Submitted:
29 July 2023
Posted:
01 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
- To examine the energy efficiency of a solid-state barocaloric cooling system designed to function as a refrigeration machine for retail of cold products needed to be kept at 278 K. The scenario is supposing to be in a hot indoor environment to examine the potentialities of the technology at extreme summer conditions (313 K). The purpose is to compare its performance with an existing vapor compression refrigerator commonly used in industrial settings. The solid-state system employs active barocaloric regeneration, using a heat-transfer fluid consisting of a 50% Ethylene-Glycol (EG) and 50% water mixture, while the solid-state refrigerant used is Acetoxy Silicone Rubber (ASR) [56]
- To assess and compare the energy efficiency of the active barocaloric cycle with that of a commercial vapor compression refrigerator of a similar size, both operating under the same working conditions.
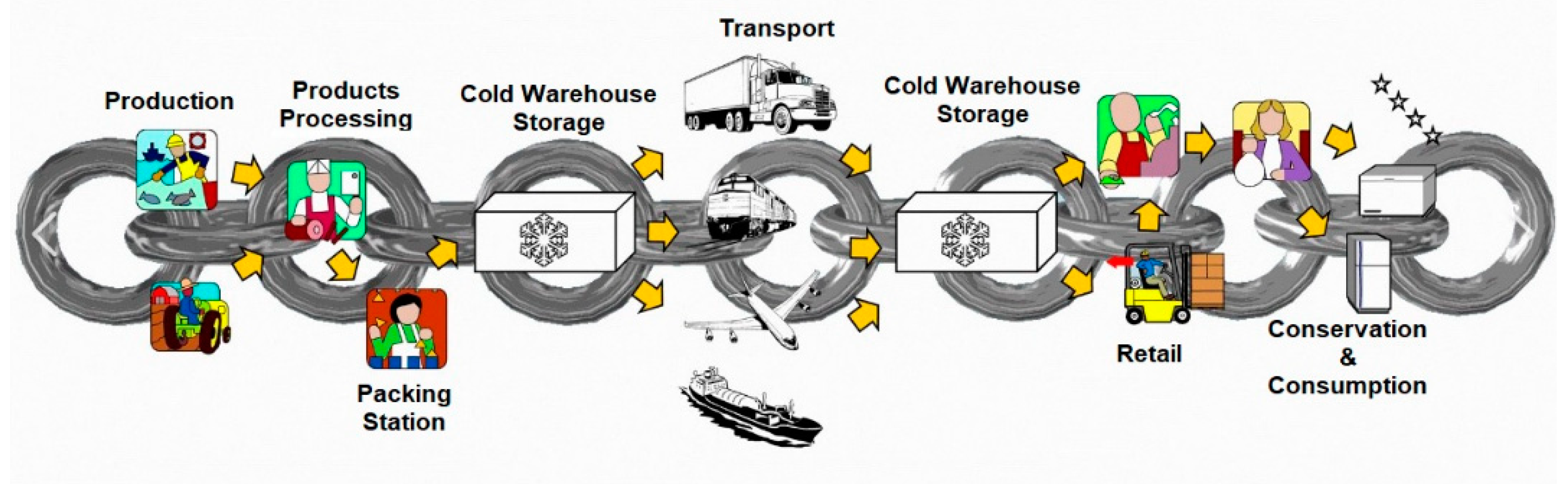
2. The project "PNRR On Foods" and the cold chain
- -
- to promote the sustainability of food production;
- -
- o increase the adherence to more sustainable dietary patterns;
- -
- to promote the sustainability of food distribution;
- -
- to increase the quality of foods and diets;
- -
- to develop smart innovative technologies for a sustainable food production and consumption;
- -
- to guarantee food safety and food security at whole population level and in specific vulnerable targets of the population.
- I.
- Production and Products Processing: measuring the temperature at the core of the product, upon leaving the packaging lines, is necessary to determine the time required to reach the temperature indicated on the packages. After physical-chemical and bacteriological checks, the thermosensitive food products - fresh or frozen - are managed in a refrigerated environment and then stored in temperature-controlled warehouses.
- II.
- Cold Warehouse Storage: the conservation of the product in the cold room guarantees the total reduction of the temperature at the core of the food product and the picking and order preparation activities can also be carried out in the same environment. On the basis of the type of product, it must also be guaranteed that the ideal temperatures and hygienic conditions of containers and packaging are maintained; the loading areas must also be at a controlled temperature and possibly adjacent to the logistics cell.
- III.
- Transport: this phase is of crucial importance for the cold chain. In fact, the vehicles must present themselves with the refrigerators already in temperature and guarantee their respect and maintenance during transport up to another point for cold warehouse storage or to the points of sale of large-scale retail trade: this occurs through the use of tools that allow operators to keep the temperature of the air in the compartment under control.
- IV.
- Retail: the products are displayed in retail outlets on special refrigerated shelves and are available for purchase by the consumer.
- V.
- Conservation and consumption: after purchasing the products, the consumer keeps them in their domestic refrigerators until they are used for consumption.
- the cleanliness and hygiene of the warehouses, equipment and transport;
- the observation of the pre-established critical limits in the various stages of the cold chain;
- the temperatures of the different actors of the cold chain.
- report on best storage and operational solutions for food waste control and reduction of the energy consumptions
- focus on the use of Phase Change materials (PCMs) for the steps of the cold chain related to: II) cold Warehouse storage; III) Transport
- the application of ecofriendly technologies alternative to vapor compression for the refrigeration systems serving the steps of the cold chain related to: IV) Retail; V) Conservation and consumption.
- Meats +5 °C +7° C
- Fish +2°C
- Fresh products +5°C
- Ice creams: -22°C
- Chocolate +18°/+20° C
3. The thermodynamic cycle based on the Barocaloric effect
3.1. The barocaloric material
3.2. Mathematical modelling
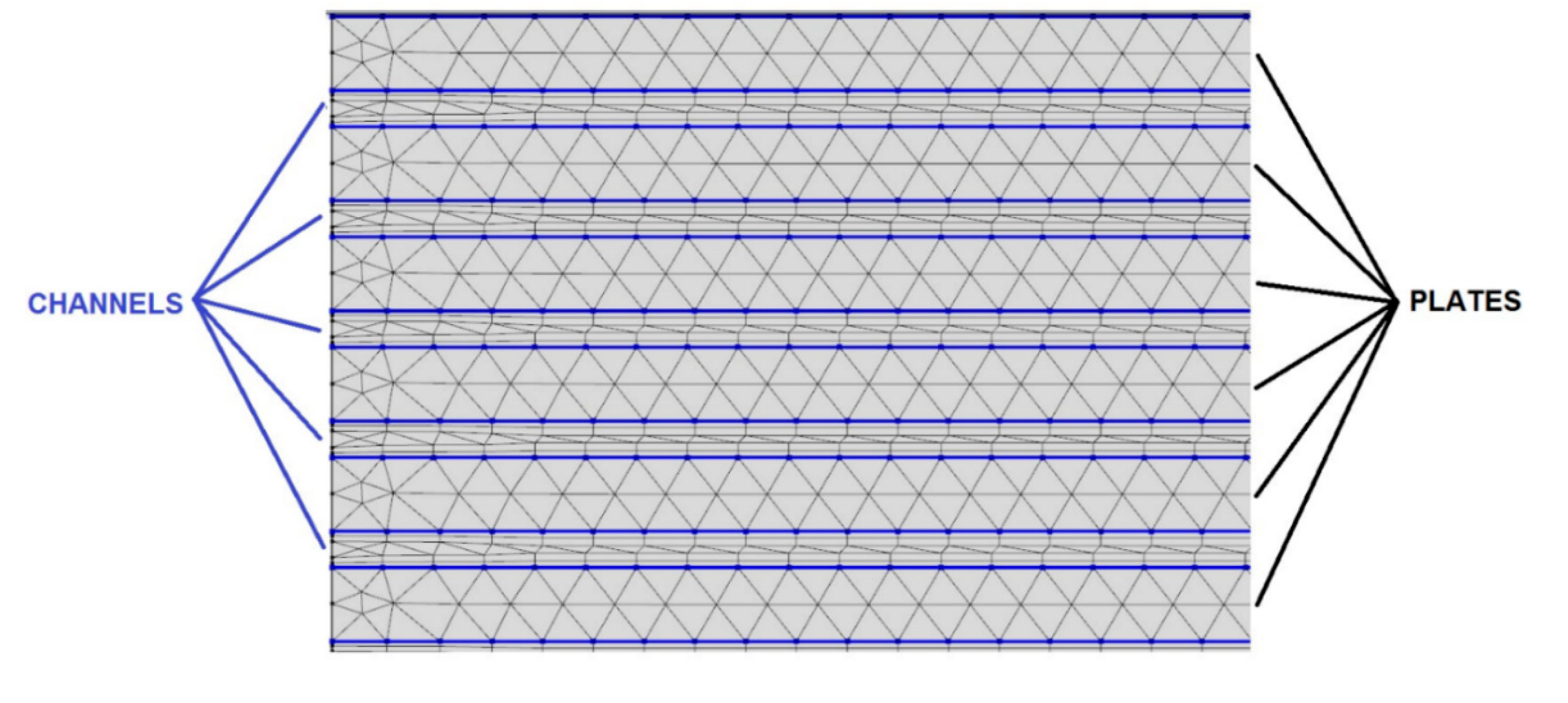
4. Thermal performance analysis
5. Results
6. Conclusion
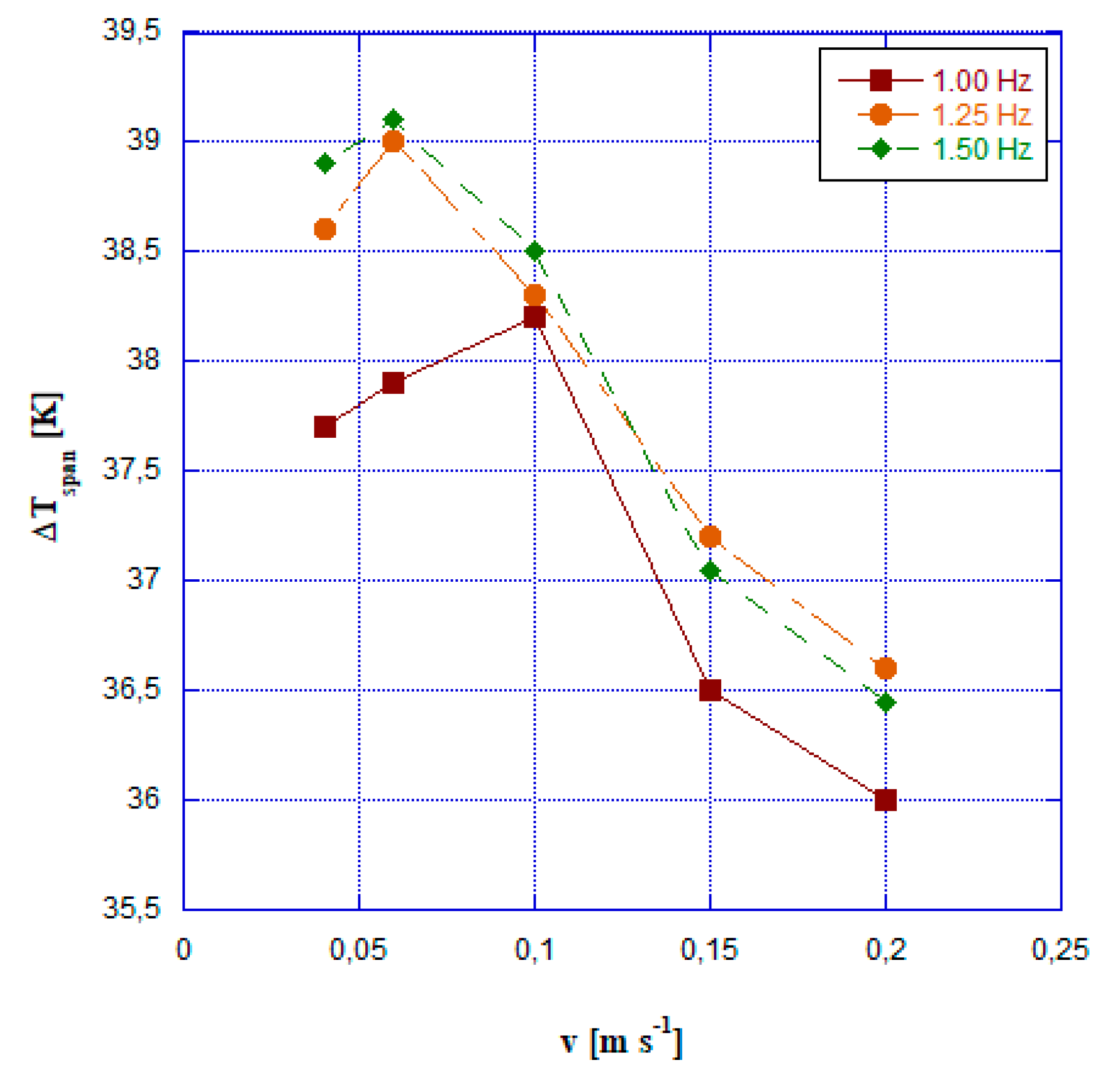
- the temperature span always exceeds 35 K and it reaches a peak of 39.2 at a speed of 0.10 m s-1 and a frequency of 1.5 Hz.
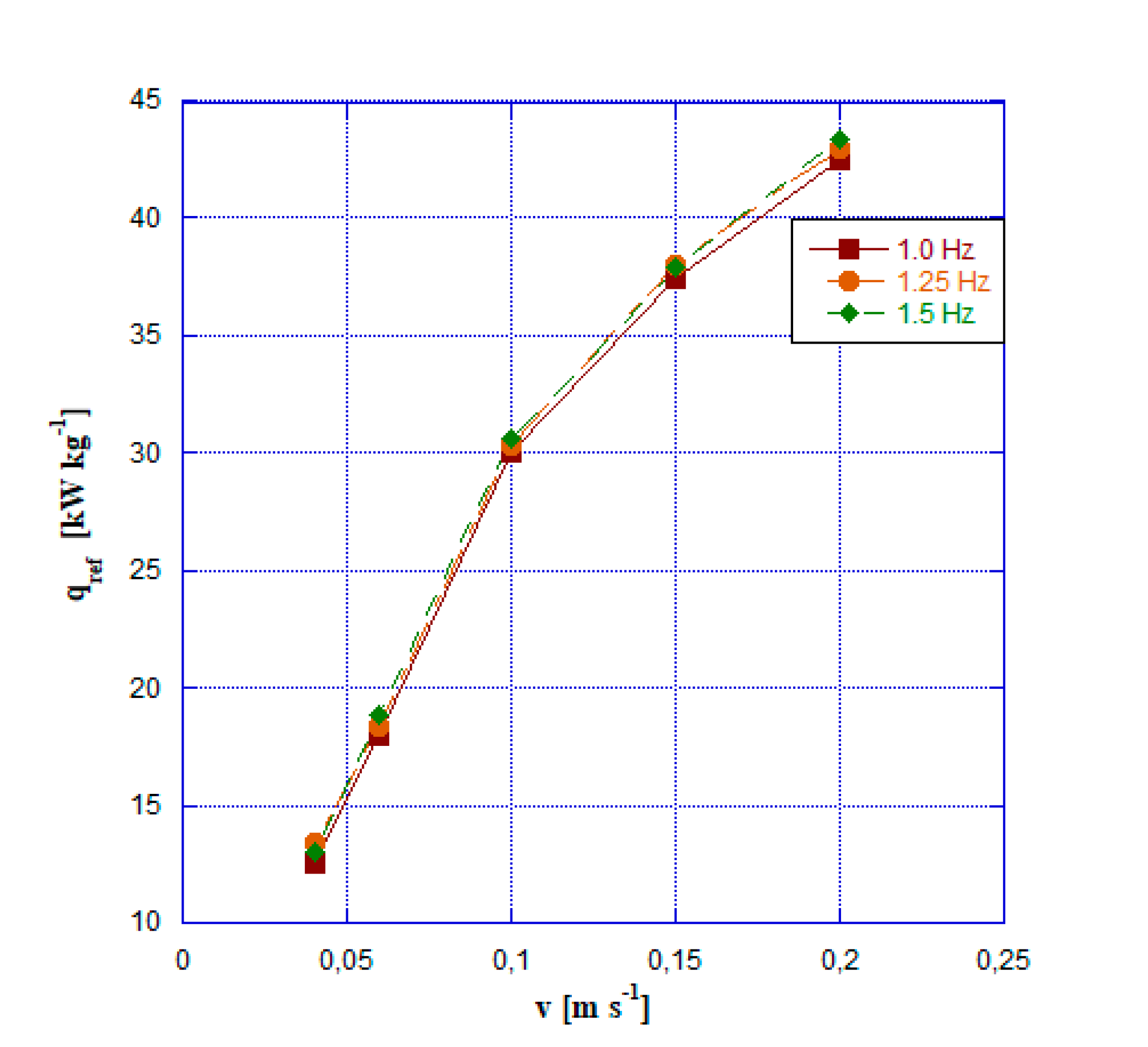
- For fluid velocity greater than 0,06 m s-1 the barocaloric system overperforms the vapor compression one in terms of cooling power. The peak of 44 kW kg-1 is reached at a speed of 0.20 m s-1 and a frequency of 1.5 Hz.
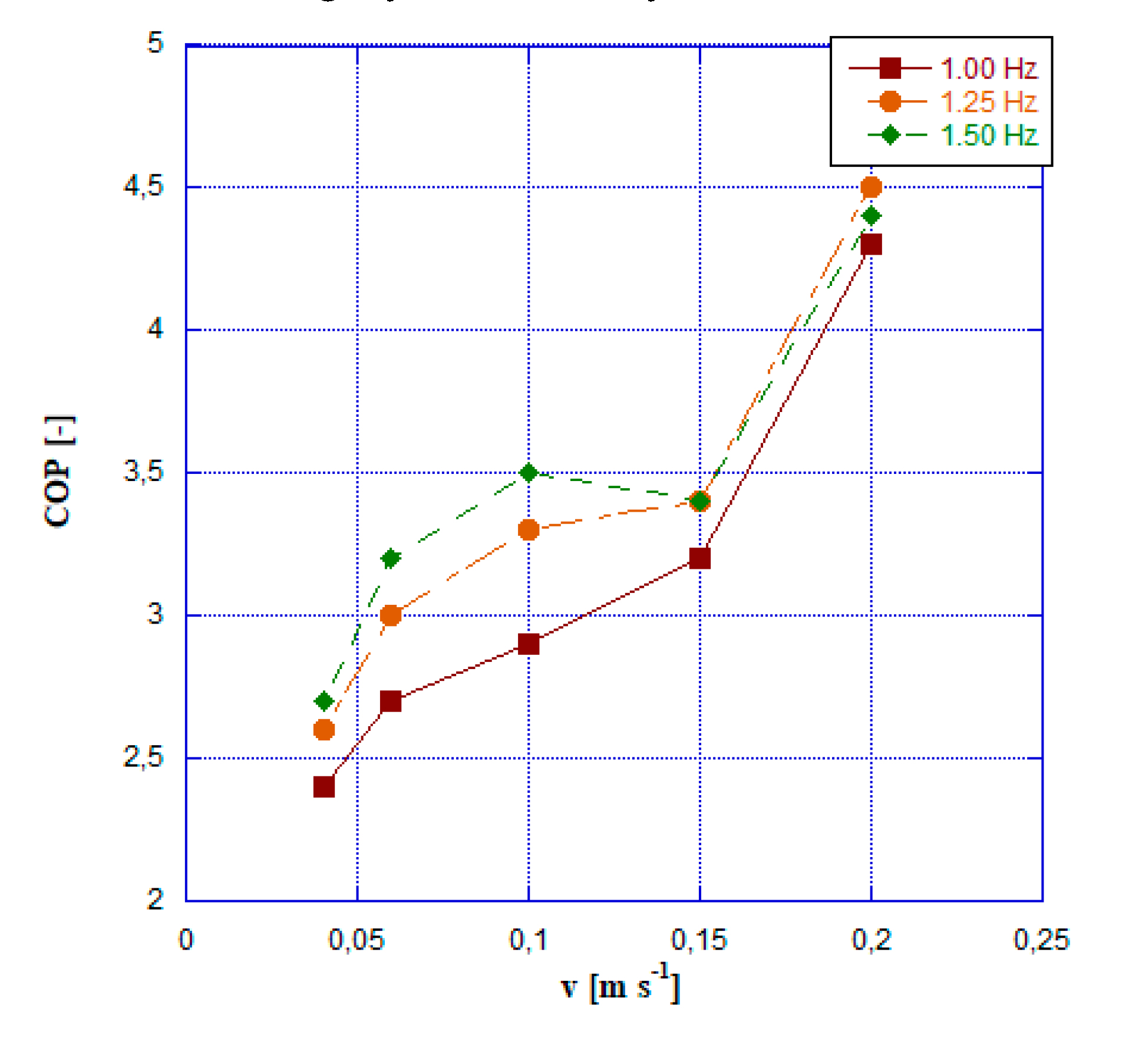
- The most favorable COP readings are observed at a frequency of 1.25 Hz and an HTF velocity of 0.2 m s-1, reaching a peak value of approximately 4.5.
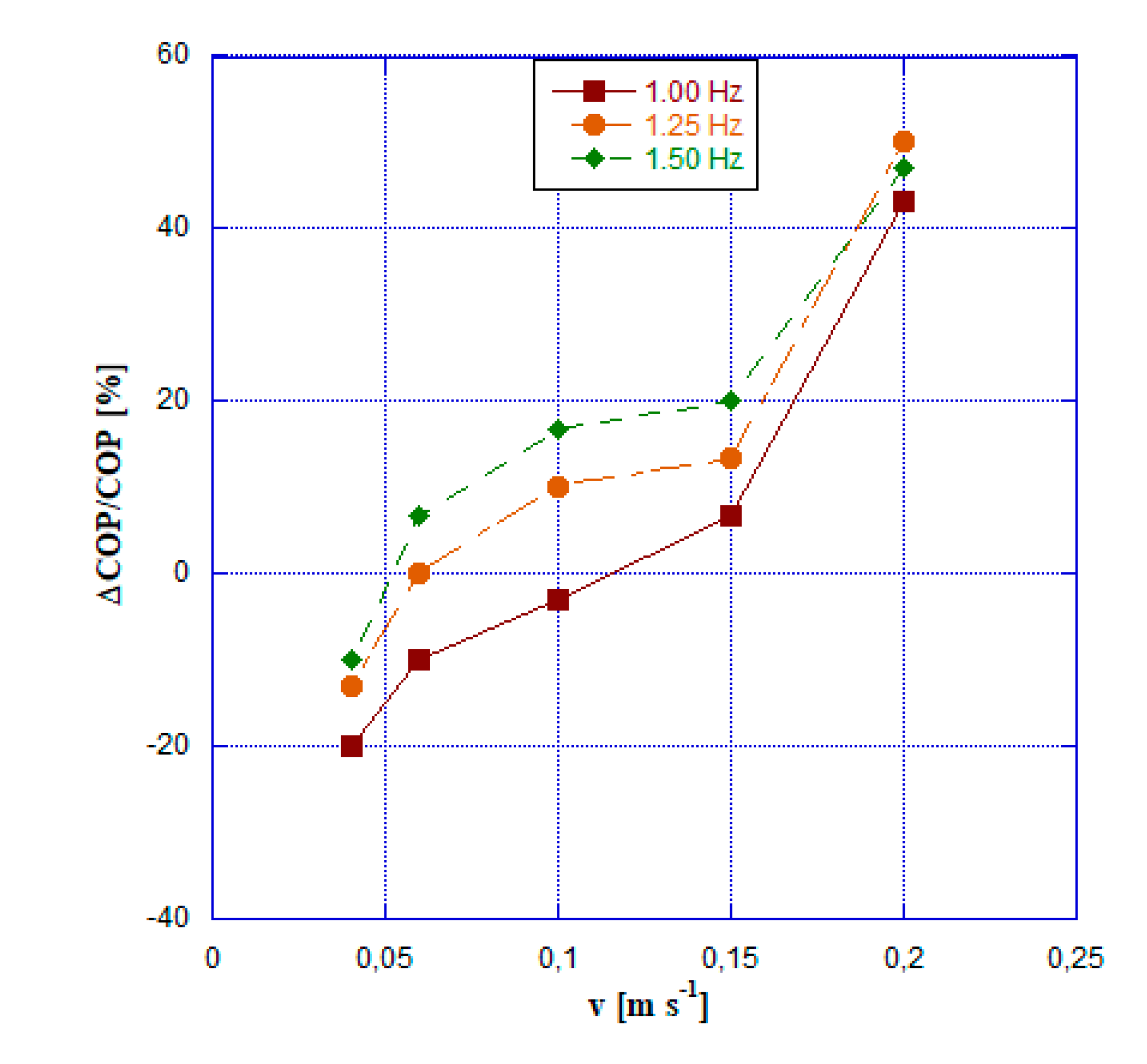
- Velocity values of the HTF equal to or greater than 0.15 m s-1 always ensure an energy saving if the domestic cooler is based on ABR cycle.
Acknowledgment
References
- Benhadid-Dib, S., & Benzaoui, A. (2012). Refrigerants and their environmental impact Substitution of hydro chlorofluorocarbon HCFC and HFC hydro fluorocarbon. Search for an adequate refrigerant. Energy Procedia, 18, 807-816.
- Aprea, C., & Greco, A. (2002). An exergetic analysis of R22 substitution. Applied Thermal Engineering, 22(13), 1455-1469.
- Aprea, C., Greco, A., & Rosato, A. (2008). Comparison of R407C and R417A heat transfer coefficients and pressure drops during flow boiling in a horizontal smooth tube. Energy Conversion and Management, 49(6), 1629-1636.
- Llopis, R. , Torrella, E., Cabello, R., & Sánchez, D. (2012). HCFC-22 replacement with drop-in and retrofit HFC refrigerants in a two-stage refrigeration plant for low temperature. International journal of refrigeration, 35(4), 810-816.
- Montreal Protocol on substances that deplete the ozone layer. United Nation Environment Program (UN), New York (NY), USA, 1987.
- James, S. J. , & James, C. J. F. R. I. (2010). The food cold-chain and climate change. Food Research International, 43(7), 1944-1956.
- Mercier, S. , Villeneuve, S., Mondor, M., & Uysal, I. (2017). Time–temperature management along the food cold chain: A review of recent developments. Comprehensive reviews in food science and food safety, 16(4), 647-667.
- Audsley, E. , Brander, M., Chatterton, J. C., Murphy-Bokern, D., Webster, C., & Williams, A. G. (2010). How low can we go? An assessment of greenhouse gas emissions from the UK food system and the scope reduction by 2050. Report for the WWF and Food Climate Research Network.
- Garnett, T. (2011). Where are the best opportunities for reducing greenhouse gas emissions in the food system (including the food chain)?. Food policy, 36, S23-S32.
- Oenema, O., Velthof, G., & Kuikman, P. (2001). Technical and policy aspects of strategies to decrease greenhouse gas emissions from agriculture. Nutrient Cycling in Agroecosystems, 60, 301-315.
- Camanzi, L., Alikadic, A., Compagnoni, L., & Merloni, E. (2017). The impact of greenhouse gas emissions in the EU food chain: A quantitative and economic assessment using an environmentally extended input-output approach. Journal of Cleaner Production, 157, 168-176.
- F. Cascetta, R. F. Cascetta, R. Di Lorenzo, S. Nardini, and L. Cirillo, "A Trnsys Simulation of a Solar-Driven Air Refrigerating System for a Low-Temperature Room of an Agro-Industry site in the Southern part of Italy," in Energy Procedia, Elsevier Ltd, Sep. 2017, pp. 329–336. [CrossRef]
- C. Aprea, F. C. Aprea, F. de Rossi, A. Greco, and C. Renno, "Refrigeration plant exergetic analysis varying the compressor ca-pacity," Int J Energy Res, vol. 27, no. 7, pp. 653–669, Jun. 2003. [CrossRef]
- "REGULATION (EC) No 852/2004 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL," 2004.
- Y. Zhang, Y. Y. Zhang, Y. Xu, R. Lu, S. Zhang, A. M. Hai, and B. Tang, "Form-stable cold storage phase change materials with durable cold insulation for cold chain logistics of food," Postharvest Biol Technol, vol. 203, p. 112409, Sep. 2023. [CrossRef]
- M. Bozorgi, P. M. Bozorgi, P. Roy, A. R. M. Siddique, K. Venkateshwar, S. Tasnim, and S. Mahmud, "Experimental investigation and life cycle assessment of a phase change material (PCM) based thermoelectric (TE) refrigerator," International Journal of Thermofluids, vol. 19, p. 100394, Aug. 2023. [CrossRef]
- Aprea, C., Greco, A., & Vanoli, G. P. (2003). Condensation heat transfer coefficients for R22 and R407C in gravity driven flow regime within a smooth horizontal tube. International journal of refrigeration, 26(4), 393-401.
- Aprea, C., De Rossi, F., & Greco, A. (2000). Experimental evaluation of R22 and R407C evaporative heat transfer coefficients in a vapour compression plant. International Journal of Refrigeration, 23(5), 366-377.
- Greco, A., Gundabattini, E., Gnanaraj, D. S., & Masselli, C. (2020). A comparative study on the performances of flat plate and evacuated tube collectors deployable in domestic solar water heating systems in different climate areas. Climate, 8(6), 78.
- D’Agostino, D., Esposito, F., Greco, A., Masselli, C., & Minichiello, F. (2020). Parametric analysis on an earth-to-air heat exchanger employed in an air conditioning system. Energies, 13(11), 292.
- D’Agostino, D., Esposito, F., Greco, A., Masselli, C., & Minichiello, F. (2020). The energy performances of a ground-to-air heat exchanger: A comparison among köppen climatic areas. Energies, 13(11), 2895.
- Greco, A. , & Masselli, C. (2020). The optimization of the thermal performances of an earth to air heat exchanger for an air conditioning system: A numerical study. Energies, 13(23), 6414.
- Cirillo, L., Greco, A., & Masselli, C. (2023). Computational investigation on daily, monthly and seasonal energy performances and economic impact through a detailed 2D FEM model of an earth to air heat exchanger coupled with an air conditioning system in a continental climate zone. Energy and Buildings, 113365.
- Fähler, S. , Rößler, U. K., Kastner, O., Eckert, J., Eggeler, G., Emmerich, H.,... & Albe, K. (2012). Caloric effects in ferroic materials: new concepts for cooling. Advanced Engineering Materials, 14(1-2), 10-19.
- P. Kabirifar, A. P. Kabirifar, A. Žerovnik, Ž. Ahčin, L. Porenta, M. Brojan, and J. Tušek, “Elastocaloric cooling: State-of-the-art and future challenges in designing regenerative elastocaloric devices,” Strojniski Vestnik/Journal of Mechanical Engineering, vol. 65, no. 11–12. Assoc. of Mechanical Eng. and Technicians of Slovenia, pp. 615–630, 2019. [CrossRef]
- Aprea, C., Greco, A., Maiorino, A., & Masselli, C. (2020). The employment of caloric-effect materials for solid-state heat pumping. International Journal of Refrigeration, 109, 1-11.
- Aprea, C., Greco, A., & Maiorino, A. (2012). Modelling an active magnetic refrigeration system: A comparison with different models of incompressible flow through a packed bed. Applied Thermal Engineering, 36, 296-306.
- D. J. Silva, J. Ventura, and J. P. Araújo, "Caloric devices: A review on numerical modeling and optimization strat-egies," International Journal of Energy Research, vol. 45, no. 13. John Wiley and Sons Ltd, pp. 18498–18539, Oct. 25, 2021. [CrossRef]
- Takeuchi, I., & Sandeman, K. (2015). Solid-state cooling with caloric materials. Physics today, 68(12), 48-54.
- L. Cirillo, A. Greco, C. Masselli, and S. Qian, “The Italian elastocaloric rotary air conditioner: Numerical modelling for optimal design and enhanced energy performances,” Thermal Science and Engineering Progress, vol. 37, Jan. 2023. [CrossRef]
- Fähler, S., & Pecharsky, V. K. (2018). Caloric effects in ferroic materials. MRS Bulletin, 43(4), 264-268.
- Kitanovski, U. Plaznik, U. Tomc, and A. Poredoš, "Present and future caloric refrigeration and heat-pump technologies," International Journal of Refrigeration, vol. 57. Elsevier Ltd, pp. 288–298, Oct. 01, 2015. [CrossRef]
- Moya, X., & Mathur, N. D. (2020). Caloric materials for cooling and heating. Science, 370(6518), 797-803.
- C. Aprea, A. C. Aprea, A. Greco, A. Maiorino, and C. Masselli, "Analyzing the energetic performances of AMR regenerator working with different magnetocaloric materials: Investigations and viewpoints," International Journal of Heat and Technology, vol. 35, no. Special Issue 1, pp. S383–S390, Sep. 2017. [CrossRef]
- Greco, A. , & Masselli, C. (2020). Electrocaloric cooling: A review of the thermodynamic cycles, materials, models, and devices. Magnetochemistry, 6(4), 67.
- Q. Li, J. Shi, D. Han, F. Du, J. Chen, and X. Qian, "Concept design and numerical evaluation of a highly efficient rotary electrocaloric refrigeration device," Appl Therm Eng, vol. 190, May 2021. 20 May. [CrossRef]
- K. Engelbrecht, "Future prospects for elastocaloric devices," JPhys Energy, vol. 1, no. 2. IOP Publishing Ltd, Jan. 01, 2019. [CrossRef]
- Cirillo, L., Farina, A. R., Greco, A., & Masselli, C. (2022). The optimization of the energy performances of a single bunch of elastocaloric elements to be employed in an experimental device. Thermal Science and Engineering Progress, 27, 101152.
- Chen, J., Lei, L., & Fang, G. (2021). Elastocaloric cooling of shape memory alloys: A review. Materials Today Communications, 28, 102706.
- Masselli, C. , Cirillo, L., & Greco, A. (2023). Cooling of electronic circuits through elastocaloric solid-state technology: A numerical analysis for the development of the CHECK TEMPERATURE prototype. Applied Thermal Engineering, 230, 120729.
- Lloveras, P. , & Tamarit, J. L. (2021). Advances and obstacles in pressure-driven solid-state cooling: A review of barocaloric materials. MRS Energy & Sustainability, 8, 3-15.
- J. Chen, K. J. Chen, K. Zhang, Q. Kan, H. Yin, and Q. Sun, “Ul-tra-high fatigue life of NiTi cylinders for compression-based elastocaloric cooling,” Appl Phys Lett, vol. 115, no. 9, Aug. 2019. [CrossRef]
- J. Tušek, K. J. Tušek, K. Engelbrecht, L. P. Mikkelsen, and N. Pryds, “Elastocaloric effect of Ni-Ti wire for application in a cooling device,” J Appl Phys, vol. 117, no. 12, Mar. 2015. [CrossRef]
- R. Wang et al., “Torsional refrigeration by twisted, coiled, and supercoiled fibers.” [Online]. Available online: http://science.sciencemag.
- L. Cirillo, A. L. Cirillo, A. Greco, and C. Masselli, “A numerical comparison among different solutions for the design of a ro-tary elastocaloric prototype,” Appl Therm Eng, vol. 228, p. 120487, Jun. 2023. [CrossRef]
- L. Cirillo, A. R. L. Cirillo, A. R. Farina, A. Greco, and C. Masselli, “Numerical optimization of a single bunch of niti wires to be placed in an elastocaloric experimental device: preliminary results,” Magnetochemistry, vol. 7, no. 5, 2021. [CrossRef]
- L. Cirillo, A. L. Cirillo, A. Greco, and C. Masselli, “Development of an electronic circuit cooling system using elastocaloric ef-fect: a FEM comparison among different configurations,” Appl Therm Eng, vol. 219, Jan. 2023. [CrossRef]
- L. Cirillo, A. L. Cirillo, A. Greco, and C. Masselli, "Cooling through barocaloric effect: A review of the state of the art up to 2022," Thermal Science and Engineering Progress, vol. 33. Elsevier Ltd, Aug. 01, 2022. [CrossRef]
- C. Aprea, A. C. Aprea, A. Greco, A. Maiorino, and C. Masselli, "The use of barocaloric effect for energy saving in a domestic refrigerator with ethylene-glycol based nanofluids: A numerical analysis and a comparison with a vapor com-pression cooler," Energy, vol. 190, Jan. 2020. [CrossRef]
- C. Aprea, A. C. Aprea, A. Greco, A. Maiorino, and C. Masselli, "A comparison between rare earth and transition metals work-ing as magnetic materials in an AMR refrigerator in the room temperature range," Appl Therm Eng, vol. 91, pp. 767–777, Dec. 2015. [CrossRef]
- X. Moya, E. X. Moya, E. Defay, V. Heine, and N. D. Mathur, "Too cool to work," Nature Physics, vol. 11, no. 3. Nature Publish-ing Group, pp. 202–205, Mar. 06, 2015. [CrossRef]
- X. Moya, S. X. Moya, S. Kar-Narayan, and N. D. Mathur, "Caloric materials near ferroic phase transitions," Nature Materials, vol. 13, no. 5. Nature Publishing Group, pp. 439–450, 2014. [CrossRef]
- Czernuszewicz, J. Kaleta, D. Lewandowski, and M. Przybylski, "An idea of the test stand for studies of mag-netobarocaloric materials properties and possibilities of their application," Physica Status Solidi (C) Current Topics in Solid State Physics, vol. 11, no. 5–6, pp. 995–999, 2014. [CrossRef]
- Czernuszewicz, J. Kaleta, M. Królewicz, D. Lewandowski, R. Mech, and P. Wiewiórski, "A test stand to study the possibility of using magnetocaloric materials for refrigerators," International Journal of Refrigeration, vol. 37, no. 1, pp. 72–77, Jan. 2014. [CrossRef]
- Aprea, A. Greco, A. Maiorino, and C. Masselli, "Solid-state refrigeration: A comparison of the energy perfor-mances of caloric materials operating in an active caloric regenerator," Energy, vol. 165, pp. 439–455, Dec. 2018. [CrossRef]
- W. Imamura, É. O. Usuda, L. S. Paixão, N. M. Bom, A. M. Gomes, and A. M. G. Carvalho, "Supergiant Barocaloric Effects in Acetoxy Silicone Rubber over a Wide Temperature Range: Great Potential for Solid-state Cooling," Chi-nese Journal of Polymer Science (English Edition), vol. 38, no. 9, pp. 999–1005, Sep. 2020. [CrossRef]
- DECRETO LEGISLATIVO 27 gennaio 1992, n. 110. Attuazione della direttiva n. 89/108/CEE in materia di alimenti surgelati destinati all'alimentazione umana. Decreto del Presidente della Repubblica.
- G. Sebald, Z. G. Sebald, Z. Xie, and D. Guyomar, "Fatigue effect of elastocaloric properties in natural rubber," Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, vol. 374, no. 2074, Aug. 2016. [CrossRef]
- S. Woo, W. D. S. Woo, W. D. Kim, S. H. Lee, B. I. Choi, and H. S. Park, "Fatigue life prediction of vulcanized natural rubber subjected to heat-aging," Procedia Eng, vol. 1, no. 1, pp. 9–12, Jul. 2009. [CrossRef]
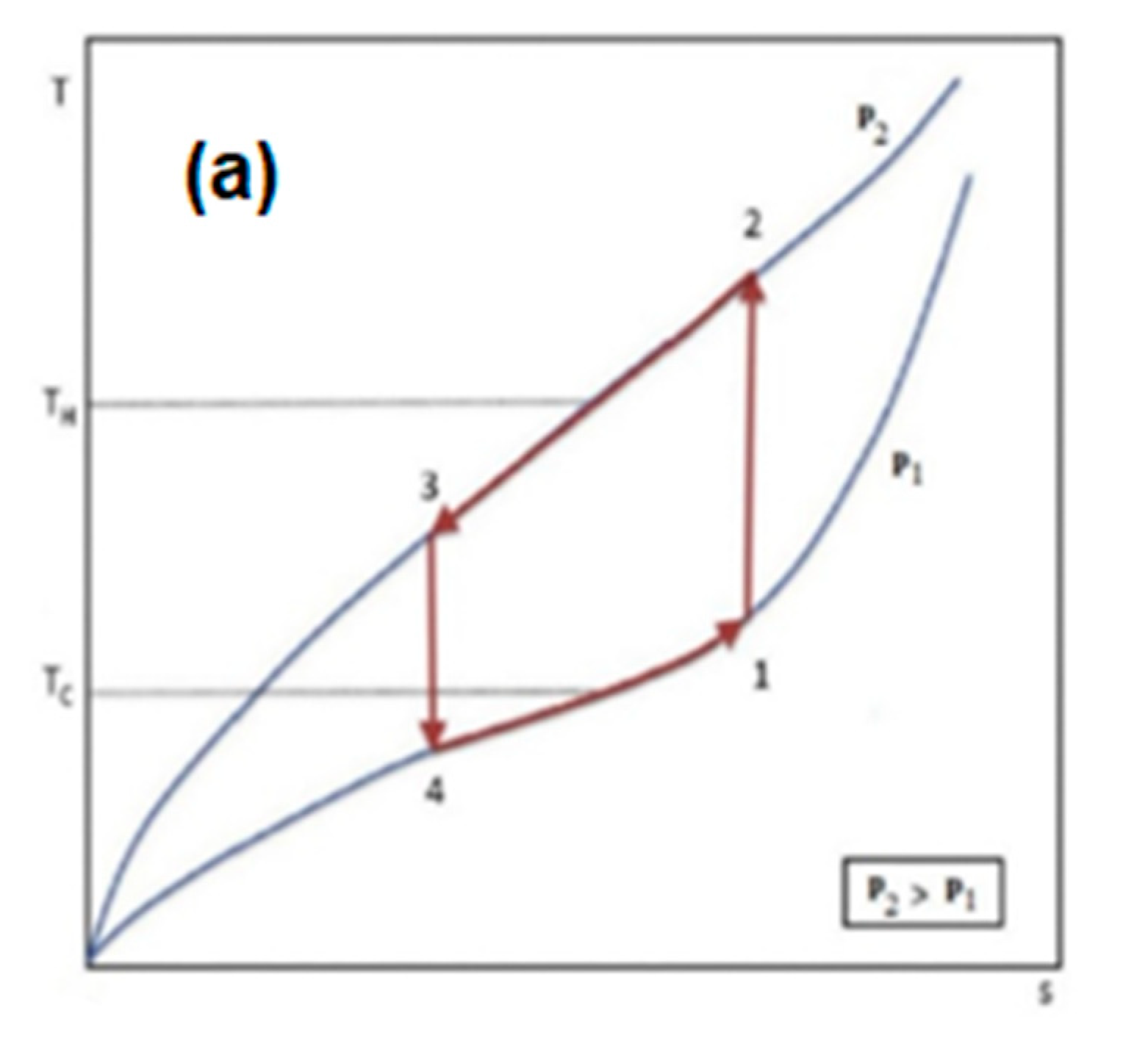
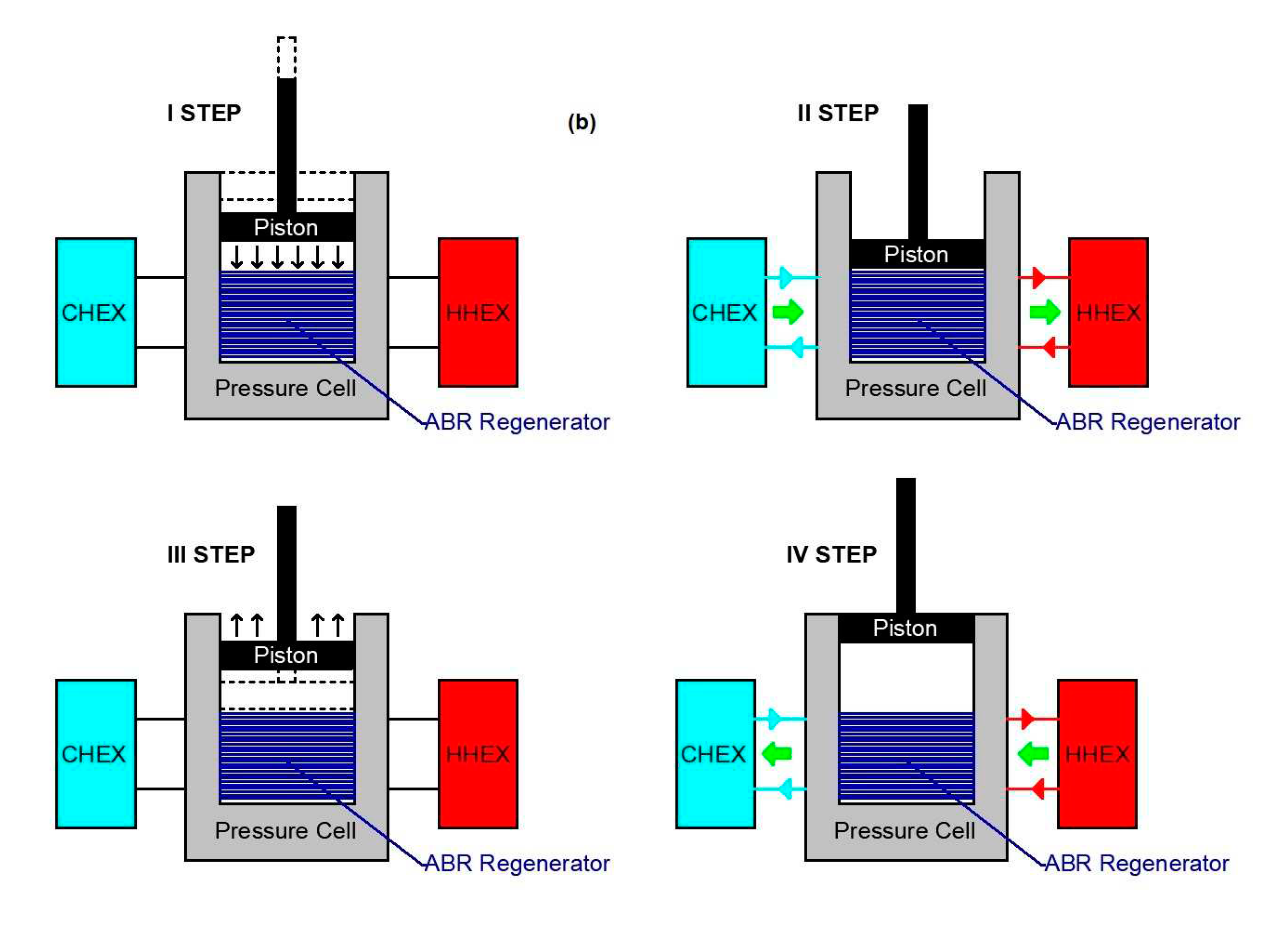
| Material | Δp [GPa] | Mathematical expression |
|---|---|---|
| ASR | 0.390 | |
| Parameter | Unit | Value |
|---|---|---|
| Tset point | K | 278 – 313 |
| f | Hz | 1.25 |
| ∆p | GPa | 0.390 |
| vHTF | m s-1 | 0.04 – 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





