1. Introduction
Polypoidal choroidal vasculopathy (PCV), a subtype of neovascular age-related macular degeneration (nAMD), is characterized by polypoidal lesions at the terminus of the branching vascular network (BVN) on indocyanine green angiography (ICGA) [
1]. The increasing use of multimodal imaging, especially optical coherence tomography (OCT) and OCT angiography (OCTA), has provided cumulative evidence that PCV is vascular and originates from the type 1 neovascular network [
2]. Recently, PCV was classified into the pachychoroid disease spectrum, characterized by Haller’s layer vessel dilatation and attenuated choriocapillaris [
3]. These related choroidal changes have a relationship with the locations of BVN ingrowth, suggesting that these pachychoroid features underlie the pathogenesis of PCV lesions [
4]. To the best of our knowledge, there have been studies on the relationship between the morphological characteristics of polypoidal lesions and visual acuity, but not on BVN. Therefore, this study investigates the relationship between BVN morphology and changes in visual acuity, as well as its associated imaging features. Given the variable size and morphology of PCV, those with massive hemorrhage or BVN extending beyond the scope of OCTA may have a different course or nature than those with PCV confined to the macular area with limited hemorrhage. Therefore, we conducted our study to PCV with limited hemorrhage and confined to the macula.
Anti-vascular endothelial growth factor (VEGF) monotherapy is the preferred treatment to achieve the best visual outcomes for PCV with as few injections as possible. However, to date, there are still lack of standard imaging parameters to determine visual and structural prognosis. Our study also analyzed the effect of anti-VEGF therapy for PCV patients and their morphological changes, and investigated the parameters influencing the prognosis of visual acuity.
2. Materials and Methods
2.1. Eligibility criteria and anti-VEGF treatment
This was a retrospective study based on real-world evidence. A medical record review of patients who were first diagnosed with PCV using ICGA was performed from January 2019 to May 2022 at the Department of Ophthalmology, the Fourth People’s Hospital of Shenyang, China Medical University.
The inclusion criteria were as follows: 1) Treatment-naive PCV diagnosis based on EVEREST criteria [
5]; 2) PCV activity was confirmed by both leakage on fluorescein angiography (FA) and exudation on OCT [
6]; 3) PCV lesion was ranged within OCTA 6x6 macular examination range; 4) macular hemorrhage did not affect BVN morphological recognition [
7]; 5) received three-plus pro re nata (3+PRN, PRN dosing with that of 3 initial monthly injections followed by PRN) [
8] intravitreal injections of anti-VEGF treatment and were followed up at one month after each injection; 6) the study eye did not reactive for at least 6 months after the last injection. No reactivation meant no intraretinal or subretinal fluid (SRF) on OCT, and no need for further anti-VEGF therapy or other treatment [
9]. The exclusion criteria were as follows: 1) any other fundus diseases that could confuse the results, such as retinal angiomatous proliferation, retinal arterial microaneurysms, retinal artery or vein occlusion; 2) previous ophthalmic intervention (such as laser, vitrectomy, or photodynamic therapy); 3) refractive medium turbidity affecting BVN morphological recognition or image discrimination; 4) patients with high pigment epithelium detachment(PED)that influences the measurement of choroidal parameters; 5) quiescent PCV and massive hemorrhage PCV (Quiescent PCV is characterized by the presence of one or several polyps in the absence of any intraretinal or subretinal fluid or hemorrhage, and usually does not need treatment [
10]. Massive hemorrhage PCV was defined as four or more disc areas with subretinal or sub-RPE hemorrhaging [
11]. 6) and patients with serious systemic diseases (such as heart or brain infarction, liver or kidney failure, infectious diseases, rheumatic diseases, blood diseases, etc.)
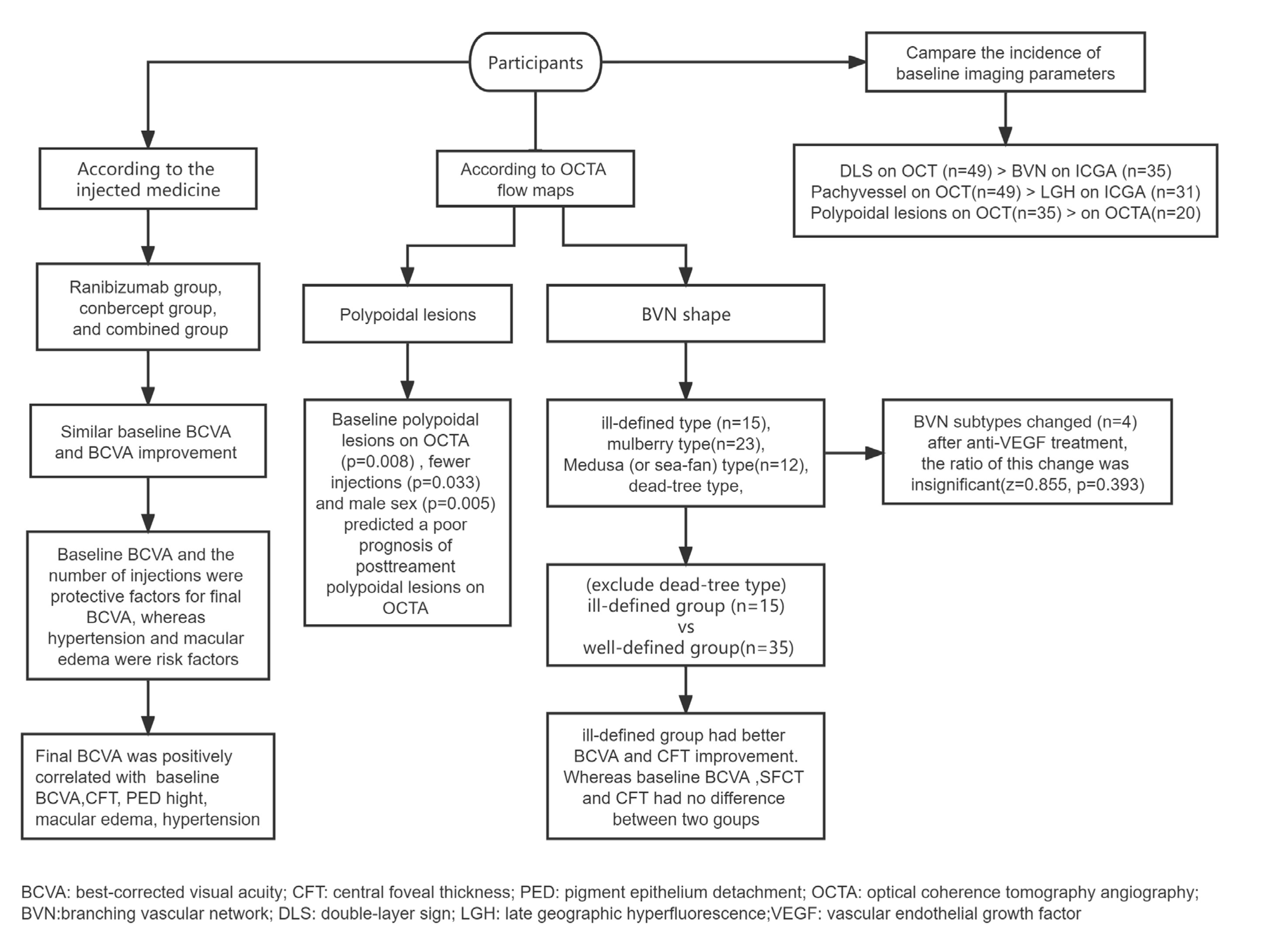
In total, 51 eyes of 51 patients satisfied all the inclusion and exclusion criteria (
Figure 1). All patients received anti-VEGF intravitreal injections using ranibizumab, conbercept, or both. Medication alterations resulted from inadequate responses defined as persistence of SRF on OCT after 3 times of the loading phase injection [
12].
2.2. Clinical measurements
All patients underwent examinations including best-corrected visual acuity (BCVA) in decimal form, fundus photography (TRC-50DX; Topcon, Tokyo, Japan), FA, ICGA (Spectralis HRA; Heidelberg Engineering, Heidelberg, Germany), enhanced depth imaging OCT (Spectralis HRA+OCT; Heidelberg Engineering), and OCTA (RTVue AngioVue System, Optovue, Inc, Fremont, CA) of a macular cube (6 × 6 mm), centred at the fovea. All data were measured by two masked retinal specialists and the average value was used for evaluation. In case of disagreement, a third senior retinal specialist made the final judgement.
We recorded the PCV-related parameters including: late geographic hyperfluorescence (LGH) on ICGA; polypoidal lesions on OCT and OCTA [
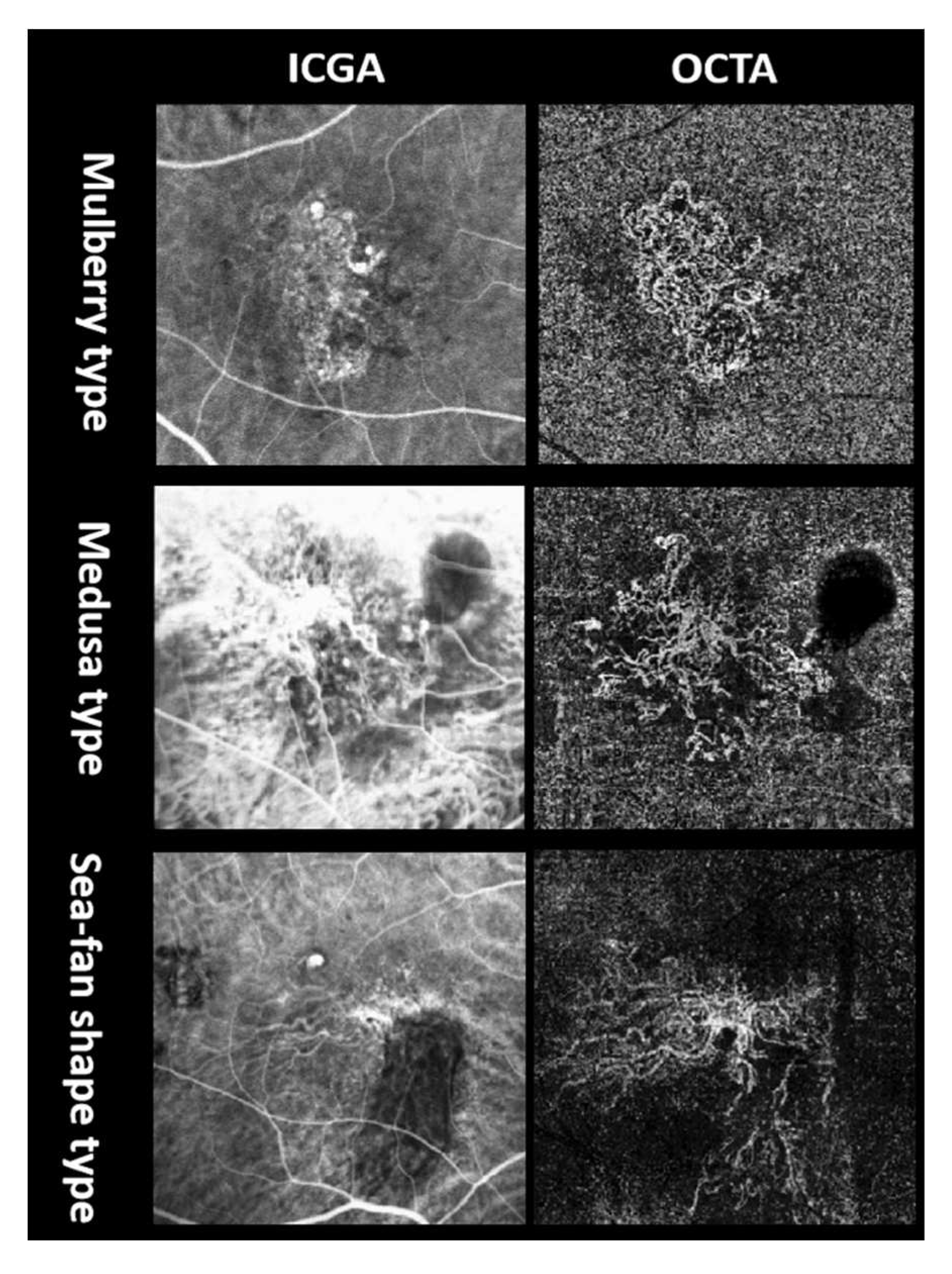
13]; BVN on ICGA and OCTA; double-layer sign (DLS) and pachyvessels on OCT; macular edema on OCT including intraretinal fluid and intraretinal cystoid regions; central foveal thickness (CFT); SRF height; SFCT; and PED height. According to the injected medicine, subgroup analysis categorized all enrolled patients into three groups: ranibizumab, conbercept, and combined groups to analyze the differences in the efficacy. According to OCTA flow maps, BVN was divided into four subtypes based on previous nAMD studies [
14,
15], including ill-defined type, Medusa (or sea-fan shape) type, mulberry type, and dead tree type (
Figure 2). Alteration among these subtypes was counted. Furthermore, mulberry type and medusa (or sea-fan shape) type are more likely to be active, whereas the rest two types are more likely to be less active. Since dead tree type is mostly the latest phase in the course of the disease, which is essentially different from the other types, it was excluded from the following comparison. We divided the remaining patients into ill-defined group and well-defined group, the latter including mulberry type and medusa (or sea-fan shape) type patients. And the differences in the parameters between the two groups were compared. The medical history of hypertension was also documented.
2.3. Statistical analyses
Statistical analyses were performed using SPSS (version 23.0; IBM Inc., Chicago, IL, USA). Means are expressed as median and interquartile range. Categorical variables were expressed as numbers. Decimal BCVA was converted to the logMAR form for statistical analysis. Differences in BCVA between different anti-VEGF injection groups were analyzed using one-way ANOVA tests. Wilcoxon matched-pair signed-rank tests were used to investigate the differences between quantitative data and ranked data. Spearman’s correlation coefficient was used to investigate the factors influencing prognosis of BCVA and polypoidal lesions on OCTA. Moreover, the factors influencing the prognosis of BCVA and polypoidal lesions on OCTA were calculated using linear regression analysis and multiple logistic regression analysis, respectively. Wilcoxon-Mann-Whitney test were used to investigate the differences of imaging parameters between ill-defined group and well-defined group. Gender between these two groups was analyzed by Chi-Square test. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Statistical significance was defined as a P-value < 0.05.
3. Results
3.1. General patient information
Excluding the patients with quiescent PCV and massive hemorrhage PCV, this study finally enrolled fifty-one PCV eyes (26 right eyes and 25 left eyes) of 51 patients with a mean age of 65.0 years (interquartile range: 62.0-71.0 years), including 27 males and 24 females, and the average number of injections was 5 times (interquartile range: 4 to 8). Three injections 3 eyes; four injections 10 eyes; five injections 17 eyes; six injections 4 eyes; seven injections 2 eyes; eight injections 5 eyes; nine injections4 eyes; ten injections 2 eyes; 11 injections2 eyes; 12 injections 1 eye; 18 injections 1 eye.
At baseline, similar BCVA was found in the ranibizumab (0.70 [interquartile range: 0.655-1.325]), conbercept (0.70 [interquartile range: 0.40-1.00]), and combined groups (0.71 [interquartile range: 0.40-1.00], F=0.355, p=0.703). Compared with the baseline (0.70 [interquartile range: 0.40-1.00]), BCVA at last visit (0.60 [interquartile range: 0.40-1.00]) was significantly improved (z=3.093, p=0.002). However, there was no difference in BCVA improvement between the ranibizumab (0.70 [interquartile range: 0.52-1.24]), conbercept (0.52 [interquartile range: 0.30-1.00]), and combined groups (0.56 [interquartile range: 0.375-0.82], F=2.438, p=0.098) at last visit. No change in the number of macular edemas on OCT. And BVN shape on OCTA changed in several patients, without statistically significant. (
Table 1)
Final BCVA was positively correlated with baseline BCVA (r=0.682, p<0.001), medical history of hypertension (r=0.288, p=0.040), presence of macular edema at baseline (r=0.540, p<0.001), CFT at baseline (r=0.415, p=0.002), PED height at baseline (r=0.370, p=0.007), presence of macular edema after anti-VEGF injections (r=0.350, p=0.012), CFT after anti-VEGF injections (r=0.364, p=0.009), and PED height after anti-VEGF injections (r=0.279, p=0.048). After adjusting for age, linear regression analysis revealed that baseline BCVA (B=0.383, 95%CI [0.193-0.572], t=4.087, p<0.001), and the number of injections (B=-0.030, 95%CI [-0.058--0.002], t=2.156, p=0.037) were protective factors for BCVA after anti-VEGF injections. In contrast, medical history of hypertension (B=0.222, 95%CI [0.068-0.375], t=2.913, p=0.0060), and presence of macular edema at baseline (B=0.297, 95%CI [0.040-0.555], t=2.330, p=0.025) were risk factors for BCVA after anti-VEGF injections.
3.2. Variation and correlation of imaging parameters
According to OCTA profile at baseline, fifteen eyes were regarded as ill-defined type, twenty-three eyes were regarded as mulberry type, twelve eyes were regarded as medusa (or sea-fan shape) type, and one eye was regarded as dead-tree type. Four participant’s BVN morphology subtypes had changed on OCTA after anti-VEGF treatment. One ill-defined type change into medusa type, one mulberry type changed into medusa type, and two mulberry type change into dead tree type. However, generally, the ratio of this change was statistically insignificant (z=0.855, p=0.393).
Fifteen eyes were assigned to the ill-defined group, while thirty-five eyes with mulberry type and medusa (or sea-fan shape) type were assigned to the well-defined group. The BCVA in the ill-defined group (-0.18 [interquartile range: -0.40 to 0.00]) was significantly improved after anti-VEGF injections, compared with that (0.00 [interquartile range: -0.15 to 0.00]) in the well-defined group (z=2.143, p=0.032). The improvement of CFT in the ill-defined group (211.00[interquartile range:73.00 to 305.00]) was more significant than that in the well-defined group (68.00[interquartile range: -14.00 to 189.00]) (z=2.371, p=0.018). However, no difference was found in age, gender, the number of injections, baseline BCVA, baseline SFCT, baseline CFT, SRF, baseline PED height, SFCT improvement, SRF improvement and PED height improvement between these two groups (
Table 2).
The polypoidal lesions on OCTA images after anti-VEGF injections were positively correlated with male patients (r=0.367, p=0.008), the presence of polypoidal lesions on OCTA images (r=0.331, p=0.018) at baseline, and the presence of polypoidal lesions on OCT images (r=0.478, p<0.001) after anti-VEGF injections. After adjusting for age, multiple logistic regression analysis showed that male sex (p=0.005), the presence of polypoidal lesions on OCTA at baseline (p=0.008), and fewer injections (p=0.033) predicted a poor prognosis of polypoidal lesions on OCTA after anti-VEGF injections (
Table 3).
After anti-VEGF therapy, the CFT was decreased significantly from 431.0 (interquartile range: 341.0-698.0) µm at baseline to 350.0 (interquartile range: 242.0-580.0) µm (z=3.923, p<0.001) at last visit. There was also a reduction in SRF from 140.0 (interquartile range: 0.0-257.0) µm at baseline to 0.0 (interquartile range: 0.0-165) µm (z=3.137, p=0.002). And the proportion of polypoidal lesions on OCT was 51.0% after anti-VEGF injections, which was significantly lower than that at baseline (z=2.183, p=0.029). The reduction in pachyvessels on OCT was also observed from the baseline (49/51) to last visit (40/51, z=3.0, p=0.003).
3.3. Comparison of the incidence of imaging parameters at baseline
In the early stages of ICGA, 68.6% of eyes with PCV showed BVN, which was lower than the detection rate of the DLS on OCT (96.1%, z=3.5, p<0.001). Similarly, 60.8% of eyes with PCV showed LGH in the late stage of ICGA, which was lower than the detection rate of choroidal pachyvessels on OCT (96.1%, z=4.025, p<0.001). According to the non-invasive detection of polypoidal lesions, OCTA had a relatively lower detection rate (20/51) than OCT (35/51, z=3.441, p=0.001). OCTA had a similar detection capability for BVN (70.6%) with ICGA (68.6%, z=0.243, p=0.808).
4. Discussion
4.1. Factors that influence the prognosis of BCVA
Our study qualitatively and quantitatively analyzed the structural changes in 51 PCV eyes of 51 patients, pre- and post-treatment using multimodal imaging. There was no difference in BCVA improvement between the different anti-VEGF injection groups, consistent with previous study [
16]. A significant trend of improving BCVA was observed compared to baseline, and the final BCVA was related to the baseline BCVA, in accordance with the LUMINOUS study [
17]. The final BCVA was also related to history of hypertension at baseline, same as reported by Gupta et al [
18]. Furthermore, pre- and post-treatment macular edema, CFT and PED height could also affect the final BCVA. Similarly, Schmidt-Erfurth et al indicated that progressive visual loss occurred in PED patients, and was induced by secondary macular edema in the neurosensory retina [
19]. Yamamoto et al also suggested that macular edema was associated with severe vision loss in eyes with end-stage PCV [
20]. Anti-VEGF therapy is known to reduce neovascularization, intra- and sub- retinal exudation. Therefore, persistent macular edema, and higher CFT and PED leads to low vision recovery.
Furthermore, the ill-defined BVN group has a better improvement of vision than the well-defined group. Similarly, Cheung et al reported that the area of BVN and age were associated with change in BCVA [
21]. The difference is that in our study, no age correlation was discovered. And in this study, BVN morphological subtypes were altered in several cases. The alteration of BVN morphological subtypes was thought to be arterialized with thicker and dilated vessel trunks. The absence of tiny branches has led to the transformation of an immature pattern into a mature pattern. The well-defined type has mostly mature dilated vessels and immature lesions which were significantly associated with choroidal neovascularization (CNV) growth [
15], whereas the ill-defined type lacks this structure which is full of tiny vessels. So, it’s reasonable to assume that the well-defined type was possibly more mature or had a longer course than the ill-defined type, result in more damage and less vision recovery. To some extent, the PCV vision prognosis could be predicted by referring to CNV morphological subtypes. Polypoidal lesions and BVN should be considered together to assess the PCV activity.
4.2. Variation and correlation of imaging parameters
On OCT imaging, SRF height, CFT, polypoidal lesions and pachyvessels were decreased significantly after treatment, similar to previous studies [
1,
22,
23]. Regression of polypoidal lesions is a significant anatomical target for the treatment of PCV, as it may have implications for long- term disease stability [
24]. Pachyvessels are representative signs of choroidal vascular hyperpermeability [
25]. The presence of hyperpermeability is a significant risk factor for the development of active PCV [
26]. The VEGF-related pachyvessels diameter change may regulate choroidal thickness and exudative change; and pachyvessels diameter may be a biomarker of exudative change [
27].
On OCTA imaging, the presence of pre-treatment polypoidal lesions on OCTA, fewer injections, and male sex predicted a poor prognosis of polypoidal lesions on OCTA after treatment. A study by Liu et al supported our observations. They reported that males with the rs17030 G allele in complement component 3 gene conferred an increased risk for PCV [
28]. Polypoidal lesion regression as a treatment goal for PCV management remains controversial. Recent clinical trials have shown improved visual acuity with inactive but persistent polypoidal lesions [
29]. Chang et al indicated that polypoidal lesions detected by OCTA were more likely to be persistent than those with a negative flow signal on OCTA [
29].
4.3. Comparison of the incidence of different imaging parameters
Comparing the incidence of baseline imaging parameters, we found that the detection rate of the DLS on OCT was higher than BVN on ICGA. In our study, BVN (68.6%) had a similar incidence with a previous study [
22]. The term BVN is commonly used to describe the whole network area or the relatively large vessels in PCV eyes [
23], which is now considered type 1 macular neovascularization [
29]. Previous histology studies showed that the space within the DLS contained macular neovascularization as well as serous fluid, exudation, thickened extracellular matrix material, or was thickened beneath the basal laminar deposits [
23,
28]. A previous study indicated that the extent of the DLS on OCT was larger than BVN on ICGA in some cases [
17]. Therefore, the detection probability of DLS is greater than BVN, although DLS usually spatially represents the area of the BVN [
16], even though they are not identical.
We found that the detection rate of pachyvessels on OCT was higher than that of LGH on ICGA, although LGH and pachyvessels are thought to be related to choroidal vascular hyperpermeability. LGH was defined as a well-demarcated geographic hyperfluorescent lesion in the late phase of ICGA [
30]. Our results were similar to those of a previous study in which LGH was noted in most PCV eyes [
31]. Kim et al found that all eyes that developed active PCV had LGH at baseline, and vice-versa [
31]. Vessel dilation may be derived from vortex venous engorgement and anastomosis, and macular choroidal vessels could not modulate pressure by vortex vein outflow, so that the regional vessel pressure could be abnormally high, which may result in vascular remodeling [
30]. Therefore, pachyvessels may be an early change that appears before the clinical manifestation of PCV, which coincides with a report by Siedlecki et al [
32].
We assessed two non-invasive examinations to detect polypoidal lesions and found OCTA had a relatively lower detection rate than OCT. Our study showed similar detection rates of polypoidal lesions with previous studies on OCT and OCTA [
33,
34]. Ring-like or bubble-like changes on OCT are highly suggestive of polypoidal lesions and are characteristic of PCV. Although slow and turbulent flow within polypoidal lesions or circulation at the periphery of an aneurysmal dilation might contribute to poor detection on OCTA [
35], these cannot fully replace ICGA. In this regard, the Asia Pacific Ocular Imaging Society PCV workgroup recently provided recommendations for the non-invasive diagnosis of PCV [
36].
This study had several limitations. This was a retrospective, single-center study with a limited sample size. Patients with large hemorrhage or PEDs were excluded so that all parameters could be compared in the same individual, therefore, the findings may primarily refer to PCV eyes with limited hemorrhage. These patients have minimal hemorrhage and are small in extent. Also, Pachyvessels did not have a size criterion, thus we just conducted a qualitative analysis based on their appearance on OCT. We abandoned the analysis of choroidal vascular density because its measurements were interfered too much by artifacts and hemorrhage. All limitations should be considered in future research.
5. Conclusions
In conclusion, our results revealed the visual and morphological changes of patients with active subfoveal circumscribed PCV. The baseline BCVA and the number of injections were protective factors for final BCVA, whereas a history of hypertension and macular edema were risk factors. This study categorized the shape of BVN. PCV patients with ill-defined BVN pattern presented better vision improvement. This study also highlighted that male sex, a smaller number of injections and the presence of polypoidal lesions on OCTA images at baseline were risk factors for the prognosis of polypoidal lesions on OCTA images.
Author Contributions
F.X.: conceptualization, methodology, software, formal analysis, writing—original draft, writing—review& editing. P. X., H.Z. and Q.W.: data curation, methodology, formal analysis, visualization. N.T.: resources, investigation, supervision, project administration. R.H.: conceptualization, software, formal analysis, writing—original draft, writing—review& editing, supervision, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from the Health Commission of Shenyang Research Foundation (No. 2020021).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board/Ethics Committee of the Fourth People’s Hospital of Shenyang (protocol code 2021-kt-005 and date of approval 2021 Oct. 20).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author and with appropriate permissions of the Fourth People’s Hospital of Shenyang. The data are not publicly available due to ethical reasons.
Acknowledgments
Thank to Bing Ma, department of clinical epidemiology and evidence-based medicine, first hospital of China medical university, Shenyang, China, for the statistical analysis.
Conflicts of Interest
The authors have no conflict of interest to declare.
Abbreviations
| PCV |
polypoidal choroidal vasculopathy |
| nAMD |
neovascular age-related macular degeneration |
| BVN |
branching vascular network |
| ICGA |
indocyanine green angiography |
| OCT |
optical coherence tomography |
| OCTA |
optical coherence tomography angiography |
| VEGF |
vascular endothelial growth factor |
| FA |
fluorescein angiography |
| PRN |
pro re nata |
| PED |
pigment epithelium detachment |
| SRF |
subretinal fluid |
| BCVA |
best-corrected visual acuity |
| LGH |
late geographic hyperfluorescence |
| SFCT |
subfoveal choroidal thickness |
| DLS |
double-layer sign |
| CFT |
central foveal thickness |
| PLs |
polypoidal lesions |
| CNV |
choroidal neovascularization |
References
- Fenner, B.J.; Cheung, C.M.G.; Sim, S.S.; Lee, W.K.; Staurenghi, G.; Lai, T.Y.Y.; Ruamviboonsuk, P.; Kokame, G.; Yanagi, Y.; Teo, K.Y.C. Evolving treatment paradigms for PCV. Eye 2021, 36, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Dansingani KK, Gal-Or O, Sadda SR, Yannuzzi LA, Freund KB. Understanding aneurysmal type 1 neovascularization (polypoidal choroidal vasculopathy): a lesson in the taxonomy of 'expanded spectra' - a review. Clin Exp Ophthalmol. 2018 Mar;46(2):189-200.
- Cheung CMG, Lee WK, Koizumi H, Dansingani K, Lai TYY, Freund KB. Pachychoroid disease. Eye (Lond). 2019 Jan;33(1):14-33.
- Lee, W.K.; Baek, J.; Dansingani, K.K.; Lee, J.H.; Freund, K.B. CHOROIDAL MORPHOLOGY IN EYES WITH POLYPOIDAL CHOROIDAL VASCULOPATHY AND NORMAL OR SUBNORMAL SUBFOVEAL CHOROIDAL THICKNESS. Retina 2016, 36, S73–S82. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.S.; Ngo, W.K.; Chen, J.P.; Tan, N.W.; Lim, T.H. EVEREST study report 2: imaging and grading protocol, and baseline characteristics of a randomised controlled trial of polypoidal choroidal vasculopathy. Br. J. Ophthalmol. 2015, 99, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Vyas, C.H.; Cheung, C.M.G.; Tan, C.; Chee, C.; Wong, K.; Jordan-Yu, J.M.N.; Wong, T.Y.; Tan, A.; Fenner, B.; Sim, S.; et al. Multicentre, randomised clinical trial comparing intravitreal aflibercept monotherapy versus aflibercept combined with reduced-fluence photodynamic therapy (RF-PDT) for the treatment of polypoidal choroidal vasculopathy. BMJ Open 2021, 11, e050252. [Google Scholar] [CrossRef]
- Koizumi, H.; Yamagishi, T.; Yamazaki, T.; Kinoshita, S. Relationship Between Clinical Characteristics of Polypoidal Choroidal Vasculopathy and Choroidal Vascular Hyperpermeability. Am. J. Ophthalmol. 2012, 155, 305–313. [Google Scholar] [CrossRef]
- Augsburger, M.; Sarra, G.-M.; Imesch, P. Treat and extend versus pro re nata regimens of ranibizumab and aflibercept in neovascular age-related macular degeneration: a comparative study. Graefe's Arch. Clin. Exp. Ophthalmol. 2019, 257, 1889–1895. [Google Scholar] [CrossRef]
- Zhao, X.-Y.M.; Luo, M.-Y.M.; Meng, L.-H.M.; Zhang, W.-F.M.; Li, B.M.; Wang, E.-Q.M.; Liu, S.-Z.; Yu, W.-H.M.; Chen, Y.-X.M. THE INCIDENCE, CHARACTERISTICS, MANAGEMENT, PROGNOSIS, AND CLASSIFICATION OF BREAKTHROUGH VITREOUS HEMORRHAGE SECONDARY TO POLYPOIDAL CHOROIDAL VASCULOPATHY. Retina 2020, 41, 1675–1685. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Yu, W.; Chen, Y. Additional file 1 of Activation of quiescent polypoidal choroidal vasculopathy after membrane peeling vitrectomy for epiretinal membrane: a case report. [CrossRef]
- Iwasaki, M.; Kobayashi, K.; Aoki, S.; Miyamoto, H.; Imaizumi, H. Comparative analysis of polypoidal choroidal vasculopathy with and without hemorrhage treated by anti-VEGF monotherapy. Graefe's Arch. Clin. Exp. Ophthalmol. 2021, 1–10. [Google Scholar] [CrossRef]
- Schworm, B.; Luft, N.; Keidel, L.F.; Herold, T.R.; Wolf, A.; Priglinger, S.G.; Siedlecki, J. Ranibizumab non-response in pachychoroid neovasculopathy: Effects of switching to aflibercept. Sci. Rep. 2020, 10, 8439. [Google Scholar] [CrossRef]
- Cheung CMG, Lai TYY, Teo K, Ruamviboonsuk P, Chen SJ, Kim JE, et al. Polypoidal Choroidal Vasculopathy: Consensus Nomenclature and Non-Indocyanine Green Angiograph Diagnostic Criteria from the Asia-Pacific Ocular Imaging Society PCV Workgroup. Ophthalmology. 2021 Mar;128(3):443-52.
- Coscas GJ, Lupidi M, Coscas F, Cagini C, Souied EH. OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY VERSUS TRADITIONAL MULTIMODAL IMAGING IN ASSESSING THE ACTIVITY OF EXUDATIVE AGE-RELATED MACULAR DEGENERATION: A New Diagnostic Challenge. Retina. 2015 Nov;35(11):2219-28.
- Karacorlu, M.; Muslubas, I.S.; Arf, S.; Hocaoglu, M.; Ersoz, M.G. Membrane patterns in eyes with choroidal neovascularization on optical coherence tomography angiography. Eye 2019, 33, 1280–1289. [Google Scholar] [CrossRef]
- Huang, Z.; Ding, Q.M.; Yan, M.; Lian, H.; Chen, Z.; Chen, X.; Song, Y. SHORT-TERM EFFICACY OF CONBERCEPT AND RANIBIZUMAB FOR POLYPOIDAL CHOROIDAL VASCULOPATHY. Retina 2019, 39, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Koh A, Lai TYY, Wei WB, Mori R, Wakiyama H, Park KH, et al. REAL-WORLD EFFECTIVENESS AND SAFETY OF RANIBIZUMAB TREATMENT IN PATIENTS WITH AND WITHOUT POLYPOIDAL CHOROIDAL VASCULOPATHY: Twelve-Month Results From the LUMINOUS Study. Retina. 2020 Aug;40(8):1529-39.
- Gupta, P.; Ting, D.S.W.; Thakku, S.G.; Wong, T.-Y.; Cheng, C.-Y.; Wong, E.; Mathur, R.; Wong, D.; Yeo, I.; Cheung, C.M.G. DETAILED CHARACTERIZATION OF CHOROIDAL MORPHOLOGIC AND VASCULAR FEATURES IN AGE-RELATED MACULAR DEGENERATION AND POLYPOIDAL CHOROIDAL VASCULOPATHY. Retina 2017, 37, 2269–2280. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Waldstein, S.M.; Deak, G.-G.; Kundi, M.; Simader, C. Pigment Epithelial Detachment Followed by Retinal Cystoid Degeneration Leads to Vision Loss in Treatment of Neovascular Age-Related Macular Degeneration. Ophthalmology 2015, 122, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto M, Tsujikawa A, Mizukami S, Miyoshi N, Yoshimura N. Cystoid macular edema in polypoidal choroidal vasculopathy viewed by a scanning laser ophthalmoscope: CME in PCV viewed by SLO. Int Ophthalmol. 2009 Dec;29(6):503-6.
- Cheung CMG, Tan CS, Patalauskaite R, Margaron P, Lai TYY. RANIBIZUMAB WITH OR WITHOUT VERTEPORFIN PHOTODYNAMIC THERAPY FOR POLYPOIDAL CHOROIDAL VASCULOPATHY: Predictors of Visual and Anatomical Response in the EVEREST II Study. Retina. 2021 Feb 1;41(2):387-92.
- Hua, R.; Duan, J.; Zhang, M. Pachychoroid Spectrum Disease: Underlying Pathology, Classification, and Phenotypes. Curr. Eye Res. 2021, 46, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Jaffe GJ, Martin DF, Toth CA, Daniel E, Maguire MG, Ying GS, et al. Macular morphology and visual acuity in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2013 Sep;120(9):1860-70.
- Li, X.; Qu, J.; Su, G.; Yu, S.; Zhang, Y.; Sadda, S.V. ; STAR Study Group The comparison of two different strategies of intravitreal conbercept for polypoidal choroidal vasculopathy in Chinese patients results from a 48-week randomized phase 4 study: STAR study. Acta Ophthalmol. 2022, 101, e327–e337. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, S.C.; Kwon, K.Y.; Lee, J.H.; Koh, H.J.; Byeon, S.H.; Kim, S.S.; Kim, M.; Lee, C.S. Subfoveal choroidal thickness as a predictor of treatment response to anti-vascular endothelial growth factor therapy for polypoidal choroidal vasculopathy. Graefe's Arch. Clin. Exp. Ophthalmol. 2015, 254, 1497–1503. [Google Scholar] [CrossRef]
- Fan, D.; Hua, R.; Li, J.Q. Different imaging characteristics between unilateral and bilateral polypoidal choroidal vasculopathy. Photodiagnosis Photodyn. Ther. 2019, 26, 1–7. [Google Scholar] [CrossRef]
- Nagai, N.; Suzuki, M.; Minami, S.; Kurihara, T.; Kamoshita, M.; Sonobe, H.; Watanabe, K.; Uchida, A.; Shinoda, H.; Tsubota, K.; et al. Dynamic changes in choroidal conditions during anti-vascular endothelial growth factor therapy in polypoidal choroidal vasculopathy. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Liu, K.; Lai, T.Y.Y.; Chiang, S.W.Y.; Chan, V.C.K.; Young, A.L.; Tam, P.O.S.; Pang, C.P.; Chen, L.J. Gender specific association of a complement component 3 polymorphism with polypoidal choroidal vasculopathy. Sci. Rep. 2014, 4, 7018–7018. [Google Scholar] [CrossRef]
- Chang, C.-J.; Huang, Y.-M.; Hsieh, M.-H.; Li, A.-F.; Chen, S.-J. Flow signal change in polyps after anti-vascular endothelial growth factor therapy. PLOS ONE 2020, 15, e0241230. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Ledesma-Gil, G.; Cheung, C.M.G. INTERVORTEX VENOUS ANASTOMOSIS IN Pachychoroid-RELATED DISORDERS. Retina 2020, 41, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.T.; Kang, S.W.; Chung, S.E.; Kong, M.G.; Kim, J.H. Development of polypoidal choroidal vasculopathy in unaffected fellow eyes. Br. J. Ophthalmol. 2012, 96, 1217–1221. [Google Scholar] [CrossRef] [PubMed]
- Siedlecki, J.; Schworm, B.; Priglinger, S.G. The Pachychoroid Disease Spectrum—and the Need for a Uniform Classification System. Ophthalmol. Retin. 2019, 3, 1013–1015. [Google Scholar] [CrossRef]
- Takayama, K.; Ito, Y.; Kaneko, H.; Kataoka, K.; Sugita, T.; Maruko, R.; Hattori, K.; Ra, E.; Haga, F.; Terasaki, H. Comparison of indocyanine green angiography and optical coherence tomographic angiography in polypoidal choroidal vasculopathy. Eye 2016, 31, 45–52. [Google Scholar] [CrossRef]
- Cheung, C.M.G.; Yanagi, Y.; Mohla, A.; Lee, S.Y.; Mathur, R.; Chan, C.M.; Yeo, I.; Wong, T.Y. CHARACTERIZATION AND DIFFERENTIATION OF POLYPOIDAL CHOROIDAL VASCULOPATHY USING SWEPT SOURCE OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY. Retina 2017, 37, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Hua R, Wang H. Dark Signals in the Choroidal Vasculature on Optical Coherence Tomography Angiography: An Artefact or Not? J Ophthalmol. 2017;2017:5498125.
- Chong Teo KY, Sadda SR, Gemmy Cheung CM, Chakravarthy U, Staurenghi G, Invernizzi A, et al. Non-ICGA treatment criteria for Suboptimal Anti-VEGF Response for Polypoidal Choroidal Vasculopathy: APOIS PCV Workgroup Report 2. Ophthalmol Retina. 2021 Oct;5(10):945-53.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).