Submitted:
29 July 2023
Posted:
01 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Greenhouse Cultivation
2.2. Substrate for Cultivation
2.3. Preparation of Indole-3-Acetic Acid Solution
2.4. Experimental Design
2.5. Agronomic Characteristics
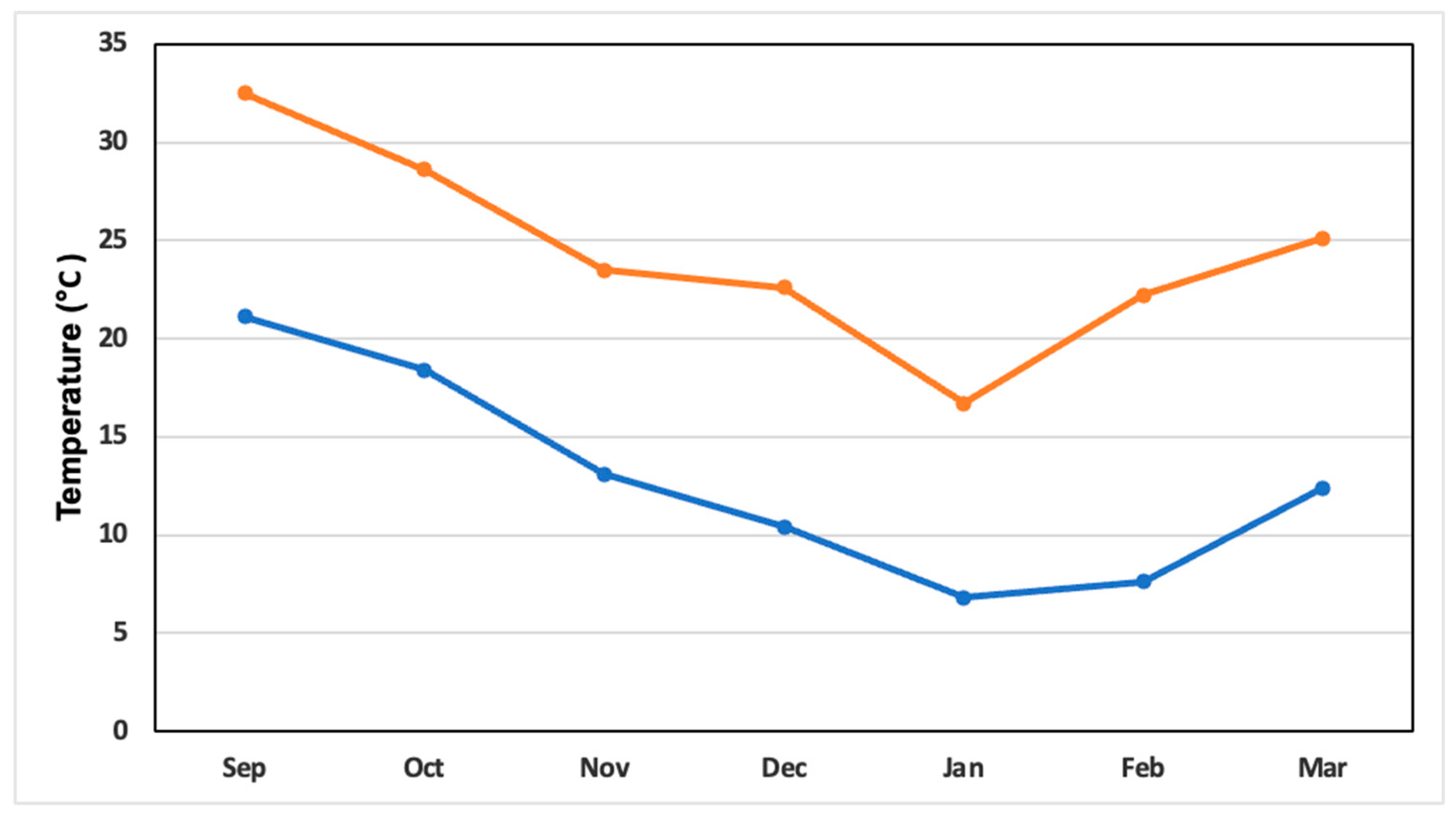
2.6. Greenhouse Climatic Variables
2.7. Determination of Growing Parameters
2.8. Chlorophyll, Carotenoids and Nitrates
2.9. Total phenolic Content and Antioxidant Activity (DPPH)
2.10. Statistical Analysis
3. Results
3.1. Effect of the Concentrations of IAA on the Onion Growth Physical Parameters
3.2. Effect of the Concentrations of IAA on Chlorophyll, Carotenoids and Nitrates
3.3. Effect of the Concentrations of IAA on Phenolic Content (DPPH)
3.4. Classification Analysis
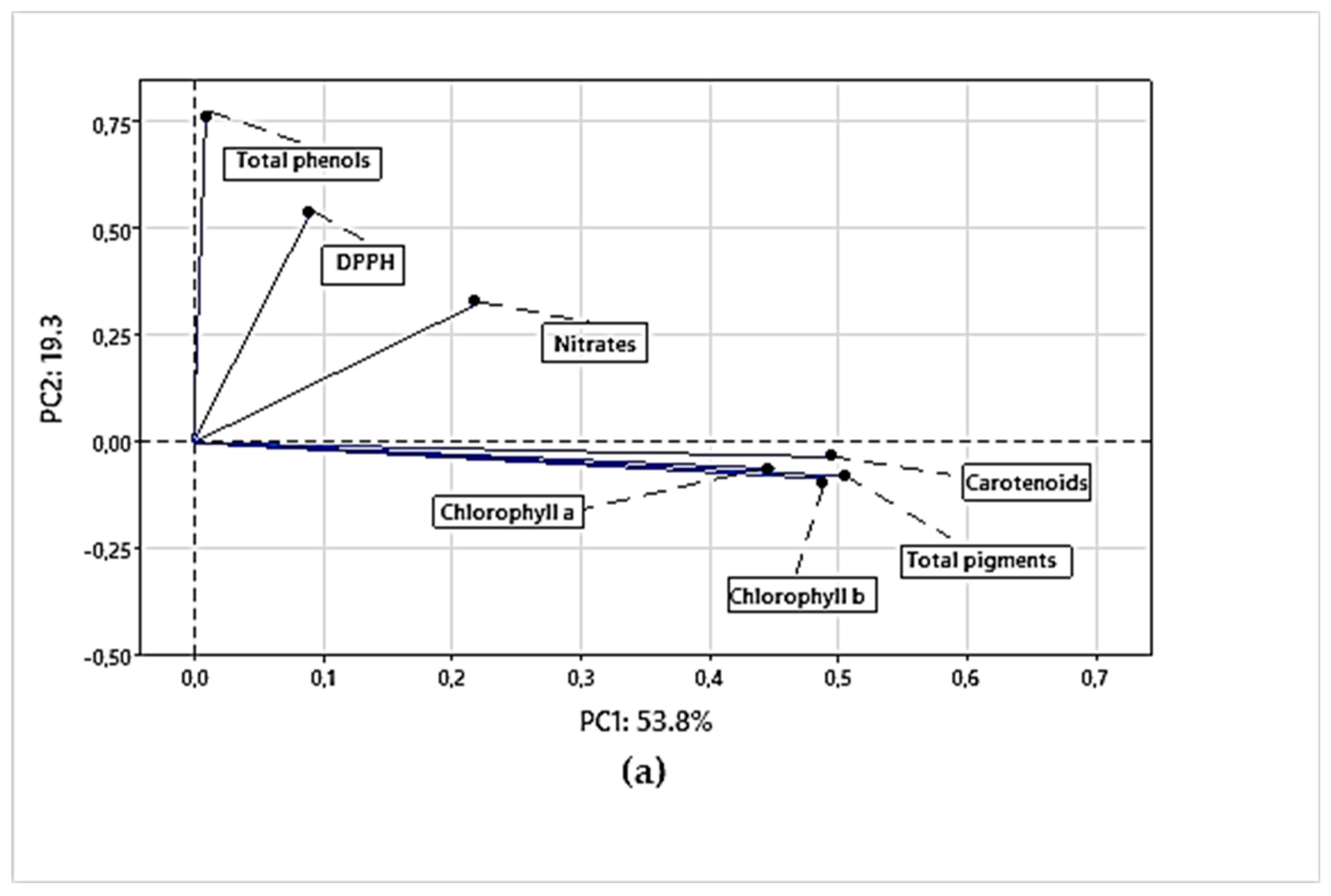
3.4.1. Principal Component Analysis (PCA) for Chemical Parameters
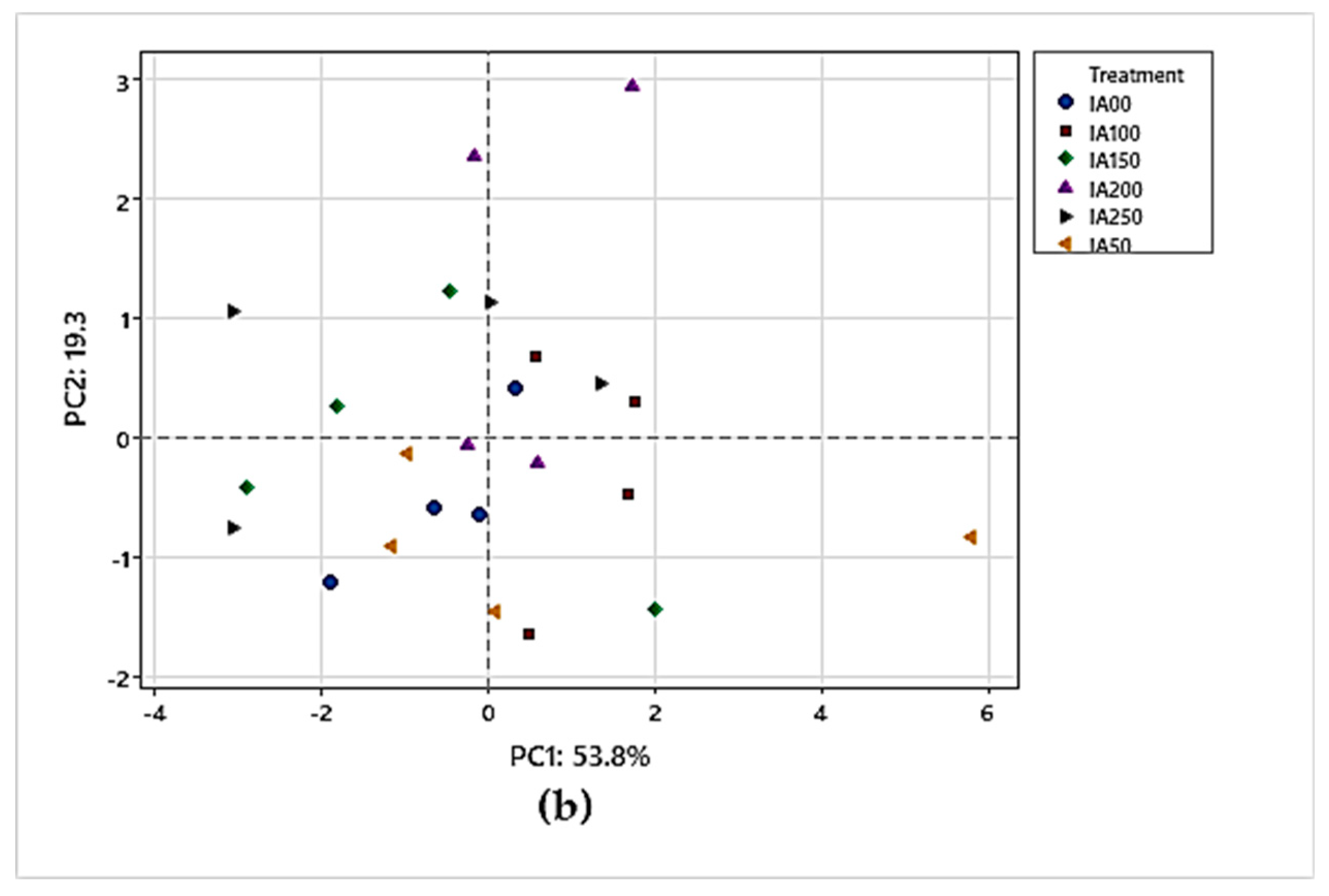
3.4.2. Principal Component Analysis (PCA) for Physical Parameters
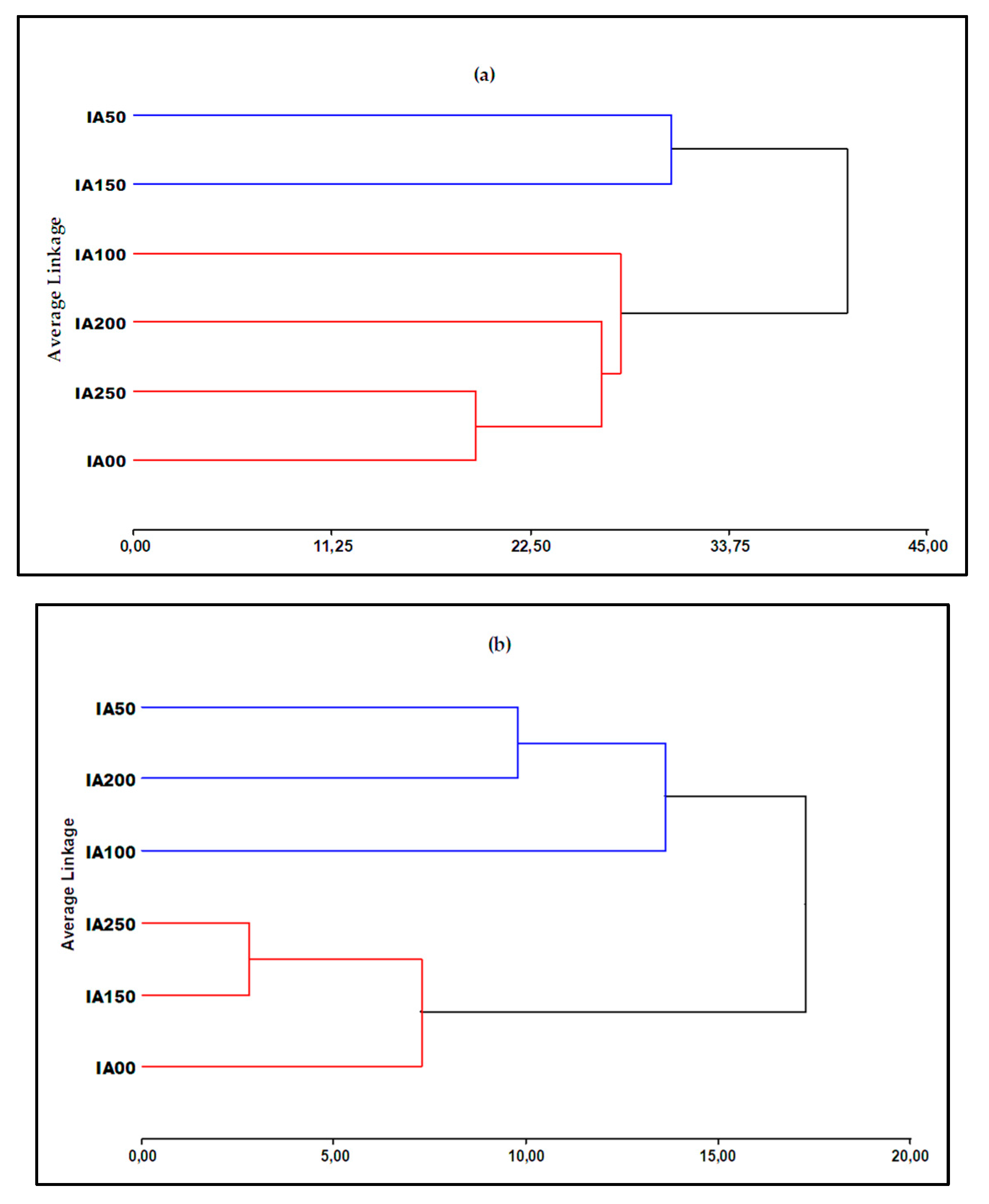
3.4.3. Combined Physical and Chemical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, K.; Mahato, N.; Nile, S.H.; Lee, E.T.; Lee, Y.R. Economical and Environmentally-Friendly Approaches for Usage of Onion (Allium Cepa L.) Waste. Food Funct. 2016, 7, 3354–3369. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowska, M.A.; Jeleń, H.H. Role of Sulfur Compounds in Vegetable and Mushroom Aroma. Molecules 2022, 27, 6116. [Google Scholar] [CrossRef] [PubMed]
- Golubkina, N.; Caruso, G. Chapter 5 - Onion. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Jaiswal, A.K., Ed.; Academic Press, 2020; pp. 73–87. ISBN 978-0-12-812780-3.
- Murtado, A.; Mubarik, N.R.; Tjahjoleksono, A. Isolation and Characterization Endophytic Bacteria as Biological Control of Fungus Colletotrichum Sp. on Onion Plants (Allium Cepa L.). IOP Conf. Ser.: Earth Environ. Sci. 2020, 457, 012043. [Google Scholar] [CrossRef]
- Nepomuceno, R.A.; Brown, C.M.B.; Mojica, P.N.; Brown, M.B. Biological Control Potential of Vesicular Arbuscular Mycorrhizal Root Inoculant (VAMRI) and Associated Phosphate Solubilizing Bacteria, Pseudochrobactrum Asaccharolyticum against Soilborne Phytopathogens of Onion (Allium Cepa L. Var. Red Creole). Archives of Phytopathology and Plant Protection 2019, 52, 714–732. [Google Scholar] [CrossRef]
- Dutta, R.; K., J.; Nadig, S.M.; Manjunathagowda, D.C.; Gurav, V.S.; Singh, M. Anthracnose of Onion (Allium Cepa L.): A Twister Disease. Pathogens 2022, 11, 884. [CrossRef] [PubMed]
- Kalman, B.; Abraham, D.; Graph, S.; Perl-Treves, R.; Meller Harel, Y.; Degani, O. Isolation and Identification of Fusarium Spp., the Causal Agents of Onion (Allium Cepa) Basal Rot in Northeastern Israel. Biology 2020, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Ratnarajah, V.R.; Gnanachelvam, N.G. Effect of Abiotic Stress on Onion Yield: A Review. 2021. [Google Scholar] [CrossRef]
- Khar, A.; Singh, H.; Verma, P. Mitigating Abiotic Stresses in Allium Under Changing Climatic Scenario. In Genomic Designing for Abiotic Stress Resistant Vegetable Crops; Kole, C., Ed.; Springer International Publishing: Cham, 2022; pp. 253–278. ISBN 978-3-031-03964-5. [Google Scholar]
- Carvalho, F.P. Agriculture, Pesticides, Food Security and Food Safety. Environmental Science & Policy 2006, 9, 685–692. [Google Scholar] [CrossRef]
- Hewett, E.W. An Overview of Preharvest Factors Influencing Postharvest Quality of Horticultural Products. International Journal of Postharvest Technology and Innovation 2006, 1, 4–15. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in Agriculture. Frontiers in Plant Science 2020, 11. [Google Scholar] [CrossRef]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Scientia Horticulturae 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Hamid, B.; Zaman, M.; Farooq, S.; Fatima, S.; Sayyed, R.Z.; Baba, Z.A.; Sheikh, T.A.; Reddy, M.S.; El Enshasy, H.; Gafur, A.; et al. Bacterial Plant Biostimulants: A Sustainable Way towards Improving Growth, Productivity, and Health of Crops. Sustainability 2021, 13, 2856. [Google Scholar] [CrossRef]
- Fu, S.-F.; Wei, J.-Y.; Chen, H.-W.; Liu, Y.-Y.; Lu, H.-Y.; Chou, J.-Y. Indole-3-Acetic Acid: A Widespread Physiological Code in Interactions of Fungi with Other Organisms. Plant Signaling & Behavior 2015, 10, e1048052. [Google Scholar] [CrossRef]
- Gray, W.M. Hormonal Regulation of Plant Growth and Development. PLOS Biology 2004, 2, e311. [Google Scholar] [CrossRef]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in Action: Signalling, Transport and the Control of Plant Growth and Development. Nat Rev Mol Cell Biol 2006, 7, 847–859. [Google Scholar] [CrossRef]
- Hagaggi, N.Sh.A.; Mohamed, A.A.A. Enhancement of Zea Mays (L.) Growth Performance Using Indole Acetic Acid Producing Endophyte Mixta Theicola Isolated from Solenostemma Argel (Hayne). South African Journal of Botany 2020, 134, 64–71. [Google Scholar] [CrossRef]
- Zhang, Y.; Paschold, A.; Marcon, C.; Liu, S.; Tai, H.; Nestler, J.; Yeh, C.-T.; Opitz, N.; Lanz, C.; Schnable, P.S.; et al. The Aux/IAA Gene Rum1 Involved in Seminal and Lateral Root Formation Controls Vascular Patterning in Maize (Zea Mays L.) Primary Roots. Journal of Experimental Botany 2014, 65, 4919–4930. [Google Scholar] [CrossRef]
- Bommineni, V.R.; Greyson, R.I. Effect of Gibberellic Acid and Indole-3-Acetic Acid on Growth and Differentiation of Cultured Ear Inflorescences of Maize (Zea Mays L.). Plant Science 1990, 68, 239–247. [Google Scholar] [CrossRef]
- Bermejo, A.; Granero, B.; Mesejo, C.; Reig, C.; Tejedo, V.; Agustí, M.; Primo-Millo, E.; Iglesias, D.J. Auxin and Gibberellin Interact in Citrus Fruit Set. J Plant Growth Regul 2018, 37, 491–501. [Google Scholar] [CrossRef]
- Masmoudi, F.; Tounsi, S.; Dunlap, C.A.; Trigui, M. Halotolerant Bacillus Spizizenii FMH45 Promoting Growth, Physiological, and Antioxidant Parameters of Tomato Plants Exposed to Salt Stress. Plant Cell Rep 2021, 40, 1199–1213. [Google Scholar] [CrossRef]
- Mir, A.R.; Siddiqui, H.; Alam, P.; Hayat, S. Foliar Spray of Auxin/IAA Modulates Photosynthesis, Elemental Composition, ROS Localization and Antioxidant Machinery to Promote Growth of Brassica Juncea. Physiol Mol Biol Plants 2020, 26, 2503–2520. [Google Scholar] [CrossRef]
- Yadav, A.N. Plant Microbiomes for Sustainable Agriculture: Current Research and Future Challenges. In Plant Microbiomes for Sustainable Agriculture; Yadav, A.N., Singh, J., Rastegari, A.A., Yadav, N., Eds.; Desarrollo Sostenible y Biodiversidad; Springer International Publishing: Cham, 2020; pp. 475–482. ISBN 978-3-030-38453-1. [Google Scholar]
- Li, Z.; Zhang, X.; Zhao, Y.; Li, Y.; Zhang, G.; Peng, Z.; Zhang, J. Enhancing Auxin Accumulation in Maize Root Tips Improves Root Growth and Dwarfs Plant Height. Plant Biotechnology Journal 2018, 16, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-W.; Lee, S.M.; Joo, S.-H.; Yun, H.S.; Lee, Y.; Kaufman, P.B.; Kirakosyan, A.; Kim, S.-H.; Nam, K.H.; Lee, J.S.; et al. Elongation and Gravitropic Responses of Arabidopsis Roots Are Regulated by Brassinolide and IAA. Plant, Cell & Environment 2007, 30, 679–689. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Abdel-Motaal, F.; El-Sayed, M.; Jogaiah, S.; Shigyo, M.; Ito, S. ichi; Tran, L.S.P. Dissection of Trichoderma Longibrachiatum-Induced Defense in Onion (Allium Cepa L.) against Fusarium Oxysporum f. Sp. Cepa by Target Metabolite Profiling. Plant Science 2016, 246, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, D.; Rupinder, S.; Ishita, W. Effect of Foliar Application of GA3 and NAA on Onion-a Review. Plant Archives 2018, 18, 1209–1214. [Google Scholar]
- Hye, M.; Haque, M.; Karim, M. Influence of Growth Regulators and Their Time of Application on Yield of Onion. Pakistan Journal of Biological Sciences 2002, 5. [Google Scholar] [CrossRef]
- Publicaciones fertilizantes. Available online: https://www.mapa.gob.es/es/agricultura/publicaciones/Publicaciones-fertilizantes.aspx (accessed on 9 June 2023).
- Ministerio de la Presidencia, Relaciones con las Cortes y Memoria Democrática Real Decreto 47/2022, de 18 de Enero, Sobre Protección de Las Aguas Contra La Contaminación Difusa Producida Por Los Nitratos Procedentes de Fuentes Agrarias; 2022; Vol. BOE-A-2022-860, pp. 5664–5684.
- LICHTENTHALER, H.K.; WELLBURN, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochemical Society Transactions 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid Colorimetric Determination of Nitrate in Plant Tissue by Nitration of Salicylic Acid. Communications in Soil Science and Plant Analysis 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Cruzado, M.; Pastor, A.; Castro, N.; Cedrón, J.C. Determinación de Compuestos Fenólicos y Actividad Antioxidante de Extractos de Alcachofa (Cynara Scolymus L.). Revista de la Sociedad Química del Perú 2013, 79, 57–63. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT - Food Science and Technology 1995, 28, 25–30. [Google Scholar] [CrossRef]
- He, Y.; Zhang, T.; Sun, Y.; Wang, X.; Cao, Q.; Fang, Z.; Chang, M.; Cai, Q.; Lou, L. Exogenous IAA Alleviates Arsenic Toxicity to Rice and Reduces Arsenic Accumulation in Rice Grains. J Plant Growth Regul 2022, 41, 734–741. [Google Scholar] [CrossRef]
- Romanov; , G. A.; Aksenova, N.P.; Konstantinova, T.N.; Golyanovskaya, S.A.; Kossmann, J.; Willmitzer, L. Effect of Indole-3-Acetic Acid and Kinetin on Tuberisation Parameters of Different Cultivars and Transgenic Lines of Potato in Vitro. Plant Growth Regulation 2000, 32, 245–251. [Google Scholar] [CrossRef]
- Hu, Q.-Q.; Shu, J.-Q.; Li, W.-M.; Wang, G.-Z. Role of Auxin and Nitrate Signaling in the Development of Root System Architecture. Frontiers in Plant Science 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Darra, B.L.; Saxena, S.N. Role of IAA on the Mineral Composition of Maize (Zea Mays) Crop under Various Osmotic Stressed Conditions. Plant Soil 1973, 38, 657–661. [Google Scholar] [CrossRef]
- Serrani, J.C.; Carrera, E.; Ruiz-Rivero, O.; Gallego-Giraldo, L.; Peres, L.E.P.; García-Martínez, J.L. Inhibition of Auxin Transport from the Ovary or from the Apical Shoot Induces Parthenocarpic Fruit-Set in Tomato Mediated by Gibberellins. Plant Physiology 2010, 153, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Pflanzen, G.; Alam, M.; Khan, M.; Khan, A.; Imtiaz, M.; Khan, A.; Naeem, M.; Haq, S.; Asim Shah Bacha, S. ; Samiullah, ·; et al. Indole-3-Acetic Acid Rescues Plant Growth and Yield of Salinity Stressed Tomato (Lycopersicon Esculentum L.). 2019. [Google Scholar] [CrossRef]
- Talukdar, M.; Swain, D.K.; Bhadoria, P.B.S. Effect of IAA and BAP Application in Varying Concentration on Seed Yield and Oil Quality of Guizotia Abyssinica (L.f.) Cass. Annals of Agricultural Sciences 2022, 67, 15–23. [Google Scholar] [CrossRef]
- Gupta, S.; Stirk, W.A.; Plačková, L.; Kulkarni, M.G.; Doležal, K.; Van Staden, J. Interactive Effects of Plant Growth-Promoting Rhizobacteria and a Seaweed Extract on the Growth and Physiology of Allium Cepa L. (Onion). Journal of Plant Physiology 2021, 262, 153437. [Google Scholar] [CrossRef]
- Kondhare, K.R.; Patil, A.B.; Giri, A.P. Auxin: An Emerging Regulator of Tuber and Storage Root Development. Plant Science 2021, 306, 110854. [Google Scholar] [CrossRef]
- Ram, M.; Khan, M.A.; Jha, P.; Khan, S.; Kiran, U.; Ahmad, M.M.; Javed, S.; Abdin, M.Z. HMG-CoA Reductase Limits Artemisinin Biosynthesis and Accumulation in Artemisia Annua L. Plants. Acta Physiol Plant 2010, 32, 859–866. [Google Scholar] [CrossRef]
- Lobo, L.L.B.; de Andrade da Silva, M.S.R.; Castellane, T.C.L.; Carvalho, R.F.; Rigobelo, E.C. Effect of Indole-3-Acetic Acid on Tomato Plant Growth. Microorganisms 2022, 10, 2212. [Google Scholar] [CrossRef]
- Spaepen, S.; Dobbelaere, S.; Croonenborghs, A.; Vanderleyden, J. Effects of Azospirillum Brasilense Indole-3-Acetic Acid Production on Inoculated Wheat Plants. Plant Soil 2008, 312, 15–23. [Google Scholar] [CrossRef]
- Wang, H.; Shan, X.; Wen, B.; Owens, G.; Fang, J.; Zhang, S. Effect of Indole-3-Acetic Acid on Lead Accumulation in Maize (Zea Mays L.) Seedlings and the Relevant Antioxidant Response. Environmental and Experimental Botany 2007, 61, 246–253. [Google Scholar] [CrossRef]
- Zhou, J.; Cheng, K.; Huang, G.; Chen, G.; Zhou, S.; Huang, Y.; Zhang, J.; Duan, H.; Fan, H. Effects of Exogenous 3-Indoleacetic Acid and Cadmium Stress on the Physiological and Biochemical Characteristics of Cinnamomum Camphora. Ecotoxicology and Environmental Safety 2020, 191, 109998. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhou, J.; Chen, K.; Wang, Y.; Ai, Y.; Zhang, C.; Zhou, S. Effect of Indole-3-Acetic Acid Supplementation on the Physiology of Lolium Perenne L. and Microbial Activity in Cadmium-Contaminated Soil. Environ Sci Pollut Res 2022, 29, 52483–52492. [Google Scholar] [CrossRef]
- Qi, B.; Zhang, X.; Mao, Z.; Qin, S.; Lv, D. Integration of Root Architecture, Root Nitrogen Metabolism, and Photosynthesis of ‘Hanfu’ Apple Trees under the Cross-Talk between Glucose and IAA. Horticultural Plant Journal 2022. [Google Scholar] [CrossRef]
- Garnica, M.; Houdusse, F.; Zamarreño, A.M.; Garcia-Mina, J.M. The Signal Effect of Nitrate Supply Enhances Active Forms of Cytokinins and Indole Acetic Content and Reduces Abscisic Acid in Wheat Plants Grown with Ammonium. Journal of Plant Physiology 2010, 167, 1264–1272. [Google Scholar] [CrossRef]
- Cantliffe, D.J. Nitrate Accumulation in Table Beets and Spinach as Affected by Nitrogen, Phosphorus, and Potassium Nutrition and Light Intensity1. Agronomy Journal 1973, 65, 563–565. [Google Scholar] [CrossRef]
- Blom-Zandstra, M. Nitrate Accumulation in Vegetables and Its Relationship to Quality. Annals of Applied Biology 1989, 115, 553–561. [Google Scholar] [CrossRef]
- Chen, B.-M.; Wang, Z.-H.; Li, S.-X.; Wang, G.-X.; Song, H.-X.; Wang, X.-N. Effects of Nitrate Supply on Plant Growth, Nitrate Accumulation, Metabolic Nitrate Concentration and Nitrate Reductase Activity in Three Leafy Vegetables. Plant Science 2004, 167, 635–643. [Google Scholar] [CrossRef]
- Hageman, R.H.; Flesher, D. Nitrate Reductase Activity in Corn Seedlings as Affected by Light and Nitrate Content of Nutrient Media 12. Plant Physiol 1960, 35, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.E. Influence of Abscisic Acid on Nitrate Accumulation and Nitrate Reductase Activity in Potato Tuber Slices. Plant and Cell Physiology 1981, 22, 1541–1551. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, X.; Guo, X.; Qi, X.; Feng, F.; Zhang, Y.; Zhao, Q.; Han, D.; Sun, H. Nitrate Modulates Lateral Root Formation by Regulating the Auxin Response and Transport in Rice. Genes 2021, 12, 850. [Google Scholar] [CrossRef]
- Atif, M.; Perveen, S.; Parveen, A.; Mahmood, S.; Saeed, M.; Zafar, S. Thiamine and Indole-3-Acetic Acid Induced Modulations in Physiological and Biochemical Characteristics of Maize (Zea Mays L.) under Arsenic Stress. Sustainability 2022, 14, 13288. [Google Scholar] [CrossRef]
- Kecis, H.; Bagues, M.; Abdelouhab, Y.; Mekircha, F.; Gali, L.; Kadi, K.; Addad, D.; Nagaz, K.; Brahmi, F.; Kouba, Y. Different Indole-3-Acetic Acid and 6 Benzyl Amino Purine Concentrations Affect Biomass, Phenolic Profile, and Bioactivity in Mentha Rotundifolia L. Food Measure 2023. [Google Scholar] [CrossRef]
- Mona, S.A.; Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Soliman, D.W.K.; Wirth, S.; Egamberdieva, D. Increased Resistance of Drought by Trichoderma Harzianum Fungal Treatment Correlates with Increased Secondary Metabolites and Proline Content. Journal of Integrative Agriculture 2017, 16, 1751–1757. [Google Scholar] [CrossRef]
- Arias, J.P.; Zapata, K.; Rojano, B.; Arias, M. Effect of Light Wavelength on Cell Growth, Content of Phenolic Compounds and Antioxidant Activity in Cell Suspension Cultures of Thevetia Peruviana. Journal of Photochemistry and Photobiology B: Biology 2016, 163, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Oueslati, S.; Ksouri, R.; Falleh, H.; Pichette, A.; Abdelly, C.; Legault, J. Phenolic Content, Antioxidant, Anti-Inflammatory and Anticancer Activities of the Edible Halophyte Suaeda Fruticosa Forssk. Food Chemistry 2012, 132, 943–947. [Google Scholar] [CrossRef]
- Mirheidari, F.; Hatami, M.; Ghorbanpour, M. Effect of Different Concentrations of IAA, GA3 and Chitosan Nano-Fiber on Physio-Morphological Characteristics and Metabolite Contents in Roselle (Hibiscus Sabdariffa L.). South African Journal of Botany 2022, 145, 323–333. [Google Scholar] [CrossRef]
- Esan, A.M.; Masisi, K.; Dada, F.A.; Olaiya, C.O. Comparative Effects of Indole Acetic Acid and Salicylic Acid on Oxidative Stress Marker and Antioxidant Potential of Okra (Abelmoschus Esculentus) Fruit under Salinity Stress. Scientia Horticulturae 2017, 216, 278–283. [Google Scholar] [CrossRef]
- Lee, S.K.; Mbwambo, Z.H.; Chung, H.; Luyengi, L.; Gamez, E.J.; Mehta, R.G.; Kinghorn, A.D.; Pezzuto, J.M. Evaluation of the Antioxidant Potential of Natural Products. Comb Chem High Throughput Screen 1998, 1, 35–46. [Google Scholar] [CrossRef]
- Subhasree, B.; Baskar, R.; Laxmi Keerthana, R.; Lijina Susan, R.; Rajasekaran, P. Evaluation of Antioxidant Potential in Selected Green Leafy Vegetables. Food Chemistry 2009, 115, 1213–1220. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Kocira, A.; Czerwińska, E.; Wójtowicz, A.; Bronowicka-Mielniczuk, U.; Koszel, M.; Findura, P. Modeling Biometric Traits, Yield and Nutritional and Antioxidant Properties of Seeds of Three Soybean Cultivars Through the Application of Biostimulant Containing Seaweed and Amino Acids. Frontiers in Plant Science 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The Role of Plant-Derived Natural Antioxidants in Reduction of Oxidative Stress. BioFactors 2022, 48, 611–633. [Google Scholar] [CrossRef]
- Andre, C.M.; Larondelle, Y.; Evers, D. Dietary Antioxidants and Oxidative Stress from a Human and Plant Perspective: A Review. Current Nutrition & Food Science 2010, 6, 2–12. [Google Scholar] [CrossRef]
- Kisiriko, M.; Anastasiadi, M.; Terry, L.A.; Yasri, A.; Beale, M.H.; Ward, J.L. Phenolics from Medicinal and Aromatic Plants: Characterisation and Potential as Biostimulants and Bioprotectants. Molecules 2021, 26, 6343. [Google Scholar] [CrossRef]
- Báidez, A.G.; Gómez, P.; Del Río, J.A.; Ortuño, A. Dysfunctionality of the Xylem in Olea Europaea L. Plants Associated with the Infection Process by Verticillium Dahliae Kleb. Role of Phenolic Compounds in Plant Defense Mechanism. J. Agric. Food Chem. 2007, 55, 3373–3377. [Google Scholar] [CrossRef]
- Schauffler, G.P.; dos Anjos Verzutti Fonseca, J.; Di Piero, R.M. Defense Mechanisms Involved in the Resistance of Maize Cultivars to Bipolaris Maydis. Eur J Plant Pathol 2022, 163, 269–277. [Google Scholar] [CrossRef]
- Basu, S.; Roychoudhury, A.; Saha, P.P.; Sengupta, D.N. Differential Antioxidative Responses of Indica Rice Cultivars to Drought Stress. Plant Growth Regul 2010, 60, 51–59. [Google Scholar] [CrossRef]
- Figueredo, E.F.; Cruz, T.A. da; Almeida, J.R. de; Batista, B.D.; Marcon, J.; Andrade, P.A.M. de; Hayashibara, C.A. de A.; Rosa, M.S.; Azevedo, J.L.; Quecine, M.C. The Key Role of Indole-3-Acetic Acid Biosynthesis by Bacillus Thuringiensis RZ2MS9 in Promoting Maize Growth Revealed by the IpdC Gene Knockout Mediated by the CRISPR-Cas9 System. Microbiological Research 2023, 266, 127218. [Google Scholar] [CrossRef]
- Park, C.H.; Yeo, H.J.; Park, Y.J.; Morgan, A.M.A.; Valan Arasu, M.; Al-Dhabi, N.A.; Park, S.U. Influence of Indole-3-Acetic Acid and Gibberellic Acid on Phenylpropanoid Accumulation in Common Buckwheat (Fagopyrum Esculentum Moench) Sprouts. Molecules 2017, 22, 374. [Google Scholar] [CrossRef] [PubMed]

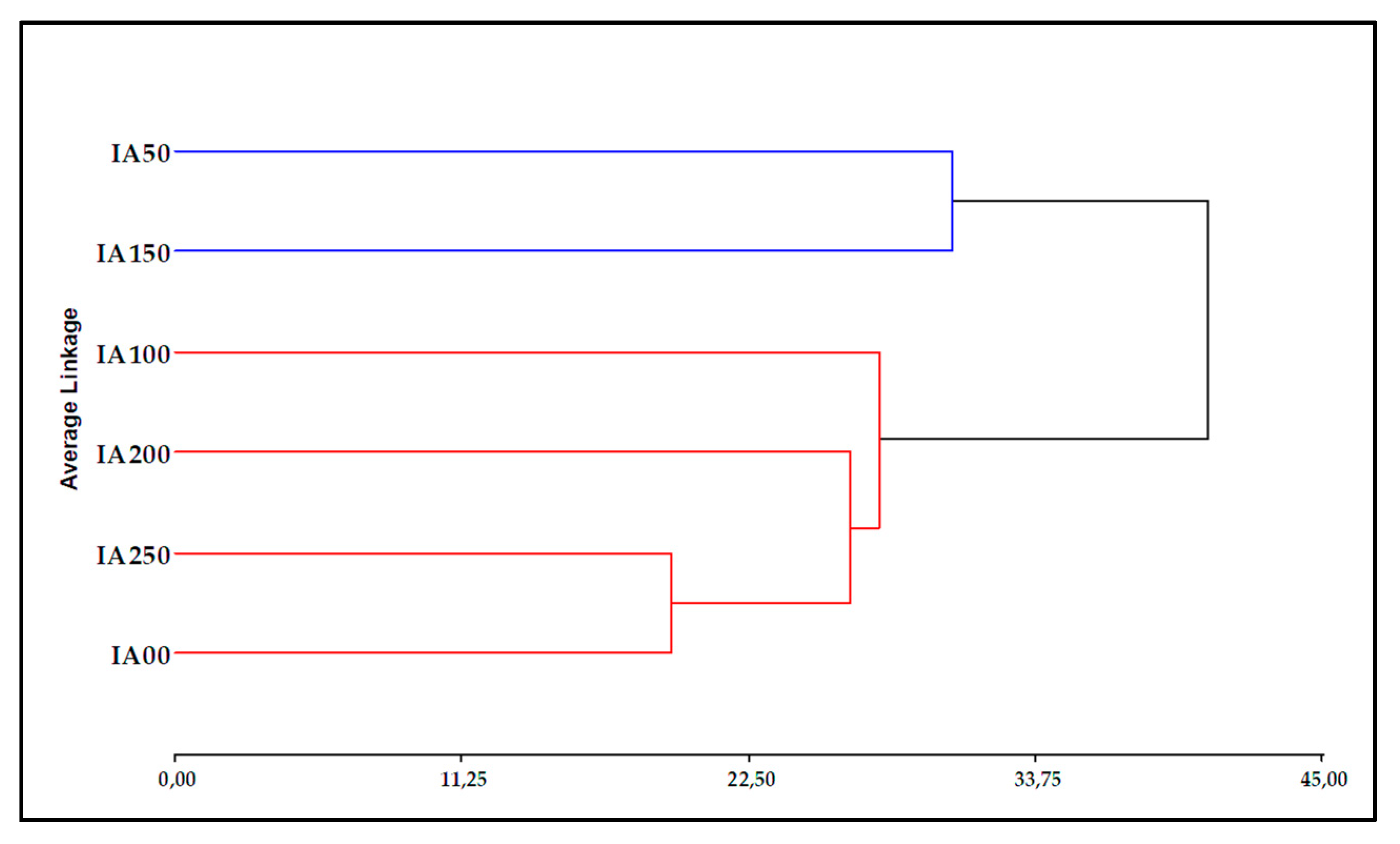
| Treatments | FW (gr) |
FR (gr) |
FF.(gr) | FB (gr) |
|---|---|---|---|---|
| IAA | ||||
| IA50 | 92.0 ± 11.6 a | 13.63 ±1.39 a | 36.47 ± 3.79 a | 3.688 ± 0.180 a |
| IA100 | 63.88 ± 3.02 ab | 9.500 ± 0.354 a | 26.25 ± 3.49 a | 3.3292 ± 0.0712 ab |
| IA150 | 79.4 ± 16.0 ab | 13.25 ± 2.89 a | 31.87 ± 7.57 a | 3.476 ± 0.186 ab |
| IA200 | 53.00 ± 8.37 b | 9.38 ± 1.01 a | 27.21 ± 6.57 a | 2.7915 ± 0.0678 c |
| IA250 | 62.5 ± 15.4 ab | 12.00 ± 3.23 a | 25.23 ± 6.75 a | 3.127 ± 0.257 bc |
| Control | ||||
| IA0 | 57.5 ± 13.4 ab | 7.75 ± 1.53 a | 24.13 ± 5.67 a | 3.090 ± 0.206 bc |
| Significance | ||||
| biostimulant concentration (B) | ns | ns | ns | * |
| Treatments | PH (cm) |
BH (cm) |
CDB (cm) |
|---|---|---|---|
| IA50 | 56.00 ±2.61 ab | 5.000 ±0.449 a | 4.025 ±0.315 a |
| IA100 | 56.50 ±2.25 ab | 4.625 ±0.492 a | 3.225 ±0.131 a |
| IA150 | 58.25 ±2.78 a | 4.800 ±0.394 a | 3.850 ±0.497 a |
| IA200 | 51.00 ±2.45 b | 5.250 ±0.250 a | 3.200 ±0.618 a |
| IA250 | 54.50 ±1.94 ab | 4.675 ±0.287 a | 3.050 ±0.429 a |
| Control | |||
| IA0 | 52.25 ±1.03 ab | 4.425 ±0.417 a | 3.075 ±0.180 a |
| Significance | |||
| biostimulant concentration (B) | ns | ns | ns |
| Treatments | RDW (gr) |
BDW (gr) |
FDW (gr) |
|---|---|---|---|
| IAA | |||
| IA50 | 0.4582 ± 0.0807 ab | 0.532 ± 0.176 a | 0.793 ±0.200 a |
| IA100 | 0.4546 ± 0.0918 ab | 0.5092 ± 0.0480 a | 0.631 ± 0.106 a |
| IA150 | 0.4364 ± 0.0751 ab | 0.797 ± 0.301 a | 0.749 ± 0.190 a |
| IA200 | 0.5508 ± 0.0626 a | 0.448 ± 0.233 a | 0.4419 ± 0.0950 a |
| IA250 | 0.5338 ± 0.0818 a | 0.498 ± 0.148 a | 0.6107 ± 0.0718 a |
| Control | |||
| IA0 | 0.3178 ± 0.0346 b | 0.517 ± 0.175 a | 0.458 ± 0.159 a |
| Significance | |||
| biostimulant concentration (B) | ns | ns | ns |
| Treatments | Chlorophyll a | Chlorophyll b | Carotenoids | Total pigments | Nitrates |
|---|---|---|---|---|---|
| (mg/ fw) | (mg/ fw) | (mg/ fw) | (mg/ fw) | (mg/ kg fw) | |
| IAA | |||||
| IA50 | 0.445 ± 0.120 a | 0.409 ± 0.131 a | 0.2006 ± 0.0587 a | 0.4473 ± 0.0384 a | 1424 ± 174 b |
| IA100 | 0.3706 ± 0.0286 a | 0.4532 ± 0.0275 a | 0.1987 ± 0.0230 a | 0.5869 ± 0.0320 a | 3734 ± 763 a |
| IA150 | 0.2941 ± 0.0595 a | 0.2983 ± 0.0739 a | 0.1253 ± 0.0533 a | 0.4193 ± 0.0943 a | 2422 ± 552 ab |
| IA200 | 0.4135 ± 0.0580 a | 0.3598 ± 0.0414 a | 0.1557 ± 0.0165 a | 0.5565 ± 0.0447 a | 2278 ± 366 ab |
| IA250 | 0.2669 ± 0.0551 a | 0.2405 ± 0.0816 a | 0.1270 ±0.0565 a | 0.3627 ± 0.0942 a | 2104 ± 619 b |
| Control | |||||
| IA0 | 0.2592 ± 0.0133 a | 0.3561 ± 0.0578 a | 0.1291 ±0.0231 a | 0.4473 ± 0.0384 a | 2072 ±371 b |
| Significance | |||||
| biostimulant concentration (B) | ns | ns | ns | ns | ns |
| Treatments | FT (mg AGE/g dm) |
DPPH (μmol TROLOX/g dm) |
|---|---|---|
| IAA | ||
| IA50 | 0.690 ± 0.269 c | 7.577 ± 0.413 ab |
| IA100 | 1.600 ± 0.597 bc | 6.007 ± 0.272 bc |
| IA150 | 2.692 ± 0.364 abc | 5.421 ± 0.925 c |
| IA200 | 3.989 ± 0.766 a | 7.94 ± 1.00 a |
| IA250 | 2.973 ±0.394 ab | 6.662 ± 0.546 abc |
| Control | ||
| IA0 | 0.635 ± 0.449 c | 7.498 ± 0.361 ab |
| Significance | ||
| biostimulant concentration (B) | * | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





