1. Introduction
In type 1 diabetes mellitus, less insulin is generated, whereas in type 2, the body has problems utilizing the insulin that is produced (Ibrahim and Hassan, 2018). According to predictions, there will be 592 million people worldwide with diabetes mellitus by 2035, making it one of the top 10 killers worldwide (Hamada et al., 2013). Serious macro and microvascular consequences of diabetes mellitus are caused by persistently insufficient glycemic control (Forbes and Cooper, 2013). Diabetes is not explicitly treated in the modern medical system. Angiotensin-converting enzyme inhibitors and anti-diabetic medications can be used alone or in combination to treat it early and stop the problem from progressing to an overt stage of nephropathy (Foggensteiner et al., 2001).
Vanillic acid - a flavoring phenolic agent with antioxidant properties - has been successfully used in treating a variety of diseases brought on by oxidative stress and reactive oxygen species (ROS), including CCl4-induced liver and kidney toxicity and streptozotocin-induced diabetic neuropathy (Khairnar et al., 2020; El Rabey et al., 2021; Alamri et al., 2022). Vanillic acid and Zn (II) complex are also used as antioxidants and anti-diabetics (Oke et al., 2021). Additionally, in streptozotocin-induced diabetic rats, it reduced the oxidative stress and inflammation brought on by diabetes (Ji et al., 2020). The antioxidant activity of silver nanoparticles (AgNPs) covered in vanillic acid has been improved in the treatment of diseases brought on by excessive oxidative stress (Zamani and Moradshahi, 2013 and El Rabey et al., 2023). Metallic and polymer nanoparticles created biologically were used for diagnostic purposes and to increase the effectiveness of medicine delivery (Jahangirian et al., 2017; Patra et al., 2018; Ioana and Cristea, 2020 and El Rabey et al., 2023).
Silver nanoparticles were also extensively used in the biomedical sector (Burdus et al., 2018), in the development of nanomedicine, and for numerous pharmacological functions (Younas et al., 2021). Silver nitrate and imipenem nanoparticles successfully battled off Pseudomonas aeruginosa, a drug-resistant bacterium (Shahbandeh et al., 2020). Additionally, Staphylococcus aureus was shielded from building a vancomycin resistance by the silver nanoparticles. Furthermore, encasing infectious germs in nanoparticles made them more vulnerable to drugs. For instance, fluconazole-loaded fungal chitosan nanoparticles were employed to make the drug-resistant Candida spp. more sensitive to fluconazole (El Rabey et al., 2019). Additionally, curcumin was added to fungal chitosan nanoparticles, increasing their anticancer potency (Almutairi et al., 2020).
This study set out to evaluate whether vanillic acid might have a hypoglycemic effect and whether AgNPs-coating might improve that impact.
2. Resources and Methodologies
2.1. Chemicals and Animals
All of the chemicals, reagents, and kits used in this inquiry were analytical grade and bought from Sigma-Aldrich, unless another vendor was recommended. (USA). However, the Sprague Dawley rats were seized from the Giza Agricultural Research Center in Egypt.
2.2. Nanoparticles of Silver Nitrate with Vanillic Acid Coating (AgNPS)
Color-mediated silver ion reactions were used to create vanillic acid, which was then coated with silver nitrate nanoparticles (Zamani and Moradshahi, 2013; El Rabey et al., 2023). 2g of vanillic acid was dissolved in a few drops of ethanol before being combined with 100 mL of distilled water. Next, 20 mg of NaNO3 was added, mixed with a stirrer, and then added to get the pH to 10.0. The mixture changed from colorless to yellow to dark brown, ensuring the formation of silver nanoparticles (El Rabey et al., 2023).
2.3. Assessment of the Properties of Synthesized Vanillic acid Coated with AgNPs
2.3.1. Visualization of the AgNPs-Coated Vanillic acid in Transmission Electron Microscopy
The produced AgNPs-coated vanillic acid’s size, shape, assembly, and purity were all analyzed using transmission electron microscopy. (TEM). The sample’s TEM grids were cleaned, cleared out with filter paper, and then placed in a few drops, each around 2 to 5 l in volume, on a parafilm sheet (Zhang et al., 2016 and El Rabey et al., 2023).
2.3.2. Examination of UV-Visible Spectroscopy
In order to identify the surface plasmon resonance, the UV-vis spectra of the generated AgNPs-coated vanillic acid nanoparticles between 300 and 800 nm were studied. (Vivek et al., 2012 and El Rabey et al., 2023).
2.4. Size and Distribution can be Determined via Dynamic Light Scattering. (DLS)
The size and distribution peak of the produced AgNPs-coated vanillic acid was measured using the dynamic light scattering (DLS) method. Before analysis, the produced vanillic acid AgNPs were diluted ten times with deionized water. Then, in a clean cuvette, 25 l of the produced AgNPs-coated vanillic acid were combined, equilibrated for 2 minutes at 20 °C, dispersed, and scaled using the particle sizing device "NICOMP Nano ZLS (Z3000 zls)" (Entegris, Germany) (Janset al., 2009 and Alamri et al., 2022).
2.5. Experimental Methods and Animals
24 of the study’s animals were housed for 14 days in standardized lab conditions at the Faculty of Pharmacy at Mansoura University, which has an approved animal house ethics program. (Approval code, 0185). Throughout the experiment, food and water were readily available. Four groups of rats were created after acclimation. As the experimental control group, the first group (G1) received one injection of 0.1 mol/l citrate buffer (pH 4.5) in the tail vein. The remaining rats were fasted for 12 hours before receiving an intravenous injection of streptozotocin (65 mg/kg bw) in a freshly made, 0.1 mol/l citrate buffer. (pH 4.5) (El Rabey et al., 2017). Blood glucose measurements showed that the start of diabetes was indicated by a level greater than 200 mg/dl. The diabetic rats were divided into three groups at random: the second group, designated G2, was left untreated as a control-positive diabetic group; the third group, designated G3, received a therapy of vanillic acid (100 mg/g bw); and the fourth group, designated G4, received a treatment of vanillic acid (100 mg/g bw) with AgNPs (Kumar et al., 2011). The length of the treatment program was four weeks.
2.6. Dissection and Blood Collection
The rats were diethyl ether-anesthetized and dissected four weeks following treatment. After being divided, the two kidneys were washed with saline solution. The second kidney was fixed in 10% formalin for histology preparations while the first kidney was kept on ice to prepare kidney tissue homogenate. Blood was collected from the heart of the sedated animals, centrifuged for five minutes at 3000 rpm to separate the plasma, then transferred to clean tubes and placed in the freezer for biochemical analysis.
2.7. Preparation of Renal Tissue Homogenate
The kidney was homogenized in phosphate buffer (pH 7.4) while it was extremely cold. The mixture was centrifuged at 4000 rpm for 15 minutes, and the supernatant that was collected was used to determine the amount of lipid peroxidation and antioxidant enzymes.
2.8. Biochemical Analyses
2.8.1. Kidney Function
Human Diagnostic Kits were used to measure the quantities of urea, creatinine, and uric acid in the blood. (Germany). Using Human Diagnostic Kits, serum potassium (K+), calcium (Ca++), and sodium (Na+) ions were also assessed. (Germany).
2.8.2. Interleukin-6 (IL-6)
Serum Interleukin-6 was determined using the MyBioSource Kit (San Diego, USA). (IL-6).
2.8.3. Immunoglobulins
A Genway Biotech kit (USA) was used in accordance with the manufacturer’s instructions to calculate immunoglobulins (IgG, IgA, and IgM) using Berne’s method from 1974.
2.8.4. Antioxidants and Lipid Peroxidation
The Biodiagnostic Kit (Egypt) was used to assess the activity of superoxide dismutase (SOD), glutathione-s-transferase (GST), and catalase (CAT) in the kidney tissue homogenate, according to Habig et al. (1974), Nishikimi et al. (1972), and Aebi (1984), respectively. Malondialdehyde (MDA) in the kidney tissue homogenate was also assessed using the same Biodiagnostic method.
2.9. Histopathology
The 10% formalin-fixed kidney underwent paraffin embedding, ethanol dehydration (in a 70, 80, and 90% series), and xylene clearing. A 5 microtomic slice was cut into microtome sections and stained with hematoxylin and eosin (H&E). (Zhang et al., 2016).
2.10. Statistical Analysis
The statistical package for the social sciences (SPSS) software, version 17.0, was used for data analysis. (Armitage and Berry, 1984). The data are presented as mean standard deviation. The significance between groups was examined using Duncan’s test and one-way analysis of variance (ANOVA).
3. Results
3.1. AgNPs-Coated Vanillic Acid
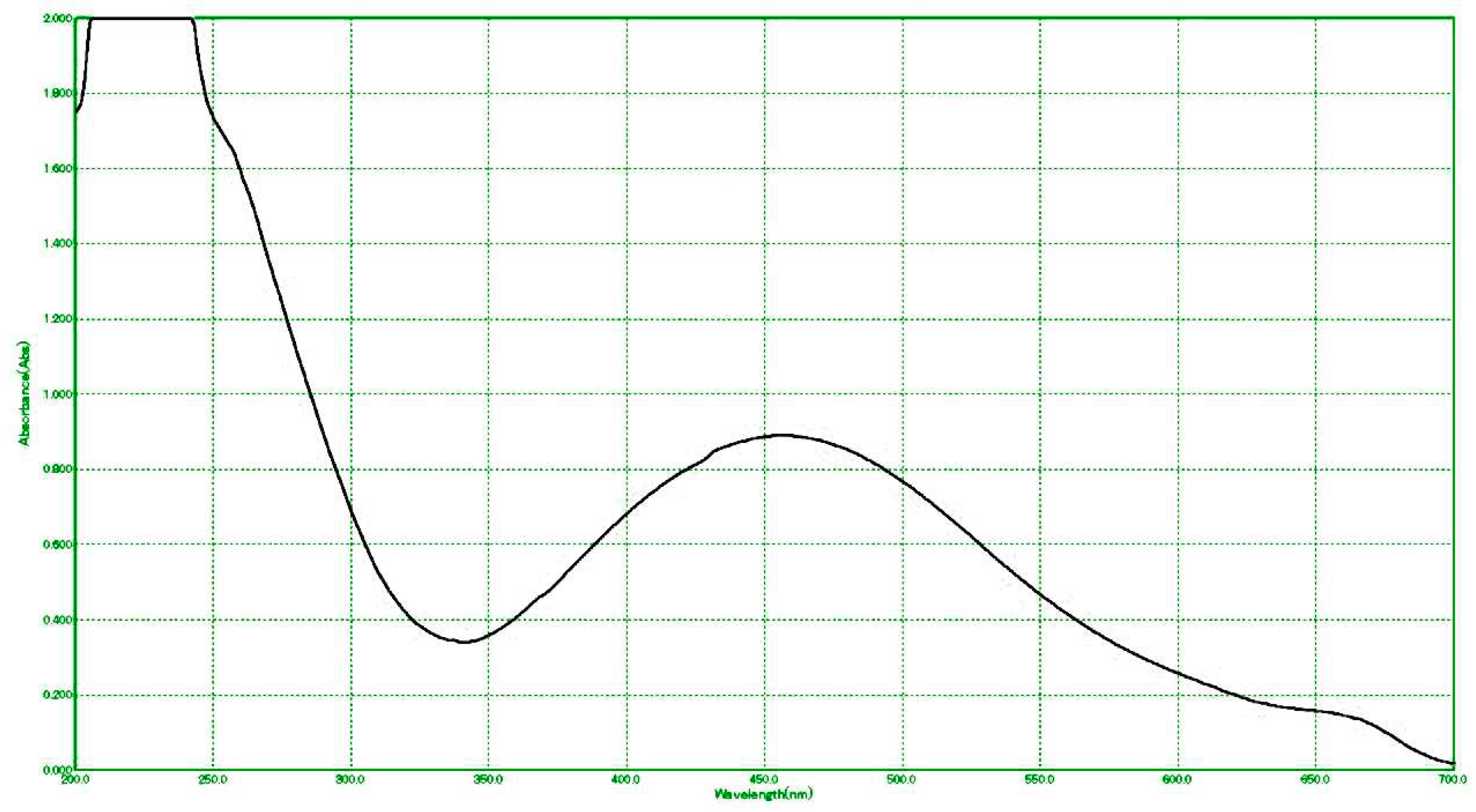
The vanillic acid solution changed color to a dark brown tint after adding silver nitrate to make the AgNPs-coated vanillic acid, indicating the formation of the silver nanoparticle. The obtained substance’s UV-vis spectrum was examined in order to confirm the creation of surface plasmon resonance (SPR)-peaking AgNPs-coated vanillic acid with 450 nm wavelengths (
Figure 1). The wavelength between 400 and 500 nm is observed to have the maximum UV-vis absorption of vanillic acid nanoparticles generated during biosynthesis.
Figure 2 displays the TEM image of vanillic acid-coated AgNPs. The size of the AgNPs – coated vanillic acid ranged from 10.1 to 15.0 nm. (Zhang et al., 2016). The structure of the synthetic vanillic acid covered with AgNPs can be observed via the oscillations of the electron plasmons on the free surface (
Figure 2). In addition, according to the results of the dynamic light scattering (DLS) investigation shown in
Figure 3, the generated AgNPs-vanillic acid had an average size of 52.3 nm.
3.2. IL-6, FBS, and HbA1c
Table 1 displays the effects of administering vanillic acid and vanillic acid coated with AgNPs to STZ-induced diabetic rats on FBS, HbA1c, and IL-6. G2 rats had greater blood levels of FBS and HbA1c than G1 rats due to STZ-induced diabetes. In contrast, diabetic rats in G3 and G4 treated with vanillic acid or vanillic acid coated with AgNPs had significantly lower blood levels of FBS, HbA1c, and IL-6. The increased levels of FBS, HbA1c, and IL-6 were more effectively reduced by treatment with vanillic acid coated with AgNPs in G4 than by therapy with ordinary vanillic acid in G3.
3.3. Antioxidant and Lipid Peroxidation
Table 2 illustrates the effect of STZ-induced diabetes on antioxidants and lipid peroxidation of renal tissue homogenate. Less GST, SOD, and CAT were generated in G2 when hyperglycemia was induced. The elevated levels of malondialdehyde (MDA) in the kidney tissue homogenate indicate that lipid peroxidation was also elevated. Following vanillic acid therapy, the antioxidant enzyme was somewhat increased and lipid peroxidation was lowered in G3. In contrast, when vanillic acid was coated with AgNPs, the antioxidant enzymes were significantly increased and the degree of lipid peroxidation was decreased in G4.
3.4. Kidney Function and Serum Electrolytes
In G2, STZ-induced diabetes was associated with significantly higher levels of urea, creatinine, uric acid, and potassium ions (K+), but lower sodium (Na+) and calcium (Ca++), as shown in
Table 3. However, diabetic rats treated with vanillic acid in G3 and those treated with vanillic acid supplemented with AgNPs in G4 had significantly higher levels of all examined kidney function indices and electrolyte levels. In addition, AgNPs-coated vanillic acid in G4 significantly improved kidney function and electrolyte levels as compared to vanillic acid in G3.
3.5. Immunoglobins
Table 4 displays that IgA, IgM, and IgG immunoglobulin G2 levels were also elevated in STZ-induced diabetes. However, the levels of these increased immunoglobins were significantly lowered when diabetic rats in groups G3 and G4 were treated with either vanillic acid or AgNPs-coated nanoparticles. AgNPs-coated vanillic acid decreased immunoglobin levels more than regular vanillic acid.
3.6. Pathology
3.6.1. Renal Tissue
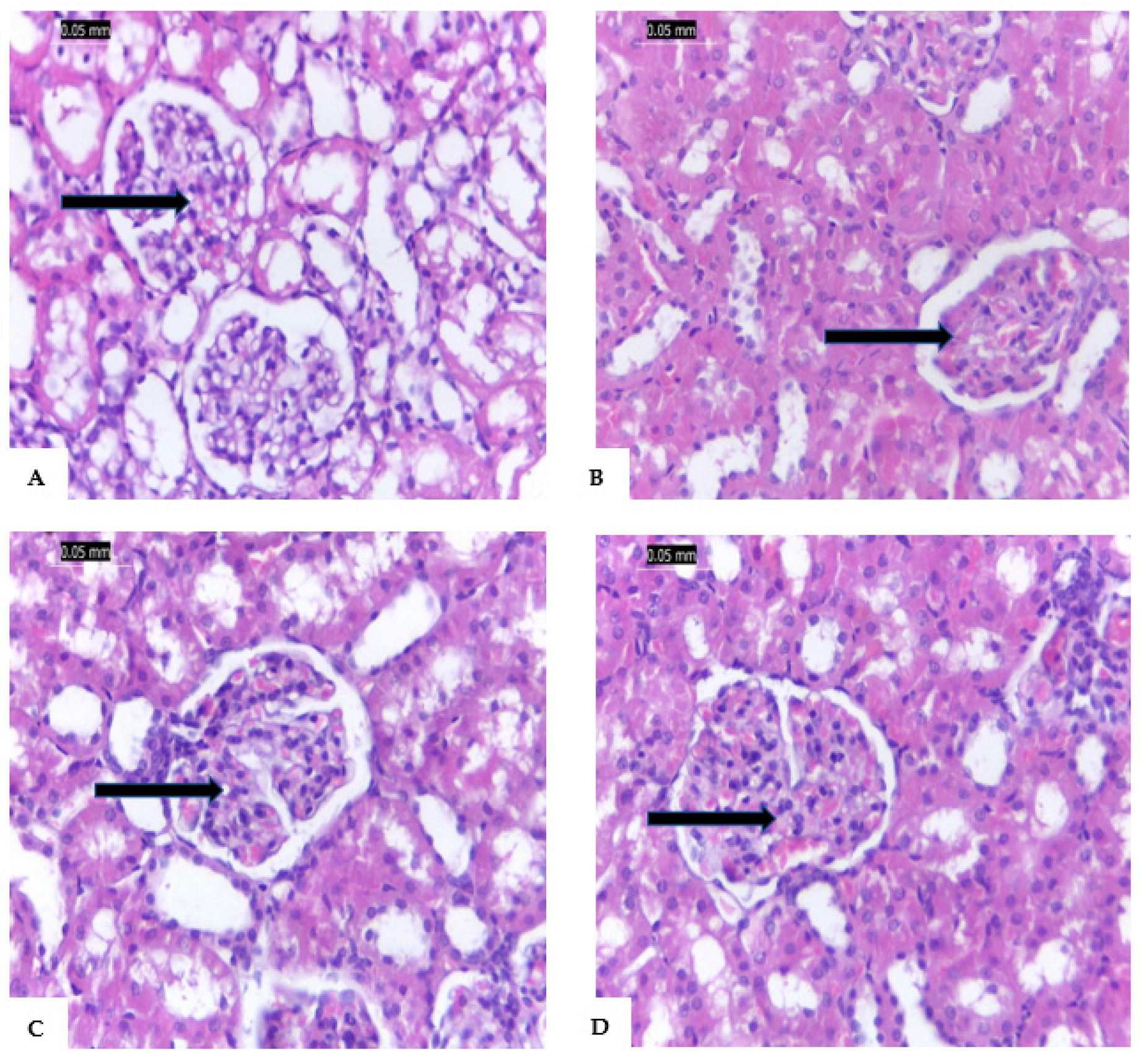
The renal tissue of G1 is shown in
Figure 4A with normal renal tissue, blood vessels, interstitial tissues, renal tubules, and living epithelium. In
Figure 4B, the renal tissues of STZ-induced diabetic rats with reduced vascular tufts, tubular shrinkage, inflammatory interstitial mononuclear infiltration, and glomerular ischemia.
Figure 4C shows that STZ-induced diabetic rats treated with vanillic acid (G3) led to the regeneration of renal tissue despite mild tubular atrophy, glomerular ischemia, and interstitial inflammation. In addition, G4 rats (STZ-induced diabetic rats treated with vanillic acid-coated AgNPs) showed renal tissues appeared almost normal and without any signs of inflammation (
Figure 4D).
3.6.2. Pancreas
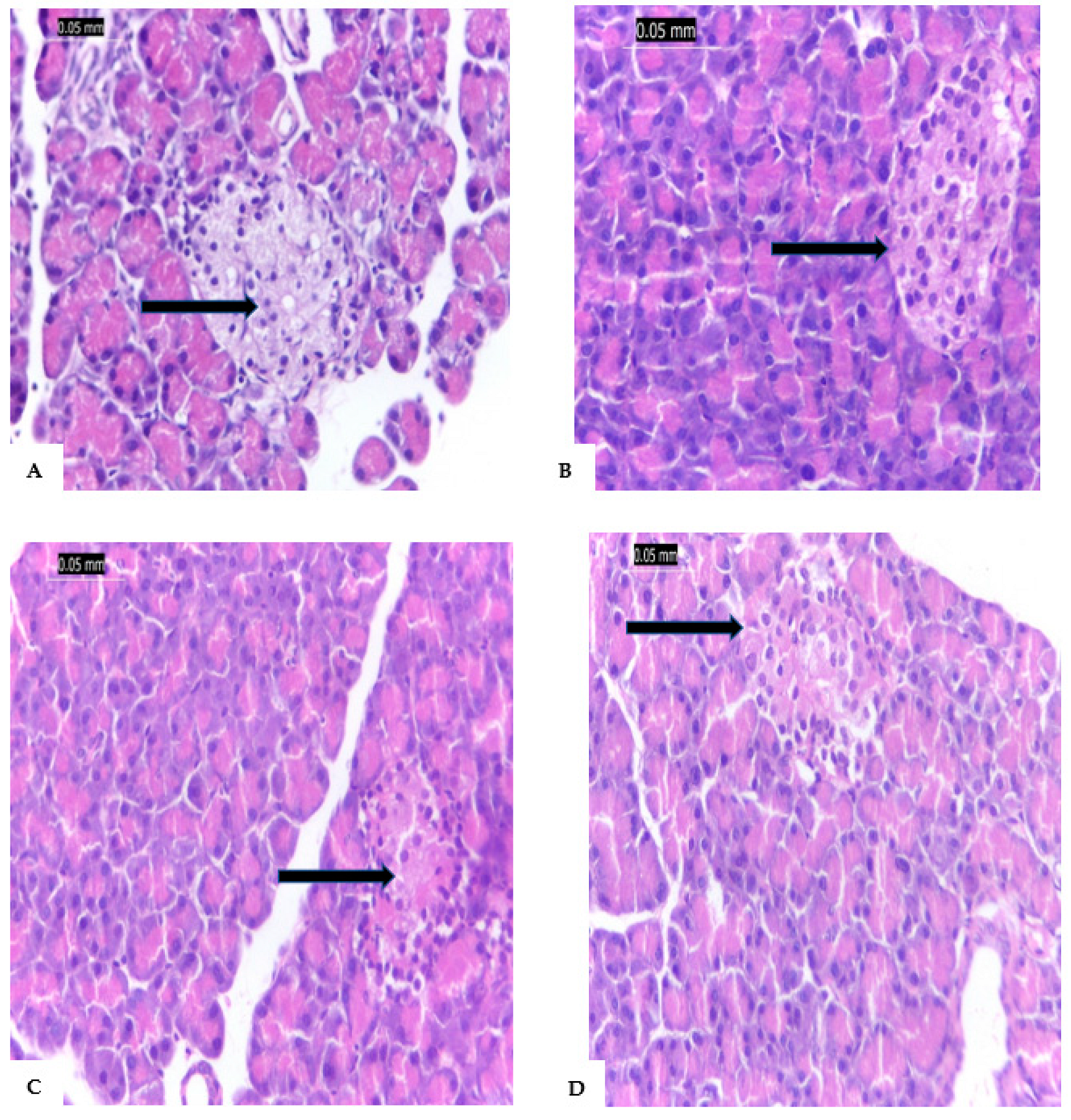
Figure 5A shows a typical pancreatic cell with acini and a typical Islet of Langerhans. The pancreatic tissues, on the other hand, were extensively damaged by the STZ injection, pancreatic tissue of G2 is shown in
Figure 5B, which showed inflammation of the corroded main channels as well as the acini, lymphocytes, and eosinophils between the islets. In G3 (
Figure 5C), the pancreatic tissue shows mild inflammatory infiltration around large ducts. Additionally, in G4, the pancreatic tissues of rats treated with AgNPs-coated vanillic acid did not exhibit any signs of inflammation in the islets or around big ducts; as a result, these tissues are essentially identical to those found in normal pancreas.
4. Discussion
AgNPs-coated vanillic acid was successfully synthesized and showed a surface plasmon resonance (SPR) peak at 450 nm which is consistent with earlier studies (Zamani and Moradshahi, 2013; El Rabey et al., 2023). Additionally, the dynamic light scattering (DLS) analysis revealed that the vanillic acid generated with AgNPs coating was, on average, 52.3 nm in size. This result is in line with (Vivek et al., 2012; Zhang et al., 2016; Alamri et al., 2022 and El Rabey et al., 2023).
The anti-diabetic and antioxidant effects of vanillic acid were demonstrated in the current investigation to have a protective effect in rats with STZ-induced DN (Vivek et al., 2012; Patra et al., 2018; Alamri et al., 2022 and El Rabey et al., 2023). According to Al-Malki and El Rabey (2015) and El Rabey et al. (2017), diabetic rats treated with vanillic acid and vanillic acid-coated AgNPs had significantly lower serum levels of FBS, HbA1c, and IL-6 than diabetic rats treated with STZ. Vanillic acid’s methoxy group, which scavenges free radicals, is what gives it its medicinal antioxidant and anti-diabetic qualities (Kumar et al., 2011; Khairnar et al., 2020; Oke et al., 2021 and Ji et al., 2021). In comparison to vanillic acid coated with silver nanoparticles (AgNPs), which improves the antioxidant and therapeutic action of vanillic acid and speeds up its delivery to the infected tissues, vanillic acid alone was less effective at lowering the elevated levels of FBS, HbA1c, and IL-6 (Kumar et al., 2011; Alamri et al., 2022 and El Rabey et al., 2023).
The decreased renal tissue homogenate GST, SOD, and CAT and higher MDA in the STZ-administered group (G2) are attributable to DNA damage from STZ-generating free radicals that permanently harm the pancreatic islets (Al-Malki and El Rabey, 2015 and El Rabey et al., 2017). While MDA was reduced and the antioxidant enzyme was only marginally increased by treatments with vanillin and AgNPs-coated vanillin. Additionally, AgNPs-coated vanillic acid significantly increased the antioxidant enzymes and decreased lipid peroxidation in the kidney tissue homogenate as compared to the positive control group and more so than vanillic acid alone. The compound’s antioxidant effect is also due to the methoxy group of vanillic acid, which scavenges free radicals, reduces oxidative stress, and subsequently reduces lipid peroxidation (Kumar et al., 2011; Oke et al., 2021 and El Rabey et al., 2023). Vanillic acid that had been coated with AgNPs also exhibited increased antioxidant activity, which increased antioxidant enzyme activity and decreased lipid peroxidation (Kumar et al., 2011 and Alamri et al., 2022).
The levels of uric acid, urea, creatinine, potassium, sodium, and calcium ions in the blood of the STZ-administered group increased while reduced due to STZ’s toxic activity on the pancreatic islets, which led to the development of diabetic nephropathy (Tavafi, 2013; El Rabey et al., 2017; Al-Malki and El Rabey, 2015). It interfered with intracellular and extracellular electrolytes in diabetic patients, resulting in nephropathy, neuropathy, and vascular issues (Al-Rubeaan et al., 2011; Wang et al., 2013 and El Rabey et al., 2017). All renal functions were enhanced by the antioxidant and anti-diabetic properties of vanillic acid and vanillic acid coated with AgNPs. This improvement significantly restored kidney function to baseline levels (Raja and Deepa, 2010; Liamis et al., 2014 and El Rabey et al., 2017). Vanillic acid was also more successful than vanillic acid alone at improving kidney function and electrolyte levels due to the ameliorating effect of covering it with AgNPs (Kumar et al., 2011 and El Rabey et al., 2023).
Al-Malki and El Rabey (2015) stated that the increased immunoglobins (IgA, IgM, and IgG) in the positive STZ-treated group were brought on by the oxidative stress produced by the accumulation of free radicals, whereas the improvement in these immunoglobulins after treatment with vanillic acid is due to the antioxidant and free radical scavenging activity of vanillic acid and its AgNPs-coated form that was reflected in the improvement (Kumar et al., 2011; Khairnar et al., 2020; Ji et al., 2020 and Oke et al., 2021). The AgNPs-coated vanillic acid improved the immunoglobin levels more than the uncoated vanillic acid did because the nano silver nitrate coating enhanced the antioxidant and anti-diabetic effects of vanillic acid (El Rabey et al., 2017; Khairnar et al., 2020and Oke et al., 2021).
The severe effects of STZ injection on kidney tissues, as shown by the glomerular ischemia with reduced vascular tufts, interstitial mononuclear inflammation, and significant tubular atrophy, are inconsistent with the fact that STZ-induced diabetes causes pathological changes to the vital organs (Ramudu et al., 2011 and Sindhu et al., 2015). The renal tissue of the STZ-induced diabetic rats, however, was significantly improved following therapy as a result of the antioxidant and free radical scavenging properties of vanillic acid (Khairnar et al., 2020; Oke et al., 2021 and El Rabey et al., 2023). Additionally, treatment of STZ-induced diabetic rats with silver nitrate nanoparticles virtually restored their renal tissues to normal with no signs of inflammation because it boosted the antioxidant and anti-diabetic activity of vanillic acid (Kumar et al., 2011; Khairnar et al., 2020 and Oke et al., 2021).
The STZ-administered group experienced a rise in free radicals and oxidative stress, which resulted in pathogenic effects on the pancreatic tissues. These effects included inflammation of the acini, lymphocytes, a visible inflammatory infiltrate, corroded large ducts, and eosinophils between islet cells (Ramudu et al., 2011 and Al-Malki and El Rabey, 2015). STZ-induced diabetes alters crucial organs. (Raja and Deepa, 2010; Ramudu et al., 2011 and El Rabey et al., 2017). When vanillic acid was administered to the diabetic rats, the methoxy group of it, which has antioxidant and free radical scavenging characteristics, significantly enhanced all altered pancreatic tissues (Khairnar et al., 2020; Oke et al., 2021 and El Rabey et al., 2017). The silver nanoparticle coating has also improved the antioxidant efficacy of vanillic acid (Kumar et al., 2011; Ahmed et al., 2014; Burdușel et al., 2018; Oke et al., 2021).
5. Conclusions
Vanillic acid reduced antihyperglycemic activity, oxidative kidney injury, and renal failure in diabetic rats. Thus, it was possible to infer vanillic acid’s nephroprotective effects in diabetic rats. Vanillic acid’s antioxidant properties may be responsible for the consequence, which leads to a disruption in the downstream inflammatory cascade and necroptotic kidney injury. The study also suggests that vanillic and phenolic acids have promise as new nephroprotective medicines. The use of vanillic acid coated with silver nanoparticles also decreased inflammation more effectively than the use of vanillic acid alone, demonstrating improved vanillic acid antioxidant activity.
Declarations of Interest
The authors declare no conflict of interest.
Data availability
All data are available upon request from the corresponding author.
List of Abbreviations
| AgNPs |
Silver nanoparticles |
| CAT |
Catalase |
| DLS |
dynamic light scattering |
| FBS |
fasting blood sugar |
| G1 |
a negative control group injection of 0.1 mol/l citrate buffer (pH 4.5). |
| G2 |
a positive control group was intravenously injected with freshly prepared streptozotocin (65 mg/kg bw) in a 0.1 mol/l citrate buffer (pH 4.5). |
| G3 |
was intravenously injected with freshly prepared streptozotocin (65 mg/kg bw) in a 0.1 mol/l citrate buffer (pH 4.5) and treated with (100 mg/g bw vanillic acid. |
| G4 |
was intravenously injected with freshly prepared streptozotocin (65 mg/kg bw) in a 0.1 mol/l citrate buffer (pH 4.5) and treated with 100 mg/g bw AgNPs-coated vanillic acid. |
| GSH |
Glutathione reduced |
| GST |
Glutathione transferase |
| HbA1c |
Glycated hemoglobin |
| IL-6 |
Interleukin -6 |
| MDA |
Malondialdehyde |
| ROS |
Reactive oxygen species |
| SOD |
Superoxide dismutase |
| SPSS |
Statistical Package for the Social Sciences |
| STZ |
Streptozotocin |
| TEM |
transmission electron microscope |
References
- Aebi, H. , 1984. Catalase in Vitro. Meth. Enzymol. 105, 121–126. [CrossRef]
- Ahmed, D. , Kumar, V., Verma, A., et al., 2014. Antidiabetic, Renal/Hepatic/Pancreas/Cardiac Protective and Antioxidant Potential of Methanol/Dichloromethane Extract of Albizzia Lebbeck Benth. Stem Bark (ALEx) on Streptozotocin-Induced Diabetic Rats. BMC Complement. Alternat. Med. 14, 243-260. [CrossRef]
- Alamri, E.S. , El Rabey, H.A., Alzahrani, OR., et al., 2022. Enhancement of the Protective Activity of Vanillic Acid against Tetrachloro-Carbon (CCl4) Hepatotoxicity in Male Rats by the Synthesis of Silver Nanoparticles (AgNPs). Molecules. 28-27(23), 8308. doi.org/10. 3390. [Google Scholar]
- Al-Malki, A.L. , El Rabey, H.A., 2015. The antidiabetic effect of low doses of Moringa oleifera Lam. seeds on streptozotocin-induced diabetes and diabetic nephropathy in male rats. Biomed Res. Int. 2015. 381040. doi.org/10. 1155. [Google Scholar]
- Almutairi, F.M. , El Rabey H.A., Tayel, A.T., et al., 2020. Augmented anticancer activity of curcumin loaded fungal chitosan nanoparticles. Int. J. Biol. Macromol. 15(155), 861-867. [CrossRef]
- Al-Rubeaan, K. , Siddiqui, K., Abu Risheh, K., et al., 2011. Correlation between Serum Electrolytes and Fasting Glucose and Hb1Ac in Saudi Diabetic Patients. Biol. Trace Elem. Res. 144, (1-3), 463-468. [CrossRef]
- Armitage, W.G. , Berry, G.Y., 1987. Statistical Methods, 7th ed.; Iowa State University: Ames, IA, USA, pp. 39–63.
- Bancroft, J.D. , Stevens, A., 1996. The haematoxylin and eosin. In Theory and Practice of Histological Techniques; Churchill Livingstone: 6th ed. London, UK.
- Berne, G. , 1974. Detection of Total IgG. Clin. Chemist. 200, 61-89. doi.org/10. 1155. [Google Scholar]
- Burdus, A. , Gherasim, O., Grumezescu, A.M., et al. Review, Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomat. 8, 681. [CrossRef]
- El Rabey, H.A. , Alamri, E.S., Alzahrani, O.R., et al., 2023. Silymarin and vanillic acid silver nanoparticles alleviate the carbon tetrachloride-induced nephrotoxicity in male rats. Int. J. Polym. Sci. 4120553. doi.org/10. 1155. [Google Scholar]
- El Rabey, H.A. , Almutairi, F.M., Alalawy, A.I., et al., 2019. Augmented control of drug-resistant Candida spp. via fluconazole loading into fungal chitosan nanoparticles. Int. J. Biol. Macromol. 141, 511-516. [CrossRef]
- El Rabey, H.A. , Al-Seeni, M.N., Bakhashwain A.S., 2017. The Antidiabetic Activity of Nigella sativa and Propolis on Streptozotocin-Induced Diabetes and Diabetic Nephropathy in Male Rats. Evidence-based Complement. Alternat. Med. 1-14. doi.org/10. 1155. [Google Scholar]
- El Rabey, H.A. , Rezk, S.M., Sakran, M.I., et al., 2021. Green coffee methanolic extract and silymarin protect against CCl4-induced hepatotoxicity in albino male rats. BMC Complement Alternat. Med. 21(1).19. [CrossRef]
- Foggensteiner, L. , Mulroy, S., Firth, J., 2001. Management of diabetic nephropathy. J. R. Soc. Med. 94, 210–217. [CrossRef]
- Forbes, J.M. , Cooper, M.E., 2013. Mechanisms of diabetic complications. Physiol. Rev. 93,137–188. doi.org/10.1152/physrev.00045. 2011. [Google Scholar]
- Habig, W. , Pabst, M., Jakoby, W., 1974. Glutathione S-Transferase: The First Enzymatic Step in Mercapturic Acid Formation. Journal of Biological Chemistry. 249, 22, 7130-7139. doi.org/10. 1016. [Google Scholar]
- Hamada, Y. , Nagasaki, H., Fuchigami, M., et al., 2013. The alpha-glucosidase inhibitor miglitol affects bile acid metabolism, ameliorating obesity and insulin resistance in diabetic mice. Metabolism. 62(5), 734–742. [CrossRef]
- Ibrahim, O.H. , Hassan, M.A., 2018. The Use of Antidiabetic Drugs in Alzheimer’s Disease, New Therapeutic Options and Future Perspective. Pharmacol. Pharm. 9, 157-174. [CrossRef]
- Ioana, G. , Cristea, D., 2020. Silver nanoparticles for delivery purposes. In book: by Masoud Mozafari; ’Nanoengineered Biomaterials for Advanced Drug Delivery, pp. 347-371. [CrossRef]
- Jahangirian, H. , Lemraski, E.G., Webster, et al., 2017. A review of drug delivery systems based on nanotechnology and green chemistry: Green nanomedicine. Int. J. Nanomed. 12, 2957–2978. [CrossRef]
- Jans, H. , Liu, X., Austin, L., Maes, G., Huo, Q., 2009. Dynamic Light Scattering as a Powerful Tool for Gold Nanoparticle Bioconjugation and Biomolecular Binding Studies. Anal. Chem. 81(22), 9425-9432.
- Ji, G. , Sun, R., Hu, H. et. al., 2020. Vanillic acid ameliorates hyperglycemia-induced oxidative stress and inflammation in streptozotocin-induced diabetic rats. J. King Saud Univ. – Sci. 32, 2905–2911. doi.org/10.1016/j.jksus.2020.04.010.
- Khairnar, S. , Pawar, S., Patil, V., Rudrapal, M., 2020. Effect of Vanillic Acid in Streptozotocin-Induced Diabetic Neuropathy. Asian J. Biol. Sci. 9(3), 306-312. [CrossRef]
- Kumar, S. , Prahalathan, P., Raja, B., 2011. Antihypertensive and antioxidant potential of vanillic acid, a phenolic compound in l-NAME-induced hypertensive rats: A dose-dependence study. Redox Rep. 16(5), 208–215. [CrossRef]
- Liamis, G. , Liberopoulos, E., Barkas, F., Elisaf, M., 2014. Diabetes Mellitus and Electrolyte Disorders. World J. Clin. Cases. 2(10), 488–496. [CrossRef]
- Nishikimi, M. , Roa, N., Yogi, K., 1972. The Occurrence of Superoxide Anion in the Reaction of Reduced Phenazine Methosulfate and Molecular Oxygen. Biochem. Biophys. Res.Commun. 46(2), 849–854. [CrossRef]
- Oke, I.M. , Ramorobi, L.M., Mashele, S.S. Vanillic acid–Zn(II) complex: a novel complex with antihyperglycaemic and anti-oxidative activity. Journal of Pharmacy and Pharmacology, (2021), Vol 73, 1703–1714.
- Patra, J.K. , Das, G., Fraceto, L.F., et al., 2018. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol. 16. 71. [CrossRef]
- Raja, B. , Deepa, M.S., 2010. The protective role of vanillic acid against acetaminophen-induced hepatotoxicity in rats. J. Pharm. Res. 3, 1480–1484. [CrossRef]
- Ramudu, S. , Korivi, M., Kesireddy, N., et al., 2011. Nephro-Protective Effects of a Ginger Extract on Cytosolic and Mitochondrial Enzymes against Streptozotocin (STZ)-Induced Diabetic Complications In Rats. Chin. H. Phys. 54(2), 79-86. [CrossRef]
- Shahbandeh, M. , Moghadam, M.T., Mirnejad, R. et al., 2020. The Efficacy of AgNO3 Nanoparticles Alone and Conjugated with Imipenem for Combating Extensively Drug-Resistant Pseudomonas aeruginosa. Int. J. Nanomed. 15, 6905-6916. [CrossRef]
- Sindhu, G. , Nishanthi, E., Sharmila, R., 2015. Nephroprotective effect of vanillic acid against cisplatin induced nephrotoxicity in wistar rats: a biochemi-cal and molecular study. Environ. Toxicol. Pharmacol. 39(1), 392–404. [CrossRef]
- Tavafi, M. , 2013. Complexity of diabetic nephropathy pathogenesis and design of investigations. J. Renal. Inj. Prev. 2(2), 61-65. [CrossRef]
- Vivek, R. , Ramar, T., Muthuchelian, K., et al., 2012. Green biosynthesis of silver nanoparticles from Annona squamosa leaf extract and it’s in vitro cytotoxic effect on MCF-7 cells. Process. Biochem. 47, 2405-2410. [CrossRef]
- Wang, S. , Hou, X., Liu, Y., et al., 2013. Serum Electrolyte Levels in Relation to Macrovascular Complications in Chinese Patients with Diabetes Mellitus. Cardiovasc. Diabetol. 12, 146-155. [CrossRef]
- Younas, M. , Ahmad, M.A., Jannat, F.T., et al., 2021. Chapter 18, “Role of silver nanoparticles in multifunctional drug, in Nanomedicine Manufacturing and Applications”. Elsevier Inc. p. 298-319.
- Zamani, H. , Moradshahi, A., 2013. Synthesis and coating of nanosilver by vanillic acid and its effects on Dunaliella salina Teod, Mol. Biol. Res. Commun. 2(3), 47-55. https://mbrc.shirazu.ac.ir/article_1571_346.
- Zhang, X. , Liu, Z., Shen, W., Gurunathan, S., 2016. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 17, 1534-1543. [CrossRef]
- Zhang, X.F. , Liu, Z.G., Shen, W., Gurunathan, S., 2016 Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. sci. 17(9), 1534. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).