Zarrin, M.; Ahmadpour, A.; Castro, N.; González-Cabrera, M.; Hernández-Castellano, L.E. Feed restriction around parturition does not affect colostrum immunoglobulin G concentration in dairy fat-tailed sheep but does affect performance and blood metabolites in newborn lambs. Journal of Dairy Science. 2023, 106(4), 2980-2988. [CrossRef]
| Direct Methods | RID ELISA STIGA |
| Indirect Methods | Refractometer Colostrometer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




